Abstract
We analyzed the impact of axonopathy-inducing agents 1,2-diacetylbenzene (1,2-DAB) and 2,5-hexanedione (2,5-HD) on membrane-bound protein disulfide isomerase (mPDI) vs. soluble PDI (sPDI), or PDI-family member thioredoxin (THX), and asked whether changes in PDI/THX were associated with production of oxidative/nitrosative species in the Sprague-Dawley rat. We show that 1,2-DAB and 2,5-HD lower the abundance of sPDI and THX. However, the protein expression of mPDI is increased in 1,2-DAB axonopathy and neuroproteins became more S-nitrosylated. The abundance of heme oxygenase-1 (HO-1) and isoforms of nitric oxide synthase (neuronal, endothelial, and inducible NOS) remained unchanged suggesting that S-nitrosylation occured via increased mPDI-transnitrosylation and/or diminished THX-denitrosylation. The transcription of PDI and glucose regulated protein-78 (GRP-78) remained unchanged indicating that post-translational modifications e.g. S-nitrosylation mediate pathogenesis of γ-diketone axonopathy. These findings open opportunities for new therapeutic testing (e.g. supplementation with denitrosylating THX) in γ-diketone-induced axonal disease.
Keywords: 1,2-diacetylbenzene; giant axonopathy; 2,5-hexanedione; protein disulfide isomerase; S-nitrosylation; thioredoxin
1. Introduction
The solvent metabolites 1,2-diacetylbenzene (1,2-DAB) or 2,5-hexanedione (2,5-HD) are γ-diketone-like compounds that react with ε-amino- or thiol-groups of lysine or cysteine moieties, respectively, of (neuro)proteins, and cause proximal (1,2-DAB) or distal (2,5-HD) neurofilament-filled axonal swellings in rodent elongated axons. Axonal atrophy and Wallerian-like degeneration occur distal to the swellings. In contrast, their respective isomers 1,3-DAB and 2,4-HD do not react with (neuro)proteins and do not cause axonopathy (1–5).
We exploit the common protein-reactive properties of 1,2-DAB and 2,5-HD to probe molecular targets and mechanisms associated with axonal disease notably giant neurofilamentous axonopathy, a pathological hallmark of a host of neurodegenerative diseases including amyotrophic lateral sclerosis (6–9). Two-dimensional differential in gel electrophoresis (2D-DIGE) and matrix-assisted laser desorption ionization time-of-flight/ tandem mass spectrometry (MALDI-TOF/MS-MS) studies have revealed that protein disulfide isomerase (PDI), a thiol-dependent protein-folding assistant (10–12), is a common target of 1,2-DAB and 2,5-HD (13, 14). In this study, we analyzed the impact of these axonopathy-inducing agents on membrane-bound PDI (mPDI) vs. soluble PDI (sPDI) or thioredoxin (THX), an enzyme that shares thiol-dependent conserved active domains with PDI-family members. In addition, we asked whether changes in PDI-family members were accompanied with overproduction of oxidative or nitrosative species, a phenomenon that is often associated with changes in PDI expression, protein misfolding, and dysfunction of the endoplasmic reticulum (ER) in certain neurodegenerative conditions (10, 12, 15–18).
We showed that 1,2-DAB and 2,5-HD lowered the protein expression of sPDI and THX in the Sprague-Dawley rat. However, they differentially altered the abundance of sPDI vs. mPDI. The protein expression of mPDI was increased following treatment with 1,2-DAB while spinal cord proteins became highly S-nitrosylated. Interestingly, the protein expression levels of heme oxygenase-1 (HO-1) and isoforms of nitric oxide synthase (NOS-1, −2, or −3) or the transcription of PDI and glucose-regulated protein 78 (GRP-78) remained unchanged suggesting no ER stress (15, 16). These findings indicate that protein S-nitrosylation associated with 1,2-DAB axonopathy results from an increase in mPDI-mediated transnitrosylation and/or a reduction in THX-denitrosylation (12, 19–22).
2. Methods
2.1. Treatment of animals
Male Sprague-Dawley rats (Charles Rivers, CA) weighing 175–225 g upon arrival were individually housed, fed a rodent chow (PMI® Nutrition International, NJ), and given water ad libitum. The animals were acclimated for 5 days in a room maintained at 20°C on a 12-h/12-h light-dark cycle prior to treatment with 1,2-DAB (99 % purity) or 2,5-HD ( 99 % purity), or their respective non-neurotoxic isomers 1,3-DAB ( 97 % purity) or 2,3-HD ( 96.5 % purity) purchased from Sigma Aldrich (Madison, WI), or vehicle (saline containing 2% acetone or 50% dimethyl sulfoxide to dissolve DAB isomers or HD isomers, respectively).
Rats (n = 5 per treatment group) were treated intraperitoneally with 20 mg/kg/day DAB isomers, or equipotent doses of HD isomers i.e. 500 mg/kg/day, or equivalent volume of vehicle, 5 days a week for up to 3 weeks. At study termination, rats ( n = 4 per treatment group) were anesthetized with 4% isofluorane (0.7 l oxygen/min), their blood collected via cardiac puncture, and decapitated prior to removal of the spinal cord as previously described (13). The spinal cord was flash frozen in liquid nitrogen and stored at −80°C prior to transcriptional and western blot studies. The remaining animals were subjected to intracardiac perfusion with cold 4% paraformaldehyde followed by 5% glutaraldehyde, each in 0.2 M sodium cacodylate buffer (pH 7.4). The spinal cords and sciatic nerves of these animals were sampled and post-fixed in excess cacodylate-buffered 1% osmium tetroxide, dehydrated, and embedded in epoxy resin. Cross sections (~900 nm) of tissues were stained with 1% toluidine blue and screened by bright-field microscopy. Thin sections (~90 nm) of regions of interest were stained with 2% uranyl acetate followed by 1% lead citrate for examination by transmission electron microscopy. Treatment and handling of rats were conducted in accordance with institutional (OHSU) guidelines for care and use of laboratory animals.
2.2. Protein immunoblots
Tissues were homogenized in ice-cold buffer (50 mM Tris-HCl, 1 mM EDTA, pH 7.4) containing a protease inhibitor mixture (Sigma Aldrich, St-Louis, MO). After centrifugation at 12,000 g for 45 min, cytosolic or membrane proteins were solubilized in an equal volume of 2x Laemmli buffer (50 mM Tris-HCl, pH6.8, 10% glycerol, 2% SDS, 10% dithiotreitol, 0.1% bromophenol blue) and boiled for 10 min. Thereafter, proteins were resolved by 8–12% denaturing SDS-polyacrylamide gels and transferred overnight to PVDF membranes (Bio-Rad Laboratories, Hercules, CA). Loading variations were ruled out after Ponceau staining. The filters were treated for 1 h with Tris-buffered saline (TBS) containing 5% dry milk and 0.1% Tween 20 to block non-specific binding, then incubated for 1 h with either a rabbit anti-PDI polyclonal antibody (1/1,000) (Stressgen, Ann Arbor, MI), or a rabbit anti-THX (1/1000) (Stressgen, MI), or a rabbit anti-heme-oxygenase-1 (HO-1, 1/500) (Stressgen, Ann Arbor, MI), or mouse anti-nitric oxide synthase (NOS-1, or −2, or −3, 1/1000) (BD Biosciences, San Diego, CA), or a rabbit anti-nitrosocysteine (1/500) (Sigma Aldrich, St-Louis, MO). The blots were subsequently probed with a goat anti-rabbit or anti-mouse horseradish peroxidase-conjugated antibody (Perkin-Elmer, Waltham, MA) diluted 1/10,000 in the same buffer. After extensive washing with TBS, peroxidase activity was detected by an enhanced chemiluminescence (ECL) detection system (GE Healthcare Life Sciences, Piscataway, NJ). The expression levels of proteins were quantified using an MCID-M1 imaging system (Imaging Research, ON, Canada).
2.3. Transcriptional studies
Gene expression for GRP-78, PDI, and β-actin (internal standard) in 1,2-DAB axonopathy, featured by intraspinal (neuronal) accumulation of proteinaceous materials, was investigated by one-step semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR). β-Actin was used as an internal standard to monitor loading variations. Total ribonucleic acid (RNA) was extracted from the lumbosacral spinal cord using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. RNA samples were resuspended in diethylpyrocarbonate-treated water, and then kept at –70°C until use. Total RNA (1 µg) was mixed with 10 mM Tris-HCl (pH 8.3), 1.5mM MgCl2, 50 mM KCl, 0.01% (w/v) bovine serum albumin, 200µM dNTPs, primers at 1 µM each, AMV reverse transcriptase (80 U/ml), Taq DNA polymerase (20 U/ml) and 50 µCi/ml [α 32P]dCTP (3000 Ci/mmol), for a total reaction volume of 50µL. The reactions were initially carried out at 50°C for 20 min followed by PCR at 95°C for 30 sec, 60°C for 45 sec and 72°C for 1 min. Amplification efficiency conditions were determined after a kinetic study to ensure that all experiments were performed in the exponential phase of amplification where PCR products remain proportional to initial template concentration. β-Actin, PDI, and GRP-78 were amplified for 18, 25, 23 cycles, respectively. After amplification, samples were electrophoresed onto 9% polyacrylamide gels, dried, and autoradiographed at –70°C with an intensifying screen. Each band was excised and Cerenkov radiation was quantified using a β-counter.
The respective forward and reverse oligonucleotide primer sequences were as follows: 5’-CCTCCCTGGAGAAGAGCTA-3’ and 5’-ACTCCTGCTTGCTGATCCAC-3’ (β-actin, 384bp), 5’-GCAAAACTGAAGGCAGAAGG-3’ and 5’-GAGTCCACCAAGGACTCT GC-3’ (PDI, 254bp), 5’-AGTGGTGGCCACTAATGGAG-3’ and 5’-CTTCAAATTTG GCCCGAGTA-3’ (GRP-78, 257bp). The specificity of the oligonucleotide primers was verified using the program BLASTN (National Center for Biotechnology Information, Bethesda, MD).
3. Results
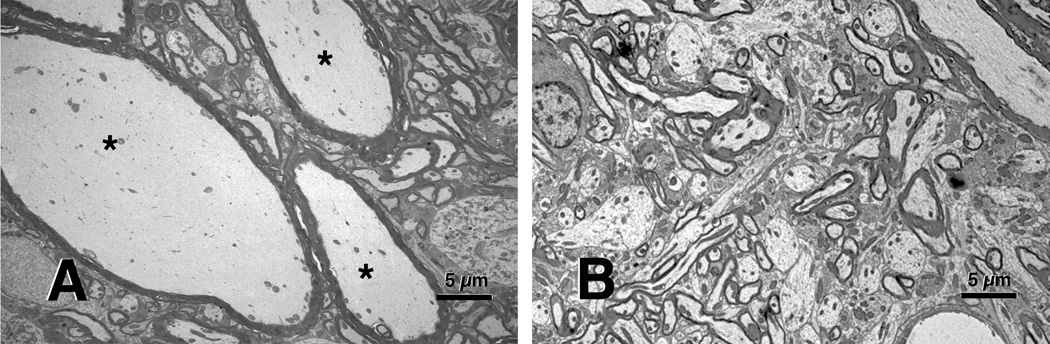
Rats treated with 1,2-DAB or 2,5-HD, but not with their respective non-axonopathic isomers or vehicle, showed a comparable degree of walking difficulty and severe neuromuscular weakness by the end of the third week of treatment. Microscopic examination of the nervous system tissues from impaired animals revealed clustering of microtubules and organelles (1,2-DAB and 2,5-HD), clumping of neurofilaments (NF) (1,2-DAB and 2,5-HD), and intraspinal 10nm-neurofilament-filled axonal swellings (1,2-DAB) (Figure 1).
Figure 1.
(A) Evidence of multiple swollen axons (*) in the lumbar anterior horn of an animal treated with 1,2-DAB. (B) Absence of similar neuropathology in a spinal cord from a rat treated with 2,5-HD. Staining made with 2% uranyl acetate followed by 1% lead citrate.
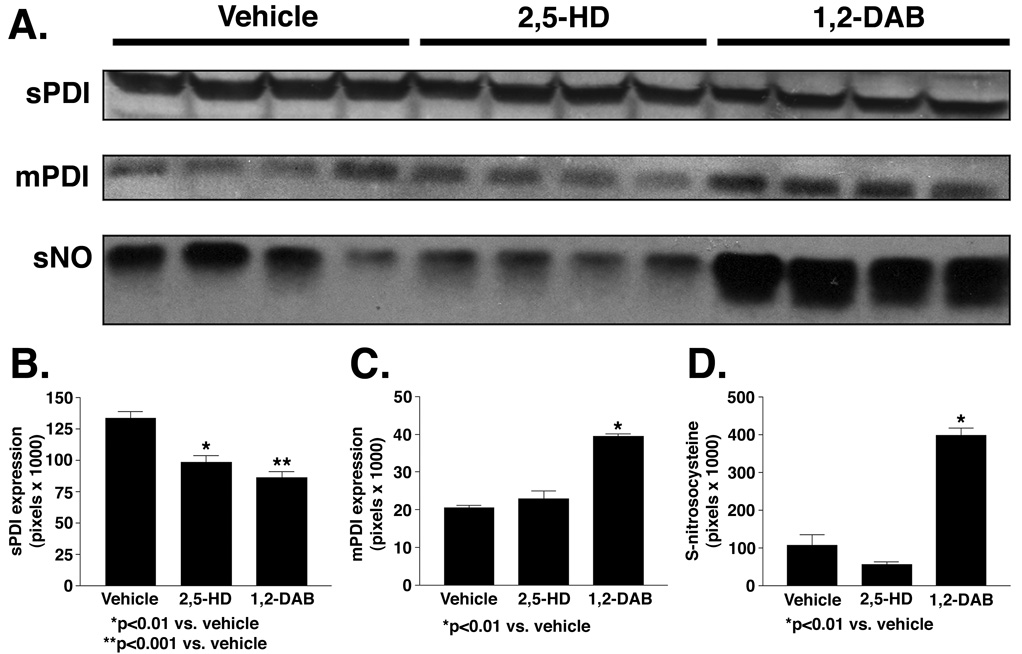
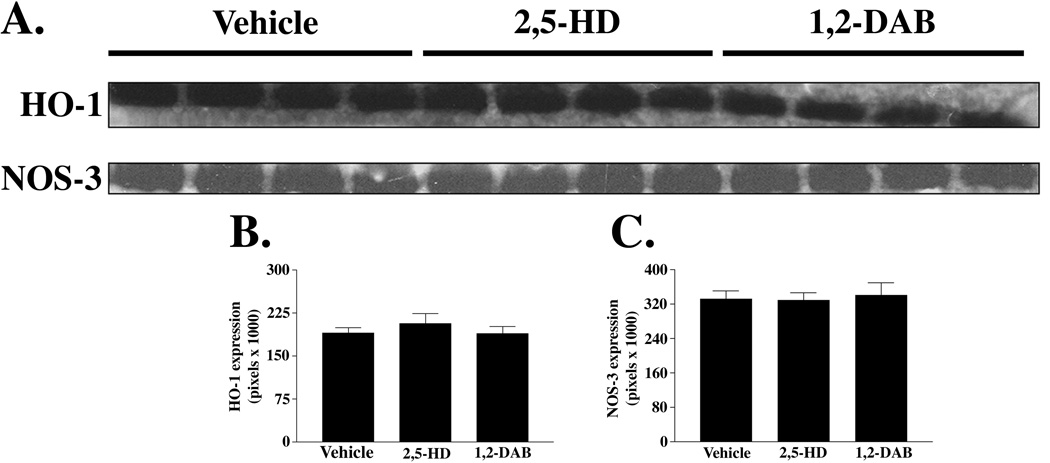
1,2-DAB and 2,5-HD significantly lowered the abundance of sPDI and THX. However, the expression of mPDI and that of S-nitrosylated (neuro)proteins were increased in lumbosacral tissues from animals treated with 1,2-DAB (Figure 2). S-nitrosylated proteins were revealed on three gel-bands with molecular weights ranging from 38 to 82 kDa. Protein expression of HO-1 and NOS-1, −2, or −3 (Figure 3) and mRNA levels of PDI or GRP-78 (not shown) remained unchanged.
Figure 2.
(A) Protein immunoblots (n = 4 per treatment group) of sPDI (58kDa), mPDI (58kDa), and S-nitrosylated proteins (~ 60 kDa) in animals treated with vehicle, 2,5-HD, or 1,2-DAB. (B) 2,5-HD and 1,2-DAB lower sPDI (25.9%, p<0.01 and 32.8%, p<0.001, respectively, relative to vehicle). (C) mPDI increased in 1,2-DAB-treated animals (92.5% relative to vehicle, p<0.01). (D) S-nitrosylated proteins significantly occur in 1,2-DAB- relative to 2,5-HD-treated animals (5.1-fold, p<0.001) or vehicle-treated animals (3.7-fold, p<0.001). Data analyzed by ANOVA followed by Tukey’s post hoc analysis. Results are expressed as mean ± standard error of the mean.
Figure 3.
(A) Protein immunoblots (n = 4 per treatment group) of HO-1 (32kDa) and representative NOS (endothelial isoform, 140kDa) in rats treated with vehicle, 2,5-HD, or 1,2-DAB. Treatment with 2,5-HD or 1,2-DAB induced no significant changes in the expression of HO-1 (p=0.6187) or NOS isoforms (e.g. NOS-3, p=0.9395) as shown in (B) and (C), respectively.
4. Discussion
We report, for the first time, a differential modulation of PDI-family members and protein S-nitrosylation in the Sprague-Dawley rat treated with γ-diketone-like 1,2-DAB or 2,5-HD. Changes in both sPDI and THX confirm that thiol-dependent PDI-family members are important players in the pathogenesis of γ-diketone axonopathy (5, 13, 14, 23). The differential pattern of mPDI expression and/or S-nitrosylation in 1,2-DAB- vs. 2,5-HD-axonopathy possibly reflect the presence (1,2-DAB) vs. the absence (2,5-HD) of proteinaceous (neurofilamentous) accumulations in the spinal cord, our tissue of interest in this study. Increase in mPDI may also result from a translocation of sPDI into membranes as part of defense mechanisms. Increased S-nitrosylation with no increase in the markers of oxidative and nitrosative stress i.e. HO-1 and NOS isoforms (1, 2, or 3), respectively, and increase in the expression mPDI concomitantly with decrease in THX, indicate that S-nitrosylation occurs via mPDI-mediated transnitrosylation and/or diminished THX-denitrosylation (12, 19–22). S-nitrosylation of selected immunoblot bands in 1,2-DAB axonopathy warrants further studies to identify those neuroproteins that became highly S-nitrosylated and explore the role of nitric oxide signaling pathways in relation to nerve fiber (axon) degeneration (15, 16).
Normal translational levels of PDI and GRP-78 and unchanged protein levels of HO-1 and NOS isoforms, suggest that S-nitrosylation does not originate from a stress of the endoplasmic reticulum (ER) (15–17). This proposal requires, however, caution for its interpretation because prolonged depletion of sPDI and/or THX may conceivably lead to decrease in protein folding capabilities within the nervous system and/or the accumulation of misfolded proteins with subsequent ER stress (15, 24–26).
In sum, while common targets of 1,2-DAB and 2,5-HD may serve as biomarkers of exposure to neurotoxic γ-diketones and hence, their respective parent neurotoxic solvents 1,2-diethylbenzene and n-hexane, this study indicates that 1,2-DAB and 2,5-HD have their mechanisms of action mediated by their differential ability to alter the abundance of PDI-family members and trigger pathologic S-nitrosylation of neuroproteins. Previous studies have demonstrated consistent neuropathological changes across species notably in the Sprague-Dawley rat and the C57BL/6 mouse (3, 4). Whether the two aforementioned species will display similar neurochemical changes upon treatment with γ-diketones have yet to be determined. Nevertheless, our findings open opportunities for testing new therapeutic means (e.g. supplementation with denitrosylating THX) in the axonal disease induced by γ-diketone-like 1,2-DAB or 2,5-HD. The two axonopathic compounds appear to be excellent tools that can be used to probe molecular mechanisms of nerve fiber (axon) degeneration.
Acknowledgements
The technical expertise of Victor Monterroso (Department of Comparative Medicine, OHSU, Portland OR) is appreciated.
Funding: National Institute of Health K01NS052183 and the Oregon Worker Benefit Fund.
References
- 1.Kim MS, Hashemi SB, Spencer PS, Sabri MI. Amino acid and protein targets of 1,2-diacetylbenzene, a potent aromatic γ-diketone that induces proximal neurofilamentous axonopathy. Toxicol Appl Pharmacol. 2002;183:55–65. doi: 10.1006/taap.2002.9456. [DOI] [PubMed] [Google Scholar]
- 2.LoPachin RM, Jortner BS, Reid ML, Das S. γ-diketone central neuropathy: quantitative morphometric analysis of axons in rat spinal cord white matter regions and nerve roots. Toxicol Appl Pharmacol. 2003;193:29–46. doi: 10.1016/j.taap.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Spencer PS, Kim MS, Sabri MI. Aromatic as well as aliphatic hydrocarbon solvent axonopathy. Int J Hyg Environ Health. 2002;205:131–136. doi: 10.1078/1438-4639-00138. [DOI] [PubMed] [Google Scholar]
- 4.Tshala-Katumbay DD, Palmer VS, Kayton RJ, Sabri MI, Spencer PS. A new murine model of giant proximal axonopathy. Acta Neuropathol. 2005;109:405–410. doi: 10.1007/s00401-005-0982-z. [DOI] [PubMed] [Google Scholar]
- 5.LoPachin RM, Barber DS, Gavin T. Molecular mechanisms of the conjugated α,β-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol Sci. 2008;104:235–249. doi: 10.1093/toxsci/kfm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Chalabi A, Miller CC. Neurofilaments and neurological disease. Bioessays. 2003;25:346–355. doi: 10.1002/bies.10251. [DOI] [PubMed] [Google Scholar]
- 7.Delisle MB, Carpenter S. Neurofibrillary axonal swellings and amyotrophic lateral sclerosis. J Neurol Sci. 1984;63:241–250. doi: 10.1016/0022-510x(84)90199-0. [DOI] [PubMed] [Google Scholar]
- 8.Hirano A, Donnenfeld H, Sasaki S, Nakano I. Fine structural observations of neurofilamentous changes in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1984;43:461–470. doi: 10.1097/00005072-198409000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto K, Hirai S, Shoji M, Senoh Y, Yamazaki T. Axonal swellings in the corticospinal tracts in amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 1990;80:222–226. doi: 10.1007/BF00308929. [DOI] [PubMed] [Google Scholar]
- 10.Appenzeller-Herzog C, Ellgaard L. The human PDI family: versatility packed into a single fold. Biochim Biophys Acta. 2008;1783:535–548. doi: 10.1016/j.bbamcr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Sevier CS, Kaiser CA. Conservation and diversity of the cellular disulfide bond formation pathways. Antioxid Redox Signal. 2006;8:797–811. doi: 10.1089/ars.2006.8.797. [DOI] [PubMed] [Google Scholar]
- 12.Turano C, Coppari S, Altieri F, Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. J Cell Physiol. 2002;193:154–163. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- 13.Tshala-Katumbay D, Monterroso V, Kayton R, Lasarev M, Sabri M, Spencer P. Probing mechanisms of axonopathy. Part I: Protein targets of 1,2-diacetylbenzene, the neurotoxic metabolite of aromatic solvent 1,2-diethylbenzene. Toxicol Sci. 2008;105:134–141. doi: 10.1093/toxsci/kfn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tshala-Katumbay D, Monterroso V, Kayton R, Lasarev M, Sabri M, Spencer P. Probing mechanisms of axonopathy. Part II: Protein targets of 2,5-hexanedione, the neurotoxic metabolite of the aliphatic solvent n-hexane. Toxicol Sci. 2009;107:482–489. doi: 10.1093/toxsci/kfn241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T, Lipton SA. Emerging roles of S-nitrosylation in protein misfolding and neurodegenerative diseases. Antioxid Redox Signal. 2008;10:87–101. doi: 10.1089/ars.2007.1858. [DOI] [PubMed] [Google Scholar]
- 16.Uehara T. Accumulation of misfolded protein through nitrosative stress linked to neurodegenerative disorders. Antioxid Redox Signal. 2007;9:597–601. doi: 10.1089/ars.2006.1517. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari DM, Soling HD. The protein disulphide-isomerase family: unravelling a string of folds. Biochem J. 1999;339(Pt 1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 19.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramachandran N, Root P, Jiang XM, Hogg PJ, Mutus B. Mechanism of transfer of NO from extracellular S-nitrosothiols into the cytosol by cell-surface protein disulfide isomerase. Proc Natl Acad Sci U S A. 2001;98:9539–9544. doi: 10.1073/pnas.171180998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zai A, Rudd MA, Scribner AW, Loscalzo J. Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide. J Clin Invest. 1999;103:393–399. doi: 10.1172/JCI4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sengupta R, Ryter SW, Zuckerbraun BS, Tzeng E, Billiar TR, Stoyanovsky DA. Thioredoxin catalyzes the denitrosation of low-molecular mass and protein S-nitrosothiols. Biochemistry. 2007;46:8472–8483. doi: 10.1021/bi700449x. [DOI] [PubMed] [Google Scholar]
- 23.Lopachin RM, Barber DS, Geohagen BC, Gavin T, He D, Das S. Structure-toxicity analysis of type-2 alkenes: in vitro neurotoxicity. Toxicol Sci. 2007;95:136–146. doi: 10.1093/toxsci/kfl127. [DOI] [PubMed] [Google Scholar]
- 24.Atkin JD, Farg MA, Turner BJ, Tomas D, Lysaght JA, Nunan J, Rembach A, Nagley P, Beart PM, Cheema SS, Horne MK. Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. J Biol Chem. 2006;281:30152–30165. doi: 10.1074/jbc.M603393200. [DOI] [PubMed] [Google Scholar]
- 25.Atkin JD, Farg MA, Walker AK, McLean C, Tomas D, Horne MK. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis. 2008;30:400–407. doi: 10.1016/j.nbd.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Ilieva EV, Ayala V, Jove M, Dalfo E, Cacabelos D, Povedano M, Bellmunt MJ, Ferrer I, Pamplona R, Portero-Otin M. Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis. Brain. 2007;130:3111–3123. doi: 10.1093/brain/awm190. [DOI] [PubMed] [Google Scholar]