Abstract
It has been shown that lipogenic enzymes, such as fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC), are highly expressed in the rodent brain during the early neonatal period and decline thereafter. However, cellular localization of these enzymes is unknown. Presently, we examined developmental changes in the levels and cellular localization of FAS and ACC, and their putative regulators, sterol-regulatory element-binding protein (SREBP)-1 and AMP-activated protein kinase (AMPK) in the mouse brain. Levels of these proteins including phosphorylated forms of ACC and AMPK decreased between postnatal day 4 (P4) and P19. Immunohistochemical studies indicated that FAS, ACC, AMPK, and SREBP-1 were expressed in neurons at P7, while FAS was found mostly in cells of oligodendrocyte lineage at P19. These studies suggest that neurons in the early neonatal brain are involved in do novo fatty acid synthesis.
Keywords: fatty acid synthase, acetyl-CoA carboxylase, AMP-activated protein kinase, developing brain, neuron, oligodendrocyte
Introduction
Lipogenesis via do novo fatty acid synthesis in the developing brain is considered important during the growth spurt and the onset of myelination because the brain at this period appears to produce all the required saturated and monounsaturated fatty acids by de novo synthesis (1, 2). It has been shown that mRNA expression of lipogenic enzymes, FAS and ACC, in the mouse brain is at a maximum at P5 and declines thereafter (3). The levels and activities of brain ACC (4) and FAS activity (5) also decrease from the early neonatal period to ~4 weeks of age in the newborn rat. However, myelin lipid synthesis, which causes major lipid accumulation in the brain, occurs between P10 and P50 with a peak at P18 (6). Therefore, in the central nervous system (CNS), levels of lipogenic enzymes are not correlated with rates of synthesis of myelin lipids, although levels of enzymes for myelin specific lipids, such as ceramide galactosyltransferase (CGT), are well linked to myelin lipid formation (7). This observation is different from that in the peripheral nervous system (PNS) where expression of FAS is the highest when active myelination occurs (8). It may be because the biosynthetic rate for myelin lipids is much greater in the PNS relative to the CNS (8). Studies on the cellular localization of lipogenic enzymes in the developing brain, which is largely unknown, would help understand developmental profiles of these enzymes.
Lipogenic enzymes, such as FAS and ACC, are known to be regulated by several factors transcriptionally and posttranscriptionally. One of such regulators is AMPK, which is known as an energy sensing protein kinase and a main regulator of glucose and lipid metabolism in peripheral tissues. AMPK activation is associated with phosphorylation of Thr172 on the catalytic α subunit of AMPK (9, 10) by AMPK kinases, such as the tumor suppressor kinase LKB1 and Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) (11). Activated AMPK phosphorylates and in turn inhibits ACC, the rate-limiting enzyme in fatty acid biosynthesis (9). Also, activation of SREBP-1, which induces expression of lipogenic enzymes such as ACC and FAS, can be both transcriptionally and posttranscriptionally regulated by AMPK in hepatic cells (12). AMPK is expressed in the brain at high levels (13). In hypothalamic neurons, ACC activity appears to be controlled by changes in AMPK activity, and ACC catalyzes formation of malonyl-CoA, which serves as the substrate for the FAS-catalyzed fatty acid formation (14). Therefore, it is possible that AMPK regulates lipogenic enzymes not only in the peripheral organs but also in the brain. Previously we have shown that ethanol exposure and nutrient deprivation affect both AMPK activities and triglyceride (TG) formation in P7 mouse brains but not in P19 mouse brains (15). These studies suggest that AMPK may affect brain lipid metabolism through regulation of lipogenic enzymes in the early postnatal period. In the present study, we examined changes in the content and cellular distribution of lipogenic enzymes, FAS and ACC, and their regulators, AMPK and SREBP-1 in the mouse brain between P4 and P19. In addition, changes in phosphorylation levels of AMPK and ACC, which indicate the activation states, were also examined. We found that neurons in the early postnatal period contained high levels of lipogenic enzymes and their regulators, which decreased thereafter.
Experimental Procedure
Animals and treatment
C57BL/6By mice between P4 and P19 were used. These mice were maintained at the Animal Facility of Nathan S. Kline Institute for Psychiatric Research. All procedures followed guidelines consistent with those developed by the National Institute of Health and the Institutional Animal Care and Use Committee of Nathan S. Kline Institute.
Western blot analysis
Western blot analyses were performed as described (15) using forebrain samples from P4, P7, P10, P13, P16, and P19 mice. Briefly, each forebrain was separately homogenized (20% w/v) in ice cold Tris-HCl (20 mM, pH 7.4) buffer containing 1% Triton X100, 0.5% CHAPS, 1mM EGTA, 1 mM EDTA, 20 mM NaF, 1 mM Na-orthovanadate, 20 mM β-glycerophosphate, 5 µM microcystin, and 1µl/ml protease inhibitor cocktail (all from Sigma, St. Louis, MO), and centrifuged at 52K × g for 30 min. Supernatants (50 µg protein) were boiled in SDS-sample buffer, separated on 10% SDS-PAGE, and blotted onto PVDF membranes. The membranes were then blocked with 5% BSA in blocking buffer (0.1% Tween 20 in Tris buffered saline, TBS) and probed with various dilutions of rabbit monoclonal anti-phospho-AMPKα (anti-pAMPKα, Thr172), rabbit monoclonal anti-AMPKα, rabbit polyclonal anti-phospho-ACC (anti-pACC, Ser79), rabbit polyclonal anti-ACC, and rabbit polyclonal anti-FAS antibodies (all from Cell Signaling Technology, Danvers, MA). Rabbit polyclonal anti-SREBP-1 and rabbit polyclonal anti-CaMKKβ antibodies (both from Santa Cruz Biotechnology, Inc. Santa Cruz, CA) were also used. Antigens were detected by the Odyssey infrared imaging system (LI-COR Inc. Nebraska, NE) using a fluorescence-labeled secondary antibody, goat anti-rabbit IgG-680 (Invitrogen, Carlsbad, CA), in blocking buffer. Intensities of desired bands were quantified using the Fujifilm Multi Gauge V2.3 software (Fujifilm USA Medical Systems, Stamford, CT, USA). The amount of protein was measured by a BCA method (Pierce, Rockford, IL).
Immunohistochemistry
Immunohistochemistry was performed as described previously (16). Mice were perfused with a solution containing 4% paraformaldehyde and 4% sucrose in cacodylate buffer (pH 7.2), and the heads were further fixed in the perfusion solution overnight. Brains were removed, transferred to phosphate buffered saline (PBS) solution, and kept at 4°C for 2–5 days until sectioned with a vibratome into 50 µm thick sections. Free-floating sections were rinsed in TBS, quenched for 10 min in methanol containing 3% hydrogen peroxide, and incubated for 1 h in a blocking solution (TBS containing 2% BSA, 0.2% milk, and 0.1% Triton X-100). This was followed by incubation overnight with primary antibodies. Anti-AMPKα, anti-pAMPKα (Thr172), anti-ACC, anti-pACC (Ser79), and anti-FAS antibodies were diluted 1:500 in a blocking solution. After rinsing with TBS, sections were incubated for 1 h with biotinylated goat anti-rabbit IgG diluted at 1: 200 in a blocking solution, rinsed with TBS, and stained with ABC reagents (Vectastain ABC Elite Kit, Vector Labs, Burlingame, CA) and a peroxidase substrate (DAB) kit (Vector Labs) following the manufacturer’s instructions. Blocking peptides for anti-AMPKα, anti-ACC and anti-FAS antibodies (Cell Signaling) and for anti-SREBP-1 antibody (Santa Cruz) were used to confirm the specificity of the antibodies. For double immunofluorescence labeling sections were permeabilized with TBS containing 3% BSA and 0.3% Triton X-100, rinsed with TBS containing 0.1% Triton X-100, and blocked with Mouse Ig Blocking Reagent (Vector) in TBS for 2h. Then sections were incubated with anti-pAMPKα (Thr172) antibody, anti-pACC, or anti-FAS antibody (X500) in TBS containing 3% BSA and 0.1% Triton X-100 overnight at 4° C, rinsed, and incubated with a mouse monoclonal anti-NeuN antibody (MAB 377, Upstate, Temecula, CA) or mouse monoclonal anti-O4 antibody (R&D Systems, Minneapolis, MN) at a dilution of 1:500 in TBS containing 3% BSA and 0.1% Triton X-100 for 2 h. After rinsing, sections were incubated for 1 h with a mixture of Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 594 goat anti-mouse IgG (Invitrogen) in TBS containing 1% BSA and 0.1% Triton X-100 at a dilution of 1:500, rinsed, mounted, and coverslipped using ProLong Gold Antifade Reagent (Invitrogen). All images were obtained through a 4X, 20X or 40X objective with a Nikon Eclipse TE2000 fluorescent microscope attached to a digital camera, DXM1200F.
Lipid Analysis
For lipid analysis, mice were decapitated and each brain was immediately soaked in ice-cold chloroform/methanol (1:1) solution. For the simultaneous separation of phospholipids and cholesterol, the lipid extract was applied to a high-performance thin layer chromatography (HPTLC) plate and developed first in chloroform/methanol/H2O (50:40:10) until the solvent front reached 5 cm from the bottom, then in cyclohexane/diethylether/acetic acid (65:35:1) until the top of the plate (9 cm from the origin), and the plates were stained with a solution consisting of 0.03% Coomassie Brilliant Blue, 20% methanol, and 0.5% acetic acid (17). For analyzing cholesterol and TG, the total lipid fraction extracted with chloroform/methanol (1:1) was applied to a HPTLC plate, and developed first in diethylether/hexane/acetic acid (35:65:2) until the solvent front reached 5 cm from the bottom, then in diethylether/hexane/acetic acid (2:98:1) until the top of the plate (18). The plates were stained with a solution consisting of 0.03% Coomassie Brilliant Blue, 20% methanol, and 0.5% acetic acid. The stained HPTLC plates including standard lipids with several concentrations were scanned by the Odyssey infrared imaging system and analyzed using Multi Gauge ver.2.0.
Statistical Analysis
All data were expressed as mean ± SEM (n = 3 to 6). Comparisons among groups were performed by one-way ANOVA with LSD post hoc tests. P< 0.05 was considered significant.
Results
Developmental profiles of the content of pAMPKα (Thr172), AMPKα, pACC (Ser79), ACC, FAS, and SREBP-1 (a precursor form) in the forebrain were examined using immunoblots of P4, P7, P10, P13, P16, and P19 mouse forebrain homogenates (Fig. 1A). Each antibody against pAMPKα, AMPKα, pACC, ACC or FAS used in this study gave one band or a double band on the blot with an approximate molecular weight close to the expected size of each protein, while anti-SREBP-1 antibody gave one band corresponding to a precursor form (~125 kDa) and several weaker bands with lower molecular weights (~68 kDa) which may include an active fragment of SREBP-1 (data not shown). Quantitative results shown in Fig.1B to D were obtained from immunoblots using 3 to 5 mice at each age and expressed as percentages of the content of P4 mice. The apparent double band of pAMPK or ACC was measured together as one band for quantification. Results showed that AMPKα, pAMPKα, ACC, and pACC all decreased between P4 and P19 (Fig. 1B and C). However, levels of pAMPKα and pACC significantly decreased after P10 and P13, respectively, while total levels of AMPKα and ACC were not significantly altered until P16, indicating that higher percentages of AMPKα and ACC were phosphorylated in the brain during the early neonatal period. The content of FAS and SREBP-1 decreased significantly after P13 (Fig. 1D). The content of CaMKKβ, which is considered one of the AMPK kinases in the brain (11), did not change significantly during the period (Fig. 1A). Thus, our results indicate that levels of FAS and ACC were high in the early neonatal period and declined in the later stages, confirming previous studies (4, 5, 7), although levels of ACC were rather constant till P13. Similar to FAS and ACC, levels of AMPK (specifically pAMPK) and SREBP-1 were higher in the early neonatal period.
Fig. 1.

Developmental changes in levels of lipogenic enzymes and the regulators. Forebrain samples were taken from P4, P7, P10, P13, P16, and P19 mice, and immunoblot analyses were carried out as described in Experimental Procedure. Fig.1A shows representative immunoblots of samples (50 µg protein) from P4-P19 mice. Figs. 1B–D show quantitative analyses of immunoblots for pAMPKα and AMPKα (B), pACC and ACC (C), and FAS and SREBP-1 (D). Data are expressed as percentages of the content of P4 mice. *Significantly (P< 0.05) different from the content of P4 mice by one-way ANOVA with LSD post hoc tests. In Fig. 1D, values at P13, P16, and P19 were significantly different from values at P4 in both FAS and SREBP-1.
These developmental changes were also observed by immunohistochemistry. Fig. 2A shows representative images of brain coronal sections including regions of the cingulate, motor, somatosensory cortices and the cingulum, corpus callosum and caudate putamen. Brain sections from P7, P10, P16, and P19 mice were stained using anti-pAMPKα, anti-pACC, or anti-FAS antibodies. When sections were treated simultaneously, sections from P7 and P10 mice were strongly stained while sections from P16 and P19 mice were scarcely stained with these antibodies. The differences in the staining intensity were observed generally in all the forebrain sections we examined although each specific region, such as the hypothalamus, has not been evaluated. In P7 brains, pAMPKα, pACC, and FAS were widely expressed in the cortex (Fig. 2A). A part of the cortex (a small rectangle area in Fig. 2A), where the staining was particularly strong, was shown in Fig. 2B as an image with higher magnification. In P19 brains, most of the cortex regions were scarcely stained with these antibodies except that there were FAS-positive cells and, to a lesser extent, pACC-positive cells near the cingulum region (a small rectangle area in Fig. 2A shown in Fig. 2B as a magnified image), and these cells were scantly spread into upper layers of the cortex. Representative images in the area of the cingulate cortex stained with anti-AMPK, anti-ACC, anti-FAS, and anti-SREBP-1 antibodies are shown in Fig. 3. Antibodies pre-treated with blocking peptides gave no staining in P7 brain sections [shown as P7 (control) in Fig. 3], confirming the specificities of these antibodies. Images in Fig. 3 showed that ACC, FAS, and SREBP-1 were expressed in cells with neuron-like morphologies at P7, although the presence of these proteins in other cell types has not been ruled out. AMPK expression indicated neuronal nuclear localization as previously reported (19). The expression patterns of AMPK, ACC, and SREBP-1 at P19 were similar to those at P7, although overall staining intensities decreased at P19. However, the distribution of FAS-positive cells changed markedly between P7 and P19. At P19, a majority of the FAS-positive cells, such as the cells indicated by arrows in Fig.3, showed smaller cell bodies than neurons. They were morphologically similar to oligodendrocytes/oligodendrocyte progenitors and were highly populated near the cingulum region (Fig. 2B). It has been reported that SREBP-1 (20) and AMPK (19, 21) are mainly localized in neurons, while ACC is localized in oligodendrocytes in the developing brain (22). In order to identify cell types that express AMPK, ACC, and FAS, double immunofluorescence labeling was used (Fig. 4). The dual labeling with anti-pAMPKα antibody and anti-NeuN antibody indicated that pAMPKα in the cortex of P7 mice was mostly localized in neurons (Fig. 4A). The distribution of AMPKα in the forebrain was similar to that of pAMPKα (data not shown). Also, Dual labeling with the anti-NeuN antibody and anti-pACC or anti-FAS antibodies shown in Fig. 4B and 4C indicated neuronal/neuropil localization of pACC and FAS at P7. In contrast to the nuclear expression of pAMPKα, pACC and FAS appeared to be expressed in the cytoplasm of neurons. Also, punctate staining was observed in sections labeled with anti-pACC antibody (Fig. 4B). The labeling pattern of ACC was similar to that of pACC, and the punctate staining was also observed (data not shown). Dual labeling in the cingulum region of P19 brains indicated that most of the FAS-positive cells were not overlapped with NeuN–positive neurons, but overlapped with O4-positive cells (Fig. 4D and E). It has been shown that O4 positive staining, which is primarily localized at the cell membrane and processes (23), is restricted to cells of the oligodendroglial lineage (24). Thus, we observed reduction in the levels of lipogenic enzymes and the regulators in the brain, especially in neurons, between P4 and P19. We then analyzed changes in lipid profiles during the same period (Fig.5). Lipids were extracted from whole brains of P4, P7, P10, P13, P16 and P19 mice, and levels of phospholipids, cholesterol, and TG were analyzed. A representative picture of lipids separated on HPTLC plates is shown in Fig. 5A. Cholesterol and phospholipids were developed on the same plate, and TG was developed on a separate plate. Quantitative results of the content of cholesterol and TG (Fig. 5B and C) were obtained from HPTLC analyses using 4 to 5 mice at each age, and expressed as mg/g wet weight. Results showed that phospholipids and cholesterol increased between P4 and P19 as expected for the myelin lipid synthesis with a peak at P18 (6, 25). However, levels of TG decreased in the brain during the period.
Fig. 2.
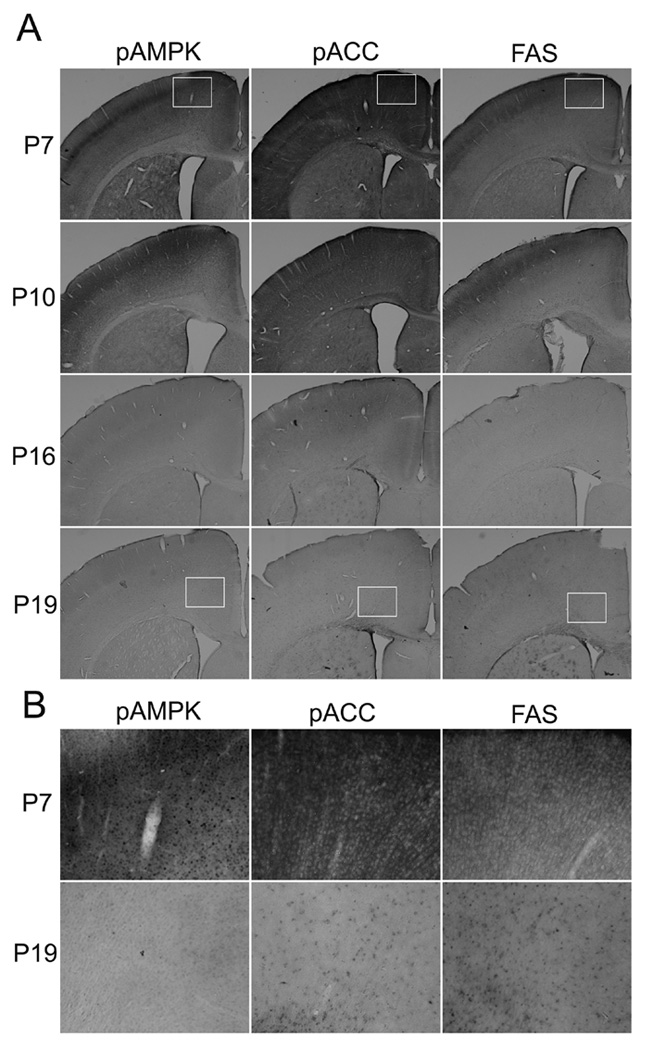
Developmental changes in immunostaining patterns of brain sections labeled with antibodies against pAMPKα (Thr172), pACC (Ser79), and FAS. In Fig.2A, brain sections from P7, P10, P16, and P19 mice were immunostained with anti-pAMPKα (Thr172) antibody, anti-pACC (Ser79) antibody or anti FAS antibody using the ABC method as described in Experimental Procedure. The size of a rectangle inserted into panels of P7 and P19 is 0.59 mm × 0.47 mm. Fig. 2B shows magnified images of the area indicated as rectangles in Fig. 2A.
Fig. 3.

Imminostaining patterns of AMPK-, ACC-, FAS-, and SREBP-1-positive cells. Brain sections from P7 and P19 were stained with anti-AMPK, anti-ACC, anti-FAS, and anti-SREBP-1 antibodies using the ABC method. For controls, the antibodies were incubated with blocking peptides for 1 h at room temperature before applying to P7 brain sections. For other experiments, the antibodies were incubated without blocking peptides for 1 h at room temperature before applying to P7 or P19 brain sections. Representative images show the cingulate cortex regions. Arrows show some of the cells morphologically similar to oligodendrocytes/oligodendrocite progenitors. The scale bar indicates 25 µm.
Fig. 4.
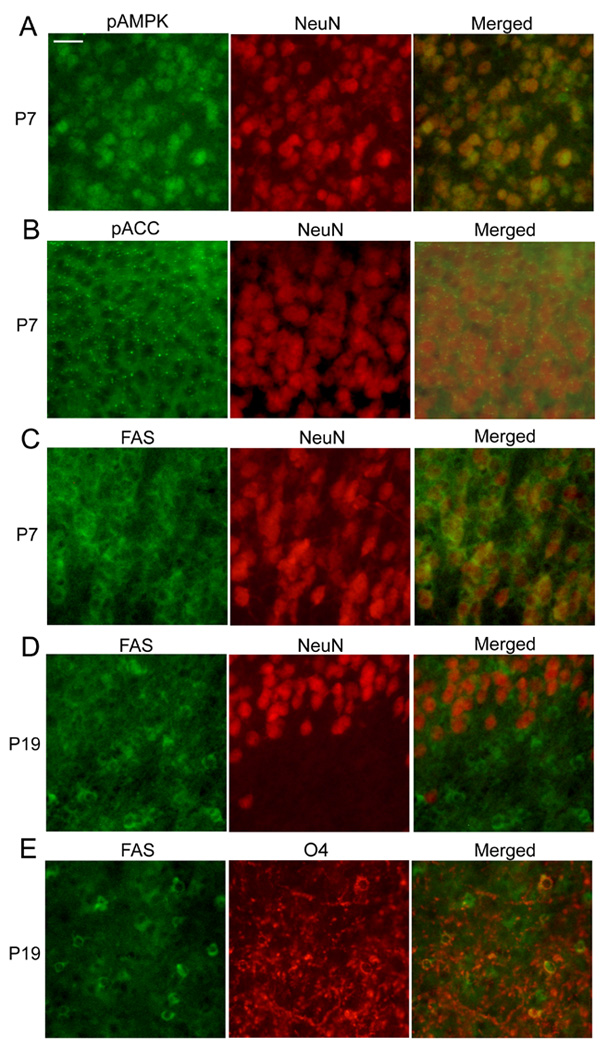
Double immunofluorescence labeling using markers for neurons (NeuN) and cells of oligodendrocyte lineage (O4). Brain sections from P7 (A-C) and P19 (D and E) mice were double-immunostained using antibodies against pAMPKα and NeuN (A), pACC and NeuN (B), FAS and NeuN (C), FAS and NeuN (D), and FAS and O4 (E). The scale bar in Fig.3A represents 25 µm.
Fig. 5.
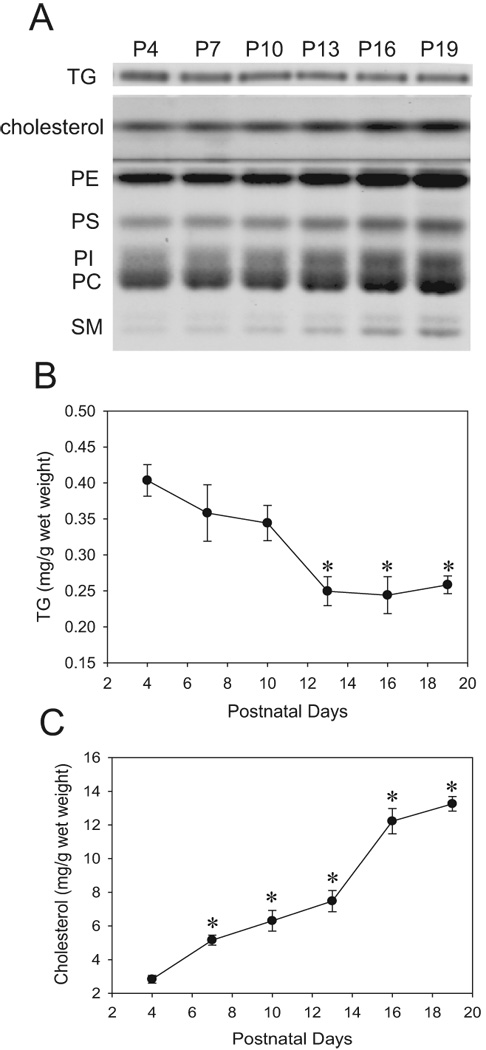
Developmental changes in the content of brain lipids. The brain lipids extracted from P4, P7, P10, P13, P16, and P19 were separated on HPTLC plates, stained with Coomassie Brilliant Blue, and analyzed as described in Experimental Procedure. Fig.1A shows a representative picture of HPTLC plates. The lipid extract from 0.25 mg (wet weight) of the brain was loaded on each lane of a HPTLC plate. Cholesterol, phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidylcholine (PC), and sphingomyelin (SM) were simultaneously separated on the same plate, and TG was separated on a different plate under different conditions as described in Experimental Procedure. Figs. 1B and 1C show quantitative results of changes in the amounts of TG (B) and cholesterol (C) obtained from HPTLC analyses. The values are expressed as mg/g wet weight of brains. * Significantly (P< 0.05) different from the content of P4 mice by one-way ANOVA with LSD post hoc tests.
Discussion
Our results showed that lipogenic enzymes, FAS and ACC, were highly expressed in mouse brains during the early neonatal period and decreased thereafter (Fig. 1 and Fig. 2), in agreement with previous studies (4, 5, 7). Our immnohistochemical studies indicate that high expression of FAS and ACC during the early neonatal period is largely due to the expression of these enzymes in neurons (Fig. 3 and Fig. 4). Considering active neural differentiation and the lack of transport of saturated and monounsaturated fatty acids from blood to the brain during the period (1, 2), lipogenesis is likely to take place in the developing brain. The high expression of FAS and ACC found in neurons indicates that lipogenesis occurs in neurons, although it is possible that other cell types, such as oligodendrocyte progenitors, are also involved in lipogenesis. The expression of pACC (an inactive form of ACC) also suggests that lipogenesis in neurons may be down-regulated at the level of ACC phosphorylation. Neuronal localization of FAS and ACC has been also reported in hypothalamic neurons in vivo and in vitro (26, 27, 28) and in several other cultured neurons (29, 30). Our immunohistochemical studies using antibodies against ACC and pACC, which react with both ACC1 and ACC2, showed punctate staining. It may suggest the presence of ACC2, because ACC2 has been shown to be associated with mitochondria (31). However, ACC in the neonatal rat brain appears to be mainly ACC1 with a molecular weight of 265 kDa (4). Although our immunoblot results gave a double band with a molecular weight close to265 kDa, an experiment using an antibody specific to ACC2 may be necessary to determine the presence or absence of ACC2. Also, further studies will be needed to establish the subcellular localization of neuronal ACC.
The neuronal expression of ACC and FAS observed at P7 decreased substantially by P19 (Fig. 3 and Fig. 4). Specifically, the expression of FAS was barely detectable in neurons at P19, when FAS expression was clearly observed in cells of oligodendrocyte lineage. The active lipogenesis in neurons may decline along with decreases in the levels of ACC and FAS, and oligodendrocytes during active myelination with a peak at P18 (6) are likely to be involved in fatty acid synthesis. Although we did not detect high ACC expression in P19 brains, ACC was expressed in the cells near the cingulum as observed for pACC expression at P19 (Fig. 2B), and it has been reported that ACC is expressed in oligodendrocytes in the rat brain during myelination (22).
We also found that AMPK and SREBP-1 were highly expressed in neurons during the early neonatal period, which agrees with previous reports showing neuronal localization of AMPK (19,21) and SREBP-1 (20) in the developing rodent brain. Specifically, the content of pAMPK and pACC was high at P4 and P7, and decreased to 20% of the amount of P4 by P19 (Fig. 1). It has been established that AMPK regulates lipogenesis and β-oxidation through modifying ACC and SREBP-1 transcriptionally and posttranscriptionally in the peripheral organs (9). The high levels of pAMPKα and pACC in neurons of the early postnatal brains (Fig. 1 and Fig. 2) imply that phosphorylation of ACC is catalyzed by pAMPK, an active form of AMPK. Such regulation of ACC by AMPK has been observed not only in the peripheral tissues but also in hypothalamic neurons, cortical neurons, and Neuro2a cells (29, 30). Our previous studies (15) have shown that ethanol decreases the content of pAMPK and pACC, and increases TG synthesis, which is considered as a marker for lipogenesis, in P7 mouse brains. Conversely, nutrient deprivation increases pAMPK and decreases TG levels in these brains. However, neither ethanol exposure nor nutrient deprivation affects levels of pAMPK and TG in P19 brains. These observations, combined with the neuronal localization of AMPK, ACC, and FAS (Fig. 3 and Fig. 4) and the high content of pAMPK and pACC in early neonatal brains (Fig. 1 and Fig. 2), support the notion that AMPK regulates lipogenesis in neurons during the early neonatal period.
Thus, our results indicate that the expression of FAS may increase in oligodendrocytes and decrease in neurons during the period of active myelination. Rapid accumulation of phospholipids and cholesterol shown in Fig. 5 appears to be correlated with the myelin formation. The content of TG peaked earlier than the active myelin formation may be correlated with earlier neuronal lipogenesis. Because the decrease in the TG level (expressed as mg/g wet weight of brains) cannot be solely explained by the increase in the proportion of myelin present in the brain samples, it is likely that TG synthesis decreases between P4 and P19. Our previous study indicates that cultured neurons synthesize TG (32). However, neuronal localization of TG remains to be confirmed in vivo. Also, the possible contribution of TG transported from the blood cannot be excluded, although the developing brain appears to produce all the required saturated and monounsaturated fatty acids by de novo synthesis (1,2).
Our studies suggest that AMPK in early postnatal stages may regulate lipid synthesis essential for cell proliferation and differentiation. However, AMPK activation during the early neonatal period may also be related to other cellular functions. AMPK activation is known to regulate intracellular signaling pathways involved in cellular survival and apoptotic cell death. It has been shown that the AMPK-activating agent AICAR protects cultured hippocampal neurons against death induced by glucose deprivation (21), and prevents ceramide synthesis and apoptosis in astrocytes (33). It is conceivable that the AMPK/ACC pathway activated in neurons in the early postnatal period may play important roles in the brain metabolism and functions. Further studies, such as gene manipulation, are necessary to confirm the involvement of AMPK in the lipid metabolism in the developing brain.
Acknowledgments
This work was supported by an NIH/NIAAA grant R01 AA015355.
References
- 1.Marbois BN, Ajie HO, Korsak RA, et al. The origin of palmitic acid in brain of the developing rat. Lipids. 1992;27:587–592. doi: 10.1007/BF02536115. [DOI] [PubMed] [Google Scholar]
- 2.Edmond J, Higa TA, Korsak RA, et al. Fatty acid transport and utilization for the developing brain. J. Neurochem. 1998;70:1227–1234. doi: 10.1046/j.1471-4159.1998.70031227.x. [DOI] [PubMed] [Google Scholar]
- 3.Garbay B, Bauxis-Lagrave S, Boiron-Sargueil F, et al. Acetyl-CoA carboxylase gene expression in the developing mouse brain. Comparison with other genes involved in lipid biosynthesis. Brain Res. Dev. Brain Res. 1997;98:197–203. doi: 10.1016/s0165-3806(96)00169-1. [DOI] [PubMed] [Google Scholar]
- 4.Spencer EB, Bianchi A, Widmer J, et al. Brain acetyl-CoA carboxylase: isozymic identification and studies of its regulation during development and altered nutrition. Biochem. Biophys. Res. Commun. 1993;192:820–825. doi: 10.1006/bbrc.1993.1488. [DOI] [PubMed] [Google Scholar]
- 5.Volpe JJ, Lyles TO, Roncari DA, et al. Fatty acid synthetase of developing brain and liver. Content, synthesis, and degradation during development. J. Biol. Chem. 1973;248:2502–2513. [PubMed] [Google Scholar]
- 6.Bourre JM, Gozlan-Devillierre N, Daudu O, et al. Is there a blood-brain relationship for saturated fatty acids during development? Biol. Neonate. 1978;34 doi: 10.1159/000241125. 182-118. [DOI] [PubMed] [Google Scholar]
- 7.Garbay B, Cassagne C. Expression of the ceramide galactosyltransferase gene during myelination of the mouse nervous system. Comparison with the genes encoding myelin basic proteins, choline kinase and CTP:phosphocholine cytidylyltransferase. Brain Res. Dev. Brain Res. 1994;83:119–124. doi: 10.1016/0165-3806(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 8.Salles J, Sargueil F, Knoll-Gellida A, et al. Fatty acid synthase expression during peripheral nervous system myelination. Brain Res. Mol. Brain Res. 2002;101:52–58. doi: 10.1016/s0169-328x(02)00161-4. [DOI] [PubMed] [Google Scholar]
- 9.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase--development of the energy sensor concept. J. Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawley SA, Davison M, Woods A, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 11.Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J. Physiol. 2006;574:85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You M, Matsumoto M, Pacold CM, et al. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 13.Stapleton D, Mitchelhill KI, Gao G, et al. Mammalian AMP-activated protein kinase subfamily. J. Biol. Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 14.Wolfgang MJ, Lane MD. The role of hypothalamic malonyl-CoA in energy homeostasis. J. Biol. Chem. 2006;281:37265–37269. doi: 10.1074/jbc.R600016200. [DOI] [PubMed] [Google Scholar]
- 15.Saito M, Chakraborty G, Mao RF, et al. Ethanol alters lipid profiles and phosphorylation status of AMP-activated protein kinase in the neonatal mouse brain. J. Neurochem. 2007;103:1208–1218. doi: 10.1111/j.1471-4159.2007.04836.x. [DOI] [PubMed] [Google Scholar]
- 16.Saito M, Mao RF, Wang R, et al. Effects of gangliosides on ethanol-induced neurodegeneration in the developing mouse brain. Alcohol Clin. Exp. Res. 2007;31:665–674. doi: 10.1111/j.1530-0277.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K, Handa S. Coomassie brilliant blue staining of lipids on thin-layer plates. Anal. Biochem. 1984;142:406–410. doi: 10.1016/0003-2697(84)90484-6. [DOI] [PubMed] [Google Scholar]
- 18.Saito M, Salgia R, Beckley R, et al. The effects of monensin on membrane lipids of cultured human skin fibroblasts. Biochim. Biophys. Acta. 1986;856:689–693. doi: 10.1016/0005-2736(86)90164-1. [DOI] [PubMed] [Google Scholar]
- 19.Turnley AM, Stapleton D, Mann RJ, et al. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J. Neurochem. 1999;72:1707–1716. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- 20.Ong WY, Hu CY, Soh YP, et al. Neuronal localization of sterol regulatory element binding protein-1 in the rodent and primate brain: a light and electron microscopic immunocytochemical study. Neuroscience. 2000;97:143–153. doi: 10.1016/s0306-4522(00)00031-2. [DOI] [PubMed] [Google Scholar]
- 21.Culmsee C, Monnig J, Kemp BE, et al. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J. Mol. Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- 22.Tansey FA, Thampy KG, Cammer W. Acetyl-CoA carboxylase in rat brain. II. Immunocytochemical localization. Brain Res. 1988;471 doi: 10.1016/0165-3806(88)90158-7. 131-128. [DOI] [PubMed] [Google Scholar]
- 23.Lin S, Rhodes PG, Lei M, et al. alpha-Phenyl-n-tert-butyl-nitrone attenuates hypoxic-ischemic white matter injury in the neonatal rat brain. Brain Res. 2004;1007:132–141. doi: 10.1016/j.brainres.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J. Neurosci. Res. 1997;47:455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Muse ED, Jurevics H, Toews AD, et al. Parameters related to lipid metabolism as markers of myelination in mouse brain. J. Neurochem. 2001;76:77–86. doi: 10.1046/j.1471-4159.2001.00015.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim EK, Miller I, Landree LE, et al. Expression of FAS within hypothalamic neurons: a model for decreased food intake after C75 treatment. Am. J. Physiol. Endocrinol. Metab. 2002;283:E867–E879. doi: 10.1152/ajpendo.00178.2002. [DOI] [PubMed] [Google Scholar]
- 27.Chakravarthy MV, Zhu Y, Lopez M, et al. Brain fatty acid synthase activates PPARalpha to maintain energy homeostasis. J Clin Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao S, Lane MD. Effect to the anorectic fatty acid synthase inhibitor C75 on neuronal activity n the hypothalamus and brainstem. Proc. Natl. Acad. Sci. USA. 2003;100:5628–5633. doi: 10.1073/pnas.1031698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landree LE, Hanlon A, Strong DW, et al. C75, a fatty acid synthase inhibitor, modulates AMP-activated protein kinase to alter neuronal energy metabolism. J. Biol. Chem. 2004;279:3817–3827. doi: 10.1074/jbc.M310991200. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Elheiga L, Brinkley WR, Zhong L, et al. The subcellular localization of acetyl-CoA carboxylase 2. Proc. Natl. Acad. Sci. U S A. 2000;97:1444–1449. doi: 10.1073/pnas.97.4.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito M, Saito M, Cooper TB, et al. Ethanol-induced changes in the content of triglycerides, ceramides, and glucosylceramides in cultured neurons. Alcohol Clin. Exp. Res. 2005;29:1374–1383. doi: 10.1097/01.alc.0000175011.22307.61. [DOI] [PubMed] [Google Scholar]
- 33.Blazquez C, Geelen MJ, Velasco G. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001;489:149–153. doi: 10.1016/s0014-5793(01)02089-0. [DOI] [PubMed] [Google Scholar]


