Abstract
Purpose
Women with reduced CYP2D6 activity have low endoxifen concentrations and likely worse long term benefits from tamoxifen. We investigated the association between CYP2D6 genotype and tamoxifen-induced hot flashes, in a prospective cohort.
Methods
We collected hot flash frequency and severity data over 12 months from 297 women initiating tamoxifen. We performed CYP2D6 genotyping using the AmpliChip CYP450 Test and correlated inherited genetic polymorphisms in CYP2D6 and tamoxifen-induced hot flashes.
Results
Intermediate metabolizers had greater mean hot flash scores after 4 months of tamoxifen therapy (44.3) compared to poor metabolizers (20.6, p=0.038) or extensive metabolizers (26.9, p=0.011). At 4 months, we observed a trend toward fewer severe hot flashes in poor metabolizers compared to intermediate plus extensive metabolizers (p=0.062).
Conclusions
CYP2D6 activity may be a modest predictive factor for tamoxifen-induced hot flashes. The presence or absence of hot flashes should not be used to determine tamoxifen's efficacy.
Keywords: breast cancer, tamoxifen, CYP2D6, hot flash, genotype
Introduction
The selective estrogen receptor modulator tamoxifen is one of the mainstays for treatment and prevention of hormone receptor positive breast cancer.[1] Tamoxifen is a pro-drug that is converted to active metabolites by cytochrome P450 (CYP) enzymes, primarily CYP2D6.[2] Endoxifen (4-hydroxy N-desmethyl tamoxifen) is believed to be a key active metabolite of tamoxifen. Endoxifen has equal affinity for ER and equal in vitro anti-cancer activity as the other primary metabolite, 4-hydroxy tamoxifen, but endoxifen is present in 5-10 fold higher concentrations than 4-hydroxy tamoxifen.[2] Most but not all studies reported to date suggested that subjects with reduced or absent CYP2D6 activity have reduced serum concentrations of endoxifen, and may have worse long term tamoxifen-associated benefits than those with normal enzyme activity.[2-10]
One of the most bothersome tamoxifen-associated toxicities is hot flashes, reported by over 50% of women.[1] A retrospective analysis has suggested that moderate or severe hot flashes are significantly less common in women homozygous for the *4 null variant of CYP2D6.[4] Because patients with reduced CYP2D6 activity have worse breast cancer outcomes and are less likely to report hot flashes, it has been suggested that presence of hot flashes during tamoxifen therapy may predict for superior breast cancer outcomes.[11,12] Based on these data, we hypothesized that inherited germline variants in the CYP2D6 gene that cause reduced or absent enzymatic activity would be associated with lower hot flash scores in tamoxifen-treated women.
Methods
We enrolled 297 women initiating tamoxifen in a prospective multicenter observational study designed to identify associations between CYP2D6 germline variants and tamoxifen-related phenotypes. Complete details regarding the design and conduct of this study and CYP2D6 genotyping methods have been previously reported.[13,14] The protocol was approved by the institutional review boards of all three participating study sites. All enrolled patients provided written informed consent.
In brief, patients initiating therapy with tamoxifen for breast cancer treatment or prevention were enrolled and treated with tamoxifen 20 mg orally per day for 12 months. Participants recorded the number of hot flashes that were mild, moderate, severe, or very severe[15] over 7 days prior to initiation of tamoxifen therapy and after 1, 4, 8, and 12 months of tamoxifen therapy. Hot flash score, which is a summary of hot flash frequency and severity, was calculated as previously described.[16]
Whole blood samples were obtained from each patient at baseline. The women underwent comprehensive genotyping for 33 CYP2D6 alleles using the AmpliChip CYP450 Test (Roche Diagnostics, Basel, Switzerland) and the xTAG CYP2D6 assay (Luminex Corp, Austin, Texas; 16 alleles).[14] Each CYP2D6 allele was assigned a value from 0 (for nonfunctional alleles) to 1 (for fully functional alleles) based on its relative activity for dextromethorphan O-demethylation.[17] For each subject, the two allele scores were summed.[18] Patients were classified as poor metabolizers (PMs) if their total score was <1, intermediate metabolizers (IMs) if the score was 1 to <2, and extensive metabolizers (EMs) if the score was ≥2.
The primary endpoint for this study was to assess the relationship between CYP2D6 germline variants and change in hot flash score during the initial 4 months of tamoxifen therapy. This time period was selected and used in all our analyses because tamoxifen serum concentrations reach steady state by 4 months, and to limit the confounding effect of concomitant medication usage and premature trial discontinuation.[13] A general linear model was used to test the differences in hot flash frequency and score among the three genotype groups. Fisher's exact test was used to compare the hot flash severity distribution of the three groups (no hot flashes, mild/moderate, and severe/very severe) between cohorts divided by CYP2D6 metabolizer status (PM versus EM/IM). The effect of CYP2D6 metabolizer status on hot flash-free survival during the first year of tamoxifen treatment was tested using Kaplan-Meier plots and log-rank tests. Time to hot flash was treated as a time-to-event outcome. Subjects with hot flashes at baseline or who were taking medications known to affect hot flashes were excluded from the survival analyses.
Results
Change in Hot Flash Score by CYP2D6 Genotype
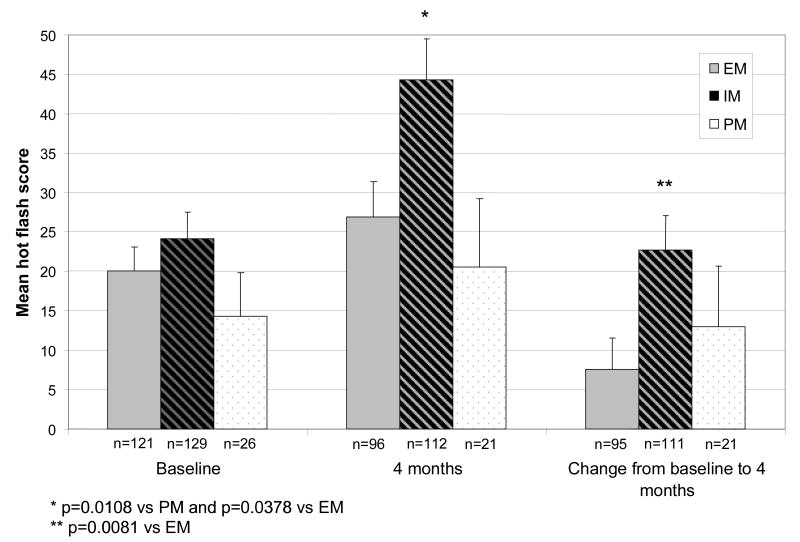
For the entire cohort, mean weekly hot flash score increased within 1 month of initiating tamoxifen therapy and remained elevated throughout treatment.[13] In an intent to treat analysis, we did not observe differences in mean absolute hot flash score at baseline among women according to CYP2D6 metabolizer status (Figure 1). At the 4 month time-point, IMs reported significantly higher mean weekly hot flash scores (44.3 ± 10.2) compared to either EMs (26.9 ± 8.8, p=0.011) or PMs (20.6 ± 16.9, p=0.038). When patients on SSRIs known to inhibit CYP2D6 were omitted from the analysis, a higher hot flash score was still noted in IMs compared to EMs (p=0.0395, data not shown).
Figure 1.
Mean weekly hot flash score at baseline, 4 months after initiating tamoxifen, and change in hot flash score from baseline to 4 months by CYP2D6 metabolizer group derived from CYP2D6 genotype for all study participants. Number of subjects evaluated at each timepoint listed below the bars. Error bars signify standard error. EM=extensive metabolizer (sold bars), IM=intermediate metabolizer (diagonal bars), PM=poor metabolizer (dotted bars).
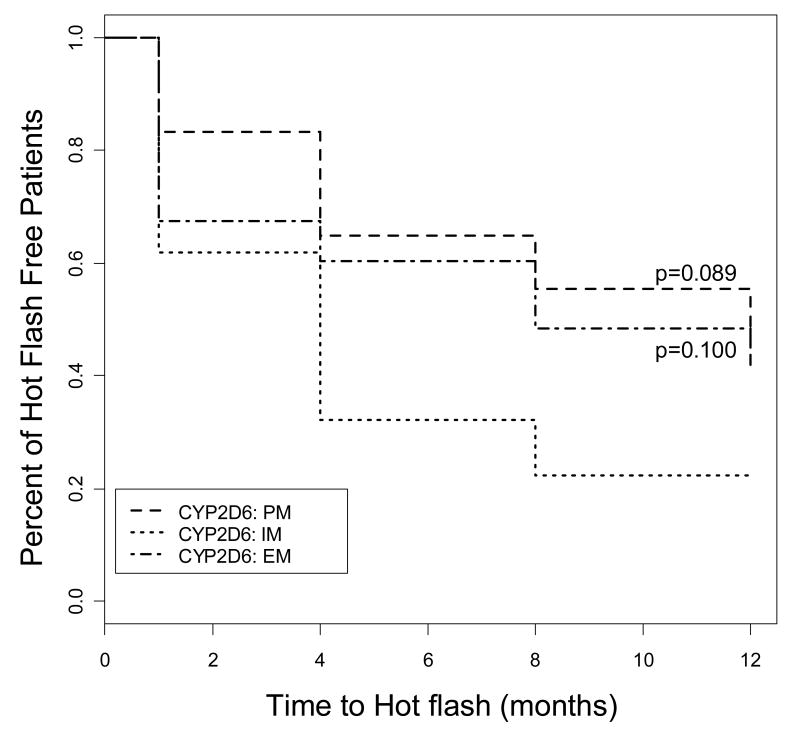
In an intent to treat analysis, we observed a significant increase in mean hot flash score in IMs (41.8 ± 6.2) compared to EMs (25.3 ± 4.7) after 4 months of tamoxifen therapy relative to baseline (p=0.040). In addition, in the subset of subjects not on concomitant medications known to treat hot flashes or to affect CYP2D6 activity at any time during study participation (n=109), we observed a trend suggesting that EMs and PMs were more likely to remain free of hot flashes during tamoxifen therapy compared to IMs (p=0.100 and p=0.089, respectively; Figure 2).
Figure 2.
Kaplan-Meier curves for the effect of CYP2D6 genotype on hot flash-free survival during the first year of tamoxifen treatment (n=109). Subjects with hot flashes at baseline or who were taking medications known to affect hot flashes or tamoxifen metabolism were excluded from the analysis. EM=extensive metabolizer, IM=intermediate metabolizer, PM=poor metabolizer. P values represent comparison of EM or PM to IM.
Change in Hot Flash Severity by CYP2D6 Genotype
Since hot flash score is the product of hot flash severity and frequency, it is possible that CYP2D6 genotype may preferentially influence hot flash severity, as has previously been reported in patients homozygous for the most prevalent CYP2D6 null variant in Caucasians, CYP2D6*4.[4] At 4 months, we observed that PMs were less likely to develop severe or very severe hot flashes compared to EMs and IMs combined (9.5% vs 29.8%, p=0.062; Table). When potential associations between CYP2D6 genotype and hot flash frequency were analyzed, the findings were similar to that seen for hot flash score (data not shown).
Table.
Mean hot flash severity at baseline and after 4 months of tamoxifen therapy by CYP2D6 metabolizer group derived from CYP2D6 genotype for all study participants.
| Severity | Month 0 | Month 4 | ||
|---|---|---|---|---|
| EMa/IMb | PMc | EM/IM | PM | |
| No Hot Flashes | 42.4% (n=106) |
53.9% (n=14) |
24.0% (n=50) |
42.9% (n=9) |
| Mild/moderate | 37.6% (n=94) |
38.5% (n=10) |
46.2% (n=96) |
47.6% (n=10) |
| Severe/very severe | 20.0% (n=50) |
7.7% (n=2) |
29.8% (n=62) |
9.5% (n=2) |
| P value | 0.285 | 0.062 | ||
EM=extensive metabolizer
IM=intermediate metabolizer
PM=poor metabolizer
Conclusions
In this prospective observational study, we detected an association between CYP2D6 intermediate metabolizer phenotype and tamoxifen-associated hot flashes (Figure 1). Though this association of hot flash score with IMs, but not EMs, is unexpected, it may reflect reduced adherence to therapy in subjects with the EM phenotype. One potential explanation is that subjects with the EM phenotype who experienced more severe hot flashes were more likely to discontinue therapy prior to the 4 month assessment, and therefore could not be included in the analysis. This explanation does not appear to be valid, however, since of the subjects for whom baseline hot flash data were available, similar numbers of EM and IM subjects were missing hot flash data at the 4 month time point (25 of 121 EMs and 30 of 129 IMs).
The strengths of this study include a prospective assessment of hot flashes using a validated hot flash diary [16] both prior to and during tamoxifen therapy. Limitations include heterogeneity of the patients with respect to menopausal status, prior chemotherapy, and concomitant medications, all of which are known to influence hot flashes.
These results differ from a recent preliminary report that EMs are more likely to experience hot flashes than PMs.[19] In that report from the Women's Healthy Eating and Living (WHEL) study, women with early stage breast cancer who had been taking tamoxifen for at least 4 months self-reported hot flash severity over the preceding 4 weeks at the time of enrollment. Our study differs from the WHEL report in multiple ways, including acquisition of hot flash data using a validated 7 day hot flash diary to capture both hot flash frequency and severity data, as well as assessment of hot flashes both prior to and during tamoxifen therapy. In addition, the use of concomitant medications that could affect CYP2D6 activity by the WHEL participants is unknown. These factors could potentially account for the different results noted in the two studies.
Our observation that PMs are less likely to report severe hot flashes than EMs and IMs are similar to those previously published in a retrospective report.[4] Two main differences between our study and the prior publication are that we performed more comprehensive CYP2D6 genotyping and we prospectively collected patient-reported hot flash frequency and severity. In summary, these results suggest that CYP2D6 activity may influence severity of tamoxifen-associated hot flashes, although it is unclear whether this CYP2D6 effect is through a differential tamoxifen metabolism or through known effects of this enzyme in the brain.[20]
Our results suggest that CYP2D6 activity is likely not the sole determinant of tamoxifen-associated hot flashes. Instead, the development of tamoxifen-associated hot flashes is likely multifactorial, including factors involved in tamoxifen metabolism as well as estrogen metabolism and signaling. Indeed, we have previously demonstrated an association between polymorphisms in the estrogen receptor beta gene ESR2 and likelihood of developing hot flashes.[13] In addition, women with hot flashes at the time of menopause have been shown to be more likely to experience tamoxifen-induced hot flashes.[21] Additional studies are required to determine the factors involved in tamoxifen-associated hot flashes and to further elucidate the relationship between hot flashes and breast cancer outcomes. Until additional data are available, clinicians should not use the presence or absence of hot flashes in tamoxifen-treated women to predict possible long term benefits related to the drug.
Acknowledgments
This work was supported in part by the Pharmacogenetics Research Network grant number U-01 GM61373 (D.A.F.), which supports the Consortium on Breast Cancer Pharmacogenomics (COBRA); National Institutes of Health Clinical Pharmacology training grant number T32-GM08425 (D.A.F.); National Institute of General Medical Sciences, National Institutes of Health grant number K24RR020815 (D.A.F.); Damon Runyon-Lilly Clinical Investigator award CI-3 from the Damon Runyon Cancer Research Foundation (V.S.); and Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes-on-Sale™ (D.F.H.) This publication was made possible by Grant Numbers M01-RR000042 (University of Michigan), M01-RR020359 (Georgetown University), and M01-RR00750 (Indiana University) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Conflicts of interest:
NLH has received research funding from AstraZeneca and Lilly Pharmaceuticals.
JMR has received research funding from Pfizer and speaking honoraria for Roche Diagnostics.
TS has received speaking honoraria for Roche Diagnostics.
AMS is a member of the speaker's bureau and receives research funding from Glaxo-Smith Kline and is a consultant to Eli Lilly and Company.
DAF is on the Scientific Advisory Board of Labcorp, Inc, is a consultant to Roche Molecular Diagnostics, and has received research funding from Pfizer and Novartis.
DFH has received research funding from AstraZeneca, Glaxo-Smith Kline, Pfizer, and Novartis.
VS has served as a consultant to Wyeth Pharmaceuticals, Concert Pharmaceuticals and JDS Pharmaceuticals, and has received research funding from Glaxo-Smith Kline, Pfizer, and Novartis.
References
- 1.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 3.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 4.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 5.Schroth W, Antoniadou L, Fritz P, et al. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol. 2007;25:5187–5193. doi: 10.1200/JCO.2007.12.2705. [DOI] [PubMed] [Google Scholar]
- 6.Lim HS, Ju Lee H, Seok Lee K, et al. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol. 2007;25:3837–3845. doi: 10.1200/JCO.2007.11.4850. [DOI] [PubMed] [Google Scholar]
- 7.Nowell S, Ahn J, Rae JM, et al. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat. 2005;91:249–258. doi: 10.1007/s10549-004-7751-x. [DOI] [PubMed] [Google Scholar]
- 8.Wegman P, Vainikka L, Stal O, et al. Genotype of metabolic enzymes and the benefit of tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res. 2005;7:R284–290. doi: 10.1186/bcr993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegman P, Elingarami S, Carstensen J, et al. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. 2007;9:R7. doi: 10.1186/bcr1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz MP, Suman V, Ames M, et al. Tamoxifen pharmacogenetics of CYP2D6, CYP2C19, and SULT1A1: long term follow-up of the North Central Cancer Treatment Group 89-30-52 adjuvant trial (abstract) Cancer Res. 2009;69(Suppl):6037. [Google Scholar]
- 11.Mortimer JE, Flatt SW, Parker BA, et al. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat. 2008;108:421–426. doi: 10.1007/s10549-007-9612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuzick J, Sestak I, Cella D, et al. Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9:1143–1148. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 13.Jin Y, Hayes DF, Li L, et al. Estrogen receptor genotypes influence hot flash prevalence and composite score before and after tamoxifen therapy. J Clin Oncol. 2008;26:5849–5854. doi: 10.1200/JCO.2008.16.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80:61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Finck G, Barton DL, Loprinzi CL, et al. Definitions of hot flashes in breast cancer survivors. J Pain Symptom Manage. 1998;16:327–333. doi: 10.1016/s0885-3924(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 16.Sloan JA, Loprinzi CL, Novotny PJ, et al. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 17.Blake MJ, Gaedigk A, Pearce RE, et al. Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin Pharmacol Ther. 2007;81:510–516. doi: 10.1038/sj.clpt.6100101. [DOI] [PubMed] [Google Scholar]
- 18.Gaedigk A, Simon SD, Pearce RE, et al. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008;83:234–242. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- 19.Madlensky L, Flatt SW, Natarajan L, et al. Hot flashes are associated with CYP2D6 genotype in breast cancer survivors taking tamoxifen [abstract] Cancer Res. 2009;69(Suppl):6045. [Google Scholar]
- 20.Snider NT, Sikora MJ, Sridar C, et al. The endocannabinoid anandamide is a substrate for the human polymorphic cytochrome P450 2D6. J Pharmacol Exp Ther. 2008;327:538–545. doi: 10.1124/jpet.108.141796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loprinzi CL, Zahasky KM, Sloan JA, et al. Tamoxifen-induced hot flashes. Clin Breast Cancer. 2000;1:52–56. doi: 10.3816/cbc.2000.n.004. [DOI] [PubMed] [Google Scholar]