Abstract
Monocytes and macrophages play active roles in atherosclerosis, a chronic inflammatory disease that is a leading cause of death in the developed world. The prevailing paradigm states that, during human atherogenesis, monocytes accumulate in the arterial intima and differentiate into macrophages, which then ingest oxidized lipoproteins, secrete a diverse array of pro-inflammatory mediators, and eventually become foam cells, the key constituents of a vulnerable plaque. Yet monocytes are heterogeneous. In the mouse, one subset (Ly-6Chi) promotes inflammation, expands in hypercholesterolemic conditions, and selectively gives rise to macrophages in atheromata. A different subset (Ly-6Clo) attenuates inflammation and promotes angiogenesis and granulation tissue formation in models of tissue injury, but its role in atherosclerosis is largely unknown. In the human, monocyte heterogeneity is preserved but it is still unresolved how subsets correspond functionally. These cells’ contradistinctive properties suggest commitment for specific function prior to infiltrating tissue. Such commitment argues for discriminate targeting of deleterious subsets while sparing host defense and repair mechanisms. In addition to advancing our understanding of atherosclerosis, the ability to target and image monocyte subsets would allow us to evaluate drugs designed to selectively inhibit monocyte subset recruitment or function, and to stratify patients at risk for developing complications such as myocardial infarction or stroke. In this review we summarize recent advances of our understanding of the behavioral heterogeneity of monocytes during disease progression, and outline emerging molecular imaging approaches to address key questions in the field.
INTRODUCTION
Atherosclerosis is a complex chronic disease and a leading cause of myocardial infarction and stroke1-4. At present, the dominant conceptual approaches to therapy involve manipulation of lipid metabolism and manipulation of inflammatory processes. Phase III clinical trials of torcetrapib, an agent that increases HDL and lowers LDL through inhibition of cholesteryl ester transfer protein (CETP), were terminated in 2006 because of increased mortality and cardiovascular events5, 6. While numerous other inhibitors, agonists, antagonists, peptidomimetics, antisense oligonucleotides, and gene-replacement therapies aimed at targeting lipoprotein biology may prove effective as therapies for atherosclerosis or its risk factors, the experience with torcetrapib, and the fact that myocardial infarction and stroke continue to claim lives, indicate an urgent need to explore alternative treatment strategies7. Targeting inflammatory processes is a prospective option; since the late 1970s8, inflammation has shaped our understanding of the disease and several agents that target leukocyte recruitment and retention are currently in preclinical trials.
Pathologically, atherosclerosis is characterized by the development of lesions, or atheromata, that affect the arterial blood vessels, typically at vessel bifurcations. The mechanisms that govern the evolution of atheromata at these ‘sites of predilection’ are complex and not yet fully understood, but they are known to involve non-laminar blood flow, lipid accumulation and oxidation, leukocyte recruitment, mobilization of smooth muscle cells, and cell apoptosis4, 9. Their particular combination gives rise to lesions that display remarkable heterogeneity. Rupture of a ‘vulnerable’ plaque may lead to myocardial infarction or stroke, and depends on the interplay between lesional composition and mechanical forces: ‘stable’ lesions with a collagen-rich thick fibrous cap and small lipid core are less prone to rupture than inflammatory lesions with a thin fibrous cap and large lipid-rich core. Shifting the balance from a vulnerable to a stable plaque is an attractive therapeutic consideration that may require reprogramming of the immune system from an inflammatory state (i.e. collagen breakdown, accelerated accumulation of inflammatory cells) to a regulatory or ’healing’, state (i.e. collagen synthesis, reduced accumulation of inflammatory cells or mobilization of cells that promote resolution of inflammation). Conceptual approaches available include targeting of cell subsets or specific molecules involved during inflammatory processes.
Monocytes and macrophages are widely regarded as key cellular protagonists of atherosclerosis. Indeed, circulating monocytes efficiently adhere to activated endothelium, infiltrate atherosclerotic lesions, become lesional macrophages, and participate decisively in the development and exacerbation of atherosclerosis4, 9, 10. Macrophages ingest oxidized lipoproteins via scavenger receptors, and thus as lipid-rich foam cells, they become part of the disease’s physical bulk. The cells also secrete inflammatory mediators that stimulate smooth muscle cell migration and proliferation and participate in plaque development, rupture, and thrombosis. From this perspective, it seems that monocyte/macrophages are categorically detrimental and their accumulation accelerates disease; their inhibition or ablation may seem, at first, as a clear and simple therapeutic objective. Nevertheless, monocytes are integral to the health of the organism. They are motile in the circulation, patrol the vasculature, replenish tissue with macrophages, and respond to injury, infection and various ‘danger signals’11, 12. Their indiscriminate targeting would interfere with normal homeostasis and immunity, and is therefore therapeutically nonviable. The discovery that monocytes are comprised of distinct subsets in human, mouse and other mammals suggests specialization of function, and has stimulated interest in approaches that discriminate between ‘harmful’ and ‘beneficial’ subsets. Also, hints regarding diversity of macrophages that populate human atheromata have surfaced over the years13, but it is unclear whether functional subpopulations in atheromata arise from differential stimuli encountered in regions of the plaque or reflect lineal predispositions that depend on programming before penetration into the plaque. Below we review the current knowledge on monocyte and macrophage heterogeneity, the tools that can be used to investigate the role of subtypes, and the emerging views of the role of these cells in atherosclerosis. Finally, we present possible diagnosis and treatment opportunities based on our improved understanding of monocyte and macrophage heterogeneity.
MONOCYTE AND MACROPHAGE HETEROGENEITY
Studies have documented monocyte heterogeneity in humans and mice. In humans, monocytes fall into at least two main subsets based on their expression of specific receptors, including CD14 and CD1614-17. In mice, monocyte subsets can be divided based on expression of Ly-6C, Gr1, CC-chemokine receptor 2 (CCR2) and CX3C-chemokine receptor 1 (CX3CR1). Ly-6Chi monocytes are Gr1+CCR2+CX3CR1lo while Ly-6Clo monocytes are Gr1- CCR2-CX3CR1hi. According to the relative expression of CCR2 and CX3CR1, they correspond to human CD14hiCD16- and CD14+CD16+ monocytes, respectively18-20. These observations indicate that it is possible to address the in vivo relevance of human heterogeneity by studying mice.
It is currently believed that different monocyte subsets reflect developmental stages with distinct physiological functions18-33 (Table 1). Ly-6Chi monocytes arise in the bone marrow and enter circulation partly via CCR234, 35. They express a number of inflammatory and proteolytic mediators, respond to inflammatory cues such as the CCR2 ligand CCL2 (also known as MCP-1), and accumulate in inflammatory sites. In the absence of inflammation, they are thought to differentiate into Ly-6Clo monocytes20, although Ly-6Chi → Ly-6Clo conversion is still under dispute36-38. Ly-6Clo monocytes appear to be anti-inflammatory, support granulation tissue formation (i.e., collagen deposition and healing), patrol the vasculature, and enter tissues very early after the onset of inflammation19, 22, 24. In the human, an imbalance in the relative proportion of subsets has been linked to several diseases17, 39-49. However, while there may be molecular agreement between human CD16+ and mouse Ly-6Clo monocytes (and conversely between human CD16- and mouse Ly-6Chi monocytes) there is currently a discrepancy as to their function; CD16+ monocytes have been called “inflammatory”. It is yet to be determined whether this contradiction reflects differences between species or incomplete discrimination between subsets.
Table 1.
Role of mouse monocyte subsets in inflammation
| Inflammation | Behavior of Ly-6Chi monocytes | Reference |
|---|---|---|
| Acute | Accumulate in peritoneum in response to thioglycollate | Geissmann et al. 2003. Immunity. 19:71 |
| Increase in number in response to Listeria monocytogenes infection | Sunderkotter et al 2004. J. Immunol. 172:4410 | |
| Transport Listeria monocytogenes to the brain | Drevets et al. 2005. J. Immunol. 172:4418 | |
| Accumulate in peritoneum in response Toxoplasma gondii infection | Robben et al. 2005. J. Exp. Med. 201:1761 | |
| Accumulate in hepatic lesions in response to Francisella tularensis infection | Rasmussen et al. 2006. Infect Immun. 74:6590 | |
| Accumulate in injured skeletal muscle | Arnold et al. 2007. J. Exp. Med. 204:1057 | |
| Increase in number in response to thermal injury | Noel et al. 2007. Shock. 28:684 | |
| Accumulate in injured myocardium & perform inflammatory and proteolytic function | Nahrendorf et al. 2007. J. Exp. Med. 204:3037 | |
| Chronic | Increase in number in response to chronic infection with Leishmania major | Sunderkotter et al 2004. J. Immunol. 172:4410 |
| Give rise to brain microglia in microgliosis | Mildner et al. 2007. Nat Neurosci. 10:1544 | |
| Increase in response to hypercholesterolemia and accumulate in atheromata | Swirski et al. 2007. J. Clin. Invest. 117:195 | |
| Accumulate in atheromata via CCR2, CCR5 and CX3CR1 | Tacke et al. 2007. J. Clin. Invest. 117:185 | |
| Inflammation | Behavior of Ly-6Clo monocytes | Reference |
|---|---|---|
| None | Accumulate in all tissues | Geissmann et al. 2003. Immunity. 19:71 |
| Patrol the vasculature | Auffray et al 2007. Science. 317:666 | |
| Acute | Accumulate very early in tissue | Auffray et al 2007. Science. 317:666 |
| Perform anti-inflammatory function, granulation tissue formation, angiogenesis | Nahrendorf et al. 2007. J. Exp. Med. 204:3037 | |
| Chronic | Accumulate in atheromata via CCR5 | Tacke et al. 2007. J. Clin. Invest. 117: 185 |
Monocytes and their progeny have long been understood as ‘plastic’ cells, capable of adapting to their local environment50-52. However, the existence of subsets in circulation raises the possibility that monocytes commit for specific function prior to tissue infiltration, and may reconcile the perhaps contradictory activities attributed to the entire monocyte repertoire. Future studies will need to determine the quality and extent of commitment, and how the local environment influences the consequent phenotype. It is conceivable that the eventual phenotype monocytes acquire in tissue depends on a sequence of ontogenically (cell dictates environment) and environmentally (environment dictates cell) integrated cues and checkpoints. The emerging picture then, positions monocyte subsets and their progeny as active participants in a vast immune regulatory network, rather than as downstream responders of ongoing inflammation. By extension, the circulatory system is a reservoir of functionally distinct components and may be an attractive target for discriminate therapeutic intervention.
It has been known for some time that tissue macrophages are also heterogeneous53, 54. ‘M1’ cells, or classically activated macrophages, are inflammatory and arise by stimulating their progenitors with inflammatory stimuli such as LPS or GM-CSF. ‘M2’ cells, or alternatively activated macrophages, display a more regulatory function and arise when progenitors are cultured with IL-4 and IL-13. In vivo, these cells may be discriminated because they express a set of unique markers. It is unknown whether Ly-6Chi monocytes selectively give rise to M1 macrophages and, conversely, whether Ly-6Clo monocytes give rise to M2 macrophages.
TOOLS TO INVESTIGATE THE ROLE OF SUBSETS
Most of our knowledge on the activity of monocyte populations has been obtained with ex vivo readouts (flow cytometry, histology), and has made use of a variety of genetically modified mice such as chemokine receptor knockout (CCR2-/-, CCR5-/-, CX3CR1-/-) or of mice reconstituted with modified bone marrow cells19, 23, 24, 27-29, 32, 34, 35, 55-59. These are useful to determine the location and phenotype of cell types at given time points. Initial investigations also focused on surrogate cell culture models that do not always reproduce the behavior of immune cells in tissues60. Because immune processes are dynamic, new tools have been developed to track cells in real time and to inform on cellular interactions, migration and delivery of effector function (Figure 1). Cellular imaging technologies have enabled the dynamic study of labeled immune cells in intact tissue environments. The imaging approaches provide either real-time microscopic cellular resolution or quantitative whole organ information61, and some have translational potential62. The techniques used to label and follow the fate of monocytes and other leukocyte populations in vivo are described below and can be broadly divided into three categories that rely on: genetic reporters, exogenous cell trackers and injectable imaging agents (Table 2).
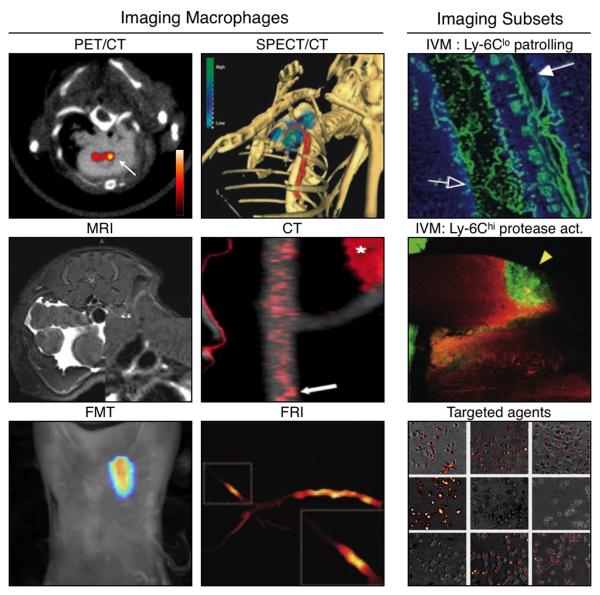
Figure 1. Molecular Imaging modalities that inform on monocyte/macrophage presence and function.
Monocyte/macrophages and associated functions have been imaged with different molecular imaging modalities at different sensitivities and resolutions. Imaging of macrophages or function has been achieved with PET/CT (image shows uptake of 64Cu targeted nanoparticles by macrophages)74; SPECT/CT (image shows accumulation of exogenously-labeled and adoptively injected 111In-monocytes)10; MRI (image shows accumulation of macrophage-targeted immunomicelles)82; CT (image shows uptake of CT-contrast agent N1177 by macrophages)83; FMT (image shows uptake of fluorescent nanoagents by macrophages; unpublished); and FRI (image shows protease-activatable regions of an excised rabbit aorta)93. To gain insight into the differential in vivo behavior of monocyte subsets, promising tools involve IVM (top image shows patrolling Ly-6Clo monocytes22; bottom image shows Ly-6Chi-associated proteolysis88) and the development of novel monocyte subset-targeted agents (image shows specificity of an agent for activated but not resting macrophages)81.
Table 2.
Imaging modalities and labeling strategies
| Imaging modality |
Resolution | Labeling approaches |
Fusion imaging |
||
|---|---|---|---|---|---|
| Genetic reporter |
Exogenous cell tracker |
Injectable targeting agent |
|||
| IVM | 1 μm | Fluorescent proteins |
No | ||
| Fluor. agents (visible, NIR) |
|||||
| Fluor. agents (visible, NIR) |
|||||
| Fiberoptics | 1-5 μm | Fluorescent proteins |
No | ||
| Fluor. agents (visible, NIR) |
|||||
| Fluor. agents (visible, NIR) |
|||||
| FMT | 1 mm | Fluor. agents (NIR) |
with CT, PET, SPECT, MRI |
||
| Fluor. agents (NIR) |
|||||
| PET | 1-2 mm | HS-VTK | with CT, FMT, SPECT, MRI |
||
| Isotope-labeled agents (64Cu) |
|||||
| Isotope-labeled agents (64Cu) |
|||||
| SPECT | 1-2 mm | Isotope-labeled agents (111In) |
with CT, FMT, PET, MRI |
||
| Isotope-labeled agents (111In) |
|||||
| MRI | 10-100 μm | (Super)para- magnetic particles |
with CT, FMT, PET, SPECT |
||
| (Super)para- magnetic particles |
|||||
IVM, Intravital microscopy; Fiberoptics, Catheter-based fiberoptic imaging; FMT, fluorescence molecular tomography; PET, position emission tomography; SPECT, single photon emission computed tomography; MRI, magnetic resonance imaging; CT, x-ray computed tomography. Fusion imaging refers to imaging modalities that can be combined. Fusion imaging is expected to help future studies aimed at better detecting cells and other biological activities as they unfold in vivo. The advantages and limitations of the imaging modalities and labeling approaches listed here are detailed in the text.
Genetic reporters are used to stably express imaging agents such as fluorescent (e.g., green fluorescent protein (GFP) and its derivatives)63, bioluminescent (e.g., luciferases)64 or other fusion proteins (e.g., herpes simplex virus-1 thymidine kinase (HSV-Tk))65. The reporter genes are typically inserted under the control of a promoter of interest. For example, mice expressing GFP under the control of the Csf1r, CD11b or Cx3cr1 promoters are available and report on monocytes and their lineage descendants19, 66. Specifically, GFP expression driven by the Cx3cr1 promoter has been used to distinguish Ly-6Chi (CX3CR1lo) from Ly-6Clo (CX3CR1hi) monocytes both ex vivo19 and in vivo22. The genetic reporter approach is particularly useful for the study of cells at microscopic resolution and offers long term tracking possibilities because the imaging agent is not diluted due to cell division. Multiphoton and confocal intravital microscopy (IVM) are methods of choice for the dynamic study of optically-labeled cells because they permit analysis at single cell resolution, in three dimensions, and at optical penetration of tissues up to 800 μm61, 67-70. Several different fluorophores can be excited at a single excitation wavelength, thus allowing the simultaneous detection of differentially tagged fluorescent objects. However, in the context of atherosclerosis, these methods will require the development of sophisticated tools, for example for tissue immobilization, because of the proximity of plaques to the beating heart. Also, current limitations of genetic reporter approaches include inadequate imaging in larger fields of view (e.g., whole body), at increased depths (e.g., >600mm), or in human patients. Bioluminescence imaging is highly sensitive but detection of target signal depends on the positioning (depth) of the emitted photons and thus does not allow absolute quantification61. Positron emission tomography (PET) imaging allows visualization of 131I— or 124I— 2′-fluoro-2′-deoxy-1-β-D-arabinofuranosyl-5-iodouracil (FIAU), a radionuclide that selectively accumulates in HSV-TK expressing cells. All the agents mentioned above have been used widely and successfully in animal models, but some are potentially immunogenic and thus have limited or no clinical translatability.
Exogenous cell trackers are being used to label purified populations of monocytes. The cells are re-injected into a recipient and tracked with appropriate imaging techniques. Exogenous trackers include a variety of optical, nuclear and magnetic resonance imaging agents. Optical imaging agents comprise fluorochromes that emit in the visible light range71, 72 (CFSE, CMTMR) and can be detected by IVM at single cell resolution near the body surface, and far-red/near-infrared agents67 (VivoTag-680, VivoTag-750) that emit light at longer wavelength and allow detection of cells deeper within the whole body (at least in small animal models). Fluorescence molecular tomography (FMT) is an imaging technology that can reconstruct three-dimensional maps of near-infrared fluorochromes in tissues based on advanced algorithms, and allows absolute quantification of target signal73. FMT can be combined with x-ray computed tomography (CT) or magnetic resonance imaging (MRI) for improved photon reconstruction and anatomic localization74, 75. Nuclear imaging agents include FDA-approved 111In-oxine10, 67, 75-77 that labels monocytes efficiently and can be detected noninvasively within the whole body by single photon emission computed tomography (SPECT) imaging76. The half-lives of the radioisotopes and cell division may limit the ability to track cells longitudinally, although we have been able to monitor leukocytes for up to 5 days when using long-lived radionuclides75. Magnetic agents include cross-linked iron oxide (CLIO) nanoparticles78 and ultrasmall superparamagnetic iron-oxide (USPIO) particles79 and Feridex80. Long-term tracking with this method may also be limited when cells divide or metabolize the agent. Coupling of the particles with fluorochromes61, 73 or radionuclides74 allows additional in vivo detection of labeled cells by optical (IVM, FMT) or nuclear (SPECT, PET) methods, respectively. Nuclear imaging offers sensitive detection irrespectively of tissue depth, but has limited spatial resolution and requires the use of radioactive agents. Conversely, MRI has lower sensitivity but can offer higher resolution. All the cell trackers mentioned above feature high cell membrane permeability, prolonged intracellular retention and low toxicity. They allow highly sensitive real-time monitoring of labeled cells across all resolution scales from microscopic to macroscopic and are potentially clinically translatable. However, one challenge associated with exogenous labels is to track cell fate and survival. For example, a signal does not distinguish between a viable labeled cell and an imaging agent that has been released (i.e., from dying cells) and sequestered elsewhere. For fluorescent agents, flow cytometry analysis and cell sorting of labeled cells can also be considered after adoptive transfer to correlate in vivo results. For example, adoptive transfer of ex vivo-labeled monocytes has shown continuous accumulation of monocytes during atherogenesis10. Accumulation can be imaged ex vivo and in vivo longitudinally, and is attenuated with statin treatment76. It remains unknown, however, whether defined monocyte subsets accumulate in specific lesions or at specific stages of lesional development, and if they contribute differentially to atherogenesis.
Injectable imaging agents are compounds that can be administered into live subjects to label cells (monocyte subsets) or molecules (monocyte-associated proteases) of interest, and that can be detected by appropriate imaging modalities. Injectable agents with magnetic properties include derivitized/functionalized CLIO and USPIO particles79, 81 and Gd3+-loaded micelles carrying specific antibodies (e.g., targeting scavenger receptors)82. Some agents are preferentially taken up, and in some cases phagocytosed, by endogenous monocyte and macrophage populations, and can be detected by MRI in atherosclerotic lesions. Iodine-containing contrast agents can also label lesional macrophages for detection with CT83, while nuclear agents such as 64Cu-labeled nanoparticles show good avidity for lesional macrophages and efficient detection by PET imaging74. Other PET tracers include 18F-FDG, which is taken up by all metabolically active cells84 and is therefore not specific to macrophages. Typically, the injectable imaging agents mentioned above label monocytes indiscriminately, and thus cannot be used to study monocyte heterogeneity. Most recently, efforts have been made to develop novel injectable agents that offer more selective targeting capabilities. The strategy employs phage libraries that are modified with various peptide affinity ligands85, 86, or nanoparticle libraries derivatized with small molecules81, and that are screened against cell populations of interest. For example, some small molecules ‘tune’ nanoparticle surfaces and can redirect uptake into specific cell subpopulations81. The technique has been used successfully to identify agents that are specific for activated versus resting macrophages81 and for M2 macrophages within the tumor-microenviroenment 87. It is hoped that such strategies will define more agents with selectivity for monocyte or macrophage subsets. Other interesting injectable imaging agents rely on detection of molecular functions such as enzymatic activity. For example, proteases such as cathepsin B, K, S and L and matrix metalloprotease (MMP)-2 and 12 are expressed abundantly during inflammation, and participate actively in tissue remodeling and plaque destabilisation88-90, as well as in allergic airway inflammation91. Protease-targeted optical reporters are based on a polymeric scaffold that consists of near-infrared fluorochromes, specific protease peptide substrates, and partially methoxypegylated graft copolymers. The sensor is injected in its inactive state in which fluorochromes are not excitable due to auto-quenching. Proteolytic cleavage of the scaffold by a specific enzyme releases the fluorochromes and results in extensive fluorescence generation locally (de-quenching). Amplification is achieved because one active enzyme moiety can activate multiple reporters73. These probes have been used to image enzyme activity by IVM in surgically exposed carotid atherosclerotic plaques88, 92, and in intact animals either by real-time near infrared catheter molecular sensing93 or by FMT and with cellular resolution by fluorescence microscopy in a variety of inflammatory conditions67, 78, 91, 94. Of note, some injectable agents confer transient background noise, and in this case it is preferable to define precise timing for imaging after injection to optimize signal-to-noise ratios. The protease-targeted optical sensors may be particularly useful to study monocyte/macrophage heterogeneity because some proteases are typically expressed at higher levels in Ly-6Chi monocytes when compared to their Ly-6Clo counterparts24, 78. Injectable imaging agents with specificity for molecular targets are under intense scrutiny because they can carry multiple reporters for imaging at different resolutions and depths, they can combine diagnostic and therapeutic interventional capabilities, and they offer the advantage of being usable in various experimental animals and in humans (Table 3).
Table 3.
Differential expression of biomarkers in subsets
| Biomarker | Cell type (mouse) | Cell type (human) | Function |
|---|---|---|---|
| CD14 | ND | CD14+CD16lo | Receptor for detection of bacterial lipopolysaccharide |
| CD16 | ND | CD14loCD16hi | Fcy receptor binds to Fc portion of IgG antibodies |
| CCR2 | Ly-6Chi | CD14+CD16lo | A chemokine receptor that promotes recruitment of Ly-6Chi monocytes to lesions in mice |
| CX3CR1 | Ly-6Clo > Ly-6Chi | CD14loCD16hi > CD14+CD16lo | A chemokine receptor that has surprisingly been reported to promote recruitment of Ly-6Chi, but not Ly-6Clo, monocytes to lesions |
| Cathepsins | Ly-6Chi > Ly-6Clo | ND | Proteolytic enzymes that remodel the extracellular matrix and decrease stability of plaques |
| MMPs | Ly-6Chi > Ly-6Clo | ND | Proteolytic enzymes that remodel the extracellular matrix and decrease stability of plaques |
| MPO | Ly-6Chi > Ly-6Clo | ND | An oxydant-generating enzyme that promotes inflammation and plaque formation and destabilization |
ND, either differences between subsets have not be determined or no clear species conservation of antigens
EMERGING VIEWS ON THE ROLE OF MONOCYTE SUBSETS IN ATHEROSCLEROSIS
The combination of imaging approaches mentioned above and classical cell and molecular biology tools have revealed new insights on the roles of monocyte subsets during disease progression (Figure 2). Several reports have shown that hypercholesterolemic mice undergo a gradual and systemic increase of Ly-6Chi monocytes. These cells adhere preferentially to activated endothelium and accumulate efficiently in progressive lesions in a CCR2, CCR5 and CX3CR1-dependent manner28, 29. The accompanying Ly-6Chi monocytosis requires elevated concentrations of cholesterol or lipid derivatives, and results from increased cell survival, continued proliferation and, possibly, impaired Ly-6Chi → Ly6Clo conversion. The presence of a cholesterol-lowering drug of the statin class reduces Ly-6Chi monocytosis and atherosclerosis28. Thus, increased serum cholesterol levels, in addition to promoting lipid deposition to lesions95 and inducing M-CSF production for local monocyte maturation into macrophages96, 97, also trigger the expansion of circulating monocytes. These findings suggest that a cholesterol-rich diet promotes murine atherogenesis, at least in part through the development of Ly-6Chi monocytosis. Ly-6Chi monocytes recruited to atherosclerotic aortas differentiate into macrophages28 although some cells may acquire a DC phenotype29. It is likely that most of the Ly-6Chi cells acquire traits of lesional M1 cells and eventually become foam cells, although decisive experimental evidence in support of this is currently lacking.
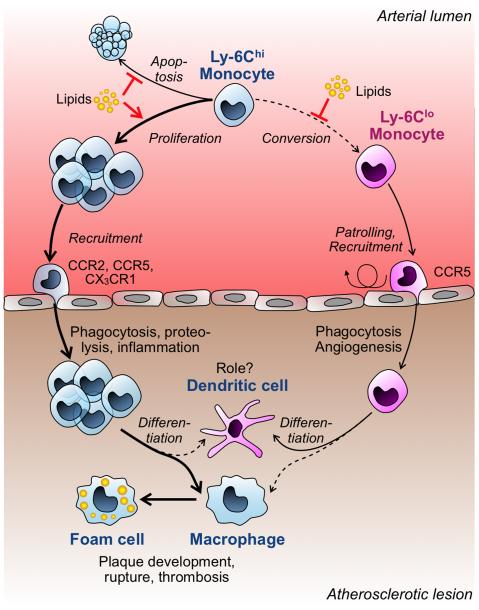
Figure 2. Model for the role of monocyte subtypes in experimental mouse atherosclerosis.
Elevated levels of cholesterol or lipid derivatives (‘Lipids’) expand the Ly-6Chi monocyte repertoire in circulation by several mechanisms that include reduction of cell apoptosis, increased proliferation and impaired Ly-6Chi → Ly-6Clo conversion. Ly-6Chi monocytes are recruited to atherosclerotic lesions via the CCR2, CCR5 and CX3CR1 chemokine receptors. The recruited cells differentiate massively into macrophages, although some cells can acquire a DC phenotype. Lesional macrophages eventually become foam cells and participate in plaque development, rupture and thrombosis. Ly-6Clo monocytes do no expand in periphery and infiltrate lesions less frequently than Ly-6Chi monocytes. Their recruitment depends on CCR2, but may not require CX3CR1. Ly-6Clo cells exhibit phagocytic and pro-angiogenic functions, and may preferentially mature into DC in lesions, however their participation in disease progression remains largely unknown.
Ly-6Clo monocytes infiltrate atherosclerotic lesions less frequently than Ly-6Chi monocytes. Their accumulation relies on CCR5, but surprisingly not on CX3CR1 as it is the case for Ly-6Chi monocytes29. There exists some controversy as to how Ly-6Clo monocytes participate in disease progression. For example, it was found that the greatest decrease of lesions occurs when all CCR2, CCR5 and CX3CR1 chemokine receptors are blocked simultaneously58, 59, indirectly suggesting that both Ly-6Chi and Ly-6Clo monocytes contribute to lesion bulk. Conversely, the findings that Ly-6Clo monocytes are less inflammatory and mediate their effects via granulation tissue formation opens the possibility that this subset promotes a more ‘stable’ lesion, if not necessarily a smaller one24, 98. Clarification of this issue may necessitate new tools for specific ablation of Ly-6Clo monocytes in lesions while sparing their Ly-6Chi counterparts. Interestingly, Ly-6Clo cells selectively upregulate CD11c and may mature to DC in lesions, as observed with in vivo labeling of monocytes with latex beads29. This suggests a role for these cells in controlling adaptive immune responses locally, although their precise functions and the types of responses that they dictate remain speculative. Finally, the recent finding that Ly-6Clo monocytes patrol the vasculature suggests these cells may control the influx of other monocytes in the growing atheromata22.
NEW CHALLENGES AND OPPORTUNITIES
A number of critical questions on monocyte subsets in experimental atherosclerosis remain (Table 4). For example, do monocyte subsets accumulate in different lesions and/or at different stages of lesional evolution? What are the functional fates of monocyte subsets in lesions? Is ablation of specific subsets a promising treatment strategy? The technical challenges that accompany these questions stem from the size and location of lesions in the body and the frequency of monocytes in the blood, and include motion artifacts, depth penetration of imaging modality and its spatial resolution and sensitivity. The questions necessitate that the tools outlined above are optimized and validated to, eventually, specifically and selectively target, enumerate, image and ablate candidate subsets. Future insight into the heterogeneous in vivo behavior of monocyte subsets will therefore require a coordinated effort of technical and biological considerations.
Table 4.
Most pressing biological and technical considerations
| Biological questions | |
|---|---|
| 1 | What is the precise fate of monocyte subsets once they have entered lesions and do they contribute differentially to disease progression? |
| 2 | Does specific ablation or inhibition of a monocyte subtype result in descreased lesion burden while sparing host defense and repair mechanisms? |
| 3 | Do the findings for mouse monocyte subsets hold true in humans? |
| Technical challenges | |
|---|---|
| 1 | Validation of intravital imaging technologies with subcellular resolution to study the complexity of the host response in target sites (atheromata, draining lymph nodes) |
| 2 | Development of new agents that permit to distinguish leukocyte subtypes (monocyte, macrophage and DC subsets, and other cells present in atheromata) |
| 3 | Development of injectable agents that target cell populations of interest, can be used in humans, and have theranostic potential |
Insights gained in experimental systems will require validation in humans, especially given that monocyte subsets in murine atherosclerosis appear to exhibit contradistinctive behavior. Studies must first determine how well murine monocyte subsets correspond to their human counterparts. Further clinical studies will examine whether monocyte subsets are prospective biomarkers of disease and whether they represent treatment targets. Imaging will likely be an important tool for these purposes. For example, some exogenous cell trackers and injectable agents that selectively image subset presence or subset-associated activity may be clinically translatable. If studies show, for example, that a particular subset associates with or predicts for complications of atherosclerosis, it may be useful to employ either ex vivo or in vivo imaging tools to non-invasively and rapidly assess a patient’s prognosis. The link between monocyte heterogeneity and disease also offers a number of treatment opportunities. These include inhibition of subset accumulation, inhibition of subset-associated function, subset ablation in blood and/or lesion, and sequestration of subsets from lesions. For example, antagonists against CCR2 may lead to better prognosis through inhibiting accumulation of CD16lo (CCR2hi) monocyte subsets. Inhibition of protease activity that associates with the inflammatory monocyte population is another opportunity for treatment. Subset depletion may be achieved (at least theoretically) by covalently coupling monocyte-specific agents to novel photosensitizers (i.e. meso-tetraphenyldichlorin) for photodynamic therapy, or to other cell death-inducing agents99. Finally, it may be possible to treat atherosclerosis by promoting efflux of particular cell types from atheromata100. Together, these methods may lead to better risk stratification and may pave the way for monocyte subset-based therapies.
ACKNOWLEDGEMENTS
Sources of Funding: This work was supported in part by NIH grants U01 HL080731, P50 CA86355, R24 CA69246 and P01-A154904 (to R.W.) and the MGH-Center for Systems Biology (to M.J.P.).
Acknowledgements: None
Footnotes
Disclosure: None
REFERENCES
- 1.Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov. 2004;3:913–925. doi: 10.1038/nrd1548. [DOI] [PubMed] [Google Scholar]
- 2.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 3.Goldschmidt-Clermont PJ, Creager MA, Losordo DW, Lam GK, Wassef M, Dzau VJ. Atherosclerosis 2005: recent discoveries and novel hypotheses. Circulation. 2005;112:3348–3353. doi: 10.1161/CIRCULATIONAHA.105.577460. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 5.Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, Duggan WT, Ruzyllo W, Bachinsky WB, Lasala GP, Tuzcu EM. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–1316. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 6.Tall AR, Yvan-Charvet L, Wang N. The failure of torcetrapib: was it the molecule or the mechanism? Arterioscler Thromb Vasc Biol. 2007;27:257–260. doi: 10.1161/01.ATV.0000256728.60226.77. [DOI] [PubMed] [Google Scholar]
- 7.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Glomset JA. The pathogenesis of atherosclerosis (second of two parts) N Engl J Med. 1976;295:420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- 9.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 10.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 12.Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37(Suppl 1):S9–17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 13.Waldo SW, Li Y, Buono C, Zhao B, Billings EM, Chang J, Kruth HS. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 15.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 16.Draude G, von Hundelshausen P, Frankenberger M, Ziegler-Heitbrock HW, Weber C. Distinct scavenger receptor expression and function in the human CD14(+)/CD16(+) monocyte subset. Am J Physiol. 1999;276:H1144–1149. doi: 10.1152/ajpheart.1999.276.4.H1144. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 18.Drevets DA, Dillon MJ, Schawang JS, Van Rooijen N, Ehrchen J, Sunderkotter C, Leenen PJ. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J Immunol. 2004;172:4418–4424. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- 19.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 20.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 21.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 23.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 24.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noel JG, Osterburg A, Wang Q, Guo X, Byrum D, Schwemberger S, Goetzman H, Caldwell CC, Ogle CK. Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock. 2007;28:684–693. doi: 10.1097/shk.0b013e31805362ed. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen JW, Cello J, Gil H, Forestal CA, Furie MB, Thanassi DG, Benach JL. Mac-1+ cells are the predominant subset in the early hepatic lesions of mice infected with Francisella tularensis. Infect Immun. 2006;74:6590–6598. doi: 10.1128/IAI.00868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robben PM, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6C hi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Manivannan A, Dawson R, Crane IJ, Mack M, Sharp P, Liversidge J. Differentiation to the CCR2+ inflammatory phenotype in vivo is a constitutive, timelimited property of blood monocytes and is independent of local inflammatory mediators. J Immunol. 2005;175:6915–6923. doi: 10.4049/jimmunol.175.10.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179:3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 34.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 35.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008 doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 37.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 40.Cannon CP, McCabe CH, Wilcox RG, Bentley JH, Braunwald E. Association of white blood cell count with increased mortality in acute myocardial infarction and unstable angina pectoris. OPUS-TIMI 16 Investigators. Am J Cardiol. 2001;87:636–639. A610. doi: 10.1016/s0002-9149(00)01444-2. [DOI] [PubMed] [Google Scholar]
- 41.Fingerle-Rowson G, Auers J, Kreuzer E, Fraunberger P, Blumenstein M, Ziegler-Heitbrock LH. Expansion of CD14+CD16+ monocytes in critically ill cardiac surgery patients. Inflammation. 1998;22:367–379. doi: 10.1023/a:1022316815196. [DOI] [PubMed] [Google Scholar]
- 42.Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med. 1974;290:1275–1278. doi: 10.1056/NEJM197406062902302. [DOI] [PubMed] [Google Scholar]
- 43.Hanai H, Iida T, Takeuchi K, Watanabe F, Yamada M, Kikuyama M, Maruyama Y, Iwaoka Y, Hirayama K, Nagata S, Takai K. Adsorptive depletion of elevated proinflammatory CD14+CD16+DR++ monocytes in patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103:1210–1216. doi: 10.1111/j.1572-0241.2007.01714.x. [DOI] [PubMed] [Google Scholar]
- 44.Horelt A, Belge KU, Steppich B, Prinz J, Ziegler-Heitbrock L. The CD14+CD16+ monocytes in erysipelas are expanded and show reduced cytokine production. Eur J Immunol. 2002;32:1319–1327. doi: 10.1002/1521-4141(200205)32:5<1319::AID-IMMU1319>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Kapinsky M, Torzewski M, Buchler C, Duong CQ, Rothe G, Schmitz G. Enzymatically degraded LDL preferentially binds to CD14(high) CD16(+) monocytes and induces foam cell formation mediated only in part by the class B scavenger-receptor CD36. Arterioscler Thromb Vasc Biol. 2001;21:1004–1010. doi: 10.1161/01.atv.21.6.1004. [DOI] [PubMed] [Google Scholar]
- 46.Rothe G, Gabriel H, Kovacs E, Klucken J, Stohr J, Kindermann W, Schmitz G. Peripheral blood mononuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1996;16:1437–1447. doi: 10.1161/01.atv.16.12.1437. [DOI] [PubMed] [Google Scholar]
- 47.Rothe G, Herr AS, Stohr J, Abletshauser C, Weidinger G, Schmitz G. A more mature phenotype of blood mononuclear phagocytes is induced by fluvastatin treatment in hypercholesterolemic patients with coronary heart disease. Atherosclerosis. 1999;144:251–261. doi: 10.1016/s0021-9150(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 48.Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, Lackner KJ, Iz M, Meyer J, Darius H, Rupprecht HJ. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 49.Todd I, Radford PM, Ziegler-Heitbrock L, Ghaemmaghami AM, Powell RJ, Tighe PJ. Elevated CD16 expression by monocytes from patients with tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum. 2007;56:4182–4188. doi: 10.1002/art.23133. [DOI] [PubMed] [Google Scholar]
- 50.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volkman A. The origin and fate of the monocyte. Ser Haematol. 1970;3:62–92. [PubMed] [Google Scholar]
- 52.Volkman A, Gowans JL. The Origin Of Macrophages From Bone Marrow In The Rat. Br J Exp Pathol. 1965;46:62–70. [PMC free article] [PubMed] [Google Scholar]
- 53.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 54.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Guo J, Van Eck M, Twisk J, Maeda N, Benson GM, Groot PH, Van Berkel TJ. Transplantation of monocyte CC-chemokine receptor 2-deficient bone marrow into ApoE3-Leiden mice inhibits atherogenesis. Arterioscler Thromb Vasc Biol. 2003;23:447–453. doi: 10.1161/01.ATV.0000058431.78833.F5. [DOI] [PubMed] [Google Scholar]
- 56.Guo J, de Waard V, Van Eck M, Hildebrand RB, van Wanrooij EJ, Kuiper J, Maeda N, Benson GM, Groot PH, Van Berkel TJ. Repopulation of apolipoprotein E knockout mice with CCR2-deficient bone marrow progenitor cells does not inhibit ongoing atherosclerotic lesion development. Arterioscler Thromb Vasc Biol. 2005;25:1014–1019. doi: 10.1161/01.ATV.0000163181.40896.42. [DOI] [PubMed] [Google Scholar]
- 57.Veillard NR, Steffens S, Pelli G, Lu B, Kwak BR, Gerard C, Charo IF, Mach F. Differential influence of chemokine receptors CCR2 and CXCR3 in development of atherosclerosis in vivo. Circulation. 2005;112:870–878. doi: 10.1161/CIRCULATIONAHA.104.520718. [DOI] [PubMed] [Google Scholar]
- 58.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 59.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2-/- mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pittet MJ, Mempel TR. Regulation of T-cell migration and effector functions: insights from in vivo imaging studies. Immunol Rev. 2008;221:107–129. doi: 10.1111/j.1600-065X.2008.00584.x. [DOI] [PubMed] [Google Scholar]
- 61.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 63.Giepmans BN, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 64.Contag PR, Olomu IN, Stevenson DK, Contag CH. Bioluminescent indicators in living mammals. Nat Med. 1998;4:245–247. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]
- 65.Koehne G, Doubrovin M, Doubrovina E, Zanzonico P, Gallardo HF, Ivanova A, Balatoni J, Teruya-Feldstein J, Heller G, May C, Ponomarev V, Ruan S, Finn R, Blasberg RG, Bornmann W, Riviere I, Sadelain M, O’Reilly RJ, Larson SM, Tjuvajev JG. Serial in vivo imaging of the targeted migration of human HSV-TK-transduced antigen-specific lymphocytes. Nat Biotechnol. 2003;21:405–413. doi: 10.1038/nbt805. [DOI] [PubMed] [Google Scholar]
- 66.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swirski FK, Berger CR, Figueiredo JL, Mempel TR, von Andrian UH, Pittet MJ, Weissleder R. A near-infrared cell tracker reagent for multiscopic in vivo imaging and quantification of leukocyte immune responses. PLoS ONE. 2007;2:e1075. doi: 10.1371/journal.pone.0001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Germain RN, Miller MJ, Dustin ML, Nussenzweig MC. Dynamic imaging of the immune system: progress, pitfalls and promise. Nat Rev Immunol. 2006;6:497–507. doi: 10.1038/nri1884. [DOI] [PubMed] [Google Scholar]
- 69.Bousso P, Robey EA. Dynamic behavior of T cells and thymocytes in lymphoid organs as revealed by two-photon microscopy. Immunity. 2004;21:349–355. doi: 10.1016/j.immuni.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Celli S, Garcia Z, Beuneu H, Bousso P. Decoding the dynamics of T cell-dendritic cell interactions in vivo. Immunol Rev. 2008;221:182–187. doi: 10.1111/j.1600-065X.2008.00588.x. [DOI] [PubMed] [Google Scholar]
- 71.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 72.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 73.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23:313–320. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- 74.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pittet MJ, Grimm J, Berger CR, Tamura T, Wojtkiewicz G, Nahrendorf M, Romero P, Swirski FK, Weissleder R. In vivo imaging of T cell delivery to tumors after adoptive transfer therapy. Proc Natl Acad Sci U S A. 2007;104:12457–12461. doi: 10.1073/pnas.0704460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kircher MF, Grimm J, Swirski FK, Libby P, Gerszten RE, Allport JR, Weissleder R. Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions. Circulation. 2008;117:388–395. doi: 10.1161/CIRCULATIONAHA.107.719765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pittet MJ, Swirski FK, Reynolds F, Josephson L, Weissleder R. Labeling of immune cells for in vivo imaging using magnetofluorescent nanoparticles. Nat Protoc. 2006;1:73–79. doi: 10.1038/nprot.2006.11. [DOI] [PubMed] [Google Scholar]
- 78.Nahrendorf M, Sosnovik DE, Waterman P, Swirski FK, Pande AN, Aikawa E, Figueiredo JL, Pittet MJ, Weissleder R. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ Res. 2007;100:1218–1225. doi: 10.1161/01.RES.0000265064.46075.31. [DOI] [PubMed] [Google Scholar]
- 79.Trivedi RA, Mallawarachi C, JM UK-I, Graves MJ, Horsley J, Goddard MJ, Brown A, Wang L, Kirkpatrick PJ, Brown J, Gillard JH. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–1606. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 80.Zelivyanskaya ML, Nelson JA, Poluektova L, Uberti M, Mellon M, Gendelman HE, Boska MD. Tracking superparamagnetic iron oxide labeled monocytes in brain by high-field magnetic resonance imaging. J Neurosci Res. 2003;73:284–295. doi: 10.1002/jnr.10693. [DOI] [PubMed] [Google Scholar]
- 81.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 82.Amirbekian V, Lipinski MJ, Briley-Saebo KC, Amirbekian S, Aguinaldo JG, Weinreb DB, Vucic E, Frias JC, Hyafil F, Mani V, Fisher EA, Fayad ZA. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A. 2007;104:961–966. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hyafil F, Cornily JC, Feig JE, Gordon R, Vucic E, Amirbekian V, Fisher EA, Fuster V, Feldman LJ, Fayad ZA. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13:636–641. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 84.Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V, Fayad ZA. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–896. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 85.Kelly K, Alencar H, Funovics M, Mahmood U, Weissleder R. Detection of invasive colon cancer using a novel, targeted, library-derived fluorescent peptide. Cancer Res. 2004;64:6247–6251. doi: 10.1158/0008-5472.CAN-04-0817. [DOI] [PubMed] [Google Scholar]
- 86.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96:327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 87.Leimgruber A, Berger C, Cortez-Retamozo V, Etzrodt M, Newton AP, Waterman P, Figueiredo JL, Kohler R, Elpek N, Mempel TR, Swirski FK, Nahrendorf M, Weissleder R, Pittet MJ. Behavior of endogenous tumor-associated macrophages assessed in vivo using a functionalized nanoparticle. Neoplasia. 2009 doi: 10.1593/neo.09356. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, Kohler RH, Shi GP, Libby P, Weissleder R. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115:2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 89.Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, Duramad P, Sun C, Zhang Y, Yang X, Peters C, Shi GP. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115:2065–2075. doi: 10.1161/CIRCULATIONAHA.107.688523. [DOI] [PubMed] [Google Scholar]
- 90.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cortez-Retamozo V, Swirski FK, Waterman P, Yuan H, Figueiredo JL, Newton AP, Upadhyay R, Vinegoni C, Kohler R, Blois J, Smith A, Nahrendorf M, Josephson L, Weissleder R, Pittet MJ. Real-time assessment of inflammation and treatment response in a mouse model of allergic airway inflammation. J Clin Invest. 2008;118:4058–4066. doi: 10.1172/JCI36335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in earlystage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 93.Jaffer FA, Vinegoni C, John MC, Aikawa E, Gold HK, Finn AV, Ntziachristos V, Libby P, Weissleder R. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118:1802–1809. doi: 10.1161/CIRCULATIONAHA.108.785881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grimm J, Kirsch DG, Windsor SD, Kim CF, Santiago PM, Ntziachristos V, Jacks T, Weissleder R. Use of gene expression profiling to direct in vivo molecular imaging of lung cancer. Proc Natl Acad Sci U S A. 2005;102:14404–14409. doi: 10.1073/pnas.0503920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parhami F, Fang ZT, Fogelman AM, Andalibi A, Territo MC, Berliner JA. Minimally modified low density lipoprotein-induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. J Clin Invest. 1993;92:471–478. doi: 10.1172/JCI116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi W, Haberland ME, Jien ML, Shih DM, Lusis AJ. Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation. 2000;102:75–81. doi: 10.1161/01.cir.102.1.75. [DOI] [PubMed] [Google Scholar]
- 98.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 99.McCarthy JR, Jaffer FA, Weissleder R. A macrophage-targeted theranostic nanoparticle for biomedical applications. Small. 2006;2:983–987. doi: 10.1002/smll.200600139. [DOI] [PubMed] [Google Scholar]
- 100.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]