Abstract
Objective
This study tests the hypothesis that S1P2R regulates expression of SMC-differentiation genes following arterial injury.
Methods and Results
Carotid ligation injury was performed in wild-type and S1P2R-null mice. At various time points after injury, expression of multiple SMC differentiation genes, myocardin, and S1P receptors (S1P1R, S1P2R, and S1P3R) was measured by quantitative PCR. These experiments demonstrate that at day 7 after injury, S1P2R specifically regulates expression of smooth muscle α-actin (SMA) and that this is not mediated by changes in expression of myocardin or any of the S1PRs. In vitro studies using carotid SMCs prepared from wild-type and S1P2R-null mice show that S1P stimulates expression of all SMC-differentiation genes tested, but S1P2R significantly regulates expression of SMA and SM22α only. Chromatin immunoprecipitation assays suggest that S1P-induced recruitment of serum response factor to the SMA promoter and enhancer largely depends on S1P2R. S1P-stimulated SMA expression requires S1P2R-dependent activation of RhoA and mobilization of calcium from intracellular stores. Chelation of calcium does not affect the activation of RhoA by S1P, whereas blockade of Rho by C3 exotoxin partially inhibits the mobilization of calcium by S1P.
Conclusions
The results of this study support the hypothesis that S1P2R regulates expression of SMA after injury. We further conclude that transcriptional regulation of SMA by S1P in vitro requires S1P2R-dependent activation of RhoA and mobilization of calcium from intracellular calcium stores.
Keywords: sphingosine-1-phosphate, S1P2R, smooth muscle actin, Rho, calcium
Restenosis is a major complication of surgical procedures performed to restore arterial blood flow. Despite all efforts, pharmacological inhibition of restenosis has not yet been achieved. In normal arteries, smooth muscle cells (SMCs) are quiescent and regulate vascular tone. After arterial injury, medial SMCs proliferate and lose expression of genes encoding the contractile apparatus, a process referred to as phenotypic modulation.1, 2 It is generally believed that decreased expression of these “differentiation genes” is instrumental for the increased proliferative potential of SMCs after injury, although the exact molecular mechanisms remain to be defined. In this study, we test the hypothesis that one of the pathways regulating phenotypic modulation after arterial injury involves the sphingosine-1-phosphate receptor-2 (S1P2R).
The bioactive sphingolipid S1P is best known for its regulation of lymphocyte egress from lymphatic organs3, 4 and its requirement for vessel formation in development.5 Both processes are mediated by S1P1R, one of the five G-protein coupled receptors that bind S1P. SMCs express three S1P receptors (S1P1R, S1P2R and S1P3R). Concomitant pharmacological blockade of S1P1R and S1P3R inhibits neointimal growth in balloon-injured rat carotid arteries6, suggesting a lesion promoting role for S1P. Consistent with this conclusion, increased S1P concentrations in human serum were found to be highly predictive for the onset and progression of coronary artery disease.7 In contrast, we have recently suggested an inhibitory role for S1P in arterial lesion growth based on our observation that S1P2R-null mice but not their wild-type littermates develop large intimal lesions after carotid injury.8 In this study, medial proliferation was increased in S1P2R-null arteries compared to wild-type arteries, indicating that S1P2R prevents SMC proliferation.8 In addition to SMC migration and proliferation, S1P has been shown to induce expression of SMC differentiation genes such as smooth muscle α-actin (SMA), and this effect has been ascribed to activation of Rho.9, 10 Activation of most promoters of SMC differentiation genes require binding of the transcription factor SRF (serum response factor) in combination with myocardin or a myocardin-related-transcription factor (MRTF) as a co-factor.1, 11, 12 Upon activation of Rho and stimulation of actin polymerization, cytosolic MRTF is released from G-actin and translocates into the nucleus. The physiological relevance of this process has been shown in proepicardial cells in which blockade of Rho kinase prevents their differentiation to coronary SMCs.13 Recently, a role for voltage-gated calcium channels (VGCCs) in the expression of SMC differentiation genes in rat SMCs has been suggested. In KCl-treated cells, blockade of VGCCs by nifedipine attenuated SMA expression.14 Moreover, S1P-induced membrane translocation of RhoA was decreased by pretreatment of SMCs with nifedipine.6
The present study tests the hypothesis that S1P2R regulates expression of SMC differentiation genes after arterial injury and investigates the potential signaling pathways involved.
Methods
Materials
S1P was purchased from Cayman and thrombin from American Diagnostica Inc. Antibodies were from Abcam (SM22α, β-actin), Cell Signaling (β-tubulin), Santa Cruz (SRF, Pol II, rabbit IgG), and Sigma (SMA, mouse IgG-1κ). The ECL-kit, horseradish peroxidase (HRP)-coupled anti-mouse antibody, and HRP-coupled protein A, and protein A sepharose beads were from GE Healthcare. C3 exotoxin was from Cytoskeleton, BAPTA-AM and thapsigargin from Calbiochem, and nifedipine from Sigma. Custom primers and probes were purchased from Invitrogen or Integrated DNA Technologies. All primer and probe sequences are shown in Table I; please see www.ahajournals.org.
Animals, surgical procedures and isolation of carotid SMCs
S1P2R-null mice and wild-type mice were kindly provided by Dr Richard L. Proia (NIH, Bethesda, MD).15 Heterozygous mice were bred to generate S1P2R-null and wild-type littermates. Genotypes were verified by polymerase chain reaction analysis using specific primers.15 The left common carotid artery of male mice (8–10 weeks old) was dissected and ligated near the carotid bifurcation as previously described.16 At the indicated time points, carotid arteries were excised and immediately frozen in liquid nitrogen or transferred to RNALater (Ambion). Carotid SMCs were isolated as previously described 17and maintained in DMEM supplemented with antibiotics (200 U/mL penicillin, 0.2 mg/mL streptomycin, all from GIBCO) and 10% fetal bovine serum (Atlantic Biologics).
Analysis of gene expression by real time PCR and Western blot analysis
Standard protocols were used to analyze gene expression by real-time PCR and Western blot analysis using preparations of total RNA and protein, respectively. Please see www.ahajournals.org for detailed methods including primer sequences (Table I).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed with minor adjustments as recently described.18 Please see www.ahajournals.org for detailed methods including primer sequences (Table II).
RhoA assay
Rho activity was measured using a commercially available kit as to the manufacturer’s instructions (Cytoskeleton).
Calcium measurement
Serum-starved SMCs were loaded with 3 µmol/L Fluo4 calcium indicator dye (Molecular Probes, Invitrogen) for 30 min and rinsed with Tyrode solution. SMCs were stimulated with S1P (1 µmol/L) or KCl (60 mmol/L) and changes in fluorescence at were imaged once every second for 150 seconds using confocal microscopy (LSM-510, Carl Zeiss; excitation: 488 nm; emission: 505–570 nm). The pixel intensities were quantified using NIH ImageJ software.
Statistics
As indicated in Figure legends, data are presented as mean +/− S.D. or S.E.M. Significance of differences between 2 groups was calculated using Student’s t-test and Bonferroni adjustments were used for multiple variant analyses. Multiple isolations of SMCs were used in all in vitro experiments.
Results
S1P2R regulates expression of SMA after arterial injury
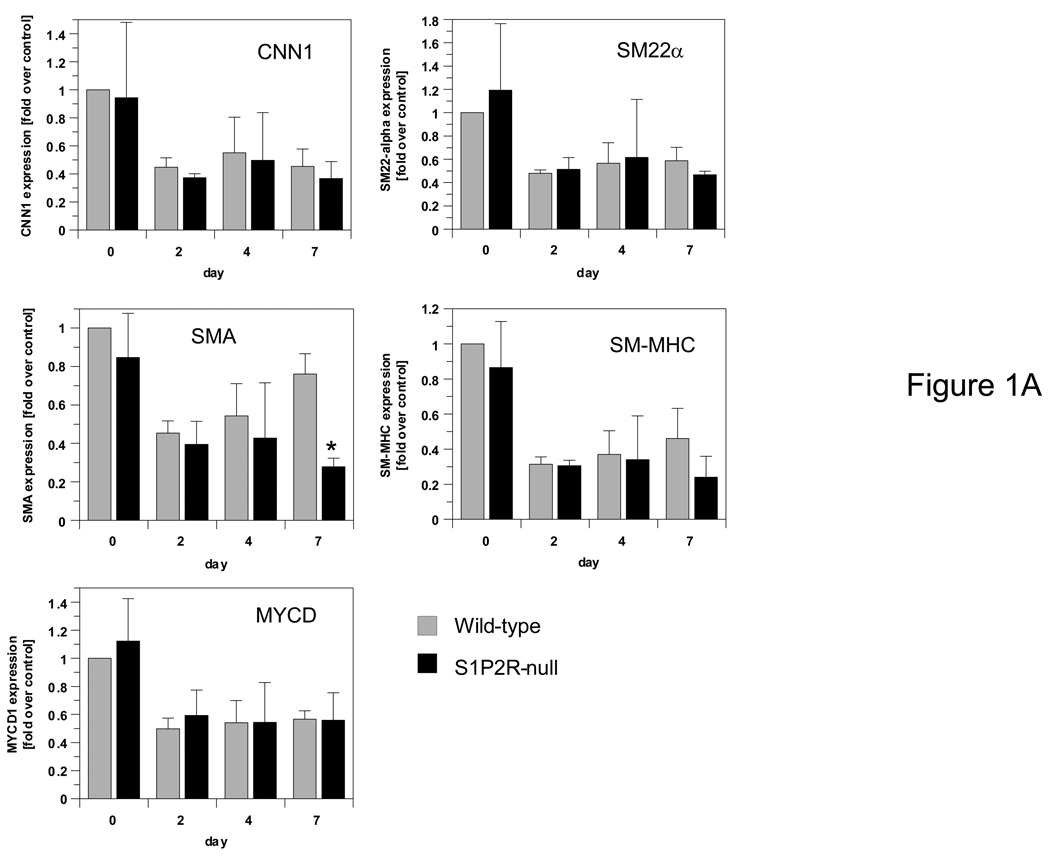
We recently reported that S1P2R-deficient arteries respond to injury with the formation of a large neointima, whereas wild-type arteries do not.8 In agreement with this observation, we observed increased medial proliferation in injured S1P2R-null arteries compared to wild-type arteries.8 To test the possibility that S1P2R regulates expression of SMC differentiation genes thereby controlling injury-induced phenotypic modulation, we measured mRNA of SMA, SM22α, calponin-1(CNN1) and smooth muscle-myosin heavy chain (SM-MHC) in carotid arteries of S1P2R-null and wild-type mice at day 0, 2, 4 and 7 after injury. Expression of each gene tested decreased with injury (Fig. 1A). A day 7, however, only SMA was significantly lower expressed in S1P2R-null arteries compared to wild-type arteries (Fig. 1A). Neither myocardin, S1P1R or S1P3R was differentially expressed between wild-type and S1P2R-null arteries following injury (Fig. 1A,B) and therefore, none of these genes is likely to mediate S1P2R-dependent SMA expression after injury. To confirm RNA expression data, we measured SMA and SM22α protein as well. Whereas SMA is present at similar levels in uninjured wild-type and S1P2R-null arteries, its expression at day 7 and 14 after injury decreased significantly in S1P2R-null arteries only (by 30%, at day 7 (Fig. I, please see www.ahajournals.org ). Such differences were not observed for SM22α (Fig. I).
Figure 1. S1P2R induces expression of SMA after arterial injury but does not affect expression of myocardin, other SMC differentiation genes, or S1P receptors.
S1P2R-null and wild-type mice underwent ligation injury of the left carotid artery. Arteries were harvested at the time points indicated. Total RNA was extracted, reverse transcribed and analyzed by qPCR for expression of calponin-1 (CNN1), SM22α, smooth muscle myosin heavy chain (SM-MHC), smooth muscle α-actin (SMA), myocardin (MYCD) (A), S1P1R, S1P2R and S1P3R (B). Data (mean +/− S.D., n=3) are expressed as fold induction over expression in wild-type at day 0.
S1P2R regulates expression of SMA in response to S1P
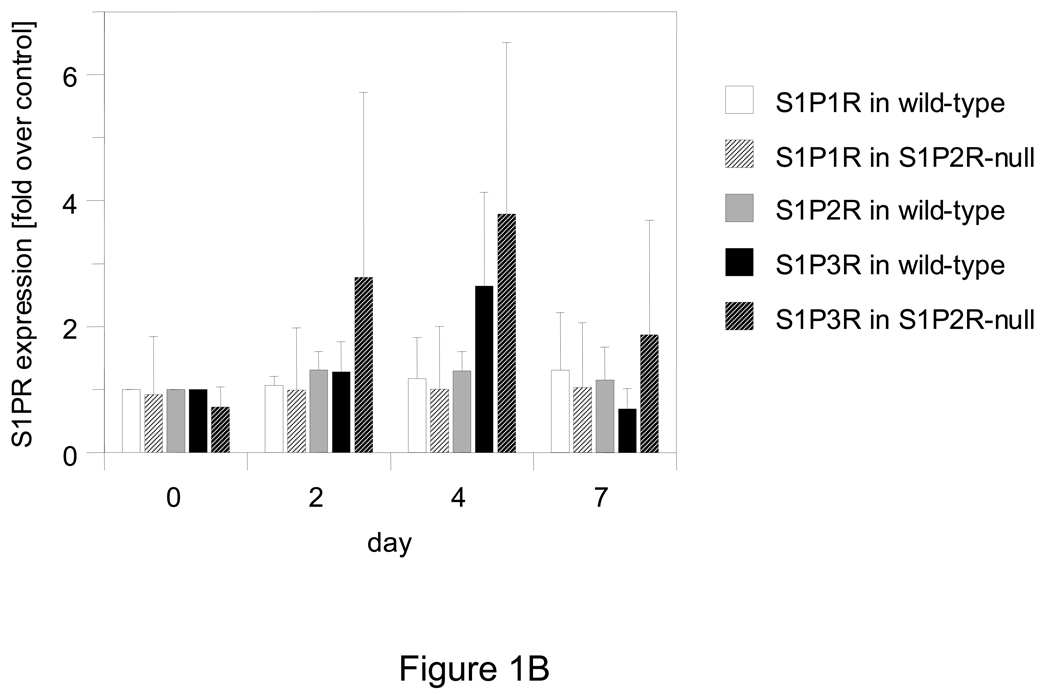
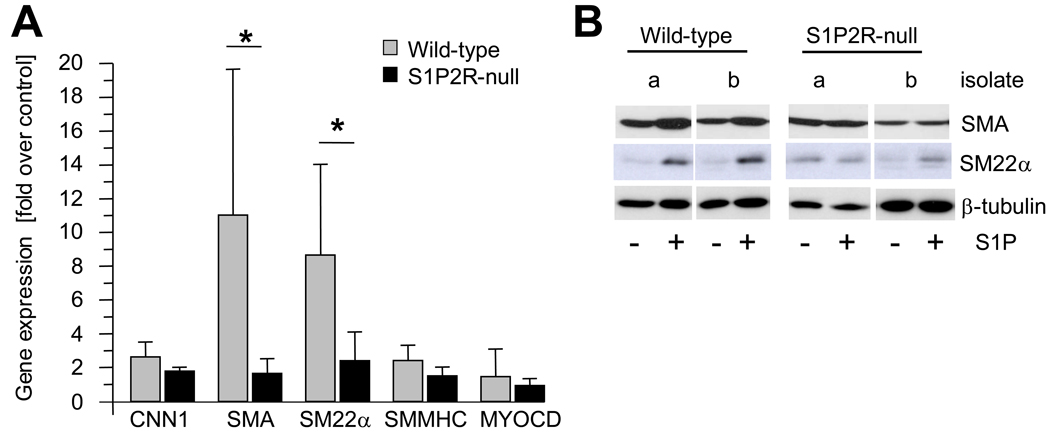
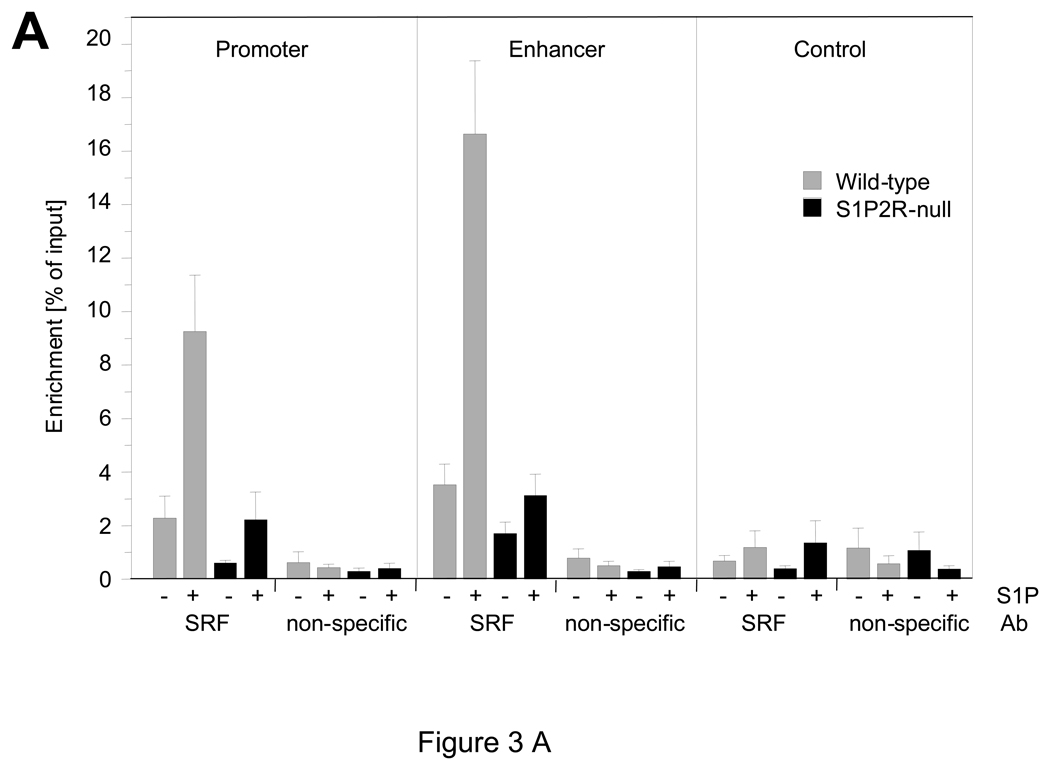
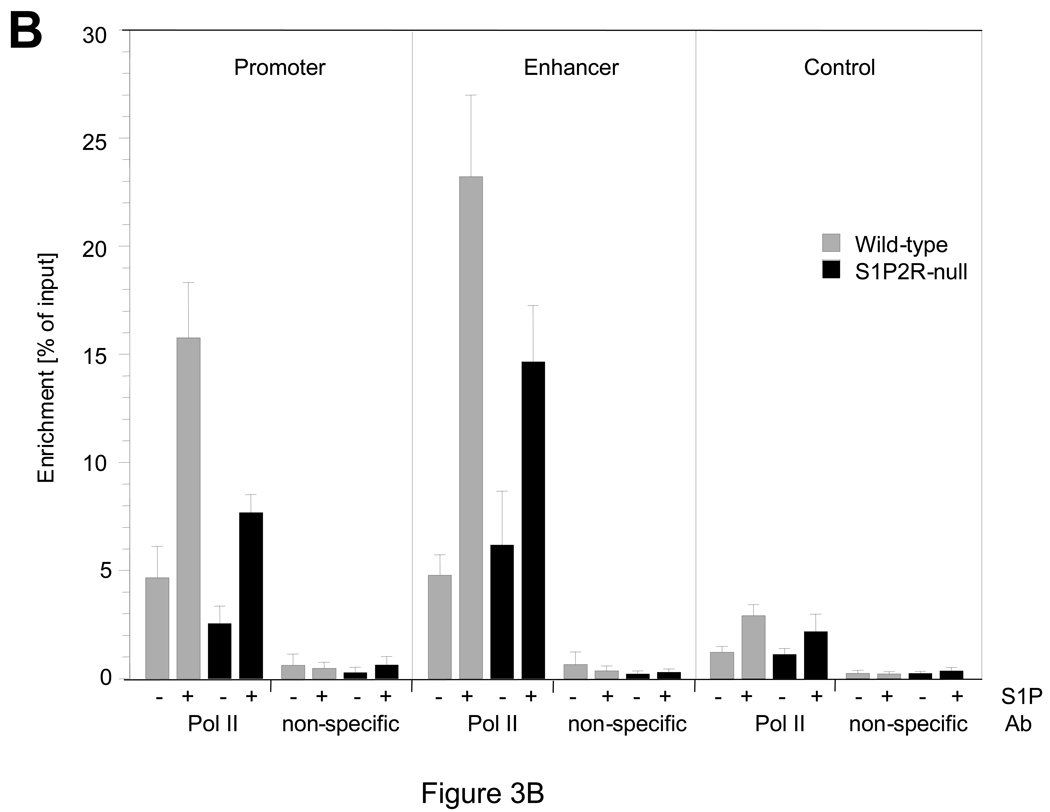
In vitro, S1P has previously been shown to stimulate SMA expression in SMCs, and a role for S1P2R and possibly S1P3R in this process has been suggested.9 Using wild-type and S1P2R-null SMCs in vitro, we found that expression of SMA, SM22α, CNN1, and SM-MHC, but not myocardin, is induced by S1P (Fig. 2A). A significant difference between wild-type and S1P2R-null SMCs, however, was observed with SMA and SM22α only, both of which were more strongly induced in wild-type SMCs (Fig. 2A). The same difference, although less pronounced, was observed for SMA and SM22α protein (Fig. 2B). As SMA expression is regulated by SRF, we performed chromatin immunoprecipitation (ChIP) assays to investigate whether SRF enrichment at the promoter or the enhancer region in the first intron of the SMA gene are regulated by S1P2R. In wild-type SMCs, S1P increased enrichment of SRF at both sites by approximately five-fold (Fig. 3A). In S1P2R-null SMCs, enrichment of SRF before or after S1P stimulation was significantly less (Fig. 3A). To demonstrate that S1P also stimulates transcription of the SMA gene, we determined binding of RNA polymerase II (Pol II) to the promoter and enhancer region. Binding of Pol II to either region was stimulated by S1P in both cell types, but markedly decreased in S1P2R-null SMCs compared to wild-type SMCs (Fig. 3B).
Figure 2. SMA expression by S1P depends on S1P2R.
Quiescent SMCs were stimulated with 1 µmol/L S1P. (A) At 3 hours after stimulation, mRNA expression of CNN1, SMA, SM22α, SM-MHC and MYOCD was measured by qPCR. Data (mean +/− S.D., n=4–5) are expressed as fold induction by S1P for wild-type and S1P2R-null SMCs, respectively. *P<0.05 (B) At 24 hours after stimulation, total cell lysates were prepared and equal amounts (1 µg/lane) subjected to SDS-PAGE followed by Western blotting. Blots were probed with antibody against SMA, SM22α and β-tubulin. Typical blots are shown for two independent isolates (a, b) for each cell type.
Figure 3. S1P regulates enrichment of SRF and polymerase II at the SMA gene in a S1P2R-dependent manner.
At 15 min after stimulation, chromatin was prepared and analyzed for binding of serum response factor (SRF, (A)) and RNA polymerase II (Pol II; (B)) to CArG domains in the promoter and enhancer of the SMA, as well as to a region approximately 6 Kb downstream of the start codon (control). Data (mean +/− S.E.M.) are presented as percent of input; n=6 for wild-type (SRF, Pol II), n=5 for S1P2R-null (SRF, Pol II) and n=3 for all non-specific antibody controls (rabbit IgG for SRF and mouse IgG-1κ for Pol II).
S1P-induced SMA expression requires Rho activation and calcium release from intracellular stores
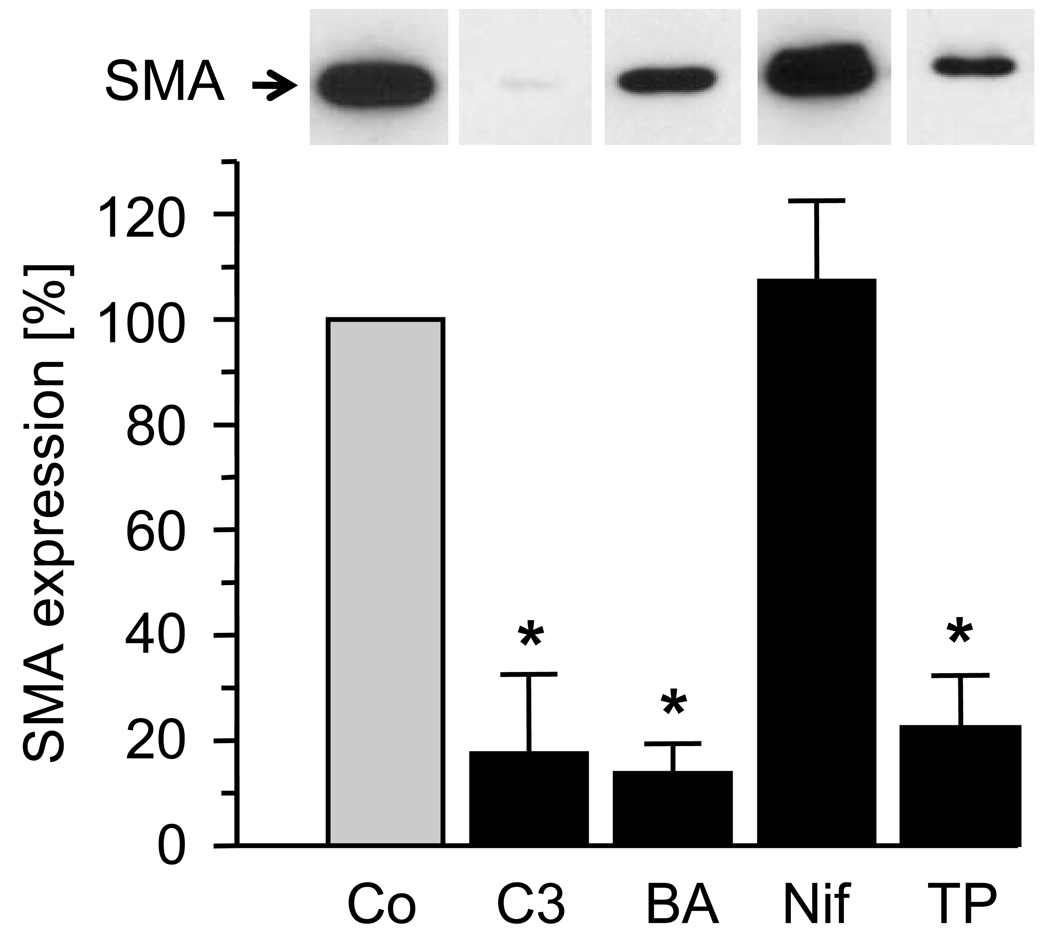
It is known that S1P activates Rho and causes an increase in intracellular calcium.19–22 We tested a role for Rho and calcium in S1P2R-dependent SMA expression by using various inhibitors. Treatment of wild-type SMCs with C3 exotoxin (a Rho inhibitor23), BAPTA-AM (calcium chelator) or thapsigargin (an inhibitor of the sarco-endoplasmic reticulum Ca2+/ATPase, SERCA), all prevented the S1P-dependent induction of SMA message and protein expression (Fig. 4). In contrast, blockade of L-type VGCCs with nifedipine had no effect on SMA expression by S1P.
Figure 4. SMA expression by S1P depends on Rho and release from intracellular stores.
Quiescent wild-type SMCs were treated with C3 exotoxin (C3, 24 hours, 2 µg/mL), BAPTA-AM (BA, 30 min, 10 µmol/L), nifedipine (Nif, 30 min, 1 µmol/L) or thapsigargin (TP, 3 µmol/L, 8 hours) before stimulation with S1P (1 µmol/L ). At 3 hours after stimulation, SMA expression was measured by qPCR. Data (mean +/− S.D., n=3) in the presence of inhibitor are expressed as percent control (vehicle). *P<0.05 (drug-treated versus control; Bonferroni adjustment was applied). Insert: At 24 hours after stimulation, protein extracts were prepared and equal amounts of extract (1 µg/lane) subjected to SDS-PAGE followed by Western blotting. Blots were probed with SMA antibody. A typical blot is shown.
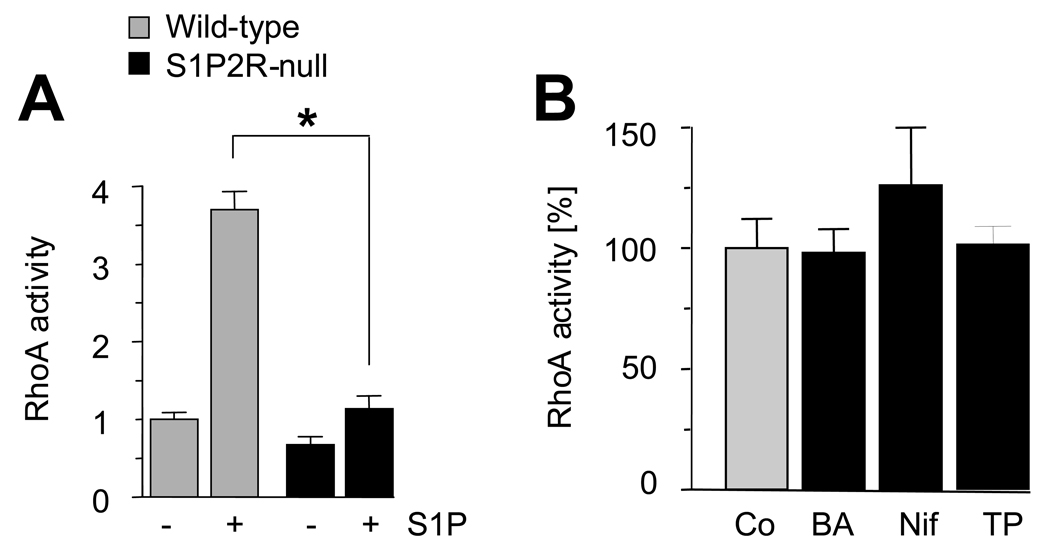
We next determined the contribution of S1P2R to activation of Rho and release of calcium by S1P. Based on the literature, S1P2R is the dominant S1P receptor to activate Rho but S1P3R might also contribute.19–22, 24 In contrast, S1P3R is generally considered the primary S1P receptor responsible for the increase of intracellular calcium in response to S1P.24 In comparison to wild-type SMCs, activation of RhoA by S1P in S1P2R-null SMCs is insignificant (Fig. 5A). As L-type VGCC-mediated calcium influx has recently been suggested to contribute to RhoA activation,6 we tested whether a calcium-dependent event is upstream of S1P-mediated activation of RhoA. This is not the case as chelating calcium does not affect activation of RhoA by S1P (Fig. 5B). Further, blockade of either SERCA by thapsigargin or L-type VGCCs by nifedipine had no effect on RhoA activity following S1P stimulation (Fig. 5B).
Figure 5. Activation of RhoA by S1P depends on S1P2R.
Quiescent SMCs (wild-type and S1P2R-null) were stimulated with 1 µmol/L S1P for 5 min before RhoA activity was measured. Data are expressed as fold stimulation over quiescent wild-type. (A) Data are presented as mean +/− S.D., n=3, 3 independent isolates were used for each cell type. *P<0.05 (B) Where indicated, wild-type cells were treated with BAPTA-AM (BA), nifedipine (Nif) or thapsigargin (TP) as described for Fig. 4 before stimulation with S1P. Data are presented as percent expression with 100% measured in the presence of vehicle (mean +/− S.D., n=3).
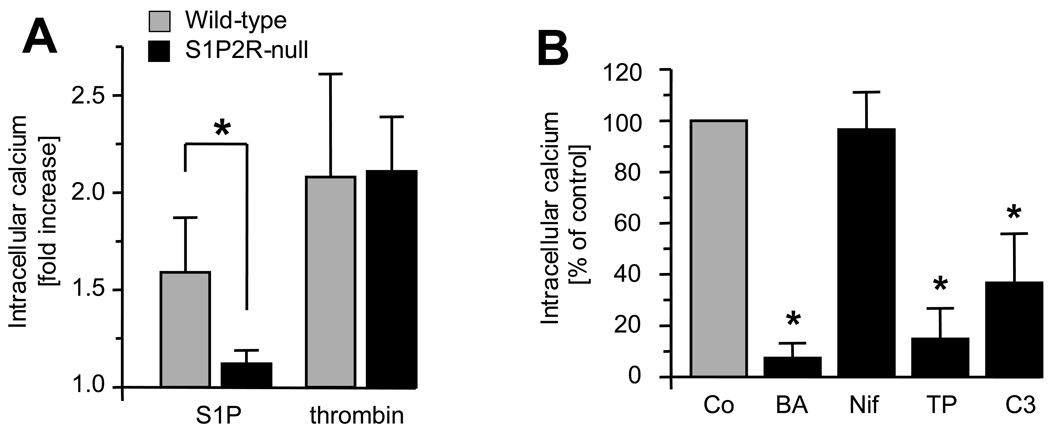
To assess the contribution of S1P2R to the increase in intracellular calcium induced by S1P, we measured calcium transients in wild-type and S1P2R-null SMCs. In average, S1P2R-null cells reached only 25% of the S1P-mediated calcium increase observed in wild-type cells (Fig. 6A), whereas, as expected, wild-type and S1P2R-null SMCs responded similarly to thrombin (Fig. 6A). The S1P-induced calcium transients were abolished by BAPTA-AM and thapsigargin but not affected by nifedipine (Fig. 6B), which is consistent with our observation that thapsigargin but not nifedipine inhibits S1P-induced expression of SMA (Fig. 4). The effects of these drugs on KCl-stimulated calcium transients in SMCs have been investigated in control experiments, which confirmed the efficacy of nifedipine (Fig. II; please see www.ahajournals.org). Further, we found that C3 exotoxin reduced the S1P-stimulated calcium release by approximately 60% suggesting that Rho regulates, at least in part, calcium transients induced by S1P (Fig. 6B).
Figure 6. S1P releases calcium from intracellular stores in a S1P2R-dependent manner.
Calcium transients were measured in quiescent wild-type and S1P2R-null SMCs following stimulation with 1 µmol/L S1P or 10 ng/mL thrombin and quantified by measuring peak height after setting the baseline to 1. Data are presented as mean +/− S.D., n=10–12, *P<0.05. (A) Quiescent wild-type cells were pretreated with BAPTA-AM (BA), nifedipine (Nif), thapsigargin (TP), or C3 as described for Fig. 4 before S1P stimulation. Data are presented as mean +/− S.D, n=4–8, *P<0.05 (drug-treated vs. control, Bonferroni adjustment was applied).
Discussion
We previously demonstrated that S1P2R-null carotid arteries respond to ligation injury with the formation of large neointimal lesions, whereas their wild-type littermates do not, suggesting that S1P2R inhibits neointimal growth.8 S1P2R blocks SMC migration by inhibiting activation of Rac.25, 26 Although this mechanism is likely to contribute to the inhibitory effect of S1P2R on neointimal formation, it does not explain our finding that medial proliferation is much higher in injured S1P2R-null carotid arteries compared to wild-type arteries.8 An inhibitory effect of S1P2R on SMC proliferation in vitro has recently been demonstrated using the S1P2R antagonist, JTE-013.6 Given the previous observations in vitro that S1P induces expression of SMC differentiation genes via a Rho-dependent pathway9, 10, and that S1P2R is the major contributor to Rho activation in response to S1P19–22, we tested the hypothesis that S1P2R regulates expression of SMC differentiation genes after arterial injury. We measured mRNA expression of CNN1, SM22α, SM-MHC and SMA after carotid injury in wild-type and S1P2R-null mice and found that at 2 days after injury, expression of all genes was decreased by approximately 50% in both animals. The only difference between wild-type and S1P2R-null animals was that re-expression of SMA at day 7 after injury only occurred in wild-type arteries (Fig. 1A and Fig. I, please see www.ahajournals.org). This was unexpected given our assumption that all SMC differentiation genes tested are similarly regulated by SRF and its cofactors of the myocardin/MRTF family. In vitro, S1P induces expression of SMA, SM22α, CNN1 and SM-MHC to different extent, and S1P2R seems to only promote the expression of SMA and SM22α (Fig. 2). Clearly, these genes are regulated by multiple pathways that are differentially activated by S1P. We do not know why SM22α is strongly induced by S1P/S1P2R in vitro, but its expression does not differ between injured wild-type and S1P2R-null arteries (compare Fig. 1A with Fig. 2). It is possible that differences emerge later after injury.
Using ChIP assays, we demonstrate that S1P2R regulates SRF binding to the regulatory CArG domains in the SMA promoter and enhancer region (Fig. 3A). A similar observation was made for Pol II which is consistent with the conclusion that S1P2R regulates SMA transcription. We do not believe that this process is regulated by changes in myocardin expression because there is no difference in myocardin mRNA levels between wild-type and S1P2R-null arteries at any timepoint after injury (0–7 days), and especially at day 7, when expression of SMA is significantly higher in wild-type arteries (Fig. 1A). As has been previously suggested by in vitro experiments, a possible mechanism for S1P2R-dependent regulation of SMA expression is the Rho-dependent translocation of the SRF co-factors MRTF-A/B from the cytosol into the nucleus.9 Whether this mechanism also regulates SMA expression by S1P2R after injury in vivo remains to be investigated.
Given a recent report in balloon-injured rat carotid arteries demonstrating that injury regulates expression of S1PRs6, we investigated whether differences in S1P1R and S1P3R expression in wild-type and S1P2R-null mice could account for the observation that S1P2R-null arteries but not wild-type arteries form intimal lesions after injury. In contrast to the rat model, we did not observe significant changes of S1PR expression after injury (Fig. 1B). At most, there is a trend that expression of S1P3R is slightly induced after injury, but this was observed in wild-type as well as in S1P2R-null arteries (Fig. 1B). It is possible that differences between the rat and mouse model regarding expression of S1PRs after injury is due to the endothelium which is removed in the rat model but present in the mouse model. Taken together, our current hypothesis is that S1P2R regulates the re-expression of SMA after injury, thereby limiting the potential of the injured artery to form an intima but we cannot rule out other mechanisms.
To elucidate S1P2R-dependent signaling pathways that regulate SMA expression, we compared the responses of wild-type and S1P2R-null SMCs to S1P. We confirmed previous suggestions that S1P2R activates RhoA, and that RhoA is critical for SMA expression by S1P9 (Fig. 4 and Fig 5). In addition, we determined that release of calcium from intracellular stores is also required for S1P-mediated expression of SMA (Fig. 4). Calcium transients following S1P stimulation have been previously reported and depending on cell type, different S1P receptors appear to be involved. Early studies using mouse embryonic fibroblasts lacking S1P2R, S1P3R, or both, suggested that calcium increase induced by S1P is IP3-dependent and that S1P3R, but not S1P2R, is involved.24 In contrast, over-expression of individual S1P receptors in HeLa cells demonstrated the ability of S1P2R and S1P3R (but not S1P1R) to increase intracellular calcium after S1P stimulation.27 Our data in SMCs indicate S1P2R is the primary receptor responsible for S1P-induced increase of cytosolic calcium (Fig. 6A). Consistent with their effects on SMA expression (Fig. 4), thapsigargin but not nifedipine blocked the increase of intracellular calcium by S1P (Fig. 6B). Moreover, blockade of Rho by C3 exotoxin was partially inhibitory suggesting that Rho regulates release of calcium from intracellular stores by S1P (Fig. 6B). In endothelial cells, Rho has been shown to mediate an association of the IP3 receptor with the transient receptor potential channel (TRPC1) thereby stimulating calcium entry following store depletion.28 Whether such a mechanism exists in SMCs remains to be investigated.
To date, a calcium dependency for SMA expression has only been reported in KCl-treated SMCs.14 This study concluded that KCl-induced cell depolarization and the subsequent influx of calcium through L-type VGCCs regulates activation of RhoA.14 A similar mechanism has recently been proposed for S1P-induced activation of RhoA, based on the observation that nifedipine blocks both translocation of RhoA to the membrane and SRF enrichment at the SMA CArG domains in response to S1P.6 This further implies that nifedipine also blocks S1P-induced SMA expression, although these data were not presented. We tested the possibility that S1P might regulate SMA expression via L-type VGCCs operating upstream of RhoA by measuring S1P-induced RhoA activation and SMA expression in the absence and the presence of BAPTA-AM or nifedipine. Somewhat surprisingly, we found that nifedipine had no effect on RhoA activation or SMA expression (Fig. 4, Fig 5), and we therefore conclude that L-type VGCCs are not required for S1P-induced SMA expression in mouse SMCs. Given this observation, we investigated whether calcium release from intracellular stores is stimulated by S1P and contributes to SMA expression. The SERCA-inhibitor thapsigargin blocked S1P-mediated SMA expression (Fig. 4) as well as the increase of intracellular calcium (Fig. 6D), but not the activation of RhoA by S1P (Fig. 5B). This apparent discrepancy in S1P utilizing different calcium stores to express SMA may be due to cell-type-specific properties as the above mentioned study6 was performed in rat aortic SMCs, whereas we used mouse carotid SMCs.
The mechanism by which calcium regulates SMA expression in SMCs is unknown. SRF is phosphorylated by calcium-dependent calmodulin kinase (CaMK) at multiple sites,29 which may directly regulate its activity.30 In cardiac myocytes, calcium/CaMK inhibits the association between SRF and a histone deacetylase thereby de-repressing SRF activity.31 Epigenetic regulation of the SMA promoter has been shown32, 33 and it is an attractive possibility that calcium regulates SRF activity by the recruitment of histone modifying enzymes.
In summary, we provide evidence that S1P/S1P2R regulates SMA expression in arteries after injury. In vitro, the extent of induction of other SRF-dependent SMC differentiation genes by S1P greatly varies and in vivo, SMA is the only SMC differentiation gene we found regulated by S1P2R. Although more work is necessary to define the role for S1P2R in the injury response, we believe that induction of SMA, and potentially additional SMC differentiation genes, contributes to the inhibition of neointimal growth by S1P2R. We further demonstrate that S1P-mediated induction of SMA expression in vitro requires S1P2R-dependent activation of RhoA and release of calcium from intracellular stores.
Supplementary Material
Acknowledgment
a) Sources of funding: NIH grants HL052459, HL070850, and HL088374. A.D.G. is a recipient of an AHA predoctoral fellowship.
b) Acknowledgement: We thank Richard Proia for the generous gift of S1P2R-null mice, Aesim Cho for mouse colony maintenance, L. Fernando Santana and Michael LaFlamme for their help with calcium measurements, Ron Seifert for his help with confocal microscopy, as well as Joel Nelson and Karol Bomsztyk for their expert advice concerning the FastChIP method.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Hao H, Gabbiani G, Bochaton-Piallat ML. Arterial smooth muscle cell heterogeneity: implications for atherosclerosis and restenosis development. Arterioscler Thromb Vasc Biol. 2003;23:1510–1520. doi: 10.1161/01.ATV.0000090130.85752.ED. [DOI] [PubMed] [Google Scholar]
- 3.Cyster JG. Chemokines, Sphingosine-1-phosphate, and Cell Migration in Secondary Lymphoid Organs. Annual Review of Immunology. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 4.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wamhoff BR, Lynch KR, Macdonald TL, Owens GK. Sphingosine-1-Phosphate Receptor Subtypes Differentially Regulate Smooth Muscle Cell Phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1454–1461. doi: 10.1161/ATVBAHA.107.159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, Gleason LA, Nakajima N, Sabbadini RA. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu T, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, Reidy MA. Sphingosine 1-Phosphate Receptor 2 Negatively Regulates Neointimal Formation in Mouse Arteries. Circ Res. 2007;101:995–1000. doi: 10.1161/CIRCRESAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 9.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- 10.Urata Y, Nishimura Y, Hirase T, Yokoyama M. Sphingosine 1-phosphate induces alpha-smooth muscle actin expression in lung fibroblasts via Rho-kinase. Kobe J Med Sci. 2005;51:17–27. [PubMed] [Google Scholar]
- 11.Wang DZ, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev. 2004;14:558–566. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Cen B, Selvaraj A, Prywes R. Myocardin/MKL family of SRF coactivators: key regulators of immediate early and muscle specific gene expression. J Cell Biochem. 2004;93:74–82. doi: 10.1002/jcb.20199. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Landerholm TE, Wei JS, Dong XR, Wu SP, Liu X, Nagata K, Inagaki M, Majesky MW. Coronary smooth muscle differentiation from proepicardial cells requires rhoA-mediated actin reorganization and p160 rho-kinase activity. Dev Biol. 2001;240:404–418. doi: 10.1006/dbio.2001.0403. [DOI] [PubMed] [Google Scholar]
- 14.Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, Owens GK. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a rho kinase/myocardin/SRF-dependent mechanism. Circ Res. 2004;95:406–414. doi: 10.1161/01.RES.0000138582.36921.9e. [DOI] [PubMed] [Google Scholar]
- 15.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 17.Cho A, Reidy MA. Matrix metalloproteinase-9 is necessary for the regulation of smooth muscle cell replication and migration after arterial injury. Circ Res. 2002;91:845–851. doi: 10.1161/01.res.0000040420.17366.2e. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JD, Denisenko O, Sova P, Bomsztyk K. Fast chromatin immunoprecipitation assay. Nucleic Acids Res. 2006;34:e2. doi: 10.1093/nar/gnj004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 20.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2002;1582:72–80. doi: 10.1016/s1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 21.Takuwa Y. Subtype-specific differential regulation of Rho family G proteins and cell migration by the Edg family sphingosine-1-phosphate receptors. Biochim Biophys Acta. 2002;1582:112–120. doi: 10.1016/s1388-1981(02)00145-2. [DOI] [PubMed] [Google Scholar]
- 22.Watterson KR, Ratz PH, Spiegel S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell Signal. 2005;17:289–298. doi: 10.1016/j.cellsig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Boquet P. Bacterial toxins inhibiting or activating small GTP-binding proteins. Ann N Y Acad Sci. 1999;886:83–90. doi: 10.1111/j.1749-6632.1999.tb09403.x. [DOI] [PubMed] [Google Scholar]
- 24.Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, Yang AH, Zhang G, Brown JH, Chun J. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J Biol Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto H, Takuwa N, Yokomizo T, Sugimoto N, Sakurada S, Shigematsu H, Takuwa Y. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol. 2000;20:9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, Matsui O, Takuwa Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- 27.Rapizzi E, Donati C, Cencetti F, Pinton P, Rizzuto R, Bruni P. Sphingosine 1-phosphate receptors modulate intracellular Ca2+ homeostasis. Biochemical and Biophysical Research Communications. 2007;353:268–274. doi: 10.1016/j.bbrc.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA Interaction with Inositol 1,4,5-Trisphosphate Receptor and Transient Receptor Potential Channel-1 Regulates Ca2+ Entry: ROLE IN SIGNALING INCREASED ENDOTHELIAL PERMEABILITY. J. Biol. Chem. 2003;278:33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- 29.Flück M, Booth FW, Waxham MN. Skeletal Muscle CaMKII Enriches in Nuclei and Phosphorylates Myogenic Factor SRF at Multiple Sites. Biochemical and Biophysical Research Communications. 2000;270:488–494. doi: 10.1006/bbrc.2000.2457. [DOI] [PubMed] [Google Scholar]
- 30.Miranti CK, Ginty DD, Huang G, Chatila T, Greenberg ME. Calcium activates serum response factor-dependent transcription by a Ras- and Elk-1-independent mechanism that involves a Ca2+/calmodulin-dependent kinase. Mol Cell Biol. 1995;15:3672–3684. doi: 10.1128/mcb.15.7.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis FJ, Gupta M, Camoretti-Mercado B, Schwartz RJ, Gupta MP. Calcium/calmodulin-dependent protein kinase activates serum response factor transcription activity by its dissociation from histone deacetylase, HDAC4. Implications in cardiac muscle gene regulation during hypertrophy. J Biol Chem. 2003;278:20047–20058. doi: 10.1074/jbc.M209998200. [DOI] [PubMed] [Google Scholar]
- 32.Cao D, Wang Z, Zhang CL, Oh J, Xing W, Li S, Richardson JA, Wang DZ, Olson EN. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol Cell Biol. 2005;25:364–376. doi: 10.1128/MCB.25.1.364-376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest. 2006;116:36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.