Abstract
Ligament graft fixation with bioabsorbable interference screws is a standard procedure in cruciate ligament replacement. Previous screw designs may resorb incompletely, and can cause osteolysis and sterile cysts despite being implanted for several years. The aim of this study was to examine the in vivo degradation and biocompatibility of the new Milagro™ interference screw (Mitek, Norderstedt, Germany). The Milagro™ interference screw is made of 30% ß-TCP (TriCalcium phosphate) and 70% PLGA (Poly-lactic-co-glycolic acid). In the period between June 2005 and February 2006, 38 patients underwent graft fixation with Milagro™ screws in our hospital. Arthroscopic ACL reconstruction was performed using hamstring tendon grafts in all the patients. MR imaging was performed on 12 randomly selected patients out of the total of 38 at 3, 6 and 12 months after surgery. During the examination, the volume loss of the screw, tunnel enlargement, presence of osteolysis, fluid lines, edema and postoperative screw replacement by bone tissue were evaluated. There was no edema or signs of inflammation around the bone tunnels. At 3, 6 and 12 months, the tibial screws showed an average volume loss of 0, 8.1% (±7.9%) and 82.6% (±17.2%, P < 0.05), respectively. The femoral screws showed volume losses of 2.5% (±2.1%), 31.3% (±21.6%) and 92.02% (±6.3%, P < 0.05), respectively. The femoral tunnel enlargement was 47.4% (±43.8%) of the original bone tunnel volume after 12 months, and the mean tunnel volume of the tibial tunnel was −9.5% (±58.1%) compared to the original tunnel. Bone ingrowth was observed in all the patients. In conclusion, the resorption behaviour of the Milagro™ screw is closely linked to the graft healing process. The screws were rapidly resorbed after 6 months and, at 12 months, only the screw remnants were detectable. Moreover, the Milagro™ screw is biocompatible and osteoconductive, promoting bone ingrowth during resorption. Tunnel enlargement is not prevented in the first months but is reduced by bone ingrowth after 12 months.
Keywords: Anterior cruciate ligament, Graft fixation, Interference screw, Milagro™ screw, Magnetic resonance imaging
Introduction
Graft fixation with interference screws is one of the standard procedures in ACL and PCL reconstruction. Owing to their sharp edges, metal interference screws can cause graft irritation [28] problems during potential revision procedures [11, 15] as well as MRI artefacts. Therefore, metal interference screws are no longer recommended for graft fixation in ACL reconstruction [28]. Revision rates of 5–10% after ACL reconstruction [15] are another argument for bioabsorbable implants.
A variety of bioabsorbable screws with different material compositions are available today, each with their advantages and disadvantages. Polylactide screws are partially resorbed (30–35%) with osteolysis observed in 16% [9] and sterile cysts in 5–10% of the cases [20] after 24 months. Polyglycolide interference screws degrade completely within 12 months but are still not replaced by bone tissue after 3 years [9]. Several authors have also reported foreign body reactions after implantation of bioabsorbable screws [4, 25]. Until now, screw replacement by bone tissue has only been sporadically observed in the long term [19]. There are various studies confirming a lack of replacement of bioabsorbable screws by bone tissue even after an extended period [1, 3, 22, 23, 26], yet, this bone ingrowth is essential in the event of a need for revision procedures [11].
According to the manufacturer, the new bioabsorbable Milagro™ interference screw for graft fixation in ACL reconstruction provides for optimized degradation and integration into the bone tissue; this would potentially diminish the above-mentioned problems and improve the clinical results of ACL reconstruction. This study investigates the degradation and integration behaviour of the Milagro™ screw in human subjects after ACL reconstruction. It is the first investigation of this implant we are aware of.
Materials and methods
Study design
Thirty-eight patients from our database of patients who underwent an ACL reconstruction between June 2005 and February 2006 were studied. MR imaging was performed on 12 randomly selected patients from this cohort at 3, 6 and 12 months after surgery.
Exclusion criteria were the presence of additional fractures around the knee joint, previous surgery on the affected knee joint, as well as ICRS grade 2, 3 or 4 cartilage lesions exceeding 5 cm2. Also excluded were patients with PCL lesions, those who had an autologous chondrocyte transplantation or mosaicplasty with more than one transplanted cylinder (or a cylinder larger than 1 cm), bone–tendon–bone grafts, those with more than 50% of the inner or outer meniscus resected and patients with axis deformities or underlying diseases that produced physical impairment.
The Milagro™ interference screw is made of 30% ß-TCP (TriCalcium phosphate) and 70% PLGA (Poly-lactic-co-glycolic acid). It is available in diameters of 7–12 mm and 23, 30 or 35 mm in length (Fig. 1). The cannulated screw can be introduced precisely over a guide wire and is inserted with a specific screwdriver.
Fig. 1.
Milagro™ interference screw (7 × 23 mm)
Surgical technique
The ACL reconstruction was performed arthroscopically by two experienced orthopaedic specialists. In 22 cases, the semitendinosus tendon alone was used as the cruciate ligament graft and, in the remaining 16 cases, the semitendinosus tendon in combination with the gracilis tendon was used. After removal, the tendons were sutured and augmented with Orthocord™ sutures (Mitek, Norderstedt, Germany) on the femoral and tibial aspects. The tibial tunnel was prepared using an alignment jig and referencing the PCL. The cortex was opened at an angle of 55° to the tibial articular surface directly above the pes anserinus. The femoral tunnel was prepared over the anteromedial portal using an alignment jig with a 5–6 mm offset—depending on the graft height—in the 1.30 or 10.30 position. After femoral screw fixation, the graft was pretensioned and the knee joint moved through its range of motion before the tibial screw was placed as close to the joint as possible. A 23-mm femoral screw and 30-mm tibial screw were used. The bone tunnel diameter was adapted to 0.5 mm of the graft height. The diameter of the interference screws was selected according to the bone tunnel diameter (0.5 mm smaller).
Radiological evaluation
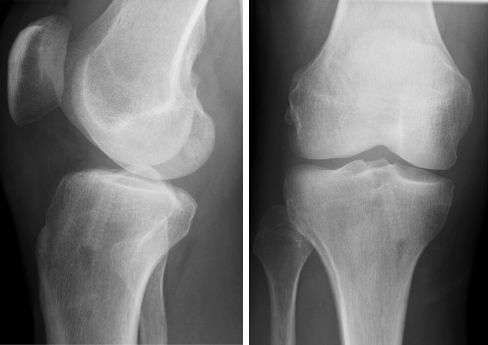
All the patients had postoperative radiographs of their knee joint taken in two planes. The positions of the bone tunnels and the Milagro™ screws were evaluated to exclude potential screw malalignment or dislocation (Fig. 2).
Fig. 2.
The position of the radiopaque Milagro™ screw can be examined on the postoperative radiograph. The dorsal position of the graft relating to the screw should be noted in the lateral view
Magnetic resonance imaging
MR imaging was performed in 12 randomly selected patients at 3, 6 and 12 months after surgery. All the MRI examinations were performed with a 1.5 Tesla MRI scanner (Magnetom Symphony and Sonata, Siemens Erlangen, Germany). A standard protocol was used for the MR studies of the knee joint. This standard protocol includes the use of a specific surface coil for knee evaluation.
Standard T1-weighted spin echo (SE) sequences in the sagittal plane, T2-weighted spin echo (SE) sequences in the sagittal plane, proton-density-weighted turbo spin echo sequences (TSE) with fat saturation, and a 3D-DESS sequence were used. The image matrix used was 512 × 256, and FOV was 180 mm in the DESS and proton-density-weighted turbo spin echo sequences, and 220 mm in the T1- and T2-weighted sequences. The section thickness was either 1.5 or 3 mm. The preoperative screw volume was calculated using the following formula: V1 = π r2 × l (“r” is the screw radius and “l” the screw length). The postoperative screw volume was approximated using the following formula: V2 = π (1/2 (d1/2 + d2/2))2 × l (d1 is the minimum screw diameter and d2 the maximum screw diameter; “l” is the maximum measurable screw length). In eight immediately postoperative MRI examinations (within 3 days after surgery), the screws were measured and the precision of the digital measurement determined in the 3D-DESS sequence.
The screw resorption rate was determined in the sagittal plane of the DESS sequence (section thickness 1.5 mm, FOV 180 mm, distance factor 20%, TR 21.9 ms, TE 5.78 ms). Bone tunnel enlargement was evaluated digitally by measuring the bone tunnel diameters perpendicular to the long axis of the tunnel in the sagittal plane. Each channel was divided into three sections, and the maximal channel diameter of each third was used to calculate the area of the channel cross section. The mean values of three measurements at the tibial and the femoral channel were calculated and compared with the original channel to evaluate bone tunnel enlargement. The diameter of the drill was used to estimate the size of the original channel.
As the distal third of the femoral tunnel and the proximal third of the tibial tunnel are important for implant fixation and for potential revision surgery, the cross-sectional areas of these regions were calculated separately.
Statistical analyses
The kinetics of bone tunnel enlargement and the rates of screw resorption were evaluated using repeated measures analysis of variance. In cases of significant results (P < 0.05), an alpha adjustment was performed by using the Bonferroni–Holm procedure for the post-hoc comparisons. Analyses were conducted with SAS 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Screw resorption
There were no fluid lines or signs of edema in the area of the bone tunnels in any of the 12 patients examined by MRI after 6 and 12 months. In one female patient, a cystic structure resembling a ‘cyclops’ lesion was observed at the site of the tibial graft insertion. However, there were no radiological signs of inflammation and the screw was not in contact with the ‘cyclops’ lesion.
MRI is able to show the screws in clear negative contrast to bone and soft tissues thereby facilitating screw measurement (Fig. 3a and b). Only in the immediate postoperative period and at 3 months was marrow edema observed around the bone tunnels in all the patients, and the same had disappeared after 6 months. There were no fluid lines around the screws at any time. The graft was also confirmed in all the patients to lie in the normal graft position.
Fig. 3.
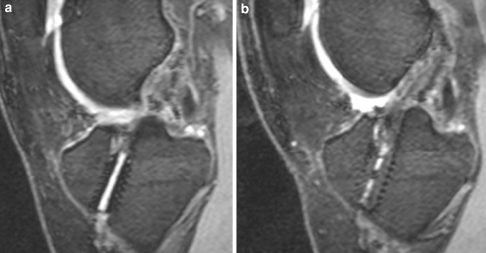
a, b 23-year-old female patient at 6 months (a) and 12 months (b) after ACL reconstruction. MR image (DESS sequence) shows the tibial screw is still visible at 6 months but at 12 months, only traces of the screw are detectable. Moreover, bone ingrowth into the screw was observed. through ingrowth of bone, the tunnel enlargement was reduced after 12 months
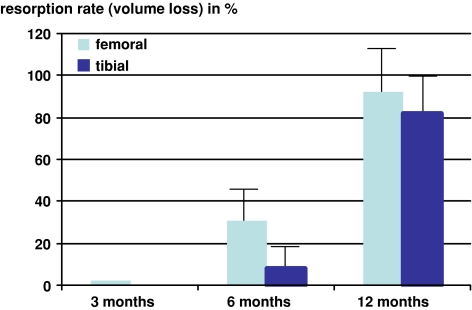
All the patients showed bone ingrowth into the resorbing screws, but no screw was completely replaced by bone. The tibial screws showed an average volume loss of 8.1% (±7.9%) at 6 months and 82.6% (±17.2%; P < 0.05) at 12 months (Fig. 4). The femoral screws showed an average volume loss of 31.26% (±21.6%) at 6 months and 92.02% (±6.3%; P < 0.05) at 12 months. Volume loss was significantly higher in the femoral than in the tibial screws after 6 months (Fig. 4).
Fig. 4.
MR image shows volume loss of the tibial and femoral Milagro™ screws measured at 3, 6 and 12 months. The femoral screws show significantly faster resorption than the tibial screws (P < 0.05). At 12 months, only traces of the screws are detectable
Bone tunnel enlargement
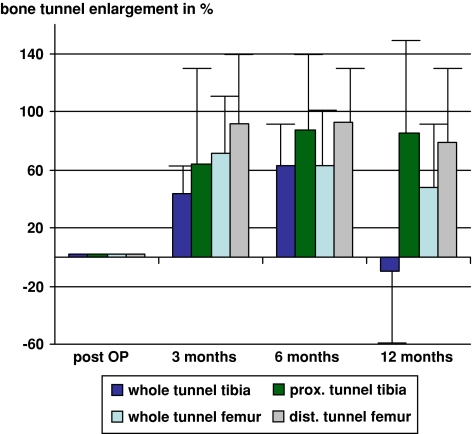
Tibial tunnel enlargement (mean value of the whole tunnel) was 43.5% (±26.1%) at 3 months, 62.7% (±30.0%) at 6 months and −9.5% (±58.1%) of the original tunnel at 12 months. Femoral tunnel enlargement (mean value of the whole tunnel) was 71.2% (±56.9%) at 3 months, 62.9% (±41.5%) at 6 months and 47.4% (±43.7%) of the original tunnel at 12 months. After 12 months, the tibial tunnel enlargement was significantly reduced compared to the channel size after 3 and 6 months (P < 0.05). After 12 months, tibial tunnel enlargement was significantly reduced compared to the femoral tunnel (P < 0.05) (Figs. 5 and 6).
Fig. 5.
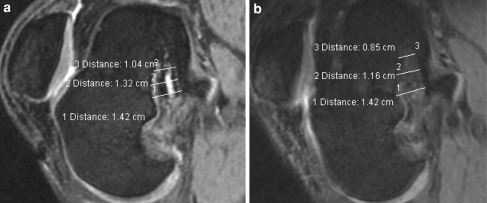
a, b MR images (DESS sequence) of the femoral bone tunnel at 6 and 12 months. The plane was chosen parallel to the femoral tunnel. Figures show the three mentioned measurements of the femoral bone tunnel. Distinct bone tunnel enlargement was first observed at 6 months and remained almost constant until 12 months. The Milagro™ screw showed clear resorption signs at 6 months and was barely detectable after 12 months
Fig. 6.
Tibial and femoral tunnel enlargement at 3, 6 and 12 months
The enlargement of the proximal third of the tibial tunnel was 85.2% (±72.6%) and in the distal third of the femoral tunnel it was 78.7% (±50.9%) (Fig. 6). In these regions close to the joint line, there was no significant decrease in the tunnel diameter over time (P > 0.05). There was a significant difference in the reduction of the tunnel diameter over time between the proximal third of the tibial tunnel and the whole tibial tunnel (P < 0.05) as well as between the distal third of the femoral tunnel and the whole femoral tunnel (P < 0.05).
Discussion
Interference screws are often used for graft fixation in ACL reconstruction. Traditional bioabsorbable interference screws are often made of absorbable polymers. In an overview of biologic implants in sports medicine, it has been shown that degradation kinetics differs substantially among more than 40 known bioabsorbable polymers [27]. Furthermore, a large variety of additional factors appear to affect degradation rates, including molecular weight, sterilization, implant size, self-reinforcement, copolymer or stereocopolymer ratios, and processing techniques [27].
The effect of the polymer type on absorption is evident in various studies. Fink et al. [10] performed CT scans on ACL/bone–patellar tendon–bone fixed with PGA and showed complete absorption by 12 months. Similarly, Lajtai et al. [16] looked at copolymer PGA/PLA screws used in ACL/bone–patellar tendon–bone on MRI and found them to be completely absorbed at 6 months. However, Drogset et al. [9] used PLA screws in ACL/bone–patellar tendon–bone and at 2 years, there was a 60% reduction volume in screws. The studies above demonstrate that in the same environment, the specific composition of screws affects the degradation process.
In addition to the polymer, the environment within which the screw is implanted affects its properties. In PLLA interference screws used for the fixation of hamstring ACL reconstructions, no radiological evidence of absorption after 4 years was found [22]. Ma et al. [18] studied PLLA interference screws at a minimum of 2 years’ follow-up after hamstring ACL reconstruction; most of the screws were partially degraded on MRI scans, but none was completely degraded at 2–4 years after surgery.
Both these studies show that the implantation of screw in hamstring tendons affects biologic properties, thus slowing down the degradation process compared to that of a bone–patellar tendon–bone.
Until now replacement of screws by bone tissue was either not observed [3, 22–24] or only sporadically observed in the long term [19]. In this study, the new Milagro™ interference screws and their degradation after ACL reconstruction were examined by means of MR imaging. Thirty percent ß-TCP was added to the poly-lactic-co-glycolic acid to accelerate resorption of the screws and induce bone ingrowth. ß-TCP is known to be osteoconductive when used as a bone substitute, and it is completely replaced by bone tissue [5]. By mixing restorable polymers and ß-TCP, the biomechanical properties, resorption behaviours and osteoconductivity of both materials have been enhanced in experimental studies [2, 20]. A similar study with a different screw (Biolok, ArthroCare, Sunnyvale, CA, USA) and different transplant was presented by Barber et al. [2]. ß-TCP–PLLA screws were used for fixation of bone–tendon–bone transplants. They concluded that the screws are osteoinductive, being partially or completely replaced by bone tissue [2].
In this study, a bioabsorbable interference screw with a different chemical composition was used for both femoral and tibial graft fixation. We believe the bioabsorbable interference screws for graft fixation have three advantages: First, the screw prevents early motion of the graft within the tunnel, a feature important for a stable healing [13]; Second, it reduces synovial fluid reflux into the bone tunnel, thus reducing possible negative effects of cytokines that may enhance bone tunnel enlargement [14]; Third, it allows fixation of the transplant close to the tunnel entrance avoiding the so-called bungee-effect [14]. Moreover, the Milagro™ screw has the advantage of being detectable using conventional radiography so that its position can be documented and monitored on the postoperative radiograph as demonstrated in this study (Fig. 2). However, according to the literature [12, 28] and confirmed by our own experience, the introduction of the femoral screw can easily cause graft irritation.
Animal models have revealed that in using hamstring tendons as cruciate ligament grafts fixed with poly-(d-,l-lactide) screws, direct contact was established between tendon and bone tunnel wall within 12 weeks [14]. Bone–tendon junction took up to 24 weeks [14]. In consideration of these results, we consider the resorption behaviour of the Milagro™ screws adapted to the healing process of the ACL-transplant. Especially during the first 3 months, when a stable fixation is required for a secure integration of the graft into the bone [17], the Milagro™ screw shows almost no resorption. Even after 6 months, only slight resorption of the tibial Milagro™ screws was observed in this study. Between 6 and 12 months—after primary graft healing—the screws were rapidly degraded with approximately 90% of the screw being resorbed after 12 months. The degradation of the tibial Milagro™ screws proceeded significantly slower as compared to the femoral screws. This difference is desirable as graft failure almost exclusively occurs in tibial screws during pullout testing [7].
In this study, degradation was evaluated using MR imaging. It is known that after ACL reconstruction there is a high correlation between histological and MRI findings [14], thereby making MR imaging a valid examination method. By comparing the actual with the measured screw diameter in the immediate postoperative period, we found a measuring accuracy of 98% from the MRI examination, and thus a greater likelihood that our data are accurate, particularly for the first 6 months. Despite the fact that the method of measuring the screw resorption after 12 months could not be validated by comparison with histological sections, we had to assume a good correlation and continued accuracy. This assumption is based on the fact that ingrowing tissue in the screws can be detected by MRI scans [14, 16]. However, Morgan et al. [21] demonstrated that a screw that appeared to have a clear outline on MRI was actually undergoing degradation. They explanted en bloc a PLA screw used in a patient with ACL/bone–patellar tendon–bone after 2.5 years. Under histological examination, the screw was found to have reduction of 75% molecular weight with implant fragmentation. However, according to Morgan et al. [21], it is possible that the resorption process of the screws in vivo precedes the morphological findings revealed by MRI. This fact should account for the interpretation of the results of this study.
Tunnel enlargement was already observed 6 weeks after ACL reconstruction [6]. The extent of tunnel enlargement depends on the type of graft fixation [6]. Surprisingly, the most extensive tunnel enlargement was observed in anatomic graft fixation [6–8]. However, the extent of the tunnel enlargement has no impact on the clinical results [6, 8]. Nevertheless, it can cause significant problems during revision surgery. We therefore measured not only the proximal parts of the tibial tunnel and the distal parts of the femoral tunnel, but also the other parts of the tunnels. In our experience, the central and peripheral parts of the tunnels are as important for revision surgery as the parts of the tunnels close to the joint line. In comparing our results with those of other studies, one has to be aware of the different techniques for measuring the bone tunnels [6–8, 18]. In order to facilitate a better comparison with other studies, we also present the results of the tunnel enlargement in the areas close to the joint. The big differences in the values of tunnel enlargement close to the joint and the enlargement of the whole tunnel is based on the fact that the tunnel in the proximal third of the tibia and the distal third of the femur is enlarged compared to the original tunnel volume. In contrast, after 12 months, the tunnel volume in the peripheral thirds especially in the tibia is reduced because Milagro™ screws were almost completely resorbed and partially replaced by bone tissue (Fig. 4). As demonstrated in this study, Milagro screws could not prevent tunnel enlargement in the first 6 months, but ingrowth of bone tissue in the screws reduced the tunnel volume significantly after 1 year. Despite bone ingrowth, enlargement could not be avoided in the tunnel region close to the joint line, even if the screw was placed close to the joint line. Data found in the literature support MRI values of tunnel enlargement of up to 75% of the original tunnel volume after a few weeks [6] and up to 100% of the original volume at 6 months [6]. Other authors also confirm duplication of tunnel volume in MR imaging at 6 months with an increase in volume of the femoral bone tunnel of 100.4% and an increase in volume of the tibial bone tunnel of 73.9% [8]. Comparing values of tunnel enlargement found in the literature with those in this study, similar values are found for the proximal parts of the tibial bone tunnel and the distal parts of the femoral bone tunnel [6–8].
Conclusion
Features of the Milagro™ screw include visibility on conventional radiographs and a resorption behaviour that appears to be adapted to the graft healing, with almost no resorption during the first 3 months and almost complete degradation at 12 months. Tunnel enlargement cannot be prevented by the use of the Milagro™ screws. However, the fact that the screws are partially replaced by bone tissue might be of advantage especially in revision surgery. Long-term clinical results have to confirm the possible advantages of the presented radiological results.
Acknowledgment
The MRI examinations were financially supported by DePuy Mitek (Norderstedt, Germany).
References
- 1.Bach FD, Carlier RY, Elis JB, Mompoint DM, Feydy A, Judet O (2002) Anterior cruciate ligament reconstruction with bioabsorbable polyglycolic acid interference screws: MR imaging follow-up. Radiology 225(2):541–550 [DOI] [PubMed]
- 2.Barber FA, Dockery WD (2008) Long-term absorption of beta-tricalcium phosphate poly-l-lactic acid interference screws. Arthroscopy 24(4):441–447 [DOI] [PubMed]
- 3.Barber FA, Dockery WD (2006) Long-term absorption of poly-l-lactic acid interference screws. Arthroscopy 22(8):820–826 [DOI] [PubMed]
- 4.Bostman O, Hirvensalo E, Makinen J, Rokkanen P (1990) Foreign body reactions to fracture fixation implants of biodegradable synthetic polymers. JBJS (Br) 72:592–596 [DOI] [PubMed]
- 5.Bodde EW, Wolke JG, Kowalski RS, Jansen JA (2007) Bone regeneration of porous beta-tricalcium phosphate (Conduittrade mark TCP) and of biphasic calcium phosphate ceramic (Biosel(R) in trabecular defects in sheep. J Biomed Mater Res A 82(3):711–722 [DOI] [PubMed]
- 6.Buelow JU, Siebold R, Ellermann A (2002) A prospective evaluation of tunnel enlargement in anterior cruciate ligament reconstruction with hamstrings: extracortical versus anatomical fixation. Knee Surg, Sports Traumatol, Arthrosc 10:80–85 [DOI] [PubMed]
- 7.Cain EL, Phillips BB, Charlebois SZ, Azar FM (2005) Effect of tibial tunnel dilation on pullout strength of semitendinosus-gracilis-graft in anterior cruciate ligament reconstruction. Orthopedics 28(8):779–783 [DOI] [PubMed]
- 8.Clathworthy MG, Annear P, Buelow JU, Bartlett RJ (1999) Tunnel widening in anterior cruciate ligament reconstruction: a prospective evaluation of hamstrings and patella tendon grafts. Knee Surg, Sports Traumatol Arthrosc 7:138–145 [DOI] [PubMed]
- 9.Drogset JO, Grontvedt T, Myhr G (2006) Magnetic resonance imaging analysis of bioabsorbable interference screws used for fixation of bone-patellar tendon-bone autografts in endoscopic reconstruction of the anterior cruciate ligament. Am J Sports Med 34(7):1164–1169 [DOI] [PubMed]
- 10.Fink C, Benedetto KP, Hackl W, Hoser C, Freund MC, Rieger M (2000) Bioabsorbable polyglyconate interference screw fixation in anterior cruciate ligament reconstruction: a prospective computed tomography-controlled study. Arthroscopy 16(5):491–498 [DOI] [PubMed]
- 11.George MS, Dunn WR, Spindler KP (2006) Current concepts review: revision anterior cruciate ligament reconstruction. Am J Sports Med 34(12):2026–2037 [DOI] [PubMed]
- 12.Herbort M, Weimann A, Zantop T, Strobel M, Raschke M, Petersen W (2006) Initial fixation strength of a new hybrid technique for femoral ACL graft fixation: the bone wedge technique. Arch Orthop Trauma Surg 127(9):769–775 [DOI] [PubMed]
- 13.Hoeher J, Moller HD, Fu FH (1998) Bone tunnel enlargement after anterior cruciate ligament reconstruction: fact or fiction? Knee Surg Sports Traumatol Arthrosc 6:231–240 [DOI] [PubMed]
- 14.Hunt P, Rehm O, Weiler A (2006) Soft tissue graft interference fit fixation: observations on graft insertion site healing and tunnel remodeling 2 years after ACL reconstruction in sheep. Knee Surg Sports Traumatol Arthrosc 14:1245–1251 [DOI] [PubMed]
- 15.Johnson D, Harner CD, Maday MG (1994) Revision anterior cruciate ligament surgery. In: Fu F, Harner CD, Vince KG (eds) Knee surgery. Williams & Wilkins, Baltimore, MD, pp 877–895
- 16.Lajtai G, Humer K, Aitzetmuller G, Unger F, Noszian I, Orthner E (1999) Serial magnetic resonance imaging evaluation of a bioabsorbable interference screw and the adjacent bone. Arthroscopy 15(5):481–488 [DOI] [PubMed]
- 17.Logan M, Williams A, Myers P (2003) Is bone tunnel osseointegration in hamstring tendon autograft anterior cruciate ligament reconstruction important? Arthroscopy 19(8):E1–E3 [DOI] [PubMed]
- 18.Ma CB, Francis K, Towers J, Irrgang J, Fu FH, Harner CH (2004) Hamstring anterior cruciate ligament reconstruction: a comparison of bioabsorbable interference screw and endobutton-post fixation. Arthroscopy 20:122–128 [DOI] [PubMed]
- 19.Macarini L, Murrone M, Marini S, Mocci A, Ettorre GC (2004) MRI in ACL reconstructive surgery with PDLLA bioabsorbable interference screws: evaluation of degradation and osteointegration processes of bioabsorbable screws. Radiol Med 107(1–2):47–57 [PubMed]
- 20.Montjovent MO, Mathieu L, Schmoekel H, Mark S, Bourban PE, Zambelli PY, Laurent-Applegate LA, Pioletti DP (2007) Repair of critical size defects in the rat cranium using ceramic-reinforced PLA scaffolds obtained by supercritical gas foaming. J Biomed Mater Res A 83(1):41–51 [DOI] [PubMed]
- 21.Morgan CD, Gehrmann RM, Jayo MJ, Johnson CS (2002) Histologic findings with a bioabsorbable anterior cruciate ligament interference screw explant after 2.5 years in vivo. Arthroscopy 18(9):E47 [DOI] [PubMed]
- 22.Radford MJ, Noakes J, Read J, Wood DG (2005) The natural history of a bioabsorbable interference screw used for anterior cruciate ligament reconstruction with a 4-strand hamstring technique. Arthroscopy 21(6):707–710 [DOI] [PubMed]
- 23.Singhal MC, Holzhauer M, Powell D, Johnson DL (2008) MRI evaluation of the tibial tunnel/screw/tendon interface after ACL reconstruction using a bioabsorbable interference screw. Orthopedics 31(6):575–579 [DOI] [PubMed]
- 24.Tecklenburg K, Burkart P, Hoser C, Rieger M, Fink C (2006) Prospective evaluation of patellar tendon graft fixation in anterior cruciate ligament reconstruction comparing composite bioabsorbable and allograft interference screws. Arthroscopy 22(9):993–999 [DOI] [PubMed]
- 25.Warden WH, Chooljian D, Jackson DW (2008) Ten-year magnetic resonance imaging follow-up of bioabsorbable poly-L-lactic acid interference screws after anterior cruciate ligament reconstruction. Arthroscopy 24(3):371–373 [DOI] [PubMed]
- 26.Warden WH, Friedman R, Teresi LM, Jackson DW (1999) Magnetic resonance imaging of bioabsorbale polylactic acid interference screws during the first 2 years after anterior cruciate ligament reconstruction. Arthroscopy 15(5):474–480 [DOI] [PubMed]
- 27.Weiler A, Hoffmann RF, Stahelin AC, Helling HJ, Sudkamp NP (2000) Biodegradable implants in sports medicine: the biological base. Arthroscopy 16:305–321 [DOI] [PubMed]
- 28.Zantop T, Weimann A, Schmidtko R, Herbort M, Raschke MJ, Petersen W (2006) Graft laceration and pullout strength of soft-tissue anterior cruciate ligament reconstruction: in vitro study comparing titanium, poly-d, l-lactide, and poly-d, l-lactide-tricalcium phosphate screws. Arthroscopy 22(11):1204–1210 [DOI] [PubMed]