Abstract
Antiretroviral-therapy has dramatically changed the course of HIV infection and HIV-infected (HIV(+)) individuals are becoming more frequently eligible for solid-organ transplantation. However, only scarce data are available on how immunosuppressive strategies relate to transplantation outcome and immune function. We determined the impact of transplantation and immune-depleting treatment on CD4+ T-cell counts, HIV-, EBV-, and CMV-viral loads and virus-specific T-cell immunity in a 1-year prospective cohort of 27 HIV(+) kidney transplant recipients. While the results show an increasing breadth and magnitude of the herpesvirus-specific cytotoxic T-cell (CTL) response over-time, they also revealed a significant depletion of polyfunctional virus-specific CTL in individuals receiving thymoglobulin as a lymphocyte-depleting treatment. The disappearance of polyfunctional CTL was accompanied by virologic EBV-reactivation events, directly linking the absence of specific polyfunctional CTL to viral reactivation. The data provide first insights into the immune-reserve in HIV+ infected transplant recipients and highlight new immunological effects of thymoglobulin treatment. Long-term studies will be needed to assess the clinical risk associated with thymoglobulin treatment, in particular with regards to EBV-associated lymphoproliferative diseases.
Keywords: HIV, kidney transplantation, T cell, Immunological monitoring
Introduction
The availability of antiretroviral therapy (ART) has changed the long-term clinical management of HIV infection and chronic renal failure is now increasingly prevalent in the HIV(+) population. In fact, in HIV(+) individuals treated with ART, chronic kidney, liver and heart diseases have replaced opportunistic malignancies and infections as the leading cause of mortality.(1–3) Chronic renal failure in HIV(+) individuals can be due to direct effects of HIV infection (HIV-associated nephropathy/HIVAN), or result from immune-complex glomerulonephritis, ART drug toxicity or co-morbid diseases such as hypertension and diabetes.(4–6) Kidney transplantation is considered the most effective treatment modality for end-stage renal disease (ESRD) and has been successfully attempted in the HIV(+) setting, notwithstanding a somewhat elevated occurrence of acute rejection episodes.(7–14) Accordingly, the use of appropriate immunosuppressive regimens in this setting is a matter of debate more so than the generally well tolerated ART. In particular, immunosuppressive (IS) regimens including thymoglobulin (ATG), have been associated with an accelerated long-term CD4+ T-cell depletion and increased risk of non-opportunistic infections and may thus hamper overall clinical outcome in this patient group.(15)
Among opportunistic complications, Epstein-Barr virus (EBV)- and Cytomegalovirus (CMV)-associated diseases are among the most dreaded ones in solid organ transplant recipients.(16–21) Although effective immune control of these pathogens has been demonstrated in healthy subjects and by adoptive T-cell transfer approaches, the knowledge on HIV-, EBV-, and CMV-specific immunity in the context of solid organ transplantation is limited and the understanding of the virus-specific immunological reserve of HIV(+) kidney transplant recipients subjected to iatrogenic IS being especially poor. However, a better understanding of factors associated with loss of immune control over viral infections may be needed to ensure best possible clinical outcome of organ transplantation and to make optimal use of available organs. By longitudinally enumerating CD4+ T-cells, quantifying HIV, EBV and CMV viral loads, and assessing the breadth, magnitude and functionality of virus-specific CD8+ T-cell responses in HIV(+) kidney transplant recipients we sought to gain insight into the complex relation between iatrogenic IS, viral pathogens and host immunity.
Material and Methods
Study participants
Twenty-seven HIV(+) transplant recipients were included in this study. Inclusion criteria included undetectable plasma HIV RNA for six months before transplantation (measured using either the Amplicor Monitor Ultrasensitive PCR assay or the bDNA Versant version 3.0 assay), CD4+ T-cell-counts >200 cells/μL, and no use of IL-2 or GM-CSF in the six months prior to transplantation. Exclusion criteria were pregnancy and significant wasting or weight loss. Of the 27 subjects, none had any other organ transplantation before enrolling into the study. Twenty-six, 18 and 6 out of the 27 transplant recipients received prophylactic antiviral medication (Ganciclovir/Acyclovir) during the first 3, the second 3 and the last 6 months of the observation period, respectively. Ganciclovir was used prominently, with only 2 and 1 patients receiving Acyclovir and both Ganciclovir and Acyclovir, respectively.
The Institutional Review Boards of the participating hospitals approved the study and all subjects provided written informed consent before recruitment.
Viral loads
Plasma CMV DNA was quantified as previously described.(22) The lower limit of detection for CMV DNA was 1000 copies/ml.(23) CMV reactivation was defined here as any single or repeated newly detectable plasma CMV viral load in latently infected individuals.
Cellular EBV viral loads were also determined using previously methods.(24, 25) The lower limit of sensitivity of this assay is 1copy EBV DNA per 105 PBMCs. Consistent with recent reports, re-activation of latent EBV-infection was defined as either single or repeated occurrence of detectable viral load (>100 copies/105 cells) in previously aviremic patients, or as a significant increase in patients that continuously had detectable EBV viral loads; an increase being considered significant if the peak value exceeded threefold standard deviation of the individual’s mean viral load (calculated without the values of the obvious viral load peak).(26)
ELISpot assays
ELISPot assays were carried out as previously described.(27, 28) Briefly, frozen PBMC-samples were thawed and plated directly, without prior in vitro expansion, into 96-well ELISpot plates with 100,000 cells per well in the presence of 10 μg/mL of peptide(s). Plates were then incubated for 16 hours and developed for the secretion of IFN-γ. Spots were counted using an AID ELISpot Reader Unit (Autoimmun Diagnostika GmbH, Strassberg, Germany). Results were expressed as spot forming cells per 1×106 input cells. Thresholds for positive responses were determined as at least 5 spots per well and spot counts exceeding the mean plus 3 standard deviations of negative control wells.
In order to identify specificities for single peptides, we carried out a 2-step ELISpot procedure. In the first step, we tested the cells for responses to a matrix of peptide pools were each peptide was present in two peptide pools. Each pool was tested in a different well on the plate and the patterns of reactive pools allowed us to predict the individual targeted peptide(s) present in these pools. To confirm the identity of the targeted epitope, a second ELISpot assay was performed, now testing the suspected reactive peptides individually. This method represents a time- and sample-saving way to test large libraries of peptides, without having to test each peptide separately.
Antigenic peptides
The peptide sets used consisted of previously described HLA class I restricted CD8+ T-cell epitopes for HIV, CMV and EBV. For HIV, all 184 optimally defined HIV-derived CD8+ T-cell epitopes listed in the 2001 edition of the Los Alamos National Laboratory HIV Immunology Database CD8+ T-cell epitope list were included.(29) The set of used EBV-derived CD8+ T-cell epitopes consisted of 92 HLA class I restricted CD8+ T-cell epitopes as recently described.(27) A set of 38 CMV-derived CD8+ T-cell epitopes was included to assess CMV-specific T-cell reactivity.(27) Given the wide promiscuity of CD8+ T-cell epitopes, all individuals were tested with the entire sets of virus-specific peptides, regardless of their HLA type and the peptides described HLA restriction,(30) using a matrix system that allowed us to get a complete screen with two rounds of ELISpot assays and minimal amounts of cells.
Multi-parameter flow cytometry
Thawed cells were washed twice and re-suspended in R10 at 106/mL and one million cells were typically used per stain. Anti-CD28 and anti-CD49d (BD Biosciences, CA) were added at a final concentration of 1μg/ml along with selected (using ELISpot results) HIV-, EBV- or CMV-derived single peptides (final concentration 2μg/ml) and incubated with the cells at 37° C for one hour. Selection of individual peptides was based on positive responses observed in the screening ELISpot data. Then, Brefeldin A (Sigma) was added (final concentration 10μg/ml) and the cells cultured for six more hours at 37° C. A UV viability dye (LIVE/DEAD® Fixable Blue Dead Cell Stain Kit, Invitrogen, CA) was then used according to the manufacturer’s protocol to be able to discriminate between viable and apoptotic cells before cells were washed twice in FACS Buffer (PBS/1% FCS) and stained with all surface antibodies (i.e. anti-CD3-Pacific Blue and anti-CD8-Pacific Orange, both from BD Biosciences) for 1 hour at 4°C. The cells were again washed twice in FACS Buffer and permeabilized using the Fix/Perm Kit from BD Biosciences following the manufacturer’s instructions. After washing, cells were incubated with 250 μL BD Fix/Perm solution for 15 min at 4°C, washed with BD PermWash solution and subsequently incubated for for 1 hour at 4°C with all intracellular antibodies (i.e. anti-IFN-γ-PE-Cy7, anti-TNF-α-Alexa 700, both from BD Biosciences, and anti-IL-2-APC from Biolegend (San Diego, CA)). After two washing steps in BD PermWash solution and an additional washing step in FACS Buffer, cells were resuspended in a small volume of FACS Buffer and placed at 4°C until use.
Statistical analysis
The breadth and magnitude of virus-specific CD8+T-cell responses as well as the CD4+ T-cell counts in study participants were compared between time points using non-parametric unpaired Kruskal-Wallis tests and non-parametric paired Wilcoxon Signed Rank tests, whereas the frequencies of individual CD8+ T-cell responses, stratified by functionality, were analyzed by Mann-Whitney U tests. All reported P values are two-sided, and P values <0.05 were considered statistically significant. Where data were missing, the number of missingobservations is indicated and no assumptions about missing data were made. GraphPad Prism (Version 5.0) and StatXact (Version 6.0) software were used for statistical analyses.
Results
Study Population, Immunosuppressive regimens, graft function and other clinical outcomes
Demographic, immunological and clinical characteristics of the study population are summarized in Table 1. A total of 27 HIV (+) kidney transplant recipients were included. At the time of transplantation, all study participants were infected with EBV and CMV infection was prevalent (25/27 [92.6%]). Allo-sensitization (defined by peak panel-reactive antibodies [PRA] >10%) was present in seven of 27 (25.9%) HIV(+) transplant recipients. Anti-retroviral therapy (ART) consisted of nucleoside/nucleotide reverse transcriptase inhibitors (RTI) and/or non-nucleoside RTI and/or protease inhibitors, mostly combined as a three-class therapy.
Table 1.
Demographic, immunological and clinical characteristics of HIV(+) renal transplant recipients
| Variable | |
|---|---|
| Patient number | 27 |
| Age – median (range) | 46 (32–71) |
| Race: | |
| -African Americans – no. (%) | 18 (66.7) |
| -Caucasian – no. (%) | 9 (33.3) |
| Male gender – no. (%) | 24 (88.8) |
| Living donor – no. (%) | 12 (44.4) |
| Epstein-Barr Virus infected – no. (%) | 27 (100) |
| Cytomegalovirus infected – no. (%) | 25 (92.6) |
| - Donor +/Recipient − | 0 (0) |
| - Donor +/Recipient + | 15 (55.5) |
| - Donor −/Recipient + | 10 (37.1) |
| - Donor −/Recipient − | 2 (7.4) |
| HLA-A/B mismatches – median (range) | 3 (0–4) |
| HLA-DR mismatches – median (range) | 2 (0–2) |
| Panel reactive Ab (50) >10% – no. (%) | 7 (25.9) |
| Cause of end stage renal disease – no. (%) | |
| Hypertensive renal disease | 9 (33.3) |
| HIV associated nephropathy | 7 (25.9) |
| Glomerulonephritis | 2 (7.4) |
| Others | 9 (33.3) |
Use of immunosuppressive and antiretroviral agents within the study and graft function of all study participants are listed in Table 2. Ten of the 27 [37.0%] transplant recipients received anti-thymocyte globulin (ATG) perioperatively (i.e. immediately prior to transplantation [n=9], or within the first 12 weeks post-transplantation [n=1]). In addition, one other patient received ATG as treatment against a suspected rejection 22 weeks after transplantation. Twenty-five of the 27 [92.6%] individuals were initiated on a standard triple immunosuppressive regimen consisting of steroids (Prednisone), a calcineurin inhibitor (Cyclosporine A [CyA] or Tacrolimus/FK506 [FK]), and a nucleotide/DNA synthesis inhibitor (Mycophenolate Mofetil [MMF] or Azathioprine). At 12 months post-transplantation, graft survival was overall 100% and graft function (glomerular filtration rate [GFR] estimated by the MDRD equation) was 60.7±23.1 mL/min/1.73m2. Seven early (< six months post-transplantation) and two late (6–12 months post-transplantation) biopsy-proven clinical rejection episodes were observed over the study timeframe. Finally, none of the patients developed lymphoproliferative diseases during the one year follow-up period.
Table 2.
Immunosuppressive regimens, graft function and anti-retroviral therapy
| Variable | |
|---|---|
| Immunosuppressive regimen – no. (%) | |
| Anti-thymocyte globulin (ATG) | 10 (37.0) |
| Simulect® (Basiliximab) | 11 (40.7) |
| At transplantation | |
| - Triple therapya | 25 (92.6) |
| - Dual therapy | 1 (3.7) |
| - Monotherapy | 1 (3.7) |
| At 6 months | |
| - Triple therapy | 23 (85.2) |
| - Dual therapy | 3 (11.1) |
| - Monotherapy | 1 (3.7) |
| At 1 year | |
| - Triple therapy | 22 (81.5) |
| - Dual therapy | 4 (14.8) |
| - Monotherapy | 1 (3.7) |
| Glomerular Filtration Rate – mL/min/1.73m2 | |
| mean±SD | 60.7±23.1 |
| 1 year | |
| Biopsy-proven clinical rejection episodes – no. (%) | |
| <6 months | 7 (25.9) |
| 6 months to 1 year | 2 (7.4) |
| Anti-retroviral therapy – no. (%) | |
| Three-class therapyb | 22 (81.5) |
| Two-class therapy | 5 (18.5) |
Immunosuppressive triple therapy consisted of Cyclosporine A (CyA) or Tacrolimus (FK), Mycophenolate Mofetil (MMF) or Azathioprine, and Prednisone (Pred). In some cases (n=10), Sirolimus supplemented or replaced either MMF or Pred. Dual therapy consisted of CyA or FK and Pred or MMF, alternatively of MMF or Azathiorpine and Sirolimus, and in one case of FK or Azathiorpine and Pred. Monotherapy consisted of either Tacrolimus, MMF, Azathioprine or Pred.
Three-class therapy consisted of nucleoside/nucleotide reverse transcriptase inhibitors (nRTI), non-nucleoside RTI (nnRTI; or in one case fusion inhibitors [FI]) and protease inhibitors (PI). Two-class therapy included nRTI and nnRTI or PI.
Transient reduction in CD4+ T-cell counts is driven by ATG treatment
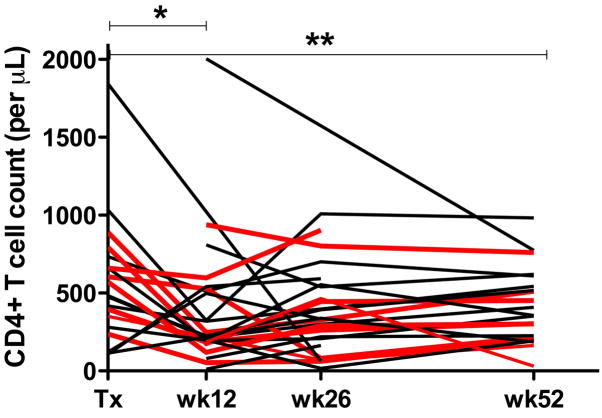
CD4+ T-cell counts were determined before and at 12, 26, and 52 weeks post-transplantation. The median CD4+ T-cell count at study entry was 483 CD4+ T-cells/ml (range 108–1843). Subsequently, a significant decrease in CD4+ T-cell counts was observed 12 weeks post-transplantation (Fig. 1). While statistically significant when compared to pre-transplantation time point for all 27 subjects, the initial decline in CD4+ T-cell counts at week 12 was largely driven by the patients treated with ATG (pre-transplant vs. week 12 CD4+ T-cell-counts in ATG-treated vs. non-ATG-treated individuals: p=0.01 [median CD4+ T-cell-counts pre- and week 12 post-transplantation: 588 and 187 cells/μl, respectively] vs. p=0.30 [median CD4+ T-cell-counts pre- and week 12 post-transplantation: 475 and 320 cells/μl, respectively]). Although most individuals recovered their CD4+ T-cell counts thereafter, their numbers remained significantly below pre-transplant levels at the end of the 1 year follow-up period (pre- vs. week 52 post-transplantation; all HIV(+) study participants: p<0.01, ATG-treated: p=0.01 [median CD4+ T-cell-counts week 52 post-transplantation: 302 cells/μl], non-ATG-treated: p=0.2 [median CD4+ T-cell-counts week 52 post-transplantation: 517 cells/μl]).
Figure 1. CD4+ T-cell-counts in HIV(+) kidney transplant recipients before and at 12, 26, and 52 weeks post-transplantation.
Counts are expressed as CD4+ T-cells per μL of peripheral blood. Each line represents one individual, red lines represent individuals that were treated with ATG. Note the significantly lower CD4+ T-cell counts at week 12 (* p<0.05) and week 52 (** p<0.01, [Wilcoxon signed rank]) compared to pre-transplantation levels.
Stable HIV viral loads and pre- versus post-transplantation HIV-specific CD8+ T-cell responses
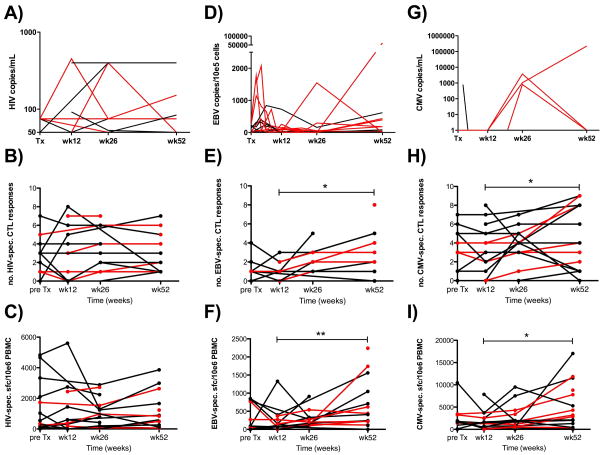
HIV viremia was well controlled in all study participants both before (inclusion criterion) and after transplantation (Fig. 2, Panel A).
Figure 2. Kinetics of viral loads, and breadth and magnitude of HIV-, EBV- and CMV-specific CD8+ T-cell responses in HIV(+) kidney transplant recipients.
Panel A) shows HIV viral loads before and after transplantation. HIV viral loads are expressed as copies per mL plasma/serum. Note that most measurements showed viremia below the detection limit of the assay (<50 copies/ml) and thus fall on the x-axis. Panel B) and C) show the ex vivo assessment of the breadth and magnitude of HIV-specific CD8+ T-cell responses before and after transplantation as assessed by ELISpot assays.. Similarly, panels D)–F) show longitudinal measurements of EBV viral loads (in copies/105 cells; median of 7 measurements per patient), and breadth and magnitude of the EBV-specific CD8+ T-cell response, respectively. Note the significant increase in both breadth (Panel E, * p<0.05) and magnitude (Panel F, ** p<0.01) of EBV-specific T-cell responses between weeks 12 and 52. Finally, panels G)–I) show CMV viral loads (expressed as copies per mL plasma; median of 7 measurements per person), and CMV-specific CD8+ T-cell responses pre- and post-transplantation, respectively. Again, note the significant increase in both breadth (Panel H, * p<0.05) and magnitude (Panel I, * p<0.05) of CMV-specific CD8+ T-cell responses between weeks 12 and 52. Each line or symbol represents a single individual; red lines and symbols identify individuals that were treated with ATG. Bold horizontal lines indicate medians.
The breadth (i.e. the number of epitopes recognized) and the magnitude (i.e. the frequency of epitope-specific T-cells, expressed as IFNγ spot forming cells per million PBMC [abbreviated here as SFC]) of the immune responses to optimally-defined CD8+ T-cell restricted epitopes, as measured by IFN-γ ELISpot, was stable between pre-transplantation time points and 12, 26 and 52 weeks post-transplant (Fig. 2, Panels B and C: median of three epitopes recognized). Only minor variations in the magnitude of these responses were observed (pre, 12 weeks, 26 weeks and 52 weeks post-transplantation medians: 540, 370, 780 and 720 SFC; Fig. 2, Panel C). No difference in response rates were detected between HIV(+) transplant recipients that were receiving ATG (median 2–3 responses over time) and those not receiving ATG (median 1.5–3 responses over time).
Post-transplantation cellular host immune responses to EBV are increased compared to baseline values
In accordance with previous reports indicating a high prevalence of EBV DNA in peripheral blood mononuclear cells and saliva of treated HIV(+) individuals, EBV DNA was detected at least once in every subject over the course of follow-up (Fig. 2, Panel D).(31, 32) Importantly, the rate detection of EBV DNA did not differ between pre- and post-transplantation PBMC samples (5/21 [23.8%] pre- vs. 47/147 [31.3%] available post-transplantation samples with detectable EBV viral load). Using the criteria described in the Material and Methods section, 8 out of 10 [80%] HIV(+) individuals treated with ATG reactivated EBV. The remaining two ATG treated individuals also showed ongoing viral replication but could not be classified formally as reactivators since pre-transplantation viral load measurements were missing. In contrast, EBV-reactivation was observed in only 1/15 [6.7%] ATG-naïve transplant recipients (P<0.001, Fig. 2, Panel D). EBV viral loads remained statistically significant higher in the ATG-treated vs. non-ATG-treated sub-groups of transplant recipients, both at week 12 and week 52 post-transplantation compared to baseline (pre-transplantation) values (p=0.007 and p=0.01, respectively, Fig. 2, Panel D, red versus black lines).
Before transplantation, EBV-specific T-cells were detected in 10/13 [76.9%] patients for whom EBV-specific immune responses could be assessed. Comparison of pre- and post-transplantation time points by non-parametric unpaired analysis of variance (ANOVA; Kruskal Wallis test) revealed no significant difference. However, excluding subjects for whom longitudinal data was unavailable, by means of a paired non-parametric analysis (Wilcoxon), revealed significantly increased breadths and magnitudes of the EBV-specific CD8+ T-cell response over time (week 12 and 52 breadth/magnitude (in SFC): 1/119 and 2/410, respectively; p=0.01 and p=0.007 for both breadth and magnitude, respectively; Fig. 2, Panels E and F). ATG-treated individuals did not differ significantly from the ATG untreated group (p>0.05).
Cellular host immune responses to CMV increase over the first year post-transplantation
CMV reactivation was detected in four of the 25 transplant recipients that were CMV infected at the pre-transplantation time point (Fig. 2, Panel G). These included 3 of the 7 [42.8%] subjects that received ATG and for whom follow-up CMV viral loads were available and 1 of the 9 [11.1%] transplant recipients that did not receive ATG (p=0.26). We then assessed the CMV-specific T-cell reactivity and pre-transplantation responses were detected in 23 [92.0%] of the 25 CMV-infected subjects. CMV-specific responses pre- and post-transplantation were again undistinguishable both in terms of breadth and magnitude by ANOVA analyses (Fig. 2, Panels H and I). Nevertheless, and analogous to the EBV-specific CD8+ T-cell response, paired analyses (Wilcoxon) on longitudinal data points from the same subjects revealed statistically significant broader and stronger responses at week 52 (breadth/magnitude: 3/2850) compared to week 12 (3/690; p=0.02 and p=0.01 for breadth and magnitude, respectively). ATG-treatment did not impact the breadth or magnitude of the CMV-specific CD8+ T-cell response.
Reduction in effector functions in virus-specific T-cell responses post-transplantation are driven by ATG and are associated with EBV reactivation episodes
To investigate the reason(s) why ATG-treated and ATG-untreated patients show such differences in control of EBV-reactivation, notwithstanding very comparable breadths and magnitudes of their EBV-specific CD8+ T-cell responses, the effector functionality of HIV-, EBV- and CMV-derived peptide specific CD8+ T-cell responses were assessed longitudinally. Although a potential association between functional competence of specific CTL populations and viral control could only be expected, if at all, in the case of EBV, for which prophylactic or therapeutic effect of anti-viral agents is less well established as for HIV and CMV, T-cell responses to HIV- and CMV (which were under efficient pharmacological control) were analyzed as well to determine the effect of ATG on anti-viral immune responses across different viruses.(33)
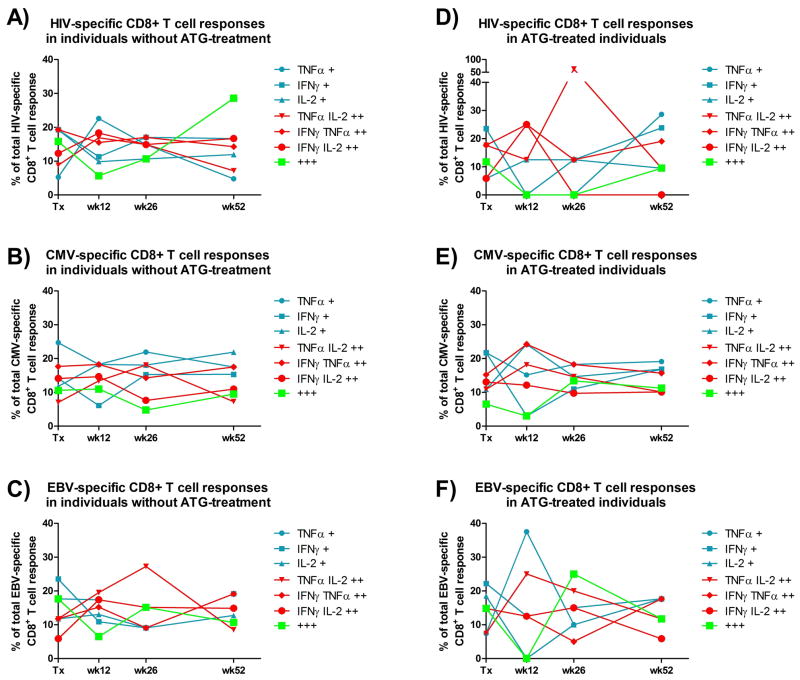
The functional analyses were based on the ability of CD8+ T-cells to react to antigen stimulation with the secretion of the cytokines IFN-γ, TNF-α and/or IL-2. First, each response was assigned one of the seven possible combinations of effector functions as listed in Figure 3 and the relative (%) contribution of each combination to the total virus-specific response across all subjects (from each group; i.e. ATG-treated an ATG-naïve transplant recipients) at any given time point was recorded. As shown in Figure 3, ATG-treatment appears to induce a more vigorous, and transiently complete, depletion of tri-functional antiviral CD8+ T-cell responses than ATG-free immunosuppressive regimen, although this comparison did not reach statistical significance. ATG treated individuals experienced a similar drop in tri-functional cells specific for CMV at week 12.
Figure 3. Impact of ATG-treatment on the functional composition of virus-specific CD8+ T-cell responses in HIV(+) kidney transplant recipients.
Panels A) and D) represent the functional composition, pre- and post-transplantation, of HIV-reactive CD8+ T-cells of ATG-treated and ATG-untreated HIV(+) kidney transplant recipients, respectively. The composition is expressed as % of total HIV specific CD8+ T-cells, i.e. encompassing all ATG-treated and -untreated recipients, respectively. Likewise, panels B) and E), and C) and F) represent the functional composition of CMV- and EBV-responsive CD8+ T-cells in patients that did or did not receive ATG-treatment, respectively.
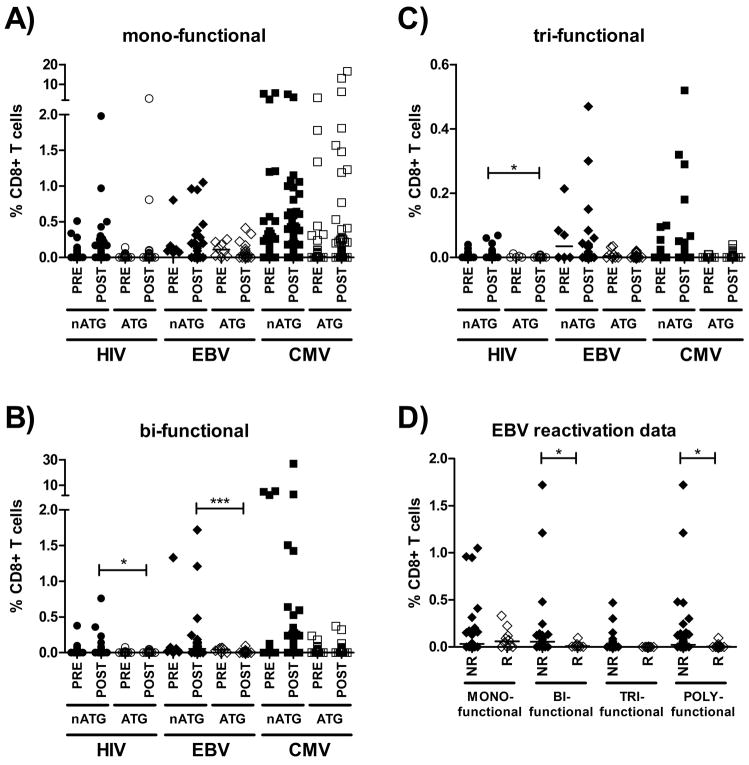
Since this analysis was designed to encompass all individual immune responses within each group and to provide an overall assessment of the effector function patterns, responses of smallest or greatest magnitude were attributed the same weight. We thus potentially overestimated the contribution of weak responses to the total virus-specific immunity. To compensate for this potential bias, we then compared all individual responses based on their effector function profile and their frequency in the CD8+ T-cell pool of each patient, for each virus separately and between pre- and post-transplantation time points (Figure 4). Responses were recorded either as mono-, bi- or tri-functional and their frequency compared between ATG-treated and ATG-untreated individuals. As shown in Figure 4, panel A, the frequencies of HIV, EBV and CMV-specific CD8+ T-cells that responded with only one detectable effector function were largely unaffected by immunosuppressive regimens, as for all viruses the ATG treated and ATG untreated individuals showed comparable magnitudes of responses pre- vs. post-transplantation. In contrast, we observed an almost complete absence of bi- and tri-functional responses in ATG-treated subjects compared to ATG-untreated individuals at post-transplantation time points (i.e. panels B and C: EBV-specific CD8+ T-cell immune responses [p<0.001] as well as bi-and tri-functional HIV-specific CD8+ T-cell responses [both p<0.05]). Importantly, ATG treated and ATG untreated individuals had comparable pre-transplantation frequencies of mono-, bi- and tri-functional CD8+ T-cells specific for HIV, EBV and CMV. To test for a possible link between the relative absence of bi- and tri-functional CD8+ T-cells and viral control, the effector function profiles of CTL specific for EBV were compared to the occurrence of reactivation of EBV-replication (Fig. 4 panel D). Individual EBV-specific CD8+ T-cells were recorded based on their number of detectable effector functions and their frequency and compared between time points with signs of viral reactivation and time points that were not followed by a reactivation event. As shown, the relative magnitude of mono-functional EBV-specific CD8+ T-cell responses did again not significantly differ between those patients that subsequently reactivated EBV-replication vs. those that did not. However, bi-functional EBV-specific immune responses of greater magnitude were detected in patients that maintained viral control vs. individuals that reactivated virus (p=0.016). Overall, ‘poly’-functional immune responses (defined as bi- or tri-functional responses) were detected less often prior to EBV-reactivation events, and if detected, had significantly lower frequencies than responses seen at time points that were not followed by viral reactivation (p=0.024).
Figure 4. Impact of ATG-treatment on the individual frequency of mono-, bi- and tri-functional HIV-, EBV- and CMV-specific CD8+ T-cells pre- and post-transplantation and the occurrence of EBV-reactivation post-transplantation in HIV(+) kidney transplant patients.
Panel A) shows individual frequencies of mono-functional) HIV-, EBV- and CMV-specific CD8+ T-cells over time in transplant recipients that were treated with or without ATG (labeled “ATG” and “non-ATG”, respectively). Similarly, panels B) and C) represent individual frequencies of bi- and tri-functional virus-specific CD8+ T-cells in the same groups. Panel D) shows individual frequencies of EBV-specific CD8+ T-cells with the indicated level of polyfunctionality that where measured at the time of an EBV-reactivation event or conversely, at time points that were not followed by any reactivation event. Horizontal lines indicate medians. * p<0.05; *** p<0.001. Closed symbols represent non-ATG-treated individuals, while open symbols represent ATG-treated individuals. Abbreviations: PRE: pre-transplantation; POST: post-transplantation; nATG: non-ATG treated individuals; ATG: ATG-treated individuals; NR: individuals for whom no EBV-reactivation was observed; R: individuals that reactivated EBV-replication.
Discussion
Despite good outcomes after solid organ transplantation in HIV(+) recipients, accepting HIV(+) individuals on transplantation waiting-lists continues to be a matter of debate.(34) In this prospective study in HIV(+) kidney transplant recipients we sought to shed light on issues related to immune competence that may influence organ allocation for transplantation in HIV(+) patients. In particular, we investigated how post-transplant immunosuppression (IS), and in particular Thymoglobulin (ATG)-treatment, impacts anti-herpesviral immunity. The advent of ART has largely contributed to the decreased occurrence of herpesvirus-related diseases; however, these remain a significant concern for HIV-infected individuals undergoing solid organ transplantation as pharmacological IS might diminish the anti-herpesviral immunity even further.(35, 36) The present study demonstrates that HIV viremia remained well-controlled and HIV-specific immune-responses were stable throughout the observation period. However, the individuals who were treated with lymphocyte-depleting regimen (ATG) show a particularly profound absence of polyfunctional virus-specific T-cells and experience the majority of viral-reactivation episodes, indicating that ATG treatment in this setting may put these individuals at a considerable risk for the development of herpesvirus-associated diseases. The short-term follow-up of our study did unfortunately not allow us to substantiate this finding clinically.
A number of previous studies on renal transplantation in the HIV(+) host have yielded promising results but were often based on highly selected individuals.(7, 8, 10, 37) By contrast, the HIV(+) population studied here was–with regards to ethnicity, level of pre-sensitization and renal pathologies–representative of HIV(+) US residents currently, or in the near future, awaiting renal replacement therapy.(38) The data demonstrate that the breadth and magnitude of the HIV specific T-cell response remained stable between pre- and up to one year post-transplantation while the herpesvirus-specific response gained in both, breadth and total magnitude. While the stable HIV-specific response may have been predicted given the well-controlled HIV viremia, the increased herpesvirus-specific immunity post-transplantation was more surprising. While this expansion may be a consequence of antigen driven activation of EBV- and CMV-specific T-cells, it is also important to note that such expansion was not limited to subjects with documented viral reactivation. Further analyses of plasma and cellular viral replication, excluding possible artifacts such as non-productively infected, apoptotic cells will be needed to better understand the balance between viral reactivation and immune reactivity. However, the present data suggest that an immunologically relevant viral replication is ongoing as comparative analyses in HIV-negative kidney transplant recipients show a slow decay in herpesvirus-specific immune responses and an absence of viral reactivation (Gasser et al, manuscript in preparation)
Naturally, the observed increased in immune reactivity does not necessarily imply improved immune control, as antigen driven T-cell responses to infection such as HIV, KSHV and other pathogens have been described.(28, 39, 40) To address whether the expanded cells were functional and whether the ATG treatment, shown to cause a more pronounced CD4+ T-cell depletion, was associated with higher frequencies of functionally impaired T-cells, we conducted flow-cytometric analyses on T-cells specific for HIV, EBV and CMV.(15, 41–43) Guided by earlier studies on T-cell function in these infections, we measured three effector functions widely used to characterize CTL-functionality, i.e. IFN-γ, TNF-α and IL-2.(44–46) Overall, the post-transplantation time points revealed T-cell populations with fewer detectable effector functions, affecting T-cells specific for all three viruses tested and suggesting that IS and/or ATG treatment may severely cripple these pre-existing immune responses. A subsequent stratification of tested subjects into ATG-treated vs. non-ATG-treated transplant recipients demonstrated that the observed effects was strongly driven by ATG treatment as ATG-treated patients consistently lacked polyfunctional anti-viral CTL responses of high frequency. Thus, while we could not detect any reduction in T-cell effector functions associated with “routine” (i.e. ATG-free) IS treatment, the data indicate that ATG treatment has rather devastating effects on T-cell functionality. Importantly, these effects were not limited to weak and borderline responses, as ATG-treatment was associated with a wide depletion of polyfunctional response of high magnitude. Linking the initial observation that EBV-reactivation was almost exclusively restricted to ATG-treated patients to the subsequent functional immune-analyses of anti-viral CTL responses, we found a direct association between the absence of polyfunctional EBV-specific CTL responses and EBV-reactivation (Fig. 4, panel D). Importantly, we have not observed the development of any lymphoproliferative disease, which represented a big concern in the beginning of the study. A longer follow-up period is however needed to properly assess the long-term consequences of EBV reactivation episodes. Although HIV- as well as CMV-specific CD8+ T-cell responses were affected by similar functional impairments, the virological consequences were very likely silenced by anti-viral treatments. Intriguingly, three out of the four patients that reactivated CMV-replication were treated with ATG, an observation that will need to be expanded to more subjects and combined with refined detection approaches to assess a potential association between ATG treatment and subclinical viral reactivation in the setting of HIV+ organ transplantation.(47, 48) Further studies will also need to include properly matched HIV-negative patients to address the role of HIV-infection in the ATG-driven loss of immune-functionality. Preliminary observations in an independently designed cohort of HIV-negative kidney recipients indicate that ATG drives, expectedly, a similar drop in total CD4+T-cell counts, and might adversely affect breadth and magnitude of herpesvirus-specific CTL as well. If confirmed and extended to functional analyses, these data would suggest that the ATG-mediated effects described here for HIV-infected individuals would likewise influence other groups of transplant recipients. However, differences between transplant centers in their inclination to ATG treatment, the readiness to include HIV-infected individuals on transplantation waiting lists and impact of the local donor/recipients ethnicities will require substantial efforts in the design and execution of well-controlled analyses of immune reactivity and outcome of transplantation in HIV+ versus HIV− organ recipients.
In summary, the present study is the first report on immunological competence in HIV-infected individuals undergoing solid organ transplantation. The data demonstrate a profound effect of ATG on T-cell function, reminiscent of the functional impairment observed in patients with persistent HIV viral load,(49) and reveal an association between T-cell functionality and viral reactivation, at least for EBV. As the loss of effector function was not limited to EBV specific T-cells, the immune suppressive treatment using ATG will likely need to be sustained by prophylactic treatment of ATG-treated HIV(+) patients for other (viral) co-pathogen and might ultimately argue in favor of non-depleting immunosuppressive regimen to be used in HIV(+) transplant recipients.
Acknowledgments
This work was supported by a grant from the Swiss Foundation for Medical and Biological Grants (to O.G.) and the Solid Organ Transplantation in HIV: Multi-Site Study (AI052748) grant funded by the National Institute of Allergy and infectious Diseases as well as R01 067077 to CB. We thank Dr. Jose Miro at Hospital Clinic in Barcelona for helpful discussions.
References
- 1.Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30(Suppl 1 1):S5–14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Selik RM, Byers RH, Jr, Dworkin MS. Trends in diseases reported on U.S. death certificates that mentioned HIV infection, 1987–1999. J Acquir Immune Defic Syndr. 2002;29(4):378–387. doi: 10.1097/00126334-200204010-00009. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Renal Data System. USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2004. [Google Scholar]
- 5.Gardenswartz MH, Lerner CW, Seligson GR, Zabetakis PM, Rotterdam H, Tapper ML, et al. Renal disease in patients with AIDS: a clinicopathologic study. Clin Nephrol. 1984;21(4):197–204. [PubMed] [Google Scholar]
- 6.Rao TK, Filippone EJ, Nicastri AD, Landesman SH, Frank E, Chen CK, et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310(11):669–673. doi: 10.1056/NEJM198403153101101. [DOI] [PubMed] [Google Scholar]
- 7.Abbott KC, Swanson SJ, Agodoa LY, Kimmel PL. Human immunodeficiency virus infection and kidney transplantation in the era of highly active antiretroviral therapy and modern immunosuppression. J Am Soc Nephrol. 2004;15(6):1633–1639. doi: 10.1097/01.asn.0000127987.19470.3a. [DOI] [PubMed] [Google Scholar]
- 8.Kumar MS, Sierka DR, Damask AM, Fyfe B, McAlack RF, Heifets M, et al. Safety and success of kidney transplantation and concomitant immunosuppression in HIV-positive patients. Kidney Int. 2005;67(4):1622–1629. doi: 10.1111/j.1523-1755.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 9.Pelletier SJ, Norman SP, Christensen LL, Stock PG, Port FK, Merion RM. Review of transplantation in HIV patients during the HAART era. Clin Transpl. 2004:63–82. [PubMed] [Google Scholar]
- 10.Qiu J, Terasaki PI, Waki K, Cai J, Gjertson DW. HIV-positive renal recipients can achieve survival rates similar to those of HIV-negative patients. Transplantation. 2006;81(12):1658–1661. doi: 10.1097/01.tp.0000226074.97314.e0. [DOI] [PubMed] [Google Scholar]
- 11.Roland ME. Solid-organ transplantation in HIV-infected patients in the potent antiretroviral therapy era. Top HIV Med. 2004;12(3):73–76. [PubMed] [Google Scholar]
- 12.Roland ME, Stock PG. Review of solid-organ transplantation in HIV-infected patients. Transplantation. 2003;75(4):425–429. doi: 10.1097/01.TP.0000046943.35335.18. [DOI] [PubMed] [Google Scholar]
- 13.Roland ME, Stock PG. Solid organ transplantation is a reality for patients with HIV infection. Curr HIV/AIDS Rep. 2006;3(3):132–138. doi: 10.1007/BF02696657. [DOI] [PubMed] [Google Scholar]
- 14.Stock P, Roland M, Carlson L, Freise C, Hirose R, Terrault N, et al. Solid organ transplantation in HIV-positive patients. Transplant Proc. 2001;33(7–8):3646–3648. doi: 10.1016/s0041-1345(01)02569-6. [DOI] [PubMed] [Google Scholar]
- 15.Carter JT, Melcher ML, Carlson LL, Roland ME, Stock PG. Thymoglobulin-associated Cd4+ T-cell depletion and infection risk in HIV-infected renal transplant recipients. Am J Transplant. 2006;6(4):753–760. doi: 10.1111/j.1600-6143.2006.01238.x. [DOI] [PubMed] [Google Scholar]
- 16.Boubenider S, Hiesse C, Goupy C, Kriaa F, Marchand S, Charpentier B. Incidence and consequences of post-transplantation lymphoproliferative disorders. J Nephrol. 1997;10(3):136–145. [PubMed] [Google Scholar]
- 17.Cockfield SM. Identifying the patient at risk for post-transplant lymphoproliferative disorder. Transpl Infect Dis. 2001;3(2):70–78. doi: 10.1034/j.1399-3062.2001.003002070.x. [DOI] [PubMed] [Google Scholar]
- 18.Drew WL. Cytomegalovirus infection in patients with AIDS. J Infect Dis. 1988;158(2):449–456. doi: 10.1093/infdis/158.2.449. [DOI] [PubMed] [Google Scholar]
- 19.Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson MA, Mills J. Serious cytomegalovirus disease in the acquired immunodeficiency syndrome (AIDS). Clinical findings, diagnosis, and treatment. Ann Intern Med. 1988;108(4):585–594. doi: 10.7326/0003-4819-108-4-585. [DOI] [PubMed] [Google Scholar]
- 21.Steininger C, Puchhammer-Stockl E, Popow-Kraupp T. Cytomegalovirus disease in the era of highly active antiretroviral therapy (HAART) J Clin Virol. 2006;37(1):1–9. doi: 10.1016/j.jcv.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 22.van Doornum GJ, Guldemeester J, Osterhaus AD, Niesters HG. Diagnosing herpesvirus infections by real-time amplification and rapid culture. J Clin Microbiol. 2003;41(2):576–580. doi: 10.1128/JCM.41.2.576-580.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanghavi SK, Abu-Elmagd K, Keightley MC, St George K, Lewandowski K, Boes SS, et al. Relationship of cytomegalovirus load assessed by real-time PCR to pp65 antigenemia in organ transplant recipients. J Clin Virol. 2008;19:19. doi: 10.1016/j.jcv.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 24.Rowe DT, Qu L, Reyes J, Jabbour N, Yunis E, Putnam P, et al. Use of quantitative competitive PCR to measure Epstein-Barr virus genome load in the peripheral blood of pediatric transplant patients with lymphoproliferative disorders. J Clin Microbiol. 1997;35(6):1612–1615. doi: 10.1128/jcm.35.6.1612-1615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadowsky RM, Laus S, Green M, Webber SA, Rowe D. Measurement of Epstein-Barr virus DNA loads in whole blood and plasma by TaqMan PCR and in peripheral blood lymphocytes by competitive PCR. J Clin Microbiol. 2003;41(11):5245–5249. doi: 10.1128/JCM.41.11.5245-5249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurmann S, Fricke L, Wagner HJ, Schlenke P, Hennig H, Steinhoff J, et al. Molecular parameters for precise diagnosis of asymptomatic Epstein-Barr virus reactivation in healthy carriers. J Clin Microbiol. 2003;41(12):5419–5428. doi: 10.1128/JCM.41.12.5419-5428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bihl FK, Loggi E, Chisholm JV, 3rd, Hewitt HS, Henry LM, Linde C, et al. Simultaneous assessment of cytotoxic T lymphocyte responses against multiple viral infections by combined usage of optimal epitope matrices, anti- CD3 mAb T-cell expansion and “RecycleSpot”. J Transl Med. 2005;3(1):20. doi: 10.1186/1479-5876-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frahm N, Korber BT, Adams CM, Szinger JJ, Draenert R, Addo MM, et al. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J Virol. 2004;78(5):2187–2200. doi: 10.1128/JVI.78.5.2187-2200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frahm N, Goulder P, Brander C. Total assessment of HIV specific CTL responses: Epitope clustering, processing preferences and the impact of HIV sequence heterogeneity. In: Korber CBB, Walker B, Koup R, Moore J, Haynes B, Meyers G, editors. HIV Molecular immunology database. Los Alamos, NM, USA: Los Alamos National Laboratory: Theoretical Biology and Biophysics; 2002. [Google Scholar]
- 30.Frahm N, Yusim K, Suscovich TJ, Adams S, Sidney J, Hraber P, et al. Extensive HLA class I allele promiscuity among viral CTL epitopes. Eur J Immunol. 2007;37(9):2419–2433. doi: 10.1002/eji.200737365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brengel-Pesce K, Morand P, Schmuck A, Bourgeat MJ, Buisson M, Bargues G, et al. Routine use of real-time quantitative PCR for laboratory diagnosis of Epstein-Barr virus infections. J Med Virol. 2002;66(3):360–369. doi: 10.1002/jmv.2153. [DOI] [PubMed] [Google Scholar]
- 32.Miller CS, Berger JR, Mootoor Y, Avdiushko SA, Zhu H, Kryscio RJ. High prevalence of multiple human herpesviruses in saliva from human immunodeficiency virus-infected persons in the era of highly active antiretroviral therapy. J Clin Microbiol. 2006;44(7):2409–2415. doi: 10.1128/JCM.00256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green M, Reyes J, Webber S, Rowe D. The role of antiviral and immunoglobulin therapy in the prevention of Epstein-Barr virus infection and post-transplant lymphoproliferative disease following solid organ transplantation. Transpl Infect Dis. 2001;3(2):97–103. doi: 10.1034/j.1399-3062.2001.003002097.x. [DOI] [PubMed] [Google Scholar]
- 34.Halpern SD, Asch DA, Shaked A, Stock PG, Blumberg E. Determinants of transplant surgeons’ willingness to provide organs to patients infected with HBV, HCV or HIV. Am J Transplant. 2005;5(6):1319–1325. doi: 10.1111/j.1600-6143.2005.00812.x. [DOI] [PubMed] [Google Scholar]
- 35.Bower M, Palmieri C, Dhillon T. AIDS-related malignancies: changing epidemiology and the impact of highly active antiretroviral therapy. Curr Opin Infect Dis. 2006;19(1):14–19. doi: 10.1097/01.qco.0000200295.30285.13. [DOI] [PubMed] [Google Scholar]
- 36.Wood C, Harrington W., Jr AIDS and associated malignancies. Cell Res. 2005;15(11–12):947–952. doi: 10.1038/sj.cr.7290372. [DOI] [PubMed] [Google Scholar]
- 37.Stock PG, Roland ME, Carlson L, Freise CE, Roberts JP, Hirose R, et al. Kidney and liver transplantation in human immunodeficiency virus-infected patients: a pilot safety and efficacy study. Transplantation. 2003;76(2):370–375. doi: 10.1097/01.TP.0000075973.73064.A6. [DOI] [PubMed] [Google Scholar]
- 38.Excerpts From the United States Renal Data System 2003 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Am J Kidney Dis. 2003;42(Supplement S5):1–230. [PubMed] [Google Scholar]
- 39.Spiegel HM, DeFalcon E, Ogg GS, Larsson M, Beadle TJ, Tao P, et al. Changes in frequency of HIV-1-specific cytotoxic T cell precursors and circulating effectors after combination antiretroviral therapy in children. J Infect Dis. 1999;180(2):359–368. doi: 10.1086/314867. [DOI] [PubMed] [Google Scholar]
- 40.Woodberry T, Suscovich TJ, Henry LM, Martin JN, Dollard S, O’Connor PG, et al. Impact of Kaposi sarcoma-associated herpesvirus (KSHV) burden and HIV coinfection on the detection of T cell responses to KSHV ORF73 and ORF65 proteins. J Infect Dis. 2005;192(4):622–629. doi: 10.1086/432103. [DOI] [PubMed] [Google Scholar]
- 41.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204(10):2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daucher M, Price DA, Brenchley JM, Lamoreaux L, Metcalf JA, Rehm C, et al. Virological outcome after structured interruption of antiretroviral therapy for human immunodeficiency virus infection is associated with the functional profile of virus-specific CD8+ T cells. J Virol. 2008;82(8):4102–4114. doi: 10.1128/JVI.02212-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makedonas G, Betts MR. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol. 2006;28(3):209–219. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 44.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bihl F, Narayan M, Chisholm JV, 3rd, Henry LM, Suscovich TJ, Brown EE, et al. Lytic and latent antigens of the human gammaherpesviruses Kaposi’s sarcoma-associated herpesvirus and Epstein-Barr virus induce T-cell responses with similar functional properties and memory phenotypes. J Virol. 2007;81(9):4904–4908. doi: 10.1128/JVI.02509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLaughlin BE, Baumgarth N, Bigos M, Roederer M, De Rosa SC, Altman JD, et al. Nine-color flow cytometry for accurate measurement of T cell subsets and cytokine responses. Part II: Panel performance across different instrument platforms. Cytometry A. 2008;73(5):411–420. doi: 10.1002/cyto.a.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nashan B, Luck R, Kliem V, Brunkhorst R, Schlitt HJ, Klempnauer J. CMV in kidney transplantation: a single center experience over 22 years. Clin Transpl. 1999:181–188. [PubMed] [Google Scholar]
- 48.Rostaing L, Crespin A, Icart J, Lloveras JJ, Durand D, Martinet O, et al. Cytomegalovirus (CMV) prophylaxis by acyclovir in pre-transplant CMV-positive renal transplant recipients. Transpl Int. 1994;7(Suppl 1 1):S331–335. doi: 10.1111/j.1432-2277.1994.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 49.Streeck H, Brumme ZL, Anastario M, Cohen KW, Jolin JS, Meier A, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5(5):e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. Jama. 1999;282(23):2220–2226. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]