Abstract
Background
Major biological and cultural innovations in late Pliocene hominin evolution are frequently linked to the spread or fluctuating presence of C4 grass in African ecosystems. Whereas the deep sea record of global climatic change provides indirect evidence for an increase in C4 vegetation with a shift towards a cooler, drier and more variable global climatic regime beginning approximately 3 million years ago (Ma), evidence for grassland-dominated ecosystems in continental Africa and hominin activities within such ecosystems have been lacking.
Methodology/Principal Findings
We report stable isotopic analyses of pedogenic carbonates and ungulate enamel, as well as faunal data from ∼2.0 Ma archeological occurrences at Kanjera South, Kenya. These document repeated hominin activities within a grassland-dominated ecosystem.
Conclusions/Significance
These data demonstrate what hitherto had been speculated based on indirect evidence: that grassland-dominated ecosystems did in fact exist during the Plio-Pleistocene, and that early Homo was active in open settings. Comparison with other Oldowan occurrences indicates that by 2.0 Ma hominins, almost certainly of the genus Homo, used a broad spectrum of habitats in East Africa, from open grassland to riparian forest. This strongly contrasts with the habitat usage of Australopithecus, and may signal an important shift in hominin landscape usage.
Introduction
The hominin fossil and archeological records of Africa exhibit substantial anatomical and behavioral change during the Plio-Pleistocene (∼1.5–3.0 Ma), including the evolution of Homo and Paranthropus, the origin of lithic technology and archeological sites, the first evidence of large mammal butchery, lower limb elongation and selection for endurance running, and thermoregulatory adaptations to hot, dry environments [1]. These evolutionary innovations have been linked consistently to novel selective pressures encountered as early hominins foraged increasingly in more open, arid woodland and grassland habitats that were replacing wooded biomes. However, the most finely resolved evidence for environmental change is not from the fossil and archeological sites themselves, but from deep sea core records that indicate drier and more variable conditions in tropical and subtropical Africa [2]–[8]. An increase in arid-adapted vegetation is also reflected by morphological changes across many African large mammal lineages and by the dispersal of the Eurasian grazer Equus across Africa ∼2.3 Ma [9], [10]. Although these data suggest that grassland-dominated ecosystems (defined here as having >75% C4 plants and a graze-dependent fauna) should be present as one extreme of the continental habitat spectrum, actual documentation of both Pliocene grasslands and of hominin activities in open habitats has until now eluded paleoanthropologists. Here we use faunal and stable isotopic evidence to demonstrate the earliest presence of a grassland-dominated ecosystem, and archeological evidence for hominin activities within this setting from the late Pliocene locality of Kanjera South, Kenya (Fig. 1). At least one species of tool-making hominin, almost certainly of the genus Homo [1], was repeatedly using this open setting. In contrast, most other Oldowan occurrences are situated in more wooded settings. These findings indicate that by ∼2.0 Ma tool-making hominins, probably early Homo, accessed and used a broad spectrum of East African habitats, from open grassland to riparian forest.
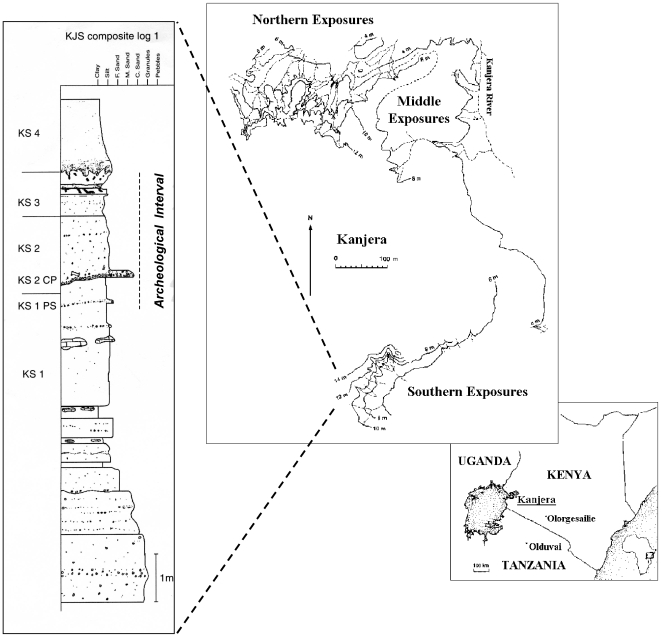
Figure 1. Placement map and stratigraphic diagram showing the location of Kanjera in southwestern Kenya and of the Southern Exposures at Kanjera.
The composite stratigraphic log shows the basal three beds of the Southern Member (KS-1 to KS-3) and the base of KS-4. Spatially associated artifacts and fossils are found as diffuse scatters and also in more vertically discrete concentrations from the top of KS-1 through KS-3, with KS-2 providing the bulk of the archeological sample.
The late Pliocene Oldowan occurrences at Kanjera South are found on the northern margins of the Homa Mountain Carbonatite Complex, Homa Peninsula, southwestern Kenya (Fig. 1). The Homa Peninsula lies within the Nyanza Rift, which presented a depositional low to the north of the site. The lithological sequence at Kanjera South consists of 6 beds of the Southern Member of the Kanjera Formation, from oldest to youngest KS-1 to KS-6 [11], [12]. Only KS-1 to KS-4 is described here since archeological occurrences are known only within this interval, from the top of Bed KS-1 through Bed KS-3.
KS-1 deposition began as a flow of pyroclastic material, possibly as a lahar, from the Homa Mountain complex in the south towards the depocenter in the Nyanza Rift graben. Lower KS-1 shows little internal stratification and no pedogenic development. In contrast, the well-bedded, better sorted and pedogenically modified upper parts of KS-1 represent reworking of the deposits by ephemeral streams running across the fan of the original pyroclastic flows. KS-2 represents a continuation of this environmental setting, with deposition by anastomosing channels flowing with intermittent, diffuse, generally low energy flow regimes and better-developed pedogenesis than KS-1.
KS-3 sees the transition to a wetter depositional environment, as evidenced by soft sediment deformation and the presence of a small channel, though stable land surfaces with pedogenesis continued to be found. KS-4 represents a continuation of this moister trend, with clays being deposited either during the transgression of a lake out of the depocenter to the north or during the formation of a wetland system. The homogeneity of the KS-4 clays favors the former interpretation, and the paleosol layers interbedded in KS-4 indicate intervals of lake regression sufficiently long for pedogenesis to take place.
A combination of biostratigraphy (co-occurrence of the equid Equus sp., the suids Metridiochoerus andrewsi and M. modestus, and the proboscidean Deinotherium sp.) as well as magnetostratigraphy (a reversed sequence in Beds KS-1 to KS-4 with the presence of the Olduvai subchron (1.95–1.77 Ma) in Beds KS-5 and KS-6) indicate that the KS-1 to KS-3 archeological occurrences date between ∼2.3 Ma (the dispersal of Equus across Africa, first occurrence of M. modestus) and 1.95 Ma (the base of the Olduvai subchron) [1], [11]. Given the apparent rapidity of deposition, an age of ∼2 Ma for the archeological occurrences seems most likely.
Except for artifacts and fauna found in thin, discontinuous conglomerate lenses, hominin activity was the primary agent of accumulation of the majority of archeological materials at this site [11], [13]. Discussion here focuses on KS-2 materials from the 169 m2 Excavation 1, which has yielded 2190 fossils and over 2471 artifacts with three dimensional coordinates from several levels within the 1.5-m-thick sequence. KS-2 accumulated rapidly and, based on the limited development of pedogenic features, likely represents decades to centuries of deposition.
Results and Discussion
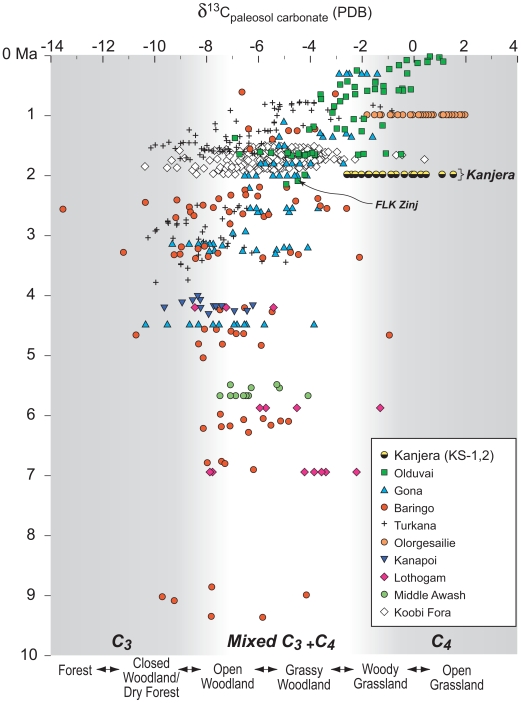
Habitats rich in plants using the C3 photosynthetic pathway, such as woodland and dry forests, are well-documented between 10 and 2 Ma in East Africa (Fig. 2). Stable isotopic analysis of pedogenic carbonates and occluded paleosol organic matter across an 130 m transect of the Kanjera South locality provide the first clear evidence of a grassland setting (>75% C4 vegetation) in this 10 million year sequence (Fig. 2, Tables S1 and S2). Evidence that grassland habitats dominated the regional ecosystem beyond the confines of our excavations is provided by large mammal frequencies, particularly of the family Bovidae, as well as the stable isotopic composition of tooth enamel from a suite of herbivorous mammals. Large mammals often range extensively during the course of a season or year [14], [15] and so can provide a sense of regional vegetation structure [16]. Predicted habitat and dietary preferences of primates, ungulates and proboscideans from Kanjera (Table S3) are based primarily on analogy with extant relatives, degree of hypsodonty, functional analysis of limbs and masticatory morphology, and stable isotopic analyses of South and East African fossil and modern fauna [9], [17]–[27]. Several taxa (crocodile, Phalacrocoracidae [cormorant], hippopotamid) reflect proximity to permanent water, perhaps a lake as suggested by KS-4. The reduncine bovids are indicative of edaphic grasslands and possibly woodland along the lake margin, whereas woodland is suggested by the presence of several tragelaphine bovid fossils, giraffe remains, and a Cercopithecus sp. monkey. The suid M. modestus may also signal woodland [18]. A Hippotragus sp. bovid fossil signals a woodland/grassland ecotone, whereas the antilopine Antidorcas recki is best associated with bushland to grassland habitats [9], [22], [24], [25]. The equids, alcelaphine bovids, and Theropithecus fossils are indicative of open, grassy environments. In spite of the range of predicted habitat preferences, taxa that preferred open, grassland habitats dominate the fauna (Tables S3 and S4). Seven hundred thirty two of the 886 fossils (82.6%) attributable to zoological family are bovids; of the 143 fossils identifiable to tribe, 132 (92%) are Antilopini or Alcelaphini, which are indicators of open grassland ecosystems in modern settings [9], [27]. The high frequency of equids in KS-2 (11.6%) is similar to the relative abundance of zebras in modern, grassland-dominated ecosystems in East Africa such as the Serengeti, Tanzania [11], [28].
Figure 2. Stable carbon isotopic composition of paleosol carbonates from the late Miocene through Pleistocene of East Africa [7], [11], [30], [33], [51]–[59].
Shaded intervals represent pedogenic nodules forming in C3 dominated and C4 dominated environments. Paleosol carbonate δ13C values of -2 or greater are approximately equivalent to floral communities with 75% or more C4 plants [31]. Intermediate values represent a mix of C3 and C4 vegetation. Plant root systems associated with KS-2 archeological site formation could have extended into the top of KS-1, so paleosol carbonate data from both KS-2 and the top of KS-1 are presented.
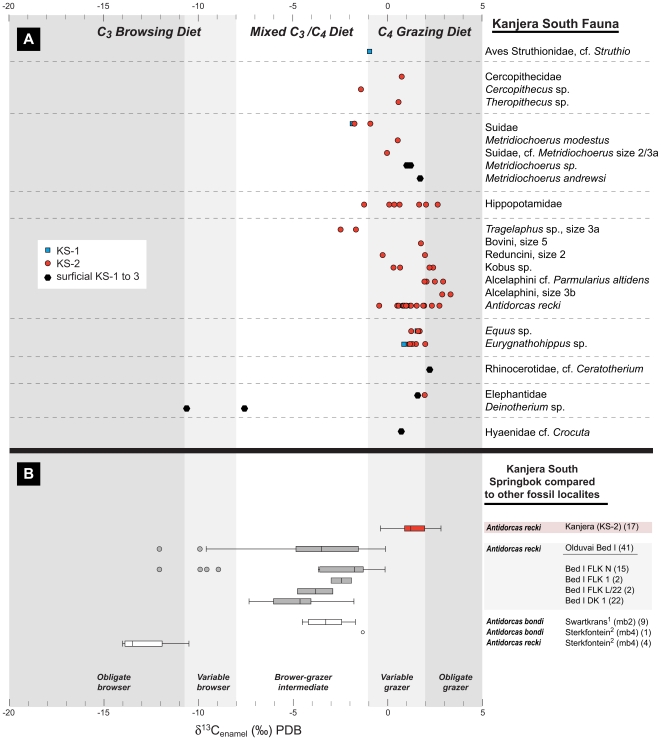
Isotopic analysis of enamel indicates that these taxa uniformly had a large amount of grass in their diets, reflecting the dominance of grass in the vegetation community (Fig. 3a) (Tables S3 and S4). This is true even for taxa that normally have a C3-rich (fruit or browse) diet (e.g., tragelaphine bovids and the monkey Cercopithecus sp.). One of the two teeth from Deinotherium, an obligate browser, has the most positive δ 13C value ever documented for this taxon [23], [29]. This indicates Deinotherium at least occasionally consumed C4 plants. The strong C4 signal occurs across the spectrum of animals found at Kanjera South, including non-dispersing taxa such as monkeys, rhinos, tragelaphine bovids, and suids. This confirms that the grassland dietary signal is not simply the result of dry season domination of the residential mammalian community by migratory grazers congregating near a permanent water source.
Figure 3. Stable carbon isotopic data of enamel from Kanjera South and other African localities.
A. Stable carbon isotopic composition of fossil mammal tooth enamel from KS-2 in Excavation 1. The KS-2 fauna is supplemented by several taxa unique to KS-1 or KS-3, or found on the surface of the KS-1 to KS-3 sequence, to provide a more complete sense of the diet of the mammalian community during the deposition of the archeological levels. The shading reflects the relative importance of C3 browse versus C4 grass in the diet, with δ13C values greater than -1 reflecting a diet with more than 75% C4 vegetation. Isotopic dietary classification follows others [23]. A probable ostrich (cf. Struthio) eggshell fragment was also analyzed. B. Box and whiskers plots of the stable carbon isotopic composition of modern and fossil gazelles from Kanjera, Bed I Olduvai Gorge, Tanzania, and Sterkfontein and Swartkrans, South Africa [60], [61]. Like many modern antilopines, Antidorcas recki was able to switch between browse and graze as necessary [9]. Numbers in parentheses after site name represent number of samples analyzed. Bed I localities are presented in stratigraphic order, from oldest (DK I, ∼1.87 Ma) to youngest (FLK NI, ∼1.78 Ma) [3].
These data provide the earliest isotopic evidence of an open habitat and a grassland-dominated ecosystem in East Africa. The presence of artifacts and archeological fauna both low and high in the KS-2 sequence and in the underlying KS-1 and overlying KS-3 indicates that hominins repeatedly visited this grass-rich area on the landscape for hundreds or even thousands of years. These data also substantively expand the known range of variation in Oldowan hominin habitat usage. Paleosol carbonate studies from the type locality of the Oldowan Industrial Complex, Olduvai Gorge, Tanzania, suggest that the Bed I and lower Bed II (∼1.7–1.87 Ma) basin margin was frequently well-wooded [30], [31]. Paleosol carbonate isotopic chemistry from the most informative Bed I archeological occurrence, FLK I Level 22 (FLK Zinj), suggests that artifacts and fossils were deposited in a grassy woodland (Fig. 2). Stable isotopic analysis of enamel samples from the extinct gazelle Antidorcas recki document an increasing amount of graze and a decreasing amount of browse in their diet through the Bed I sequence (Fig. 3b), consistent with a drying trend noted by other lines of paleontological and geological evidence [24], [28], [32]. Enamel samples of A. recki from FLK Zinj suggest a mixed diet of browse and grass, whereas Kanjera A. recki was predominantly grazing. Antidorcas recki individuals from the relatively arid, upper portion of the Bed I sequence have more negative δ13C values than those in the Kanjera KS-2 sample, suggesting that there was a greater proportion of open habitat at Kanjera than at any time during the deposition of Bed I Olduvai. Isotopic data at or in the vicinity of other Oldowan occurrences, including the oldest archeological sites (2.5–2.6 Ma) at Gona, Ethiopia [33] and 1.75–2.0 Ma occurrences at Koobi Fora, Kenya [34] indicate hominin activities in habitat mosaics that on average had 50% C3 vegetation (Fig. 2). Pollen data from Gona is concordant with a well-wooded setting for hominin site activities [35]. Vegetation mosaics including substantive woodland components are also suggested for Pliocene and early Pleistocene Oldowan sites in West Turkana, Kenya, and the Shungura Formation, Ethiopia [36], [37]. Finally, Oldowan hominin activities in a riparian forest setting are suggested by paleoenvironmental evidence from the Koobi Fora Formation in the Turkana basin, Kenya, at ∼2.0 Ma [38].
These findings indicate that by ∼2.0 Ma Oldowan hominins had access to and used a broad spectrum of East African habitats, from open grassland to riparian forest. Stone tool manufacture and archeological site formation at this time is most likely attributable to the genus Homo. Associations between H. habilis sensu lato (here including H. habilis and H. rudolfensis) and stone tools are known in the geological record by 2.3 Ma [39], [40]. The single definitive stone tool user in the Plio-Pleistocene, H. erectus (here including H. ergaster), appears in Africa by 1.8 Ma [41]. Overlap in size, cranial morphology, and cranial scaling between H. habilis and H. erectus suggest a close phylogenetic relationship between the two species [42]–[44], and support the idea that late Pliocene stone tool use was part of the behavioral repertoire of the evolving Homo lineage. Brain size expansion and masticatory changes in the Homo lineage have plausibly been linked to stone tool-dependent foraging [1].
Paranthropus, also known prior to 2.0 Ma, has been argued to have made stone tools based on hand bone morphology, and its stratigraphic association with Oldowan artifacts in eastern and southern Africa [45]. However, the developmental investment in very large jaws and cheek teeth seen in Paranthropus would have been unnecessary if a stone tool kit allowing extra-oral processing of food was in use. Moreover, there is no perceptible change in the archeological record after Paranthropus goes extinct, as might be expected if two parallel tool traditions, one formed by Homo, the other by Paranthropus, were in place during the late Pliocene and early Pleistocene [1]. While Paranthropus may have used a non-lithic technology [46], it is unlikely to have formed the Oldowan occurrences under consideration here.
The breadth of habitat use inferred for early Homo by 2.0 Ma contrasts strongly with that of Australopithecus, a precursor to Homo, for which heterogeneous environments, all with significant woodland or forest components, are documented consistently [23], [47]. The ∼1.5 Ma H. erectus skeleton from Nariokotome, Kenya, signals adaptive shifts in hominin mobility, foraging, and thermoregulation towards the increased use of open, hot, and dry environments [48]–[50]. These shifts are anticipated by the recurrent use of open habitats by early Homo at Kanjera. The Kanjera data do not, however, necessarily indicate that early Homo used open habitats in preference to wooded ones. Combined evidence from Oldowan sites suggests that early Homo was flexible in its habitat use, and that the capacity to extract resources from a range of open and more wooded environments was a vital component of its adaptation.
Methods
Excavation 1 was carried out within a grid of 169 1m×1m squares, excavated in 5 cm spits following site stratigraphy. Fossil and artifact-bearing horizons were dug with awls and dental picks. A Topcon total station was used for the precise determination of specimen N, E, and Z coordinates and in contour mapping. Object dip and orientation was measured with a Brunton compass. Sediments were dry sieved through 1 mm mesh. Sedimentary, taphonomic, and zooarcheological analyses indicate that the site assemblages formed predominantly through hominin activity [11], [13]. In KS-1, KS-2 PS, and KS-3 there is a clear spatial relationship between the artifacts and fauna. Many objects are outsized clasts relative to grain size; a diverse array of skeletal parts exhibiting a range of hydraulic transport potentials have been recovered; artifact and fossil refits have been made; and both percussion marks and cut marks have been found on bones.
Isotopic Analysis of Paleosol Carbonates
Pedogenic carbonates used in this analysis exhibited microstructure consistent with in situ formation without subsequent recrystallization. The difference in the δ 13C of occluded organic matter and pedogenic carbonate fits theoretical predictions for diagenetically unaltered materials. Diagenetic carbonate cements from the Kanjera Formation have negative δ 13C values (Table S1), so that the positive signal reported here is unlikely to have resulted through diagenetic alteration.
Paleosol carbonate samples were washed in double distilled water and dried. The outer layers of the carbonate nodules were removed using a dental burr and discarded. The inner part of each carbonate nodule was crushed in an agate mortar and each sample was split into two aliquots, the first treated with 2% NaOHCl solution at 60°C for 24 hours to remove any organic contamination. The second aliquot was treated with 1M HCl until no reaction was observed and the remaining organic matter was washed to neutrality and freeze-dried. Organic samples were analyzed using flash combustion CF-IRMS. Samples were combusted in a Carlo Erba 1108 sample converter and the evolved gas was analyzed in a Europa Geo 20/20 gas source mass spectrometer at the University of Oxford. Results are reported using the standard delta per mil (‰) notation relative to the VPDB international standard (Table S2). International and in-house standards analyzed along with the organic samples gave standard deviation of ±0.4‰ for carbon.
Isotopic Analysis of Enamel
Tooth enamel samples were carefully cleaned using an aluminium oxide air abrasive system to remove any adhering sediment and cementum. The outer surface of the enamel was abraded further, removing the outermost portion that was most likely to be diagenetically altered. Samples were then extracted from the cleaned enamel using a 0.5 mm diamond dental burr. Samples were ground and homogenized using an agate mortar. Powdered enamel samples were treated with 2% NaOHCl solution at 60°C for 24 hours to remove any organic contamination. Samples were then washed with double distilled water and treated with 0.1M CH3COOH at 25°C for 6 hours under vacuum to remove any secondary carbonate contamination. Samples were rinsed to neutrality and dried. All enamel samples for isotopic analysis were reacted with 100% phosphoric acid at 90°C in a common acid bath system. The evolved CO2 was pre-concentrated using a cold finger system and was analyzed at the University of Oxford using a VG Prism gas source isotope ratio mass spectrometer running in dual inlet mode. Results are reported using the standard delta per mil (‰) notation relative to the VPDB international standard. International and in-house standards analyzed along with the enamel samples gave standard deviations of ±0.08‰ for carbon and ±0.12‰ for oxygen.
Supporting Information
Isotopic data from diagenetic sparry, pendant and poikilotopic calcite cements from KS-1 and KS-2, and from samples of carbonatite from the Homa Mountain carbonatite complex.
(0.03 MB DOC)
Paleosol Carbonate Isotopic Data.
(0.04 MB DOC)
Vertebrate taxon list from KS-2, Excavation 1. Isotopic dietary classification of Kanjera mammalian fossils follows others (23) using the isotopic data presented in Table S4. Obligate grazers and obligate browsers consume an almost exclusive (>95%) C4 or C3 diet, respectively. Variable grazers and variable browsers consume a predominantly (75–95%) C4 or C3 diet, respectively. Brower-grazer intermediate refers to taxa consuming a mix of C4 and C3 vegetation.
(0.04 MB DOC)
Stable isotopic composition of fossil eggshell and tooth enamel from Excavation 1.
(0.13 MB DOC)
Acknowledgments
We thank the Office of the President, Republic of Kenya, and the National Museums of Kenya for permission and support in conducting the field and laboratory studies described here. This research was conducted under the co-operative agreement between the National Museums of Kenya and the Smithsonian Institution. Logistical support was provided by the Human Origins Program of the Smithsonian Institution.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Funding from the L. S. B. Leakey Foundation, the Leverhulme Trust, the National Geographic Society, the National Science Foundation, the Professional Staff Congress-City University of New York Research Award Program, and the Wenner-Gren Foundation for Kanjera field and laboratory research is gratefully acknowledged. Logistical support was provided by the Human Origins Program of the Smithsonian Institution. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Plummer TW. Flaked stones and old bones: biological and cultural evolution at the dawn of technology. Yrbk Phys Anthropol. 2004;47:118–164. doi: 10.1002/ajpa.20157. [DOI] [PubMed] [Google Scholar]
- 2.deMenocal PB. African climate change and faunal evolution during the Pliocene-Pleistocene. Earth Planetary Sci Lett. 2004;220:3–24. [Google Scholar]
- 3.Potts R. Environmental hypotheses of hominin evolution. Yrbk Phys Anthropol. 1998;41:93–136. doi: 10.1002/(sici)1096-8644(1998)107:27+<93::aid-ajpa5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Feakins SJ, deMenocal PB, Eglinton TI. Biomarker records of late Neogene changes in northeast African vegetation. Geol. 2005;33:977–980. [Google Scholar]
- 5.Kingston JD. Early human evolutionary paleoecology. Yrbk Phys Anthropol. 2007;50:20–58. [Google Scholar]
- 6.Potts R. Environmental hypotheses of Pliocene human evolution. In: Bobe R, Alemseged Z, Behrensmeyer AK, editors. Hominin environments in the East African Pliocene: an assessment of the faunal evidence. Dordrecht: Springer; 2007. pp. 25–50. [Google Scholar]
- 7.Wynn JG. Influence of Plio-Pleistocene aridification on human evolution: evidence from paleosols of the Turkana Basin, Kenya. Amer J Phys Anthropol. 2004;123:106–118. doi: 10.1002/ajpa.10317. [DOI] [PubMed] [Google Scholar]
- 8.Vrba ES. On the connections between paleoclimate and evolution. In: Vrba ES, Denton GH, Partridge TC, Buckle LH, editors. Paleoclimate and evolution with emphasis on human origins. New Haven: Yale University Press; 1995. pp. 24–45. [Google Scholar]
- 9.Bobe R, Behrensmeyer AK. The expansion of grassland ecosystems in Africa in relation to mammalian evolution and the origin of the genus Homo. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;207:399–420. [Google Scholar]
- 10.Turner A, Wood B. Comparative palaeontological context for the evolution of the early hominid masticatory system. J Hum Evol. 1993;24:301–318. [Google Scholar]
- 11.Plummer TW, Bishop LC, Ditchfield P, Hicks J. Research on late Pliocene Oldowan sites at Kanjera South, Kenya. J Hum Evol. 1999;36:151–170. doi: 10.1006/jhev.1998.0256. [DOI] [PubMed] [Google Scholar]
- 12.Behrensmeyer AK, Potts R, Plummer T, Tauxe L, Opdyke N, et al. The Pleistocene locality of Kanjera, Western Kenya: stratigraphy, chronology and paleoenvironments. J Hum Evol. 1995;29:247–274. [Google Scholar]
- 13.Ferraro JV. Broken bones and shattered stones: on the foraging ecology of Oldowan hominins. PhD dissertation. 2007. University of California Los Angeles, Los Angeles, California.
- 14.Estes RD. Berkeley: University of California Press; 1991. The Behavior Guide to African Mammals. [Google Scholar]
- 15.Kingdon J. San Diego: Academic Press; 1997. The Kingdon Field Guide to African Mammals. [Google Scholar]
- 16.Shipman P, Harris JM. Habitat preference and paleoecology of Australopithecus boisei in eastern Africa. In: Grine F, editor. The evolutionary history of the “robust” Australopithecines. New York: Aldine de Gruyter; 1998. pp. 343–380. [Google Scholar]
- 17.Bernor RL, Armour-Chelu M. Toward an evolutionary history of African hipparionine horses. In: Bromage TG, Schrenk F, editors. African biogeography, climate change and human evolution. New York: Oxford University Press; 1999. pp. 189–215. [Google Scholar]
- 18.Bishop LC. Suid paleoecology and habitat preference at African Pliocene and Pleistocene hominid localities. In: Bromage TG, Schrenk F, editors. African biogeography, climate change and human evolution. Oxford University Press; 1999. pp. 216–225. [Google Scholar]
- 19.Cerling TE, Harris JM, Passey BH. Diets of East African Bovidae based on stable isotope analysis. J Mammal. 2003;84:456–470. [Google Scholar]
- 20.Harris JM, Cerling TE. Dietary adaptations of extant and Neogene African suids. J Zool London. 2002;256:45–54. [Google Scholar]
- 21.Cerling TE, Harris JM, MacFadden BJ, Leakey MG, Quade J, et al. Global vegetation change through the Miocene/Pliocene boundary. Nature. 1997;389:153–158. [Google Scholar]
- 22.Kappelman J, Plummer TW, Bishop LC, Duncan A, Appleton S. Bovids as indicators of Plio-Pleistocene paleoenvironments in East Africa. J Hum Evol. 1997;32:229–256. doi: 10.1006/jhev.1996.0105. [DOI] [PubMed] [Google Scholar]
- 23.Kingston JD, Harrison T. Isotopic dietary reconstructions of Pliocene herbivores at Laetoli: implications for early hominin paleoecology. Palaeogeogr Palaeoclimatol Palaeoecol. 2007;243:272–306. [Google Scholar]
- 24.Plummer TW, Bishop LC. Hominid paleoecology at Olduvai Gorge, Tanzania as indicated by antelope remains. J Hum Evol. 1994;27:47–75. [Google Scholar]
- 25.Spencer L. Dietary adaptations of Plio-Pleistocene Bovidae: implications for hominid habitat use. J Hum Evol. 1997;32:201–228. doi: 10.1006/jhev.1996.0102. [DOI] [PubMed] [Google Scholar]
- 26.Sponheimer M, Lee-Thorp JA. Using carbon isotope data of fossil bovid communities for paleoenvironmental reconstruction. S Afr J Sci. 2003;99:273–275. [Google Scholar]
- 27.Vrba ES. The significance of bovid remains as indicators of environment and predation patterns. In: Behrensmeyer AK, Hill AP, editors. Fossils in the making. University of Chicago Press; 1980. pp. 247–272. [Google Scholar]
- 28.Potts R. New York: Aldine De Gruyter; 1988. Early hominid activities at Olduvai.407p [Google Scholar]
- 29.Cerling TE, Harris JM, Leakey MG. Browsing and grazing in elephants: the isotope record of modern and fossil proboscideans. Oecologia. 1999;120:364–374. doi: 10.1007/s004420050869. [DOI] [PubMed] [Google Scholar]
- 30.Sikes NE. Early hominid habitat preferences in East Africa: paleosol carbon isotopic evidence. J Hum Evol. 1994;27:25–45. [Google Scholar]
- 31.Sikes NE, Ashley GM. Stable isotopes of pedogenic carbonates as indicators of paleoecology in the Plio-Pleistocene (upper Bed I), western margin of the Olduvai Basin, Tanzania. J Hum Evol. 2007;53:574–594. doi: 10.1016/j.jhevol.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Bamford MK, Stanistreet IG, Stollhofen H, Alber RM. Late Pliocene grassland from Olduvai Gorge, Tanzania. Palaeogeogr Palaeoclimatol Palaeoecol. 2008;257:280–293. [Google Scholar]
- 33.Quade J, Levin N, Semaw S, Stout D, Renne P, et al. Paleoenvironments of the earliest stone toolmakers, Gona, Ethiopia. GSA Bull. 2004;116:1529–1544. [Google Scholar]
- 34.Quinn RL, Lepre CJ, Wright JD, Feibel CS. Paleogeographic variations of pedogenic carbonate δ13C values from Koobi Fora, Kenya: implications for floral compositions of Plio-Pleistocene hominin environments. J Hum Evol. 2007;53:560–573. doi: 10.1016/j.jhevol.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 35.López-Sáez JA, Domínguez-Rodrigo M. Palynology of OGS-6a and OGS-7, two new 2.6 Ma archaeological sites from Gona, Afar, Ethiopia: insights on aspects of Late Pliocene habitats and the beginnings of stone tool use. Geobios. 2009;42:503–511. [Google Scholar]
- 36.Howell FC, Haesaerts P, Heinzelin J. Depositional environments, archeological occurrences and hominids from Members E and F of the Shungura Formation (Omo basin, Ethiopia). J Hum Evol. 1987;16:665–700. [Google Scholar]
- 37.Prat S, Brugal J-P, Tiercelin J-J, Barrat J-A, Bohn M, et al. First occurrence of early Homo in the Nachukui Formation (West Turkana, Kenya) at 2.3–2.4 Myr. J Hum Evol. 2005;49:230–240. doi: 10.1016/j.jhevol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Braun DR, Harris JWK, McCoy JT, Quinn RL, Bamford M, et al. Pliocene hominin behavioral adaptations: new evidence from the Koobi Fora Formation. Paleoanthropol. 2006;A14 [Google Scholar]
- 39.Kimbel WH. The origin of Homo. In: Grine FE, Fleagle JG, Leakey RE, editors. The first humans-origin and early evolution of the genus Homo. Netherlands: Springer; 2009. pp. 31–38. [Google Scholar]
- 40.Roche H, Blumenschine RJ, Shea JJ. Origins and adaptations of early Homo: what archeology tells us. In: Grine FE, Fleagle JG, Leakey RE, editors. The first humans-origin and early evolution of the genus Homo. Netherlands: Springer; 2009. pp. 135–147. [Google Scholar]
- 41.Wood B. Oxford: Clarendon Press; 1991. Koobi Fora research project volume 4: Hominid cranial remains from Koobi Fora.466p [Google Scholar]
- 42.Anton SC. Framing the Question: Diet and Evolution in Early Homo. In: Vinyard C, Ravosa MJ, Wall C, editors. Primate Craniofacial Function and Biology. Netherlands: Springer; 2008. pp. 443–482. [Google Scholar]
- 43.Lieberman DE, Wood BA, Pilbeam DR. Homoplasy and early Homo: an analysis of the evolutionary relationships of H. habilis sensu stricto and H. rudolfensis. J Hum Evol. 1996;30:97–120. [Google Scholar]
- 44.Rightmire GP, Lordkipanidze D. Comparisons of early Pleistocene skulls from East Africa and the Georgian Caucasus: evidence bearing on the origin and systematics of genus Homo. In: Grine FE, Fleagle JG, Leakey RE, editors. The first humans-origin and early evolution of the genus Homo. Netherlands: Springer; 2009. pp. 39–48. [Google Scholar]
- 45.Susman RL. Who made the Oldowan tools? Fossil evidence for tool behavior in Plio-Pleistocene Hominids. J Anthro Res. 1991;47:129–151. [Google Scholar]
- 46.D’Errico F, Backwell L. Assessing the function of early hominin bone tools. J Archaeol Sci. 2009;36:1764–1773. [Google Scholar]
- 47.Reed KE. Early hominid evolution and ecological change through the African Plio-Pleistocene. J Hum Evol. 1997;32:289–322. doi: 10.1006/jhev.1996.0106. [DOI] [PubMed] [Google Scholar]
- 48.Bramble DM, Leiberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler P. The thermoregulatory advantages of hominid bipedalism in open equatorial environments: the contribution of convective heat loss and cutaneous evaporative cooling. J Hum Evol. 1991;21:107–115. [Google Scholar]
- 50.Walker A, Leakey R. Cambridge: Harvard University press; 1993. The Nariokotome Homo erectus skeleton.457p [Google Scholar]
- 51.Sikes NE, Potts R, Behrensmeyer AK. Early Pleistocene habitat in Member 1 Olorgesailie based on paleosol stable isotopes. J Hum Evol. 1999;37:721–746. doi: 10.1006/jhev.1999.0343. [DOI] [PubMed] [Google Scholar]
- 52.Cerling TE, Bowman JR, O'Neil JR. An isotopic study of a fluvial-lacustrine sequence: the PlioPleistocene Koobi Fora Formation, East Africa. Palaeogeogr Palaeocl. 1988;63:335–356. [Google Scholar]
- 53.Levin NE, Cerling TE, Passey BH, Harris JM, Ehleringer JR. A stable isotope aridity index for terrestrial environments. PNAS. 2006;103:11201–11205. doi: 10.1073/pnas.0604719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kingston JD. Environmental determinants in early hominid evolution: issues and evidence from the Tugen Hills, Kenya. In: Andrews P, Banham P, editors. Late Cenozoic Environments and Hominid Evolution: a tribute to Bill Bishop. London: Geological Society; 1999. pp. 69–84. [Google Scholar]
- 55.Kingston JD, Marino BD, Hill A. Isotopic evidence for Neogene hominid paleoenvironments in the Kenya Rift Valley. Science. 1994;264:955–959. doi: 10.1126/science.264.5161.955. [DOI] [PubMed] [Google Scholar]
- 56.Cerling TE, Harris JM, Leakey MG. Isotope paleoecology of the Nawata and Nachukui Formations at Lothagam, Turkana Basin, Kenya. In: Leakey MG, Harris JM, editors. Lothagam: The dawn of humanity in Eastern Africa. New York: Columbia University Press; 2003. pp. 605–624. [Google Scholar]
- 57.WoldeGabriel G, Haile-Selassie Y, Renne PR, Hart WK, Ambrose SH, Asfaw B, Heiken G, White T. Geology and palaeontology of the late Miocene Middle Awash valley, Afar rift, Ethiopia. Nature. 2001;412:17–178. doi: 10.1038/35084058. [DOI] [PubMed] [Google Scholar]
- 58.Wynn JG. Paleosols, stable carbon isotopes, and paleoenvironmental interpretation of Kanapoi, Northern Kenya. J Hum Evol. 2000;39:411–432. doi: 10.1006/jhev.2000.0431. [DOI] [PubMed] [Google Scholar]
- 59.Cerling TE, Hay RL. An isotopic study of paleosol carbonates from Olduvai Gorge. Quat Res. 1986;25:63–78. [Google Scholar]
- 60.Lee-Thorp JA, van der Merwe NJ, Brain CK. Diet of Australopithecus robustus at Swartkrans from stable carbon isotopic analysis. J Hum Evol. 1994;27:361–372. [Google Scholar]
- 61.van der Merwe NJ, Thackeray JF, Lee-Thorp JA, Luyt J. The carbon isotope ecology and diet of Australopithecus africanus at Sterkfontein, South Africa. J Hum Evol. 2003;44:581–597. doi: 10.1016/s0047-2484(03)00050-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Isotopic data from diagenetic sparry, pendant and poikilotopic calcite cements from KS-1 and KS-2, and from samples of carbonatite from the Homa Mountain carbonatite complex.
(0.03 MB DOC)
Paleosol Carbonate Isotopic Data.
(0.04 MB DOC)
Vertebrate taxon list from KS-2, Excavation 1. Isotopic dietary classification of Kanjera mammalian fossils follows others (23) using the isotopic data presented in Table S4. Obligate grazers and obligate browsers consume an almost exclusive (>95%) C4 or C3 diet, respectively. Variable grazers and variable browsers consume a predominantly (75–95%) C4 or C3 diet, respectively. Brower-grazer intermediate refers to taxa consuming a mix of C4 and C3 vegetation.
(0.04 MB DOC)
Stable isotopic composition of fossil eggshell and tooth enamel from Excavation 1.
(0.13 MB DOC)