Abstract
We hypothesized that religiosity, a set of traits variably expressed in the population, is modulated by neuroanatomical variability. We tested this idea by determining whether aspects of religiosity were predicted by variability in regional cortical volume. We performed structural magnetic resonance imaging of the brain in 40 healthy adult participants who reported different degrees and patterns of religiosity on a survey. We identified four Principal Components of religiosity by Factor Analysis of the survey items and associated them with regional cortical volumes measured by voxel-based morphometry. Experiencing an intimate relationship with God and engaging in religious behavior was associated with increased volume of R middle temporal cortex, BA 21. Experiencing fear of God was associated with decreased volume of L precuneus and L orbitofrontal cortex BA 11. A cluster of traits related with pragmatism and doubting God's existence was associated with increased volume of the R precuneus. Variability in religiosity of upbringing was not associated with variability in cortical volume of any region. Therefore, key aspects of religiosity are associated with cortical volume differences. This conclusion complements our prior functional neuroimaging findings in elucidating the proximate causes of religion in the brain.
Introduction
Religious behavior is a uniquely human phenomenon without accepted animal equivalents [1], [2], present in all modern cultures and evident in archaeology from all periods of human history and pre-history [2], [3]. For an explanation of religion, we turn to Tinbergen's distinction between ultimate (or evolutionary) and proximate (causal and developmental) explanations [4]. Much of the scientific literature on religion attempts to create ad hoc explanations and explain the observed range of religious phenomena, in light of evolutionary benefits at the personal and community levels [1], [2]. Other models draw analogies between the religion phenotype and that of adaptive animal behaviors [1], [2], [5] or simulate religious behavior de nova, using pre-specified parameters [2], [6]. While these approaches bring certain benefits, they nevertheless lack a clear understanding of the proximate neural circuitry that supports religious beliefs. Cognitive Neuroscience attempts to create an explanatory schema that includes both proximate and ultimate causes, by linking the emergence of religion in our ancestors with the development of novel cognitive processes, such as Theory of Mind (ToM) [7], [8], social cognition [1], [7], [8] and symbolic language [3], which, in turn, have different evolutionary origins [1] and presumably resulted from expansion of specific brain regions (such as the prefrontal cortex, PFC, the precuneus, the temporal lobe, etc) [7]. (In this article, we use the term “religion” or “religious behavior” to designate shared beliefs, practices and experiences regarding supernatural agents. We use the term “religiosity” to designate a set of psychological and behavioral traits related to adoption of religious beliefs and engagement in religious behavior. We use the term “God” to refer to the target of shared religious commitment in Western traditions. For simplicity, and accuracy, we use the pronoun “He” to denote this target, because God is typically described in these traditions as personal, gendered, and male.)
In order to discover the proximate causes of religion in the brain, our group initially focused on religious beliefs and their cognitive architecture. We found that religious beliefs (at least in the North American Western traditions we studied) are organized cognitively along three dimensions, which constitute a conceptual space. The first two reflect perceptions of 1) Gods' involvement and 2) God's emotion. A third reflects judgment over 3) the source of religious knowledge ranging from doctrinal to experiential [9]. The neural correlates of these dimensions were found to be brain networks which had been previously implicated in understanding agents' actions and intent-related ToM (for Dimension 1), emotion-related ToM and emotional regulation (for Dimension 2) and abstract semantic processing and imagery (for Dimension 3) [9]. By differentially engaging these networks, individuals construct religious belief representations, which are subsequently adopted or rejected based upon cognitive-emotional interactions within the anterior insulae [9]. Religious belief systems presumably interact with other belief systems, social values [10] and morals, and help determine long-term goal selection, behavioral control and emotional balance [11].
Other functional neuroimaging studies have sought to discover the neural correlates of various religious acts or practices, which presumably recreate religious experiences inside the scanner. In these studies, subjects have performed (among other tasks) formalized and improvised praying [12], [13], “mystical” praying [14] and meditation [15]. These studies have reported several functional brain correlates to these practices, with variable engagement of both subcortical [12] and cortical areas which had been previously implicated in social cognition [13]. Their findings have not yet been integrated in any comprehensive neural-cognitive model for religious experience. Nevertheless, the variability of their results is informative by itself, since it seemingly rules out that any single area is modularly or modally specific to religious experience (a so-called “God-spot”).
A strategy, for discovering the proximate causes of complex cognitive functions, which, in turn, may inform novel evolutionary explanations, is to associate variability at the level of the behavioral phenotype and (structural or functional) variability at the level of the likely proximate causes (such as at the levels of genetics and/or the brain). For instance, such an approach has been used to discover the proximate causes of emotional and non-emotional memory [16], [17], [18], [19] and face recognition [20], [21]. Religion seems suitable for such an approach, since religiosity varies widely among modern humans, a fact attributable to environmental and genetic factors [22], [23] and to its interaction with other personality and social behavior traits [11], [24], [25]. In this study, we hypothesized that religiosity is tied to neuroanatomical variability and tested this idea by determining whether components of religiosity were predicted by variability in regional cortical volume measured by magnetic resonance imaging (MRI).
Forty healthy participants with varying patterns of religiosity participated in this and an already published parallel functional MRI (fMRI) study [9]. The purpose of the fMRI study was to identify cognitive processes and brain networks engaged by exposure to a range of religious beliefs. In the VBM study reported below, on the other hand, we assumed that religiosity patterns are based on clusters of personality traits that influence cognitive strategies and behavior over time. For measurement of these traits, we relied on participants' self-reporting about their current religious experience and behavior, their religious upbringing, and about aspects of their worldview (See Supporting Table S1). All survey items were presented as 7-point visual analog Likert scales and subjects had to report the degree to which they currently experience (or had experienced during their upbringing) the content of the item. These items were subsequently entered in a Principal Component (PC) Factor Analysis (FA) to identify common themes underlying their responses. Then, to identify regions of gray matter whose volume is associated with these traits, a VBM analysis of gray matter was performed with the identified principal components as regressors.
Results
Factor Analysis (Table 1)
Table 1. The rotated correlation matrix of the original survey items and the four Principal Components (PCs).
| Principal Components | ||||
| Survey Item | PC1 | PC2 | PC3 | PC4 |
| Degree of current religiosity | .906 | |||
| Degree of current religious participation | .917 | |||
| Current frequency of praying | .918 | |||
| Current frequency of praying in private | .830 | |||
| Current frequency of reading of Scripture | .852 | |||
| Degree to which religion influences important decisions | .803 | .347 | ||
| Belief in Life after Death | .778 | .427 | ||
| Belief in Heaven | .798 | .418 | ||
| Belief in Hell | .792 | .312 | .308 | |
| Belief in a personal God | .765 | .337 | ||
| Perception of God’s Love | .875 | |||
| Praying for forgiveness of sins | .866 | |||
| Fear of God’s anger | .911 | |||
| Seeking God’s will | .939 | |||
| Perception of God’s proximity | .911 | |||
| Perception of God’s awareness | .917 | |||
| Perception of God’s friendship | .911 | |||
| Perception of God’s fellowship | .941 | |||
| Relying of God for important decisions | .915 | |||
| Doubting God’s existence | −.617 | −.561 | −.318 | |
| Religiosity during upbringing | .829 | |||
| Religious participation during upbringing | .881 | |||
| Praying during upbringing | .899 | |||
| Endorsement of “Eat, drink and be merry, because tomorrow we die” | −.816 | |||
| Belief that humanity is equally good and bad | −.829 | |||
| Belief that values are relative depending on the situation | −.604 | −.385 | ||
| Belief that life has an ultimate purpose | .776 | |||
Four Principal Components (PCs) were identified; they can be best explained by those survey items with the highest and most exclusive loadings. Items suggesting both an intimate relationship with God (such as experiencing God's fellowship and seeking his will) and engagement in religious behavior (such as prayer and religious participation) loaded on the 1st PC (PC1). Items referring to religiosity of upbringing loaded positively and an item implying moral relativism loaded negatively on the 2nd PC (PC2). Doubting God's existence, endorsement of “Eat, drink and be merry, because tomorrow we die” [a saying epitomizing the Epicurean and Hedonistic philosophic traditions, which advocated a worldview contrasting sharply with the one advocated by the Judeo-Christian religious tradition], and considering humanity as equally good and bad, loaded negatively on the 3rd PC (PC3). Therefore, PC3 can be best understood by these negative correlations with elements of non-religious pragmatism. Finally, fear of God's anger loaded on the 4th PC (PC4).
VBM (Table 2)
Table 2. Cortical areas whose volume is associated with the PCs.
| Regressor | Voxel coordinates | Z value | Cluster size | t-statistics significance | Brodmann area | Localization |
| PC1: Experiencing an intimate relationship with God (positive correlation) | (58, 6, −20) | 4.01 | 382 | Cluster level correction (p = 0.030) | BA 21 | R middle temporal gyrus |
| (54, 12, −26) | 3.85 | Above cluster | Cluster level correction (p = 0.030) | BA 21 | R temporal pole | |
| PC2: Religiosity of upbringing | No areas exceeded threshold | |||||
| -PC3: Non-religious pragmatism (positive correlation) | (14, −68, 32) | 4.58 | 574 | Cluster level correction (p = 0.005) | BA 7 | R precuneus |
| FWE correction (p = 0.075) | ||||||
| (20, −56, 20) | 3.79 | Above cluster | Cluster level correction (p = 0.005 | BA 17 | R calcarine gyrus | |
| PC4: Experiencing fear of God’s anger (negative correlation) | (−10, −66, 52) | 4.18 | 464 | Cluster level correction (p = 0.014) | BA 7 | L precuneus |
| (−32, 62, −10) | 3.87 | 351 | Cluster level correction (p = 0.041) | BA 11 | L orbitofrontal cortex |
PC1
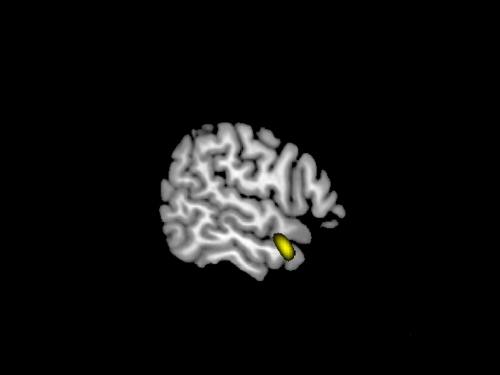
Experiencing an intimate relationship with God positively correlated with cortical volume of BA 21 at the R middle temporal gyrus (MTG) and its extension at the temporal pole (Fig. 1).
Figure 1. Experiencing an intimate relationship with God (PC1) positively correlated with cortical volume at the R middle temporal gyrus (MTG), BA 21, extending to the temporal pole.
Threshold was set to p<0.001 uncorrected for visualization.
PC2
There were no cortical areas whose volume correlated with religiosity of upbringing.
- PC3
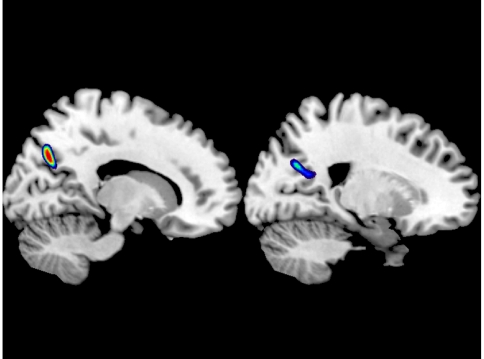
Non-religious pragmatism (the inverse of PC3) positively correlated with cortical volume at the R precuneus, BA 7 and the R calcarine gyrus, BA 17 (Fig. 2). (Alternatively stated, PC3 negatively correlated with cortical volume at the R precuneus, BA 7 and the R calcarine gyrus, BA 17).
Figure 2. Non-religious pragmatism (the inverse of PC3) positively correlated with cortical volume at the R precuneus, BA 7 and the R calcarine gyrus, BA 17.
Threshold was set to p<0.001 uncorrected for visualization.
PC4
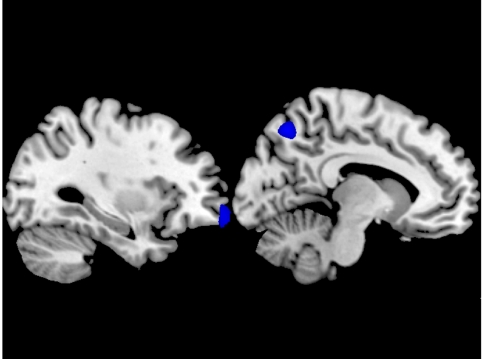
Experiencing fear of God's anger negatively correlated with cortical volume at the L precuneus, BA 7 and the L orbitofrontal cortex, BA 11. In other words, increased cortical volume in these areas predicted non-threatening God-related beliefs (Fig. 3).
Figure 3. Experiencing fear of God's anger negatively correlated with cortical volume at the L precuneus, BA 7 and the L orbitofrontal cortex, BA 11.
Threshold was set to p<0.001 uncorrected for visualization.
Discussion
In this study we identified structural brain variability associated with aspects of long-term religiosity. By defining brain regions that are tied to religion-related psychological and behavioral traits, we complemented our prior fMRI findings of networks involved in navigating the conceptual space of religious beliefs (and adopting those beliefs). The two methods assessed different levels of brain organization (functional recruitment during task performance vs. structural changes resulting from brain development and plasticity). Combined, their findings advance our understanding of the proximate causes of religion in the brain.
Relationships between regional cortical thickness or volume (measured with an unbiased and automated data analysis system) and cognitive task performance have been validated for a range of tasks [26]. Here, we show that such relationships exist in relation to the traits represented by the PCs of religiosity. These relationships, though, need not be considered as simple linear ones (i.e. increased brain volume resulting from/leading to trait manifestation), similarly to linear relationships demonstrable for simple tasks (i.e. increased brain volume resulting from/leading to improved task performance) [26], [27]. Both religious belief and religious practice functionally engage areas which are more broadly involved in social cognitive processing [9], [13]. Such areas are not selectively engaged by specific tasks or tied to unique brain functions [28], unlike the highly specialized motor and visuospatial areas or the hippocampus, the cortical density of which has so far been shown to change with practice [29], [30], [31], [32]. (Changes captured by MRI presumably represent changes in synaptic density [33], myelination within the grey matter [34] and other histologic alterations.) Nevertheless, the effect of personality traits (such as religiosity) can be viewed as selectively engaging complex sets of cognitive processes and representations over time, in response to similar situational demands [35]. Such selective and repeated engagement of networks involved in social cognition [28] may also result in practice-related changes in them. Some evidence for this exists in relation to certain personality traits [36] and complex cognitive practices, such as meditation [37]. Moreover, any practice-induced cortical changes do not rule out the possibility of pre-existing innate regional cortical differences predisposing people to certain traits and behaviors [29]. (Processes involving the white matter, such as myelination and synaptogenesis, are known to underlie the expansion of cognitive functionality in humans, from developmental [34] as well as from evolutionary perspectives [38]. This study was not designed to assess any micro-structural white matter changes or their relationship to religiosity.)
In relation to the FA results, it is interesting to note that items referring to religious behavior (such as praying and religious participation) clustered in PC1 with items referring to an intimate relationship with God (such as experiencing God's fellowship), rather than in PC4 with items reflecting fear of God. Whether this results from a pattern of religion based on non-threatening God representations which has prevailed in modern western society or is a human universal is an open question.
PC1, both reflecting intimacy with God and predicting religious behavior, correlated with cortical volume of the R MTG, BA 21. The R temporal lobe has long been suggested as a locus for religion in the brain [39]. In our parallel fMRI study, R BA 20 and 21 were engaged by doctrinal religious knowledge referring to the most abstract attributes of God [9]. Moreover, the R MTG, BA 21 and R IFG, BA 45, were co-activated as part of a network which was thought to mediate ToM in regards to God's intent and emotion [40], [41] and relate these to one's self [9]. This co-activation may be explained by the fact that the MTG (and neighboring lateral temporal areas) is (are) strongly interconnected in humans with the inferior frontal gyrus (IFG) via the arcuate fasciculus; phylogenetically, this neural network is considered crucial for the emergence of uniquely human cognitive abilities (such as symbolic language [42], [43]). In particular, the R MTG is important for self-processing (underscored, for instance, by its depressed activity in depersonalization disorder) [44], monitoring the status of intimate relationships (such as a mother's with her own child) [45] and setting boundaries between representations of intimate others (such as one's own mother) and one's self [46]. Therefore, by evolution of this area and its connections, a personal relationship with God as an intimate other may have become possible, allowing modern humans to experience this bonding– which they variably do. There is evidence that the R MTG is indeed structurally variable in modern humans and this variability has important clinical implications: R MTG volume is decreased in adolescent and first-episode schizophrenia patients [47], [48] and increased in obsessive compulsive disorder (OCD) patients [49]. We speculate that the range of R MTG volumes can be viewed as a spectrum, in which high R MTG volume is associated with stereotyped and ritualistic behavior, high-normal volume is associated with religious behavior (which, we should note, is by definition ritualistic), low-normal volume is associated with non-religiosity, and pathologically low volume is associated with schizophrenia (in which disorganized behavior and aberrant religiosity, with blurred boundaries between the self and God, may occur).
Experiencing fear of God's anger (PC4) was negatively correlated with volume of BA 11 and L precuneus, BA 7. BA 11 consists of phylogenetically newer granular cortex, associated with our ability for emotion-related ToM (also termed cognitive empathy) [50]. This area also exerts an emotional regulatory effect [51], inhibiting excessive emotional responses to negative stimuli to prevent them from interfering with performance under regular conditions [52], being itself inhibited in the face of grave danger [53]. In regards to the role of precuneus in religion, we have already proposed that it helps relate the representation of God to the self [9], [54] and, on that basis, we interpret its activation by personal praying in devout Christians [13] and the finding of the current study that increased volume of the L precuneus prevents fear of God (suggesting a enhanced ability to relate God to the self). The precuneus may also help provide context to the religious experience by retrieving memories and relating them to current situations [54], [55]. Therefore, people with lower cortical volumes in BAs 7 and 11 may be prone to a fear-based approach to God because of being compromised in representing the intentions and emotional disposition of God [50], regulating their emotions and modulating fearful responses towards a perceived powerful agent [56], as well as because of deficient engagement in personal (conversational) prayer with Him [13].
Moreover, an enhanced ability to switch between different perspectives to address moral dilemmas [54], [57] may explain why people with increased R precuneus volume tend to consider humanity as both good and bad and moral values relative (items loading on PC3, see Table 1). We speculate that people with increased R precuneate volume may also place an emphasis on worldly experiences over the inner life of imagination [58], which, in turn, may predispose them to adopt a non-religious life stance.
This study is correlational; therefore, it does not imply causality. Moreover, it was performed in adults. Subjects may have been predisposed to follow specific patterns of religious behavior by their individual brain development or their religious behavior may have contributed to volume changes of certain brain areas. Regardless, the fact that there is no brain area correlating with religiosity of upbringing (PC2) argues against religious nurture independently accounting for regional brain variability. Therefore, religiosity in adult life may reflect innate “susceptibilities”, perhaps genetic or early developmental, which are non-modifiable during upbringing, or any initial effect of religious upbringing may be dissipated by experiences in later life.
The brain areas identified in this and the parallel fMRI studies are not unique to processing religion, but play major roles in social cognition. This implies that religious beliefs and behavior emerged not as sui generis evolutionary adaptations, but as an extension (some would say “by product”) of social cognition and behavior. Furthermore, the current study suggests that evolution of certain areas that advanced understanding and empathy towards our fellow human beings (such as BA 7, 11 and 21) may, at the same time, have allowed for a relationship with a perceived supernatural agent (God) based on intimacy rather than fear. The idea that how you relate to “thy God” parallels how you behave to “thy neighbor” is a long-cherished claim of many religions (and is backed by some empirical evidence [11], [59]). In this study, we see that this link is elaborated in the cognitive and neural foundations of religion.
Methods
The Neuroscience Institutional Review Board of the National Institutes of Health has approved this research. Informed written consent has been obtained and all clinical investigation has been conducted according to the principles expressed in the Declaration of Helsinki.
Healthy right-handed healthy adults were recruited through posting of the study on the website of the National Institutes of Health and word of mouth. Subjects provided medical history and underwent a screening neurological exam with a neurologist. Subjects with neurological or psychiatric disease or not able to undergo MRI were excluded from participation. In particular, we exclusion subjects with psychiatric diseases or first degree relatives of psychiatric patients, as well as of those with deleterious habits, such as alcoholism or substance abuse. No requirement for participation was made in regards to religiosity. Forty eligible subjects (20 women and 20 men; mean age = 35.7; mean years of education = 17.5) were, then, subjected to structural (as well as functional [9]) brain MRI and completed a survey on their religiosity.
The correlation matrix of survey items was subjected to Principal Components (PC) Factor Analysis using SPSS 17.0. Factors were extracted based on Eigenvalue <1. A varimax rotation with Kaiser normalization was applied to the solution to minimize the number of variables that have high loadings on each PC and to simplify the interpretation of the PC. Anderson-Rubin factor scores for the PCs were calculated for each subject. These scores are produced so that they have a mean of 0, a standard deviation of 1 and are uncorrelated.
A 3T GE MRI scanner (GE Medical Systems, Milwaukee, WI) and an 8-channel head coil were used to acquire high-resolution T1-weighted 3-dimensional magnetization-prepared rapid gradient-echo structural images. We used the unified segmentation algorithm [60], [61] implemented on SPM5 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, UCL) to acquire images of gray matter, white matter and cerebrospinal fluid. We then performed modulated normalization of the gray matter images and smoothing with a 8-mm full-width at half-maximum filter. The resulting images were entered in a multiple regression analysis model, with the factor scores for the four PCs as covariates of interest. Moreover, age, gender, education and total intracranial volume were included in the model as covariates of no-interest, since we wanted to control for their effect on regional grey matter volume. In particular, age was an important confounder and, therefore, variance associated with it needed to be accounted for, since it is known to affect cognitive performance across a range of tasks, cortical grey density and volume throughout life [34], [62], [63], [64], [65], [66], and may also be related with changes in religiosity. Design orthogonality was calculated in SPM5 and no collinearity among the PCs or among the PCs and the other covariates was observed. Global effects for gray matter were calculated and global normalization with Analysis of Covariance (ANCOVA) was performed to localize regions where the trends in GM volume differ from global GM effects [67]. The effect of each covariate of interest was individually assessed at the whole brain level. The uncorrected statistical threshold for voxels at the whole brain level was set to p<0.001, with a minimum cluster size of 300, and, for detected clusters, cluster-level correction was implemented. Non-Stationary Cluster Extent Correction, which corrects for non-isotropic smoothness of VBM data [68] was also applied.
This study is correlational, bridging distant levels of biological organization (i.e. the cellular and tissue level, to the degree that its features are captured by MRI, and that of long-term behavior). The observed effects could be mediated by behavioral confounders influencing intermediate levels of organization (altering, for instance, patterns of self-care or habits, such as alcohol drinking). Such intermediate levels include the levels of the organ (brain), the organ system (nervous system), and the organism. In our study design and analysis, we control for many of these confounders. First, we excluded subjects with neurological or psychiatric diseases (or first degree relatives of psychiatric patients), as well as of those with deleterious habits, such as alcoholism or substance abuse, given their relationship with religiosity [69], [70] and their effect on regional grey matter [71], [72]. Second, we controlled for the effects of the important biological confounders of age, gender and total intracranial volume (a surrogate for genetic and environmental factors influencing global brain development) by including them as covariates in the model. Third, we included as covariate the important social confounder of education (which presumably induces widespread brain reorganization). Fourth (and perhaps most importantly), we calculated the global mean for grey matter for each subject, as a surrogate for any confounder's global effect on grey matter volume, and performed global normalization using an Analysis of Variance approach. This way we identified regions where the trends in grey matter volume differ from the total grey matter volume [67]. Admittedly, we did not correct for the potential confounding effect of general health (including vascular disease, diabetes etc). Nevertheless, we do not believe that their effect raises serious doubts over the validity of the findings: associations between general health and religiosity exist, but are generally indirect [73], [74] and there is no reason to suspect that their effect in regards to cortical volume is regionally specific.
Supporting Information
Participants' religiosity survey
(0.05 MB DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The Intramural Research Program of the National Institute on Aging (NIA/NIH) and the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NINDS/NIH) supported this research. The funders had no role in study design, data collection and analysis and preparation of the manuscript. The manuscript underwent a process of being cleared for publication by the NIA and NINDS, according to the relevant rules of these Institutes.
References
- 1.Boyer P, Bergstrom B. Evolutionary Perspectives on Religion. Annual Review of Anthropology. 2008;37:111–130. [Google Scholar]
- 2.Bulbulia J. The cognitive and evolutionary psychology of religion. Biology and Philosophy. 2004;19:655–686. [Google Scholar]
- 3.Wade N. New York: Penguin Press.; 2006. Before the dawn: recovering the lost history of our ancestors.312 [Google Scholar]
- 4.Tinbergen N. On aims and methods of Ethology (Reprinted from Zeitschrift fur Tierpsychologie, vol 20, pg 410, 1963). Animal Biology. 2005;55:297–321. [Google Scholar]
- 5.Bulbulia J. Religious Costs as Adaptations that Signal Altruistic Intention. Evolution and Cognition. 2004;10:19–42. [Google Scholar]
- 6.Dawkins R. xxiii. Oxford; New York: Oxford University Press.; 2006. The selfish gene.360 [Google Scholar]
- 7.Boyer P. Religious thought and behaviour as by-products of brain function. Trends Cogn Sci. 2003;7:119–124. doi: 10.1016/s1364-6613(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 8.Boyer P. Religion: Bound to believe? Nature. 2008;455:1038–1039. doi: 10.1038/4551038a. [DOI] [PubMed] [Google Scholar]
- 9.Kapogiannis D, Barbey AK, Su M, Zamboni G, Krueger F, et al. Cognitive and neural foundations of religious belief. Proc Natl Acad Sci U S A. 2009;106:4876–4881. doi: 10.1073/pnas.0811717106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zahn R, Moll J, Paiva M, Garrido G, Krueger F, et al. The neural basis of human social values: evidence from functional MRI. Cereb Cortex. 2009;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough ME, Willoughby BLB. Religion, Self-Regulation, and Self-Control: Associations, Explanations, and Implications. Psychological Bulletin. 2009;135:69–93. doi: 10.1037/a0014213. [DOI] [PubMed] [Google Scholar]
- 12.Schjodt U, Stodkilde-Jorgensen H, Geertz AW, Roepstorff A. Rewarding prayers. Neurosci Lett. 2008;443:165–168. doi: 10.1016/j.neulet.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 13.Schjoedt U, Stodkilde-Jorgensen H, Geertz AW, Roepstorff A. Highly religious participants recruit areas of social cognition in personal prayer. Soc Cogn Affect Neurosci. 2009;4:199–207. doi: 10.1093/scan/nsn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beauregard M, Paquette V. Neural correlates of a mystical experience in Carmelite nuns. Neurosci Lett. 2006;405:186–190. doi: 10.1016/j.neulet.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 15.Newberg A, Alavi A, Baime M, Pourdehnad M, Santanna J, et al. The measurement of regional cerebral blood flow during the complex cognitive task of meditation: a preliminary SPECT study. Psychiatry Res. 2001;106:113–122. doi: 10.1016/s0925-4927(01)00074-9. [DOI] [PubMed] [Google Scholar]
- 16.de Quervain DJ, Henke K, Aerni A, Coluccia D, Wollmer MA, et al. A functional genetic variation of the 5-HT2a receptor affects human memory. Nat Neurosci. 2003;6:1141–1142. doi: 10.1038/nn1146. [DOI] [PubMed] [Google Scholar]
- 17.de Quervain DJ, Kolassa IT, Ertl V, Onyut PL, Neuner F, et al. A deletion variant of the alpha2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat Neurosci. 2007;10:1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- 18.Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, et al. Common Kibra alleles are associated with human memory performance. Science. 2006;314:475–478. doi: 10.1126/science.1129837. [DOI] [PubMed] [Google Scholar]
- 19.de Quervain DJ, Papassotiropoulos A. Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. Proc Natl Acad Sci U S A. 2006;103:4270–4274. doi: 10.1073/pnas.0510212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennerknecht I, Ho NY, Wong VCN. Prevalence of Hereditary Prosopagnosia (HPA) in Hong Kong Chinese Population. American Journal of Medical Genetics Part A. 2008;146A:2863–2870. doi: 10.1002/ajmg.a.32552. [DOI] [PubMed] [Google Scholar]
- 21.Van den Stock J, van de Riet WA, Righart R, de Gelder B. Neural correlates of perceiving emotional faces and bodies in developmental prosopagnosia: an event-related fMRI-study. PLoS ONE. 2008;3:e3195. doi: 10.1371/journal.pone.0003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchard TJ, Jr, McGue M, Lykken D, Tellegen A. Intrinsic and extrinsic religiousness: genetic and environmental influences and personality correlates. Twin Res. 1999;2:88–98. doi: 10.1375/136905299320565951. [DOI] [PubMed] [Google Scholar]
- 23.Koenig LB, McGue M, Krueger RF, Bouchard TJ., Jr Genetic and environmental influences on religiousness: findings for retrospective and current religiousness ratings. J Pers. 2005;73:471–488. doi: 10.1111/j.1467-6494.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 24.Koenig LB, McGue M, Krueger RF, Bouchard TJ. Examining the relationship between religiousness and prosocial and antisocial behavior: Genetic versus environmental mediation. Behavior Genetics. 2003;33:708–709. [Google Scholar]
- 25.Koenig LB, McGue M, Krueger RF, Bouchard TJ. Religiousness, antisocial behavior, and altruism: Genetic and environmental mediation. Journal of Personality. 2007;75:265–290. doi: 10.1111/j.1467-6494.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 26.Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, et al. Detection of cortical thickness correlates of cognitive performance: Reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39:10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walhovd KB, Fjell AM, Dale AM, Fischl B, Quinn BT, et al. Regional cortical thickness matters in recall after months more than minutes. Neuroimage. 2006;31:1343–1351. doi: 10.1016/j.neuroimage.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Krueger F, Barbey AK, Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends Cogn Sci. 2009;13:103–109. doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlaug G. The brain of musicians. A model for functional and structural adaptation. Ann N Y Acad Sci. 2001;930:281–299. [PubMed] [Google Scholar]
- 31.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 2006;16:1091–1101. doi: 10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- 33.Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 35.Mischel W. Toward an integrative science of the person. Annu Rev Psychol. 2004;55:1–22. doi: 10.1146/annurev.psych.55.042902.130709. [DOI] [PubMed] [Google Scholar]
- 36.Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, et al. Neuroanatomical correlates of extraversion and neuroticism. Cereb Cortex. 2006;16:1809–1819. doi: 10.1093/cercor/bhj118. [DOI] [PubMed] [Google Scholar]
- 37.Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuster JM. xvi. Oxford; New York: Oxford University Press; 2003. Cortex and mind: unifying cognition.294 [Google Scholar]
- 39.Persinger MA. Religious and mystical experiences as artifacts of temporal lobe function: a general hypothesis. Percept Mot Skills. 1983;57:1255–1262. doi: 10.2466/pms.1983.57.3f.1255. [DOI] [PubMed] [Google Scholar]
- 40.Lissek S, Peters S, Fuchs N, Witthaus H, Nicolas V, et al. Cooperation and deception recruit different subsets of the theory-of-mind network. PLoS ONE. 2008;3:e2023. doi: 10.1371/journal.pone.0002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang AT, Lee SS, Sigman M, Dapretto M. Reading Affect in the Face and Voice. Arch Gen Psychiatry. 2007;64:698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, et al. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci. 2008;11:426–428. doi: 10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
- 43.Glasser MF, Rilling JK. DTI tractography of the human brain's language pathways. Cereb Cortex. 2008;18:2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- 44.Simeon D, Guralnik O, Hazlett EA, Spiegel-Cohen J, Hollander E, et al. Feeling unreal: a PET study of depersonalization disorder. Am J Psychiatry. 2000;157:1782–1788. doi: 10.1176/appi.ajp.157.11.1782. [DOI] [PubMed] [Google Scholar]
- 45.Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother's response to infant's attachment behaviors. Biol Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 46.Vanderwal T, Hunyadi E, Grupe DW, Connors CM, Schultz RT. Self, mother and abstract other: an fMRI study of reflective social processing. Neuroimage. 2008;41:1437–1446. doi: 10.1016/j.neuroimage.2008.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lui S, Deng W, Huang X, Jiang L, Ma X, et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166:196–205. doi: 10.1176/appi.ajp.2008.08020183. [DOI] [PubMed] [Google Scholar]
- 48.Spencer MD, Moorhead TW, McIntosh AM, Stanfield AC, Muir WJ, et al. Grey matter correlates of early psychotic symptoms in adolescents at enhanced risk of psychosis: a voxel-based study. Neuroimage. 2007;35:1181–1191. doi: 10.1016/j.neuroimage.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Narayan VM, Narr KL, Phillips OR, Thompson PM, Toga AW, et al. Greater regional cortical gray matter thickness in obsessive-compulsive disorder. Neuroreport. 2008;19:1551–1555. doi: 10.1097/WNR.0b013e3283112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2008 doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- 51.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, et al. The neural system that bridges reward and cognition in humans: an fMRI study. Proc Natl Acad Sci U S A. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brannan S, Liotti M, Egan G, Shade R, Madden L, et al. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proc Natl Acad Sci U S A. 2001;98:2029–2034. doi: 10.1073/pnas.98.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 55.Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage. 2005;27:824–834. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 57.Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: FMRI correlates of valence, emotional intensity, and control in the processing of attitudes. J Cogn Neurosci. 2004;16:1717–1729. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- 58.Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McNamara P. Religion and the Frontal lobes. In: Andresen J, editor. Religion in mind: cognitive perspectives on religious belief, ritual, and experience. Cambridge, UK; New York, NY: Cambridge University Press; 2001. pp. 236–256. [Google Scholar]
- 60.Friston KJ. vii. London: Academic.; 2007. Statistical parametric mapping: the analysis of functional brain images. p. 647 p., [632] p. of plates p. [Google Scholar]
- 61.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 62.Walhovd KB, Fjell AM, Dale AM, McEvoy LK, Brewer J, et al. Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270; discussion 1275–1268. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 64.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Fischl B, et al. Size does matter in the long run: hippocampal and cortical volume predict recall across weeks. Neurology. 2004;63:1193–1197. doi: 10.1212/01.wnl.0000140489.33249.95. [DOI] [PubMed] [Google Scholar]
- 65.Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, et al. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 67.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 68.Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 69.Koenig HG. Research on religion, spirituality, and mental health: a review. Can J Psychiatry. 2009;54:283–291. doi: 10.1177/070674370905400502. [DOI] [PubMed] [Google Scholar]
- 70.Moreira-Almeida A, Neto FL, Koenig HG. Religiousness and mental health: a review. Rev Bras Psiquiatr. 2006;28:242–250. doi: 10.1590/s1516-44462006000300018. [DOI] [PubMed] [Google Scholar]
- 71.Goghari VM, Rehm K, Carter CS, MacDonald AW. Sulcal thickness as a vulnerability indicator for schizophrenia. Br J Psychiatry. 2007;191:229–233. doi: 10.1192/bjp.bp.106.034595. [DOI] [PubMed] [Google Scholar]
- 72.Goghari VM, Rehm K, Carter CS, MacDonald AW., 3rd Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb Cortex. 2007;17:415–424. doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- 73.Koenig LB, Vaillant GE. A prospective study of church attendance and health over the lifespan. Health Psychol. 2009;28:117–124. doi: 10.1037/a0012984. [DOI] [PubMed] [Google Scholar]
- 74.Mueller PS, Plevak DJ, Rummans TA. Religious involvement, spirituality, and medicine: implications for clinical practice. Mayo Clin Proc. 2001;76:1225–1235. doi: 10.4065/76.12.1225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Participants' religiosity survey
(0.05 MB DOC)