Abstract
Individuals with neurofibromatosis type 1 (NF1) are prone to develop optic pathway gliomas that can result in significant visual impairment. To explore the cellular basis for the reduced visual function resulting from optic glioma formation, we employed a genetically engineered mouse model of Nf1 optic glioma (Nf1+/−GFAPCKO mice). We performed multi-modal functional and structural analyses both before and after the appearance of macroscopic tumors. At 6 weeks of age, prior to obvious glioma formation, Nf1+/−GFAPCKO mice had decreased visual evoked potential amplitudes and increased optic nerve axon calibers. By 3 months of age, Nf1+/−GFAPCKO mice exhibited pronounced optic nerve axonopathy and apoptosis of neurons in the retinal ganglion cell layer. Magnetic resonance diffusion tensor imaging showed a progressive increase in radial diffusivity between 6 weeks and 6 months of age in the optic nerve proximal to the tumor indicating ongoing deterioration of axons. These data suggest that optic glioma formation results in early axonal disorganization and damage that culminates in retinal ganglion cell death. The Nf1+/−GFAPCKO mice provide a useful model for defining mechanisms of visual abnormalities in children with NF1 and lay the foundations for future interventional studies aimed at reducing visual loss.
Keywords: Apoptosis, Magnetic resonance imaging, Neurofibromatosis-1, Optic pathway glioma, Retinal ganglion cell, Visual evoked potential
INTRODUCTION
Individuals with the inherited cancer predisposition syndrome, neurofibromatosis type 1 (NF1), develop tumors that involve both the central and peripheral nervous systems (1, 2). The most common central nervous system tumor is a glial neoplasm that arises along the optic pathway. Optic pathway gliomas (OPGs) are low-grade glial fibrillary acidic protein (GFAP)-immunoreactive tumors (3) that typically affect the prechiasmatic optic nerves and chiasm (4–6). NF1-associated OPGs usually arise in children within the first decade of life, most often in preschool age children (7). Because of their location along the optic pathway, the typical presenting sign is visual impairment, and 25% to 40% of children with these tumors have decreased visual acuity at the time of initial OPG diagnosis (8).
Several barriers limit our ability to improve the clinical outcome for children with NF1-associated OPG. First, accurate methods for assessing visual function in very young children are required. Visual screening in the greatest at-risk population of children with NF1 (infants and toddlers) is often challenging using standard methods employed for school-age children (6). This has prompted clinicians to evaluate other modalities including visual evoked potentials (VEPs) for identifying children with NF1-associated OPG visual deficits (9, 10). Moreover, prognostic markers of tumor growth are lacking. In this regard, the radiographic appearance of an OPG does not predict its individual clinical behavior and there is no correlation between tumor size or contrast enhancement and visual function (11, 12). To provide such predictive information, diffusion-based magnetic resonance imaging (MRI) has been suggested as a potential method for assessing high-grade glioma growth (13–15) but has not been examined in low-grade brain tumors. Despite the ability to arrest tumor growth using chemotherapy in 60% to 80% of children with NF1-associated OPG, few patients exhibit improved visual acuity (16). The lack of visual improvement raises the possibility that the neuronal damage secondary to optic glioma formation is not reversible.
To gain insight into the cellular pathogenesis of NF1-associated OPG, we employed a strain of genetically engineered mice (GEM) in which all cells are heterozygous for a targeted mutation in the Nf1 gene (Nf1+/−), except for cells of glial origin in which neurofibromin expression was completely ablated by Cre-mediated Nf1 inactivation in GFAP+ (Nf1+/−GFAPCKO mice) (17). Nearly 100% of these GEM develop astrocytic neoplasms with low proliferative indices involving the prechiasmatic optic nerves and chiasm. Herein, we employ this unique mouse model to assess optic pathway pathology using multiple modalities, including visual physiology, diffusion-based MRI, and transmission electron microscopy. We found that Nf1 mutant mice with optic gliomas have reduced VEP amplitudes and increased radial diffusivity in the optic nerve, enlarged optic nerve axons, and retinal ganglion cell (RGC) loss. These observations suggest that the Nf1 optic glioma GEM model may be an excellent experimental platform to understand the critical relationship between glioma formation and neuronal dysfunction relevant to visual loss in children with NF1-associated OPG.
MATERIALS AND METHODS
Mice
Nf1+/−GFAPCKO mice (17) were generated by successive interbreeding of Nf1+/−, Nf1flox/flox (18), and GFAP-Cre mice (19). Age-matched C57BL/6, Nf1flox/flox or Nf1flox/wt animals were analyzed as wild-type controls. GFAP-Cre animals were also mated with R26R-EYFP mice (20) to detect Cre-mediated recombination in vivo. All mice were maintained on a C57BL/6 background.
Visual Evoked Potential and Electroretinogram Measurements
Visual evoked potentials (VEPs) (21) and full-field electroretinograms (ERGs) (22) were recorded on a UTAS-E 3000 Visual Electrodiagnostic System (LKC Technologies, Gaithersburg, MD). For all electrophysiologic measurements, mice were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (15 mg/kg). Body temperature was maintained between 36° and 37°C throughout the recordings with a heating pad and monitored using a rectal temperature probe. Corneal anesthesia was achieved with 1% proparicaine and the pupils were dilated for recordings with 1% atropine. Corneas were kept moist with application of 1% carboxymethyl-cellulose and the placement of clear contact lenses (Metro Optics, Austin, TX), which stayed in place throughout the procedure.
For VEP measurements, stainless steel needle electrodes were placed as follows: recording electrode on the scalp over the visual cortex, reference electrode in the skin of the left ear, and ground electrode in the base of the tail. Brief white flashes at 0.2 log cd sec m−2 were delivered via a Ganzfeld sphere on a dark background. For each trial, 80 consecutive flashes were averaged at 1.9 Hz.
For ERG recordings, mice were dark adapted overnight and prepared for recordings under infrared illumination. A 2-mm-diameter stainless steel loop positioned gently on the cornea by a micromanipulator served as the recording electrode. The reference needle electrode was placed under the skin between the eyes, and the ground needle electrode was placed at the base of the tail. Stimuli were brief white flashes delivered via a Ganzfeld integrating sphere and signals were recorded with band-pass settings of 0.3 to 500 Hz. After a 10-minute stabilization period, an 11-step scotopic intensity series (−3.60 to 0.875 log cd-s/m2 stimulus) was recorded, which included rod-specific and scotopic bright flash responses. After a 10-minute light adaptation period on a steady white background (2.30 log cd/m2), a 5-step photopic intensity series was recorded (0.0 to 2.82 log cd-s/m2 stimulus). Scotopic and photopic b-wave amplitudes and scotopic a-wave amplitudes were recorded for all flash intensities.
Morphometry of Optic Nerve Axons
Mice were perfused with Ringer's solution followed by 1.5% paraformaldehyde and 2.5% glutaraldehyde in 0.1M cacodylate buffer (pH 7.4). Both retrobulbar optic nerve segments from each mouse were post-fixed in 1% -buffered OsO4, dehydrated through an ethanol/acetone series and embedded in Durcupan (Electron Microscopy Sciences, Hatfield, PA) for light and electron microscopy. Transverse semi-thin sections (0.5 μm) obtained at a 1-mm distance behind the eyeballs were stained with paraphenylenediamine (1% in isopropanol/methanol; 30 minutes) to optimize contrast of the myelinated fibers for automated axon counts. Mosaic images of paraphenylenediamine-stained nerve cross-sections were produced on an Olympus BX51WI Microscope at 100x equipped with a mechanical stage and Microbrightfield Neurolucida software and captured with an Optronics Microfire (1600×1200) CCD camera (0.075 μm/pixel). Post-processing with an Adobe Photoshop action script sequence (levels, curves, contrast, sharpening, and posterization) provided high contrast 2-bit renditions of the original images (Supplemental Fig. 1). A MatLab script was used to segment these images and to automate counts and size determinations for all the axons in each nerve. The measurements excluded the myelin sheath and used eccentricity and size criteria to eliminate glial and vascular profiles. Axon sizes (areas) were mapped into bins to assess size distributions of the axonal populations by area. Axonal profiles were also examined by transmission electron microscopy (JEOL-1200) in thin sections obtained from selected specimens.
Diffusion Tensor Imaging of the Optic Nerve
Images were collected in an Oxford Instruments 4.7-Tesla magnet equipped with 15-cm inner diameter, actively shielded gradient coils and interfaced with a Varian INOVA console, as described previously (17). Diffusion tensor imaging data were acquired using a conventional spin-echo imaging sequence, modified by the addition of a Stejskal-Tanner diffusion-sensitizing gradient pair. Six images with different gradient directions were acquired with a b value of 785 s/mm2, together with a reference spin-echo (b = 0) image. Slice thickness was 0.5 mm with a field of view of 1.5 cm × 1.5 cm2. The 3 primary diffusivities, λ1 > λ2 > λ3, were calculated by diagonalization of the diffusion tensor using software written in Matlab. These primary parameters were combined into relative anisotropy and radial diffusivity (the mean of λ2 and λ3) (23).
Immunofluorescence and TUNEL Staining
Mice were perfused transcardially with PBS and 4% paraformaldehyde in PBS. Following overnight post-fixation at 4°C, eyes and optic nerves were either transferred to 70% ethanol solution prior to paraffin embedding or into 30% sucrose for 36 hours before embedding into OCT medium and snap freezing. For neurofilament staining, 20-μm cryostat sections were incubated with solutions in the following order: 10% normal donkey serum, mouse monoclonal anti-Neurofilament 68 antibody (Sigma, St. Louis, MO) and fluorescent anti-mouse secondary antibody in PBS. Finally, sections were stained with Hoechst (Molecular Probes, Eugene, OR) and mounted onto glass slides. Images were acquired with confocal microscopy (Olympus FluoView1000; Olympus, Tokyo, Japan). TUNEL and immunofluorescence double labeling was employed on 5 μm paraffin sections. First, NeuN (Chemicon, Temecula, CA), MAP2 (PharMingen, San Diego, CA), glutamine synthetase (Millipore, Billerica, MA), or cyclin D3 (Cell Signaling, Beverly, MA) staining was performed using Alexa568 conjugated secondary antibodies (Molecular Probes), as described previously (24). Next, the same sections were TUNEL stained according to the manufacturer's instructions (Roche Diagnostics, Nutley, NJ). Finally, slides were coverslipped with DAPI-containing mounting medium. The percentage of TUNEL+ cells in the ganglion cell layer was counted on three consecutive paraffin sections. For the GFAP-Cre; R26R-EYFP retina immunofluorescence staining was performed as described previously (24).
Statistical Analysis
Each experiment was performed with at least four animals from two or more independent litters. Statistical significance (p < 0.05) was determined by unpaired t-test (with Welch's Correction) using GraphPad Prism 4.0 software (GraphPad, Inc., San Diego, CA).
RESULTS
Nf1+/−GFAPCKO Mice Exhibit Reduced Visual Evoked Potentials
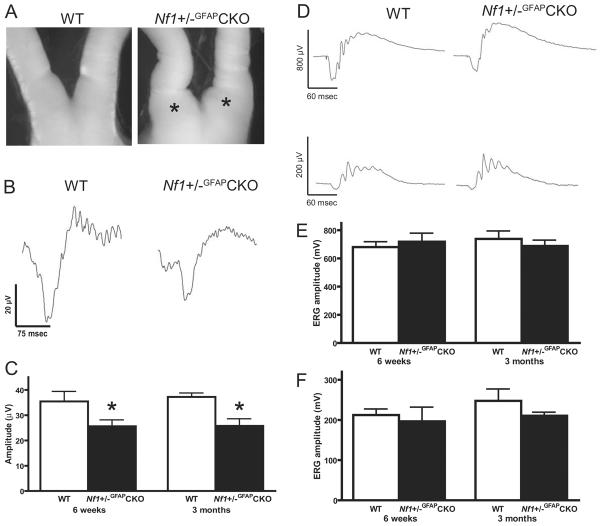
Visual acuity in children with NF1-associated OPG is usually assessed using standardized eye charts, including Teller acuity, Lea figure, HOTV matching, and Snellen testing modalities (6). While these measurements provide reliable information about visual function, they are not applicable for visual testing in rodents. Instead, we chose to employ electrophysiological assessments of neuronal function, as have been used in children with NF1-associated OPG (9, 25). Visual evoked potentials and electroretinograms were recorded in WT and Nf1+/−GFAPCKO mice at 6 weeks and 3 months of age. These specific time points were chosen to reflect a period of early tumor evolution in which glial hyperplasia, neovascularization and microglial infiltration are seen but gross histologic, pathologic and neuroimaging evidence of a glioma is lacking (6 weeks of age) and a time point when an obvious glioma is detected by gross examination (Fig. 1A), pathologic features (e.g. nuclear atypia) and magnetic resonance imaging (26). At both 6 weeks and 3 months, VEP recordings revealed significant decreases in amplitudes, with more modest increases in latency, in Nf1+/−GFAPCKO compared to wild-type mice (Fig. 1B, C). In contrast, there was no change in the amplitudes or latencies of either the photopic or scotopic full-field ERGs (Fig. 1D–F). These observations indicate that visual function is impaired in this Nf1 mouse OPG model at an early stage of tumor development.
Figure 1.
Visual evoked potentials (VEPs) are impaired in Nf1+/−GFAPCKO mice. (A) Loss of neurofibromin expression in astrocytes of Nf1+/− mice (Nf1+/−GFAPCKO mice) results in optic gliomas involving the prechiasmatic optic nerves and chiasm. By 3 months of age, large focal enlargements of these regions are seen in Nf1+/−GFAPCKO mice (asterisks), but not in control (WT) mice. (B) Representative VEP traces from 6-week-old WT and Nf1+/−GFAPCKO mice show decreased amplitudes and increased latencies at 6 weeks and 3 months of age. (C) There is a significant reduction in the VEP amplitudes at both 6 weeks and 3 months of age in Nf1+/−GFAPCKO mice. (D) Representative traces from scotopic (upper row) and photopic (lower row) electroretinogram (ERG) recordings from 3-month-old mice. E, There was no significant difference in the amplitude of scotopic b-waves measured in Nf1+/−GFAPCKO mice compared to WT mice at either age. F, Photopic amplitudes showed no significant change between Nf1+/−GFAPCKO and WT mice at either age. Data presented are the mean and standard error of the mean (SEM). Asterisks denote significant differences (p < 0.05).
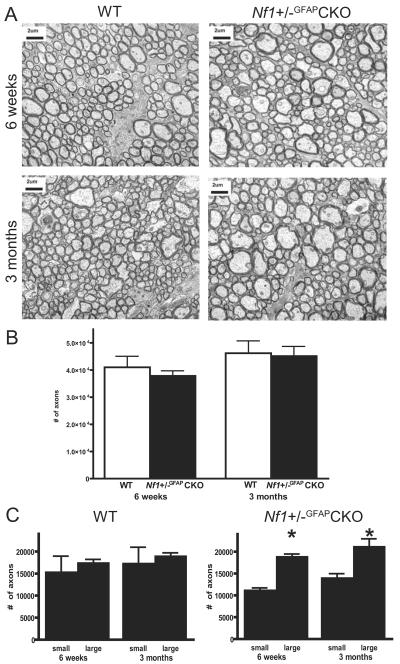
Nf1+/−GFAPCKO Optic Nerves Have Increased Numbers of Large Diameter Axons
Next, we sought to identify structural correlates for the impaired visual function inNf1+/−GFAPCKO mice using automated axon counting on paraphenylenediamine stained semi-thin cross sections (Supplementary Fig. 1). There was no loss of axons at either 6 weeks or 3 months of age by automated axon counting (Fig. 2B). At both ages, however, there was an increase in the proportion of axons with larger (>0.25 μm2) calibers (Fig. 2A). There was no difference between the number of smaller (<0.15 μm2) and larger nerve fibers in WT mice (Fig. 2C). These results suggest that the impairment of visual function in Nf1+/−GFAPCKO mice is associated with ultrastructural changes in optic nerve axons as early as 6 weeks of age.
Figure 2.
Altered morphology of retrobulbar optic nerve axons. (A) Cross-sectional electron photomicrographs display an increased percentage of large caliber axons in Nf1+/−GFAPCKO mice anterior (>3 mm away) to the tumor site at 6 weeks and 3 months of age. (B) Automated total axon counts and size determinations were obtained from high-resolution (100x) mosaic images of paraphenylene-diamine-stained, semi-thin sections. No changes in total numbers of axons were observed at either 6 weeks or 3 months of age. (C) The distribution of axon diameters is shifted in Nf1+/−GFAPCKO optic nerves, resulting in a decrease in the number of smaller axons (<0.15 μm2) and an increase in the number of larger axons (>0.25 μm2) (p < 0.05). Columns and error bars represent the mean ± SEM.
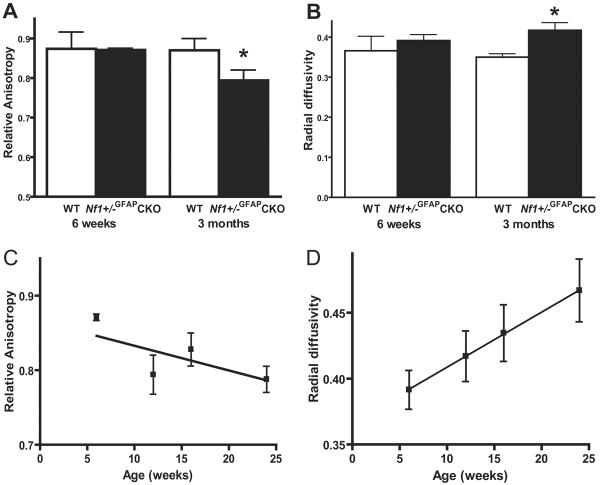
Nf1+/−GFAPCKO Mice Exhibit Progressive Increases in Optic Nerve Water Diffusivity by MRI
We next wished to determine whether we could employ diffusion tensor imaging to investigate axonal integrity and diffusivity in mice with optic gliomas. In contrast to the visual physiology or ultrastructural findings, there was no change in relative anisotropy (RA) or radial diffusivity (RD) in Nf1+/−GFAPCKO mice at 6 weeks of age compared to WT mice (Fig. 3A, B) but at 3 months of age Nf1+/−GFAPCKO mice displayed significantly decreased RA and greater RD values compared to WT mice. Although the RA and RD values did not change significantly in WT mice between 3 and 6 months of age (data not shown), the radial diffusivity progressively increased in Nf1+/−GFAPCKO mice at both 4 and 6 months of age (Fig. 3D), which likely reflects ongoing changes in water diffusion in these tumors resulting from disturbances in the integrity of the nerve and its axons.
Figure 3.
Magnetic resonance diffusion tensor imaging demonstrates a progressive increase in radial diffusivity in the Nf1+/−GFAPCKO mice. (A) Relative anisotropy in the retrobulbar optic nerve is decreased in Nf1+/−GFAPCKO mice compared to age-matched wild type (WT) mice. (B) At the same time, radial diffusivity is significantly greater in Nf1+/−GFAPCKO mouse optic nerves compared to WT controls. (C) The relative anisotropy is further decreased by 6 months of age in Nf1+/−GFAPCKO mice. (D) The radial diffusivity continues to increase at 4 and 6 months of age. Data are presented as the mean ± SEM. Asterisks denote statistically significant differences (p < 0.05).
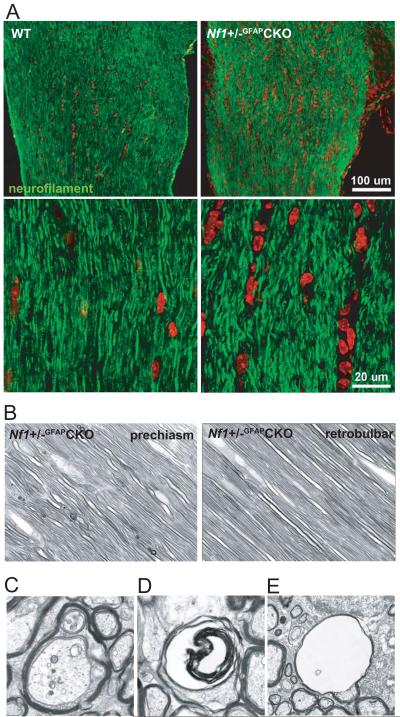
Early Changes in Prechiasmatic Optic Nerve Fiber Alignment in Nf1+/−GFAPCKO Mice
To define the effect of tumor formation in the prechiasmatic optic nerve on axonal organization, we next performed neurofilament staining on longitudinal sections of the optic nerve. Interestingly, the alignment of optic nerve axons is disorganized in the region of the developing tumor (prechiasmatic optic nerve) of 6-week-old Nf1+/−GFAPCKO mice (Fig. 4A). This early appearance of improper alignment of fibers is also seen on longitudinal sections of paraphenylenediamine-stained optic nerves (Fig. 4B). Importantly, the retrobulbar portion of the optic nerve did not show similar alterations in the organization of axonal processes. In addition, sporadic irregularities in axonal morphology in Nf1+/−GFAPCKO but not WT mice were observed at 6 weeks (data not shown). These alterations were, however, more prominent in tumor-bearing mice and included multiple neurites in a single myelin sheath (Fig. 4C), lamellar bodies in degenerating axonal spaces (Fig. 4D), and voids with no axoplasm (Fig. 4E). These findings indicate that alterations of optic nerve fiber alignment occur early during optic glioma evolution and progress from initial changes in the region of the developing tumor to involve more distant sites along the optic nerve over time.
Figure 4.
Axonal fiber alignment is altered in the prechiasmatic optic nerves of the Nf1+/−GFAPCKO mice. (A) Neurofilament staining (green) highlights the irregular axonal organization in longitudinal sections of the prechiasmatic optic nerve in 5-week-old Nf1+/−GFAPCKO mice. labeling of cell nuclei is shown in red. (B) Paraphenylenediamine-stained semi-thin sections show occasional irregular alignment of fibers outlined by their myelin sheaths in the prechiasmatic optic nerve of Nf1+/−GFAPCKO mice. Lamellar spheroids are also present. Fusiform shapes of astrocytes and their nuclei are interspersed among the fibers. However, no axonal alterations were seen in the retrobulbar portion of the optic nerve in 6-week-old Nf1+/−GFAPCKO mice. (C–E) Later, in tumor-bearing Nf1+/−GFAPCKO mice, these alterations are more prominent, and include multiple neurites in one myelin sheath (C), lamellar bodies in degenerating axonal spaces (D) and voids with no axoplasm (E) as demonstrated by transmission electron microscopy. Original magnifications: B, 1000x; C, D, 6000x; E, 3000x.
Nf1+/−GFAPCKO Mice Develop Progressive Loss of Retinal Ganglion Cells
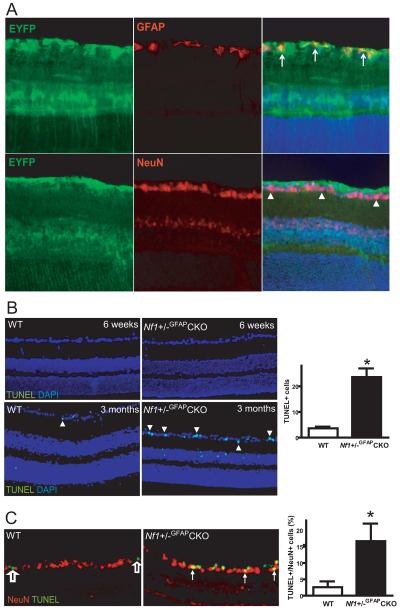
While GFAP is typically regarded as a glial protein, there is evidence from numerous laboratories that GFAP-Cre strains may result in Cre-mediated excision in neuronal populations (27, 28). To exclude the possibility that the VEP and axonal abnormalities observed in Nf1+/−GFAPCKO mice reflected Cre activity in RGCs, we employed a Cre reporter line (20): Rosa-YFP; GFAP-Cre mice exhibit YFP expression exclusively in cells in which Cre is expressed. In these studies, YFP expression was observed in GFAP+ cells in the retina, including astrocytic cells of the optic nerve layer and Müller glial cells spanning through the entire retina. We found no evidence of Cre-mediated recombination in NeuN+ neurons (Fig. 5A).
Figure 5.
Retinal ganglion cell death is seen in Nf1+/−GFAPCKO mice. (A) Immunostaining of retinas from GFAP-Cre; Rosa-EYFP reporter mice demonstrates that recombination occurred in glial cells, but not in neuronal cells. EYFP immunoreactivity (green) is found in astrocytic cells of the optic fiber layer and in scattered Müller glial cells, which overlaps with the GFAP staining (red) in the optic fiber layer (arrows). No EYFP expressing cells were double-labeled with NeuN (red) in the retinal ganglion cell layer (arrowheads). DAPI staining of nuclei is blue. (B) TUNEL staining (green) demonstrates increased apoptosis in the retinal ganglion cell layer of 3-month-old Nf1+/−GFAPCKO mice compared to control mice (arrowheads indicate TUNEL+ nuclei). No apoptotic cells were detected in 6-week-old mice of either genotype. (C) The majority of the apoptotic cells in Nf1+/−GFAPCKO mice are NeuN+ (solid arrows). In contrast, the few apoptotic cells in the 3-month-old WT mice are not NeuN+ (empty arrows). The percentage of TUNEL+, NeuN+ double-labeled cells was increased in Nf1+/−GFAPCKO mice. Data are presented as the mean ± SEM. Asterisks denote statistically significant differences (p < 0.05). Original magnifications: A, B, 100x; C, 200x.
To determine whether the VEP, MRI, and ultrastructural abnormalities observed in Nf1+/−GFAPCKO mice might reflect irreversible loss (apoptosis) of RGCs, we performed TUNEL staining. At 6 weeks of age, there were no apoptotic nuclei in any cell layer of the retina in either Nf1+/−GFAPCKO or WT mice (Fig. 5B). By 3 months of age, however, we found a ~4-fold increase in the percent of TUNEL+ cells in the ganglion cell layer in Nf1+/−GFAPCKO mice compared to age-matched WT controls (Fig. 5B). This elevated level of apoptosis was not observed in mice lacking Nf1 expression in GFAP+ cells only (Nf1GFAPCKO mice without optic glioma) or in Nf1+/− mice at 3 months of age (data not shown).
To identify which cell types in the retinal ganglion layer had undergone apoptosis, we performed double-labeling immunofluorescence using glial and neuronal markers. In these studies, apoptosis was confined to neurons, as indicated by TUNEL/NeuN (Fig. 5C) or TUNEL/MAP2 double labeling (data not shown). Approximately 15% of neurons in the retinal ganglion cell layer were TUNEL-positive (Fig. 5C). In contrast, Müller glial cell bodies in the inner granular cell layer did not contain TUNEL-stained nuclei, as assessed by cyclin D3/TUNEL or glutamine synthetase/TUNEL double labeling (data not shown).
While the vast majority of the TUNEL+ cells were neurons, we observed apoptotic cells in the inner nuclear layer, which most likely represent amacrine cells. Since amacrine cells are known to have reduced NeuN expression (29), these neurons would not be identified by NeuN/TUNEL double labeling. Based on the density of neurons in the ganglion cell layer (~8200 cells/mm2) versus the inner nuclear layer (~100,541 cells/mm2), containing ~41% retinal ganglion cells and ~39% amacrine cells, respectively (30), we estimate that fewer than 2% of the amacrine cells are TUNEL+. In contrast, a significantly larger proportion of the ganglion cells are undergoing apoptosis.
Collectively, these observations indicate that RGC apoptosis results from optic glioma formation and is not a cell-autonomous effect of reduced or absent Nf1 expression in neurons or glia, respectively.
DISCUSSION
Genetically engineered mouse (GEM) models of NF-1 have been widely used to identify the key signaling pathways that regulate tumor growth and to perform preclinical therapeutic testing (31, 32). Recently, Nf1 GEM models have also been exploited to define the contribution of cells in the tumor microenvironment to tumor formation and growth. These studies have highlighted the critical relationships that exist between stroma cell types (e.g. microglia and mast cells) and nervous system tumor development and growth (33–35). In contrast, no studies to date have employed Nf1 GEM to define the impact of tumor formation on normal nervous system function. The availability of robust and accurate mouse models of Nf1 optic glioma now position us to directly address the cellular and molecular basis of visual loss resulting from tumor formation and growth.
In this report, we used multiple complementary technologies to define the neuronal abnormalities that develop as a result of optic glioma formation in Nf1+/−GFAPCKO mice. First, we showed that visual evoked response amplitudes were diminished early during the course of optic glioma formation. The reduced amplitudes most likely reflect decreased numbers of conducting axons, rather than a demyelinating process, a hypothesis supported by an increase in the number of enlarged axons as well as neuronal disorganization observed in Nf1+/−GFAPCKO mice at 6 weeks of age. In contrast, no ERG abnormalities were observed, suggesting minimal impairment in photoreceptor or bipolar cell function in Nf1+/−GFAPCKO mice. Collectively, these observations suggest that VEPs may accurately detect visual dysfunction early in the course of disease and might be a predictor of risk of tumor formation; however, the lack of a temporal correlation between visual physiology measurements and ultrastructural changes may limit the utility of this modality for monitoring tumor growth and response to therapy. Future studies are planned using optometry as a method for assessing vision in these GEM (36).
Second, transmission electron microscopy and automatic cell counting were used to define the ultrastructural changes that result from OPG formation. The presence of increased axonal diameters in the optic nerves of Nf1+/−GFAPCKO mice suggests a degenerative process affecting axons. This progressive axonal degeneration is further supported by the finding that axonal disorganization is first observed in the region in which OPG develop in these mice (o/e/. the prechiasmatic optic nerves and chiasm), followed only later by changes in the retro-orbital optic nerve segments, and finally by death of neurons in the retina ganglion layer. This axonopathic process is most likely not cell autonomous since retinal ganglion layer cell death is not observed in Nf1+/− mice or mice lacking Nf1 expression in GFAP+ cells. We favor the interpretation that neuronal cell death reflects the process of tumor formation that disrupts the normal stabilizing and metabotropic relationships between axons and supportive cells in the prechiasmatic optic nerve region.
Third, we sought to employ diffusion-based MRI to examine changes in axonal integrity in the intact animal. Using diffusion tensor imaging (DTI) to detect alterations in water movement (37, 38), we found a time-dependent decrease in relative anisotropy and increase in radial diffusivity in Nf1+/−GFAPCKO mouse optic nerves. These changes are not obvious at 6 weeks of age but they are detected by 2 to 3 months of age (26). The strong correlation between the ultrastructural abnormalities observed by electron microscopy and the diffusion changes detected by MRI suggests that DTI might be useful for assessing the effect of tumor progression on axonal and myelin integrity. Future studies will be required to determine whether diffusion-based imaging methods reflect or predict visual deterioration in children with symptomatic NF1-associated OPG.
Finally, our studies suggest the presence of bi-directional communication between neoplastic cells and non-neoplastic cells in areas involved by and adjacent to the tumor. We have previously identified stromal cell types (microglia) and growth factors (CXCL12 and hyaluronidase) that influence optic glioma growth in Nf1+/−GFAPCKO mice, implying that the tumor microenvironment dictates tumor growth (33, 39). The current study highlights the impact of tumor formation on the non-neoplastic cell types present in the region of the developing tumor. We show that only Nf1+/−GFAPCKO mice exhibit retinal ganglion cell apoptosis. This observation suggests that tumor development is required to initiate the cascade of degenerative events that culminate in permanent cell death and vision loss. Alternatively, it is possible that Nf1-deficient neoplastic glia elaborate pro-apoptotic signals that uniquely affect Nf1+/− neurons. In this regard, some studies have demonstrated that Müller glia and astrocytes can reduce excitotoxic RGC death (40, 41), whereas others have shown that astrocytes do not enhance RGC survival in vitro (42, 43). It is possible that neurofibromin loss in astrocytes either leads to the generation of pro-apoptotic factors or results in impaired glia-mediated neuronal survival, either of which contributes to increased Nf1+/− RGC death. We are currently exploring these possibilities using astrocyte-RGC co-culture experiments in vitro.
In summary, using multiple complementary modalities we have shown that OPG formation in mice results in altered visual function. We observed decreased VEP responses and a shift towards larger caliber axons prior to macroscopic optic glioma formation, whereas, after optic gliomas were obvious, we found progressively increasing radial diffusivities on MRI and ultrastructural damage in the optic nerve axons, culminating in retinal ganglion cell death. These findings highlight the utility of GEM models of human cancer for recapitulating some of the functional abnormalities seen in children with similar tumors as well as providing important insights into their pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ryan Emnett and Emily Barr for expert technical assistance, and Dr. Bryan Smith for the development of the Matlab program for axon analysis. We also thank Dr. Frank Costantini (Columbia University) for generously providing the R26R-EYFP mice.
This work was partially funded by grants from the NCI Mouse Models of Human Cancers Consortium to D.H.G. and M.H.E. (UO1-CA84314), NS054629 to D.H.G, RR004050 to M.H.E, the Horncrest Foundation (M.A.B.), the NIH Vision Core P30 (EY-02687), and a Small Animal Imaging Resource Program grant (U24 CA83060) from the NCI/NIH. B.H. was supported by a nested post-doctoral fellowship from the DOD (W81XWH061022).
REFERENCES
- 1.Friedman JM, Gutmann DH, MacCollin M, Riccardi VM, editors. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. 3rd edition Johns Hopkins University Press; Baltimore, MA: 1999. [Google Scholar]
- 2.Riccardi VM. Neurofibromatosis: Past, present, and future. N Engl J Med. 1991;324:1283–85. doi: 10.1056/NEJM199105023241812. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. WHO Press; Geneva: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Listernick R, Charrow J, Greenwald MJ, et al. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989;114:788–92. doi: 10.1016/s0022-3476(89)80137-4. [DOI] [PubMed] [Google Scholar]
- 5.Habiby R, Silverman B, Listernick R, et al. Precocious puberty in children with neurofibromatosis type 1. J Pediatr. 1995;126:364–67. doi: 10.1016/s0022-3476(95)70449-3. [DOI] [PubMed] [Google Scholar]
- 6.Listernick R, Ferner RE, Liu GT, et al. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61:189–98. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janss A, Hiehle JF, Jr., Yachnis AT. Neurofibromatosis type 1. Med Pediatr Oncol. 1995;25:213–22. doi: 10.1002/mpo.2950250310. [DOI] [PubMed] [Google Scholar]
- 8.Listernick R, Charrow J, Greenwald M, et al. Natural history of optic pathway tumors in children with neurofibromatosis type 1: A longitudinal study. J Pediatr. 1994;125:63–66. doi: 10.1016/s0022-3476(94)70122-9. [DOI] [PubMed] [Google Scholar]
- 9.Wolsey DH, Larson SA, Creel D, et al. Can screening for optic nerve gliomas in patients with neurofibromatosis type I be performed with visual-evoked potential testing? J AAPOS. 2006;10:307–11. doi: 10.1016/j.jaapos.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Trisciuzzi MT, Riccardi R, Piccardi M, et al. A fast visual evoked potential method for functional assessment and follow-up of childhood optic gliomas. Clin Neurophysiol. 2004;115:217–26. doi: 10.1016/s1388-2457(03)00282-7. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez FJ, Perry A, Gutmann DH, et al. Gliomas in neurofibromatosis type 1: A clinicopathologic study of 100 patients. J Neuropathol Exp Neurol. 2008;67:240–49. doi: 10.1097/NEN.0b013e318165eb75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornreich L, Blaser S, Schwarz M, et al. Optic pathway glioma: Correlation of imaging findings with the presence of neurofibromatosis. AJNR Am J Neuroradiol. 2001;22:1963–69. [PMC free article] [PubMed] [Google Scholar]
- 13.Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol. 2007;25:4104–9. doi: 10.1200/JCO.2007.11.9610. [DOI] [PubMed] [Google Scholar]
- 14.Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–36. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 15.Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A. 2005;102:5524–29. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalla Via P, Opocher E, Pinello ML, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Oncol. 2007;9:430–37. doi: 10.1215/15228517-2007-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajenaru ML, Hernandez MR, Perry A, et al. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–77. [PubMed] [Google Scholar]
- 18.Zhu Y, Romero MI, Ghosh P, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–76. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajenaru ML, Zhu Y, Hedrick NM, et al. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol. 2002;22:5100–13. doi: 10.1128/MCB.22.14.5100-5113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridder WH, 3rd, Nusinowitz S. The visual evoked potential in the mouse--origins and response characteristics. Vision Res. 2006;46:902–13. doi: 10.1016/j.visres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Brantley MA, Jr., Jain S, Barr EE, et al. Neurturin-mediated ret activation is required for retinal function. J Neurosci. 2008;28:4123–35. doi: 10.1523/JNEUROSCI.0249-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 24.Hegedus B, Dasgupta B, Shin JE, et al. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1:443–57. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Falsini B, Ziccardi L, Lazzareschi I, et al. Longitudinal assessment of childhood optic gliomas: relationship between flicker visual evoked potentials and magnetic resonance imaging findings. J Neurooncol. 2008;88:87–96. doi: 10.1007/s11060-008-9537-1. [DOI] [PubMed] [Google Scholar]
- 26.Bajenaru ML, Garbow JR, Perry A, et al. Natural history of neurofibromatosis 1-associated optic nerve glioma in mice. Ann Neurol. 2005;57:119–27. doi: 10.1002/ana.20337. [DOI] [PubMed] [Google Scholar]
- 27.Fraser MM, Zhu X, Kwon CH, et al. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64:7773–79. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Harada T, Liu L, et al. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005;132:5577–88. doi: 10.1242/dev.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raymond ID, Vila A, Huynh UC, et al. Cyan fluorescent protein expression in ganglion and amacrine cells in a thy1-CFP transgenic mouse retina. Mol Vis. 2008;14:1559–74. [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–46. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dasgupta B, Yi Y, Chen DY, et al. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65:2755–60. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- 32.Hegedus B, Banerjee D, Yeh TH, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68:1520–28. doi: 10.1158/0008-5472.CAN-07-5916. [DOI] [PubMed] [Google Scholar]
- 33.Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16:1098–1112. doi: 10.1093/hmg/ddm059. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Y, Ghosh P, Charnay P, et al. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–22. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang FC, Ingram DA, Chen S, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/−- and c-kit-dependent bone marrow. Cell. 2008;135:437–48. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prusky GT, Alam NM, Beekman S, et al. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–16. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 37.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 38.Sun SW, Liang HF, Le TQ, et al. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage. 2006;32:1195–1204. doi: 10.1016/j.neuroimage.2006.04.212. [DOI] [PubMed] [Google Scholar]
- 39.Warrington NM, Woerner BM, Daginakatte GC, et al. Spatiotemporal differences in cxcl12 expression and cyclic amp underlie the unique pattern of optic glioma growth in neurofibromatosis type 1. Cancer Res. 2007;67:8588–95. doi: 10.1158/0008-5472.CAN-06-2220. [DOI] [PubMed] [Google Scholar]
- 40.Kitano S, Morgan J, Caprioli J. Hypoxic and excitotoxic damage to cultured rat retinal ganglion cells. Exp Eye Res. 1996;63:105–12. doi: 10.1006/exer.1996.0096. [DOI] [PubMed] [Google Scholar]
- 41.de Melo Reis RA, Cabral-da-Silva MC, et al. Muller glia factors induce survival and neuritogenesis of peripheral and central neurons. Brain Res. 2008;1205:1–11. doi: 10.1016/j.brainres.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 42.Baehr M, Bunge RP. Growth of adult rat retinal ganglion cell neurites on astrocytes. Glia. 1990;3:293–300. doi: 10.1002/glia.440030409. [DOI] [PubMed] [Google Scholar]
- 43.McCaffery CA, Raju TR, Bennett MR. Effects of cultured astroglia on the survival of neonatal rat retinal ganglion cells in vitro. Dev Biol. 1984;104:441–48. doi: 10.1016/0012-1606(84)90100-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.