Abstract
Purpose
To evaluate the feasibility of performing robot assisted partial nephrectomy in patients with multiple renal masses and to examine the results of our initial experiences.
Materials and Methods
We reviewed the records of 10 patients with multiple renal masses who underwent attempted robot assisted partial nephrectomy within the past 2 years. Demographic information as well as intraoperative, perioperative, and renal functional outcome data of these patients were reviewed.
Results
A total of 24 tumors in 9 patients were removed with robotic assistance. There was 1 open conversion with successful completion of partial nephrectomy. 70% (7 of 10) of patients were affected with a known hereditary renal cancer syndrome, while the remaining patients had multifocal disease with unknown germline genetic alterations. A frozen section from the tumor bed was evaluated in 5 of 10 cases and was negative in each case. 1 patient experienced post operative urinary leak resolving on post operative day 9 without intervention. Twenty-two of the 24 masses resected robotically were malignant. Our most recent 3 patients underwent successful partial nephrectomy without hilar clamping obviating the need for warm ischemia. Overall renal function was unchanged at the most recent follow up with only minimal decrease in differential function of the operated kidney.
Conclusions
Robot assisted partial nephrectomy for multiple renal masses is feasible in our early experience. Patient selection is paramount for successful minimally invasive surgery. Robot assisted partial nephrectomy without hilar clamping, especially in the hereditary patient population in which repeat ipsilateral partial nephrectomy may be anticipated, appears promising but requires further evaluation.
Keywords: Robot assisted, DaVinci, partial nephrectomy, feasibility, outcomes
It has been shown that partial nephrectomy provides cancer control equal to that of radical nephrectomy in selected patients with renal neoplasms.1 Laparoscopic partial nephrectomy was introduced in 1993 and more recently robot assisted partial nephrectomy has been described as a minimally invasive alternative to open partial nephrectomy.2,3 Initially introduced to excise small exophytic renal masses, these techniques have evolved into procedures duplicating open surgery and have facilitated the safe and accurate removal of larger and more endophytic lesions.4 Not much is known, however, about the role of laparoscopy for management of multifocal renal masses.
It has been shown that the incidence of multifocal disease ranges from 6–25% in the sporadic population.5 Multifocality is considerably higher in the hereditary renal cancer population, such as in patients with von Hippel-Lindau, hereditary papillary renal carcinoma and Birt-Hogg-Dubé. Even in high volume centers with laparoscopic expertise, the constraints of warm ischemia time needed for resection of multiple tumors typically directs the surgeon towards an open approach. Additionally, in the hereditary kidney population or in patients with multifocal renal masses, the known risk of recurrence or de novo tumor formation is much greater than in the general population. This frequently requires repeat intervention associated with higher morbidity, and it has underscored our quest towards maximizing tumor removal while minimizing hilar dissection and clamping in the open as well as the laparoscopic setting. In the current study we present our initial experience with robot assisted partial nephrectomy for 2 or more ipsilateral renal masses to evaluate the utility of minimally invasive surgery in the management of multifocal renal disease.
MATERIALS AND METHODS
Between March 2007 and December 2008, 10 robot assisted partial nephrectomies for multiple tumors on the same renal unit were attempted. All patients were evaluated on a protocol approved by the institutional review board. 7 of 10 patients (70%) had known hereditary conditions predisposing them to risk for the development of renal tumors: 4 were affected with von Hippel-Lindau, 2 with Birt-Hogg-Dubé, and 1 with Hereditary Papillary Renal Carcinoma (HPRC), while the rest had a multifocal disease with unknown germline genetic alteration. Data were obtained from reviews of operative reports, pathology reports, history and physical examination at admission, discharge, and anesthesia records.
Operative data is reported on patients in whom robot assisted partial nephrectomy was evaluated. As previously defined by Finley et al, exophytic tumors were those in which the lesion extended more than 60% off the surface of the kidney, endophytic tumors had less than 40% of the lesion off the surface of the kidney, and mesophytic tumors were between 40–60% off the natural border of the kidney.6 Preoperative evaluation included abdominal and chest imaging, routine laboratory analysis, and a nuclear renal scan. All studies were repeated at a 3 month follow up visit. Functional outcomes were assessed using serum creatinine, 24-hour urine creatinine clearance, eGFR (as calculated by MDRD equation) and MAG-3 nuclear renogram.
Technique
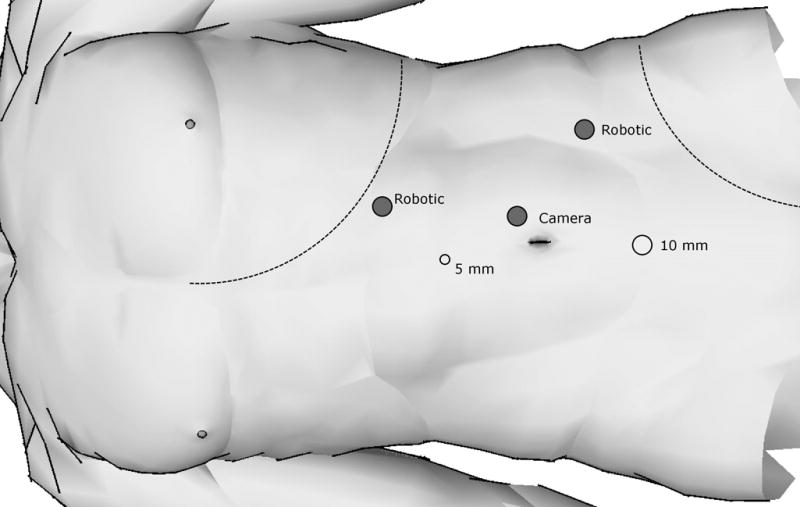
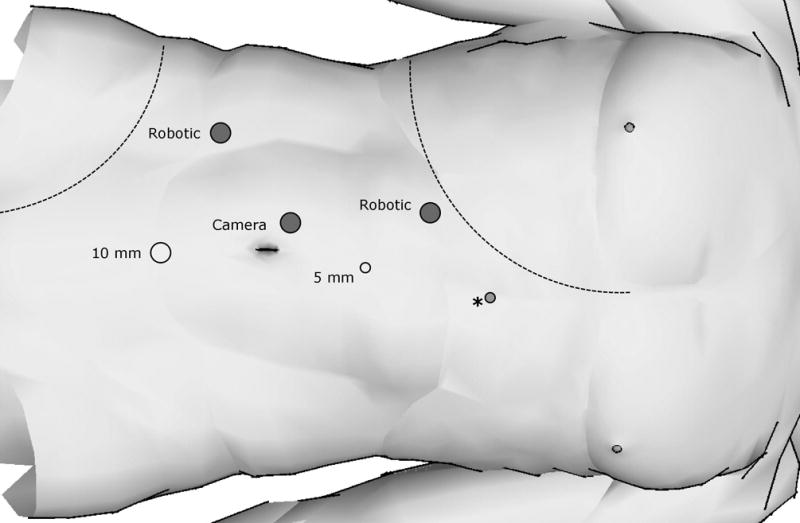
Cystoscopy and preplaced ureteral catheter in supine position was established in 8 of 10 patients at surgeon’s discretion in anticipation of collecting system entry. All patients were then placed in modified flank position with the robot to be docked over the ipsilateral shoulder. A standard three arm DaVinci surgical system (Intuitive Surgical, Sunnyvale, CA, USA) was used for all cases. A transperitoneal approach was used for all cases. Pneumoperitoneum at 15 mmHg was achieved using a Veress needle and was well tolerated by all patients. 5 ports were used for left sided surgeries and 6 ports were placed for right sided surgery for the addition of a 5mm liver retractor instrument. Port placement for robot assisted partial nephrectomy is demonstrated in figures 1a and 1b. The 30 degree down camera lens was used for the duration of the operation. After reflection of the colon, the kidney was mobilized and the hilum was identified and partially dissected to allow the placement of a laparoscopic bulldog or Satinsky, if necessary. Laparoscopic ultrasound guidance was used routinely in each case to define tumor number and extent. Surgical extirpation was initiated beginning with smaller exophytic lesions and progressing to larger and deeper tumors. All tumors were resected by a technique previously described for hereditary renal disease at the National Institutes of Health.7 Briefly, the tumor’s pseudocapsule was identified and a plane between the tumor and renal parenchyma was developed. The tumor was then inspected intracorporally to ensure an intact pseudocapsule. The resection site base was not routinely cauterized. A permanent or frozen biopsy of the resection base was performed at the surgeon’s discretion. Initially in our experience, the hilum was occluded with laparoscopic bulldog clamps or Satinsky prior to resection of the largest tumor. More recently, however, hilar occlusion occurred only when bleeding from tumor extraction became excessive or limited the ability to see planes of dissection. Retrograde injection of a diluted methylene blue solution in instances of preplaced ureteral catheter facilitated potential repair by allowing precise visualization of any collecting system injury. This was closed separately with running 3-0 vicryl suture. Renorrhaphy was performed using interrupted capsular 2-0 vicryl sutures preplaced with 10mm Hem-o-lock® (Teleflex, Research Triangle Park, NC) and Lapra-TY II® (Ethicon, Cincinnati, OH) clips on one end. The defect was filled with Surgicel® cigar shaped bolsters and hemostatic sealant prior to using the robotic needle driver to slide Hem-o-lock® and Lapra-TY II® clips on the opposite end down the suture to a desired tension. Once renorrhaphy was complete, the clamps (if used) were removed and the defect was inspected for hemostasis. A 15mm Jackson-Pratt drain was placed upon completion of all cases.
Figure 1.
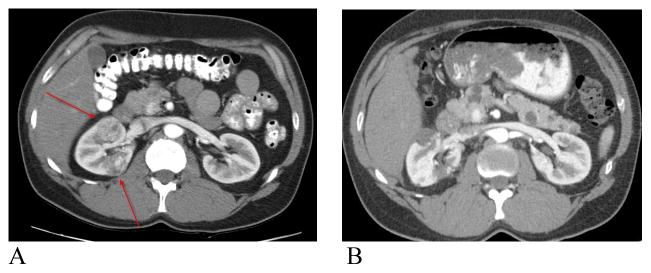
Pre-operative and post-operative CT scan in VHL patient with two large endophytic tumors. A) Pre- operative CT scan. Arrows demonstrating renal lesions B) Post-operative CT scan at 3 months demonstrating usual post operative changes.
RESULTS
Patient characteristics and operative data are shown in table 1. A total of 24 tumors were removed in 9 patients. Average follow up was 9.4 months. The mean age of the patients was 50 years (range 26–69) and 50% were male. 70% of the patients had known hereditary syndromes. Mean procedure length was 257 minutes (range 195–330). 66% of the patients (6 of 9) required hilar clamping with a mean ischemia time of 17.6 minutes including the off-clamp cohort (range 0–45). Median warm ischemia times in cases where clamping was performed was 29.6 minutes (range 19–48). Median ischemia time per tumor resected for the entire cohort was calculated to be 6.6 minutes (159 minutes for 24 tumors), while median ischemia time per tumor resected in those patients with hilar ischemia was 9.9 minutes (159 minutes for 16 tumors). Mean intra-operative blood loss was 360cc (range 100–500) and no patient required blood transfusion. All patients had at least 2 or more tumors removed with the mean number of tumors 2.7 (2–4) and the mean size of 2.3 cm (0.5–5.5). Frozen sections of the base were performed in 5 out of 10 patients and were negative in each instance. There were no losses of renal units and one conversion to open surgery. Our single conversion occurred early in the procedure secondary to bleeding. The decision was made to avoid hilar clamping early in the case prior to resection of larger and deeper masses. Therefore, the case was converted to an open partial nephrectomy with a total of 8 tumors resected. There were no intra-operative complications in our series. A single urinary leak was recorded managed expectantly with extended JP drainage. The patient did not require ureteral stenting and the leak resolved on post operative day 9. Average length of stay was 4 days (range 2–6). Tumor pathology, number, size, location, depth, and utilization of ischemia are listed in table 2. The majority of tumors removed were endophytic 75%, with the remaining 25% divided evenly between mesophytic and exophytic tumors. Of the 24 tumors resected 22 were malignant on final pathology.
Table 1.
Patient characteristics and operative data
| Number of Patients | 10 |
| Total # tumors | 24 |
| Malignancy of tumors (%) | 22 (92) |
| Laterality, Right/Left | 7/3 |
| Mean follow-up, months (range) | 9.4 (0–20) |
| Known hereditary syndrome (%) | 7 (70) |
| Age (range) | 50 (26–69) |
| Mean # tumors per patient (range) | 2.7 (2–4) |
| Mean size, cm (range) | 2.3 (0.5–5.5) |
| Mean ischemia time, minutes (range) | 17.6 (0–45) |
| Mean operative time, minutes (range) | 257 (195–330) |
| Mean estimated blood loss, ml (range) | 360 (100–500) |
| Number of peri-operative complications | 1 |
| Intra-operative conversions | 1 |
| Median length of stay, days (range) | 4 (2–6) |
Table 2.
Tumor characteristics and utilization of ischemia for resection
| Patient (N=9) | Tumor # | Size (cm) | Location* | Depth | % of tumor outside renal cortical surface | Path** | Warm Ischemia (min) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 0.8 | MP | Endophytic | 0% | Hybrid | 0 |
| 2 | 1.1 | MP | Endophytic | 0% | Hybrid | 0 | |
| 3 | 2.6 | UP | Endophytic | 0% | Hybrid | 24 | |
| 2 | 1 | 1.2 | UP | Endophytic | 0% | Hybrid | 48(Total) |
| 2 | 1.3 | UP | Endophytic | 0% | Hybrid | ||
| 3 | 2.1 | LP | Endophytic | 10% | Hybrid | ||
| 4 | 2.9 | UP | Exophytic | 90% | Hybrid | ||
| 3 | 1 | 0.8 | UP | Endophytic | 0% | AML | 0 |
| 2 | 3.0 | MP | Endophytic | 0% | Onco | 26 | |
| 4 | 1 | 0.5 | MP | Endophytic | 0% | Pap 1 | 41(Total) |
| 2 | 5.5 | MP | Endophytic | 0% | Pap 1 | ||
| 5 | 1 | 1.2 | UP | Mesophytic | 50% | Clear | 0 |
| 2 | 1.6 | UP | Endophytic | 0% | Clear | 0 | |
| 3 | 4.4 | LP | Exophytic | 80% | Clear | 19 | |
| 6 | 1 | 2.5 | MP | Endophytic | 20% | Clear | 0 |
| 2 | 3.5 | MP | Endophytic | 10% | Clear | 0 | |
| 7 | 1 | 1.0 | MP | Endophytic | 10% | Clear | 0 |
| 2 | 1.4 | UP | Mesophytic | 50% | Clear | 0 | |
| 3 | 2.0 | LP | Endophytic | 10% | Clear | 0 | |
| 4 | 3.6 | UP | Mesophytic | 50% | Clear | 0 | |
| 8 | 1 | 1.1 | UP | Endophytic | 0% | Pap 1 | 0 |
| 2 | 2.6 | UP | Endophytic | 30% | Pap 1 | 20 | |
| 9 | 1 | 3.3 | UP | Endophytic | 0% | Hybrid | 0 |
| 2 | 1.8 | MP | Exophytic | 80% | Hybrid | 9 |
Renal functional outcomes are listed in table 3. The total eGFR and differential renal function of the operated kidney were decreased by an average of 5 ml/min and 7.4%, respectively.
Table 3.
Renal functional outcomes
| Patient N=9 | Ischemia time (min) | PreOp serum Cr | PostOp serum Cr | PreOp eGFR * | PostOp eGFR * | PreOP renal scan (%) | PostOP renal scan (%) | Diff. (%)** |
|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 0.9 | 0.9 | 91 | 91 | 59 | 51 | −8 |
| 2 | 48 | 0.8 | 0.8 | 128 | 120 | 50 | 46 | −4 |
| 3 | 26 | 0.8 | 0.9 | 75 | 73 | 57 | 43 | −14 |
| 4 | 41 | 1.0 | 1.1 | 71 | 65 | 49 | 43 | −6 |
| 5 | 19 | 0.7 | 0.8 | 101 | 86 | 59 | 53 | −6 |
| 6 | 20 | 1.0 | 1.1 | 84 | 74 | 50 | 42 | −8 |
| 7 | 0 | 1.1 | 1.1 | 80 | 80 | 55 | 49 | −6 |
| 8 | 0 | 0.7 | 0.6 | 144 | 144 | 47 | NA | NA |
| 9 | 0 | 0.8 | 0.8 | 74 | 72 | 50 | NA | NA |
| Mean | 19.7 | 0.86 | 0.9 | 94 | 89 | 53% | 48% | −7.4 |
DISCUSSION
Hereditary kidney cancer differs from sporadic cases in that tumors are more frequently bilateral, multifocal and occur at an earlier age.8 Historically, patients with hereditary renal disease are closely monitored and progress to surgery when the largest tumor reaches 3cm in size.7 This patient population represents an absolute indication for nephron sparing surgery due to their high rates of tumor recurrence, need for repeat partial nephrectomy, and risk of long term renal replacement therapy. Nephron preservation is especially important with patients who present with multifocal ipsilateral disease since they are at a 5 times greater risk for contralateral recurrence compared to patients with unilateral renal mass.9
Whether partial nephrectomy is performed using an open or laparoscopic approach the goals of the surgery remain identical: oncologic efficacy, renal preservation, and early convalescence. Although initially only small, solitary, and exophytic tumors were selected for laparoscopic removal, refinements of laparoscopic technique and the recent introduction of robotic assisted surgery have facilitated a minimally invasive approach in the treatment of more complex renal masses.4
In this series we were able to perform partial nephrectomy with resection of up to 4 tumors from a single renal unit using robotic assistance. Several observations support the utilization of the robot for these complex surgeries. First, while solitary tumors in the sporadic population typically require a 1–5mm surgical margin of normal parenchyma, our patient cohort is typically managed with resection of the tumor by maintaining the plane between the normal renal parenchyma and the tumor’s pseudocapsule. This technique has been previously described and used for many years in our open series providing excellent oncologic outcomes.7 The addition of the robotic wristed articulation has facilitated navigation of these challenging dissection planes allowing these tumors to be excised safely and precisely. Second, advantages of pneumoperitoneum may have decreased the blood loss and allowed the potential for “off clamp” partial nephrectomy thus mimicking our open technique. In fact, our present results compare favorably with regards to estimated blood loss from earlier series of open partial nephrectomies, albeit the number of tumors resected in earlier series were greater.7 Also, avoidance of renal clamping may be beneficial for renal preservation as well as the prevention of post operative bleeding by performing tumor resection with immediate repair of the bleeding vessels. Additionally, minimal dissection of the renal hilum may decrease some of the vascular complications observed in our repeat and salvage partial nephrectomy series performed for this patient population.10,11 Third, since the majority of the tumors in this series were endophytic (75%) they required precise intracorporeal suturing potentially aided with robotic assisted surgery compared with traditional laparoscopy. Finally, because many patients from our cohort will require repeat intervention in the future, we anticipate that reoperation after a minimally invasive approach may be technically easier than reoperation after open surgery. Although there is no published data on this subject in the urologic literature, bariatric surgeons have described a 2-step staged laparoscopic approach lending credence to this argument.12
There is limited published data on minimally invasive surgery for multiple ipsilateral renal masses. Gill et al published a 4.5% incidence of 2 or more detected tumors in the same kidney and treated 13 patients with minimally invasive techniques.13 Out of these 13 patients, only 2 had laparoscopic partial nephrectomy for multiple tumors during the same procedure. 8 of 13 patients had 1 or all of their tumors treated with cryotherapy. In a more recent study from the same institution there were 27 patients with multiple ipsilateral renal tumors treated either with laparoscopic partial nephrectomy or cryoablation with equivalent early oncologic and functional outcomes.14 Nevertheless, enthusiasm for applying ablative technologies towards patients with multifocal disease and those at risk of recurrence or de novo tumor formation should probably be approached with caution. A recent retrospective series from Cleveland Clinic reported on 10 patients with ablative failure after previous cyrotherapy or RFA, with only 2 undergoing successful subsequent partial nephrectomy.15 Although there may be a role for ablation in select patients, surgical extirpation for multiple renal masses may allow for the best chance of the longest intervention-free interval.16 We hope that robotic assistance may decrease morbidity not only during the initial surgery but also for the repeat intervention frequently needed in the hereditary and multifocal renal disease population.
Although our cohort of patients with multifocal disease is unique, comparison of our series to the largest experiences of robot assisted partial nephrectomy in the sporadic population demonstrates similar outcomes. Ho et al. reported on 20 consecutive cases with an average tumor size of 3cm average warm ischemia time of 21.7 minutes and an average blood loss of 189 mililiters.17 In that series there were no intraoperative complications and all patients had a solitary lesion removed. Of importance, 60% (12 of 20) tumors removed were exophytic. Benway et al. published a series of 50 consecutive robot assisted partial nephrectomies for a single tumor with a mean tumor size of 2.5cm with mean warm ischemia time of 17.8 minutes and an average blood loss of 140 mililiters.18 In their series, 34% of tumors removed were benign, the complication rate was 10%, and there was one positive margin. In comparison, our cohort of multiple tumors had a higher degree of malignancy (92%) and endophytic locations (75%) while tumor size, warm ischemia times, and complication rates were comparable.
Multifocal renal tumors have been reported in 6.5–25% of patients undergoing nephrectomy for renal masses and remain a difficult diagnostic and therapeutic challenge to the urologist.5 A large multi-institutional study of over 10,000 patients with renal cell carcinoma reported that multifocality was found in 54% of bilateral disease in the general population19. Unfortunately, many of these patients will undergo radical or even bilateral nephrectomy. At our institution we traditionally manage patients with hereditary renal syndromes and multifocal renal tumors in a similar fashion by performing open partial nephrectomies.7 Justification for our aggressive approach is founded on the patients’ desire to avoid renal replacement therapy, inconsequential outcomes of renal transplantation, and emerging data supporting renal insufficiency as an independent risk factor for cardiovascular disease, hospitalization, and all-cause mortality.20 Preservation of renal function is the first impetus behind performing partial nephrectomy. Initial concerns with the utilization of minimally invasive techniques for complex partial nephrectomy rest on the foundation that prolonged warm ischemia times may adversely affect renal function. The historical duration time of safe warm ischemia was thought to be 30 minutes, while Kavoussi et al suggested that ischemia times of 30–55 minutes may not be clinically relevant by demonstrating minimal changes in pre and post operative creatinine values in 118 patients undergoing lap partial nephrectomy.21 In their study, all patients had 2 functioning renal units which may explain these findings. In another study from Desai et al, the use of nuclear renal scan demonstrated that 39 minutes of warm ischemia during laparoscopic partial nephrectomy resulted in a 29% reduction of function of the operated renal unit.22 A decrease of differential function of 7.4% in our present series is indeed smaller than the 29% described by Desai and colleagues, and may be related to a few possible reasons: shorter mean ischemia time (17.6 versus 39 minutes), timing of renogram (3 months versus 1 months), and the difference in extirpation technique (resection at the level of pseudocapsule versus healthy margin). Despite these differences the message remains unchanged: decreasing the ischemia time is beneficial for renal preservation.
While renal hilar occlusion and its accepted effects of warm ischemia is standard practice in minimally invasive nephron sparing surgery, off clamp robot assisted partial nephrectomy may still allow excellent visualization and preservation of the appropriate plane of dissection. In our 3 most recent patients, we were able to perform precise tumor excision, pelvicalyceal suture repair and renal reconstruction without hilar clamping. In these 3 patients, a total of 8 tumors were removed successfully, duplicating our open technique. Estimated mean blood loss was 467cc compared to a mean of 314cc in the group undergoing resection during hilar clamping. Figure 2 demonstrates pre and post operative CT images of a 33 year old VHL patient with 2 large endophytic renal masses who underwent successful off clamp robotic assisted partial nephrectomy. This operative strategy may potentially avoid the cumulative renal injury that inevitably occurs with prolonged hilar ischemia while still providing the patient the benefits of minimally invasive surgery.
Figure 2.
The current study has inherent limitations. It is a retrospective study and our sample size is small. Functional and oncologic outcomes are not mature. Furthermore, this series represents a highly selective group of patients who were offered the option of robotic assisted partial nephrectomy. Multiple factors including tumor location, size, number, renal function, and prior interventions were considered prior to selecting our surgical approach. In this initial series, no patient had had prior ipsilateral renal or adrenal surgery, although presently we are evaluating the feasibility of robotic partial nephrectomy after prior ipsilateral surgery. We also acknowledge that a considerable experience of the team in performing these cases via open or laparoscopic approach has contributed to the successful execution of these complex surgeries.
Additionally, we recognize that the difference in extirpation strategy may have influenced our ability to remove many of these multifocal tumors in a minimally invasive setting, especially those resected without hilar occlusion. Although these results may not necessarily translate to the treatment of patients with a single sporadic tumor, they may translate to treating patients with sporadic multifocal tumors without known familial disorder. Performing a partial nephrectomy with a “healthy” margin in this population may not be feasible or would incur an unforgivable ischemic time resulting in a high likelihood of significant loss of a function on the operated renal unit. Regardless of treatment strategy, this study provides information about the feasibility and early outcome of robot assisted partial nephrectomy for multifocal tumors in the ipsilateral kidney.
CONCLUSIONS
Robotic assisted partial nephrectomy for multiple renal masses is safe and feasible in our early experience. Renal functional outcome is minimally affected with limited follow up in this cohort. Patient selection is paramount to ensuring a successful surgical outcome. Robotic partial nephrectomy without hilar clamping, especially in the young hereditary renal tumor patient in which repeat ipsilateral partial nephrectomy may be anticipated, appears promising but requires further evaluation.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations
- VHL
von-Hippel Lindau
- HPRC
Hereditary Papillary Renal Carcinoma
- eGFR
estimated glomerular filtration rate
- MDRD
modification of diet in renal disease formula
- MAG-3
mercapto acetyl tryglycine renal scan
- RFA
radiofrequency ablation
References
- 1.Fergany AF, Saad IR, Woo L, Novick AC. Open partial nephrectomy for tumor in a solitary kidney: experience with 400 cases. J Urol. 2006;175:1630. doi: 10.1016/S0022-5347(05)00991-2. [DOI] [PubMed] [Google Scholar]
- 2.Winfield HN, Donovan JF, Godet AS, Clayman RV. Laparoscopic partial nephrectomy: initial case report for benign disease. J Endourol. 1993;7:521. doi: 10.1089/end.1993.7.521. [DOI] [PubMed] [Google Scholar]
- 3.Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, Peschel R. Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urology. 2004;64:914. doi: 10.1016/j.urology.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 4.Rogers CG, Metwalli A, Blatt AM, Bratslavsky G, Menon M, Linehan WM, et al. Robotic Partial Nephrectomy for Renal Hilar Tumors: A Multi-Institutional Analysis. J Urol. 2008 doi: 10.1016/j.juro.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltaci S, Orhan D, Soyupek S, Beduk Y, Tulunay O, Gogus O. Influence of tumor stage, size, grade, vascular involvement, histological cell type and histological pattern on multifocality of renal cell carcinoma. J Urol. 2000;164:36. [PubMed] [Google Scholar]
- 6.Finley DS, Lee DI, Eichel L, Uribe CA, McDougall EM, Clayman RV. Fibrin glue-oxidized cellulose sandwich for laparoscopic wedge resection of small renal lesions. J Urol. 2005;173:1477. doi: 10.1097/01.ju.0000154165.12738.7f. [DOI] [PubMed] [Google Scholar]
- 7.Herring JC, Enquist EG, Chernoff A, Linehan WM, Choyke PL, Walther MM. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma: 10-year experience. J Urol. 2001;165:777. [PubMed] [Google Scholar]
- 8.Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170:2163. doi: 10.1097/01.ju.0000096060.92397.ed. [DOI] [PubMed] [Google Scholar]
- 9.Blute ML, Thibault GP, Leibovich BC, Cheville JC, Lohse CM, Zincke H. Multiple ipsilateral renal tumors discovered at planned nephron sparing surgery: importance of tumor histology and risk of metachronous recurrence. J Urol. 2003;170:760. doi: 10.1097/01.ju.0000081422.47894.e6. [DOI] [PubMed] [Google Scholar]
- 10.Johnson A, Sudarshan S, Liu J, Linehan WM, Pinto PA, Bratslavsky G. Feasibility and outcomes of repeat partial nephrectomy. J Urol. 2008;180:89. doi: 10.1016/j.juro.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bratslavsky G, Liu JJ, Johnson AD, Sudarshan S, Choyke PL, Linehan WM, et al. Salvage partial nephrectomy for hereditary renal cancer: feasibility and outcomes. J Urol. 2008;179:67. doi: 10.1016/j.juro.2007.08.150. [DOI] [PubMed] [Google Scholar]
- 12.Peterli R, Wolnerhanssen BK, Peters T, Kern B, Ackermann C, von Flue M. Prospective study of a two-stage operative concept in the treatment of morbid obesity: primary lap-band followed if needed by sleeve gastrectomy with duodenal switch. Obes Surg. 2007;17:334. doi: 10.1007/s11695-007-9061-y. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg AP, Kilciler M, Abreu SC, Ramani AP, Ng C, Desai MM, et al. Laparoscopic nephron-sparing surgery for two or more ipsilateral renal tumors. Urology. 2004;64:255. doi: 10.1016/j.urology.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Lin YC, Turna B, Frota R, Aron M, Haber GP, Kamoi K, et al. Laparoscopic partial nephrectomy versus laparoscopic cryoablation for multiple ipsilateral renal tumors. Eur Urol. 2008;53:1210. doi: 10.1016/j.eururo.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen CT, Lane BR, Kaouk JH, Hegarty N, Gill IS, Novick AC, et al. Surgical salvage of renal cell carcinoma recurrence after thermal ablative therapy. J Urol. 2008;180:104. doi: 10.1016/j.juro.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 16.Kunkle DA, Uzzo RG. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer. 2008;113:2671. doi: 10.1002/cncr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho H, Schwentner C, Neururer R, Steiner H, Bartsch G, Peschel R. Robotic-assisted laparoscopic partial nephrectomy: surgical technique and clinical outcomes at 1 year. BJU Int. 2009;103:663. doi: 10.1111/j.1464-410X.2008.08060.x. [DOI] [PubMed] [Google Scholar]
- 18.Benway BM, Wang AJ, Cabello JM, Bhayani SB. Robotic Partial Nephrectomy with Sliding-Clip Renorrhaphy: Technique and Outcomes. Eur Urol. 2009 doi: 10.1016/j.eururo.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Klatte T, Wunderlich H, Patard JJ, Kleid MD, Lam JS, Junker K, et al. Clinicopathological features and prognosis of synchronous bilateral renal cell carcinoma: an international multicentre experience. BJU Int. 2007;100:21. doi: 10.1111/j.1464-410X.2007.06877.x. [DOI] [PubMed] [Google Scholar]
- 20.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 21.Bhayani SB, Rha KH, Pinto PA, Ong AM, Allaf ME, Trock BJ, et al. Laparoscopic partial nephrectomy: effect of warm ischemia on serum creatinine. J Urol. 2004;172:1264. doi: 10.1097/01.ju.0000138187.56050.20. [DOI] [PubMed] [Google Scholar]
- 22.Desai MM, Gill IS, Ramani AP, Spaliviero M, Rybicki L, Kaouk JH. The impact of warm ischaemia on renal function after laparoscopic partial nephrectomy. BJU Int. 2005;95:377. doi: 10.1111/j.1464-410X.2005.05304.x. [DOI] [PubMed] [Google Scholar]