Abstract
Objective
To compare the chondrogenic potential of adult equine mesenchymal stem cells derived from bone marrow (MSCs) or adipose tissue (ASCs).
Study Design
In vitro experimental study.
Animals
Adult Thoroughbred horses (n = 11).
Methods
BM (5 horses; mean [± SD] age, 4 ± 1.4 years) or adipose tissue (6 horses; mean age, 3.5 ± 1.1 years) samples were obtained. Cryopreserved MSCs and ASCs were used for pellet cultures in stromal medium (C) or induced into chondrogenesis ± transforming growth factor-3 (TGFβ3) and bone morphogenic factor-6 (BMP-6). Pellets harvested after 3, 7, 14, and 21 days were examined for cross-sectional size and tissue composition (hematoxylin and eosin), glycosaminoglycan (GAG) staining (Alcian blue), collagen type II immunohistochemistry, and by transmission electron microscopy. Pellet GAG and total DNA content were measured using dimethylmethylene blue and Hoechst DNA assays.
Results
Collagen type II synthesis was predominantly observed in MSC pellets from Day 7 onward. Unlike ASC cultures, MSC pellets had hyaline-like matrix by Day 14. GAG deposition occurred earlier in MSC cultures compared with ASC cultures and growth factors enhanced both MSC GAG concentrations (P<.0001) and MSC pellet size (P<.004) after 2 weeks in culture.
Conclusion
Equine MSCs have superior chondrogenic potential compared with ASCs and the equine ASC growth factor response suggests possible differences compared with other species.
Clinical Relevance
Elucidation of equine ASC and MSC receptor profiles will enhance the use of these cells in regenerative cartilage repair.
Introduction
Mesenchymal stem cells derived from bone marrow (marrow-derived stem cells, MSC) and from adipose tissue (adipose-derived stem cells, ASC) are the 2 most common equine stem cell types currently used for cell therapeutic approaches to regenerative tissue repair. Both cell types are readily accessible in the horse for isolation, enrichment, and expansion. The multipotentiality of both cell types for adipogenesis and osteogenesis has been documented.1–5 Whereas in vitro chondrogenic potential has been described for equine MSCs1,2,6–11 little attention has been directed to equine ASCs.10 We have shown inherent differences between MSCs4 and ASCs5 in cell frequency and in vitro growth characteristics, as well as ability to express alkaline phosphatase and produce bone nodules during osteogenesis.
In rodent models, Im et al12 showed that ALP staining and the amount of mineralized matrix deposition during osteogenesis was greater for MSCs compared with ASCs, and that ASCs appeared to have reduced chondrogenic potential relative to MSCs, based on the quantity of matrix production and cell morphology. Bone morphogenic proteins (BMP) are a subgroup of the transforming growth factor (TGF) superfamily and act as signaling factors that regulate cartilage and bone formation. Human ASCs have reduced expression of BMP-2, -4, and -6 relative to MSCs and do not express TGFβ receptor-1 mRNA.13 Consequently, greater concentrations of TGFβ did not enhance chondrogenesis of ASCs and only in combination with BMP-6 did ASCs express gene profiles similar to differentiated MSCs. BMP-6 also enhances in vitro chondrogenesis of MSCs.14,15 TGFβ1 used in combination with insulin-like growth factor-1 appears to enhance chondrogenesis in equine MSC cultures based on proteoglycan formation and procollagen type II mRNA synthesis.7
Based on human and rodent models, it is important to evaluate potential differences between equine MSCs and ASCs and to establish which cells may be optimal for specific regenerative tissue applications. Thus our purpose was to compare the chondrogenic potential of equine ASCs and MSCs pellet cultures in the presence or absence of a robust growth factor stimulus and to establish potential differences in their extracellular matrix composition.
Materials and Methods
Materials
All chemical reagents were obtained from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific International Inc. (Hampton, NH) unless otherwise noted.
Horses
Subcutaneous adipose tissue was harvested from the region above the dorsal gluteal muscle from 6 young Thoroughbred geldings (mean ± SD age, 3.5 ± 1.1 years) and sternal BM was collected from another 5 Thoroughbred geldings (4 ± 1.4 years).
Cell Culture Studies
Bone Marrow (BM) Aspiration
The methods used for BM aspiration16 and MSC isolation6,17 have been reported. Briefly, young horses were selected from the research herd and after sedation with detomidine HCl (0.04mg/kg intravenously [IV]) the sternum was aseptically prepared and local anesthetic (2% Lidocaine, 3 mL) infiltrated into the subcutaneous tissue. A BM aspirate (10 mL) was collected into heparinized syringes (300U/10 mL BM aspirate) using a 10 g, 3 in. Silverman BM biopsy needle. MSC isolation and expansion was performed immediately after tissue harvest.
MSC Isolation Method
BM aspirates were diluted 1:3 with stromal medium consisting of DMEM-Ham's F12 medium (vol/vol, 1:1; HyClone, Logan, UT), supplemented with a 1% antibiotic/antimycotic solution (MP Biomedicals, Irvine, CA) and 10% characterized fetal bovine serum (HyClone) and layered over Ficoll-Paque® PLUS (Stem Cell Technologies, Vancouver, Canada). This medium was also used as a control medium for the experiments described below. Nucleated cells in the BM aspirate were fractionated over a Ficoll density gradient by centrifugation at 350 × g for 30 minutes at 4°C. The MSC-enriched cell population above the Ficoll layer was then aspirated and washed in calcium and magnesium-free Dulbecco's balanced salt solution by further centrifugation at 260 × g for 5 minutes at 4°C. The washed pellet was then resuspended in stromal culture medium. MSCs were expanded in primary culture (P0) to 80% confluence and then stored after cryopreservation as described below.
Adipose Tissue Collection
Horses were sedated with detomidine HCl (0.04mg/kg IV) and butorphanol (0.01mg/kg IV), the area over the dorsal gluteal muscles was aseptically prepared, and skin and subcutaneous tissues were desensitized by local infiltration of 2% lidocaine using an inverted L-block. A 10–15 cm incision was made parallel and ∼15 cm abaxial to the vertebral column. Adipose tissue (∼ 15 mL) was harvested over the superficial gluteal fascia for ASC isolation. The skin incision was apposed with nylon suture material.
ASC Isolation Method
The ASC isolation procedure was based on a technique previously reported for human ASC isolation.18 Adipose tissue was minced with a surgical blade, washed, and briefly agitated with an equal volume of phosphate-buffered saline (PBS) solution to promote separation into 2 phases. The upper phase consisted of the minced and washed adipose tissue. The liquid infranatant containing hemopoietic cells suspended in PBS was discarded. The adipose tissue was then digested in an equal volume of a filtered PBS solution containing 1% bovine serum albumin (BSA Type V; Sigma-Aldrich) and 0.1% of collagenase (Type I; Worthington Biochemical, Lakewood, NJ) with continuously shaking at 37°C for ∼ 50 minutes. The sample was centrifuged at 260 × g for 5 minutes. To complete stromal cell separation from primary adipocytes, the sample was briefly and vigorously agitated and then centrifuged at 260 × g for 5 minutes. After discarding the supernatant containing oil, primary adipocytes, and collagenase solution, the stromal–vascular fraction pellet containing the nucleated cell portion of the adipose tissue harvest including the ASCs was then cultured in stromal medium. ASCs were expanded in primary culture (P0) and then stored after cryopreservation as described below.
Cryopreservation of Cells
Post-thaw cell viability is reported to depend on storage concentration and hence all cells were frozen at 0.5 × 106 cells/mL after expansion in P0.19 The cryopreservation medium contained 80% fetal calf serum, 10% DMEM, and 10% dimethylsulfoxide.19 Cells were placed into a 5100 Cryo 11°C Freezing Container (Wessington Cryogenics, Houghton-le-Spring, UK) for 24 hours at −80°C before transfer to liquid nitrogen.
Chondrogenesis and Pellet Cultures
For chondrogenesis experiments, primary cells (P0) were thawed and expanded (P1) to obtain ∼ 12 × 106 cells for subsequent P2 pellet cultures (n = 30; Table 1). Cells were trypsinized and aliquots of 0.25 × 106 cells (P2) were placed into racked microtubes (ISC BioExpress, Kaysville, UT) tubes and centrifuged for 5 minutes at 240 × g. The resulting pellets were then cultured and induced into chondrogenesis using DMEM/high glucose (10%), 1% antibiotic/antifungal solution, dexamethasone (100 nM), ascorbic acid 2-phosphate (50g/mL) and ITS+ (culture supplement containing bovine insulin, transferrin, selenous acid, linoleic acid, and BSA; BD Biosciences, Bedford, MA) with or without TGFβ3 (human recombinant TGFβ3, R&D Systems Inc., Minneapolis, MN) (10 ng/mL) and BMP factor-6 (human recombinant BMP-6, R&D Systems Inc.) (10 ng/mL).
Table 1.
Chondrogenesis: Experimental Design
| Time Course | Analyses | C | CH | CH–GF | ASC Donors | MSC Donors |
|---|---|---|---|---|---|---|
| Day 3 | Histology/immunohistochemistry* | 1 | 1 | 1 | 6 | 5 |
| Day 7 | Histology/immunohistochemistry | 1 | 1 | 1 | 6 | 5 |
| Biochemical & Pellet Size Analysis | 2 | 2 | 2 | 6 | 5 | |
| Day 14 | Histology/immunohistochemistry | 1 | 1 | 1 | 6 | 5 |
| Biochemical & Pellet Size Analysis | 2 | 2 | 2 | 6 | 5 | |
| Day 21 | Histology/immunohistochemistry | 1 | 1 | 1 | 6 | 5 |
| Biochemical & Pellet Size Analysis | 2 | 2 | 2 | 6 | 5 | |
| Transmission electron microscopy† | 3 | 3 | ||||
| Total pellets | 10 | 10 | 10 |
Immunohistochemistry was performed on tissue from 3 donors for both MSC and ASC pellet cultures.
Paraffin-embedded pellets used for histology were recovered and processed for TEM.
Cell lines from 5 donors were used for MSC pellet cultures and from 6 donors for ASC pellet cultures. Thirty pellets/donor/cell type (MSC or ACS) were grown. Pellets were analyzed individually for histologic examination and pooled by day (2 pellets/donor) for the biochemical analysis. Two alcian blue-stained sections/pellet for all donors were used to measure cross-sectional pellet size. Pellets were divided into 3 treatment groups: stromal medium (C), chondrogenesis medium (CH), and chondrogenesis medium plus TGFβ3 and BMP-6 (CH-GF). The medium was changed every third day. Pellets were harvested at Days 3, 7, 14, and 21 in culture and then prepared for compositional studies.
ASC, adipose-derived stem cells; MSC, mesenchymal stem cells; TGFβ3, transforming growth factor-3; BMP, bone morphogenic factor-6; TEM, transmission electron microscopy.
The 3 treatment groups were: stromal medium (C), chondrogenesis medium (CH), and chondrogenesis medium plus TGFβ3 and BMP-6 (CHGF). The medium was changed every second day in all cultures. Pellet cultures were terminated at Days 3, 7, 14, or 21 and then prepared for compositional studies. The experimental design for the chondrogenesis study is shown in Table 1.
Compositional Analysis
Pellets were either papain digested for quantification of total DNA and glycosaminoglycans (GAG) or fixed in 10% formalin and subsequently embedded in paraffin for staining and evaluation with light microscopy.
Histology
Multiple sections of all pellets were stained with Alcian blue (pH 2.5) to evaluate GAG deposition and selected pellets sections were stained with hematoxylin and eosin (H&E) to evaluate pellet morphology. Representative sections of the ASC and MSC pellet cultures and their treatment groups were read and evaluated by a pathologist (D.B.P.).
Immunohistochemistry
Immunostaining was performed using an automated immunostainer (Dako Corp., Carpinteria, CA). Paraffin-embedded sections were deparaffinized and rehydrated. Endogenous peroxidase activity was quenched with H2O2 (3.0%). Tissue sections were pretreated with proteinase K (Dako Corp.) for antigen retrieval, blocked with horse serum (30 minutes) and incubated with the primary antibody for 30 minutes at 251°C. Mouse monoclonal antibody CIIC120 (Developmental Studies Hybridoma Bank, The University of Iowa, Iowa City, IA), reported to cross-react against equine collagen type II,10 was diluted 1:5. After rinsing, slides were incubated with horseradish peroxidase-labeled polymer (avidin- and biotin-free) conjugated to goat anti-mouse IgG (EnVision, Dako Corp.). Peroxidase activity was detected using the NovaRED substrate kit (Vector Laboratories Inc., Burlingame, CA), and tissues were counterstained with Mayer's hematoxylin. For each tissue, a conditioned medium negative control that did not contain primary antibody was evaluated and no staining was detected. Paraffin-embedded cartilage from the proximal third tarsus (T3) of a 3-year-old Thoroughbred gelding was used as a positive control.
Transmission Electron Microscopy (TEM)
For TEM tissue examination, representative samples taken from 3 donors of Day 21 MSC and ASC pellet cultures (C, CH, and CHGF) were used. Paraffin-embedded pellets were deparaffinized, rehydrated with water and then fixed, processed, and embedded in an epon–araldite resin as previously reported.21 Thin sections were prepared with an MT XL microtome (RMC Products, Tucson, AZ), stained with uranyl acetate and lead citrate, and observed with an electron optical microscope (JEM-1011, Jeol Ltd., Lewisville, TX). Paraffin-embedded articular T3 cartilage was processed in the same manner and used as a positive control.
Total DNA Quantification
Total DNA within the pellets was assessed with the Hoechst assay.22 Briefly, papain digested pellet DNA concentration was assessed against a calf thymus DNA standard curve after preparation in an assay solution (2 M NaCl, 50 mM sodium monobasic phosphate, pH 7.4) containing the Hoechst 33258 dye. Pellet total DNA was measured with a spectrophotometer (Synergy HT, Bio-Tek Instruments Inc., Winooski, VT) and used to normalize GAG concentrations.
GAG Quantification
Pellets were digested in 100 μg/mL of papain in 0.4 mM sodium acetate (pH 6.8), 10 mM EDTA, 200 mM l-cysteine at 60°C for ∼ 24 hours. GAG synthesis within the pellets was measured by dimethylmethylene blue assay as previously described, using chondroitin sulfate C from shark cartilage as a standard.23
Cross-Sectional Pellet Size
The areas of 2 different cross-sections of all Alcian blue-stained pellets from all donors were measured using Image Pro Software (Cybernetics Inc., Bethesda, MD) after calibration with a micrometer.
Statistical Analysis
Data were analyzed using ANOVA with a split plot arrangement of treatments. The main plot was arranged as a 2 by 3 factorial including 2 cell types (MSC, ASC), 3 treatments (C, CH, CHGF) and the cell type by treatment interactions. The error term used for the main plot was donor within cell type by treatment. The subplot factors included day, day by cell, day by treatment, and day by cell by treatment interactions. Tukey's test was used for post hoc main effect comparisons and the t-test was used for pair-wise comparisons of the least square means to examine interaction effects. All statistical analyses were performed using Proc GLM (SAS 9.1.2, SAS Institute Inc., Cary, NC) and the type I error was maintained at α = 0.05 for all comparisons. Data are reported as arithmetic mean ± SEM error in all figures.
Results
H&E and Alcian Blue Histology
H&E-stained pellets cultured from MSCs had a hyaline cartilage-like morphology by Day 14 (Fig 1) based on characteristic lacunae formation containing round chondrocytes typically associated with maintenance of chondrogenesis.24 Pellets grown from ASCs had an immature fibroblastic tissue appearance for the duration of the study. MSC pellets had consistently more intense GAG staining than ASC pellets (Fig 2), whereas mild staining was noted as early as Day 3 in MSC pellets treated with growth factors, but was not apparent in ASCs until Day 14. Under chondrogenic conditions without added growth factors, GAG staining was less intense for both cell types but MSC differentiated pellets had GAG staining by Day 7, a week earlier than for ASCs.
Fig 1.

ASC and MSC pellet cultures (hematoxylin and eosin [H&E] stain) had an initial peripheral layer formation and maturation by Day 14 in form of a deeper palisading and perpendicular arrangement of cells. Only MSC-grown pellets produced hyaline cartilage by Day 14. The appearance of these pellets showed the characteristic lacunae formation containing chondrocytes. Pellets grown from ASCs never showed evidence of hyaline cartilage formation but rather maintained the immature fibroblastic tissue appearance. ASC, adipose-derived stem cells; MSC, bone marrow-derived stem cell.
Fig 2.

ASC and MSC pellet cultures (Alcian blue stain). Pellets cultured from MSCs had consistently more intense staining than those cultured from ASCs. Mild GAG staining was notable as early as Day 3 in MSC pellets treated with BMP-6 and TGFβ3 but not in ASCs until Day 14 under the same conditions. Chondrogenic conditions without added growth factors led to less intense GAG staining for both cell types but MSC differentiated pellets had GAG staining by Day 7, a week earlier than in ASCs. The ASC-CH and ASC-CHGF pellets of Day 14 pellet show evidence of an edge artifact. ASC, adipose-derived stem cells; MSC, bone marrow-derived stem cell; TGFβ3, transforming growth factor-3; GAG, glycosaminoglycan.
ASCs under chondrogenic conditions without growth factors pellets had fibrillar matrix by Day 7. The outer pellet layers were often composed of cohesive and spindeloid cells arranged parallel to the surface varying in thickness from 3 to 10 cells. Inner layers tended to palisade and were variably arranged perpendicular to the surface. Under growth factor treatment, the matrix density of the outer cell layer was increased by Day 14. Pellets had a broad external zone of spindeloid cells arranged parallel to the surface and ∼ 150–200 μm in thickness. By Day 21 only one of the ASC pellets had moderate amounts of dense hyaline extracellular matrix. Moderate Alcian blue staining of extracellular matrix by Day 14 suggested GAG expression in the stroma.
MSC pellets without growth factor treatment developed several layers of spindle cells arranged parallel to the surface by Day 3. Palisading spindle cells were arranged perpendicular to the surface. No extracellular matrix was seen without growth factors; however, mild Alcian blue staining was noted in the peripheral zone in CHGF MSC pellets. Within 4 days the outer zone had spindle cells layers varying from 3 to 10 cells in thickness, arranged parallel to the surface surrounded by moderate fibrillar extracellular matrix. There was no Alcian blue staining of the outer zone of Day 7 MSCs pellets but moderate staining of the matrix in the deeper layers. Partial chondroid differentiation was noted, characterized by abundant extracellular matrix with round to stellate cells, often located within the lacunae and organized into rows perpendicular to the surface. Intense Alcian blue staining was observed in the deeper cartilaginous appearing zone by Day 14 and most of the pellet had prominent chondroid differentiation. The matrix was eosinophilic near the surface but the thick chondroid differentiated zone appeared hyaline-like, typical of cartilaginous matrix and was only very mildly birefringent. Cells were round to oval and positioned within lacunae. Day 21 MSC pellets had a similar appearance but much of the matrix was birefringent under polarized light. There was evidence of variable central necrosis in most pellets of both cell types by Day 14.
Collagen Type II Immunohistochemistry
Collagen type II expression was noted by Day 7 in MSC pellets when cultured with BMP-6 and TGFβ3 (Fig 3). No evidence of collagen staining was observed in ASCs under the same conditions. MSC pellet cultures under chondrogenic conditions without growth factors had mild staining by Day 14 whereas ASC pellets again did not express collagen type II. The positive controls of paraffin-embedded cartilage sections stained with similar intensity for collagen type II as the pellets.
Fig 3.

Collagen type II immunohistochemistry staining of ASC and MSC pellets. There is convincing collagen type II expression in MSC pellets when cultured with BMP-6 and TGFβ3. ASC pellets had no evidence of collagen staining under the same conditions and are therefore not shown. Pellet cultures under chondrogenic conditions without growth factors had mild staining by Day 14 whereas ASC pellets again did not express antigen for the collagen type II antibody. All pellets were exposed to conditioned medium serving as negative control for unspecific staining and mature cartilage from the proximal surface of the third tarsus (T3) was used as positive control (data not shown). ASC, adipose-derived stem cells; MSC, bone marrow-derived stem cell; TGFβ3, transforming growth factor-3.
TEM
ASC pellets (CH and CHGF treated) from only 1 of 3 donors developed appreciable amounts of collagen fibrils, whereas all examined MSC pellets (CH and CHGF; n = 3 donors) had abundant collagen fibrils. MSC pellet cultures formed more fibrils and had more cohesive areas of extracellular matrix compared with ASC pellets. Collagen fibrils were virtually undetectable in control (C) cultures. It was notable that pellets had a random arrangement of fibrils similar to the transitional zone in articular cartilage (Fig 4E). Areas of linear fibril arrangement were seen in pellet cultures of both cell types but it was not possible to determine from which pellet layers they originated. MSC pellets (Fig 4D) appeared to have thinner collagen fibrils compared with articular cartilage (Fig 4E) as well as less interfibrillar extracellular matrix. However, compared with ASC pellets (Fig 4B). MSC fibrils seemed thicker and more mature. No compositional differences were discernable under TEM evaluation between CH and CHGF treatments.
Fig 4.

Transmission electron microscopy. Sections of ASC cultures (CHGF) shown in panel A and B clearly show collagen fibrils at × 40 and × 80k magnification, respectively. Panel C and D represent MSC pellets (CHGF) which had a similarly random fibril arrangement as the image of the transitional zone of equine articular cartilage (panel E, × 40k magnification) but less interfibrillar extracellular matrix. Panel D (MSC pellet at × 80k magnification) shows more mature and thicker fibril structure compared with that of ASC pellets (panel B, × 80k magnification) but MSC fibrils still appear to have a smaller diameter compared with articular cartilage fibrils (panel F, × 80k magnification). ASC, adipose-derived stem cells; MSC, bone marrow-derived stem cell.
DNA Concentration
DNA concentrations were significantly larger in all Day 7 cultures compared with Days 14 and 21 (P<.001; Fig 5). DNA concentrations were significantly lower for Day 7 control cultures compared with those treated under chondrogenic conditions with and without BMP-6 and TGFβ3 for both ASC and MSC cultures (P<.005).
Fig 5.

Total DNA concentrations. Among all treatment groups, total DNA content of all Day 7 pellets was significantly greater than that of the Days 14 or 21 pellets whereby “a” and “d” indicate significance at P<.0001 and P<.001, respectively. DNA content of Day 7 ASC and MSC pellets under chondrogenic conditions (± growth factors) was larger compared with control pellets, whereby significance is indicated by “b” (ASC, P<.005) and “c” (MSC, P<.0001), respectively. Data are represented as the arithmetic mean ± SE of the pooled DNA of 2 pellets/treatment (ASC cultures, n = 6; MSC cultures, n = 5). ASC, adipose-derived stem cells; MSC, bone marrow-derived stem cell.
GAG Concentration
GAG concentrations (Fig 6) normalized to total DNA concentrations were not significantly different for any cultures at Day 7. On Day 14, MSCs treated with growth factors showed significantly greater GAG concentrations than all other treatments for both cell types (P<.0001). On Day 21 MSCs cultured under chondrogenic conditions with and without growth factors expressed higher GAG concentrations compared with ASCs grown under the same conditions (ASC-CH versus MSC-CH, P<.005; ASC-CHGF versus MSC-CH, P<.008; ASC-CHGF versus MSC-CHGF, P<.0001).
Fig 6.
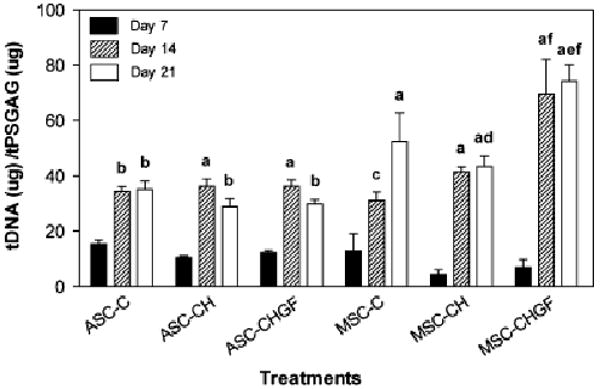
Total GAG concentrations. Relative GAG concentrations, pooled from 2 pellets/treatment and corrected by total DNA, were not significantly different for any cultures on Day 7. However, GAG concentrations were significantly larger in all pellets by Days 14 and 21 compared with Day 7. The symbols “a” (P<.0001), “b” (P<.005), and “c” (P<.03) denote the significance of these differences. Growth factor addition to MSC pellet cultures resulted in greater GAG concentrations by Day 21 compared with culture under control and chondrogenic conditions (“e” indicates P<.005). Under chondrogenic conditions alone (without growth factors) greater GAG concentrations were seen in MSC cultures compared with ASC cultures by Day 21 (“d” indicates P<.008). The addition of growth factors resulted in significantly greater amounts of GAG concentrations as early as Day 14 when comparing ASC and MSC cultures (“f” indicates P<.0001). Data are represented as the arithmetic mean ± SE (ASC cultures, n = 6; MSC cultures, n = 5). ASC, adipose-derived stem cells; MSC, bone marrow-derived stem cell; GAG, glycosaminoglycan.
Pellet Cross-Sectional Measurements
Cross-sectional pellet size of ASC-derived pellets (Fig 7) did not vary with any of the treatments, with the exception of CH between Days 3 and 21 (P<.007). Pellet sizes grown from CHGF-treated MSCs were significantly larger than CH-treated pellets on Days 14 (P<.004) and 21 (P<.0001). Significant differences in CHGF treatments in MSC cultures were found between Days 21 and 3 (P<.0001), 7 (P<.005), and 14 (P<.005). MSC pellets were also larger compared with ASC pellets on Days 7 (P<.008), 14 (P<.0001), and 21 (P<.0001) after culture under CHGF conditions. MSC pellet size of CH-treated cultures decreased significantly (P<.02) between Days 7 and 21. A trend in decreasing pellet size was also noted for ASCs but was not significant. Pellet sizes were not assessed for control cultures because the lack of structural integrity caused a progressive loss of cell material in time despite careful media changes. The correlation between GAG corrected for DNA and pellet cross-sectional area approached significance (P = .076).
Fig 7.

Pellet cross-sectional measurements. ASC-derived pellet cultures grown with or without growth factors had no differences in cross-sectional pellet areas. Pellet sizes grown from MSCs were significantly different between CH and CHGF treatments on Days 14 (P<.004) and 21 (P<.0001). On Day 7 significant differences were seen in CHGF-treated ASC and MSC pellets (P<.008). Day 21 MSC pellets treated with growth factor (CHGF) were significantly different from those of Days 3 (P<.0001), 7 (P<.005), and 14 (P<.005). On Day 7, CH treatments between MSC and ASC grown pellets also were significantly different (P = .013); however, comparing CHGF treatments between the 2 cell types showed larger MSC pellets sizes on Days 7 (P<.008), 14 (P<.0001), and 21 (P<.0001). Cross-sectional areas of pellets grown in stromal medium (C) were not measured because of a lack of characteristic pellet structure. Data are represented as the arithmetic mean ± SE (ASC cultures, n = 6; MSC cultures, n = 5). ASC, adipose-derived stem cells; MSC, bone marrow-derived stem cell.
Discussion
Use of stem cells in equine veterinary medicine is expanding to applications beyond tendon and ligament repair. MSCs injected directly into joints have significantly contributed to the healing process of experimentally induced meniscal lesions in sheep, rats, and dogs.25–28 In experimentally induced equine femoropatellar joint lesions, in vivo implantation of MSCs within autogenous fibrin plugs yielded increased fibrous tissue deposition that contained predominately collagen type II.29; however, this was only evident 30 days after implantation whereas 7 months later, control and treated lesions were indistinguishable.29
The in vitro chondrogenic potential of equine MSCs has been documented in monolayer cultures6,7,30; three-dimensional fibrin disks7; and pellet cultures.2,3,8,9 Our work confirms and extends the work of Kisiday et al,10 which examined chondrogenesis in a comparative study of equine adipose tissue and BM-derived progenitor cells.10 Mesenchymal stromal cells were cultured in agarose and self-assembling peptide hydrogels with or without the TGFβ1 yielding results consistent with our data and thus demonstrating superiority of BM MSCs in their chondrogenic potential. Similar to our study, equine ASCs were able to synthesize extracellular matrix in the form of proteoglycans and GAGs, yet unlike BM MSCs, the adipose tissue-derived progenitor cells did not demonstrate any collagen type II expression during the 3-week culture period. However, ASCs responded to TGFβ1 treatment with elevated levels of hydroxyproline and GAG production in peptide hydrogel cultures.10 These results suggest that equine ASCs may express receptors to TGFβ1 or at least may respond to the growth factor through alternate pathways.
Differences in chondrogenic potential between the 2 equine cell types are consistent with studies on human and rodent adipose and marrow-derived mesenchymal progenitor cells.12,13,31,32 However, Hennig et al13 recently showed that human ASCs do not express significant levels of TGFβ receptor-I mRNA, suggesting that human ASCs lack receptors for TGFβ1. Human ASCs did not respond significantly to culture supplementation of TGFβ1 at 10 ng/mL or higher concentrations, levels comparable to those used in both in our study and that of Kisiday. However, endoglin (CD105), a component of the TGFβ types I and II receptor complex,33 was expressed on both human ASCs34 and MSCs35 as part of their surface marker profile. Reports have suggested that dexamethasone36 in chondrogenic protocols may suppress any stimulatory effects of TGFβ1 and this may contribute to the observed tissue-dependent differences between MSCs.
The growth factor TGFβ3 has been used in several studies of human MSCs to enhance chondrogenesis because of its putative role in the regulation of cell adhesion molecules, cytokines, and cytokine receptor synthesis.37–39 Estes et al40 have shown that BMP-6 and TGFβ3 in human ASC-derived alginate bead cultures controlled chondrogenic induction and that relative to Day 0 these growth factors increased mRNA levels of collagen type II (COL2A1) by 38- and 42-fold, individually, and by 56-fold when used in combination. The same study also showed that TGFβ3 had significant effects on collagen type × (COL10A1), suggesting a role in hypertrophic collagen synthesis.
Consistent with Estes et al40 but in contrast to our own findings in an equine model, Hennig et al13 found that addition of BMP-6 resulted in similar chondrogenic induction of human ASCs and MSCs. Our data suggest that the receptor profiles of ASCs might differ between humans and horses. If the work by Awad et al36 on human ASCs is extrapolated to the equine ASC model, then the relatively higher concentration of dexamethasone (100nM) we used compared with Hennig (20 nM) may have inhibited chondrogenic differentiation and account for the diminished ASC response compared with BM MSCs. Regardless, our results indicate that there are inherent differences between these 2 equine cell types. Further investigations will be necessary to determine whether the cytokine receptor profiles of equine ASCs and BM MSCs differ and if this could account for their differential chondrogenic responses.
Immunohistochemical staining of collagen type II in MSC pellets demonstrated a pattern whereby signal was apparent within the zone below the superficial spindeloid cells layers and diminished toward the center of the pellet. This pattern may be related to oxygen diffusion and may reflect the location of an oxygen tension gradient particularly suitable for chondrocytic synthesis of collagen type II fibrils. It is known that articular cartilage is bathed in joint fluid with a relatively low oxygen concentration (∼ 5%).41 Proliferation of human ASCs is inhibited at oxygen tension of ∼ 5% but the rate of protein synthesis will increase by 2-fold and that of collagen synthesis by 3-fold and GAG synthesis and lactate production are increased as well41. Also, both cartilage thickness and cell density affect oxygen tension of in vitro cartilage sections42 and oxygen tension has been associated with the regulation of chondrogenic induction and a concomitant decrease in cell proliferation.24 Thus, oxygen tension may play a role in the current study and merits further investigation.
Inadequate nutrient diffusion to the center of the pellet is a potential cause of the central necrosis observed in the larger MSC pellets by Day 14. Because in vivo cartilage is dependent on mechanical compression to facilitate diffusion of nutrients, it is possible that the lack of mechanical stimulation of the differentiating pellet matrix under static culture conditions may lead to a reduction in nutrient diffusion.
Pellet morphology of equine ASC and MSC cultures differed considerably. Of note was the significant reduction of MSC pellet sizes under chondrogenic (CH) conditions during the 2nd week in culture. Human chondrocytes43 and MSCs44 express the contractile protein, α-smooth muscle actin (SMA). Kinner et al44 reported that SMA-expressing cells were found predominately in the peripheral layer of pellets containing type II collagen. The stimulation of cell cultures with TGFβ1 was shown to up-regulate SMA expression44 as well as contractility.45 Based on this work it as been speculated that SMA contraction may be responsible for the spherical pellet structure and cellular signaling leading to chondrogenesis after in vitro induction.44 Scaffold designs with variable resistance to cell-mediated contraction have been used to show that chondrocytic cell phenotype and collagen type II synthesis was superior in scaffolds permissive to contraction.46 Tissue remodeling, contraction, and cartilage synthesis is enhanced by treating canine chondrocytes seeded onto collagen type II-GAG scaffolds with fibroblast growth factor-2 (FGF-2).47 FGF-2 has also recently been shown to enhance chondrogenesis in equine MSCs because of an anabolic effect on GAG content and collagen type II expression.9 Consistent with these reports, the smaller pellet size in MSC chondrogenic cultures on Day 14 in our study coincided with the appearance of hyaline-like cartilaginous tissue shown in Fig 1. ASC pellets, however, had a similar but nonsignificant trend in size reduction suggesting some degree of tissue remodeling consistent with GAG deposition and traces of collagen type II fiber synthesis observed using TEM.
The BMP-6 and TGFβ3 combination had an anabolic effect on MSC pellet size. A recent report comparing human synovium- and BM-derived mesenchymal stromal cells also demonstrated an increase in synovial MSC pellet size when BMP-2 was combined with TGFβ3 under chondrogenic culture conditions.48 Like we observed in equine pellets, total DNA content of human MSC pellets decreased.48 In contrast to our equine study, these authors used BMP-2 at higher concentrations (500 ng/mL), whereas TGFβ3 concentrations were comparable. It is possible that the concentration of human recombinant BMP-6 (10 ng/mL) we used was a suboptimal stimulus for induction of equine ASC chondrogenesis, although it was sufficient for equine MSC differentiation. At this time, there is limited information concerning the use of BMP-6 for ASC chondrogenesis outside the human model. Further work is required to examine how BMPs and TGFβ synergize to promote pellet hypertrophy and extracellular matrix production.
The lack of the characteristic compact structure of pellets grown in stromal medium (C) prevented the measurement of their cross-sectional area. Failure to form a sufficiently robust extracellular matrix rendered the control pellets susceptible to cell loss during media changes. Only a few cultures in the control medium (C) formed pellets that remained intact for the duration the study; of these, most were derived from MSCs. Cellular loss in the control cultures because of media change is believed to account for the reduction in DNA content seen on Day 7 in control pellets. The ∼ 3-fold reduction in total DNA content evident in CH and CHGF cultures after Day 7 was attributed to the progressive central necrosis in pellets of both cell types. The lack of differences among the normalized GAG concentrations between controls and the treated pellet cultures is thought to relate to relative differences in DNA and measured GAG concentrations between control cultures and treated cultures. Additional variation in the data may be because MSCs and ASCs were isolated from 2 different populations of horses.
In summary, we found that equine BM-derived MSCs had superior chondrogenic potential compared with ASCs in the presence of stimulatory growth factors. Whereas easy access to ASCs in the horse makes these cells an attractive choice for tissue engineering purposes, further investigations will be necessary before they can be used clinically. These may include comparisons of the TGFβ cytokine receptor profiles and the characteristics of equine ASC and MSC extracellular matrix deposition (collagens I, II, and X) in models combining chondrogenic and osteogenic differentiation conditions.49
Acknowledgments
The authors thank the Grayson-Jockey Club Research Foundation, the Pennington Biomedical Research Foundation, the Clinical Nutrition Research Unit (NIDDK) at the Pennington Biomedical Research Center, and the Louisiana State University Equine Health Studies Program for financial support of this project. Additionally, we would like to thank Dr. Barbara Gawronska-Kosak and Dr. Bradley Estes for their advice and technical instruction as well as Mr. Hal Halloway and Mrs. Julie Millard for their technical help with histology and immunohistochemistry.
Study conducted at the Pennington Biomedical Research Center and the School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA. Funded and supported in part by the Grayson-Jockey Club Research Foundation Inc., the LSU Equine Health Studies Program, and the Pennington Biomedical Research Foundation.
References
- 1.Koerner J, Nesic D, Romero JD, et al. Equine peripheral blood-derived progenitors in comparison to bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:1613–1619. doi: 10.1634/stemcells.2005-0264. [DOI] [PubMed] [Google Scholar]
- 2.Arnhold SJ, Goletz I, Klein H, et al. Isolation and characterization of bone marrow-derived equine mesenchymal stem cells. Am J Vet Res. 2007;68:1095–1105. doi: 10.2460/ajvr.68.10.1095. [DOI] [PubMed] [Google Scholar]
- 3.Giovannini S, Brehm W, Mainil-Varlet P, et al. Multilineage differentiation potential of equine blood-derived fibroblast-like cells. Differentiation. 2008;76:118–129. doi: 10.1111/j.1432-0436.2007.00207.x. [DOI] [PubMed] [Google Scholar]
- 4.Vidal MA, Kilroy GE, Johnson JR, et al. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: adipogenic and osteogenic capacity. Vet Surg. 2006;35:601–610. doi: 10.1111/j.1532-950X.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 5.Vidal MA, Kilroy GE, Lopez MJ, et al. Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg. 2007;36:613–622. doi: 10.1111/j.1532-950X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 6.Fortier LA, Nixon AJ, Williams J, et al. Isolation and chondrocytic differentiation of equine bone marrow-derived mesenchymal stem cells. Am J Vet Res. 1998;59:1182–1187. [PubMed] [Google Scholar]
- 7.Worster AA, Brower-Toland BD, Fortier LA, et al. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19:738–749. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 8.Hegewald AA, Ringe J, Bartel J, et al. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004;36:431–438. doi: 10.1016/j.tice.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Stewart AA, Byron CR, Pondenis H, et al. Effect of fibroblast growth factor-2 on equine mesenchymal stem cell monolayer expansion and chondrogenesis. Am J Vet Res. 2007;68:941–945. doi: 10.2460/ajvr.68.9.941. [DOI] [PubMed] [Google Scholar]
- 10.Kisiday JD, Kopesky PW, Evans CH, et al. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. J Orthop Res. 2008;26:322–331. doi: 10.1002/jor.20508. [DOI] [PubMed] [Google Scholar]
- 11.Giovannini S, Brehm W, Mainil-Varlet P, et al. Multilineage differentiation potential of equine blood-derived fibroblast-like cells. Differentiation. 2008;76:118–129. doi: 10.1111/j.1432-0436.2007.00207.x. [DOI] [PubMed] [Google Scholar]
- 12.Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13:845–853. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Hennig T, Lorenz H, Thiel A, et al. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 14.Sekiya I, Larson BL, Vuoristo JT, et al. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320:269–276. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 15.Indrawattana N, Chen G, Tadokoro M, et al. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 16.Orsini JA, Divers TJ. Manual of Equine Emergencies: Treatment and Procedures. 1. Philadelphia, PA: Saunders; 1998. [Google Scholar]
- 17.Smith RK, Korda M, Blunn GW, et al. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J. 2003;35:99–102. doi: 10.2746/042516403775467388. [DOI] [PubMed] [Google Scholar]
- 18.Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 19.Goh BC, Thirumala S, Kilroy G, et al. Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med. 2007;1:322–324. doi: 10.1002/term.35. [DOI] [PubMed] [Google Scholar]
- 20.Holmdahl R, Rubin K, Klareskog L, et al. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986;29:400–410. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- 21.Sokolova YY, Fuxa JR, Borkhsenious ON. The nature of Thelohania solenopsae (Microsporidia) cysts in abdomens of red imported fire ants, Solenopsis invicta. J Invertebr Pathol. 2005;90:24–31. doi: 10.1016/j.jip.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 23.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 24.Guilak F, Awad HA, Fermor B, et al. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41:389–399. [PubMed] [Google Scholar]
- 25.Murphy JM, Fink DJ, Hunziker EB, et al. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 26.Agung M, Ochi M, Yanada S, et al. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc. 2006;14:1307–1314. doi: 10.1007/s00167-006-0124-8. [DOI] [PubMed] [Google Scholar]
- 27.Izuta Y, Ochi M, Adachi N, et al. Meniscal repair using bone marrow-derived mesenchymal stem cells: experimental study using green fluorescent protein transgenic rats. Knee. 2005;12:217–223. doi: 10.1016/j.knee.2001.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki T, Deie M, Shinomiya R, et al. Meniscal regeneration using tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. J Biomed Mater Res A. 2005;75:23–30. doi: 10.1002/jbm.a.30369. [DOI] [PubMed] [Google Scholar]
- 29.Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25:913–925. doi: 10.1002/jor.20382. [DOI] [PubMed] [Google Scholar]
- 30.Worster AA, Nixon AJ, Brower-Toland BD, et al. Effect of transforming growth factor beta1 on chondrogenic differentiation of cultured equine mesenchymal stem cells. Am J Vet Res. 2000;61:1003–1010. doi: 10.2460/ajvr.2000.61.1003. [DOI] [PubMed] [Google Scholar]
- 31.Winter A, Breit S, Parsch D, et al. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–429. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 33.Robledo MM, Hidalgo A, Lastres P, et al. Characterization of TGF-beta 1-binding proteins in human bone marrow stromal cells. Br J Haematol. 1996;93:507–514. doi: 10.1046/j.1365-2141.1996.d01-1698.x. [DOI] [PubMed] [Google Scholar]
- 34.Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 35.Barry FP, Boynton RE, Haynesworth S, et al. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem Biophys Res Commun. 1999;265:134–139. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- 36.Awad HA, Halvorsen YD, Gimble JM, et al. Effects of transforming growth factor beta1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Eng. 2003;9:1301–1312. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 37.Mackay AM, Beck SC, Murphy JM, et al. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 38.Ponticiello MS, Schinagl RM, Kadiyala S, et al. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res. 2000;52:246–255. doi: 10.1002/1097-4636(200011)52:2<246::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 39.Barry F, Boynton RE, Liu B, et al. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 40.Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54:1222–1232. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- 41.Wang DW, Fermor B, Gimble JM, et al. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184–191. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 42.Zhou S, Cui Z, Urban JP. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50:3915–3924. doi: 10.1002/art.20675. [DOI] [PubMed] [Google Scholar]
- 43.Kinner B, Spector M. Smooth muscle actin expression by human articular chondrocytes and their contraction of a collagen-glycosaminoglycan matrix in vitro. J Orthop Res. 2001;19:233–241. doi: 10.1016/S0736-0266(00)00081-4. [DOI] [PubMed] [Google Scholar]
- 44.Kinner B, Zaleskas JM, Spector M. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res. 2002;278:72–83. doi: 10.1006/excr.2002.5561. [DOI] [PubMed] [Google Scholar]
- 45.Zaleskas JM, Kinner B, Freyman TM, et al. Growth factor regulation of smooth muscle actin expression and contraction of human articular chondrocytes and meniscal cells in a collagen-GAG matrix. Exp Cell Res. 2001;270:21–31. doi: 10.1006/excr.2001.5325. [DOI] [PubMed] [Google Scholar]
- 46.Vickers SM, Squitieri LS, Spector M. Effects of cross-linking type II collagen-GAG scaffolds on chondrogenesis in vitro: dynamic pore reduction promotes cartilage formation. Tissue Eng. 2006;12:1345–1355. doi: 10.1089/ten.2006.12.1345. [DOI] [PubMed] [Google Scholar]
- 47.Veilleux N, Spector M. Effects of FGF-2 and IGF-1 on adult canine articular chondrocytes in type II collagen-glycosaminoglycan scaffolds in vitro. Osteoarthritis Cartilage. 2005;13:278–286. doi: 10.1016/j.joca.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Shirasawa S, Sekiya I, Sakaguchi Y, et al. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97:84–97. doi: 10.1002/jcb.20546. [DOI] [PubMed] [Google Scholar]
- 49.Muraglia A, Corsi A, Riminucci M, et al. Formation of a chondro-osseous rudiment in micromass cultures of human bone-marrow stromal cells. J Cell Sci. 2003;116:2949–2955. doi: 10.1242/jcs.00527. [DOI] [PubMed] [Google Scholar]


