Abstract
Gastric reflexes are mediated mainly by vago-vagal reflex circuits in the caudal medulla. Despite the fact that brainstem vago-vagal circuitry remains intact after spinal cord injury (SCI), patients with SCI at the cervical level most often present gastric stasis with an increased risk of reflux and aspiration of gastric contents. Using a miniature strain gauge sutured to the gastric surface; we tested gastric motility and reflexive gastric relaxation following oesophageal distension (oesophageal-gastric relaxation reflex) in animals 3 days after a severe spinal contusion at either the third or ninth thoracic spinal segment (acute T3- or T9 SCI, respectively). Both basal gastric motility and the oesophageal-gastric relaxation reflex were significantly diminished in animals with T3 SCI. Conversely, both basal gastric motility and the oesophageal-gastric relaxation reflex were not significantly reduced in T9 SCI animals compared to controls. The reduced gastric motility and oesophageal-gastric reflex in T3 SCI rats was not ameliorated by celiac sympathectomy. Our results show that gastric stasis following acute SCI is independent of altered spinal sympathetic input to the stomach caudal to the lesion. Our data suggest that SCI may alter the sensitivity of vagal reflex function, perhaps by interrupting ascending spinosolitary input to brainstem vagal nuclei.
Keywords: gastric motility, neurotrauma, receptive relaxation reflex, vagovagal reflex
Vago-vagal reflex circuits in the medulla mediate digestive processes originating in regions of the digestive tract extending from the oral cavity to the proximal third of the colon (reviewed in Ref.1) Unlike the extrinsic control of the intestines, gastric reflexes are dominated by vago-vagal reflex control.2–10 A simplified description of gastric vago-vagal neurocircuitry (Fig. 1) starts with general visceral afferent signals carried by the vagus nerve (cranial nerve X) to second-order neurons in the nucleus tractus solitarius (NTS). The main projections from the NTS to preganglionic neurons of the dorsal motor nucleus of the vagus (DMV) regulate gastric motility through the activation of competing and antagonistic cholinergic (excitatory) and non-adrenergic non-cholinergic (NANC; inhibitory) postganglionic projections.11 Vagally-mediated gastric relaxation can thus be achieved via a withdrawal of excitatory cholinergic tone, activation of an inhibitory NANC pathway or a combined effect upon both pathways.2–10,12,13
Figure 1.
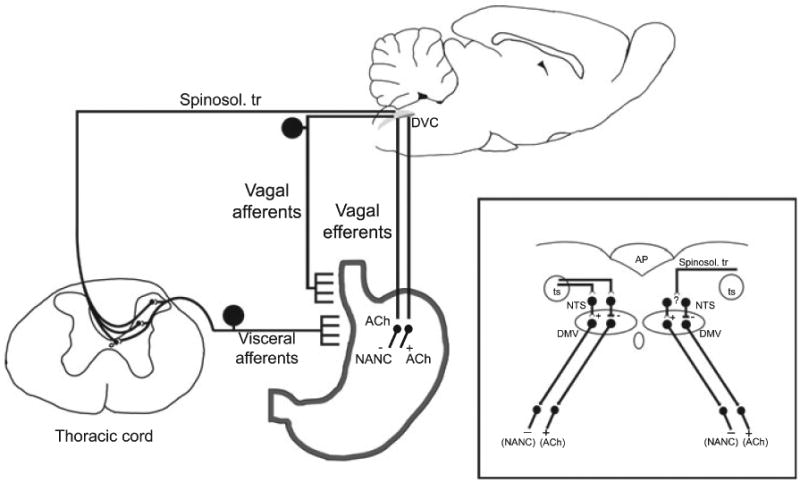
A schematic representation of both the vago-vagal parasympathetic neurocircuitry responsible for controlling gastric reflex function and the proposed spinosolitary neurocircuitry damaged by SCI. Afferent signals from the digestive tract (e.g. stretch receptors from the oesophageal wall) are relayed to the dorsal vagal complex (DVC) in the caudal medulla through the tractus solitarius (ts, inset). The DVC consists of second order neurons within the nucleus tractus solitarius (NTS, inset) that, in turn, make synaptic contacts with the dorsal motor nucleus of the vagus (DMV, inset) utilizing either GABA, glutamate or norepinephrine neurotransmission. Gastric relaxation can be achieved either by withdrawal of cholinergic (+ACh) tone or activation of non-adrenergic, non-cholinergic (-NANC) vagal neurotransmission to the gastric smooth muscle. Visceral afferent input to the spinal cord via the splanchnic nerve provides input to spinosolitary tract (Spinosol.tr) cells of origin in Lamina I, V, and X throughout the thoraco-lumbar spinal cord. The spinosolitary tract fibers course within the dorsal and dorsolateral funiculi (not shown) and enter the NTS dorsolaterally to the tractus solitarius.62
In patients with cervical spinal cord injury (SCI), dysphagia, gastrointestinal (GI) stasis, gastric hypersecretion, gastric dilation, nausea and emesis are observed immediately after injury.14,15 The high degree of post-SCI gastric disturbances requires aggressive total enteral nutrition through enteric feeding tubes, parenteral feeding or occasionally, more invasive GI intervention.16 However, a significant risk of vomiting and pulmonary aspiration accompanies severe and prolonged gastric stasis, often necessitating intensive management of the airway, and limits the appropriateness of some enteral feeding strategies.14,17 Furthermore, GI function may remain diminished long after SCI.18–26 In brief, acute failure to regulate gastric function is a major cause of post-SCI morbidity and mortality and may persist to affect long-term quality of life and nutritional status.27
In an experimental model, spinal cord transection rostral to the fourth thoracic segment inhibited gastric emptying and duodenal transit in rats fed a liquid diet.28 However, spinal transection does not accurately model most human SCI, whereby the spinal cord is contused rather than transected. Recently, a study of contusion injury of the lower thoracic spinal cord (tenth thoracic segment) also identified reduced gastric emptying as measured using the same liquid diet.29 In both studies, measures of gastric emptying did not distinguish gastric dysfunction into the two fundamentally different types of gastric paralysis: (i) flaccid, analogous to the relaxation of the stomach in a pre-emetic state; or (ii) spastic, akin to vagotomy. To overcome this shortcoming, we used miniaturized strain gauges to resolve measures of gastric motility and reflexive relaxation.30–32
The oesophageal-gastric relaxation reflex (aka. the receptive relaxation reflex) allows the stomach to receive ingested food by producing a relaxation in response to oesophageal distension during swallowing.33 Detailed strain gauge-based evidence shows that the oesophageal-gastric reflex is controlled exclusively by vagal neurocircuitry.13,34,35 The vagal afferent limb of the reflex consists of low threshold mechanoreceptors36 in the oesophagus that terminate within the sub nucleus centralis of the NTS (NTSc).34,35,37,38 Efferent projections from the NTSc to the DMV motoneurons lead to gastric relaxation.
Post-SCI gastric disturbances present an intriguing paradox since vago-vagal neurocircuits regulating gastric reflexes are located in the brainstem and remain intact following SCI. The aims of our study were to assess the gastric motility and gastric relaxation during the oesophageal-gastric reflex following acute, controlled, high level (third thoracic segment) or lower level (ninth thoracic segment) SCI; and, to determine the role of vago-vagal and sympathetic pathways in the observed post-injury reduction of gastric motility and relaxation in response to oesophageal distension.
Methods
All procedures followed National Institutes of Health guidelines for the use of animals in research and were performed under the approval of the Pennington Biomedical Research Center Animal Care and Use Committee. Experiments were performed on male Wistar rats (n = 40) initially weighing 175–200 g (Harlan, Indianapolis, IN, USA). Animals were maintained in a temperature-controlled room on a 12 : 12-h light-dark cycle with unrestricted access to food and water. Animals were pair-housed until surgery, after which animals were housed singly and observed daily.
SCI surgery and care
Rats were randomly assigned to one of the following groups: (i) a spinal laminectomy without SCI (sham); (ii) laminectomy with spinal contusion injury centered at the third thoracic segment (T3); and (iii) laminectomy with spinal contusion injury at T9. Animals were anesthetized with Nembutal (60 mg·kg−1, i.p., Abbott Laboratories, Chicago, IL, USA). Supplemental doses of anaesthetic were given as necessary to maintain a deep level of anaesthesia and topical 2% lidocaine was administered to all wound margins as a supplement to general anaesthesia. Prior to any surgical manipulation, the animal was administered buprenorphine (0.1 mg kg−1, i.p., Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA, USA) to alleviate post-operative pain, and antibiotics (Baytril, 2.5 mg kg−1 s.q., Bayer, Shawnee Mission KS) to reduce postsurgical infection. The spinal cord was exposed through a midline incision of the back at the appropriate level of the vertebral column and muscles were detached from the vertebrae. Subsequently, the T2 or T8 spinous process was removed with fine tipped rongeurs to expose the dura overlying the spinal cord surface (T3 or T9, respectively). Animals receiving a T3 or T9 spinal contusion injury (T3 SCI or T9 SCI, respectively) were placed in the clamps of the Infinite Horizon controlled impact device (Precision Systems and Instrumentation, LLC, Lexington, KY, USA) and a rapid 300 kDyne displacement of the cord and overlying dura was performed. Procedures for sham animals were the same except that the spinal cord and surrounding dura were not disturbed following laminectomy. Once the surgical procedure was complete, the muscle tissue was sutured and the skin closed with wound clips. Animals were placed in an incubation chamber maintained at 37 °C for recovery.
Chronic care of injured animals followed procedures described previously.39 Body weights were recorded once daily and laboratory chow was weighed in the morning to determine 24 h food intake. Animals with excessive waste chow that could not be recovered from the cage bottom were not included in food intake analysis. Two days following surgery, animals were fasted for 24 h with free access to water and the range of hindlimb movement was assessed during open-field locomotor testing.40
Gastrointestinal studies
Animals were deeply anesthetized with thiobutabarbitol (Inactin; Sigma, St Louis, MO, USA; 100–150 mg kg−1 IP) which does not affect brainstem autonomic function,41 intubated with a tracheal catheter and a laparotomy was made. In a subset of T3 SCI animals, the celiac-superior mesenteric ganglion complex was excised [celiac ganglionectomy (CGx)] prior to attaching the strain gauge to the stomach. Briefly, CGx was achieved by gently deflecting the intestines laterally to expose the abdominal aorta at the level of the superior mesenteric and celiac arteries. Using blunt dissection with the aid of a surgical dissecting scope, the unpaired celiac ganglion was isolated from the surrounding connective tissue and then excised. The retracted viscera were returned to the original location and the instrumentation of the stomach proceeded as described below. In control animals the ganglion was visually confirmed and no further actions were performed. Next, the stomach was isolated within the abdominal cavity and placed upon a gauze pad that had been moistened with prewarmed saline. A 6 × 8 mm encapsulated miniature strain gauge (RB Products, Minneapolis, MN, USA) was aligned with the circular smooth muscle fibers and sutured to the region of the gastric corpus, the abdominal incision and the wound margins were closed with suture. The strain gauge signal was amplified (QuantaMetrics EXP CLSG-2, Newton, PA) and recorded on a Grass polygraph (Model 79, Quincy, MA, USA) or a PC using Axotape software (Axon Instruments, Union City, CA, USA). In order to eliminate a potential confounding variable, any animals that still retained food within the stomach after the 24 h fast were excluded from the study.
Oesophageal distension balloons were fabricated from biomedical silicon tubing (ID: 0.76 mm; OD: 1.7 mm) that had been sealed at one end with silicone dental impression material and stretched so as to distend to 3 mm in diameter over a 5 mm length when filled with 100 μL of water. The oesophageal balloon was placed orally in the oesophagus of the rat approximately 1 cm above the oesophageal hiatus.
Animals were placed in a stereotaxic frame, and rectal temperature was monitored with a thermometer and maintained at 37 ± 1 °C using a feedback-controlled warming pad (TCAT 2LV, Physitemp Instruments, Clifton, NJ, USA). Following 1 h of stabilization, a 10 min period of motility was recorded on the polygraph prior to any experimental manipulation in order to verify the consistency of the motility trace over time. Afterward, the oesophageal distension balloon was filled with 100 μL of water for 1 min, and then released. Three oesophageal distensions were produced, each separated by a 30 min rest period.
Histology
At the conclusion of testing, animals were sacrificed by transcardial perfusion with a bolus of lidocaine and heparin followed immediately by cold isotonic phosphate buffered saline followed by 4% paraformaldehyde. The spinal cord lesion site was removed and postfixed overnight in refrigerated 4% paraformaldehyde, and then cryoprotected by a subsequent overnight immersion in a 30% sucrose solution before cutting frontally at 40 μm thickness (Microm HM560, Richard-Allan Scientific, Kalamazoo MI, USA). Tissues were stained with luxol fast blue (LFB) and counter-stained with cresyl violet to show myelinated fibres and cell bodies, respectively.
Stained slides were digitally imaged on a Nikon Labophot-2 light microscope, imported into Adobe Photoshop and contrast digitally enhanced to allow identification of spared white matter. The proximity of the T3 lesion centre to the cervical enlargement precluded an appropriate determination of spinal cord cross-sectional area in tissue rostral to the injury (as described in Ref.42,43). Therefore, the cross-sectional area of the intact spinal cords at T3 and T9 of comparably sized animals were determined for normalization purposes. Luxol fast blue-stained myelin in injured tissue was then expressed as a percent of the total cross-sectional area as would be predicted by the intact tissue.
Data collection and analysis
Locomotor testing was performed by scoring the range and frequency of hindlimb joint movement and coordination with the widely used Basso, Beattie, Bresnahan (BBB) locomotor rating scale.40 The BBB scale rates locomotion on an ordinal nonlinear 21-point rating from 0 (no hindlimb movement) to 21 (normal locomotion). This test assessed the extent of contusion injury, showing deficits and partial recovery. Mean energy intake (MEI) represents the daily average of kilocalories consumed per 100 g of body weight for each 2 day period of monitored feeding. Baseline gastric motility was calculated from raw polygraph traces record using the formula originally reported by Ormsbee and Bass.44 Briefly, presuming that a 0 mV signal from the strain gauge was indicative of no gastric motility, sinusoidal signals with amplitude from 6–13 mV were ranked as a 1, signals from 14–25 mV were ranked as a 2, 26–50 mV as a 4 and signals greater than 50 mV were ranked as an 8. Basal motility index represents the sum total of ranked motility peaks over the 10 min testing period. Gastric relaxation evoked by distension of the oesophageal balloon was measured in triplicate by determining the change in signal from the strain gauge before and during the 1 min inflation of the balloon.
Data is presented as mean ± standard error. Differences between BBB locomotor score, basal gastric motility and gastroinhibition for the subject groups were evaluated by a one-way anova using spss for Windows (SPSS Inc., Chicago, IL, USA), followed by the Dunnett post-hoc test. Significance was set at P < 0.05.
Results
A group of three animals that had received a spinal contusion injury were excluded from final analysis on the basis that their stomachs contained chow at the time of experimentation despite the 24 h fast.
Histological and behavioural assessment of SCI
The mean area of white matter at the T3 spinal segment of sham animals (n = 10) was 3.02 ± 0.05 mm2. Since no significant differences were observed in the mean area of white matter of rats that were ultimately ganglionectomized (n = 9) or sham ganglionectomized (n = 8), the scores were combined for analysis. The area of white matter at the lesion epicentre of T3 SCI rats (n = 17) was 0.64 ± 0.04 mm2. The mean area of white matter at the T9 spinal segment of sham animals was 3.82 ± 0.12 mm2 whereas white matter at the lesion epicentre of T9 SCI rats (n = 10) was 0.87 ± 0.14 mm2. When white matter is expressed as percentage of spared tissue, the lesion epicentre of T3 SCI rats retained ∼16% of white mater and the lesion centre of T9 SCI rats retained ∼18% of white matter (Fig. 2).
Figure 2.
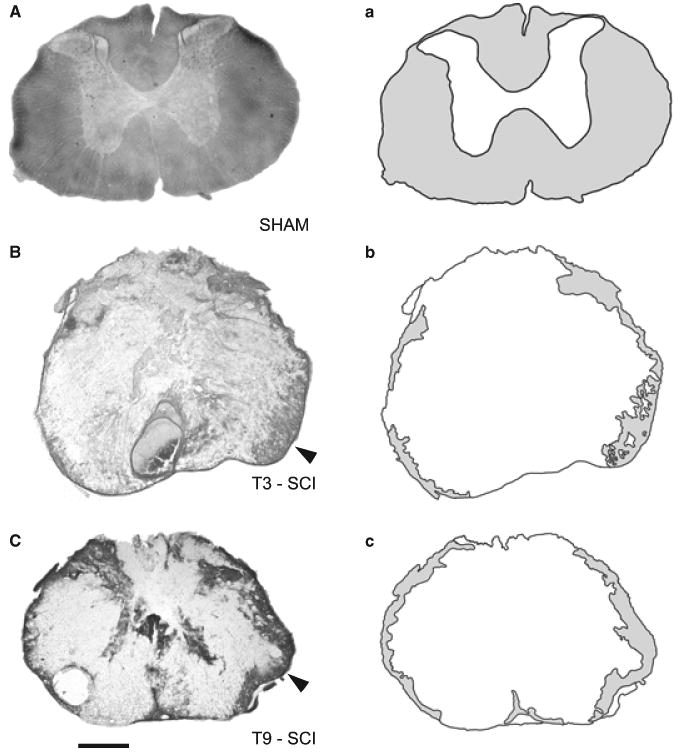
Photomicrographs (left column) and camera lucida renderings (right column) showing (A) the uninjured T3 spinal cord of a surgical control (sham), (B) the lesion epicentre of a T3 spinal contusion injury (T3 SCI) and (C) the lesion epicentre of a T9 contusion spinal injury (T9 SCI). In both contusion injuries, luxol fast blue staining of intact white matter was limited to the peripheral rim of the tissue section with the majority of tissue found in the ventrolateral funiculus (arrow, white mater represented as light gray shaded regions in a, b, and c). The majority of the remaining tissue section consisted of cellular debris from white and gray matter and post-traumatic deposits of red blood cells. All tissue was collected from rats 3 days after surgery. Calibration 200 μm.
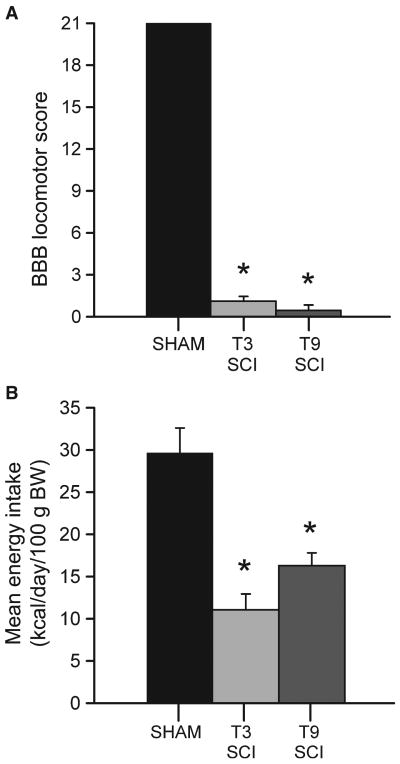
Locomotor function was not impaired following surgery in sham animals (n = 10) since all sham animals scored the maximum 21 points on the BBB scale (Fig. 3A). As no significant differences were observed in the BBB score of rats that were ultimately ganglionectomized (n = 9) or sham ganglionectomized (n = 8), the scores were combined for analysis. The BBB score of the combined cohort (17 total rats) of T3 SCI animals (1.11 ± 0.35 points) was not statistically different from T9 SCI rats (n = 10) which scored 0.45 ± 0.39 points after 3 days recovery. Comparisons between sham animals and both injury-groups were indicative of significantly compromised hind-limb function 3 days after surgery (F(2,35) = 1014.991, P < 0.05). These post-injury BBB scores indicate that movement was limited to single joint, or no hind-limb joint movement.
Figure 3.
(A) Graphic summary of the mean locomotor rating scores (±SEM) of rats tested 3 days following surgery. Hind-limb function was significantly compromised in both spinal cord injury groups with no differences between injury groups. (*P < 0.05). (B) Mean energy intake of standard laboratory chow, calculated as kcal consumed/day/100 g of body weight (BW) for post-operative food intake is significantly lower in all spinal contusion injury rats compared to controls (*P < 0.05) but did not display any significant difference between levels of injury.
SCI decreases spontaneous oral intake of food
The MEI for control animals (n = 6) was 29.64 ± 2.96 kcal day−1 100 g body weight−1. The MEI of T3 SCI and T9 SCI animals was significantly lower than controls (F(2,29) = 16.65, P < 0.05). In fact, T3 SCI animals (n = 16) had an MEI of 11.07 ± 1.87 kcal - day−1 100 g body weight−1, while T9 SCI rats had an MEI was 16.30 ± 1.51 (Fig. 3B). These data indicate that SCI groups were ingesting similar quantities of food and were not significantly different from each other with regard to overall energy balance.
SCI decreases gastric reflex function
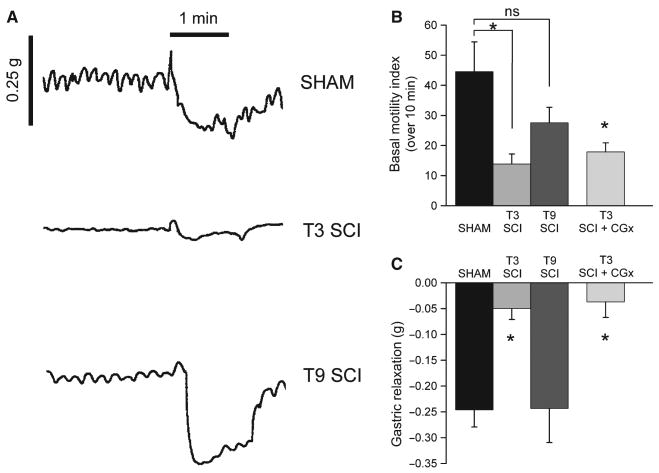
The gastric motility index, as measured by strain gauge output, in sham animals 3 days after surgery (n = 10) was 49.7 ± 13.1 (Fig. 4A,B). The motility index was significantly reduced (F(3,33) = 4.181) in T3 SCI rats (n = 8; motility index = 13.9 ± 3.3, P < 0.05; Fig. 4B). The motility index in T9 SCI rats (n = 10) displayed a non-significant decrease to 27.6 ± 5.1 (P > 0.05 vs control; Fig. 4B). In the same groups of animals, oesophageal distension in sham animals induced a gastric relaxation of −0.26 ± 0.05 g (Fig. 4A,C). The gastric relaxation induced by oesophageal distension was significantly reduced in rats that had SCI at T3 (−0.06 ± 0.04 g; F(3,33) = 5.568, P < 0.05; Fig. 4C) while the oesophageal-gastric relaxation reflex of T9 SCI animals was −0.24 ± 0.07 g (P > 0.05 vs control; Fig. 4C). These data indicate that T3 SCI produces a significant reduction in both basal gastric motility and the gastric relaxation following oesophageal distension.
Figure 4.
(A) Representative original polygraph records of gastric motility and tone from fasted animals 3 days after surgery. Trace includes 2 min of basal gastric motility prior to 1 min of balloon distension of the oesophagus (denoted by horizontal bar) followed by 1 min of recovery. Note that oesophageal distension reduced gastric motility and elicited gastric relaxation in sham as well as T9 SCI rats. Conversely, T3 SCI had no gastric motility and oesophageal distension did not induce gastric relaxation. (B) Mean basal motility index prior to oesophageal stimulation. Basal motility index was significantly reduced in T3 SCI, but not in T9 SCI, when compared to controls (sham). (C) Gastric relaxation reflex during balloon distension of the oesophagus. Gastric relaxation following oesophageal distension was significantly impaired in T3-injured rats when compared to both sham and T9 SCI rats. For both measures (basal motility and gastric relaxation), celiac ganglionectomy did not ameliorate the post-injury gastric dysreflexia in T3 SCI rats (*P < 0.05).
Celiac ganglionectomy does not relieve post-SCI gastroparesis
The motility index of T3 SCI rats with an intact celiac ganglion was 13.9 ± 3.3 (n = 8) and T3 SCI rats with CGx was 17.9 ± 3.0 (n = 9). T3 SCI rats with CGx remained significantly lower than uninjured controls (F(3,33) = 4.181, P < 0.05 T3 SCI + CGx vs control; Fig. 4B). Similarly, gastric relaxation during the oesophageal-gastric relaxation reflex of T3 SCI rats with an intact celiac ganglion was −0.05 ± 0.04 g while in T3 SCI animals + CGx the gastric relaxation was −0.04 ± 0.03 g (P < 0.05, Fig. 4C). These data indicate that the significant reduction in both basal gastric motility and the gastric relaxation induced by esophageal distension in T3 SCI rats is not sympathetically mediated.
Discussion
In the present study we demonstrate that (i) high thoracic (T3) SCI results in an impairment of gastric motility; (ii) T3 SCI disrupts the oesophageal-gastric relaxation reflex; (iii) SCI at T9 does not disrupt motility nor the oesophageal-gastric relaxation reflex and (iv) the loss of gastric motility and the gastric relaxation induced by oesophageal distension after T3 SCI is likely due to alterations in vagally-mediated reflex function, not to sympathetically-mediated impairment. The disruption of the oesophageal-gastric relaxation reflex and the absence of a post-SCI sympathetic component to gastric stasis suggest SCI may alter the sensitivity (gain) of brainstem vagal neurocircuitry. Damage to ascending spinal-solitary afferents may be one mechanism involved in this impairment.
Our conclusions are based upon several lines of evidence. From an anatomical perspective, the T3 SCI injury produced in these experiments can be considered functionally similar to complete T3 spinal cord transection in that very few LFB-labelled fibres are observed traversing the perimeter of the T3 SCI lesion epicentre. Within the 3-day post-injury time period, both T3- and T9 SCI animals had similar damage to both gray and white matter at the lesion epicentre and similar deficits in the extent of hind limb functionality as measured by open field locomotion.40 Though we observed nearly identical anatomical and locomotor deficits following T3 and T9 SCI, impaired gastric motility and reflex relaxation following oesophageal distension was observed only in T3 SCI. These data suggest that neither hind limb paralysis nor the cascade of post-injury inflammatory processes associated with severe traumatic injury (see45) are responsible for the observed impairment of gastric motility and reflex relaxation.
Our overnight feeding data demonstrate that SCI animals were spontaneously feeding, although the caloric intake was significantly reduced. There were no differences in caloric intake between T3 and T9 SCI rats, indicating that alterations in gastric reflexes in T3 SCI animals were not a result of reduced food consumption over the 2 days prior to testing. Furthermore, we observed several instances in which the stomach of overnight fasted SCI rats still retained a large volume of laboratory chow. Conversely, control animals that had been fasted overnight did not retain any laboratory chow within their stomachs. The reduced gastric emptying of awake rats reported in other studies28,29 coupled with our observations of the gastric retention of chow 24 h after fasting indicates that GI functions were impaired in our 3 day SCI animals prior to anaesthesia and provide a further validation for our model and methods.
It is well known that the stomach is under dual control by the sympathetic and parasympathetic nervous systems, though the vagal parasympathetic control of the stomach is widely regarded as imparting functional dominance.1 However, SCI produces an immediate loss of tonic, descending control of sympathetic preganglionic neurons in the intermediolateral cell column (IML) of the thoracolumbar spinal cord 46 and basal sympathetic activity is diminished after SCI in unanaesthetized rats.47 Sympathetic preganglionic neurons in the T6-T9 spinal segments provide cholinergic drive to neurons in the celiac ganglion. These postganglionic neurons, in turn, supply adrenergic (inhibitory) input to the stomach, the magnitude of which is directly proportional to the level of vagal input.48 It is possible, thus, to hypothesize that the loss of spinal function below the T3 injury induces a shift in the sympathetic neural control of gastric motility, thereby enhancing sympathetic contribution. However, we show that elimination of the sympathetic drive by ganglionectomy49 does not alter the gastroparesis induced by T3 SCI. Similar conclusions were also drawn by Gondim and colleagues50 following pharmacological blockade of autonomic transmission in 1 day spinalized rats.
Spinal cord injury impacts cardiovascular reflex control51 and noxious visceral stimuli after SCI can trigger paroxysmal hypertension.52–54 In particular, Leal and colleagues report that paroxysmal hypertension occurs as early as 1 day post-SCI in response to noxious gastric distension.54 Heart rate and mean arterial pressure were not monitored in our studies, and oesophageal distension may have triggered autonomic dysreflexia, leading to reduced gastric motility. However, the oesophageal distension stimulus we use has been reported to exert a transmural oesophageal pressure of 14–18 mmHg34 which is a pressure range sufficient to stimulate low-threshold vagal mechanoreceptors but not spinal nociceptors.55–57 Due to the low threshold distension used in this study, we do not feel that oesophageal distension-induced hypertension is likely to occur in our SCI model, though this requires further investigation.
Since gastric reflexes were evaluated in anesthetized animals, one could expect different effects on gastric reflexes through an interaction of anaesthesia and overall hemodynamic status.58 In principle, thiobutabarbitol does not alter brainstem autonomic function, as measured by cardiovascular function,41 though there may be differential effects upon other autonomic reflexes that remain to be identified. However, the post-SCI deficits in gastric emptying of a phenol red solution are collected in unanaesthetized animals28,29 and imply a reduction in motility that is in agreement with our findings. Furthermore, our reported reduction in food intake also indirectly supports our observations, since a reduction in gastric motility is likely to trigger earlier (volumetric) satiety signals in the SCI rats.59
Since vago-vagal neurocircuitry remains intact after SCI, but we still observe a remarkable impairment of vagally-mediated gastric reflexive functions, our data, and that reported previously,28,29 suggest that a spinosolitary pathway was interrupted by SCI. Gastrointestinal afferent fibres have been reported in splanchnic nerves.60 The majority of the cell bodies of visceral afferent fibres reside in thoracolumbar (T4-L2) ganglia61 and visceral peripheral input within the sympathetic nerves may converge upon lamina I and V of the dorsal horn and provide spinal input to the brainstem.62 Furthermore, viscero-spinal inputs to the NTS are relayed through identified spinosolitary pathways.63 These second-order neurons originate throughout the length of the spinal cord, with clusters in Lamina I, V, X but also intermingled with preganglionic neurons in the intermediolateral cell column.64 It is possible that injury at T3, or above, interrupts the majority of the ascending pathway provided by these second-order cells. Indeed, in our SCI animals we observed a central core lesion that pervaded extensively into the surrounding white matter whereby the dorsal and dorsolateral funiculi where spinosolitary fibres mainly ascend,64,65 in particular, were extensively damaged. Our observations of reduced basal gastric motility after SCI suggest a relevant role of these spinosolitary afferent fibres, though changes in the overall gain of vagal gastric neurocircuitry following the loss of spinosolitary input remains to be determined.
In conclusion, we have shown a significant impairment of gastric reflexive functions in a rat model of SCI. To the best of our knowledge, there are few explanations available that specifically and mechanistically connect traumatic spinal insults with the post-injury alterations in receptive relaxation, gastric motility and emptying. We would like to propose that impairment of a spinosolitary pathway, as seen in our model of high thoracic SCI, is a causative factor in gastric stasis observed in many SCI patients. The recent observation that spinal cord transection increases GI permeability to electrolytes and water66 further highlights the magnitude of the GI pathophysiology after SCI. In addition to the profound effects upon the delivery of chyme to the duodenum, fluid and electrolyte balance, SCI may alter extraction of nutrients in the small intestine. Restoration of function to these compromised mechanisms is of immediate clinical relevance after SCI as the recovery of normal gastric motility, transit, and absorption is essential for appropriate long-term health and nutritional management strategies.
Acknowledgments
The authors extend a special note of thanks to Drs R. Alberto Travagli, Kirsteen Browning and Stefany Primeaux for their commentary during the preparation of the manuscript. We thank Emily Creekmore for her assistance with data collection and analysis. We thank Dr Barry Robert, Cynthia Kloster, Hsin (Frank) Hsu and Shannon Sterba for their technical assistance. Grants: This work was supported by NINDS grant #49177 and the Pennington Foundation.
References
- 1.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahamsson H. Studies on the inhibitory nervous control of gastric motility. Acta Physiol Scand Suppl. 1973;390:1–38. [PubMed] [Google Scholar]
- 3.Abrahamsson H, Jansson G. Elicitation of reflex vagal relaxation of the stomach from pharynx and esophagus in the cat. Acta Physiol Scand. 1969;77:172–8. doi: 10.1111/j.1748-1716.1969.tb04561.x. [DOI] [PubMed] [Google Scholar]
- 4.McCann MJ, Rogers RC. Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. J Physiol. 1992;453:401–11. doi: 10.1113/jphysiol.1992.sp019235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCann MJ, Rogers RC. Functional and Chemical Anatomy of a Gastric Vago-Vagal Reflex. In: Wingate DL, editor. Innervation of the Gut: Pathophysiological Implications. Boca Raton: CRC Press; 1994. pp. 81–92. [Google Scholar]
- 6.Gillis RA, Quest JA, Pagani FD, Norman WP. Handbook of Physiology. The Gastrointestinal System. Motility and Circulation. Bethesda: American Physiological Society; 1989. Control centers in the central nervous system for regulating gastrointestinal motility; pp. 621–83. [Google Scholar]
- 7.Krowicki ZK, Sivarao DV, Abrahams TP, Hornby PJ. Excitation of dorsal motor vagal neurons evokes non-nicotinic receptor-mediated gastric relaxation. J Auton Nerv Syst. 1999;77:83–9. [PubMed] [Google Scholar]
- 8.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997;504(Pt 2):479–88. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansson G. Vago-vagal reflex relaxation of the stomach in the cat. Acta Physiol Scand. 1969;75:245–52. doi: 10.1111/j.1748-1716.1969.tb04376.x. [DOI] [PubMed] [Google Scholar]
- 10.Rogers RC, McTigue DM, Hermann GE. Vagal control of digestion: modulation by central neural and peripheral endocrine factors. Neurosci Biobehav Rev. 1996;20:57–66. doi: 10.1016/0149-7634(95)00040-l. [DOI] [PubMed] [Google Scholar]
- 11.Travagli RA, Browning KN, Gillis RA. Studies of the dorsal motor nucleus of the vagus, a major CNS center for controlling vagal outflow to the upper gastrointestinal tract. In: Quigley EM, Pfeiffer RF, editors. Neurogastroenterology. Philadelphia: Butterworth-Heinemann; 2004. [Google Scholar]
- 12.Takahashi T, Owyang C. Vagal control of nitric oxide and vasoactive intestinal polypeptide release in the regulation of gastric relaxation in rat. J Physiol. 1995;484(Pt 2):481–92. doi: 10.1113/jphysiol.1995.sp020680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermann GE, Travagli RA, Rogers RC. Esophageal-gastric relaxation reflex in rat: dual control of peripheral nitrergic and cholinergic transmission. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1570–6. doi: 10.1152/ajpregu.00717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirshblum SC, Groah SL, McKinley WO, Gittler MS, Stiens SA. Spinal cord injury medicine. 1. Etiology, classification, and acute medical management. Arch Phys Med Rehabil. 2002;3(Suppl 1):S50–8. doi: 10.1053/apmr.2002.32156. [DOI] [PubMed] [Google Scholar]
- 15.Wolf C, Meiners TH. Dysphagia in patients with acute cervical spinal cord injury. Spinal Cord. 2003;41:347–53. doi: 10.1038/sj.sc.3101440. [DOI] [PubMed] [Google Scholar]
- 16.Dwyer KM, Watts DD, Thurber JS, Benoit RS, Fakhry SM. Percutaneous endoscopic gastrostomy: the preferred method of elective feeding tube placement in trauma patients. J Trauma. 2002;52:26–32. doi: 10.1097/00005373-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Oakley PA, Coleman NA, Morrison PJ. Intensive care of the trauma patient. Resuscitation. 2001;48:37–46. doi: 10.1016/s0300-9572(00)00316-6. [DOI] [PubMed] [Google Scholar]
- 18.Cosman BC, Stone JM, Perkash I. Gastrointestinal complications of chronic spinal cord injury. J Am Paraplegia Soc. 1991;14:175–81. doi: 10.1080/01952307.1991.11735850. [DOI] [PubMed] [Google Scholar]
- 19.Berlly MH, Wilmot CB. Acute abdominal emergencies during the first four weeks after spinal cord injury. Arch Phys Med Rehabil. 1984;65:687–90. [PubMed] [Google Scholar]
- 20.Fealey RD, Szurszewski JH, Merritt JL, DiMagno EP. Effect of traumatic spinal cord transection on human upper gastrointestinal motility and gastric emptying. Gastroenterology. 1984;87:69–75. [PubMed] [Google Scholar]
- 21.Kao CH, Ho YJ, Changlai SP, Ding HJ. Gastric emptying in spinal cord injury patients. Dig Dis Sci. 1999;44:1512–5. doi: 10.1023/a:1026690305537. [DOI] [PubMed] [Google Scholar]
- 22.Kewalramani LS. Neurogenic gastroduodenal ulceration and bleeding associated with spinal cord injuries. J Trauma. 1979;19:259–65. doi: 10.1097/00005373-197904000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Nino-Murcia M, Friedland GW. Functional abnormalities of the gastrointestinal tract in patients with spinal cord injuries: Evaluation with imaging procedures. Am J Roentgenol. 1991;158:279–81. doi: 10.2214/ajr.158.2.1729781. [DOI] [PubMed] [Google Scholar]
- 24.Rajendran SK, Reiser JR, Bauman W, Zhang RL, Gordon SK, Korsten MA. Gastrointestinal transit after spinal cord injury: Effect of cisapride. Am J Gastroenterol. 1992;87:1614–7. [PubMed] [Google Scholar]
- 25.Segal JL, Milne N, Brunnemann SR. Gastric emptying is impaired in patients with spinal cord injury. Am J Gastroenterol. 1995;90:466–70. [PubMed] [Google Scholar]
- 26.Stinneford JG, Keshavarzian A, Nemchausky BA, Doria MI, Durkin M. Esophagitis and esophageal motor abnormalities in patients with chronic spinal cord injuries. Paraplegia. 1993;31:384–92. doi: 10.1038/sc.1993.64. [DOI] [PubMed] [Google Scholar]
- 27.Primeaux SD, Tong M, Holmes GM. Effects of chronic spinal cord injury on body weight and body composition in rats fed a standard chow diet. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1102–9. doi: 10.1152/ajpregu.00224.2007. [DOI] [PubMed] [Google Scholar]
- 28.Gondim FA, de Alencar HM, Rodrigues M, da Graca L, dos Santos R, Rola FH. Complete cervical or thoracic spinal cord transections delay gastric emptying and gastrointestinal transit of liquid in awake rats. Spinal Cord. 1999;37:793–9. doi: 10.1038/sj.sc.3100923. [DOI] [PubMed] [Google Scholar]
- 29.Kabatas S, Yu D, He XD, et al. Neural and anatomical abnormalities of the gastrointestinal system resulting from contusion spinal cord injury. Neuroscience. 2008;154:1627–38. doi: 10.1016/j.neuroscience.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 30.Krantis A, Mattar K, Glasgow I. Rat gastroduodenal motility in vivo: interaction of GABA and VIP in control of spontaneous relaxations. Am J Physiol Gastrointest Liver Physiol. 1998;275:G897–903. doi: 10.1152/ajpgi.1998.275.5.G897. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda H, Tsuchida D, Koda K, Miyazaki M, Pappas TN, Takahashi T. Impaired gastric motor activity after abdominal surgery in rats. Neurogastroenterol Motil. 2005;17:245–50. doi: 10.1111/j.1365-2982.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- 32.Hermann GE, Rogers RC. Tumor necrosis factor-α in the dorsal vagal complex suppresses gastric motility. Neuroimmunomodulation. 1995;2:74–81. doi: 10.1159/000096874. [DOI] [PubMed] [Google Scholar]
- 33.Canon WC, Lieb CW. The receptive relaxation of the stomach. Am J Physiol. 1911;29:267–73. [Google Scholar]
- 34.Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J Physiol. 1999;514(Pt 2):369–83. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol. 2003;285:R479–89. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sengupta JN. An overview of esophageal sensory receptors. Am J Med. 2000;108(Suppl 4a):87S–9S. doi: 10.1016/s0002-9343(99)00344-7. [DOI] [PubMed] [Google Scholar]
- 37.Lu WY, Bieger D. Vagovagal reflex motility patterns of the rat esophagus. Am J Physiol Regul Integr Comp Physiol. 1998;274(5 Pt 2):R1425–35. doi: 10.1152/ajpregu.1998.274.5.R1425. [DOI] [PubMed] [Google Scholar]
- 38.Lu WY, Bieger D. Vagal afferent transmission in the NTS mediating reflex responses of the rat esophagus. Am J Physiol Regul Integr Comp Physiol. 1998;274(5 Pt 2):R1436–45. doi: 10.1152/ajpregu.1998.274.5.R1436. [DOI] [PubMed] [Google Scholar]
- 39.Holmes GM, Van Meter MJ, Bresnahan JC, Beattie MS. Serotonergic fiber sprouting to external anal sphincter motoneurons after spinal cord contusion. Exp Neurol. 2005;193:29–42. doi: 10.1016/j.expneurol.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 41.Buelke-Sam J, Holson JF, Bazare JJ, Young JF. Comparative stability of physiological parameters during sustained anesthesia in rats. Lab Anim Sci. 1978;28:157–62. [PubMed] [Google Scholar]
- 42.Rabchevsky AG, Fugaccia I, Turner AF, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Exp Neurol. 2000;164:280–91. doi: 10.1006/exnr.2000.7399. [DOI] [PubMed] [Google Scholar]
- 43.Rabchevsky AG, Fugaccia I, Fletcher-Turner A, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances tissue sparing and functional recovery following moderate spinal cord injury. J Neurotrauma. 1999;16:817–30. doi: 10.1089/neu.1999.16.817. [DOI] [PubMed] [Google Scholar]
- 44.Ormsbee HS, Bass P. Gastroduodenal motor gradients in the dog after pyloroplasty. Am J Physiol. 1976;230:389–97. doi: 10.1152/ajplegacy.1976.230.2.389. [DOI] [PubMed] [Google Scholar]
- 45.Bramlett HM, Dietrich WD. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–41. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- 46.Zagon A, Smith AD. Monosynaptic projections from the rostral ventrolateral medulla oblongata to identified sympathetic preganglionic neurons. Neuroscience. 1993;54:729–43. doi: 10.1016/0306-4522(93)90243-9. [DOI] [PubMed] [Google Scholar]
- 47.Maiorov DN, Weaver LC, Krassioukov AV. Relationship between sympathetic activity and aterial pressure in conscious spinal rats. Am J Physiol Heart Circ Physiol. 1997;272:H625–31. doi: 10.1152/ajpheart.1997.272.2.H625. [DOI] [PubMed] [Google Scholar]
- 48.Roman C, Gonella J. Extrinsic control of digestive tract motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 2. New York: Raven Press; 1987. pp. 507–53. [Google Scholar]
- 49.Yamada M, Terayama R, Bando Y, Kasai S, Yoshida S. Regeneration of the abdominal postganglionic sympathetic system. Neurosci Res. 2006;54:261–8. doi: 10.1016/j.neures.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Gondim FA, Rodrigues CL, da Graca JR, et al. Neural mechanisms involved in the delay of gastric emptying and gastrointestinal transit of liquid after thoracic spinal cord transection in awake rats. Auton Neurosci. 2001;87:52–8. doi: 10.1016/s1566-0702(00)00261-7. [DOI] [PubMed] [Google Scholar]
- 51.Weaver LC, Marsh DR, Gris D, Brown A, Dekaban GA. Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention. Prog Brain Res. 2006;152:245–63. doi: 10.1016/S0079-6123(05)52016-8. [DOI] [PubMed] [Google Scholar]
- 52.Rabchevsky AG. Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury. Prog Brain Res. 2005;152:265–74. doi: 10.1016/S0079-6123(05)52017-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laird AS, Carrive P, Waite PM. Cardiovascular and temperature changes in spinal cord injured rats at rest and during autonomic dysreflexia. J Physiol. 2006;577(Pt 2):539–48. doi: 10.1113/jphysiol.2006.116301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leal PR, Cruz PR, Lopes AC, Jr, et al. A new model of autonomic dysreflexia induced by gastric distension in the spinal cord-transected rat. Auton Neurosci. 2008;141:66–72. doi: 10.1016/j.autneu.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol. 1989;61:1001–10. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- 56.Euchner-Wamser I, Sengupta JN, Gebhart GF, Meller ST. Characterization of responses of T2-T4 spinal cord neurons to esophageal distension in the rat. J Neurophysiol. 1993;69:868–83. doi: 10.1152/jn.1993.69.3.868. [DOI] [PubMed] [Google Scholar]
- 57.Qin C, Chandler MJ, Foreman RD. Afferent pathways and responses of T3-T4 spinal neurons to cervical and thoracic esophageal distensions in rats. Auton Neurosci. 2003;109:10–20. doi: 10.1016/j.autneu.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Leal PR, Lima RC, Jr, Lopes AC, Jr, et al. Haemodynamic changes after spinal cord transection are anaesthetic agent dependent. Auton Autacoid Pharmacol. 2007;27:167–71. doi: 10.1111/j.1474-8673.2007.00408.x. [DOI] [PubMed] [Google Scholar]
- 59.Powley TL, Phillips RJ. Gastric satiation is volumetric, intestinal satiation is nutritive. Physiol Behav. 2004;82:69–74. doi: 10.1016/j.physbeh.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 60.Ozaki N, Gebhart GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1449–59. doi: 10.1152/ajpgi.2001.281.6.G1449. [DOI] [PubMed] [Google Scholar]
- 61.Neuhuber W, Niederle B. Spinal ganglion cells innervating the stomach of the rat as demonstrated by somatopetal transport of horseradish peroxidase (HRP) Anat Embryol (Berl) 1979;155:355–62. doi: 10.1007/BF00317648. [DOI] [PubMed] [Google Scholar]
- 62.Renehan WE, Zhang X, Beierwaltes WH, Fogel R. Neurons in the dorsal motor nucleus of the vagus may integrate vagal and spinal information from the GI tract. Am J Physiol Gastrointest Liver Physiol. 1995;268:G780–90. doi: 10.1152/ajpgi.1995.268.5.G780. [DOI] [PubMed] [Google Scholar]
- 63.Menetrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol. 1987;255:439–50. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- 64.Menetrey D, de Pommery J. Origins of Spinal Ascending Pathways that Reach Central Areas Involved in Visceroception and Visceronociception in the Rat. Eur J Neurosci. 1991;3:249–59. doi: 10.1111/j.1460-9568.1991.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 65.Gamboa-Esteves FO, Tavares I, Almeida A, Batten TF, McWilliam PN, Lima D. Projection sites of superficial and deep spinal dorsal horn cells in the nucleus tractus solitarii of the rat. Brain Res. 2001;921:195–205. doi: 10.1016/s0006-8993(01)03118-3. [DOI] [PubMed] [Google Scholar]
- 66.Medeiros BA, dos Santos CL, Palheta RC, Jr, et al. Spinal cord transection modifies ileal fluid and electrolyte transport in rats. Auton Neurosci. 2008;139:24–9. doi: 10.1016/j.autneu.2007.12.003. [DOI] [PubMed] [Google Scholar]