Abstract
AIM: To investigate the effects of interferon-alpha (IFN-α) to restrain the growth and invasive potential of hepatocellular carcinoma (HCC) induced by hepatitis B virus (HBV) X protein.
METHODS: The pcDNA3.1-HBx plasmid was transfected into Chang cells by Lipofectamine in vitro, and Chang/HBx was co-cultured with IFN-α. Cell survival growth curve and clonogenicity assay were used to test the growth potential of Chang/pcDNA3.1, Chang/HBx and IFN-α-Chang/HBx in vitro. Growth assay in nude mice was used to detect the growth potential of Chang/pcDNA3.1, Chang/HBx and IFN-α-Chang/HBx in vivo. Wound healing and transwell migration assays were used to detect the invasive ability of Chang/pcDNA3.1, Chang/HBx and IFN-α-Chang/HBx.
RESULTS: Compared with CCL13 cells transfected with pcDNA3.1, CCL13 with stable expression of hepatitis B virus X protein showed the characteristics of malignant cells with high capability of growth and invasion by detecting their growth curves, colony forming efficiency, wound healing , transwell migration assays and growth assays in nude mice. Its capability of growth and invasion could be controlled by IFN-α.
CONCLUSION: IFN-α can restrain the growth and invasive potential of HCC cells induced by HBx protein, which has provided an experimental basis for IFN-α therapy of HCC.
Keywords: Hepatitis B virus X protein, Interferon-alpha, Hepatocellular carcinoma, Growth, Invasion
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most prevalent malignant tumors in Asia, especially in China, where hepatitis B virus (HBV) is the major etiologic factor for it[1-3]. The HBV X protein (HBx), a small regulatory protein, that is required for the establishment of viral infection[4,5], is believed to contribute to the development of HCC [6-8].
Interferon-alpha (IFN-α) has been used clinically for the treatment of viral infections[9-11] and malignances[12-14], for it plays an important role in both antiviral and antitumor host defenses. IFN-α also delays or prevents HCC in patients with HBV-related cirrhosis[15]. Therefore, IFN-α may exhibit an antitumor activity in HBV-related HCC through inhibiting the HBx-mediated cellular responses.
In this study, we aimed to explore the strong effect of IFN-α to restrain the growth and invasive potential of HCC induced by HBx protein through transfection of the HBx gene expression to human hepatoma cells.
MATERIALS AND METHODS
Cell lines and interferon
pcDNA3.1-HBx Chang liver cells (Chang/HBx) and pcDNA3.1 Chang liver cells (Chang/pcDNA3.1) were kindly donated by Liver Cancer Laboratory, Department of Surgery, Xiangya Hospital, Central South University (Changsha, Hunan Province, China). The IFN-α was purchased from Roche Pharmaceuticals Ltd. (Guangxi, China).
Cell culture and passage
The Chang/HBx and Chang/pcDNA3.1 were cultured and subcultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and incubated in 5% CO2 at 37°C for further research. The IFN-α concentration in the nutritive medium of IFN-α-Chang/HBx was 1000 ku/L.
Growth curve
The growth curve was designed to compare the proliferative ability of Chang/HBx cells with that of Chang/HBx cells cocultured with IFN-α and Chang/ pcDNBA3.1 cells. First, 2 × 104 cells per well were placed into 24-well plates. Next, the cells in 3 wells were counted daily during the 8 d of culture for evaluation of cell proliferation. Finally, the viability of the cells was determined by Trypan blue exclusion.
Clonogenic assay
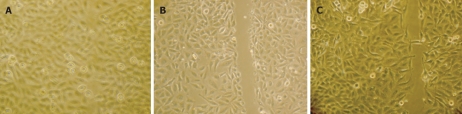
Chang/HBx, IFN-α-Chang/HBx and Chang/pcDNA3.1 cells were plated onto 60-mm Petri dishes at a density of 2 × 103 cells per dish and cultured in conditioned medium for 2 wk. The formed colonies were stained with Giemsa to allow calculation of their average colony-forming efficiency.
Wound healing assays
Six-well plates (Costar) were coated overnight with 10 mg/mL fibronectin (Becton Dickinson) in phosphate-buffered saline (PBS) and blocked with 1% bovine serum albumin (Sigma) in DMEM. Chang/HBx, IFN-α-Chang/HBx and Chang/pcDNA3.1 cells were suspended with 0.05% trypsin and 5.3 mmol/L ethylene diaminetetraacetic acid (EDTA) (Gibco) and then counted. Next, 5 × 105 cells per well were plated in serum-free DMEM with applicable inhibitors or DMSO for 3 h. Monolayer cultures were wounded with a P-200 pipette tip, and the medium was replaced with DMEM, 5% fetal bovine serum, and 2 mg/mL G418. Cells were photographed at a magnification of × 20 adjacent to a reference line etched onto the bottom of the plate. Cells were allowed to grow and migrate for 24 h at 37°C in 5% CO2 before being photographed a second time. DMSO levels were adjusted to be equal in all wells within each experiment and were never higher than 0.5% DMSO. Each experiment was performed in triplicate and repeated three times.
Transwell migration assay
Matrigel-coated filter inserts with 8-μm pores that fit into 24-well invasion chambers were obtained from Becton Dickinson. Chang/HBx cells, IFN-α-Chang/HBx cells, and Chang/pcDNA3.1 cells were detached from the tissue culture plates, washed, resuspended in conditioned medium (5 × 104 cells/200 μL), and then added to the upper compartment of the invasion chamber with or without plasmin (1.8 μg). Conditioned medium (500 μL) was added to the lower compartment of the invasion chamber. The Matrigel invasion chambers were incubated at 37°C for 24 h in 5% CO2. After incubation, the filter inserts were removed from the wells, and the cells on the upper side of the filter were removed using cotton swabs. The filters were fixed, mounted, and stained according to the manufacturer’s instructions. The cells that invaded through the Matrigel were counted on the underside of the filter. Three to five invasion chambers were used for each experimental condition. The values obtained were calculated by averaging the total number of cells from three filters.
Growth assays in nude mice
Male BALB/C-nu/nu athymic nude mice were obtained from Shanghai Laboratory Animal Center (China) at 4 wk of age. The Chang/HBx, IFN-α-Chang/HBx and Chang/pcDNA3.1 cells were collected by trypsinization and were washed with D-Hank’s solution. To produce experimental metastasis, 1 × 107 cells from each culture were injected into the right forelimb armpit of the mice. IFN-α was administrated subcutaneously at dosage of 1.5 × 10 U/kg per day in the group of IFN-α-Chang/HBx cells. After 2 wk, 2 mice from each group were killed and analyzed per week. Neoplasms were removed, rinsed in ice-cold PBS, fixed, stained with hematoxylin and eosin, and photographed.
Statistical analysis
All the data were presented as the mean ± SE. The significance of differences from the control values was determined with Student’s t test or the χ2 test. P < 0.05 was considered statistically significant.
RESULTS
Cell survival growth curve and clonogenicity assay
The cell survival growth curve of Chang/HBx cells showed faster growth than that of Chang/pcDNA3.1 cells and IFN-α-Chang/HBx cells (Figure 1). Their average colony-forming efficiency was also significantly different (Figure 2). These findings showed that Chang/HBx was more proliferative than Chang/pcDNA3.1 and IFN-α-Chang/HBx cells.
Figure 1.
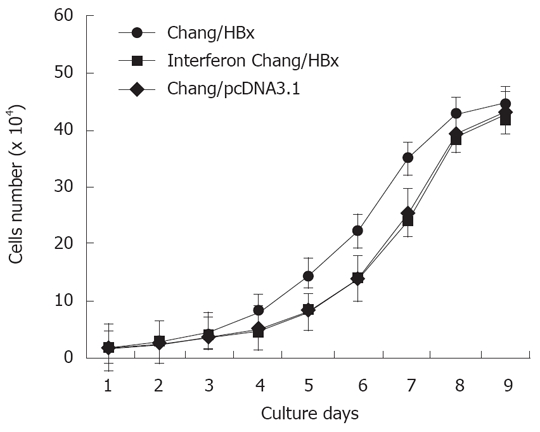
The growth curve of Chang/HBx cells shows a shift to the left from that of Chang/pcDNA3.1 and IFN-α-Chang/HBx cells.
Figure 2.
Cell proliferative ability comparisons. The average colony-forming efficiency of Chang/HBx (A) was 29.3 ± 4.5, which was significantly different from that of Chang/pcDNA3.1 (B) (12.8 ± 2.6) and IFN-α-Chang/HBx (C) cells (13.5 ± 2.3) (P < 0.05).
HBx promotes invasion of chang cells in vitro
The wound-healing assay showed that Chang/HBx cells had a higher ability to heal themselves than the control cells and IFN-α-Chang/HBx cells (Figure 3).
Figure 3.
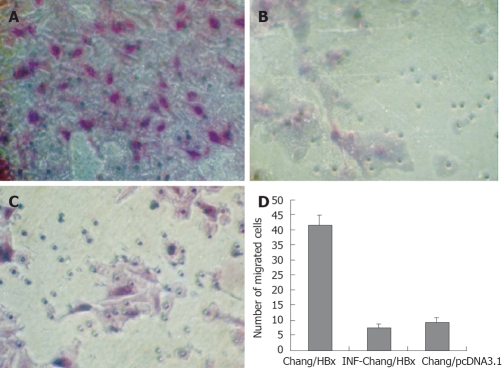
HBx promotes the invasive capacity of Chang liver cells in vitro. Wound-healing assays were performed at 0 and 24 h and observed under a phase-contrast microscope (× 100). A: Chang liver cells with pcDNA3.1-HBx were disrupted, and wound healing occurred after 24 h; B: IFN-α-Chang/HBx cells were disrupted, and the wound did not heal completely after 24 h; C: Chang cells with pcDNA3.1 were disrupted, and the wound did not heal completely after 24 h.
In the transwell migration assay, edge collection phenomena was found on cells in the transwell chamber, with few cells in the center of the wells; therefore, we selected superior, inferior, left, right, and center sites so as to represent the migration assay correctly. The assay showed that the Chang/HBx cells also had greater migration ability than the control cells and IFN-α-Chang/HBx cells (P < 0.05) (Figure 4A-C). The average number of Chang/HBx cells that migrated through the Matrigel was 41.6 ± 3.1 cells/site compared with 7.4 ± 1.2 cells/site in the control cells and 9.2 ± 1.6 cells/site in IFN-α-Chang/HBx cells (Figure 4D).
Figure 4.
HBx promotes the invasive capacity of Chang liver cells in vitro. A: Chang cells transfected with pcDNA3.1-HBx invaded through the Matrigel were counted on the underside of the transwell filter and compared with Chang/HBx cells (B) and control cells Chang/pcDNA3.1 (C) (× 200). There was no difference between IFN-α-Chang/HBx cells and Chang/pcDNA3.1; D: The average number of migrated cells per site seen under a high-power microscope (× 400) was 41.6 ± 3.1 for the transfected Chang/HBx cells and 7.4 ± 1.2 for IFN-α-Chang/HBx cells and 9.2 ± 1.6 for the control Chang/pcDNA3.1 cells.
IFN-α inhibits proliferation of chang/HBx cells in vivo
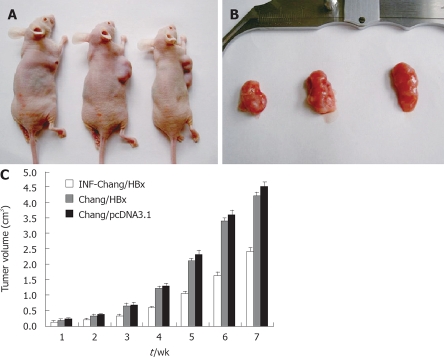
The growth assay in nude mice showed a 100% growth rate of neoplasms after inoculation of the HCC cells. The average latency period was 5 d, and the growth rate was 65.5 ± 7.6 mm3/d. There was no distant metastasis from the transfectant. The sizes of the neoplasms from IFN-α-Chang/HBx were obviously smaller than the tumors of Chang/HBx injected mice (P < 0.05), and the neoplasms from Chang/HBx grew more slowly than those from Chang/pcDNA3.1 (P < 0.05), but there was no statistical difference between them in our test. Although Chang liver cells contained the Hela marker, the formed tumor indeed was hepatoma (Figure 5), which could progress to HCC.
Figure 5.
A: A nude mouse migration model with pcDNA3.1-HBx Chang cells, IFN-α-Chang/HBx cells and Chang/pcDNA3.1 cells were constructed on the 7th wk; B: The volume of neoplasms in the three groups were observed. The sizes of the hepatomas from Chang/pcDNA3.1 and IFN-α-Chang/HBx injected nude mice were obviously larger than the tumors of Chang/HBx injected mice; C: Neoplasm growth in the Chang/HBx-inoculated nude mice compared between IFN-α-Chang/HBx and the control Chang/pcDNA3.1-inoculated mice.
DISCUSSION
Interferons are a family of cytokine that are produced by cells of the immune system. Three classes of interferons have been identified: α, β and γ. Each class has different effects though their activities overlap. The interferons direct the attack of the immune system on viruses, bacteria, tumors and other foreign substances that may invade the body[16]. IFN-α is known to have antiproliferative effects on human HCC cells, both in vivo and in vitro[17,18]. The effectiveness of IFN-α in treating HCC patients has been reported to be positive in a few previous clinical trials[19-21]. It was also shown that IFN-α delayed or prevented HCC in patients with HBV-related cirrhosis[15]. In addition, combination of IFN-α and 5-FU significantly prolonged the survival rate of patients with HCC[22]. These observations suggest that IFN-α has a capability of anticancer agents against HCC. But the precise mechanisms remain poorly understood. To investigate weather IFN-α could regulate the function of HBx protein and inhibit the growth and invasion potential of HBV-related HCC cells, we investigated Chang hepatocytes encoding HBx gene and observed that the transfected cells had acquired growth and invasive properties in vitro, but these properties could be controlled by IFN-α effectively.
Through cell survival growth curve and clonogenic assays, we found that Chang cells encoding HBx had higher dynamics for growth than the Chang cells. These cells also showed multilaminar growth. Because they had lost contact inhibition completely, their colony-forming ability was greater than that of the parental cell line. However, the growth and colony-forming ability of Chang-HBx could be inhibited by IFN-α obviously.
Our study also showed that HBx-transfected Chang had higher wound healing ability than the control cells. The transwell migration assay showed that Chang/HBx had a more obviously invasive ability than the control cells. But IFN-α could inhibit these malignant biologic behavior of Chang/HBx induced by HBx protein.
Nude mice were used as the mimic system in our study for observing the effects of cell tumorigenicity of HBx protein in Chang liver cells in vivo. However, in the growth assays of nude mice, we found that Chang/HBx cells grew more slowly than Chang/pcDNA3.1 cells. At the same time, Chang/HBx cells treated with IFN-α were found growing more slowly than Chang/HBx cells without IFN-α treatment, which inhibited the tumorigenicity of Chang/HBx cells.
Some studies have shown that HBx can induce tumor proliferation and invasion through down-regulating the function of P53[23,24], up-regulating the expression of survivin[25], activating with several important cell signaling pathways, including PKB/Akt[26], RAS/RAF/MAPK[27], wnt/β-catenin[28] and NF-κB[29]. It has also proved that IFN-α can induce p53-dependent apoptosis[30], down-regulate the expression of survivin[31] and inactive NF-κB[32] respectively. It is possible that IFN-α could control the growth and invasive potential of HBV-related HCC by inhibiting the function of HBx. The reason why Chang/HBx cells grew more slowly than Chang/pcDNA3.1 cells, is the growth of Chang/HBx cells in nude mice need more nutrient than Chang/pcDNA3.1 cells, however, as the early tumorigenesis of Chang/HBx cells formed, the nutrient was not sufficient and the cells might enter into rest phase as proliferation ceases.
In conclusion, IFN-α could inhibit the growth and invasive potential of Chang cells induced by HBx. IFN-α is a main drug for type B hepatitis and can delay and prevent HBV-related cirrhosis effectively. Postoperative IFN-α therapy can improve the survival of patients with HBV-related HCC. IFN-α therapy might be a very important means for preventing and curing HBV-related HCC. Our study provides a new evidence for this.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world, especially in China. About 53% of HCC cases in the world are related to hepatitis B virus (HBV) infection. Chronic HBV infection is a leading risk factor for HCC. It has been proved that IFN-α could control the infection of HBV, prevent HBV-related cirrhosis, lower tumor recurrence rate after surgical resection or ablation of HCC and prolong the survival rate of patients with HCC. IFN-α therapy has been applied to treat HBV-related HCC as an effective drug, but its precise mechanism remains poorly understood.
Research frontiers
Some studies have proved that IFN-α could inhibit the growth of HCC cells in vivo and in vitro. It could regulate expression of VEGF, bFGF and angiopoietins and inhibit tumor angiogenesis in human HCC cells. But there are few reports about the impact of IFN-α on the malignant characteristics of HCC cells induced by HBx protein.
Innovations and breakthroughs
Our results reveal that the liver cell lines CCL13 containing HBx gene expression have the characteristics of malignant cells, which was verified by means of detecting their growth curve, colony forming efficiency, wound healing, transwell migration assays and growth assay in nude mice. All of these could be controlled by IFN-α.
Applications
IFN-α therapy is a very important means for preventing and curing HBV-related HCC. This study provides some experimental evidence for it.
Terminology
Interferons are natural proteins produced by the cells of the immune system of most vertebrates in response to challenges by foreign agents such as viruses, parasites and tumor cells. Three classes of interferons have been identified: α, β and γ. The interferons direct the attack of immune system on viruses, bacteria, tumors and other foreign substances that may invade the body. The JAK-STAT signaling pathway is the most specific and commonly accepted IFN signaling pathway.
Peer review
This is an interesting article which investigated the effect of IFN-α on the malignant characters of human liver cell lines CCL13 containing HBx gene expression. The design of experiment is novel and the result is significant. It would be helpful to explore the mechanism of IFN-α therapy for HCC and direct the clinical therapy of HCC.
Footnotes
Supported by The grant from Natural Science Foundation of Guangxi Zhuang Autonomous Region, No. 0542068
Peer reviewer: Yogesh K Chawla, PhD, Professor, Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
S- Editor Li DL L- Editor Ma JY E- Editor Ma WH
References
- 1.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Shibuya K, Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ. Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer. 2002;2:37. doi: 10.1186/1471-2407-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoulim F, Saputelli J, Seeger C. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J Virol. 1994;68:2026–2030. doi: 10.1128/jvi.68.3.2026-2030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melegari M, Wolf SK, Schneider RJ. Hepatitis B virus DNA replication is coordinated by core protein serine phospho-rylation and HBx expression. J Virol. 2005;79:9810–9820. doi: 10.1128/JVI.79.15.9810-9820.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan HL, Sung JJ. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis. 2006;26:153–161. doi: 10.1055/s-2006-939753. [DOI] [PubMed] [Google Scholar]
- 7.Koike K, Tsutsumi T, Fujie H, Shintani Y, Kyoji M. Molecular mechanism of viral hepatocarcinogenesis. Oncology. 2002;62 Suppl 1:29–37. doi: 10.1159/000048273. [DOI] [PubMed] [Google Scholar]
- 8.Staib F, Hussain SP, Hofseth LJ, Wang XW, Harris CC. TP53 and liver carcinogenesis. Hum Mutat. 2003;21:201–216. doi: 10.1002/humu.10176. [DOI] [PubMed] [Google Scholar]
- 9.Sene D, Touitou V, Bodaghi B, Saadoun D, Perlemuter G, Cassoux N, Piette JC, Hoang PL, Cacoub P. Intraocular complications of IFN-alpha and ribavirin therapy in patients with chronic viral hepatitis C. World J Gastroenterol. 2007;13:3137–3140. doi: 10.3748/wjg.v13.i22.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YF, Zhao W, Zhong YD, Yang YJ, Shen L, Zhang N, Huang P. Comparison of the efficacy of thymosin alpha-1 and interferon alpha in the treatment of chronic hepatitis B: a meta-analysis. Antiviral Res. 2008;77:136–141. doi: 10.1016/j.antiviral.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Ueno T, Mitsuishi T, Kimura Y, Kato T, Hasegawa H, Katano H, Sata T, Kurane S, Kawana S. Immune reconsti-tution inflammatory syndrome associated with Kaposi’s sarcoma: successful treatment with interferon-alpha. Eur J Dermatol. 2007;17:539–540. doi: 10.1684/ejd.2007.0274. [DOI] [PubMed] [Google Scholar]
- 12.Kujawski LA, Talpaz M. The role of interferon-alpha in the treatment of chronic myeloid leukemia. Cytokine Growth Factor Rev. 2007;18:459–471. doi: 10.1016/j.cytogfr.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Amato RJ, Mohammad T. Interferon-alpha plus capecitabine and thalidomide in patients with metastatic renal cell cancer. J Exp Ther Oncol. 2008;7:41–47. [PubMed] [Google Scholar]
- 14.Nagano H, Miyamoto A, Wada H, Ota H, Marubashi S, Takeda Y, Dono K, Umeshita K, Sakon M, Monden M. Interferon-alpha and 5-fluorouracil combination therapy after palliative hepatic resection in patients with advanced hepatocellular carcinoma, portal venous tumor thrombus in the major trunk, and multiple nodules. Cancer. 2007;110:2493–2501. doi: 10.1002/cncr.23033. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Fukuda M, Koida I, Arase Y, Chayama K, Murashima N, et al. Interferon decreases hepatocellular carcinogenesis in patients with cirrhosis caused by the hepatitis B virus: a pilot study. Cancer. 1998;82:827–835. doi: 10.1002/(sici)1097-0142(19980301)82:5<827::aid-cncr5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Chelbi-Alix MK, Wietzerbin J. Interferon, a growing cytokine family: 50 years of interferon research. Biochimie. 2007;89:713–718. doi: 10.1016/j.biochi.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Kondo M, Nagano H, Wada H, Damdinsuren B, Yamamoto H, Hiraoka N, Eguchi H, Miyamoto A, Yamamoto T, Ota H, et al. Combination of IFN-alpha and 5-fluorouracil induces apoptosis through IFN-alpha/beta receptor in human hepatocellular carcinoma cells. Clin Cancer Res. 2005;11:1277–1286. [PubMed] [Google Scholar]
- 18.Hagiwara S, Kudo M, Nakatani T, Sakaguchi Y, Nagashima M, Fukuta N, Kimura M, Hayakawa S, Munakata H. Combination therapy with PEG-IFN-alpha and 5-FU inhibits HepG2 tumour cell growth in nude mice by apoptosis of p53. Br J Cancer. 2007;97:1532–1537. doi: 10.1038/sj.bjc.6604058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer M, Runkel L, Schaller H. HBx protein of hepatitis B virus interacts with the C-terminal portion of a novel human proteasome alpha-subunit. Virus Genes. 1995;10:99–102. doi: 10.1007/BF01724303. [DOI] [PubMed] [Google Scholar]
- 20.Sun HC, Tang ZY, Wang L, Qin LX, Ma ZC, Ye QH, Zhang BH, Qian YB, Wu ZQ, Fan J, et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol. 2006;132:458–465. doi: 10.1007/s00432-006-0091-y. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura M, Nagano H, Marubashi S, Miyamoto A, Takeda Y, Kobayashi S, Wada H, Noda T, Dono K, Umeshita K, et al. Pilot study of combination chemotherapy of S-1, a novel oral DPD inhibitor, and interferon-alpha for advanced hepatocellular carcinoma with extrahepatic metastasis. Cancer. 2008;112:1765–1771. doi: 10.1002/cncr.23356. [DOI] [PubMed] [Google Scholar]
- 22.Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, Tateishi R, Teratani T, Shiina S, Omata M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106:1990–1997. doi: 10.1002/cncr.21832. [DOI] [PubMed] [Google Scholar]
- 23.Chung TW, Lee YC, Ko JH, Kim CH. Hepatitis B Virus X protein modulates the expression of PTEN by inhibiting the function of p53, a transcriptional activator in liver cells. Cancer Res. 2003;63:3453–3458. [PubMed] [Google Scholar]
- 24.Hsieh JL, Wu CL, Lee CH, Shiau AL. Hepatitis B virus X protein sensitizes hepatocellular carcinoma cells to cytolysis induced by E1B-deleted adenovirus through the disruption of p53 function. Clin Cancer Res. 2003;9:338–345. [PubMed] [Google Scholar]
- 25.Zhang X, Dong N, Yin L, Cai N, Ma H, You J, Zhang H, Wang H, He R, Ye L. Hepatitis B virus X protein upregulates survivin expression in hepatoma tissues. J Med Virol. 2005;77:374–381. doi: 10.1002/jmv.20466. [DOI] [PubMed] [Google Scholar]
- 26.Shih WL, Kuo ML, Chuang SE, Cheng AL, Doong SL. Hepatitis B virus X protein inhibits transforming growth factor-beta -induced apoptosis through the activation of phosphatidylinositol 3-kinase pathway. J Biol Chem. 2000;275:25858–25864. doi: 10.1074/jbc.M003578200. [DOI] [PubMed] [Google Scholar]
- 27.Yun C, Cho H, Kim SJ, Lee JH, Park SY, Chan GK, Cho H. Mitotic aberration coupled with centrosome amplification is induced by hepatitis B virus X oncoprotein via the Ras-mitogen-activated protein/extracellular signal-regulated kinase-mitogen-activated protein pathway. Mol Cancer Res. 2004;2:159–169. [PubMed] [Google Scholar]
- 28.Cha MY, Kim CM, Park YM, Ryu WS. Hepatitis B virus X protein is essential for the activation of Wnt/beta-catenin signaling in hepatoma cells. Hepatology. 2004;39:1683–1693. doi: 10.1002/hep.20245. [DOI] [PubMed] [Google Scholar]
- 29.Wang T, Wang Y, Wu MC, Guan XY, Yin ZF. Activating mechanism of transcriptor NF-kappaB regulated by hepatitis B virus X protein in hepatocellular carcinoma. World J Gastroenterol. 2004;10:356–360. doi: 10.3748/wjg.v10.i3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan XW, Zhu XF, Huang XF, Sheng PY, He AS, Yang ZB, Deng R, Feng GK, Liao WM. Interferon-alpha enhances sensitivity of human osteosarcoma U2OS cells to doxorubicin by p53-dependent apoptosis. Acta Pharmacol Sin. 2007;28:1835–1841. doi: 10.1111/j.1745-7254.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- 31.Thyrell L, Arulampalam V, Hjortsberg L, Farnebo M, Grander D, Pokrovskaja Tamm K. Interferon alpha induces cell death through interference with interleukin 6 signaling and inhibition of STAT3 activity. Exp Cell Res. 2007;313:4015–4024. doi: 10.1016/j.yexcr.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Shigeno M, Nakao K, Ichikawa T, Suzuki K, Kawakami A, Abiru S, Miyazoe S, Nakagawa Y, Ishikawa H, Hamasaki K, et al. Interferon-alpha sensitizes human hepatoma cells to TRAIL-induced apoptosis through DR5 upregulation and NF-kappa B inactivation. Oncogene. 2003;22:1653–1662. doi: 10.1038/sj.onc.1206139. [DOI] [PubMed] [Google Scholar]