Abstract
The altered permeability characteristics of erythrocytes infected with malaria parasites have been a source of interest for over 30 years. Recent electrophysiological studies have provided strong evidence that these changes reflect transmembrane transport through ion channels in the host erythrocyte plasma membrane. However, conflicting results and differing interpretations of the data have led to confusion in this field. In an effort to unravel these issues, the groups involved recently came together for a week of discussion and experimentation. In this article, the various models for altered transport are reviewed, together with the areas of consensus in the field and those that require a better understanding.
Keywords: Patch-clamp, Ion channels, New permeability pathways, PSAC, Plasmodium, Oxidation
It has been known for several decades that Plasmodium falciparum-infected erythrocytes exhibit increased permeability to a wide range of structurally unrelated solutes as the internal parasite matures. These changes are thought to be important for the survival of the parasite. They may be involved in nutrient uptake, metabolite removal, volume regulation and/or modification of erythrocyte cytosolic ion concentrations (e.g. increased cytosolic Na+, which is normally low). It is, therefore, not surprising that the pathway(s) that underlie these changes have been identified as potentially important chemotherapeutic antimalarial targets.
Extensive characterization of parasite-induced permeability changes began in the 1970's through studies using radio-tracer uptake and haemolysis assays. Tracer uptake provides a direct measure of the transport of selected radiolabelled solutes; haemolysis of erythrocytes in isotonic solutions of permeant solutes is an indirect, but potentially quantitative marker of transport. On the basis of experiments carried out using these techniques, Ginsburg and co-workers (1983) suggested that the increased uptake of various solutes into the infected cell might be accounted for by novel pore-like transport pathways, terming these the `new permeability pathways', or NPP. Kirk and co-workers (1994), using pharmacological approaches with the same techniques, postulated that the NPP are a single class of anion-selective channels, also capable of transporting electroneutral and cationic solutes at lower rates.
The possibility that the NPP are ion channels led, at the turn of the century, to the first successful electrophysiological (patch-clamp) study of P. falciparum-infected human erythrocytes. Desai et al. (2000) reported the presence in the infected erythrocyte membrane of a single, novel anion channel type, the `plasmodial surface anion channel' (PSAC). However, in subsequent patch-clamp studies a number of other groups reported a range of somewhat different findings, leading to controversy in this field. This was the reason members of seven international research groups gathered in Washington DC at a workshop organised by Dr Sanjay Desai.
Before discussing the findings of the different groups, it is worth noting how the nomenclature in this field has developed. The term NPP has evolved to cover any pore or channel-like transport mechanisms that alter the permeability of the host plasma membrane as the internal parasite matures. Because there may be multiple distinct transport mechanisms induced by the parasite, more specific nomenclature for individual ion channels has been proposed in some cases (e.g. PSAC).
Patch-clamp methods are used to study transmembrane transport of ions directly by measuring the associated electrical currents. Measurement of these small currents is only possible with small bore pipettes fabricated from borosilicate or quartz. The pipettes are filled with electrolyte solutions to permit communication with electrical amplifiers that can detect the currents associated with ion movement. Importantly, the tip of the pipette must be pristine to allow very high resistance seals to be formed between the pipette and the biological membrane under study. These seals, formed through poorly understood chemical reactions between the pipette and the cell membrane, are critical for the detection of the currents produced by the movement of ions across the cell membrane. Without high quality seals, much of the measured current will be due to ions leaking between the pipette and bath solutions without crossing the cell membrane. Under optimal conditions, it is possible to measure transport-associated currents below 10-12 amperes, a value that corresponds to the movement of only 6,000 monovalent ions/ms. This very high resolution enables the measurement of currents passing through individual ion channel molecules.
Another important feature of the patch-clamp method is that the investigator can control the electrical potential difference across the membrane (known as the membrane potential or Vm). Because the imposed Vm is an important driving force for the movement of ions, this ability permits rigorous examination of ion channel selectivity (i.e. which ions are able to permeate), gating (i.e. how ion channels open and close), and transport rates. As with other aspects of patch-clamp, confidence in the imposed Vm depends on paying attention to errors that can arise from the network of resistive and capacitive elements made up of the cell, the pipette, the solutions, and the electronic equipment.
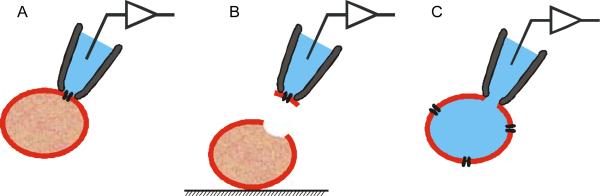
Of the five standard patch-clamp configurations, the three that have been used to characterise P. falciparum-infected erythrocytes are the cell-attached, the excised inside-out and the whole-cell configurations (Fig. 1). The cell-attached configuration is attained after formation of a seal on an intact cell. This configuration is used to measure the activity of individual ion channels in the patch of membrane encompassed by the pipette tip. The excised inside-out configuration is attained by pulling a cell-attached pipette away from the cell, exposing the intracellular membrane surface to the defined bathing solution. This configuration can also be used to measure single channel activity and has the advantage of permitting controlled application of solutions to the intracellular face of the channels under investigation. The whole-cell configuration begins from the cell-attached configuration and is attained by rupturing the membrane patch under the pipette tip with mechanical or electrical stress. This configuration is used to measure the sum of all ionic transport activity across the surface membrane of the cell, and permits control of both intracellular and extracellular solutions. In the whole-cell and the excised inside-out configurations, cytosolic factors are effectively lost due to dilution by the pipette and bath solutions, respectively.
Fig. 1.
Current consensus within the field regarding the altered permeability of Plasmodium falciparum-infected human erythrocytes.
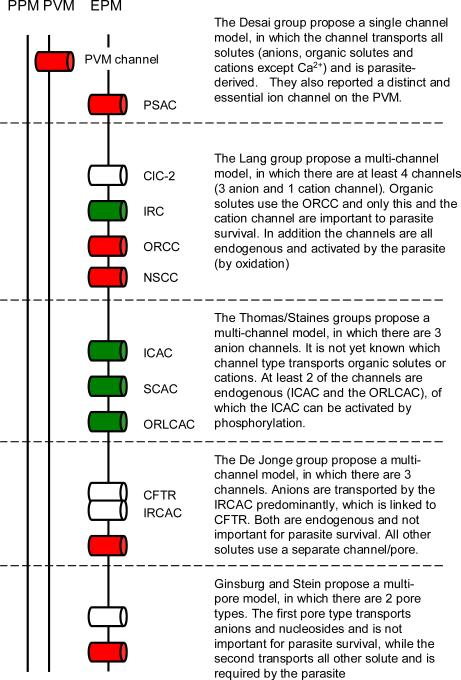
In their original study, Desai and co-workers (2000) of the NIH, Rockville, MD, USA showed that mature P. falciparum-infected erythrocytes exhibit whole-cell currents some 100- to 150-fold larger than those measured on uninfected erythrocytes. These currents were smaller at positive Vm than at negative values, despite equal and opposite driving forces for ion movement (a phenomenon sometimes referred to as `inward rectification'). Their studies revealed a strong preference for anions over cations, a selectivity sequence of l- > Br- > Cl-, and pharmacological properties very similar to those seen previously in tracer flux and haemolysis studies (Supplementary Table 1). Desai et al. also performed cell-attached patch-clamp studies and identified the unusual ion channel, PSAC, which they linked to the increased whole-cell conductance. Under their conditions, PSAC exhibited fast flickering between open and closed states with significantly fewer and shorter openings at positive Vm, consistent with the inward rectifying whole-cell currents (Supplementary Table 2). Desai and colleagues have not seen PSAC on uninfected erythrocytes under any conditions. They also found parasite-strainspecific (and host-cell-independent) variation in the electrophysiological characteristics of PSAC and interpreted this observation as evidence for the channel being parasite-encoded (Alkhalil et al., 2004). Additional support for the involvement of parasite genetic elements in the formation of the NPP has subsequently been published by Lingelbach and colleagues (Baumeister et al., 2006). Fig. 2 presents a graphical representation of the model proposed by the Desai and colleagues (as well as those proposed by others, as described below) for the altered permeability of P. falciparum-infected erythrocytes.
Fig. 2.
Patch-clamp configurations used to characterise channel activity in Plasmodium falciparum-infected human erythrocytes. (A) The cell-attached configuration. (B) The inside-out configuration. (C) The whole-cell configuration. Note that the erythrocytes depicted in the figure are not infected.
In a subsequent study involving groups in Roscoff, France and Oxford, UK, Egee and co-workers (2002) reported whole-cell currents similar to those described above, exhibiting both inward rectification and comparable magnitudes (Supplementary Table1). However, in this and a subsequent report by the French group (Bouyer et al., 2006), there were a number of important differences from the original study of Desai et al. These differences were seen in both cell-attached and excised patch measurements, as well as in uninfected erythrocytes. The single channel studies of infected cells identified three distinct anion channel types: an intermediate, a small and an outwardly rectifying, large conductance anion channel (Supplementary Table 2). The intermediate conductance anion channel has some similarities to PSAC as described by the Desai lab in that it exhibited fewer and shorter openings at positive Vm and flickering gating, but with significantly longer open and closed durations than seen by Desai et al. It also had an approximately five-fold higher single channel conductance (leading to a lower estimated channel copy number than reported by the Desai group (200-300 versus 1,000-2,000 copies per cell) to account for the similar magnitudes of whole-cell conductance) and showed significant differences in levels of inhibition by certain antagonists (the possible reasons for these discrepancies are discussed in detail below). Perhaps most importantly, similar channel activity to the intermediate and the outward rectifying, large conductance anion channels could be observed on uninfected erythrocytes, with the former induced by either membrane stretch or protein kinases. They thus proposed that the channels underlying the increased conductance of the infected erythrocyte are predominantly endogenous human ion channels (activated by the parasite). It is not yet clear which (if any) of the ion channels identified by Thomas' group might be associated with the increased permeability of the infected erythrocyte membrane to organic solutes.
In another 2002 study (Huber et al., 2002), and in a number of subsequent studies (reviewed in Huber et al., 2005), the group of Lang in Tuebingen, Germany, obtained quite different results. Their whole-cell measurements (single channel studies were not performed) suggest at least four separate malaria-induced conductance pathways, three permeable to anions and one permeable to cations (Supplementary Table 1 and Fig. 2). Two of the anion conductances described by this group exhibit inward rectification; the third exhibits outward rectification (i.e. greater currents at positive Vm than at negative values). The group subsequently identified differences in the pharmacological and selectivity properties of these conductances, as well as showing that one of the inward rectifying anion currents is attributable to the swelling-activated chloride channel ClC-2. On the basis of studies of the effects of organic solutes on whole-cell currents, this group has proposed that the channel type underlying the outward rectifying conductance is also responsible for organic solute transport across the infected cell membrane. In addition, this group found that conductances similar to each of those seen in infected cells can be activated in uninfected erythrocytes by oxidation, pointing towards modified endogenous channels as the basis of the altered permeability.
Staines et al. have published two studies (the first in collaboration with the French and German groups) examining the effects of the different experimental conditions used by the various groups, both in preparing erythrocytes and in executing the patch-clamp experiments. The first report (Staines et al., 2003) went some way to resolving the growing number of inconsistencies, showing that small amounts of serum left behind from in vitro culturing of the parasites (as occurred in the protocols used by Lang and colleagues) led to increased current activation at both positive and negative Vm in infected erythrocytes. Furthermore, the holding potential (i.e. the Vm applied to the cell between patch-clamp recordings) had a marked effect on whole-cell currents. Negative holding potentials (as used by Lang and colleagues) led to time-dependent inactivation of currents at negative Vm. Therefore, it was suggested that, combined, these two conditional issues account for the outward rectifying conductance reported by the Lang group. In the second study (Staines et al., 2006), transport of the electroneutral organic solute, sorbitol, was found to increase slightly in infected erythrocytes after gross membrane depolarisation (i.e. making the Vm positive). From these data, Staines et al. concluded that the pathways underlying the transport of organic solutes was not inward rectifying, at least under the conditions tested (by definition such channels exhibit reduced transport at positive Vm). The transport of sorbitol in infected erythrocytes, like anion conductance, was increased upon addition of extracellular serum. Additionally, this study found that conditions used during tracer flux and haemolysis assays (namely increased erythrocyte density and the presence of significant levels of lysed erythrocytes compared with patch-clamp protocols) affected patch-clamp derived data. This study raises significant questions about the extent to which data derived by different transport measurement techniques can be compared.
Finally, a study from De Jonge and colleagues (Verloo et al., 2004) in Rotterdam, Holland, also described inward rectifying whole-cell anion currents in P. falciparum-infected erythrocytes with similarities to those in previous studies (Supplementary Table 1). Somewhat surprisingly, however, infected erythrocytes containing very immature parasites (at a stage at which there has previously been reported to be no significant increase in the erythrocyte membrane permeability) also exhibited similar currents. This group also found that: (i) the increased whole-cell conductance was absent if parasites were cultured in erythrocytes from donors with cystic fibrosis (homozygous for a deletion at position F508 in the human cystic fibrosis transmembrane regulator (CFTR), a chloride channel, which has been localised to the erythrocyte plasma membrane (Lange et al., 2006)); (ii) cells which lacked the parasite-induced conductance nevertheless underwent osmotic lysis (in several solutes) at a similar rate as infected cells from normal donors; and (iii) parasites grew normally in erythrocytes from cystic fibrosis patients. On the basis of these observations, it was concluded that the increased anion conductances measured by patch-clamp are not associated with organic solute permeability changes and are not required for parasite survival. Interestingly, the inwardly rectifying anion conductance could also be activated in uninfected erythrocytes by cell shrinkage (in the presence of adenosine 5'-triphosphate (ATP) in the pipette), consistent with an endogenous origin, but not in erythrocytes from cystic fibrosis patients. In addition, they reported a separate linear CFTR-like current in uninfected erythrocytes (distinct from the inward rectifying current and again absent in erythrocytes from cystic fibrosis patients), suggesting that the channel type underlying the inward rectifying conductance is functionally dependent on CFTR (at least under their experimental conditions) but constitutes a separate entity.
In addition to the patch-clamp studies described above, Ginsburg and Stein (2004) performed a biophysical analysis of all transport data derived from tracer flux and haemolysis assays. From this, they concluded that the mechanism underlying the altered permeability in P. falciparum-infected erythrocytes consists of two types of channels or pores, with one low-copy number (four per cell) type mediating the transport of organic solutes and cations and a second high-copy number (300-400 per cell) type mediating the transport of anions and nucleobases (with the predicted copy numbers in reasonable agreement with the copy numbers reported by the Thomas group for the outward rectifying large conductance anion channel (< 10 per cell) and the intermediate conductance anion channel (200-300 copies per cell). It was argued by the authors that the high copy number pathway is not required for parasite survival (Fig. 2).
To summarise, in the 6 years since the first patch-clamp study of P. falciparum-infected erythrocytes, there have been many papers in this area. There is consensus that the parasitized erythrocyte has a dramatically increased conductance relative to uninfected erythrocytes. However, we do not agree on the number of channel types underlying the increased conductance, the electrophysiological characteristics of these channels, the origin of the channels (parasite or host), and the relationship (if any) between these channels and the increased permeability of organic solutes, including essential nutrients and metabolic wastes. This was the background to the July 2006 workshop.
At the workshop each day began with one or more detailed talks, followed by substantial blocks of time set aside for hands-on experiments. The unique opportunity for workers from different laboratories around the world to carry out experiments together proved extremely valuable. Tsione Solomon used the transmittance assay developed in the Desai lab to demonstrate sorbitol-induced lysis and inhibition by furosemide and 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB). Abdulnaser Alkhalil, Godfrey Lisk and Ajay Pillai each obtained whole-cell and cell-attached recordings consistent with PSAC activity and demonstrated inhibition by antagonists as described in their papers. Stephan Huber reproduced NPPB-sensitive sorbitol-induced lysis of uninfected erythrocytes after oxidation (linking oxidation-induced conductances to the transport of uncharged solutes). This was an important phenomenon to demonstrate because several groups have not succeeded in reproducing these effects of oxidation. In addition, he showed that the outwardly rectifying chloride current, previously reported by his group, was sensitive to the newly identified PSAC inhibitor dantrolene. Henry Staines demonstrated the effect of serum on whole-cell currents. All of the participants were able to observe the data acquisition and the subsequent analyses of recorded files. This hands-on part of the workshop showed clearly that the discrepancies between the various publications in the field are not the result of selection bias or gross artefacts in one or more laboratory's recordings.
During the talks, the experimental conditions used by each group were critically evaluated to explore to what extent technical issues might account for the discrepancies in the obtained results. Specific conditions addressed included the solutions used in the bath and pipette compartments (e.g. their osmolarity, concentrations of divalent cations and whether physiological modulators such as ATP are present), acceptable thresholds for the electrical resistance of the seal formed between the membrane and the pipette, whether perfusion around the patched erythrocyte is detrimental, the source and age of donor erythrocytes used, composition and geometry of the fabricated patch pipettes, patch-clamp equipment from different vendors, and conditions for recording currents such as duration of pulses and level of filtering. This is a field where numerous experimental details can markedly influence measurements. Several methodological differences in how the various laboratories have performed measurements were identified in our discussions, and some of the more important of these are noted below. However, the implications of these differences will still need more thorough experimental evaluation.
With regard to whole-cell measurements in uninfected and infected erythrocytes, there was general agreement between the attendees. All studies reported inwardly rectifying conductances in P. falciparum-infected erythrocytes of similar magnitude, selectivity and pharmacology (Supplementary Table 1). Where different data were reported, experimental conditions could be identified which explained the new results (e.g. the measurement of shrinkage induced inward rectifying currents in uninfected erythrocytes requires the presence of ATP within the pipette solution, while the measurement of ClC-2 derived currents requires cell swelling). In the course of discussions, groups were also asked to clarify the use of certain conditions. In particular, the Lang group were questioned about the use of negative holding potentials. Stephan Huber explained that, in their hands, negative holding potentials aid seal formation and stability and help to identify serum-dependent currents, as the inward fraction of these currents are susceptible to time-dependent inactivation (leading to the outward rectifying conductances they have charcterised). Unresolved whole-cell measurement issues surrounded some of the work presented by Patrick Verloo and Hugo De Jonge. Firstly, their data suggest that inward rectifying conductances are activated before increases in organic solute permeability; this contrasts with unpublished data from the Desai laboratory. Second, their data suggest inward rectifying conductances are not observed in infected erythrocytes from cystic fibrosis patients that carry a key mutation in CFTR, which they reported is required for activation of the channel type underlying the inward rectifying conductances (see above). A subsequent publication from the Desai laboratory found no effect of the same host mutations on PSAC activity. In an effort to resolve these differences, the groups involved suggested additional experiments that each might conduct, although it was not possible to perform these during the workshop.
Some of the most animated discussions revolved around conditions required for single channel measurements. During his talk, Sanjay Desai discussed concerns others have regarding the conditions his lab uses to study PSAC, the only anion channel seen by his group (Supplementary Table 2). Due to PSAC's small conductance and fast gating, Desai et al. insist on high seal resistances (> 100 GΩ), supra-physiological Cl- concentrations (in excess of 1 M), and perfusion only under stringent conditions. They found that seal resistances and perfusion conditions acceptable for recordings on other cell types often damage the fragile seals on human erythrocytes. Several groups criticized these conditions as excessively non-physiological, arguing that the use of a hypertonic solution leads to severe cell shrinkage, with subsequent changes in several homeostatic variables, and possible channel inhibition and/or saturation caused by the high ionic conditions. In responding, Dr Desai highlighted the close correlation between the characteristics of PSAC measured under hypertonic conditions and those seen using radio-tracer uptake and haemolysis assays, as well as in electrophysiological experiments, at more physiological osmolarities. The debate was also tempered by the recognition by all present that any solution or voltage protocol used in a patch-clamp experiment may alter ion channel behaviour.
In contrast to the single channel type reported by Desai's group, Serge Thomas' group reported three anion channel types in infected erythrocytes (Supplementary Table 2). During their talk, Serge Thomas and Stephane Egee described their strategy of obtaining data under conditions as close as possible to physiological conditions. To achieve this, they used isotonic solutions, more physiologically relevant Vm values (in the range of -20 mV to +20 mV) and the cell-attached configuration (which preserves the integrity of the cell and is therefore considered the most physiological, provided that the pipette is filled with an appropriate saline solution) during their recent single channel study of infected erythrocytes (Bouyer et al., 2006). They presented data corresponding to the tiny channel activity measured at and around the spontaneous Vm of infected erythrocytes, with the high signal-to-noise ratios required for robust data analysis. They suggested three possible reasons for the differences between their results and those of the Desai group (not withstanding the issues raised over the use of supra-physiological conditions, as discussed above). Firstly, it was suggested that the high resistance seals used by the Desai laboratory (> 100 GΩ) might lead to selection bias (with the activity of some channel types stopping the generation of these high seals). Thomas' group use conditions that generate 4-20 GΩ seals, which is generally agreed to be sufficient to accurately measure the size of currents they have reported. Second, Thomas' group record channel activity under each and every experimental condition for up to 3 min compared with only seconds in the Desai group. Third, Thomas' group filter their raw data to a greater degree than those of the Desai group (to remove high frequency noise), which allows their recorded channel activity to be analysed with greater ease. They also recounted some unpublished observations that the age and storage conditions of uninfected erythrocytes (although not relating to infected erythrocytes) can affect the measured single channel activity, with fresh cells the preferred choice.
The number of differences in conditions identified between groups prompted the suggestion that each group should provide more detailed experimental methodology in the future. It also led to the obvious question of whether all the groups should adopt the same experimental conditions. While some workers thought this might be beneficial, others opposed this notion because not all of the proposed channels can be detected under any single experimental condition and because it would hinder future mechanistic progress in this field. Attempting to overcome this impasse, Serge Thomas and Hagai Ginsburg suggested that workers should use their preferred conditions, but at the end of the study also examine their new findings with a proposed “calibration” protocol (see Supplementary Protocol file). While open to the idea of a “calibration” protocol, Desai and colleagues do not accept the proposed protocol because: i) their transport studies show no effect of erythrocyte age or storage conditions; ii) in their laboratory, a 4-20 GΩ seal resistance does not permit rigorous data analysis; iii) recordings taken with large voltages applied to the erythrocyte membrane for extended periods (1 min in the proposed protocol) damage seal quality. Notwithstanding these issues, we all agreed that if conflicting data are identified, then some effort should be put into identifying why this occurs (by altering the conditions used and/or via collaboration with other groups).
The workshop was considered a success by all present (Text Box 1 shows the current consensus). The meeting brought together, for the first time, members of all of the groups that have published patch-clamp studies of the malaria parasite-infected erythrocyte, as well as a number of others with a longstanding interest in the field. It provided each group with an opportunity to observe experimentation by other laboratories, and identified important experimental variables that contribute to the differing observations and interpretations that have been reported. Possibly the most important achievement was to provide the groups involved with important insights into how they might replicate each others' work (in particular, allowing them to try to reproduce the effects of key modulators that activate channels on uninfected erythrocytes; i.e. oxidation, membrane stretch, protein kinases, etc.).
Methodological issues aside, there are fundamental biological issues that need to be resolved:
What is the molecular nature of the pathway(s) underlying the altered permeability of P. falciparum-infected erythrocytes? Is there one discrete transport mechanism or are there multiple separate pathways? Are they derived from the parasite, the host, or a combination of the two? As discussed above, Desai and colleagues have argued for a single, parasite-encoded channel type, whereas other groups maintain that there are several channel types, which are primarily endogenous to the host erythrocyte.
Do any or all of the currents measured using patch-clamp methods represent the electrophysiological correlate of the organic solute pathways or are they, as suggested by some, simply a by-product of erythrocyte invasion?
What are the physiological roles of the channel(s)/pathway(s) induced by the parasite in the host cell membrane? How crucial are they for the survival of the parasite? Are they feasible drug targets?
Definitive answers to these questions await the identification of the molecular components of the pathway(s) and this is the major goal of those working in the field. Nevertheless, continuing functional studies using available methods, as well as new methods that may be developed, should lead to the clarification of at least some of the mechanistic uncertainties, as well as shedding new light on the biological role and therapeutic potential of the parasite-induced permeabilities.
Supplementary Material
Fig. 3.
Models for the altered permeability of Plasmodium falciparum-infected human erythrocytes. Depicted are the parasite plasma membrane (PPM), a second membrane that surrounds the parasite, termed the parasitophorous vacuole membrane (PVM), and the erythrocyte plasma membrane (EPM). Channels/pores are represented by cylinders in the appropriate membrane, where red channels/pores are proposed to be important for parasite survival, the white channels/pores are unlikely to be essential for the parasite, and in the case of the green channels/pores there is no evidence one way or the other. PSAC, plasmodial surface anion channel; CUC-2, chloride channel family member 2; IRC, inward rectifying channel; ORCC, outward rectifying conductance channel; NSCC, nonspecific cation channel; ICAC, intermediate conductance anion channel; SCAC, small conductance anion channel; ORLCAC, outwardly rectifying large conductance anion channel; CFTR, cystic fibrosis transmembrane regulator; IRCAC, inwardly rectifying conductance anion channel.
Acknowledgments
We are grateful to Robert Gwadz, April Ward, Katherine Desai, Bob Gray, and NIAID administrative staff for the many hours of work that were needed for the success of our workshop. This workshop was supported by the Intramural Research Program of the NIH, Office of Rare Diseases and NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkhalil A, Cohn JV, Wagner MA, Cabrera JS, Rajapandi T, Desai SA. Plasmodium falciparum likely encodes the principal anion channel on infected human erythrocytes. Blood. 2004;104:4279–4286. doi: 10.1182/blood-2004-05-2047. [DOI] [PubMed] [Google Scholar]
- Baumeister S, Winterberg M, Duranton C, Huber SM, Lang F, Kirk K, Lingelbach K. Evidence for the involvement of Plasmodium falciparum proteins in the formation of new permeability pathways in the erythrocyte membrane. Mol. Microbiol. 2006;60:493–504. doi: 10.1111/j.1365-2958.2006.05112.x. [DOI] [PubMed] [Google Scholar]
- Bouyer G, Egee S, Thomas SL. Three types of spontaneously active anionic channels in malaria-infected human red blood cells. Blood Cells Mol. Dis. 2006;36:248–254. doi: 10.1016/j.bcmd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Desai SA, Bezrukov SM, Zimmerberg J. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature. 2000;406:1001–1005. doi: 10.1038/35023000. [DOI] [PubMed] [Google Scholar]
- Egee S, Lapaix F, Decherf G, Staines HM, Ellory JC, Doerig C, Thomas SL. A stretch-activated anion channel is up-regulated by the malaria parasite Plasmodium falciparum. J. Physiol. 2002;542:795–801. doi: 10.1113/jphysiol.2002.022970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H, Krugliak M, Eidelman O, Cabantchik ZI. New permeability pathways induced in membranes of Plasmodium falciparum infected erythrocytes. Mol. Biochem. Parasitol. 1983;8:177–190. doi: 10.1016/0166-6851(83)90008-7. [DOI] [PubMed] [Google Scholar]
- Ginsburg H, Stein WD. The new permeability pathways induced by the malaria parasite in the membrane of the infected erythrocyte: comparison of results using different experimental techniques. J. Membr. Biol. 2004;197:113–134. doi: 10.1007/s00232-003-0646-7. [DOI] [PubMed] [Google Scholar]
- Huber SM, Uhlemann AC, Gamper NL, Duranton C, Kremsner PG, Lang F. Plasmodium falciparum activates endogenous Cl channels of human erythrocytes by membrane oxidation. EMBO J. 2002;21:22–30. doi: 10.1093/emboj/21.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SM, Duranton C, Lang F. Patch-clamp analysis of the "new permeability pathways" in malaria-infected erythrocytes. Int. Rev. Cytol. 2005;246:59–134. doi: 10.1016/S0074-7696(05)46003-9. [DOI] [PubMed] [Google Scholar]
- Kirk K, Horner HA, Elford BC, Ellory JC, Newbold CI. Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J. Biol. Chem. 1994;269:3339–3347. [PubMed] [Google Scholar]
- Lange T, Jungmann P, Haberle J, Falk S, Duebbers A, Bruns R, Ebner A, Hinterdorfer P, Oberleithner H, Schillers H. Reduced number of CFTR molecules in erythrocyte plasma membrane of cystic fibrosis patients. Mol. Membr. Biol. 2006;23:317–323. doi: 10.1080/09687860600738304. [DOI] [PubMed] [Google Scholar]
- Staines HM, Powell T, Ellory JC, Egee S, Lapaix F, Decherf G, Thomas SL, Duranton C, Lang F, Huber SM. Modulation of whole-cell currents in Plasmodium falciparum-infected human red blood cells by holding potential and serum. J. Physiol. 2003;552:177–183. doi: 10.1113/jphysiol.2003.051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines HM, Ashmore S, Felgate H, Moore J, Powell T, Ellory JC. Solute transport via the new permeability pathways in Plasmodium falciparum-infected human red blood cells is not consistent with a simple single channel model. Blood. 2006 doi: 10.1182/blood-2006-02-001693. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verloo P, Kocken CH, Van der Wel A, Tilly BC, Hogema BM, Sinaasappel M, Thomas AW, De Jonge HR. Plasmodium falciparum-activated chloride channels are defective in erythrocytes from cystic fibrosis patients. J. Biol. Chem. 2004;279:10316–10322. doi: 10.1074/jbc.M311540200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.