Abstract
Sporothrix schenckii is known to produce DHN melanin on both conidial and yeast cells, however little information is available regarding the factors inducing fungal melanization. We evaluated whether culture conditions influenced melanization of 25 Brazilian S. schenckii strains and one control strain (ATCC 10212). Tested conditions included different media, pH, temperature, incubation time, glucose concentrations, and presence or absence of tricyclazole or L-DOPA. Melanization was reduced on Sabouraud compared to defined chemical medium. The majority of strains produced small amounts of melanin at 37°C and none melanized at basic pH. Increased glucose concentrations did not inhibit melanization, rather increasing glucose enhanced pigment production in 27% of strains. Melanin synthesis was also enhanced by the addition of L-DOPA and its addition to medium with tricyclazole, an inhibitor of melanin synthesis, resulted in fungal melanization, including hyphal melanin production. Our results suggest that different S. schenckii strains have distinct control of melanization and that this fungus can use phenolic compounds to enhance melanization in vitro.
Keywords: Sporothrix schenckii, melanin, culture conditions
1. Introduction
Melanins are hydrophobic polymers of high molecular weight, composed by phenolic and indolic compounds [1,2] produced by organisms in all Kingdoms [3,4]. They are typically black or dark brown in color and their molecular structures are diverse [1]. Melanins are also insoluble in aqueous or organic fluids, resistant to concentrated acid, and susceptible to bleaching by oxidizing agents [2]. Electron spin resonance characteristics have been used to define pigments with stable organic free radicals as melanins [4]. Several fungi can produce melanin and functions of this pigment are related to survival under diverse environmental and host conditions [1,3]. Melanization can also decrease fungal susceptibility to antifungal drugs [2,6,7].
Sporothrix schenckii is the etiological agent of sporotrichosis, a subcutaneous mycosis with a worldwide distribution that can affect humans and other animals [8,9]. This dimorphic fungus is mycelial in the environment [10]. S. schenckii produces melanin via the 1,8-dihydroxynaphthalene (DHN) pentaketide pathway on conidia, but not on hyphae [11]. Importantly, yeast cells can also produce melanin vitro and during mammalian infection [12]. Melanization of S. schenckii yeast cells reduces their phagocytosis by macrophages, which contributes to the development of infection [11]. The importance of melanin on the pathogenesis of sporotrichosis is also supported by the fact that melanized S. schenckii strains appear to cause infection more easily than strains that produce low amounts of this pigment [13]. However, little information is available regarding many aspects of melanin production by S. schenckii and the participation of this virulence factor on the pathogenesis of sporotrichosis. Here we describe several factors that modulate melanin production by S. schenckii and also show that the fungus can utilize exogenous phenolic substrate to enhance melanin production in all morphological forms.
2. Material and methods
2.1. Strains
Twenty six S. schenckii strains maintained at the fungal culture collection of the Laboratorio de Micologia/IPEC/Fiocruz (a Brazilian reference center for diagnosis of Mycosis) were used in this study. One of them was isolated in the United States (ATCC 10212) and was used as a control. The other 25 were isolated from patients in Brazil, with 20 strains from patients infected by scratches or bites of cats and another 5 strains from patients infected by soil or plant handling at Espirito Santo State (Table 1). All strains were identified by morphological characteristics at both 25°C and 37°C, according to criteria previously described [10].
Table 1.
Profile of melanization and procedence of 26 S. schenckii strains used in this study
| Strain | Days to start melanization | Degree of melanizationa | pH | Glucose | ||||
|---|---|---|---|---|---|---|---|---|
| Sabb | MMc | MMLDd | Sab | MM | MMLD | enhancement | ||
| United States | ||||||||
| ATCC 10212 | NMe | NM | 6 | − | − | ++ | None | No |
| Espírito Santo State, Brazil | ||||||||
| IPEC 23249 | NM | NM | 10 | − | − | + | None | 37°C |
| IPEC 23250 | NM | NM | 12 | − | − | + | None | 37°C |
| IPEC 23251 | 9 | 7 | 4 | + | + | + | Acid | No |
| IPEC 23252 | NM | NM | 15 | − | − | + | None | 37°C |
| IPEC 23253 | 3 | 2 | 2 | +++ | +++ | +++ | A/Nf | No |
| Rio de Janeiro State, Brazil | ||||||||
| IPEC 17307 | 7 | 2 | 2 | +++ | +++ | +++ | A/N | 22 and 30°C |
| IPEC 17331 | 7 | 2 | 2 | ++ | +++ | +++ | Acid | No |
| IPEC 17521 | 15 | 11 | 12 | + | + | ++ | A/N | No |
| IPEC 17585 | 8 | 3 | 3 | +++ | +++ | +++ | A/N | No |
| IPEC 17608 | 13 | 6 | 7 | +++ | +++ | +++ | Acid | 22 and 30°C |
| IPEC 17692 | 30 | 6 | 6 | + | + | + | Neutral | No |
| IPEC 17786A | 16 | 10 | 2 | + | + | +++ | A/N | No |
| IPEC 17786B | NM | NM | 12 | − | − | + | None | No |
| IPEC 17920 | 2 | 2 | 2 | ++ | +++ | +++ | A/N | No |
| IPEC 18202 | 2 | 2 | 2 | ++ | +++ | +++ | A/N | 22 and 30°C |
| IPEC 18782A | 18 | 4 | 2 | + | +++ | +++ | A/N | 22 and 30°C |
| IPEC 18782B | NM | NM | 12 | − | − | + | None | No |
| IPEC 22582 | 15 | 5 | 3 | + | ++ | +++ | Neutral | 22 and 30°C |
| IPEC 25374 | 29 | 14 | 6 | + | + | ++ | Neutral | No |
| IPEC 25644 | 9 | 6 | 6 | +++ | +++ | ++ | A/N | No |
| IPEC 25758 | 7 | 2 | 2 | + | +++ | +++ | A/N | 22 and 30°C |
| IPEC 25976 | 9 | 3 | 8 | ++ | ++ | ++ | A/N | No |
| IPEC 26034 | 10 | 3 | 3 | +++ | +++ | +++ | Neutral | No |
| IPEC 26156 | 17 | 10 | 9 | + | +++ | +++ | Acid | 22 and 30°C |
| IPEC 26449 | 2 | 3 | 3 | +++ | +++ | +++ | Neutral | 22 and 30°C |
white colonies (-), pale brown colonies (+), dark brown colonies (++) and black colonies (+++)
Sabouraud Dextrose Agar
Minimal medium
Minimal medium with L-DOPA
No melanization after 30 days
Acid and neutral
2.2. Culture conditions
To check melanization on different media, the strains were plated on Sabouraud Dextrose Agar (10 g/liter neopeptone and 40 g/liter of dextrose with 20 g/liter agar), minimal medium (15 mM glucose, 10 mM MgSO4, 29.4 mM K2HPO4, 13 mM glycine, and 3.0 mM thiamine, pH 5.5), and minimal medium with 1mM L-3,4-dihydroxyphenylalanine (L-DOPA). Plates were incubated at 22°C and observed daily for melanin production. The degree of melanization was scored as (-) if the colonies were white, (+) if the colonies were pale brown, (++) if the colonies were dark brown and (+++) if black colonies were black.
2.3. Influence of temperature, glucose concentration and pH
The strains were plated on minimal medium agar with different glucose conditions (0, 0.3, 0.5, 1.0, 2.0, 4.0, 8.0 and 10.0%w/v) at different temperatures (22, 30 and 37°C). Minimal medium agar plates with pH 4.5, 5.5, 7.0 and 8.0 were also incubated at 22°C. After 15 days the degree of melanin produced on each medium was determined.
2.5. Influence of tricyclazole
Tricyclazole (5-methyl-1,2,4-triazol[3,4] benzothiazole) was dissolved in 0.6% ethanol (EtOH) and added to minimal medium agar [14]. Agar plates with 1, 4, 8 and 16 mg/L tricyclazole were prepared. Tricyclazole inhibits DHN melanin formation by blocking the formation of scytalone. Controls without tricyclazole were used to test the effects of EtOH on melanin synthesis and consisted of minimal medium with 0.6% EtOH. The strains plated on all media were incubated in the dark at 22°C for 15 days and assessed for melanin production.
2.6. Non-denaturing gel electrophoresis
The enzymatic ability of S. schenckii to convert L-DOPA on melanin was detected using 10% PAGE under non-denaturing conditions. Cytoplasmic extracts of S. schenckii (ATCC 10212, IPEC 23252, IPEC 22582 and IPEC 26449) were extracted by mechanical disruption of mycelial and yeast cells, and centrifugation at 5,000g for 30 min at 4°C. Gels were loaded with 300 ug of antigen. Extracts boiled for 10 min was used as the negative control. The electrophoresis was conducted at 30V for 18 h followed by immersion of the gels in 1 mM L-DOPA dissolved in citrate phosphate bu ffer (0.1 M citric acid, 0.2 M Na2HPO4, pH 6.0) and incubated at room temperature (RT) with gentle agitation. After overnight incubation, gels were checked for the presence of bands compatible with L-DOPA polymerization.
2.7. Production of melanin particles
Since some strains failed to macroscopically melanize in the conditions described above, melanin particles of these strains grown on minimal medium at 30°C and 37°C were generated. Additionally, to analyze melanin particles grown in the presence or absence of tricyclazole and/or L-DOPA, S. schenckii strain IPEC 26449 was used. This strain was chosen because it produces high amounts of melanin on minimal medium agar plates. The mycelium phase was incubated in 100 mL minimal medium, minimal medium with L-DOPA, minimal medium with tricyclazole (8mg/L) and minimal medium with L-DOPA and tricyclazole at 30°C for 10 days, under shaking (150 rpm). Yeast cells of the strains were inoculated in the same mediums at 37°C. Cell-associated melanin particles were isolated by the methodology previously described by Alviano and coworkers [15]. The generated particles were examined under light microscopy using an Olympus AX 70 microscope.
2.8. EPR analysis
Particles recovered from pigmented cells were analyzed by electron paramagnetic resonance (EPR) using a Gunn diode as the microwave source. EPR spectra were obtained with a Varian E112X-Band model spectrometer. The parameters for EPR were as follows: modulation frequency, 9.07 GHz; modulation amplitude, 1,633.0 G; center field, 3,240.0 G; sweep width, 80.0 G; microwave frequency, 9.2995 GHz; microwave power, 0.20 mW; and temperature, 77 K. As controls, we used Cryptococcus neoformans cells grown in medium without L-DOPA (nonpigmented cells) and L-DOPA C. neoformans melanin particles.
2.9. Melanin ELISA
Fifty ug of melanin-like particles isolated from S. schenckii strain 26449 under all culture conditions were coated in triplicate onto 96-well plates, blocked with 200 ul of Superblock Blocking Buffer in PBS (Pierce, Rockford, IL), incubated with antibodies (pool of sera from 40 patients with culture confirmed sporotrichosis and pooled control serum from 40 patients with no history of mycoses), conjugate [alkaline phosphatase-conjugated goat anti-human immunoglobulin G (Southern Biotech, Birmingham, AL, USA)], added enzyme substrate [1 mg/mL pNPP in 0.1 M glycine buffer (pH 10.4)] followed by addition of 3 M NaOH. A405 nm was measured with an ELISA plate reader (Bio-Tek model μQuant). Additional negative controls consisted either of uncoated wells or coated wells in which the primary antibody was omitted. Sepia and synthetic melanins (Sigma) were used as controls.
2.10. Statistics
Graphs and determination of median values were made with Sigma Plot 2000 software. Student’s t test was performed using Prism 3.0 software.
3. Results
3.1. Melanization on different media
To determine rates of melanization, 26 S. schenckii strains were plated on Sabouraud Dextrose Agar, minimal medium and minimal medium with L-DOPA at 22°C. The strains had diverse rates of pigment production and these varied with the medium used. In general, melanization on Sabouraud occurred later than in the other media. The median time for melanization on Sabouraud, minimal medium and minimal medium with L-DOPA was 14, 6 and 5 days, respectively. Some strains start to produce melanin early, but do not produce heavily melanized colonies. In contrast, some strains are delayed in melanization though they eventually form very dark colonies. The degree and rate of melanization for each strain after 30 days is shown in Table 1. The Sporothrix strains with the greatest amount of melanin include one from Brazilian Espirito Santo State and 13 strains from Rio de Janeiro. Strains unable to produce visible pigmented colonies on media without L-DOPA after 30 days of incubation include the control strain, 3 strains from Espírito Santo State and 2 strains from Rio de Janeiro. Other strains produced intermediate amounts of melanin.
3.2. Melanization at different temperatures
All the strains were inoculated on minimal medium at temperatures of 22° C, 30°C and 37°C and melanin production was determined 30 days later. The degree of melanization of the strains incubated at 30°C was similar to that incubated at 22°C (Table 1). Only 23% (n=6) of strains produced pigmented colonies at 37°C, 3 from Rio de Janeiro (IPEC 17307, IPEC 17521 and IPEC 25976) and 3 from Espirito Santo (IPEC 23249, IPEC 23250 and IPEC 23251). Some of these strains were unable to produce melanin at 30°C (Table 1).
3.3. Melanization at different glucose concentrations
Glucose was essential for melanization on minimal medium and overall growth was severely restricted in the absence of glucose. However, the majority of strains (n=18, 69%) grew and melanized similarly at glucose concentrations ≥ 0.3% w/v after 15 days of incubation. Strain IPEC 23250 produced melanized colonies at glucose concentrations ≥ 2%w/v, strain IPEC 17786B melanized in media with ≥ 8% glucose, and pigmentation occurred in strain IPEC 17521 only in 10% glucose. Four strains did not melanize on any glucose concentration tested at 22°C. These strains include the control strain (ATCC 10212), and 3 Brazilian strains (IPEC 23249, IPEC 23252, IPEC 18782B). Table 1 shows the melanin production according to temperature and glucose concentration.In 8 strains (31%) the melanin production at 22°C and at 30°C was enhanced in a glucose concentration dependent manner. For 3 strains (12%), this phenomenon occurred at 37°C but not at 22 or 30°C.
3.4. Effect of pH on melanization
Overall, basic pH (8.0) inhibited melanin production. It was observed that the same 6 S. schenckii strains unable to melanize on minimal medium without L-DOPA also produced albino colonies over the pH range examined. Eleven strains (42%), all but one from Rio de Janeiro, had the same profile of melanin production on both neutral and acidic pH. Five strain (19%), all from Rio de Janeiro, produced more melanin on neutral pH (7.0) and 4 (15%), one from Espirito Santo and the other 3 from Rio de Janeiro, synthesized more melanin on pH 4.0 and 5.0.
3.5. Melanization in the presence of tricyclazole
The tricyclazole concentrations tested inhibited the production of black melanin in all strains. Instead, a reddish pigment was seen on the cultures. Ethanol did not have any effect on S. schenckii melanization, since melanization was similar when the fungi were grown with or without 0.6% EtOH (data not shown).
3.6. Melanin particles from albino isolates
We generated melanin particles of 3 strains that did not produce visibly melanized colonies on any tested L-DOPA free culture conditions (ATCC 10212, IPEC 17786B and IPEC 18782B) to determine if they were truly melanin deficient. These 3 strains were able to produce small amounts of dark particles after protease, denaturant and hot acid treatment when cultured at 30°C that were similar in size and shape as S. schenckii conidia (Fig. 1A). These strains produced melanin particles when cultured at 37°C as well (Fig. 1B), and the amount of melanin particles for the yeast phase was visibly higher than for the mycelial phase.
Figure 1.
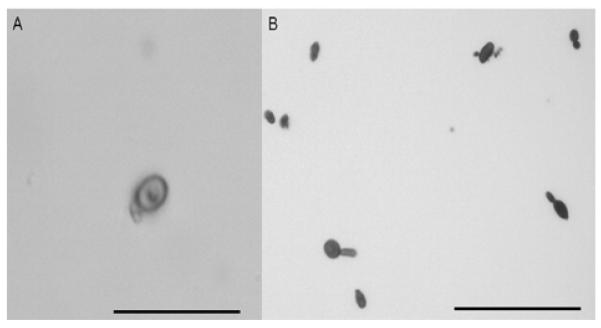
Melanin particles generated from albino colonies after incubation on minimal medium broth. (A) conidial ghost of 18782B strain, magnification 1000X. (B) yeast particles of 18782B strain, magnification 400X. Bars, 10 μm.
3.7. S. schenckii can use L-DOPA for melanogenesis
S. schenckii strains grown in medium supplemented with L-DOPA were, in general, darker than cultures grown in medium without this phenolic compound, even in the presence of elevated concentration of tricyclazole (Fig. 2A). The biochemical machinery to metabolize L-DOPA is S. schenckii were evaluated in a non-denaturing gel incubated with L-DOPA. Extracts from mycelia and yeast similarly produced the formation of dark bands on the gels consistent with polymerized L-DOPA melanin (Fig. 2B). Boiling the extracts abrogated enzymatic activity.
Figure 2.
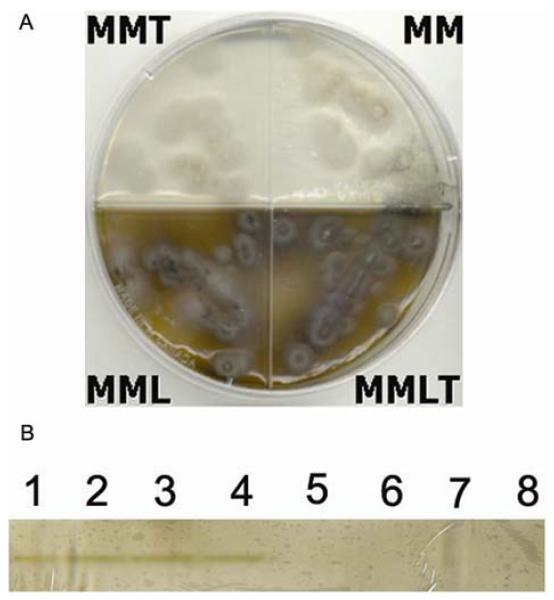
L-DOPA can be used for melanogenesis by S. schenckii. (A) S. schenckii strain IPEC 23252 on minimal medium (MM), minimal medium with tricyclazole (MMT), minimal medium with L-DOPA (MML) and minimal medium with L-DOPA and tricyclazole (MMLT). (B) PAGE of native S. schenckii mycelial antigens (1 to 4) showing a dark band compatible with L-DOPA polymerization, when antigens are boiled (5 to 8) this band disappears. 1 and 5: ATCC 10212, 2 and 6: IPEC 23252, 3 and 7: IPEC 22582, 4 and 8: IPEC 26449. Yeast antigens of the same strains produced a similar reaction.
3.8. Melanin particles in presence of L-DOPA and tricyclazole
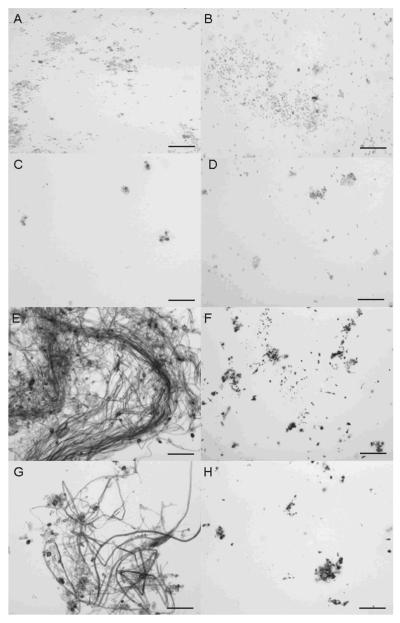
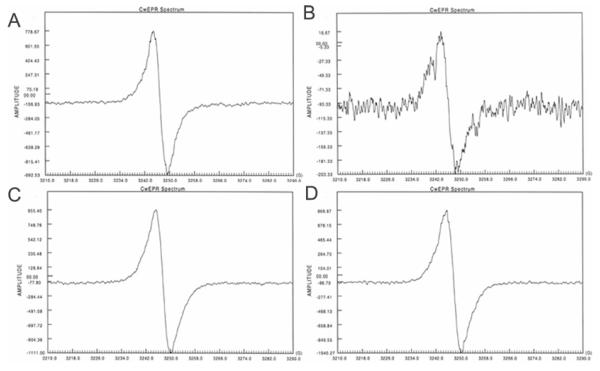
Melanin particles were obtained from S. schenckii 26449 conidia and yeast treated with enzymes and hot acid (Fig. 3A and B), whereas hyphae were completely solubilized. The addition of tricyclazole to the medium significantly reduced the quantity of particles isolated and the particles were lighter in color and dysmorphic (Fig. 3C and D) compared to particles obtained in the absence of tricyclazole. In contrast, numerous dark particles were recovered from both hyphae, conidia and yeast forms of S. schenckii grown in minimal medium with L-DOPA (Fig. 3E and F). Adding tricyclazole to the cultures with L-DOPA did not significantly impact the appearance of particles obtained from either mycelial or yeast forms of S. schenckii (Fig. 3G and H). All particles produced a signal indicative of a stable free-radical population (Fig. 4), although the intensity of the signal was lower for particles obtained from cultures with tricyclazole only and it was higher for particles generated from cultures with L-DOPA. Hence, all of the isolated particles met EPR criteria for classification as melanin-like pigments [5].
Figure 3.
Melanin particles of mycelial (A, C, E and G) and yeast forms (B, D, F and H) of S. schenckii 26449 strain grown on minimal medium (A and B), minimal medium with 8.0 mg/L tricyclazole (C and D), minimal medium with L-DOPA (E and F) and minimal medium with tricyclazole and L-DOPA (G and H). Magnification 400X. Bars, 10μ
Figure 4.
EPR analyses of mycelial S. schenckii melanin particles generated on minimal medium (A), minimal medium with 8.0 mg/L tricyclazole (B), minimal medium with L-DOPA (C) and minimal medium with tricyclazole C and L-DOPA (D). Note that amplitude of signals is lower on (B) and higher on (C) and (D). The spectrum on (B) was recorded at maximum gain in an effort to identify a signal and hence has more background noises. Acid-resistant S. schenckii particles derived from yeast forms had similar spectra to mycelial particles generated from the same culture medium.
3.9. Immunological reactivity of melanin-like particles
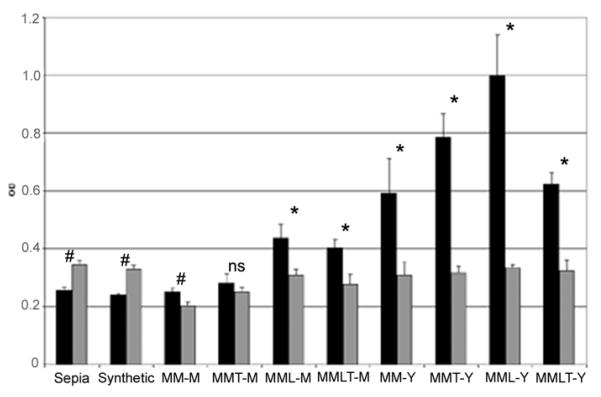
Since structures of polymerized melanins are still unknown, an ELISA technique was applied to examine differences in immunological reactivity. Sepia melanin, synthetic melanin and melanin-like particles generated from S. schenckii strain 26449 were tested with sera from normal human subjects and from patients with sporotrichosis (Fig. 5). Both Sepia and synthetic melanin react with sera from both normal human subject and sporotrichosis patients group but the absorbance was higher for pooled normal human sera. In contrast, the part icles obtained from S. schenckii consistently reacted more significantly and intensely with sporotrichosis sera than the control sera except for mycelial particles generated in minimal medium with tricyclazole (t=1.49, P=0.12). Mean absorbances for yeast particles generated were higher than absorbances for mycelial particles (t=7.63, P<0.0001). We also observed that immunological reactivity of particles generated on minimal medium with L-DOPA compared to particles generated on L-DOPA free medium was higher for both mycelial and yeast forms (t=7.25, P=0.003 and t=4.4, P=0.005 respectively).
Figure 5.
ELISA reactivities of sera from patients with sporotrichosis (Sporo) and from normal human subjects (NHS) diluted 1:100, with Sepia and synthetic melanins and with the following eight different types of S. schenckii melanin-particles (50 μg/well): mycelium grown in minimal medium (MM-M), mycelium grown on minimal medium with tricyclazole (MMT-M), mycelium grown on minimal medium with L-DOPA (MML-M), mycelium grown on minimal medium with tricyclazole and L-DOPA (MMLT-M), yeast grown on minimal medium (MM-Y), yeast grown on minimal medium with tricyclazole (MMT-Y), yeast grown on minimal medium with L-DOPA (MML-Y) and yeast grown on minimal medium with L-DOPA and tricyclazole (MMLT-Y). OD, optical density. * P < 0.0001; # P < 0.05; ns: not significant.
4. Discussion
Melanization is associated with virulence for the human pathogenic fungi S. schenckii and the fungus produces melanin in vivo and in vitro [11,12]. In this study, we analyzed the effect of culture condition on pigment production by this fungus and demonstrated that melanin production is variable among strains. Basic pH and the presence of tricyclazole (inhibitor of DHN melanin synthesis) were the only conditions that consistently impaired melanin production. Melanization on Sabouraud was also delayed and quantitatively less compared to minimal medium with or without L-DOPA. Interestingly, more strains produced melanin at 30°C compared to 37°C. Since there is no data available regarding S. schenckii melanization during mycelial to yeast transition that occurs around 37°C, this difference can be related to changes in morphology of fungal cells or even to differences in the mechanisms of melanization.
Melanogenesis in C. neoformans is repressed by high concentrations of glucose [16]. S. schenckii grown on minimal medium (0.3% w/v glucos e) produced more melanin compared to cells grown on Sabouraud (4.0%w/v glucose). Glucose did not impede melanin synthesis. The majority of strains produced black pigmented colonies at all glucose concentrations. Hence, the differences in melanization on Sabouraud and mi nimal medium are not related to carbohydrate concentration, but to other characteristics of these media. Furthermore, melanin production in several strains was actually enhanced with glucose, in a concentration dependent manner. The distinct behavior between C. neoformans and S. schenckii may be attributed to the different metabolic pathways used to produce melanin. S. schenckii can produce melanin by the DHN pathway and it has been proposed that, if the tricarboxylic acid cycle is saturated, acetyl-CoA units generated after glycolysis can be converted into pentaketides that can be used to synthesize DHN-melanin [17]. Thus, this metabolic pathway may participate in the augmented production of melanin in some strains under high glucose conditions. However, there are likely to be additional factors, since other S. schenckii strains did not manifest this behavior.
S. schenckii is able to grow over a broad pH range, from 3.0 to 12.5 [18]. Three different patterns of melanin production for the different tested pHs were observed. One group of strains produced more melanin on neutral pH (23% of strains), another group did not differ in melanin production at acidic or neutral pH (42% of strains) and a third group (15% Rof strains) produced higher amounts of black pigment under acidic conditions. In general, most plants and some soil types exist at an environmental pH around 7.0, which is approximately the pH of most anatomical sites of mammalian bodies. Therefore S. schenckii’s capacity of producing melanin over a broad pH range, especially on neutral pH is advantageous for survival on environmental and pathogenic conditions.
Five strains failed to produce visible amounts of melanin on the culture conditions used on our study. C. neoformans melanin deficient mutants have been described [19], thus we investigated if these white colony forming S. schenckii strains were melanin mutants by generating melanin particles from mycelial and yeast forms of them. All of the visually albino isolates were able to produce small amounts of mycelial melanin particles, showing they have the machinery to produce melanin. It is possible that there are additional structural aberrancies (such as cell wall or secretion defects) in these strains that impede the polymerization of melanin. For instance, Candida albicans makes melanin, but it is not apparently well polymerized on the cell wall [20]. Furthermore, normal amounts of yeast phase derived melanin particles were produced by the albino mycelial S. schenckii strains, proving that the strains were capable of synthesizing this virulence factor under their pathogenic morphological phase. Therefore, mechanisms of control of melanin synthesis at mycelial and yeast phases on this fungus appear to be distinct. Since melanin production during S. schenckii mammalian infection is important to the pathogenesis of sporotrichosis [12], normal production of melanin in the yeast phase participates in the induction of infection, even if it fails to produce melanin in the mycelial form.
During the experiments with different culture media, we found that several strains produced more melanin on L-DOPA supplemented medium. For this reason, melanin particles generated from medium with and without L-DOPA were studied. Our results strongly suggest that S. schenckii can utilize L-DOPA to enhance melanin production because the melanin deficient producing strains were able to produce pigment on this medium. Melanin particles generated from L-DOPA cultures were darker than particles generated from cultures without this compound, production of melanin was not suppressed in medium with L-DOPA and tricyclazole, and laccase-like activity was detected on cytoplasmatic proteins of both mycelial and yeast forms of S. schenckii. It has been described a phenoloxidase activity in S. schenckii [18], and the growth of a black colony on Bird Seed Agar was considered as indicative of phenoloxidase activity, without making distinction between DHN melanin and melanin derived from exogenous phenolic compounds. Our results now show that S. schenckii can use L-DOPA, and probably other phenolic compounds, for melanin synthesis. We previously suggested that the ability of S. schenckii cells to produce melanin when grown in minimal medium indicates that this fungus has the enzymes necessary to synthesize precursors required for the formation of melanin in addition to any mechanism which utilizes exogenous phenolic compounds [12], and our new results strongly support this statement.
Melanin production by S. schenckii was first described on conidial cells [11] and then on yeast cells [12]. In both studies, no melanin was synthesized on hyphal forms of this fungus. In this work, melanin particles derived from hyphae grown in L-DOPA medium was isolated. This provides additional evidence in support of L-DOPA melanin synthesis in S. schenckii and that the pathway for eumelanin production is distinct from the DHN processes. To our knowledge, this is the first report of melanin production by hyphal forms of S. schenckii.
There are several implications for the ability of this fungus to use L-DOPA in melanin synthesis, conferring advantages to survival especially during infection of mammals or other hosts. For instance, S. schenckii can infect the central nervous system [9,21], where large amounts of L-DOPA are produced by neurons, so yeast forms of this fungus could potentially use this compound to enhance melanin production. During cutaneous sporotrichosis, S. schenckii can utilize L-DOPA or other phenolic compounds associated with melanin production by melanocytes in skin to augment the production of the virulence-associated pigments.
An ELISA technique [22] was used to evaluate antigenic differences between the melanin particles generated. In general, mean absorbance values for all melanin particles probed with sera from sporotrichosis patients were significantly higher compared to values obtained using pooled normal human. Absorbances were even higher when the sporotrichosis sera was tested against L-DOPA derived particles from S. schenkii. This could be due to the fact of these particles presented more antigenic determinants than those generated without L-DOPA or that antibodies in sera from infected patients Pare directed to epitopes of the melanin expressed during infection that react preferentially with L-DOPA melanin. This second hypothesis also suggests that L-DOPA melanin is produced in vivo by S. schenckii.
In conclusion, S. schenckii has several mechanisms to control melanin production that can be affected by various factors such as pH and carbohydrate concentration. Also, these mechanisms appear to be differentially regulated on mycelial and yeast forms of the fungus. As with C. neoformans [16,23] and P. brasiliensis [24], S. schenckii can utilize phenolic compounds to augment melanin production, which may be associated with a concomitant increase in protection against unfavorable conditions in both the environmental and during infection.
Acknowledgements
R. A. P. was supported by an Interhemispheric Research Training Grant in Infectious Diseases, Fogarty International Center (NIH D43-TW007129). J. D. N. is supported in part by NIH AI52733 and AI056070-01A2, and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI-51519). R. M. Z. O. is in part supported by CNPq 306288/2006-0 and FAPERJ E26/111.619/2008. We thank Gary Gerfen for assistance with EPR experiments and Michael Ferustein for his collaboration with the culture experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gomez BL, Nosanchuk JD. Melanin and fungi. Curr. Opin. Infect. Dis. 2003;16:91–96. doi: 10.1097/00001432-200304000-00005. [DOI] [PubMed] [Google Scholar]
- [2].Nosanchuk JD, Casadevall A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 2006;50:3519–3528. doi: 10.1128/AAC.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nosanchuk JD, Casadevall A. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 2003;5:203–223. doi: 10.1046/j.1462-5814.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- [4].Riley PA. Melanin. Int. J. Biochem. Cell Biol. 1997;29:1235–1239. doi: 10.1016/s1357-2725(97)00013-7. [DOI] [PubMed] [Google Scholar]
- [5].Enochs WS, Nilges MJ, Swartz HM. A standardized test for the identification and characterization of melanins using electron paramagnetic (EPR) spectroscopy. Pigment Cell Res. 1993;6:91–99. doi: 10.1111/j.1600-0749.1993.tb00587.x. [DOI] [PubMed] [Google Scholar]
- [6].van de Sande WW, de Kat J, Coppens J, Ahmed A, Fahal A, Verbrugh H, van Belkum A. Melanin biosynthesis in Madurella mycetomatis and its effect on susceptibility to itraconazole and ketoconazole. Microbes Infect. 2007;9:1114–1123. doi: 10.1016/j.micinf.2007.05.015. [DOI] [PubMed] [Google Scholar]
- [7].van Duin D, Casadevall A, Nosanchuk JD. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob. Agents Chemother. 2002;46:3394–3400. doi: 10.1128/AAC.46.11.3394-3400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cheatwood JL, Jacobson ER, May PG, Farrel TM, Homer BL, Samuelson DA, Kimbrough JW. An outbreak of fungal dermatitis and stomatitis in a free-ranging population of pigmy rattlesnakes (Sistrurus miliarius barbouri) in Florida. J. Wildl Dis. 2003;39:329–337. doi: 10.7589/0090-3558-39.2.329. [DOI] [PubMed] [Google Scholar]
- [9].Ramos-e-Silva M, Vasconcellos C, Carneiro S, Cestari T. Sporotrichosis. Clin. Dermatol. 2007;25:181–187. doi: 10.1016/j.clindermatol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- [10].Dixon DM, Salkin IF, Duncan RA, Hurd NJ, Haines JH, Kemna ME, Coles FB. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of Sporotrichosis. J. Clin. Microbiol. 1991;29:1106–1113. doi: 10.1128/jcm.29.6.1106-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Romero-Martinez R, Wheeler M, Guerrero-Plata A, Rico G, Torres-Guerrero H. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect. Immun. 2000;68:3696–3703. doi: 10.1128/iai.68.6.3696-3703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Morris-Jones R, Youngchim S, Gomez BL, Aisen P, Hay RJ, Nosanchuk JD, Casadevall A, Hamilton AJ. Synthesis of melanin-like pigments by Sporothrix schenckii in vitro and during mammalian infection. Infect. Immun. 2003;71:4026–4033. doi: 10.1128/IAI.71.7.4026-4033.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cooper CR, Dixon DM, Salkin IF. Laboratory-acquired sporotrichosis. J. Med. Vet. Mycol. 1992;30:169–171. doi: 10.1080/02681219280000221. [DOI] [PubMed] [Google Scholar]
- [14].Mares D, Romagnoli C, Andreotti E, Manfrini M, Vicentini CB. Synthesis and antifungal action of new tricyclazole analogues. J. Agric. Food Chem. 2004;52:2003–2009. doi: 10.1021/jf030695y. [DOI] [PubMed] [Google Scholar]
- [15].Alviano DS, Franzen AJ, Travassos LR, Holandino C, Rpzental S, Ejzemberg R, Alviano CS, Rodrigues ML. Melanin from Fonseacaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect. Immun. 2004;72:229–237. doi: 10.1128/IAI.72.1.229-237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Frases S, Salazar A, Dadachova E, Casadevall A. Cryptococcus neoformans can utilize the bacterial melanin precursor homogentisic acid for fungal melanogenesis. Appl. Environ. Microbiol. 2007;73:615–621. doi: 10.1128/AEM.01947-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee JK, Jung HM, Kim SY. 1,8-dihydroxynaphthalene (DHN)-melanin biosynthesis inhibitors increase erythritol production in Torula corallina, and DHN-melanin inhibits erythrose reductase. Appl. Environ. Microbiol. 2003;69:3427–3434. doi: 10.1128/AEM.69.6.3427-3434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ghosh A, Maity PK, Hemashettar BM, Sharma VK, Chakrabarti A. Physiological characters of Sporothrix schenckii isolates. Mycoses. 2002;45:449–454. [PubMed] [Google Scholar]
- [19].Mandal P, Banerjee U, Casadevall A, Nosanchuk JD. Dual infections with pigmented and albino strains of Cryptococcus neoformans in patients with or without human immunodeficiency virus infection in India. J. Clin. Microbiol. 2005;43:4766–4772. doi: 10.1128/JCM.43.9.4766-4772.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morris-Jones R, Gomez BL, Diez S, Uran M, Morris-Jones SD, Casadevall A, Nosanchuk JD, Hamilton AJ. Synthesis of melanin pigment by Candida albicans in vitro and during infection. Infect Immun. 2005;73:6147–6150. doi: 10.1128/IAI.73.9.6147-6150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vilela R, Souza GF, Fernandes-Cota G, Mendoza L. Cutaneous and meningeal sporotrichosis in a HIV patient. Rev. Iberoam. Micol. 2007;24:161–163. doi: 10.1016/s1130-1406(07)70035-9. [DOI] [PubMed] [Google Scholar]
- [22].Rosas AL, Nosanchuk JD, Gomez BL, Edens WA, Henson JM, Casadevall A. Isolation and serological analyses of fungal melanins. J. Immunol. Methods. 2000;244:69–80. doi: 10.1016/s0022-1759(00)00255-6. [DOI] [PubMed] [Google Scholar]
- [23].Eisenman HC, Mues M, Weber SE, Frases S, Chaskes S, Gerfen G, Casadevall A. Cryptococcus neoformans laccase catalyses melanin synthesis from both D- and L-DOPA. Microbiology. 2007;153:3954–3962. doi: 10.1099/mic.0.2007/011049-0. [DOI] [PubMed] [Google Scholar]
- [24].Silva MB, Marques AF, Nosanchuk JD, Casadevall A, Travassos LR, Taborda CP. Melanin in the dimorphic fungal pathogen Paracoccidioides brasiliensis: effects on phagocytosis, intracellular resistance and drug susceptibility. Microbes Infect. 2006;8:197–205. doi: 10.1016/j.micinf.2005.06.018. [DOI] [PubMed] [Google Scholar]