Abstract
Multipotent cells that can give rise to bone, cartilage, fat, connective tissue, skeletal and cardiac muscle are termed mesenchymal stem cells (MSCs). These cells were first identified in the bone marrow, distinct from blood-forming stem cells. Based on the embryologic derivation, availability, and various pro-regenerative characteristics, research exploring their use in cell therapy shows great promise for patients with degenerative muscle diseases and a number of other conditions. In this review, the authors explore the potential for MSC therapy in the emerging field of regenerative medicine with a focus on treatment for Duchenne muscular dystrophy (DMD).
Introduction
DMD is a progressive, lethal, X-linked disease of skeletal and cardiac muscle affecting nearly 1 in 3500 males born each year in the US. DMD is caused by mutation of the dystrophin gene (2.4 megabases – the largest known gene) that, together with its location at Xp21 provides a vulnerable target for new mutations. The cardiac and skeletal muscles of DMD patients are deficient in the dystrophin gene product, a 427-kD protein found primarily in the outer cell membrane in cardiac and skeletal muscle (1). Without dystrophin in the outer membrane, the muscle fiber is particularly vulnerable to damage from normal daily activities (2). As a result, damaged DMD muscle fibers eventually succumb to injury (3). Normally, muscle damage is repaired by resident muscle stem cells (satellite cells) (4). However, continuous cycles of damage eventually overwhelms the capacity for regeneration, potentially due to impaired ability of muscle satellite cells (5). To address this progressive and ultimately fatal degeneration in DMD muscles, intense research efforts are aimed at tilting the balance in favor of regeneration. Stem cell transplantation therapy may offer one approach to enhance the regenerative ability of damaged and degenerating muscle cells in patients with DMD.
Pharmacologic agents
New technologies in the emerging field of regenerative medicine and stem-cell therapy are currently under development in the United States by more than 100 companies investing nearly 850 million dollars in 2007 with over 55 products in either clinical or preclinical trials (6). Some of these new technologies in the field of stem cell therapy will undoubtedly trickle down to benefit patients with DMD. For now, the major therapeutic strategies for DMD patients (Table 1) pharmacologic agents, gene therapy and stem cell therapy. These agents include corticosteroids (7), calcium-regulating agents (8), myostatin inhibitors (9, 10), stop codon suppressing agents (11–13), protease inhibitors (14–16), and others (17, 18). Most pharmacologic agents are not capable of providing a cure for the disease because they do not correct the underlying genetic defect that causes dystrophin deficiency in DMD patients. Correction of genetic defects in DMD patients will most likely be accomplished by gene- or cell-based strategies, or by a combination of both pharmacologic and genetic strategies (19, 20). Cell-based therapies (given with immunosuppressive agents) targeted toward replacement of dystrophin-deficient muscle cells have been reported in pre-clinical settings with success (21). The following section introduces the general concepts behind gene- and cell-based strategies.
Table 1. Overview of major therapeutic strategies for DMD patients.
| Pharmacologic agents | |
|---|---|
| Corticosteroids (prednisone, prednisolone) | |
| Calcium-regulating agents (ace-inhibitors, calcium channel blockers) | |
| Myostatin inhibitors (myostatin antibodies, follistatin derivatives) | |
| Stop codon suppressing agents (PTC 124, aminoglycosides) | |
| Protease inhibitors (leupeptin) | |
| Others (anti-inflammatory agents, L-arginine, nitric oxide-releasing agents, beta-blockers) | |
| Gene transfer | Micro or mini dystrophin, anti-sense oligonucleotides |
| Gene modification | Utrophin |
| Cell therapy | Myoblasts, satellite cells, SP cells, meso-angioblasts, adult stem cells, pericytes, mesenchymal stem cells, embryonic stem cells |
Gene transfer
Most gene therapy strategies for DMD are aimed at delivering coding regions of dystrophin or the dystrophin-related gene, utrophin, using viral vectors such as the adeno-associated virus (AAV)(22). These vectors deliver gene sequences to skeletal muscle and heart using the host’s own cellular machinery to generate and replace deficient proteins. A potential drawback to viral vectors or recombinant gene products is that they can activate immune reactions in DMD patients who are naïve to dystrophin. Strategies to reduce the vector load are being explored by testing different vector serotypes and infusion methods (23). For example, vector-less delivery of small DNA/RNA fragments called antisense oligonucleotides (AOs) may offer one solution to vector-induced immune response. The majority of DMD patients have deletions caused by errors in the large dystrophin gene leading to disruption of the open reading frame causing dystrophin deficiency. AOs can correct the reading frame of some, but not all dystrophin transcripts, yielding a truncated, but functional dystrophin gene product. A pilot clinical trial of AOs in four Dutch DMD patients was recently reported with promising results (24).
Gene modification
As an alternate gene therapy approach to dystrophin replacement in DMD patients, another approach involves over-expression of utrophin (25–30). In preclinical DMD animal models, over-expression of utrophin, a gene product with structural and functional similarities to dystrophin, resulted in reduced muscle pathology. For example, in mdx mice, force development and resistance to mechanical stretch recovered to about 80% of normal following utrophin over-expression (31). In newborn GRMD dogs, intramuscular injection of an adenoviral vector expressing a synthetic utrophin led to reduced fibrosis and increased expression of dystrophin-associated proteins (32). These results suggest that gene modification of utrophin expression can functionally compensate for lack of dystrophin in dystrophin-deficient muscles.
Cell therapy
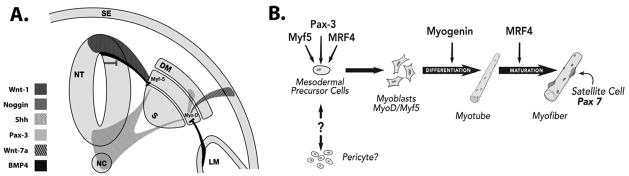
Diseased tissue may be regenerated in vivo by transplantation of healthy cells that can extensively replicate. Progenitor and stem cells have this intrinsic ability, and are used in certain clinical settings to enhance or restore damaged tissue. In attempts to regenerate muscle cells replete with dystrophin in the muscles of patients with dystrophin deficiency, several types of muscle-derived cell transplantation strategies have been tested in animals and in a few DMD patients. A muscle precursor cell, known as the myoblast (Figure 1), was one of the first cell types explored in DMD studies. Previous attempts to transplant myoblasts with intramuscular injections have not accomplished markedly effective results because of rapid death of most injected myoblasts and the failure of injected myoblasts to migrate more than ~0.5 mm away from the injection site. Satellite cells (skeletal muscle precursors) have also been explored as a potential cell source for dystrophin replacement therapy. The muscle satellite cell is positioned between the plasma membrane and the surrounding basal membrane of adult skeletal muscle fibers, and expresses CD34, Pax 3 and Pax 7 (Figure 1).
Figure 1. Stem cells in skeletal muscle development.
A. In the developing embryo, muscle formation is regulated by signaling pathways on either side of the notochord (NC). Within the somite (S) are the sclerotome and dermomyotome (DM). Signals from the notochord, neural tube (NT) and surface ectoderm (SE) begin events that lead to myogenic differentiation. The ventral neural tube and notochord produce Sonic hedgehog (Shh), whereas the dorsal neural tube produces Wnt-1.
B. Pax-3, Myf-5 and MRF4 (Myf-6) activate MyoD in mesodermal precursor cells committing them to the myogenic lineage.
Developmental biology of muscle
The embryology of skeletal muscle development is of interest to cell therapy applications because of similarities with adult stem cell fate and function (33). As embryonic stem cells become differentiated specialized cell types, they advance along a spectrum of potency (34): totipotent cells (that give rise to an entire organism); pluripotent cells (that produce all three embryonic germ layers); multipotent cells (that become various cell types within a single germ layer); and unipotent cells (that only form cells of a single lineage) (35). The three embryonic germ layers are the ectoderm, from which cells such as neurons and epidermis are derived; the endoderm, from which beta cells of the pancreas, and hepatocytes, are derived; and the mesoderm, from which cardiac and skeletal muscle and red blood cells derive.
Myogenesis is regulated by complex signaling pathways originating from tissues surrounding the paraxial mesoderm (Figure 1). Mesoderm contains mesenchyme, a primordial tissue consisting of mesenchymal cells in a gelatinous support material. Beginning in the 3rd to 4th week of gestation, the paraxial mesoderm segments into somites (paired epithelial cell masses). Within the somite are two specialized areas known as the sclerotome and the dermomyotome. The ventrally located sclerotome will give rise to cartilage and bone while the dorsally located dermomyotome (DM) will give rise to skin and skeletal muscle. With the exception of the head, all skeletal muscle in mammals arises from the mesenchymal cells of the somite. Signals from the notochord, neural tube and surface ectoderm initiate events that lead to myogenic differentiation. Together, both Shh and Wnt-1 (Figure 1A) are essential to the early activation of Myf5 to induce myogenesis (36–38). Myf-5 is expressed by cells in the epaxial domain of the myotome which give rise to back muscles. Members of the Wnt family also mediate the signals that induce and maintain the dermomyotome (39). Additionally, Shh and Wnt-1 together can induce Pax-3 expression in the dermomyotome (40), a key regulator in the specification of cells that will form skeletal muscle (41). Pax-3, a member of the Myo-D family of basic helix-loop-helix transcription factors (MyoD, Myf5, myogenin and MRF4), acts synergistically with the myocyte enhancer factor 2 (MEF2) to control skeletal muscle formation (42). Upregulation of MyoD and Myf5 are critical to directing cells to the myogenic lineage (43, 44). Pax-3, Myf-5, Myf-6 (MRF4) and Wnt-7a (Figure 1B) can all initiate the transcription of MyoD(45). Premature differentiation is antagonized by BMP4, from the lateral mesoderm (LM) and dorsal neural tube. BMP4 inhibits MyoD and Myf5 in Pax-3-expressing cells of the dermomyotome. This effect is counteracted by Noggin from the dorsal neural tube, (46) which is in turn produced by Wnt1.
Upon activation, satellite cells may also express MyoD, Myf-5, and M-Cadherin. These muscle precursor cells have the ability to expand when transplanted to form functional Pax 7+ satellite cells with the capacity to regenerate damaged skeletal muscle in vivo. Technical hurdles for muscle precursor-based cell strategies include harvesting, maintenance, and expansion of cells for clinical use. In the sections that follow, we provide background information about stem cell therapy and discuss the potential for stem cells derived from a variety of sources as one promising avenue for the treatment of degenerative muscle diseases, such as DMD.
Adult stem and progenitor cells
An adult stem cell may be defined as any stem cell that corresponds to a point in development subsequent to formation of the inner cell mass (or the epiblast) of embryos at the blastocyst stage prior to gastrulation. In other words, an adult stem cell is a stem cell found at any point in development beyond the stage of pluripotent cells of an early embryo. Use of adult stem cell sources bypasses the ethical debate surrounding embryonic stem cells (47). Adult stem cells are relatively abundant during fetal development and persist in tissues throughout adult life. Although the mechanism that determines the outcome of stem cell division is controversial (48, 49), it is clear that maintenance of a stem cell reserve is important to ensure the capacity for regeneration in the face of injury or disease (50). Therapeutic use of adult stem cells dates back to the first bone marrow transplant in 1956(51). Early suggestions supporting the existence of cells that are capable of reconstituting the blood system came from experience with persons exposed to lethal doses of radiation during World War II.
Self-renewal and multipotency, remain generally accepted as the defining features of stem cells. For example, hematopoietic stem cells can completely self-renew and repopulate both lymphoid and myeloid blood cells of a lethally irradiated mouse. Single stem cells from such rescued animals can reconstitute irradiated hosts over multiple passages (52, 53). Human hematopoietic stem cells also have been characterized with similar properties to those in mice (54). They can be enriched using antibodies to surface markers such as CD34 and CD133, and can reconstitute the bone marrow and spleen of irradiated mice (severely immunodeficient mutant strains that do not reject human cells). In addition to bone marrow, umbilical cord blood has proven to be a valuable source of hematopoietic stem cells for therapeutic applications (55, 56). Similar to stem cells in the bone marrow, the presence of stem cells in the intestinal mucosa was inferred initially from studies of recovery from radiation damage (57). Labeling experiments showed that these stem cells are located near the base of crypts in the small and large intestine, and give rise to the various mature cells of the gut. Recently, improved sorting and laser capture technologies have begun to describe the phenotype of these intestinal stem cells (58, 59).
The presence of stem cells in adult tissues such as the skin (60) has become widely accepted. The general model is that adult stem cells, found in special niches, serve as a self-renewing population responsible for tissue restoration. Stem cells give rise to progenitor cells, which can multiply but are unable to indefinitely self-renew or to completely reconstitute a tissue. The progenitors, in turn, are precursors of terminally differentiated cells that generally no longer divide (61, 62). Operationally, it can often prove difficult to distinguish between stem and progenitor cells, as adequate long-term in vivo reconstitution assays are not always available. Both stem and progenitor cells are likely to have clinical usefulness.
Stem and progenitor cells are found in tissues and organs in which cells normally turn over much more slowly than in the bone marrow, gut, or skin. These tissues include skeletal muscle, liver, kidney, blood vessels, and many more (63, 64). Remarkably, even specialized cell types that for many years were believed incapable of any degree of restoration in adults, such as neurons of the central nervous system and cardiac myocytes, now appear to contain reserves of corresponding stem cells that persist throughout adult life (65, 66). A specific portion of the mesoderm, the mesenchyme, consists of loosely packed, unspecialized cells set in a gelatinous ground substance, from which connective tissue, bone, cartilage, and the circulatory and musculoskeletal systems develop. The characteristic features and clinical utility of these unspecialized cells are the topic of intense research interests as described in the following sections.
Mesenchymal stem cells (MSCs)
These multipotent cells capable of forming bone, cartilage, fat, connective tissue and muscle were first identified as a stromal population in the bone marrow, distinct from hematopoietic stem cells (8, 10, 13, 14). However, similar cells have been found throughout the body, and new evidence indicates that MSCs are derived from perivascular stem cells associated with blood vessels (67). While many adult stem cells appear specific to the tissue or organ from which they are obtained, others are more broadly distributed and also may have less restricted differentiation potential. Examples of MSCs with great clinical potential are the MSCs derived from fat tissue obtained by liposuction (68) and the MSC derived from placental tissue. Although termed “stem cells”, MSCs do not display indefinite self-renewal capacity, and cannot be routinely expanded in culture beyond approximately seven or eight passages. A single bone marrow donation can yield thousands of potential clinical doses of MSCs, but not an unlimited supply. A subset of MSCs from bone marrow appears to have great capacity to proliferate and also to differentiate beyond the mesenchymal cell lineages. This MSC subset has been termed multipotent adult progenitor cell (MAPC). The MAPCs been reported to differentiate in vitro into numerous tissue types and also to contribute to a wide array of adult tissues and cell types after injection into a developing embryo at the blastocyst stage (14).
Characterization of MSCs
By drawing parallels with the properties of the hematopoietic stem cell machinery, Caplan (69, 70) hypothesized that similar regenerative mechanisms operate in other, nonhematopoietic, tissues and organs. Prior to this theory, several scientists observed that bone marrow may contain multipotent precursors. For example, bone marrow may be a source of nonhematopoietic cells (71), and bone marrow-derived, plastic-adherent cells can form osteocytes and chondrocytes (72). More recent work (73) defined MSCs based on their ability to form osteocytes, chondrocytes, and adipocytes using assays such as alkaline phosphatase, Collagen type II, and Oil Red O staining, respectively. Analysis of marker gene expression reinforced histological findings (74). Since those observations, technology has advanced so that putative MSCs can be prospectively identified by fluorescence-activated cell sorting (FACS). While there is debate over the precise definition of MSCs, and thus the optimal panel of surface markers for FACS to characterize MSC, a recent study demonstrated that cells with MSC-like properties all have perivascular origin (75). MSCs from various sources, including bone marrow, are typically identified based on markers such as CD44, CD73, CD90, and CD105(76–84).
Muscle satellite cells and MSCs
Emerging findings present a picture that support for the idea (85) that all MSCs are closely associated with blood vessels—and thus offer evidence that MSCs can be found throughout the body, in virtually every tissue with a blood supply (86, 87). Furthermore, it is becoming clear that stem cells from sources other than muscle may enter the muscle satellite cell niche (Figure 1)(88). Two lines of evidence suggest that satellite cells are not limited to their traditionally accepted niche under the basal lamina (89). First, the observation that migration of satellite cells occur (90, 91) suggests that satellite cells can be found in the interstitial space. Second, several reports (92–97) have detailed that satellite cells, or cells with satellite cell-like regenerative potential, derive from stem cells associated with adult vessels (pericytes, Figure 1). Additionally, myogenic stem cells and endothelial stem cells may share a common embryonic precursor (98). The debate over what constitutes a satellite cell might be resolved if the definition of satellite cell is revisited; clearly, defining a satellite cell based on anatomic location and expression of surface markers is inadequate. Because the functional nature of all regenerative cells is to respond dynamically when needed, their location and their expression of surface markers is not fixed (99). Thus, regenerative satellite cells, their origin, and the very nature of MSCs blur the functional definition of these two cell types.
Expansion of MSCs in culture
Some adult stem and progenitor cell populations have proven difficult to expand in culture. For example, despite the long history of studying hematopoietic stem cells, there has been little success in expanding their numbers in the laboratory. Other stem cell populations, such as satellite cells of skeletal muscle, rapidly multiply in culture, but then show diminished regenerative capacity when transplanted in vivo (100). Nonetheless, some cell populations that must include adult stem and progenitor cells have been expanded sufficiently for certain clinical applications. Examples include cells from human foreskin used for living skin products (101) and urothelial and smooth muscle cells used in tissue-engineered bladder augmentation (102). Furthermore, certain purified stem cell populations have been grown continuously in culture for many months, an observation that may be viewed as ‘unlimited’ self-renewal. Expanding adult stem cells can be accomplished by enrichment of rare cells using selection with antibodies to surface markers, and the use of serum-free defined medium, carefully chosen growth factors, and, in some cases, extracellular matrix components. For example, neural stem cells can be isolated from brain tissue by positive selection for CD133 and negative selection for markers found on contaminating cell types (103). Neural stem cells, when cultured with fibroblast and epidermal growth factors, grow as “neurospheres”, and maintain a significant proportion of multipotent stem cells able to give rise to neurons, astrocytes, and oligodendrocytes (104, 105). Recently, conditions were reported for the isolation of human hepatic stem cells by selection for the epithelial cell adhesion molecule (EpCAM). These cells were capable of expansion in culture for over 150 population doublings (106–108). Hepatic stem cells are precursors to hepatocytes and bile duct epithelium, and may have broader potential to generate other endodermal cell types. Spermatogonial stem cells provide another example of a growing list of adult stem cells (albeit for a germ cell fate) that can be expanded in large numbers (109). Hepatic and spermatogonial stem cells provide some clues that MSCs may be capable of expansion in large numbers for clinical use.
Muscle formation by MSCs
Because multipotent MSCs can not only form bone, cartilage, fat, but also muscle tissue, attempts are underway to exploit these properties for clinical use in muscle diseases. Given the ability of MSCs to form muscle (110–113), our research interest has recently focused on use of these cells in pre-clinical models of DMD (Figure 2). In a pilot study, gastrocnemius muscles of nude mice were injected with either 10 uL phosphate-buffered saline (PBS) or the snake venom cardiotoxin (CTX, 10−5 M) to induce muscle injury. No differences were observed on magnetic resonance imaging (MRI) between PBS and CTX-injected muscles, indicating that CTX-injury does not create confounding background on MRI. To inject cells in mouse arterial vessels, a catheter was advanced from the insertion site to the bifurcation of the abdominal aorta, and the femoral artery was briefly tied with suture. Mice were injected first with CTX to induce muscle injury, then subsequently injected intra-arterially with 5 × 105 labeled CD146+/CD34− perivascular stem cells and scanned by MRI 24 hours post injection. Results suggest that perivascular stem cells home to the site of CTX-induced muscle injury within 24 hours of intravascular injection in mice. Additional studies in large animal models of DMD are needed to explore the potential of these cells to regenerate damaged muscle.
Figure 2. Perivascular stem cells home to the site of muscle injury.
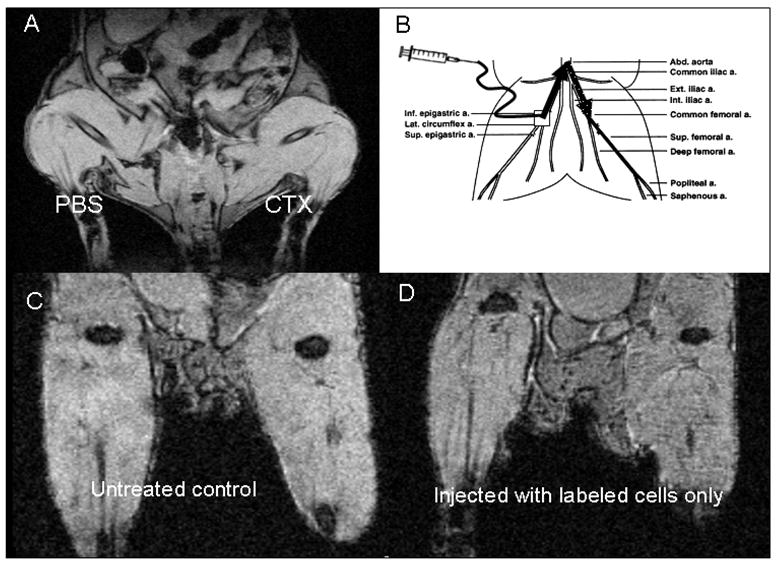
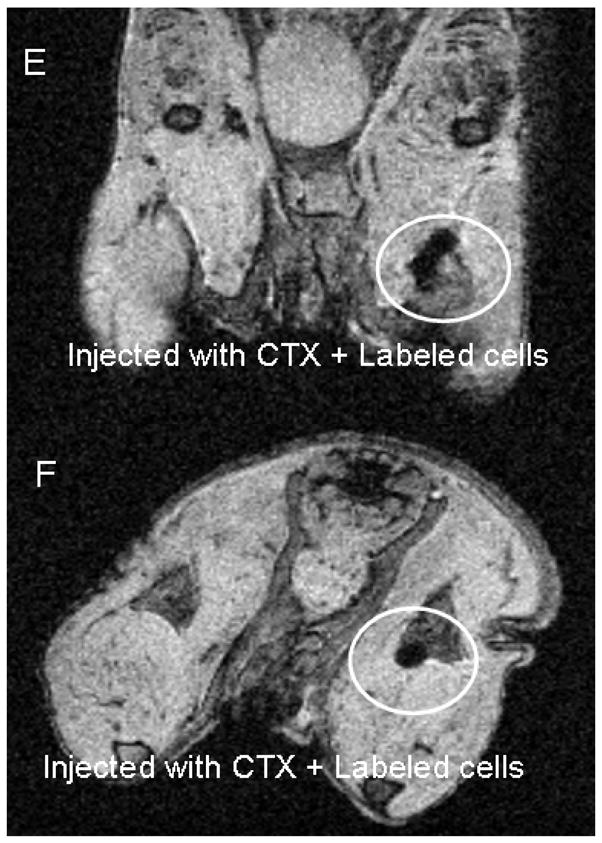
A. MRI of mouse following intramuscular injection of phosphate-buffered saline (PBS) or cadiotoxin (CTX) B. Femoral artery injection method in mice. Square indicates catheter insertion site, solid arrow indicates catheter path, dotted arrow indicates path of injected cells. C. Untreated control mouse. D. Mouse injected with iron oxide-labeled perivascular stem cells. E. and F. Mouse injected first with CTX, then with labeled cells. Circled area indicates region of labeled cells. Coronal and axial views shown in E and F, respectively.
Other investigators have shown that transplanted MSCs can act to regenerate new tissue in dystrophic muscle cells and replace expression of dystrophin in muscles of the dystrophin-deficient mdx mouse. Moreover, a type of perivascular cell, known as the meso-angioblast, was shown to dramatically restore dystrophin expression in the dystrophin-deficient GRMD dog (114). This exciting discovery demonstrated that cell therapy for DMD patients holds tremendous promise. However, the process of cell transplantation, migration, and engraftment is generally inefficient when comparing the number of cells transplanted with the number of newly-regenerated muscle cells. A solution to enhancing the efficient transduction of MSC into muscle cells will most likely come from exploiting the underlying biology that controls the stepwise myogenic process that normally regulates the generation of fully differentiated adult muscle cells from their undifferentiated fetal predecessors.
It is generally accepted that MSCs are capable of forming cell types of their own origin (115). In other words, clones of MSC derived from mesenchymal tissue are capable of forming chondrocytes, osteoblasts and adipocytes. Thus, the multipotent ability of MSC to form these three distinct cell types remains the criterion standard by which MSCs are distinguished from cells with unipotent ability (capable of giving rise to only one cell type). More recently, investigators have discovered that MSCs are capable of greater differentiation ability than initially thought. Indeed, MSCs are capable of forming muscle cells after treatment with either one, or a combination of the following: 5-azacytidine (a demethylating agent), hydrocortisone (116), dexamethasone, acorbic acid, growth factors, or when MSC are co-cultured (117, 118) or exposed to media (119) from immortalized mouse skeletal muscle (C2C12) cells. Table 2 summarizes various media recipes for inducing MSCs to form muscle cells.
Table 2. Myogenic Induction Media for MSC.
| Origin of MSC | Media | Serum | Supplementation |
|---|---|---|---|
| Adipose tissue (120) | DMEM | 10% FBS, 5% HS | 50 μM hydrocortisone, 1% anitibiotic/antimycotic |
| Bone marrow (121) | DMEM | 10% FBS, 5% HS | 10 μmol/L 5-azacytidine |
| Amniotic membrane (77) | DMEM | 5% FBS, 40% MCDB-201; ITS-LA BSA 100X | 10−8M dexamethasone, 10−4M ascorbic-acid-2- phosphate, 10 ng/ml bFGF, 10 ng/ml VEGF, 10 ng/ml IGF-1 |
| Embryonic stem cells (122) | Alpha MEM | 3% HS, 1% FBS | Co-culture with C2C12 cells |
| Fetal blood (119) | Myoblast- conditioned media | None | None |
| Fetal blood (123) | Galectin- enriched media* | None | *100–1,000 ng/ml galectin-1 |
| Adipose tissue (124) | DMEM high glucose | 20% FBS | Co-culture with primary myoblasts, 2 mM L- glutamine, 1% penicillin-streptomycin |
| Umbilical cord blood (125, 126) | DMEM | 10% FBS, 5% HS, | 0.1μM dexamethasone, 50 μM hydrocortiosne |
| Fetal aorta (127) | EBM-GM | None | 50 ng/ml platelet-derived growth factor (PDGF)-ββ |
| Bone marrow (128) | DMEM | 2% HS | Wnt3a conditioned media |
| 10T1/2 cells (129) | DMEM | 10% FBS | 20 μM 5-azacytidine, 0–300 nM testosterone |
DMEM: Dulbecco’s modified Eagle’s medium; FBS: Fetal bovine serum; HS: horse serum; MCDB-201: chick fibroblast basal medium; ITS-LA BSA: insulin, transferrin, selenic acid-linoleic acid-bovine serum albumin; VEGF: vascular endothelial growth factor; IGF-1: insulin-like growth factor; bFGF: basic fibroblast growth factor; EBM-GM: endothelial basal medium growth medium.
The full myogenic potential of MSCs and their ability to respond to myogenic cues in vivo to form functional contracting myotubes is largely unknown. In vitro experiments that explore the ability of MSCs to form muscle may not directly translate into clinical benefit for DMD patients. However, in vitro experiments may offer some clues as to how MSCs respond to external cues (130) provided by the in vivo milieu following transplantation. Besides appropriate induction media, MSCs require an environment with specific physical characteristics, such as elasticity (131). A soft matrix was found to favor MSC differentiation into neuronal-like cells, rigid matrix favored osteogenesis, and moderate elasticity favored muscle formation (132). Cell density also impacts MSC differentiation (133).
Table 3 provides a summary of the various donor sources that may be used to derive MSCs for clinical use in degenerative muscle diseases. Judging from the many potential sources of MSCs, large numbers of cells can be isolated immediately from fresh tissue. Promising tissue sources include the placenta, which is normally discarded following birth, and fat tissue discarded following liposuction procedures (lipoaspirate) (134).
Table 3. Tissue sources of MSCs.
| Source | Relevance to DMD |
|---|---|
| Adipose tissue | MSCs, cultured in specific media formulations, can be induced to form muscle cells. MSCs also display similar potential when introduced in vivo, into injured mouse muscle (135–140). When FLK-1+ MSCs, derived from human adipose tissue, were injected i.m. into a mouse model of muscular dystrophy, a decrease in serum CK was noted, as well as an increase in sarcolemmal dystrophin expression. Of note, no rejection was reported despite the fact that the cells were derived from human tissue (141). Similar experiments were performed earlier (142) which also suggested that MSCs derived from human adipose tissue display immune privilege. |
| Amniotic fluid | Several investigators (143–146) have used amniotic fluid-derived MSCs for regenerative medicine and cellular therapeutics. Following routine amniocentesis, samples are processed to yield MSCs, which proliferate quickly and have multilineage potential. The ease of collection and the therapeutic applicability of these cells holds clinical promise. |
| Amniotic membranes | The use of the amniotic membrane in surgery has a long history (147). Various groups (77, 148) have described methods for successful isolation of human amniotic membrane-derived MSCs. The expression of surface markers from cells derived from amniotic membranes likely changes over time similar to the changes observed in amniotic fluid-derived stem cells (149, 150). However, if amniotic membranes are used to obtain MSCs at a given time point, (at term, for example) it is likely that some surface markers are the same as MSCs derived from bone marrow and other tissues. Our group is currently investigating amniotic membrane-derived MSCs in a pre-clinical model of DMD. |
| Bone marrow | The best defined source of MSCs is the bone marrow, and marrow-derived MSCs are capable of forming muscle cells (151). Bone marrow-derived MSCs are capable of more than 70 population doublings (152) and retain multilineage potential even when expanded in vitro (153). Muscle cells formed from bone marrow-derived MSC have been observed without the use of demethylation reagents (154). Detailed reviews of bone marrow-derived MSCs are available elsewhere (155, 156). |
| Ear | Ear punches, which are a part of a routine marking procedure of live mice, were shown to contain MSCs with the ability to form muscle cells in vitro (157). Use of these cells in DMD research has not been reported. |
| Fetal blood | Rapid progress in imaging and thin-guage fetoscopy has allowed the collection of fetal blood. For example, Chan et al (158) collected first trimester fetal blood by ultrasound-guided cardiac aspiration, and exposed cultured MSCs to various media formulations including 5-azacytidine, conditioned media, dexamethasone and hydrocortisone. They observed either massive cell death or a lack of muscle marker expression using these myogenic induction methods. However, when galectin-1 was added to the media, they observed multinucleated myotubes positive for desmin staining. Interestingly, when the human fetal blood-derived MSCs were injected into immunodeficient mouse muscular dystrophy muscles, no spectrin-positive nor desmin-positive cell clusters were observed unless there was galectin-1 pretreatment. Later work by the same laboratory showed that intrauterine transplantation of human fetal blood-derived MSCs into immunocompetent mdx mice lead to widespread engraftment, although the levels of engraftment were low (159). |
| Skeletal muscle blood vessels | Perivascular stem cells (pericytes) can be isolated from skeletal muscle biopsies from both healthy individuals and DMD patients (160). Because MSCs are found throughout the body, it is thought that MSCs are derived from perivascular stem cells associated with blood vessels. Regardless of the donor population, pericytes have an anatomical niche distinct from satellite cells. Satellite cells reside inside the basal lamina of muscle cells, while pericytes are located underneath the basal lamina of small vessels. Intra-arterial delivery of a type of perivascular stem cell, known as a meso-angioblast, was shown to ameliorate severe muscle pathology observed in the dystrophin-deficient GRMD dog (21). This exciting discovery may represent one of the most significant breakthroughs in DMD cell-based research yet reported. |
| Synovial membranes | Early work determined that MSCs can be isolated from human synovial membranes, and that these cells were expandable while maintaining pluripotency in culture (161). Human synovial membrane MSCs (hSM-MSCs) injected into skeletal muscle of nude mice or immunosuppressed muscular dystrophy mice, the injected cells engrafted into the satellite cell niche (160). Additionally, hSM-MSC homed to cardiotoxin-injured muscle when injected into blood vessels. When administered intramuscularly to immunosuppressed muscular dystrophy mice, transplanted cells restored human dystrophin expression, and reduced the amount of immature muscle fibers. Finally, hSM-MSC partially rescued expression of mechano growth factor (MGF) in the muscular dystrophy mouse, providing some explanation for the amelioration of dystrophic muscle pathology. |
Key properties of MSC as agents for muscle repair
Immune function
Immunomodulatory properties of MSCs have implications in the treatment of inflammation (162–168) and may also indicate that immunosuppression is not always necessary following MSC transplantation (169). Mechanisms underlying an immune “privilege” of MSCs have been reviewed (170). Furthermore, an “immune phenotype” of MSCs has been described by the cell markers: MHC I+, MHC II−, CD40−, CD80−, and CD44+, CD73+, CD90+, CD105+ (171). An immune phenotype may also render some MSCs capable of intra-species transplantation (xenografts). Indeed, engraftment of human MSCs in rat models of brain injury (172), stroke (173), and myocardial infarction (174) have been described. Anti-inflammatory properties of MSCs (175, 176) support the idea that immuno-regulatory and trophic actions are central to their potential to limit fibrotic scarring. Whether or not MSCs have an anti-fibrotic effect in the cardiac and skeletal muscle tissue of DMD patients is not currently known.
Potential use of MSC transplantation for gene transfer in DMD patients
In addition to the ability of MSCs to regenerate tissue (177–188), MSCs can be used as vectors for gene delivery (189): By integrating into the host tissue, transplanted MSCs carrying modified genetic material can deliver gene products (190). However, clinical success using gene therapy for muscle diseases will require stable expression of any therapeutic gene administered. One of the important technical hurdles to overcome for successful gene therapy in DMD patients is the efficient penetration of genetic material into muscle. Efficient penetration is often limited by the nature of the cells that were directly injected. One possible solution to this problem is to genetically correct the patient’s own stem or progenitor cells in vitro and subsequently transplant engineered cells back into the patient’s diseased muscle in vivo. This approach has been used in pre-clinical experiments by a number of groups; Kobinger and colleagues targeted muscle satellite cells with a lentiviral vector encoding minidystrophin in dystrophin-deficient skeletal muscle of mdx mice (191). Bertoni and Rando (192) used RNA-DNA oligonucleotides to induce repair of the dystrophin gene in mdx mice. Bujold et al (193) used an HSV-1 vector to transduce mdx muscle cells. Li et al (194) used lentiviral vectors to transduce adult progenitor cells (MAPC) in vitro with minidystrophin vectors. Quenneville et al (195) used muscle precursor cells (MPC) genetically altered with lentiviral vectors carrying either a micro-dystrophin expression cassette or used an exon-skipping strategy. Taken together, this line of research indicates the various ways to genetically alter stem or progenitor cells for gene delivery in diseased muscle. The efficiency of the process remains one of the major obstacles, but technological advances, such as the use of fusion proteins, like the VP22 tegument protein of herpes simplex virus type 1 (HSV-1)(196) may offer solutions.
Clinical delivery of MSCs
A key property of MSC transplantation for patients with DMD is the ability of transplanted cells to engraft into muscle tissue after systemic delivery. MSCs normally mobilize in the blood in response to skeletal muscle injury (197), and a line of homing/migration/engraftment studies suggest that MSCs delivered systemically can “home” to the site of injury (198–203). Of clinical interest, intra-arterial delivery of MSCs (Figure 2) has been described (204–210). Similarities exist with respect to the chemo-attractive mechanisms used by endothelial progenitor cells (211); these mechanisms include secretion of a “homing signal” such as SDF-1 or MCP-3, and surface expression of CXCR4 receptors on MSCs (212, 213). Future experiments aimed at exploiting these homing signals may be needed to enhance the clinical delivery of MSCs into diseased cardiac and skeletal muscles.
Compatibility of transplanted MSCs
Allogeneic, and even xenogenic compatibility of donor MSCs appears to be possible (214) possibly because of their intrinsic immunosuppressive properties. Of particular interest, immunosuppressive properties of cells derived from amniotic membrane might explain the success of transplanted tissue allografts used in some corneal surgeries (215). A combined procedure, termed, “allograft limbal transplantation and amniotic membrane transplantation (ALT-AMT)” was first tested in rabbits over a decade ago (216); pre-dating this, amnion (the external membrane surrounding the fetus) was used as a biologic surgical dressing (217). Specific mechanisms for immune suppression observed with amnionic membrane-derived stem cells was initially thought to result from inhibition of T cells by indolamine 2,3-dioxygenase, a product secreted by MSCs (218, 219). More recent evidence suggests that this mechanism may be modulated by chemokines, such as SDF-1(220), TNF-α, and IFN-γ (221). An interaction between chemokines and nitric oxide (NO) may also be involved. Blood vessel formation (222) that involves regulation of NO has been described in the muscles of mdx mice. Indeed, increasing levels of NO appear to mitigate dystrophic muscle pathology observed in DMD and also enhances the migration of transplanted stem cells (223).
Effects of rehabilitation techniques on stem cell engraftment
In an effort to manipulate the in vivo cellular milieu, rehabilitation techniques such as exercise and neuromuscular electrical stimulation (NMES) have been investigated as a means to optimize stem cell transplantation in animal models of DMD. It was recently shown in mouse models that the addition of a treadmill running protocol following systemic delivery of bone marrow-derived MSCs enhanced donor cell contribution to muscle fiber regeneration (224). Changes were evident after just 1 week of training. In a similar study, stem cell engraftment increased in mdx mice following a protocol of muscle overloading (225). Increased stem cell engraftment was directly correlated with a loading-induced increase in the density of skeletal muscle vessels. More importantly, transplanted cells led to an increase in muscle regeneration; this increase was associated with increased overload resistance training. Together, emerging data suggest that physical therapeutics may play an important role in stem cell engraftment for patients with degenerative muscle diseases such as DMD.
Summary and conclusions
The availability of MSCs, their regenerative properties, and the ability to systemically deliver these cells fulfill several criteria required for successful clinical application. While MSC therapy shows promise as a strategy to ameliorate muscle degeneration caused by DMD, a better understanding of MSC characteristics and their contribution to growth and repair is needed to optimize and exploit their clinical potential.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hoffman EP, Brown RH, Jr, Kunkel LM. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Petrof BJ. Mol Cell Biochem. 1998;179:111–123. doi: 10.1023/a:1006812004945. [DOI] [PubMed] [Google Scholar]
- 3.Petrof BJ. Am J Phys Med Rehabil. 2002;81:S162–S174. doi: 10.1097/00002060-200211001-00017. [DOI] [PubMed] [Google Scholar]
- 4.MAURO A. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oexle K, Kohlschutter A. Neuropediatrics. 2001;32:123–129. doi: 10.1055/s-2001-16613. [DOI] [PubMed] [Google Scholar]
- 6.Lysaght MJ, Jaklenec A, Deweerd E. Tissue Eng Part A. 2008;14:305–315. doi: 10.1089/tea.2007.0267. [DOI] [PubMed] [Google Scholar]
- 7.Daftary AS, Crisanti M, Kalra M, Wong B, Amin R. Pediatrics. 2007;119:e320–e324. doi: 10.1542/peds.2006-1400. [DOI] [PubMed] [Google Scholar]
- 8.Williams IA, Allen DG. Am J Physiol Heart Circ Physiol. 2007;292:H846–H855. doi: 10.1152/ajpheart.00688.2006. [DOI] [PubMed] [Google Scholar]
- 9.Nakatani M, Takehara Y, Sugino H, Matsumoto M, Hashimoto O, Hasegawa Y, Murakami T, Uezumi A, Takeda S, Noji S, et al. FASEB J. 2008;22:477–487. doi: 10.1096/fj.07-8673com. [DOI] [PubMed] [Google Scholar]
- 10.Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, Flanigan KM, Pestronk A, Tawil R, Wolfe GI, et al. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 11.Hamed SA. IDrugs. 2006;9:783–789. [PubMed] [Google Scholar]
- 12.Hirawat S, Welch EM, Elfring GL, Northcutt VJ, Paushkin S, Hwang S, Leonard EM, Almstead NG, Ju W, Peltz SW, et al. J Clin Pharmacol. 2007;47:430–444. doi: 10.1177/0091270006297140. [DOI] [PubMed] [Google Scholar]
- 13.Wilton S. Neuromuscul Disord. 2007;17:719–720. doi: 10.1016/j.nmd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Badalamente MA, Stracher A. Muscle Nerve. 2000;23:106–111. doi: 10.1002/(sici)1097-4598(200001)23:1<106::aid-mus14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Nonaka I, Ishiura S, Takagi A, Sugita H. Acta Neuropathol. 1982;58:279–285. doi: 10.1007/BF00688610. [DOI] [PubMed] [Google Scholar]
- 16.Stracher A. Ann N Y Acad Sci. 1999;884:52–59. doi: 10.1111/j.1749-6632.1999.tb08635.x. [DOI] [PubMed] [Google Scholar]
- 17.Kajimoto H, Ishigaki K, Okumura K, Tomimatsu H, Nakazawa M, Saito K, Osawa M, Nakanishi T. Circ J. 2006;70:991–994. doi: 10.1253/circj.70.991. [DOI] [PubMed] [Google Scholar]
- 18.Tidball JG, Wehling-Henricks M. J Appl Physiol. 2007;102:1677–1686. doi: 10.1152/japplphysiol.01145.2006. [DOI] [PubMed] [Google Scholar]
- 19.Brunelli S, Sciorati C, D’Antona G, Innocenzi A, Covarello D, Galvez BG, Perrotta C, Monopoli A, Sanvito F, Bottinelli R, et al. Proc Natl Acad Sci U S A. 2007;104:264–269. doi: 10.1073/pnas.0608277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou GQ, Xie HQ, Zhang SZ, Yang ZM. Chin Med J (Engl ) 2006;119:1381–1391. [PubMed] [Google Scholar]
- 21.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, et al. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 22.Duan D. Hum Mol Genet. 2006;15(Spec No 2):R253–R261. doi: 10.1093/hmg/ddl180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodino-Klapac LR, Janssen PM, Montgomery CL, Coley BD, Chicoine LG, Clark KR, Mendell JR. J Transl Med. 2007;5:45. doi: 10.1186/1479-5876-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, artsma-Rus A, Bremmer-Bout M, den Dunnen JT, Koop K, van der Kooi AJ, Goemans NM, et al. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 25.Mattei E, Corbi N, Di Certo MG, Strimpakos G, Severini C, Onori A, Desantis A, Libri V, Buontempo S, Floridi A, et al. PLoS ONE. 2007;2:e774. doi: 10.1371/journal.pone.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bostick B, Yue Y, Long C, Duan D. Circ Res. 2008;102:121–130. doi: 10.1161/CIRCRESAHA.107.162982. [DOI] [PubMed] [Google Scholar]
- 27.Cerletti M, Negri T, Cozzi F, Colpo R, Andreetta F, Croci D, Davies KE, Cornelio F, Pozza O, Karpati G, et al. Gene Ther. 2003;10:750–757. doi: 10.1038/sj.gt.3301941. [DOI] [PubMed] [Google Scholar]
- 28.Gillis JM. Acta Neurol Belg. 2000;100:146–150. [PubMed] [Google Scholar]
- 29.Hirst RC, McCullagh KJ, Davies KE. Acta Myol. 2005;24:209–216. [PubMed] [Google Scholar]
- 30.Odom GL, Gregorevic P, Chamberlain JS. Biochim Biophys Acta. 2007;1772:243–262. doi: 10.1016/j.bbadis.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillis JM. Acta Neurol Belg. 2000;100:146–150. [PubMed] [Google Scholar]
- 32.Cerletti M, Negri T, Cozzi F, Colpo R, Andreetta F, Croci D, Davies KE, Cornelio F, Pozza O, Karpati G, et al. Gene Ther. 2003;10:750–757. doi: 10.1038/sj.gt.3301941. [DOI] [PubMed] [Google Scholar]
- 33.Laird DJ, von Andrian UH, Wagers AJ. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 34.Price FD, Kuroda K, Rudnicki MA. Biochim Biophys Acta. 2007;1772:272–283. doi: 10.1016/j.bbadis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Huard J, Cao B, Qu-Petersen Z. Birth Defects Res C Embryo Today. 2003;69:230–237. doi: 10.1002/bdrc.10020. [DOI] [PubMed] [Google Scholar]
- 36.Borycki AG, Brunk B, Tajbakhsh S, Buckingham M, Chiang C, Emerson CP., Jr Development. 1999;126:4053–4063. doi: 10.1242/dev.126.18.4053. [DOI] [PubMed] [Google Scholar]
- 37.Cossu G. J Clin Invest. 2004;114:1540–1543. doi: 10.1172/JCI23733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maltin CA, Delday MI, Sinclair KD, Steven J, Sneddon AA. Reproduction. 2001;122:359–374. doi: 10.1530/rep.0.1220359. [DOI] [PubMed] [Google Scholar]
- 39.Christ B, Brand-Saberi B. Int J Dev Biol. 2002;46:905–914. [PubMed] [Google Scholar]
- 40.Maltin CA, Delday MI, Sinclair KD, Steven J, Sneddon AA. Reproduction. 2001;122:359–374. doi: 10.1530/rep.0.1220359. [DOI] [PubMed] [Google Scholar]
- 41.Relaix F. Cell Mol Life Sci. 2006;63:1221–1225. doi: 10.1007/s00018-006-6015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haberland M, Arnold MA, McAnally J, Phan D, Kim Y, Olson EN. Mol Cell Biol. 2007;27:518–525. doi: 10.1128/MCB.01415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckingham M. Curr Biol. 1994;4:61–63. doi: 10.1016/s0960-9822(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 44.Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, Montarras D, Rocancourt D, Relaix F. J Anat. 2003;202:59–68. doi: 10.1046/j.1469-7580.2003.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi X, Garry DJ. Genes Dev. 2006;20:1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 46.Cossu G, Borello U. EMBO J. 1999;18:6867–6872. doi: 10.1093/emboj/18.24.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, Shun CT, Yen ML, Lee MC, Chen YC. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- 48.Wagers AJ, Christensen JL, Weissman IL. Gene Ther. 2002;9:606–612. doi: 10.1038/sj.gt.3301717. [DOI] [PubMed] [Google Scholar]
- 49.Kuang S, Gillespie MA, Rudnicki MA. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Caplan AI. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 51.THOMAS ED, LOCHTE HL, Jr, LU WC, FERREBEE JW. N Engl J Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 52.Ema H, Morita Y, Yamazaki S, Matsubara A, Seita J, Tadokoro Y, Kondo H, Takano H, Nakauchi H. Nat Protoc. 2006;1:2979–2987. doi: 10.1038/nprot.2006.447. [DOI] [PubMed] [Google Scholar]
- 53.Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, Weissman IL, Contag CH. Proc Natl Acad Sci U S A. 2004;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bryder D, Rossi DJ, Weissman IL. Am J Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schoemans H, Theunissen K, Maertens J, Boogaerts M, Verfaillie C, Wagner J. Bone Marrow Transplant. 2006;38:83–93. doi: 10.1038/sj.bmt.1705403. [DOI] [PubMed] [Google Scholar]
- 56.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Proc Natl Acad Sci U S A. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potten CS, Loeffler M. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 58.Giannakis M, Stappenbeck TS, Mills JC, Leip DG, Lovett M, Clifton SW, Ippolito JE, Glasscock JI, Arumugam M, Brent MR, et al. J Biol Chem. 2006;281:11292–11300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 59.Dekaney CM, Rodriguez JM, Graul MC, Henning SJ. Gastroenterology. 2005;129:1567–1580. doi: 10.1053/j.gastro.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 60.Blanpain C, Fuchs E. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weissman IL, Anderson DJ, Gage F. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 62.Metcalf D. Stem Cells. 2007;25:2390–2395. doi: 10.1634/stemcells.2007-0544. [DOI] [PubMed] [Google Scholar]
- 63.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 64.Presnell SC, Petersen B, Heidaran M. Semin Cell Dev Biol. 2002;13:369–376. doi: 10.1016/s1084952102000939. [DOI] [PubMed] [Google Scholar]
- 65.Gage FH. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 66.Anversa P, Kajstura J, Leri A, Bolli R. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 67.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Gimble J, Guilak F. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 69.Caplan AI. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 70.Caplan AI. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 71.Prockop DJ. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 72.Friedenstein AJ, Gorskaja JF, Kulagina NN. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 73.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 74.Pittenger M, Vanguri P, Simonetti D, Young R. J Musculoskelet Neuronal Interact. 2002;2:309–320. [PubMed] [Google Scholar]
- 75.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 76.Aggarwal S, Pittenger MF. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 77.Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, Lanzoni G, Cantoni S, Cavallini C, Bianchi F, et al. BMC Dev Biol. 2007;7:11. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fox JM, Chamberlain G, Ashton BA, Middleton J. Br J Haematol. 2007;137:491–502. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- 79.Gawronska-Kozak B, Manuel JA, Prpic V. J Cell Biochem. 2007;102:122–135. doi: 10.1002/jcb.21286. [DOI] [PubMed] [Google Scholar]
- 80.Jackson L, Jones DR, Scotting P, Sottile V. J Postgrad Med. 2007;53:121–127. doi: 10.4103/0022-3859.32215. [DOI] [PubMed] [Google Scholar]
- 81.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 82.Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B, Villageois A, Bagnis C, Breittmayer JP, Groux H, et al. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schrepfer S, Deuse T, Lange C, Katzenberg R, Reichenspurner H, Robbins RC, Pelletier MP. Stem Cells Dev. 2007;16:105–107. doi: 10.1089/scd.2006.0041. [DOI] [PubMed] [Google Scholar]
- 84.Soncini M, Vertua E, Gibelli L, Zorzi F, Denegri M, Albertini A, Wengler GS, Parolini O. J Tissue Eng Regen Med. 2007;1:296–305. doi: 10.1002/term.40. [DOI] [PubMed] [Google Scholar]
- 85.Tavian M, Peault B. Int J Dev Biol. 2005;49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- 86.Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, et al. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 87.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 89.MAURO A. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hughes SM, Blau HM. Nature. 1990;345:350–353. doi: 10.1038/345350a0. [DOI] [PubMed] [Google Scholar]
- 91.Yokota T, Lu QL, Morgan JE, Davies KE, Fisher R, Takeda S, Partridge TA. J Cell Sci. 2006;119:2679–2687. doi: 10.1242/jcs.03000. [DOI] [PubMed] [Google Scholar]
- 92.Christov C, Chretien F, bou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, et al. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De AL, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella De Angelis MG, Ponzetto C, Cossu G. J Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 95.Ramirez M, Lucia A, Gomez-Gallego F, Esteve Lanao J, Perez-Martinez A, Foster C, Andreu AL, Martin MA, Madero L, Arenas J, et al. Br J Sports Med. 2006;40:719–722. doi: 10.1136/bjsm.2006.028639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, et al. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 97.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 98.Kardon G, Campbell JK, Tabin CJ. Dev Cell. 2002;3:533–545. doi: 10.1016/s1534-5807(02)00291-5. [DOI] [PubMed] [Google Scholar]
- 99.Chamberlain G, Fox J, Ashton B, Middleton J. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 100.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 101.Parenteau NL, Nolte CM, Bilbo P, Rosenberg M, Wilkins LM, Johnson EW, Watson S, Mason VS, Bell E. J Cell Biochem. 1991;45:245–251. doi: 10.1002/jcb.240450304. [DOI] [PubMed] [Google Scholar]
- 102.Oberpenning F, Meng J, Yoo JJ, Atala A. Nat Biotechnol. 1999;17:149–155. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 103.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Proc Natl Acad Sci U S A. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gage FH. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 105.Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, van der KD. Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 106.Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, et al. J Exp Med. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wauthier E, Schmelzer E, Turner W, Zhang L, LeCluyse E, Ruiz J, Turner R, Furth ME, Kubota H, Lozoya O, et al. Methods Cell Biol. 2008;86:137–225. doi: 10.1016/S0091-679X(08)00008-3. [DOI] [PubMed] [Google Scholar]
- 108.Turner WS, Seagle C, Galanko JA, Favorov O, Prestwich GD, Macdonald JM, Reid LM. Stem Cells. 2008;26:1547–1555. doi: 10.1634/stemcells.2007-0863. [DOI] [PubMed] [Google Scholar]
- 109.Kubota H, Avarbock MR, Brinster RL. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pittenger M, Vanguri P, Simonetti D, Young R. J Musculoskelet Neuronal Interact. 2002;2:309–320. [PubMed] [Google Scholar]
- 111.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 112.Prockop DJ. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 113.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 114.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, et al. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 115.Jackson L, Jones DR, Scotting P, Sottile V. J Postgrad Med. 2007;53:121–127. doi: 10.4103/0022-3859.32215. [DOI] [PubMed] [Google Scholar]
- 116.Mizuno H, Hyakusoku H. J Nippon Med Sch. 2003;70:300–306. doi: 10.1272/jnms.70.300. [DOI] [PubMed] [Google Scholar]
- 117.Barberi T, Willis LM, Socci ND, Studer L. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wise CJ, Watt DJ, Jones GE. J Cell Biochem. 1996;61:363–374. doi: 10.1002/(SICI)1097-4644(19960601)61:3%3C363::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 119.Chan J, O’Donoghue K, de la FJ, Roberts IA, Kumar S, Morgan JE, Fisk NM. Stem Cells. 2005;23:93–102. doi: 10.1634/stemcells.2004-0138. [DOI] [PubMed] [Google Scholar]
- 120.Mizuno H, Hyakusoku H. J Nippon Med Sch. 2003;70:300–306. doi: 10.1272/jnms.70.300. [DOI] [PubMed] [Google Scholar]
- 121.Li Y, Zhang C, Xiong F, Yu MJ, Peng FL, Shang YC, Zhao CP, Xu YF, Liu ZS, Zhou C, et al. BMC Cell Biol. 2008;9:24. doi: 10.1186/1471-2121-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 123.Chan J, O’Donoghue K, Gavina M, Torrente Y, Kennea N, Mehmet H, Stewart H, Watt DJ, Morgan JE, Fisk NM. Stem Cells. 2006;24:1879–1891. doi: 10.1634/stemcells.2005-0564. [DOI] [PubMed] [Google Scholar]
- 124.Di RG, Iachininoto MG, Tritarelli A, Straino S, Zacheo A, Germani A, Crea F, Capogrossi MC. J Cell Sci. 2006;119:2945–2952. doi: 10.1242/jcs.03029. [DOI] [PubMed] [Google Scholar]
- 125.Gang EJ, Jeong JA, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. Stem Cells. 2004;22:617–624. doi: 10.1634/stemcells.22-4-617. [DOI] [PubMed] [Google Scholar]
- 126.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 127.Invernici G, Emanueli C, Madeddu P, Cristini S, Gadau S, Benetti A, Ciusani E, Stassi G, Siragusa M, Nicosia R, et al. Am J Pathol. 2007;170:1879–1892. doi: 10.2353/ajpath.2007.060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shang YC, Wang SH, Xiong F, Zhao CP, Peng FN, Feng SW, Li MS, Li Y, Zhang C. Acta Pharmacol Sin. 2007;28:1761–1774. doi: 10.1111/j.1745-7254.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- 129.Singh R, Artaza JN, Taylor WE, Gonzalez Cadavid NF, Bhasin S. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 130.Shefer G, Wleklinski Lee M, Yablonka Reuveni Z. J Cell Sci. 2004;117:5393–5404. doi: 10.1242/jcs.01419. [DOI] [PubMed] [Google Scholar]
- 131.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 132.Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE. J Musculoskelet Neuronal Interact. 2007;7:335. [PubMed] [Google Scholar]
- 133.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 134.Liu Y, Yan X, Sun Z, Chen B, Han Q, Li J, Zhao RC. Stem Cells Dev. 2007;16:695–706. doi: 10.1089/scd.2006.0118. [DOI] [PubMed] [Google Scholar]
- 135.Bossolasco P, Corti S, Strazzer S, Borsotti C, Del BR, Fortunato F, Salani S, Quirici N, Bertolini F, Gobbi A, et al. Exp Cell Res. 2004;295:66–78. doi: 10.1016/j.yexcr.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 136.Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 137.Ferrari G, Cusella De AG, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 138.Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B, Villageois A, Bagnis C, Breittmayer JP, Groux H, et al. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wakitani S, Saito T, Caplan AI. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 140.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 141.Liu Y, Yan X, Sun Z, Chen B, Han Q, Li J, Zhao RC. Stem Cells Dev. 2007;16:695–706. doi: 10.1089/scd.2006.0118. [DOI] [PubMed] [Google Scholar]
- 142.Rodriguez AM, Pisani D, Dechesne CA, Turc Carel C, Kurzenne JY, Wdziekonski B, Villageois A, Bagnis C, Breittmayer JP, Groux H, et al. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De CP, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, et al. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 144.In ‘t Anker PS, Scherjon SA, Kleijburg-van der KC, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH. Blood. 2003;102:1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 145.Kaviani A, Perry TE, Dzakovic A, Jennings RW, Ziegler MM, Fauza DO. J Pediatr Surg. 2001;36:1662–1665. doi: 10.1053/jpsu.2001.27945. [DOI] [PubMed] [Google Scholar]
- 146.Kunisaki SM, Armant M, Kao GS, Stevenson K, Kim H, Fauza DO. J Pediatr Surg. 2007;42:974–979. doi: 10.1016/j.jpedsurg.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 147.Trelford JD, Trelford-Sauder M. Am J Obstet Gynecol. 1979;134:833–845. doi: 10.1016/0002-9378(79)90957-8. [DOI] [PubMed] [Google Scholar]
- 148.Soncini M, Vertua E, Gibelli L, Zorzi F, Denegri M, Albertini A, Wengler GS, Parolini O. J Tissue Eng Regen Med. 2007;1:296–305. doi: 10.1002/term.40. [DOI] [PubMed] [Google Scholar]
- 149.Bili C, Divane A, Apessos A, Konstantinos T, Apostolos A, Ioannis B, Periklis T, Florentin L. Prenat Diagn. 2002;22:360–365. doi: 10.1002/pd.301. [DOI] [PubMed] [Google Scholar]
- 150.Torricelli F, Brizzi L, Bernabei PA, Gheri G, Di LS, Nutini L, Lisi E, Di TM, Cariati E. Ital J Anat Embryol. 1993;98:119–126. [PubMed] [Google Scholar]
- 151.Wakitani S, Saito T, Caplan AI. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 152.Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 153.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 154.Muguruma Y, Reyes M, Nakamura Y, Sato T, Matsuzawa H, Miyatake H, Akatsuka A, Itoh J, Yahata T, Ando K, et al. Exp Hematol. 2003;31:1323–1330. doi: 10.1016/j.exphem.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 155.Bianco P, Gehron RP. J Clin Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bianco P, Robey PG, Simmons PJ. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gawronska-Kozak B, Manuel JA, Prpic V. J Cell Biochem. 2007;102:122–135. doi: 10.1002/jcb.21286. [DOI] [PubMed] [Google Scholar]
- 158.Chan J, O’Donoghue K, Gavina M, Torrente Y, Kennea N, Mehmet H, Stewart H, Watt DJ, Morgan JE, Fisk NM. Stem Cells. 2006;24:1879–1891. doi: 10.1634/stemcells.2005-0564. [DOI] [PubMed] [Google Scholar]
- 159.Chan J, Waddington SN, O’Donoghue K, Kurata H, Guillot PV, Gotherstrom C, Themis M, Morgan JE, Fisk NM. Stem Cells. 2007;25:875–884. doi: 10.1634/stemcells.2006-0694. [DOI] [PubMed] [Google Scholar]
- 160.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 161.De BC, Dell’Accio F, Tylzanowski P, Luyten FP. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 162.Aggarwal S, Pittenger MF. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 163.Caplan AI. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 164.Chamberlain G, Fox J, Ashton B, Middleton J. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 165.Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Stem Cells. 2006;24:2466–2477. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 166.Nauta AJ, Fibbe WE. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 167.Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, van GM, Stadler G, Redl H, Gabriel C. Tissue Eng. 2007;13:1173–1183. doi: 10.1089/ten.2006.0313. [DOI] [PubMed] [Google Scholar]
- 168.Wolf D, Wolf AM. Lancet. 2008;371:1553–1554. doi: 10.1016/S0140-6736(08)60666-2. [DOI] [PubMed] [Google Scholar]
- 169.Le BK, Tammik C, Rosendahl K, Zetterberg E, Ringden O. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 170.Niederkorn JY. Nat Immunol. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 171.Chamberlain G, Fox J, Ashton B, Middleton J. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 172.Mahmood A, Lu D, Lu M, Chopp M. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 173.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 174.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Am J Physiol Heart Circ Physiol. 2006;290:H2196–H2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 175.Iyer SS, Rojas M. Expert Opin Biol Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 176.Uccelli A, Pistoia V, Moretta L. Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 177.Chan J, Waddington SN, O’Donoghue K, Kurata H, Guillot PV, Gotherstrom C, Themis M, Morgan JE, Fisk NM. Stem Cells. 2007;25:875–884. doi: 10.1634/stemcells.2006-0694. [DOI] [PubMed] [Google Scholar]
- 178.Ferrari G, Cusella-De AG, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 179.Gawronska-Kozak B, Manuel JA, Prpic V. J Cell Biochem. 2007;102:122–135. doi: 10.1002/jcb.21286. [DOI] [PubMed] [Google Scholar]
- 180.Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Biol Reprod. 2007;77:577–588. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 181.Jackson L, Jones DR, Scotting P, Sottile V. J Postgrad Med. 2007;53:121–127. doi: 10.4103/0022-3859.32215. [DOI] [PubMed] [Google Scholar]
- 182.Kunisaki SM, Armant M, Kao GS, Stevenson K, Kim H, Fauza DO. J Pediatr Surg. 2007;42:974–979. doi: 10.1016/j.jpedsurg.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 183.Liu Y, Yan X, Sun Z, Chen B, Han Q, Li J, Zhao RC. Stem Cells Dev. 2007;16:695–706. doi: 10.1089/scd.2006.0118. [DOI] [PubMed] [Google Scholar]
- 184.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 185.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, et al. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B, Villageois A, Bagnis C, Breittmayer JP, Groux H, et al. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tamagawa T, Oi S, Ishiwata I, Ishikawa H, Nakamura Y. Hum Cell. 2007;20:77–84. doi: 10.1111/j.1749-0774.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 188.Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T. Transplantation. 2005;79:528–535. doi: 10.1097/01.tp.0000149503.92433.39. [DOI] [PubMed] [Google Scholar]
- 189.Goncalves MA, Swildens J, Holkers M, Narain A, van Nierop GP, van de Watering MJ, Knaan-Shanzer S, de Vries AA. Mol Ther. 2008;16:741–748. doi: 10.1038/mt.2008.16. [DOI] [PubMed] [Google Scholar]
- 190.Laird DJ, von Andrian UH, Wagers AJ. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 191.Kobinger GP, Louboutin JP, Barton ER, Sweeney HL, Wilson JM. Hum Gene Ther. 2003;14:1441–1449. doi: 10.1089/104303403769211655. [DOI] [PubMed] [Google Scholar]
- 192.Bertoni C, Rando TA. Hum Gene Ther. 2002;13:707–718. doi: 10.1089/104303402317322276. [DOI] [PubMed] [Google Scholar]
- 193.Bujold M, Caron N, Camiran G, Mukherjee S, Allen PD, Tremblay JP, Wang Y. Cell Transplant. 2002;11:759–767. [PubMed] [Google Scholar]
- 194.Li S, Kimura E, Fall BM, Reyes M, Angello JC, Welikson R, Hauschka SD, Chamberlain JS. Gene Ther. 2005;12:1099–1108. doi: 10.1038/sj.gt.3302505. [DOI] [PubMed] [Google Scholar]
- 195.Quenneville SP, Chapdelaine P, Skuk D, Paradis M, Goulet M, Rousseau J, Xiao X, Garcia L, Tremblay JP. Mol Ther. 2007;15:431–438. doi: 10.1038/sj.mt.6300047. [DOI] [PubMed] [Google Scholar]
- 196.Xiong F, Xiao S, Peng F, Zheng H, Yu M, Ruan Y, Li W, Shang Y, Zhao C, Zhou W, et al. Hum Gene Ther. 2007;18:490–501. doi: 10.1089/hum.2006.155. [DOI] [PubMed] [Google Scholar]
- 197.Ramirez M, Lucia A, Gomez-Gallego F, Esteve Lanao J, Perez Martinez A, Foster C, Andreu AL, Martin MA, Madero L, Arenas J, et al. Br J Sports Med. 2006;40:719–722. doi: 10.1136/bjsm.2006.028639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, Arienti D, Calamani F, Zatti D, Paul P, et al. Transplantation. 2004;78:1439–1448. doi: 10.1097/01.tp.0000144606.84234.49. [DOI] [PubMed] [Google Scholar]
- 199.Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 200.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, McCarthy PM, Penn MS. Stem Cells. 2007;25:245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 201.Seeger FH, Zeiher AM, Dimmeler S. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S110–S113. doi: 10.1038/ncpcardio0734. [DOI] [PubMed] [Google Scholar]
- 202.Shyu WC, Lee YJ, Liu DD, Lin SZ, Li H. Front Biosci. 2006;11:899–907. doi: 10.2741/1846. [DOI] [PubMed] [Google Scholar]
- 203.Voermans C, van Hennik PB, van der Schoot CE. J Hematother Stem Cell Res. 2001;10:725–738. doi: 10.1089/152581601317210827. [DOI] [PubMed] [Google Scholar]
- 204.Bachrach E, Perez AL, Choi YH, Illigens BM, Jun SJ, del NP, McGowan FX, Li S, Flint A, Chamberlain J, et al. Muscle Nerve. 2006;34:44–52. doi: 10.1002/mus.20560. [DOI] [PubMed] [Google Scholar]
- 205.Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, et al. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 206.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, et al. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 207.Torrente Y, Tremblay JP, Pisati F, Belicchi M, Rossi B, Sironi M, Fortunato F, El FM, D’Angelo MG, Caron NJ, et al. J Cell Biol. 2001;152:335–348. doi: 10.1083/jcb.152.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Torrente Y, Camirand G, Pisati F, Belicchi M, Rossi B, Colombo F, El FM, Caron NJ, Issekutz AC, Constantin G, et al. J Cell Biol. 2003;162:511–520. doi: 10.1083/jcb.200210006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, et al. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 210.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, et al. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 211.Urbich C, Dimmeler S. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 212.Hogaboam CM, Carpenter KJ, Schuh JM, Proudfoot AA, Bridger G, Buckland KF. Pharmacol Ther. 2005;107:314–328. doi: 10.1016/j.pharmthera.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 213.Laird DJ, von Andrian UH, Wagers AJ. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 214.Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, Arienti D, Calamani F, Zatti D, Paul P, et al. Transplantation. 2004;78:1439–1448. doi: 10.1097/01.tp.0000144606.84234.49. [DOI] [PubMed] [Google Scholar]
- 215.Ueta M, Kweon MN, Sano Y, Sotozono C, Yamada J, Koizumi N, Kiyono H, Kinoshita S. Clin Exp Immunol. 2002;129:464–470. doi: 10.1046/j.1365-2249.2002.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kim JC, Tseng SC. Cornea. 1995;14:473–484. [PubMed] [Google Scholar]
- 217.Trelford JD, Trelford Sauder M. Am J Obstet Gynecol. 1979;134:833–845. doi: 10.1016/0002-9378(79)90957-8. [DOI] [PubMed] [Google Scholar]
- 218.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 219.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 220.Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, Constantin G, Torrente Y, Cossu G. J Cell Biol. 2006;174:231–243. doi: 10.1083/jcb.200512085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 222.Straino S, Germani A, Di CA, Porcelli D, De MR, Mangoni A, Napolitano M, Martelli F, Biglioli P, Capogrossi MC. Circulation. 2004;110:3341–3348. doi: 10.1161/01.CIR.0000147776.50787.74. [DOI] [PubMed] [Google Scholar]
- 223.Brunelli S, Sciorati C, D’Antona G, Innocenzi A, Covarello D, Galvez BG, Perrotta C, Monopoli A, Sanvito F, Bottinelli R, et al. Proc Natl Acad Sci U S A. 2007;104:264–269. doi: 10.1073/pnas.0608277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Palermo AT, LaBarge MA, Doyonnas R, Pomerantz J, Blau HM. Dev Biol. 2005;279:336–344. doi: 10.1016/j.ydbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 225.Ambrosio F, Ferrari RJ, Fitzgerald GK, Carvell GE, Boninger ML, Huard J. 2008 [Google Scholar]