Summary
Ventricular chamber morphogenesis, first manifested by trabeculae formation, is crucial for cardiac function and embryonic viability and depends on cellular interactions between endocardium and myocardium. We show that ventricular Notch1 activity is highest at presumptive trabecular endocardium. RBPJk and Notch1 mutants show impaired trabeculation and marker expression, attenuated EphrinB2, NRG1 and BMP10 expression and signaling and decreased myocardial proliferation. Functional and molecular analyses show that Notch inhibition prevents EphrinB2 expression and that EphrinB2 is a direct Notch target acting upstream of NRG1 in the ventricles. However, BMP10 levels are found to be independent of both EphrinB2 and NRG1 during trabeculation. Accordingly, exogenous BMP10 rescues the myocardial proliferative defect of in vitro cultured RBPJk mutants, while exogenous NRG1 rescues differentiation in parallel. We suggest that during trabeculation Notch independently regulates cardiomyocyte proliferation and differentiation, two exquisitely balanced processes whose perturbation may result in congenital heart disease.
Keywords: Notch, cellular communication, proliferation, differentiation, endocardium, myocardium, chamber development, trabeculation, EphrinB2, NRG1, BMP10
Introduction
Ventricular chamber formation is essential to heart development and adult cardiac function (Moorman and Christoffels, 2003). Maintaining the balance between cardiomyocyte proliferation and differentiation is critical in this process, as heart muscle cells must multiply rapidly to sustain hemodynamic pressure and progress in their maturation (Moorman and Christoffels, 2003). The trabeculae, which are highly organized sheets of cardiomyocytes forming muscular ridges lined by endocardial cells (Ben-Shachar et al., 1985), form as a result of interactions between primitive myocardium and endocardium at the end of cardiac looping (E9.0-9.5 in mouse). These structures are a constant feature of the early vertebrate ventricular chambers, and represent the first and most characteristic sign of ventricular differentiation (Sedmera et al., 1997). Trabeculae gradually become part of papillary muscles, the interventricular septum, and cardiac conduction system cells. Failure to do so may result in congenital heart disease (CHD).
Not much is known about the genetic circuitry and molecular regulation of ventricular trabeculation. Only a few pathways such as NRG1/ErbB2, 4 (Gassmann et al., 1995; Lee et al., 1995; Meyer and Birchmeier, 1995), EphrinB2/EphB4 (Wang et al., 1998; Gerety et al., 1999) or BMP10 (Chen et al., 2004) have been shown to be involved in this process. However, it is not clear how these different molecular mechanisms are related, and intense research is required to understand the complexity underlying ventricular morphogenesis (Moorman and Christoffels, 2003).
Notch is a local signaling pathway that regulates embryonic cell fate determination, differentiation and patterning (reviewed in Artavanis-Tsakonas et al., 1999). Notch constitute an evolutionarily conserved group of type I transmembrane receptors (Notch1-Notch4 in mammals), with a large extracellular region and an intracellular domain (NICD), including two nuclear localization signals and a transactivation region. The extracellular region of Notch can interact with membrane-bound ligands of the Delta or Serrate/Jagged families. A ligand binding-induced signal is delivered to the nucleus in a process involving proteolytic cleavage of the receptor by γ-secretase activity and nuclear translocation of NICD (Kopan, 2002). In the nucleus, NICD heterodimerizes with the RBPJK/CBF1/Su(H) effector transcription factor (Fortini and Artavanis-Tsakonas, 1994), converting it from a repressor to an activator. Notch target genes include those encoding basic helix-loop-helix (bHLH) transcription factors of the Hes and HRT/Hey/Herp families (Iso et al., 2003).
Specific Notch ligands, receptors and targets are expressed in the heart from early developmental stages. Delta4 (Krebs et al., 2000), Notch1 (Del Amo et al., 1992) and Notch4 (Uyttendaele et al., 1996) are transcribed in endocardium from gastrulation onwards, whereas Jag1 and Notch2, show restricted expression in myocardium from mid-gestation (Loomes et al., 1999; McCright et al., 2002). HRT1 and HRT2 show chamber-specific expression in endocardium and myocardium (Nakagawa et al., 1999). Notch is required for endocardial differentiation and formation of the cardiac valve primordia (Timmerman et al., 2004). In Xenopus myocardium Notch appears to inhibit cardiomyogenesis (Rones et al., 2000; Schroeder et al., 2003), and manipulation of Notch signaling in vitro is consistent with this finding (Schroeder et al., 2003; Nemir et al., 2006). Further in vivo studies indicate that Notch signaling inhibits myocardial differentiation (Watanabe et al., 2006) and participates in cardiac patterning (Rutenberg et al., 2006), although its mechanism of action is not well understood. HRT2 appears to be an important Notch mediator in the heart (reviewed in Kokubo et al., 2005), but additional targets remain to be identified.
In this study we have addressed the role of Notch in ventricular chamber development. We find that Notch1 activity is highest at presumptive trabecular endocardium and as development proceeds, concentrates at the base of trabeculae. RBPJk and Notch1 mutants show impaired trabeculation and marker expression, attenuated EphrinB2, NRG1 and BMP10 expression and signaling and decreased myocardial proliferation. Functional and molecular analyses show that Notch inhibition prevents EphrinB2 expression and that EphrinB2 is a direct Notch target acting upstream of NRG1 in the ventricles. However, BMP10 levels are found to be independent of both EphrinB2 and NRG1 during trabeculation. Accordingly, exogenous BMP10 rescues the myocardial proliferative defect of in vitro cultured RBPJk mutants, while exogenous NRG1 rescues their differentiation defect. We suggest that Notch coordinates an interaction between ventricular endocardium and myocardium that is critical for trabeculation, through two distinct Notch-dependent processes: 1) the transition of primitive myocardial epithelium to trabecular and compact myocardium, mediated by the EphrinB2-dependent endocardial paracrine factor NRG1, and 2) the maintenance of a BMP10-dependent proliferating cardiomyocyte population in the trabeculae. We discuss the role of Notch signaling in regulating cardiomyocyte proliferation/differentiation equilibrium, and suggest that altered Notch signaling may directly relate to CHD involving defective cardiomyogenesis, as seen in human cardiac valve development (Garg et al., 2005).
Results
Endocardial Notch signaling in trabecular development
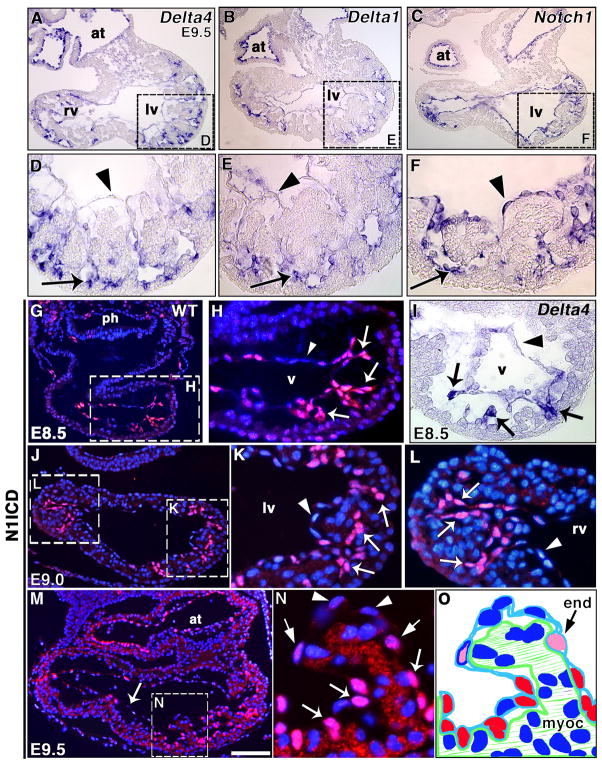
Delta4 and Notch1 mRNA are expressed in E8.5 endocardium (Timmerman et al., 2004). At E9.5, Delta4 and Delta1 levels were particularly high in endocardium at the base of ventricular trabeculae (Fig. 1A, B, D, E). In contrast, Notch1 transcription appeared uniform throughout ventricular endocardium (Fig. 1C, F).
Figure 1. Notch is active in the developing trabeculae.
(A–F) Delta4, Delta1 and Notch1 transcription in E9.5 wt heart by WISH. Delta4 (A, D) and Delta1 (B, E) transcribe preferentially in the endocardium at the base of trabeculae (arrows); little or no transcript is detected in distal endocardium (arrowheads). Notch1 (C, F) is uniformly transcribed in ventricular endocardium (arrow and arrowhead). (G–N) N1ICD staining (red), nuclei counterstained with DAPI (blue). (G, H) General heart view at E8.5 (G) and detail of ventricle (H). Note strong N1ICD expression in endocardium at the base of presumptive trabeculae (arrows) and no signal in distal endocardium (arrowhead). (I) Delta4 transcription at the base of nascent trabeculae (arrows) at E8.5. (J) E9.0 general heart view; note strong N1ICD staining in both ventricles. (K, L) Left (K) and right (L) ventricles with strong N1ICD staining in endocardium at the base of trabeculae (arrows), and no signal in distal endocardium (arrowhead). (M) E9.5 general heart view, strong N1ICD expression in ventricles and atrium, weaker expression in interventricular endocardium (arrow). (N) Detail of trabecula in left ventricle. Note strong N1ICD expression at the base (arrows) and progressively reduced signal at the middle (thin arrows) and distal tip (arrowheads). (O) Schematic representation of the trabecula shown in (N). The red color indicates strong N1ICD staining and the pink color indicates weak staining. at, atrium; end, endocardium; myoc, myocardium; ph, pharynx; lv, left ventricle; rv, right ventricle; v, ventricle. All panels are transverse sections. Scale bar, 100 μm in A–C, G, J, M; 25 μm in D–F, H, I, K, L; 80 μm in G; 15 μm in N.
To ascertain whether Delta4 and Delta1 ventricular transcription indicates the site of Notch activation, we stained E8.5-E9.5 embryos using an antibody to the activated form of Notch1 (N1ICD). At E8.5, N1ICD was strongly expressed in the endocardium of the prospective trabecular region (Fig. 1G, H), overlapping with Delta4 at this stage (Fig. 1I). At E9.0, endocardial N1ICD expression was stronger at the base of developing trabeculae and reduced at the distal end (Fig. 1J, K, L). At E9.5, N1ICD was predominantly expressed in the endocardium at the base of trabeculae (Fig. 1M, N), and to a lesser extent in interventricular endocardium (Fig. 1M). High-resolution fluorescence and confocal image analysis showed that N1ICD endocardial staining reduced progressively from the base to the distal end of trabeculae (Fig. 1N, O and Suppl. Fig. 1 and movie). These results indicated active endocardial Notch signaling prior to and during ventricular trabeculation, leading us to study whether this process was impaired in Notch pathway mutants.
Notch pathway mutants show defective trabeculation and marker expression
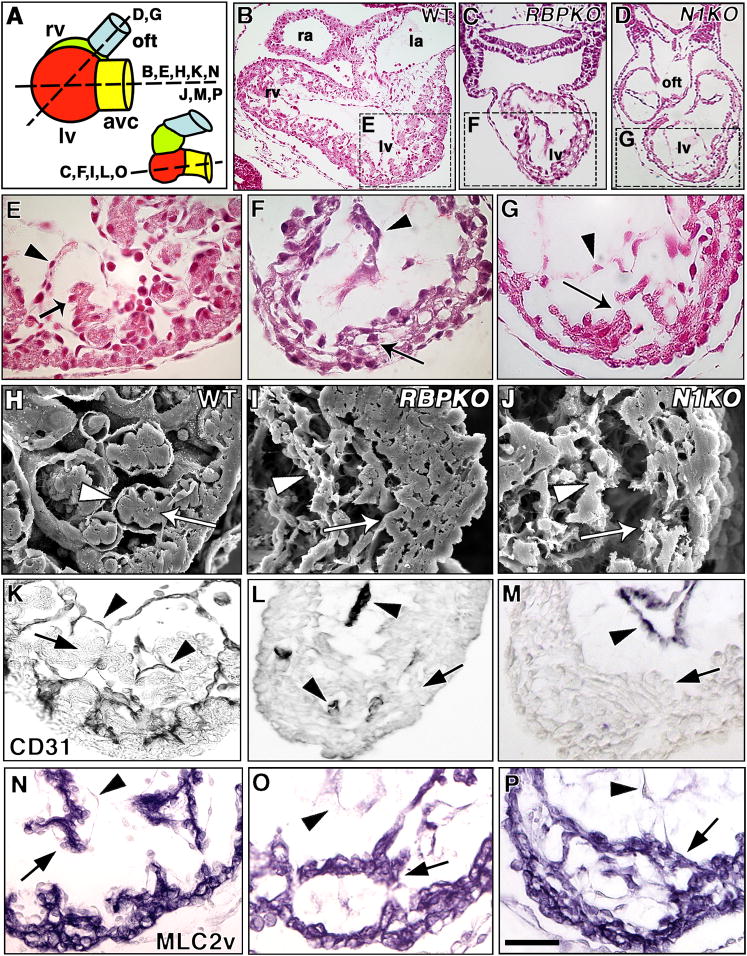
We have studied standard targeted Delta1 (Hrabe de Angelis et al., 1997), Notch1 (Conlon et al., 1995) and RBPJk mutants mutants (Oka et al., 1995). In addition, we have compared our data with results obtained with conditional Notch1 (Radtke et al., 1999) and RBPJk (Han et al., 2002) alleles deleted in the endocardium by breeding them with Tie2-CRE/+ transgenic mice (Kisanuki et al., 2001). We analyzed serial sections of E8.5-E10.5 Notch1 and RBPJk mutants for ventricular abnormalities (Fig. 2B–P; planes of section in Fig. 2A). In E9.5 wt embryos, both left and right ventricles showed developing trabeculae growing towards the ventricular lumen (Fig. 2B, E). In contrast, RBPJk (Fig. 2C, F) and, to a lesser extent, Notch1 mutants (Fig. 2D, G) had poorly developed trabeculae and a less-structured myocardium. We found no obvious cardiac defects in Delta1 mutants at E9.5 (not shown), suggesting functional redundancy among these ligands.
Figure 2. Defective trabeculation in E9.5 Notch1 and RBPJk mutants.
(A) Schematic representation of E9.5 wt or Notch1 (left) and RBPJk mutant hearts (right), depicting a lateral view of cardiac chambers. Section levels shown in (B–P) are indicated. In RBPJk mutants, the ventricles are aligned along the A–P axis. avc, AV canal; oft, outflow tract. (B–G) H+E-stained transverse sections and (H–J) SEM photomicrographs. (B, C, D) General views of representative wt (B), RBPJk (C) and Notch1 (D) mutants at the level of ventricles. Details of wt left ventricle (E, H), note developing trabeculae with myocardium (E, H, arrows) and endocardium (arrowheads); left ventricles of RBPJk (F, I) and Notch1 (G, J) mutants. RBPJk and Notch1 embryos show collapsed endocardium (arrowheads), disorganized myocardium (arrows) and poorly developed trabeculae. (K–M) Endocardial CD31/PECAM staining (arrowheads) in the left ventricle of wt (K), RBPJk (L) and Notch1 (M) embryos. In wt trabeculae the myocardium (arrow) is surrounded by endocardium (arrowhead). Arrowheads in (L, M) show endocardium and arrows indicate myocardium. (N-P) MLC2v staining in wt (N), RBPJk (O) and Notch1 (P) embryos show MLC2v expression throughout myocardium including trabeculae (arrow). Arrowheads indicate endocardium. la, left atrium; oft, outflow tract; ra, right atrium. Scale bar, 100 μm in B–D; 25 μm in D–P.
Scanning electron microscopy (SEM) revealed well-organized trabeculae in wt embryos, with close apposition of endocardium and myocardium (Fig. 2H). However, in RBPJk (Fig. 2I) and Notch1 (Fig. 2J) mutants, the endocardium did not surround the myocardium, which showed irregular thickness. RBPJk mutants had a more severe phenotype than Notch1 mutants, suggesting functional redundancy of Notch receptors in trabeculation. CD31/PECAM expression revealed a close endocardium:myocardium apposition in the developing trabeculae in wt embryos (Fig. 2K), whereas there were abnormally few contacts between these tissues in RBPJk and Notch1 mutants (Fig. 2L, M). To determine whether ventricular myocardium cell fate determination was affected in Notch mutants, we analyzed expression of the contractility marker MLC2v. E9.5 wt embryos showed MLC2v expression in developing compact zone myocardium and trabeculae (Fig. 2N). Likewise, RBPJk (Fig. 2O) and Notch1 mutants (Fig. 2P) expressed MLC2v throughout the myocardium, including the poorly developed trabeculae. Other early myocardial differentiation markers such as α-cardiac actin, smooth muscle α-actin and Irx4 were expressed in RBPJk mutants (Suppl. Fig. 2), indicating that early ventricular myocardial differentiation was unaffected.
To study the consequences of Notch deletion on ventricular patterning and trabeculation, we used WISH and semiquantitative RT-PCR to analyze expression of endocardium (Irx5) and myocardium (HOP, Irx3, PEG1) trabecular markers. Suppl. Fig. 3 shows that expression of these genes was severely reduced in RBPJk mutants, indicating that disruption of Notch ligand/receptor interactions between endocardial cells affects interactions with myocardial cells and impairs trabecular differentiation. As communication between endocardium and myocardium is critical for trabeculation (Meyer and Birchmeier, 1995), we studied the signaling pathways involved in this process.
Defective BMP10 signaling and cardiomyocyte proliferation in Notch pathway mutants
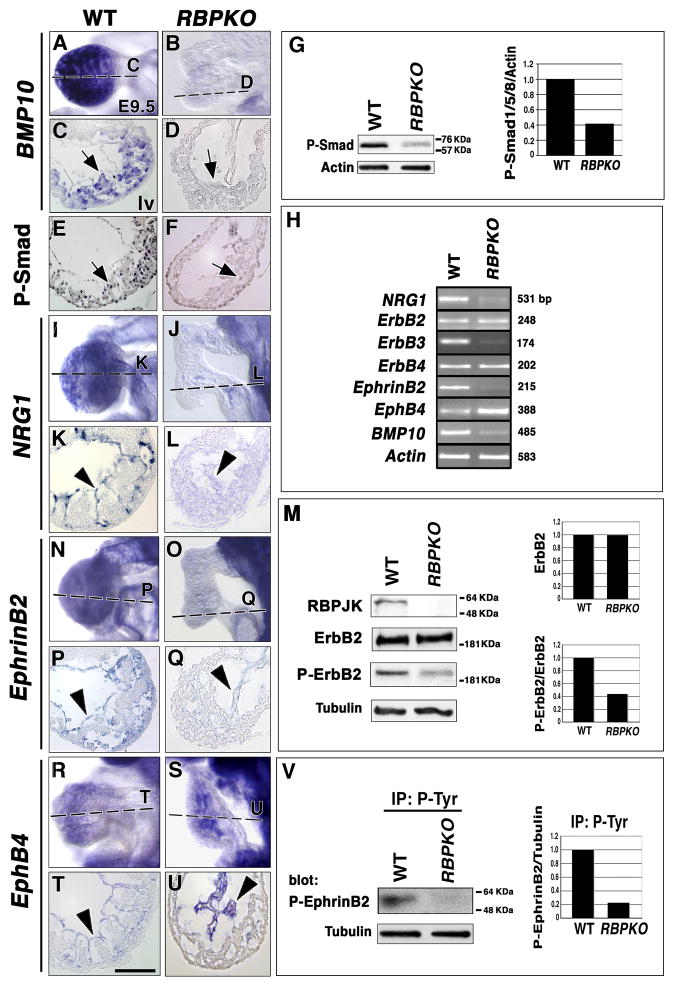
The cytokine BMP10 is expressed in trabecular myocardium at E9.5 (Fig. 3A, C and (Chen et al., 2004). BMP10 mutants show impaired ventricular trabeculation, caused by defective cardiomyocyte proliferation (Chen et al., 2004). BMP10 transcription was greatly reduced in RBPJk mutants as WISH (Fig. 3B, D) and RT-PCR analysis (Fig. 3H) showed, indicating that Notch is required for BMP10 expression. To assess the BMP10 signaling reduction in RBPJk mutants, we used an antibody specific for phosphorylated Smad1/5/8 proteins, which transduce the BMP10 signal to the nucleus (Mazerbourg et al., 2005). Phospho-Smad1/5/8 expression in ventricular myocardium (Fig. 3E) was reduced in RBPJk mutants (Fig. 3F). In contrast, similar phospho-Smad1/5/8 staining was observed in the outflow tract region of E9.5 wt (Suppl. Fig. 4A) and RBPJk mutants (Suppl. Fig. 4B), presumably because other TGFβ family members such as BMP2 (Mazerbourg et al., 2005) activate Smad1/5/8 in this region. To quantify chamber-specific differences between wt and RBPJk mutants in phospho-Smad1/5/8 expression, we carefully separated the ventricles from the outflow tract and atrio-ventricular (AV) canal regions. Ventricle-specific Western blot revealed a marked (60%) reduction in phospho-Smad1/5/8 expression in RBPJk mutants (Fig. 3G), indicating that BMP10-dependent signaling was affected in this tissue.
Figure 3. Impaired BMP10, NRG1 and EphrinB2 expression and activity in RBPJk mutants.
BMP10 transcription in E9.5 wt trabecular myocardium (A, C) is reduced in RBPJk mutants (B, D). P–Smad1/5/8 ventricular expression in wt myocardium (E, arrow) is reduced in RBPJk mutants (F, arrow). (G) Left: Western blot with anti-P-Smad1/5/8 antibody in wt and RBPJk mutant ventricles. Right: Ratio of P-Smad1/5/8 expression to actin. (H) Semiquantitative RT-PCR analysis in E9.5 wt and RBPJk mutant hearts. wt endocardial NRG1 expression (I, K) is reduced in RBPJk mutants (J, L). (M) Western blot of E9.5 cardiac extracts shows no RBPJK protein expression, relatively normal ErbB2 receptor levels and reduced activated ErbB2 receptor levels (P-ErbB2) in RBPJk mutants. Top right: Ratio of total ErbB2 levels in wt and mutants. Bottom right: Ratio of P-ErbB2 to total ErbB2 levels. (N, P) EphrinB2 expression in wt endocardium is greatly reduced in RBPJk mutants (O, Q). Endocardial EphB4 expression (R, T) is increased in RBPJK mutants (S, U). (V) Immunoprecipitation with pTyr and western blot with anti-phosphorylated EphrinB2 antibody. Right: Normalized phospho-EphrinB2 levels. In (G, M, V) representative results from three experiments with two independent sets of extracts are shown. Arrows in (C–F) show myocardium and arrowheads endocardium. All sections show left ventricle. Scale bar, 25 μm.
To assess whether BMP10 down-regulation affected cell proliferation in Notch mutants, we analyzed both the S (measuring BrdU incorporation into DNA) and mitosis phases of the cell cycle (using an anti-phospho histone H3 -PHH3- antibody). At E9.5, numerous BrdU+ cells were found in wt myocardium (35% of total cells; n= 10 embryos; Suppl. Fig. 4C, E), whereas RBPJk mutants showed a drastic reduction in BrdU+ cells (<15%; n = 6; Suppl. Fig. 4D, E). Approximately 6–8% of nuclei in wt myocardium were PHH3+ (n=6; Suppl. Fig. 4F, H), whereas only 2% of nuclei stained positive in RBPJk mutants (n=4; Suppl. Fig. 4G, H). The predominance of BrdU+ myocardial cells in the E10.5 compact zone myocardium (Suppl. Fig. 5A, B) correlated with endocardial N1ICD+ cells at the base of trabeculae (Suppl. Fig. 5C, D), suggesting that endocardial Notch signaling is required for cardiomyocyte proliferation. As BMP10 antagonizes the cell cycle kinase inhibitor p57Kip2 (Chen et al., 2004), we tested whether its expression was increased in RBPJk mutants. p57Kip2 was expressed primarily in endocardium of wt embryos, and few p57Kip2+ cells were detected in myocardium (n=5; Suppl. Fig. 4I, K and Chen et al., 2004). RBPJk mutants showed a 40% increase in p57Kip2 expression (n=5; Suppl. Fig. 4J, K), consistent with their reduced myocardial BMP10 expression (Fig. 3B, D). Our data indicate that Notch is required to sustain cardiomyocyte proliferation -via BMP10- during trabeculation.
Defective NRG1 and EphrinB2 signaling in Notch pathway mutants
The signaling pathway represented by the ligand NRG1 and the ErbB receptors is essential for trabeculation (Gassmann et al., 1995; Lee et al., 1995; Meyer and Birchmeier, 1995). NRG1 acts as a paracrine signal in the myocardium leading to ErbB4-ErbB2 dimerization and stimulation of ErbB2 intrinsic tyrosine kinase activity (Falls, 2003). wt E9.5 NRG1 expression in ventricular endocardium (Fig. 3I, K, H and Meyer and Birchmeier, 1995), was greatly reduced in RBPJk mutants (Fig. 3J, L, H). In contrast, ErbB2 and ErbB4 were normally expressed in myocardium of Notch mutants (Fig. 3H and not shown). ErbB2 also heterodimerizes with ErbB3 (Falls, 2003), which is exclusively expressed in AV canal endocardial cushion mesenchyme (Meyer and Birchmeier, 1995 and Suppl. Fig. 6A, C, E). ErbB3 is required for endocardial cushion development (Erickson et al., 1997), similarly to Notch (Timmerman et al., 2004), and ErbB3 expression was reduced in AV canal of RBPJk mutants (Fig. 3H and Suppl. Fig. 6B, D, F). NRG1 pathway activation was assessed by Western blot. E9.5 wt and RBPJk mutant ventricles showed similar total ErbB2 levels (Fig. 3M). In contrast, extracts probed with anti-phospho-ErbB2 antibody showed a marked reduction (60%) in the amount of phosphorylated ErbB2 in RBPJk mutants (Fig. 3M). These results indicated that Notch is required for endocardial NRG1 expression.
The EphrinB2/EphB4 membrane-bound ligand/receptor system is required for cardiovascular development, including trabeculation (Wang et al., 1998; Gerety et al., 1999). Both EphrinB2 (Fig. 3N, P and Wang et al., 1998) and EphB4 (Fig. 3R, T and Gerety et al., 1999) are expressed in wt endocardium. EphrinB2 expression was drastically reduced in RBPJk mutants (Fig. 3O, Q, H), whereas EphB4 expression was increased (Fig. 3S, U, H), indicating that Notch is needed for endocardial EphrinB2 and EphB4 expression. During vascular development, EphrinB2 marks arterial and EphB4 vein endothelium (Wang et al., 1998). The observation that Notch mutants show loss of EphrinB2 and increased EphB4 expression suggests that the endocardial default phenotype, like that of endothelium, would be “vein”.
We examined EphrinB2 signaling activity using ventricular extracts precipitated with anti-pTyr and probed with anti-phospho-EphrinB2 antibody. The results showed severe reduction (80%) of phospho-EphrinB2 levels in RBPJk mutants (Fig. 3V), most likely as a consequence of reduced EphrinB2 expression. These data indicated that cardiac NRG1- and EphrinB2-dependent signaling is greatly reduced in RBPJk mutants.
Endocardial-specific Notch1 or RBPJk deletion impairs BMP10, NRG1 and EphrinB2 expression
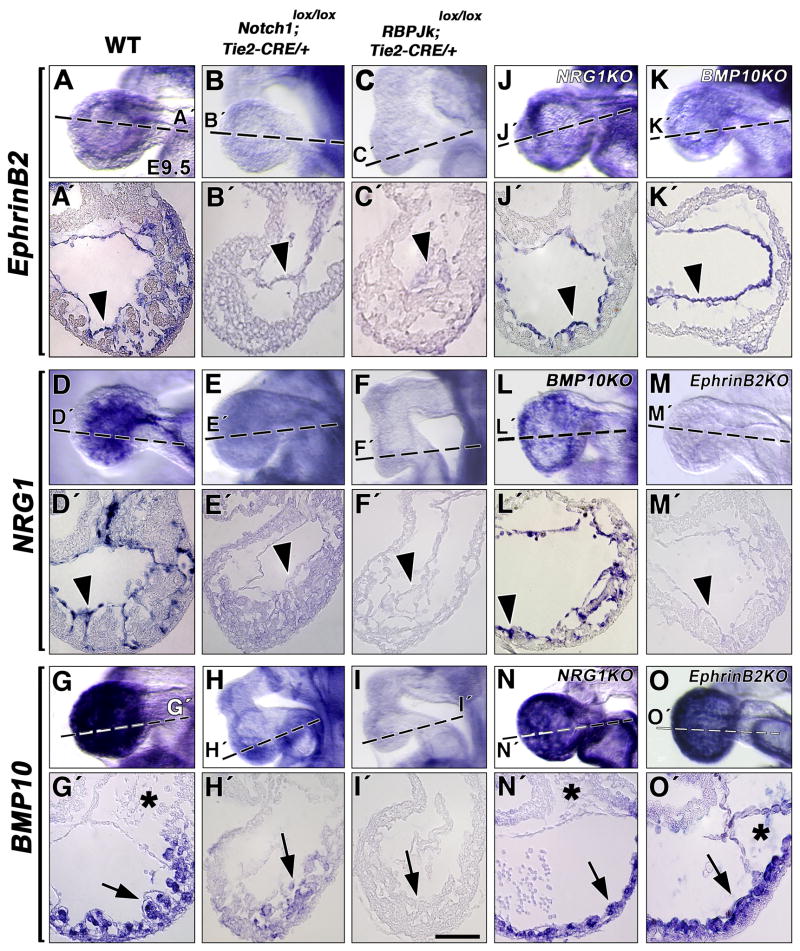
To demonstrate that the molecular phenotypes observed were caused specifically by the abrogation of Notch1 endocardial function, we deleted Notch1 or RBPJk in the endocardium, using conditional Notch1 (Radtke et al., 1999) and RBPJk (Han et al., 2002) lines, that we bred with Tie2-CRE mice (Kisanuki et al., 2001). WISH analysis of E9.5 embryos showed normal endocardial EphrinB2 expression in wt embryos (Fig. 4A, A′); in contrast, N1lox/N1lox; Tie2-CRE/+ (n=5; Fig. 4B, B′) and RBPJklox/RBPJklox; Tie2-CRE/+ (n=4; Fig. 4C, C′) mutants showed severely reduced EphrinB2 expression. Comparison of NRG1 transcription in wt (Fig. 4D, D′), N1lox/N1lox; Tie2-CRE/+ (n=5; Fig. 4E, E′) and RBPJklox/RBPJklox; Tie2-CRE/+ (n=4; Fig. 4F, F′) embryos showed a strong reduction in both mutants, more drastic in RBPJklox/RBPJklox; Tie2-CRE/+embryos (Fig. 4F′). The reduction of BMP10 transcription in trabecular myocardium (Fig. 4G, G′) was milder in N1lox/N1lox; Tie2-CRE/+ embryos (4 out of 6 embryos; Fig. 4H, H′) than in RBPJklox/RBPJklox; Tie2-CRE/+ mutants (n=4; Fig. 4I, I′), suggesting functional redundancy at the receptor level. These results demonstrate that the molecular phenotypes observed in standard RBPJk and Notch1 mutants are due to the abrogation of Notch1-dependent signaling in the endocardium.
Figure 4. Expression analysis in endocardial-specific Notch mutants and standard targeted EphrinB2, NRG1 and BMP10 mutants.
WISH, E9.5. (A, A′) EphrinB2 expression in wt embryos; reduction in Notch1lox/lox; Tie2-CRE (B, B′) and RBPJklox/lox; Tie2-CRE (C, C′) mutants. (D, D′) NRG1 expression in wt; reduction in Notch1lox/lox; Tie2-CRE (E, E′) and RBPJklox/lox; Tie2-CRE (F, F′) mutants. (G, G′) BMP10 expression in wt; reduction in Notch1lox/lox; Tie2-CRE (H, H′) and RBPJklox/lox; Tie2-CRE (I, I′) mutants. Normal EphrinB2 transcription in E9.5 NRG1 (J, J′) and BMP10 mutants (K, K′). Normal NRG1 expression in BMP10 mutants (L, L′) and marked reduction in EphrinB2 mutants (M, M′). BMP10 expression in NRG1 (N, N′) and EphrinB2 (O, O′) mutants. Arrowheads point to endocardium and arrows to myocardium. Asterisks indicate endocardial cushion of the AV canal, devoid of mesenchymal cells in EphrinB2 mutants. Scale bar, 25 m.
NRG1 acts downstream of EphrinB2 and independently of BMP10 during trabeculation
We have shown that cardiac BMP10, NRG1 and EphrinB2 expression depends on Notch signaling. To determine whether these molecules function in a common pathway during trabeculation, we first analyzed by WISH the onset of their expression during cardiogenesis. We detected EphrinB2 (Suppl. Fig. 7A) and NRG1 (Suppl. Fig. 7B) endocardial expression in early E8.0 (six-somites) wt embryos. In contrast, BMP10 transcription was not detectable in the E8.0 heart (Suppl. Fig. 7C). At E9.0 (twelve-somites), endocardial EphrinB2 (Suppl. Fig. 7D) and NRG1 (Suppl. Fig. 7E) expression was maintained and we could barely detect BMP10 in myocardium (Suppl. Fig. 7F).
Then, we analyzed EphrinB2, NRG1 and BMP10 expression in the appropriate mutant backgrounds. Endocardial EphrinB2 expression (Fig. 4A, A′) was normal in NRG1 (n= 6; Fig. 4J, J′) and BMP10 (n= 4; Fig. 4K, K′) mutants. Likewise, endocardial NRG1 expression (Fig. 4D, D′) was unaffected in BMP10 mutants (n= 4; Fig. 4L, L′). In contrast, NRG1 expression was severely reduced in EphrinB2 mutants (n =6; Fig. 4M, M′), indicating that its transcription depends on EphrinB2 but not on BMP10. Normal BMP10 transcription (Fig. 4G, G′) in NRG1 (n = 5; Fig. 4N, N′) and EphrinB2 (n = 6; Fig. 4O, O′) mutants indicated that its expression does not depend on NRG1 and EphrinB2. These results, together with the early (E8.0) cardiac expression of EphrinB2 and NRG1 but not BMP10, indicated that EphrinB2 acts upstream of NRG1 and that BMP10 is independent of both pathways during trabeculation.
Normal cellular proliferation and impaired myocardial differentiation in EphrinB2 and NRG1 mutants
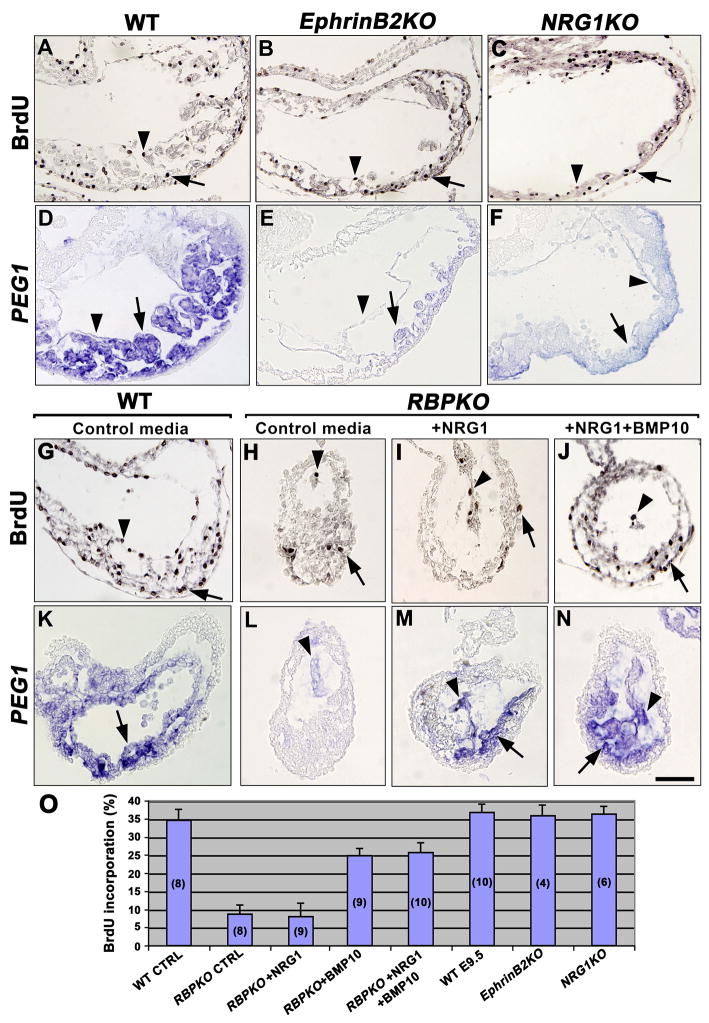
As both EphrinB2 and NRG1 have been shown to depend on Notch for their expression, an analysis of proliferation and differentiation in EphrinB2 and NRG1 mutants was required. Ventricular cell proliferation measured by BrdU incorporation was similar in wt (n=10; 35%; Fig. 5A, O and Suppl. Fig. 4C, E) was relatively normal in EphrinB2 (n=4; Fig. 5B, O) and NRG1 mutants (n=6; Fig. 5C, O). Ventricular differentiation was examined by WISH using a PEG1 probe. PEG1 expression in trabecular myocardium and endocardium (Fig. 5D and (King et al., 2002), was markedly reduced in EphrinB2 (n=4; Fig. 5E) and NRG1 mutants (n=6; Fig. 5F), suggesting that both EphrinB2 and NRG1 are required for ventricular myocardial differentiation.
Figure 5. Cardiac proliferation and marker analysis of EphrinB2 and NRG1 mutants and in vitro cytokine rescue experiment with RBPJk mutants.
(A–C) BrdU staining in endocardium and myocardium of E9.5 wt (A), EphrinB2 (B) and NRG1 (C) mutants. Positive cells in endocardium are indicated with arrowheads and with arrows in myocardium. (D–F) PEG1 expression in trabecular myocardium and endocardium of wt embryo (D), and severe reduction in EphrinB2 (E) and NRG1 (F) mutants. Arrowheads indicate endocardium and arrows myocardium. (G–J) BrdU staining in wt (G) and RBPJk mutants cultured in control media (H), media with NRG1 (I) and media with NRG1 plus BMP10 (J). (K–N) PEG1 expression in wt (K) and RBPJk mutants cultured in control media (L), media with NRG1 (M) and media with NRG1 plus BMP10 (N). (O) Quantification of BrdU incorporation. The values are means of BrdU-positive nuclei. The number of embryos analyzed is indicated in parenthesis inside of bars. Scale bar, 40 μm.
NRG1 rescues differentiation while BMP10 rescues proliferation defects of cultured Notch pathway mutants
To test whether the secreted NRG1 and BMP10 are the effectors of pathways involved in ventricular myocardium proliferation and differentiation, we cultured whole E8.5 RBPJk mutant embryos from heterozygous intercrosses in normal (control) media, NRG1-containing media, BMP10-containing media or media containing NRG1 plus BMP10. After 19hs of culture and BrdU addition, we processed the embryos for proliferation or WISH analysis. Fig. 5G, O shows extensive BrdU incorporation in the ventricle of wt cultured embryos (approximately 35%; n=8), similar to our in vivo observations (Fig. 5A and Suppl. Fig. 4C, E). As previously described (Suppl. Fig. 4D, E), RBPJk mutants cultured in control media showed reduced BrdU incorporation (<10%; n=8; Fig. 5H, O), similar to embryos cultured in NRG1-containing media (<10%; n=9; Fig. 5I, O). In contrast, RBPJk mutants cultured in media with NRG1 plus BMP10 showed a clear increase in proliferation (27%; n=10; Fig. 5J, O). Similar results were obtained with RBPJk mutants cultured with BMP10 alone (25%; n=9; Fig. 5O and not shown), suggesting that the effect was BMP10-dependent. We then examined expression of the ventricular differentiation marker PEG1 in cultured embryos. PEG1 trabecular myocardial and endocardial expression in cultured wt embryos (Fig. 5K; 8 out of 8 embryos) was severely reduced in RBPJk mutants cultured in control media (Fig. 5L; 7 out of 7), as observed in vivo (Suppl. Fig. 3N, P, Q). In contrast, RBPJk mutants cultured in NRG1-containing media (Fig. 5M; 6 out of 8) or in media containing both cytokines (Fig. 5N; 7 out of 9), showed normal PEG1 expression, suggesting that the effect was NRG1-dependent. These results indicate that the myocardial proliferation defect of Notch mutants is caused by their reduced BMP10 expression, while the differentiation defect is due to the low NRG1 levels.
EphrinB2 is a N1ICD/RBPJK target
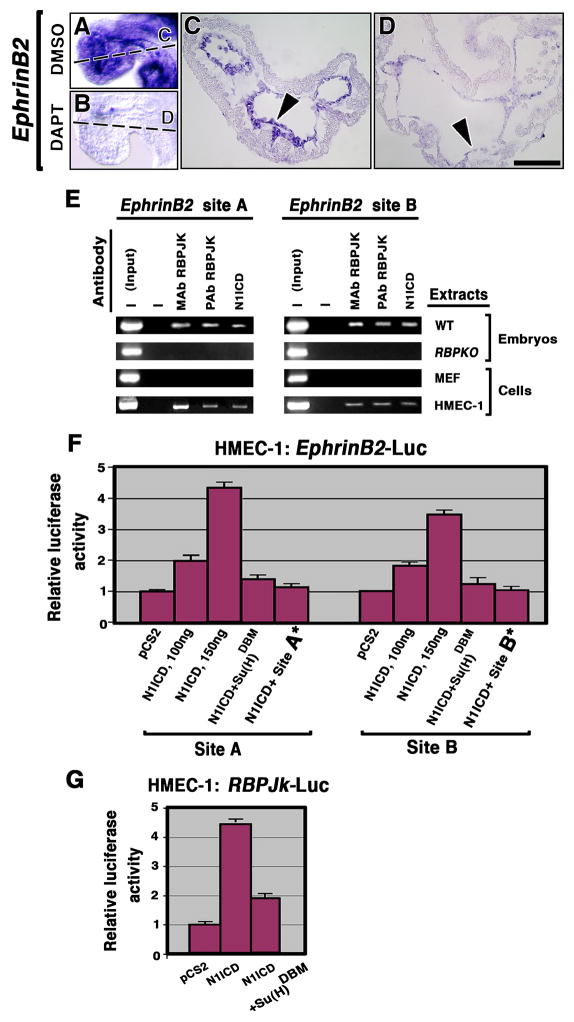
To further substantiate the relationship of Notch with the EphrinB2, NRG1 and BMP10 pathways, we cultured E8.5 wt embryos in media containing DMSO or DAPT, a γ-secretase inhibitor (Dovey et al., 2001). After 16 h culture, no obvious effect was observed on NRG1 or BMP10 expression in DAPT-treated and DMSO control embryos (Suppl. Fig. 8). In contrast, whereas DMSO-cultured embryos showed normal EphrinB2 expression (n = 26; Fig. 6A, C), DAPT-treated embryos showed a drastic reduction in EphrinB2 expression (n = 10; Fig. 6B, D), suggesting a direct dependence of EphrinB2 expression on Notch activity. The persistence of NRG1 and BMP10 expression in DAPT-cultured embryos may be due to the relatively short period of culture, which may have prevented us from detecting changes in expression that may indirectly depend on Notch.
Figure 6. EphrinB2 is a Notch target in the endocardium.
(A–D) WISH showing EphrinB2 expression in E9.5 DMSO control (A, C, arrowhead) and severe reduction in DAPT-treated embryos (B, D, arrowhead). (E) ChIP assays. Chromatin from E9.5 wt and RBPJk mutant hearts, MEF and HMEC-1 cells was immunoprecipitated with monoclonal (MAb) or polyclonal (PAb) antibodies against RBPJK or N1ICD. The EphrinB2 genomic DNA was analyzed for RBPJK binding sites A and B. “Input” corresponds to PCR products generated using DNA from non-immunoprecipitated chromatin as a template. —, no antibody was added to the reaction mixture. (F) Luciferase reporter assays. HMEC-1 cells were co-transfected with Renilla luciferase plasmid and 100 or 150ng of N1ICD, Su(H)DBM effector plasmid or a control plasmid (empty pCS2 vector), in combination with a reporter plasmid containing wt RBPJK binding sites (A or B) or mutated ones (A* or B*). (G) HMEC-1 cells transfected with empty vector, N1ICD or Su(H)DBM and RBPJk reporter plasmid. After normalization to Renilla luciferase activity, firefly luciferase activity relative to that of control plasmid was calculated for each reporter. Relative values (mean+SD) from at least four independent experiments performed in triplicate are represented by the bars in the bar chart. Scale bar, 25 μm (C, D).
To test whether Notch1/RBPJK controls EphrinB2 expression directly, we carried out in silico analysis of this locus (Suppl. Fig. 9). We identified two RBPJK core consensus binding sites (GTGGGAA; Bailey and Posakony, 1995) that are evolutionarily conserved among mouse, rat, monkey and humans. The first site (A) is located within the first intron, close to exon 2, and the second site (B) is at the beginning of intron 2 (Suppl. Fig. 9). We performed chromatin immunoprecipitation (ChIP) assays with one anti-N1ICD antibody and two anti-RBPJK antibodies (see Experimental Procedures), using nuclear extracts from E9.5 wt and RBPJk hearts. We used the human microvasculature endothelial cell line HMEC-1 as in vitro model, and mouse embryonic fibroblasts (MEF) as negative controls, as they express no detectable EphrinB2 (not shown). The different antibodies immunoprecipitated chromatin from wt embryos and HMEC-1 cells extracts, but not from RBPJk mutants or MEF control extracts (Fig. 6E). These results demonstrated specific binding of the N1ICD/RBPJK complex to these two sites in the EphrinB2 genomic DNA.
To confirm that N1ICD/RBPJK binding to these sites activates EphrinB2 expression, we performed luciferase reporter assays using HMEC-1 cells. Two different EphrinB2 genomic fragments spanning either site A or site B, were cloned upstream of the firefly luciferase gene. HMEC-1 cells transfected with empty vector (pCS2) showed basal luciferase activity with both site A and site B reporter constructs (Fig. 6F). In contrast, N1ICD was able to transiently activate this EphrinB2 reporter constructs up to 4.5-fold in a dose-dependent fashion in HMEC-1 cells (Fig. 6F; P<0.001; Student’s t test). To demonstrate that this effect was N1ICD/RBPJK-dependent, we used a dominant negative RBPJK (Su(H)DBM) that binds to NICD but is unable to bind to DNA, blocking NICD/RBPJK-mediated gene activation (Wettstein et al., 1997). Cotransfection of N1ICD and Su(H)DBM caused a 75–80% reduction in luciferase activity in both sites (Fig. 6F). To confirm that transcriptional activation occurred specifically via sites A and B, we introduced a single nucleotide mutation (GTGGCAA) in both sites (A* or B*). This has been shown to reduce drastically RBPJK binding to DNA (Tun et al., 1994). Cotransfection of N1ICD and site A* or site B* caused an almost complete reduction in luciferase activity (Fig. 6F).
As a control of N1ICD activity, HMEC-1 cells were transfected with a RBPJK-Luc reporter construct (McKenzie et al., 2005), leading to a 4.5-fold increase in luciferase activity compared to control cells transfected with empty vector, and to a 65–70% reduction of activity upon cotransfection with Su(H)DBM (Fig. 6G). The results showed that these two RBPJK binding sites in the EphrinB2 genomic DNA are functional and activate EphrinB2 transcription. Together our data demonstrate that EphrinB2 is a direct N1ICD/RBPJK target.
Discussion
In this study we show that Notch is crucial for ventricular trabeculation and chamber development. EphrinB2 is a direct endocardial Notch target during this process that also requires the Notch-dependent activity of BMP10 and NRG1, to promote proliferation and differentiation of ventricular myocardium.
Preferential Notch signaling in the trabeculae is evidenced by restricted Delta1 and Delta4 transcription, and N1ICD expression, in endocardium at the base of the developing trabeculae. Signal is reduced at the distal end of trabeculae, suggesting that endocardial Delta-Notch expression is activated by local cues from the underlying myocardium; the reduction of ligand expression in endocardium of Notch mutants (not shown and Timmerman et al., 2004) suggests that Notch signals in the ventricles by lateral induction (Lewis, 1998), and that N1ICD-expressing endocardial cells behave as a developmental field.
The idea that Notch plays a role in ventricular development is supported by the trabeculation-defective phenotype of standard and endocardial–specific Notch1 and RBPJk mutants. Molecular analysis indicates that Notch1 signaling is required for ventricular myocardial differentiation. Interestingly, constitutive cardiac Notch1 activation driven by Mesp1-CRE leads to impaired ventricular myocardium maturation and inhibition of cardiomyocyte differentiation (Watanabe et al., 2006). This result might be thought to be in conflict with our data, but as the authors argue, it may be consequence of ectopic Notch1 myocardial expression in these mice which may generate additional secondary signals that would inhibit cardiomyocyte differentiation (Watanabe et al., 2006).
What are the Notch targets in the heart? Ventricular expression of the HRT1 and HRT2 transcription factors (Nakagawa et al., 1999) is unaffected in RBPJk and Notch1 mutants (see Suppl. Fig. 10), suggesting that other Notch-dependent genes may be responsible for the regulation of ventricular morphogenesis.
Notch mutants display defective expression of three signaling pathways that are not classic Notch targets: BMP10, EphrinB2 and NRG1. BMP10 is required for cardiomyocyte proliferation (Chen et al., 2004). The reduced BMP10 expression in both standard and endocardial-specific Notch mutants, suggested that Notch and BMP10 interact during cardiogenesis. Ventricular BMP10 activity measured by Smad1/5/8 phosphorylation was clearly reduced in RBPJk mutants. Low BMP10 signaling was accompanied by reduced cardiomyocyte proliferation and increased myocardial expression of the cell cycle inhibitor p57Kip2. Defective cardiac cellular proliferation was rescued culturing RBPJk mutants in BMP10-conditioned media (Suppl. Fig. 5J), suggesting that in trabecular myocardium Notch modulates proliferation via BMP10. Proliferation and differentiation, mutually exclusive in skeletal muscle cells (Parker et al., 1995), are intimately linked in cardiomyocytes. Our data indicate that Notch mutant cardiomyocytes exit cell cycle prematurely and do not progress in their differentiation towards trabecular muscle.
NRG1 expression and activity was markedly reduced in Notch1 and RBPJk embryos, indicating that Notch and NRG1/ErbB signaling interact during trabecular development. NRG1 promotes in vitro proliferation of neonatal cardiomyocytes (Zhao et al., 1998), but in a whole-mouse embryo culture system NRG1 induces trabeculation (Hertig et al., 1999), or cardiac conduction system development (Rentschler et al., 2002), without increasing proliferation. Concurring with these results, we rescued the myocardial differentiation defect of RBPJk mutants by culturing them in NRG1-containing media (Fig. 5M, N).
Cardiac EphrinB2/EphB4 expression and activity was impaired in Notch mutants and culture of wt embryos with DAPT, specifically reduced EphrinB2 transcription. Our ChIP assays demonstrated that N1ICD/RBPJK binds to two conserved RBPJK responsive elements within the EphrinB2 genomic DNA, an association that is lost in vivo in RBPJk mutants. Reporter assays with endothelial cells showed that N1ICD specifically activated luciferase expression downstream of these two sites, demonstrating that EphrinB2 is a direct transcriptional target of N1ICD/RBPJK.
How are these signaling mechanisms related? Analysis of EphrinB2, NRG1 and BMP10 mutants revealed that cardiac NRG1 transcription was reduced in EphrinB2 mutants while BMP10 mutants showed normal EphrinB2 and NRG1 expression. These results indicate that during trabeculation endocardial EphrinB2 acts upstream of NRG1, and that both molecules act independently of BMP10. Thus, NRG1 could be the secreted “missing molecule” linking EphrinB2/EphB4 endocardial signaling to the specific changes described in trabeculating myocardial cells (Hertig et al., 1999; Rentschler et al., 2002).
We suggest that during trabeculation Notch links endocardial with myocardial signaling via its direct effect on the EphrinB2/EphB4 pathway, which is required for endocardial NRG1 production and subsequent ErbB2/B4 activation in myocardial cells. Notch1 is active in endocardium and BMP10 is expressed in myocardium, suggesting that Notch1 is required for production of a soluble endocardial signal, which induces myocardial BMP10 expression. NRG1 was a candidate, but BMP10 expression is normal in NRG1 mutants (Fig. 4N, N′), indicating that the NRG1/ErbB pathway does not connect Notch and BMP10 during trabeculation.
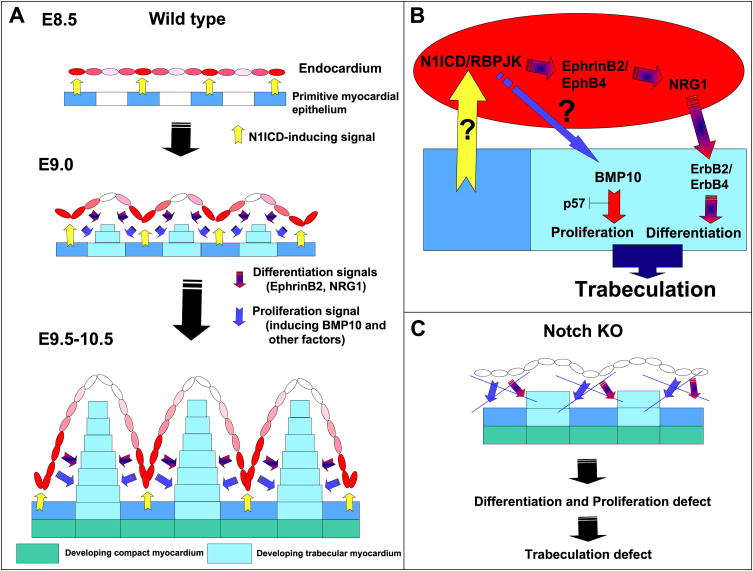
Our data show that Notch mediates an endocardium-myocardium interaction critical for trabeculation and ventricular chamber morphogenesis, identifying two distinct Notch-dependent processes (summarized in Fig. 7): 1) the transition of primitive myocardial epithelium to trabecular and compact myocardium (EphrinB2- and NRG1-dependent), 2) the maintenance of a proliferating trabecular cardiomyocyte population (BMP10-dependent) during this transition. In agreement with the proposed crucial role for Notch in trabeculation, constitutive cardiac Notch1 activation leads to a complex phenotype, including ectopic trabeculation in AV myocardium (Watanabe et al., 2006).
Figure 7. A model for Notch activity in ventricular development.
(A) E8.5 wt embryo. A myocardial cue (yellow) leads to N1ICD expression (red) in specific endocardial regions. At E9.0, N1ICD/RBPJK activate endocardial EphrinB2 leading to NRG1 expression. NRG1 activates the ErbB2/B4 receptors in myocardium to induce trabecular muscle differentiation. As the trabecular ridge develops, the endocardium separates from the myocardial N1ICD-inducing cue, and N1ICD is down-regulated (pink) at the tip of the trabeculae. At E9.5-10.5, N1ICD is higher in the endocardium at the base of the trabeculae, and the ventricular myocardium has differentiated into compact zone (green) and trabecular myocardium (blue) regions. The different intensity of N1ICD expression (pink or red) represents the predominant N1ICD activation at the base of trabeculae in response to a spatially restricted myocardial cue. (B) Molecular pathways downstream of Notch during trabeculation. (C) In Notch mutants, proliferation and differentiation signals are disrupted and trabeculation is impaired.
The less dense ventricular compact zone myocardium in Notch mutants is reminiscent of a CHD termed isolated ventricular non-compaction (IVNC; Jenni et al., 2001), a genetically heterogeneous, congenital disorder characterized by altered myocardial structure. It is tempting to speculate that Notch signaling may be altered in infants with conditions including malseptation, abnormal valve development (defective papillary muscle formation) or conduction system defects —all of which are related to abnormal trabeculation— or in adults as a response to pathological hypertrophic signals.
Experimental procedures
Mouse genotyping
For details see Suppl. Information.
Histology, in situ hybridization and immunohistochemistry
Hematoxylin/eosin (H+E) staining was performed by standard methods. Whole-mount in situ hybridization (WISH) and sectioning were as described (de la Pompa et al., 1997). Details for probes will be provided on request.
For details on immunohistochemistry see Suppl. Information.
Scanning electron microscopy
Mouse embryos were fixed and processed according to standard protocols.
Short-term whole mouse embryo culture and DAPT treatment
E8.5 wild-type mouse embryos were dissected at room temperature in DMEM/FBS (1:1) leaving all membranes intact. Embryos were cultured in 3-cm tissue culture dishes containing 1 ml of 1% agarose to avoid embryo attachment; 2 ml of medium [DMEM/FBS (1:1) plus antibiotics and 50 μM DAPT (γ-secretase inhibitor IX; 565770, Calbiochem)] or DMSO was added to the hardened agarose. Embryos were cultured (37°C, 5% CO2) for 16 h, then dissected in PBS, fixed in 4% PFA, and analyzed by WISH.
Short-term whole mouse embryo culture in cytokines-containing media, proliferation and marker analysis of RBPJk mutants
E8.5 wt and RBPJk mutant embryos were cultured overnight as described above in 2ml of BMP10-conditioned media plus 2.5×10−8M NRG1 (396-HB/CF; R&D Systems). After overnight culture (16hs), BrdU was added to the media at a final concentration of 30ug/ml and the embryos were incubated for an additional 3h, followed by fixation and processing for immunostaining or WISH. BMP10 conditioned-media was prepared according to (Chen et al., 2004).
Analysis of P-Smad1/5/8, ErbB2 and EphrinB2 phosphorylation
Two independent protein extracts, from 25 E9.5 wt or RBPJk mutant ventricles each were prepared. Ventricles were sonicated in RIPA lysis buffer (50 mM Tris-HCl pH 7.5, 0.5% deoxycholate, 150 mM NaCl, 1% NP40, 0.1% SDS, protease and phosphatase inhibitors). Protein concentration was determined by BCA assays (Pierce) and samples were stored at −80°C. Extracts were immunoprecipitated with an antibody against phosphorylated tyrosine residues (V2171, Promega) (16 h, 4°C). Precipitates or whole extracts were separated by 8–10% SDS-PAGE and transferred to Immobilon-P membranes (Millipore). Western blot was performed with antibodies to ErbB2 receptor (SC-284, Santa Cruz Biotechnology), phosphorylated-ErbB2 (06-229; Upstate Biotechnology), phosphorylated EphrinB2 (3481; Cell Signaling) and phospho-Smad1/Smad5/Smad8 (9511; Cell Signaling); for RBPJK, we used the T6719 rat monoclonal antibody (Hamaguchi et al., 1992). ECL (Amersham) was used to visualize immunoreactive bands, whose intensity was quantified from digital photo-images with Photoshop.
Chromatin immunoprecipitation (ChIP) assays
Before crosslinking, embryos were pipetted gently to disaggregate and homogenize tissue. Chromatin was sheared to an average length of 0.4–0.8 kb. We used polyclonal (de la Pompa et al., 1997) and monoclonal (K0043, (Sakai et al., 1995), antibodies to RBPJK and Notch (Cell Signaling). PCR amplifications were performed in 25 μl with primers specific for the promoter analyzed. PCR amplification sensitivity was evaluated on serial dilutions of total DNA collected after sonication (input fraction). Amplified DNA was separated on 2% agarose gels and visualized with ethidium bromide. Two sets of primers were used to amplify approximately 200 bp of DNA sequences containing the two conserved RBPJK-binding sites of the mouse EphrinB2 gene: forward EphrinB2_bsA, 5′-AAC AGC GCA TGG AAA CTA CC-3′ and reverse EphrinB2_bsA, 5′-CCT TCC TGG GTC TCT TAG GC-3′ and forward EphrinB2_bsB 5′-ACC TAG GGC AAG TGG GAA CT-3′ and reverse EphrinB2_bsB 5′-CAG TGT TGG GCA GAC TGC TA-3′. PCR amplification was carried out with a variable number of cycles (94°C for 30 sec, 60°C for 30 sec, 72°C for 30 sec). For HMEC-1 cells, primers were designed against the highly conserved binding site 1 of the human EphrinB2 promoter. Forward human EphrinB2_bsA, 5′-CAT CAA CAG CAC AGG GAA AC-3′ and reverse human EphrinB2_bsA, 5′-CTC TTC CTC CGT GGT GAG TC-3′. PCR amplification was carried out with a variable number of cycles (94°C for 30 sec, 59°C for 30 sec, 72°C for 30 sec).
Cell culture, transfections, site directed mutagenesis and luciferase assays
For luciferase assays, we generated a reporter construct by cloning a 600bp and 800 bp DNA fragments containing RBPJK binding sites A or B in the mouse EphrinB2 gene into the KpnI and XhoI sites of the pGL3 promoter luciferase plasmid (Promega). The sequence of the primers for site A was: 5′ATG GTA CCA GGA GAG GGC TCT TCA TCT TGC AG-3′ (forward, KpnI site underlined) and 5′-ATC TCG AGG CCT TGT CCG GGT AGA AAT CTA AA-3′ (reverse, XhoI site underlined). For site B the primers sequence was (5′to 3′): ATG GTA CCC AAA TGC CAC ATA GAC TAA GAA AC (forward) and ATC TCG AGG GCC ACT GTA GTC AGA AGC ATA (reverse). Mutations were introduced into these two sites using the QuikChange Site-Directed Mutagenesis kit from Stratagene (La Jolla, CA, USA) according to the manufacturer’s instructions. We introduced a single nucleotide mutation (G to C, underlined) in the two RBPJK binding sites, what has been shown to reduce drastically binding activity (Hamaguchi et al., 1992). The primers used to mutate site A were: GGT TAA AAA ATA ACA AAC AGG TGG CAA GGT CTG ACT CCC CAC CAG GGG (forward) and CCC CTG GTG GGG AGT CAG ACC TTG CCA CCT GTT TGT TAT TTT TTA ACC (reverse). For site B the primers were: CCT AGC TTC CTA CCT AGG GCA AGT GGC AAC TTG GTA AAC ATT CAA GGT TTA ACC (forward) and GGT TAA ACC TTG AAT GTT TAC CAA GTT GCC ACT TGC CCT AGG TAG GAA GCT AGG (reverse). The mutated fragments were re-cloned into the pGL3 vector and the introduction of mutations were verified by DNA sequencing. Luciferase reporter assays were performed with HMEC-1 cells. HMEC-1 cells were grown in endothelial cell basal medium-2 (EBM-2 Cambrex, CC-3156) supplemented with EGM 2 (Cambrex, CC-4176) and 8%FBS. The cells were seeded in 24-well plates at a density of 5 × 104 cells/well. Equal amounts (150ng) of the different EphrinB2 luciferase constructs [wt site A, wt site B, mutant site A (*) or mutant site B (*)], or RBPJk luciferase plasmids, 150 ng of pCS2 empty vector, vector encoding N1ICD or Su(H)DBM and 10 ng renilla luciferase were transfected in triplicate (Fugene6, Roche Diagnostics). pGL3 promoter vector was added when necessary to keep DNA amount constant. After 24 h, lysates were made and luciferase activity measured using the Dual Luciferase Assay kit (Promega) in a FB15 luminometer (Zylux, Oak Ridge, TN, USA).
RNA isolation and semiquantitative RT-PCR
E8.5-E9.5 wt and RBPJk mutant embryos were dissected in ice-cold PBS and the heart separated from the rest of the body and purified using Trizol (Invitrogen). First-strand cDNA synthesis was performed using a First Strand cDNA synthesis kit (Amersham), with 1 μg total RNA per reaction (for primers and conditions, see Suppl. Table). Amplified PCR products were cloned in the PCRII-TOPO vector (Invitrogen) and sequenced. PCR products were quantitated by phosphorimager analysis (Bio-Rad). For primers details see Suppl. Table.
Statistics
All results are expressed as means + SD. An unpaired two-tailed t test was performed to assess differences between two groups. A P value of <0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank A.M. Pendas for the EphrinB2 sequence analysis, T. Honjo and F. Radtke for the RBPJk and Notch1 floxed lines, M. Yanagisawa for the Tie2-CRE line, V. Christoffels for Irx3 and Irx5 probes, D. Franco for anti-MLC2v antibody, C. Bernabeu for the HMEC-1 cells, D. Sedmera and D. Stainier for critical reading of the manuscript and C. Mark for editorial assistance. JGB was partially supported by a basic and clinical research fellowship from the Spanish Society for Cardiology. This work was funded by grant SAF2004-05204 (Spanish Ministry of Education and Science) and GR/SAL/0851/2004 (Regional Government of Madrid) to JLdlP. The Department of Immunology and Oncology was founded and is supported by the Spanish National Research Council (CSIC) and by Pfizer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Bailey AM, Posakony JW. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar G, Arcilla RA, Lucas RV, Manasek FJ. Ventricular trabeculations in the chick embryo heart and their contribution to ventricular and muscular septal development. Circ Res. 1985;57:759–766. doi: 10.1161/01.res.57.5.759. [DOI] [PubMed] [Google Scholar]
- Chen H, Shi S, Acosta L, Li W, Lu J, Bao S, Chen Z, Yang Z, Schneider MD, Chien KR, et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131:2219–2231. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, Conlon RA. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- Del Amo FF, Smith DE, Swiatek PJ, Gendron-Maguire M, Greenspan RJ, McMahon AP, Gridley T. Expression pattern of Motch, a mouse homolog of Drosophila Notch, suggests an important role in early postimplantation mouse development. Development. 1992;115:737–744. doi: 10.1242/dev.115.3.737. [DOI] [PubMed] [Google Scholar]
- Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- Erickson SL, O’Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, Lu LH, Moore MW. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Fortini ME, Artavanis-Tsakonas S. The suppressor of hairless protein participates in notch receptor signaling. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y, Yamamoto Y, Iwanari H, Maruyama S, Furukawa T, Matsunami N, Honjo T. Biochemical and immunological characterization of the DNA binding protein (RBP-J kappa) to mouse J kappa recombination signal sequence. J Biochem (Tokyo) 1992;112:314–320. doi: 10.1093/oxfordjournals.jbchem.a123898. [DOI] [PubMed] [Google Scholar]
- Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- Hertig CM, Kubalak SW, Wang Y, Chien KR. Synergistic roles of neuregulin-1 and insulin-like growth factor-I in activation of the phosphatidylinositol 3-kinase pathway and cardiac chamber morphogenesis. J Biol Chem. 1999;274:37362–37369. doi: 10.1074/jbc.274.52.37362. [DOI] [PubMed] [Google Scholar]
- Hrabe de Angelis M, McIntyre Jn, Gossler A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature. 1997;386:717–721. doi: 10.1038/386717a0. [DOI] [PubMed] [Google Scholar]
- Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666–671. doi: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Bland Y, Webb S, Barton S, Brown NA. Expression of Peg1 (Mest) in the developing mouse heart: involvement in trabeculation. Dev Dyn. 2002;225:212–215. doi: 10.1002/dvdy.10142. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kokubo H, Miyagawa-Tomita S, Johnson RL. Hesr, a Mediator of the Notch Signaling, Functions in Heart and Vessel Development. Trends Cardiovasc Med. 2005;15:190–194. doi: 10.1016/j.tcm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Kopan R. Notch: a membrane-bound transcription factor. J Cell Sci. 2002;115:1095–1097. doi: 10.1242/jcs.115.6.1095. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- Loomes KM, Underkoffler LA, Morabito J, Gottlieb S, Piccoli DA, Spinner NB, Baldwin HS, Oakey RJ. The expression of Jagged1 in the developing mammalian heart correlates with cardiovascular disease in Alagille syndrome. Hum Mol Genet. 1999;8:2443–2449. doi: 10.1093/hmg/8.13.2443. [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Sangkuhl K, Luo CW, Sudo S, Klein C, Hsueh AJ. Identification of receptors and signaling pathways for orphan bone morphogenetic protein/growth differentiation factor ligands based on genomic analyses. J Biol Chem. 2005;280:32122–32132. doi: 10.1074/jbc.M504629200. [DOI] [PubMed] [Google Scholar]
- McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- McKenzie GJ, Stevenson P, Ward G, Papadia S, Bading H, Chawla S, Privalsky M, Hardingham GE. Nuclear Ca2+ and CaM kinase IV specify hormonal- and Notch-responsiveness. J Neurochem. 2005;93:171–185. doi: 10.1111/j.1471-4159.2005.03010.x. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Moorman AF, Christoffels VM. Cardiac chamber formation: development, genes, and evolution. Physiol Rev. 2003;83:1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- Nakagawa O, Nakagawa M, Richardson JA, Olson EN, Srivastava D. HRT1, HRT2, and HRT3: a new subclass of bHLH transcription factors marking specific cardiac, somitic, and pharyngeal arch segments. Dev Biol. 1999;216:72–84. doi: 10.1006/dbio.1999.9454. [DOI] [PubMed] [Google Scholar]
- Nemir M, Croquelois A, Pedrazzini T, Radtke F. Induction of cardiogenesis in embryonic stem cells via downregulation of Notch1 signaling. Circ Res. 2006;98:1471–1478. doi: 10.1161/01.RES.0000226497.52052.2a. [DOI] [PubMed] [Google Scholar]
- Oka C, Nakano T, Wakeham A, de la Pompa JL, Mori C, Sakai T, Okazaki S, Kawaichi M, Shiota K, Mak TW, Honjo T. Disruption of the mouse RBP-J kappa gene results in early embryonic death. Development. 1995;121:3291–3301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- Parker SB, Eichele G, Zhang P, Rawls A, Sands AT, Bradley A, Olson EN, Harper JW, Elledge SJ. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267:1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE, Fishman GI. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci U S A. 2002;99:10464–10469. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rones MS, McLaughlin KA, Raffin M, Mercola M. Serrate and Notch specify cell fates in the heart field by suppressing cardiomyogenesis. Development. 2000;127:3865–3876. doi: 10.1242/dev.127.17.3865. [DOI] [PubMed] [Google Scholar]
- Rutenberg JB, Fischer A, Jia H, Gessler M, Zhong TP, Mercola M. Developmental patterning of the cardiac atrioventricular canal by Notch and Hairy-related transcription factors. Development. 2006;133:4381–4390. doi: 10.1242/dev.02607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Furukawa T, Iwanari H, Oka C, Nakano T, Kawaichi M, Honjo T. Loss of immunostaining of the RBP-J kappa transcription factor upon F9 cell differentiation induced by retinoic acid. J Biochem (Tokyo) 1995;118:621–628. doi: 10.1093/oxfordjournals.jbchem.a124955. [DOI] [PubMed] [Google Scholar]
- Schroeder T, Fraser ST, Ogawa M, Nishikawa S, Oka C, Bornkamm GW, Nishikawa S, Honjo T, Just U. Recombination signal sequence-binding protein Jkappa alters mesodermal cell fate decisions by suppressing cardiomyogenesis. Proc Natl Acad Sci U S A. 2003;100:4018–4023. doi: 10.1073/pnas.0438008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Hu N, Clark EB. Developmental changes in the myocardial architecture of the chick. Anat Rec. 1997;248:421–432. doi: 10.1002/(SICI)1097-0185(199707)248:3<421::AID-AR15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development. 1996;122:2251–2259. doi: 10.1242/dev.122.7.2251. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4 [see comments] Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kokubo H, Miyagawa-Tomita S, Endo M, Igarashi K, Aisaki KI, Kanno J, Saga Y. Activation of Notch1 signaling in cardiogenic mesoderm induces abnormal heart morphogenesis in mouse. Development. 2006;133:1625–1634. doi: 10.1242/dev.02344. [DOI] [PubMed] [Google Scholar]
- Wettstein DA, Turner DL, Kintner C. The Xenopus homolog of Drosophila Suppressor of Hairless mediates Notch signaling during primary neurogenesis. Development. 1997;124:693–702. doi: 10.1242/dev.124.3.693. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.