Abstract
Cancer stem cells (CSCs) have been defined as a unique subpopulation in tumors that possess the ability to initiate tumor growth and sustain tumor self-renewal. Although the evidence has been provided to support the existence of CSCs in various solid tumors, the identity of gastric CSCs has not been reported. In this study, we have identified gastric cancer-initiating cells from a panel of human gastric cancer cell lines using cell surface marker CD44. Among six gastric cancer cell lines, three lines MKN-45, MKN-74, and NCI-N87 had a sizeable subpopulation of CD44(+) cells, and these cells showed spheroid colony formation in serum-free media in vitro as well as tumorigenic ability when injected into stomach and skin of severe combined immunodeficient (SCID) mice in vivo. The CD44(+) gastric cancer cells showed the stem cell properties of self-renewal and the ability to form differentiated progeny and gave rise to CD44(−) cells. CD44 knockdown by short hairpin RNA resulted in much reduced spheroid colony formation and smaller tumor production in SCID mice, and the CD44(−) populations had significantly reduced tumorigenic ability in vitro and in vivo. Other potential CSC markers, such as CD24, CD133, CD166, stage-specific embryonic antigen-1 (SSEA-1), and SSEA-4, or sorting for side population did not show any correlation with tumorigenicity in vitro or in vivo. The CD44(+) gastric cancer cells showed increased resistance for chemotherapy- or radiation-induced cell death. These results support the existence of gastric CSCs and may provide novel approaches to the diagnosis and treatment of gastric cancer.
Keywords: Neoplastic stem cell biology, CD44, Severe combined immunodeficiency mice, Serum-free media
Introduction
Gastric adenocarcinoma remains the fourth most common cancer and the second leading cause of cancer-related mortality in the world. Although gastric cancer was the leading cause of cancer death in the United States as recently as 1930, with incidence rates in male exceeding 45 per 100,000, a decline in both incidence and mortality, particularly for distal tumors, has been observed in this country over the past 70 years [1, 2]. The risk of developing gastric adenocarcinoma is strongly associated with Helicobacter pylori infection, which is gradually disappearing from Western societies. Despite the overall decline in gastric cancer prevalence, the treatment of stomach cancer remains a challenging problem because the surgical resection is still the primary curative modality, although many patients who undergo a resection develop regional or distant recurrences and the overall 5-year survival rate for gastric cancer patients remains around 20% in the Western countries [2].
Interest in gastric cancer stem cells (CSCs) has arisen in the broader context of the CSC hypothesis, which first appeared more than a century ago when a number of European pathologists observed that tumors were composed of a heterogeneous mixture of partially differentiated cell types, similar in many respects to a normal organ [3, 4]. The laboratory group led by John E. Dick first demonstrated the existence of CSCs more than a decade ago when they proved the hypothesis to be largely true for human acute myeloid leukemia [5, 6]. The “leukemic stem cell,” which was defined by specific markers of CD34+CD38−, could serially reproduce the disease in immunodeficient mice, demonstrating properties of longevity and self-renewal. This finding was subsequently verified in breast [7] and brain tumors [8]. Despite some limitations, the growth of a subset of tumor cells (typically less than 5% of total tumor cells) with defined markers in immunodeficient mice has become the “gold standard” for identifying a CSC [9] in other solid tumors including prostate cancer [10], melanoma [11, 12], colon [13–15], liver [16, 17], pancreatic cancer [18, 19], head and neck [20], and lung cancer [21]. In some of these studies, as few as 100 cells of the CSC subpopulation induced tumor growth in immunodeficient mice. In addition, it should be noted that there exist some discrepancies for CSC markers among different groups [11–19], and few studies have examined specific markers in both human and murine models of disease. At a recent American Association of Cancer Research Workshop, a working group used the available data to create a consensus definition of the CSC as “cells within a tumor that possess the capacity for self-renewal and that can cause the heterogeneous lineages of cancer cells that constitute the tumor” [22].
This new paradigm has remarkable implications for cancer therapy because it suggests that our current therapies are more successful at eradicating non-CSCs than CSCs [9, 23]. Consequently, the purification and characterization of CSCs could lead to the identification of better targets for therapeutic intervention. With respect to gastric cancer, previous studies have not yet defined and characterized CSCs for this solid tumor. Thus, in this study, we have analyzed gastric cancer cell lines with defined surface markers and have identified the existence of gastric cancer initiating cells in the CD44(+) population. The CD44-positive gastric cancer cells showed the properties of self-renewal and the ability to produce differentiated progeny, consistent with the CSC phenotype. In addition, the CD44(+) gastric cancer cells demonstrated properties of chemo- and radio-resistance, which likely accounts for the resistance of this tumor type to standard treatment protocols. These data may emphasize the necessity of novel therapeutic approaches targeted toward CSCs to achieve better clinical outcomes for patients with gastric cancer.
Materials and Methods
Cell Culture
Human gastric cancer cell lines, AGS and NCI-N87, were purchased from American Type Culture Collection (Manassas, VA, http://www.atcc.org). MKN-28, MKN-45, MKN-74, and KATO-III cells were purchased from Riken (Ibaraki, Japan; http://www.brc.riken.go.jp/lab/cell/english). AGS cells were maintained in Dulbecco’s modified Eagle’s medium, while the other cell lines were maintained in Royal Park Memorial Institute (RPMI). All cell culture media were supplemented with 10% fetal bovine serum (FBS), 10 mM HEPES, and 1% of penicillin–streptomycin (all from Invitrogen, Carlsbad, CA, http://www.invitrogen.com).
Flow Cytometry Analysis and Fluorescence-Activated Cell Sorting
For surface marker analysis by flow cytometry, 70%--90% confluent cells in a 100-mm cell plate (5–10 million cells per plate) were washed once with phosphate-buffered saline(−), and then cells were dissociated from plates using Trypsin-EDTA (Invitrogen) or nonenzymatic solution Cellstripper (Mediatech Inc., Manassas, VA; http://www.cellgro.com) and centrifuged. Cell pellets were resuspended and incubated for 30 min at room temperature with 100-fold dilution of following antibodies: anti-CD44-fluorescein isothiocyanate rat monoclonal (clone IM7; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com), anti-CD44-allophycocyanin (APC) mouse monoclonal (clone G44-26; BD Biosciences), anti-CD24-phycoerythrin (PE) (BD Biosciences), anti-CD133/1-APC (Miltenyi Biotec, Auburn, CA, http://www.miltenyibiotec.com), anti-CD166-PE (BD Biosciences), anti-stage-specific embryonic antigen-1 (SSEA-1)-PE (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com), and anti-SSEA-4-PE (R&D Systems Inc.). Then samples were stained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) with a final concentration of 2 ng/ml with fluorescence-activated cell sorter (FACS)-LSRII flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com). The results were analyzed using software FlowJo version 7.2.4 (Tree Star Inc., Ashland, OR; http://www.treestar.com). For FACS cell sorting, 5–10 million cells were collected and stained as described earlier and sorted using FACS BDAria (Becton, Dickinson and Company). The purity of sorted cells was estimated to be more than 94%.
Spheroid Colony Formation Assay
FACS-sorted human gastric cancer cells were inoculated in each well (10 cells per well or otherwise indicated) of ultra-low-attachment 96-well plates (Corning Life Sciences, Acton, MA, http://www.corning.com/lifesciences) supplemented with 100–200 μl of RPMI-1640 medium (Invitrogen) plus 10 mM HEPES, human recombinant epidermal growth factor (EGF) (Invitrogen) at the concentration of 20 ng/ml, and human recombinant basic fibroblast growth factor (bFGF) (Invitrogen) at the concentration of 10 ng/ml. The cell viability was assessed by staining with trypan blue (Sigma-Aldrich) at several time points. After 4 or 5 weeks, each well was examined using light microscope and total well numbers with spheroid colonies were counted. Images of the spheroid colonies were recorded using light and fluorescence dual microscopy ECLIPSE TE-2000U (Nikon Instruments Inc., Melville, NY; http://www.nikoninstruments.com) in phase-contrast mode and SPOT RT-KE digital camera (Diagnostics Instruments Inc., Sterling Heights, MI; http://www.diaginc.com). Images were acquired and converted to TIFF files using SPOT software (version 4.6).
In Vivo Tumorigenicity in Severe Combined Immunodeficient Mice
FACS-sorted human gastric cancer cell lines were suspended in sterile RPMI-1640 supplemented with 10% FBS. Eight- to 12-week-old ICR-severe combined immunodeficient (SCID) mice or Rag2/gammaC double knockout mice (both from Taconic, Hudson, NY; http://www.taconic.com) from selected experiments were anesthetized using a small animal anesthesia system supplied with oxygen (2 l/min) and isoflurane. A 5-mm incision was then made in the skin overlying the midabdomen, and the stomach was exposed. Then the cancer cells were injected into the serous side of the stomach using a handmade glass micropipette at the dose of around 30,000 cells per site or as otherwise indicated. The skin incision was closed with absorbable suture. Also the same number of cancer cells were injected subcutaneously in the midline of the animal dorsum. Mice were monitored weekly for tumor growth for up to 16 weeks.
CD44 Knockdown by Lentivirus-Mediated Short Hairpin RNA
CD44 knockdown in the gastric cancer cell lines was performed by infection with a lentivirus that expresses human CD44-specific short hairpin RNA (shRNA) as previously described [24]. Briefly, the lentivirus vector plasmid encoding human CD44-specific shRNA (Sigma-Aldrich) was transfected with capsule and packaging plasmids using Superfect (Qiagen, Valencia, CA, http://www.qiagen.com) into HEK293T cells, and after 48 hours, supernatant was collected and used as infection solution without enrichment. Among the five predesigned target sequences for human CD44, the following sequence was used in this experiment: 5′-GCCCTATTAGTGATTTCCAAA-3′. The scramble shRNA obtained from Addgene (Cambridge, MA; http://www.addgene.org) was used as control. The efficiency of infection of the cells was evaluated by a green fluorescent protein-expressing lentivirus. Forty-eight to 72 hours after viral infection, the cells were treated with medium containing puromycin (5 μg/ml; Sigma-Aldrich) to remove noninfected cells. The CD44 knockdown was confirmed by FACS analysis as described in the previous section.
Chemoresistance and Radioresistance Experiments
The CD44(+) and (−) fractions from FACS-sorted MKN-74 cells were inoculated into 12-well plates (10,000 cells per well) in triplicate on the day prior to testing. Each well was supplied with RPMI-1640 medium containing 10% FBS, along with a chemotherapy reagent such as 10 mM 5-fluorouracil (5-FU) and 200 μM of etoposide (VP-16) (both Sigma-Aldrich) or no drug as control. The appropriate medium for each well was changed after 3 days from initial treatment. The number of viable cells was evaluated after 6 days from initial treatment using the Cell Counting Kit-8 (Dojindo, Rockville, MD, http://www.dojindo.com) following the manufacturer’s instructions, and the optical absorbance at wavelength 450 nm was measured for the supernatant of each well using the plate reader Multiskan EX (Thermo Fisher Scientific Inc., Waltham, MA; http://www.thermofisher.com).
In radioresistance experiment, the CD44(+) and (−) fractions from FACS-sorted MKN45 cells were inoculated into six-well plates (50,000 cells per well) in triplicate on the day prior to testing. Each plate was irradiated with 3 and 6 Gy of radiation, respectively (0.86 Gy/min), using a Cesium 137 irradiator Gammacell 40 (Nordion, Ottawa, ON, Canada; http://www.mds.nordion.com). The number of viable cells was evaluated using the Cell Counting Kit-8 as described earlier.
Mouse and Human Gastric Cancer Samples
The insulin-gastrin (INS-GAS) transgenic mice (FVB/N background), previously described [25], were bred under specific pathogen free condition. Animals were housed in microisolator, solid-bottomed polycarbonate cages, fed a commercially prepared pellet diet, and given water ad libitum. INS-GAS mice at 2 or 3 months of age were inoculated with Helicobacter felis (H. felis; ATCC 49179) three times every other day over the course of a week with 100 million colony forming units. All experiments were approved by the Institutional Animal Care and Use Committee of Columbia University Medical Center. Six gastric cancer samples obtained from the Department of Surgery, Hyogo College of Medicine (Nishinomiya City, Hyogo prefecture, Japan; http://www.corp.hyo-med.ac.jp/english), were analyzed in this study. All of the tumors were confirmed as gastric adenocarcinoma by expert human pathologists in the Clinical Pathology Department of the Institute. Written informed consent for this research study was obtained from the patients prior to surgery with approval by the Institutional Review Board of the Institute.
Immunohistochemical Staining
Tissues were fixed in 10% formalin, embedded in paraffin, and processed by standard histological methods. From each selected paraffin block, 5-μm serial sections were cut. Immunohistochemical studies were performed with avidin–biotin–peroxidase complex kits (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) according to the manufacturer’s instructions. The following primary antibodies were used: anti-mouse CD44 (dilution 1:30, rat anti-mouse clone IM7; BD Biosciences), anti-human-specific CD44 (dilution 1:100, mouse anti-human; BD Biosciences), anti-mouse CD24 (dilution 1:800, rat anti-mouse; Abcam, Cambridge, U.K., http://www.abcam.com), anti-human-specific CD24 (1:100, mouse anti-human; BD Biosciences), and anti-human-specific epithelial membrane antigen (EMA; 1:100, mouse anti-human; DAKO, Carpinteria, CA, http://www.dako.com). Primary antibodies were incubated at room temperature for 1 hour, or overnight, in a humidified chamber. Diaminobenzidine (Vector Laboratories) was used as the chromogen and slides were counterstained with Mayer’s hematoxylin.
Statistics
Experiments presented in the figures are representative of two or three different repetitions. The data are presented as the mean values ± standard derivation. Comparisons between groups are evaluated by Student’s t test. Values of p < .01 or p < .05 are considered to be statistically significant as indicated, respectively.
Results
Surface Marker Expression Profile and In Vivo Tumorigenicity of Human Gastric Cancer Cell Lines
First, we analyzed the expression patterns of possible candidate cell surface markers for CSCs by using FACS for the following six human gastric cancer cell lines: AGS, KATO-III, MKN-28, MKN-45, MKN-74, NCI-N-87 (hereinafter N-87). Based on previous published reports regarding CSCs in solid tumors [7–21], the following markers were studied: CD24, CD44, CD133, and CD166. In addition, we also investigated the embryonic stem cell markers SSEA-1 and SSEA-4 [26]. The results of the FACS studies for CD44 and CD24 are shown in Figure 1A. MKN-45 and MKN-74 cells showed a high level of expression of CD44, with up to 94% of cells expressing CD44, while N-87 cells showed as little as 5% expression and MKN-28 cells showed no expression of this marker. KATO-III cells alone expressed CD133, and only KATO-III and MKN-28 cells expressed CD166 (Fig. 1A). CD44 expression was confirmed using two different clones of anti-CD44 antibodies (see Methods and Materials section). As previously reported [27], CD44 is the receptor for hyaluronic acid (HA), one of the extracellular matrix components, and we confirmed CD44 expressed in these cell lines bound to HA (supporting information Fig. S1). With respect to the effect of cell dissociation methods on the cell surface marker analysis, we compared three different methods, trypsin-EDTA, nonenzymatic solution, and mechanical dissociation using cell scraper (see Methods and Materials section), and we found that trypsin-EDTA and nonenzymatic solution had the same result, while mechanical dissociation significantly reduced the percentage of the marker positive cells (data not shown). Therefore, we used trypsin-EDTA as standard cell dissociation method in all other experiments. A summary of the results of the surface marker analysis is shown in Table 1. Overall, the panel of cell lines demonstrated significant heterogeneity in their pattern of marker expression.
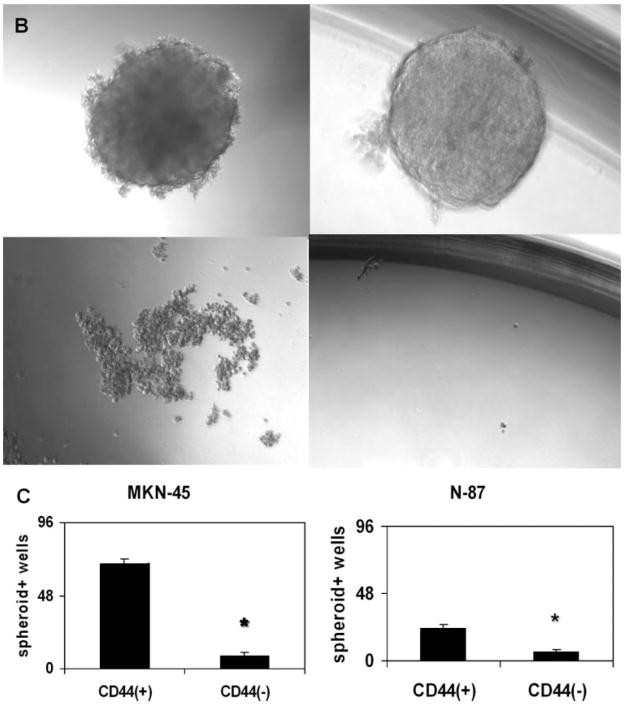
Figure 1.
Analysis of cell surface markers and spheroid colony formation assay. (A): Fluorescence-activated cell sorter (FACS) analysis of candidate surface markers for gastric cancer stem cells CD44 and CD24. Top left: Analysis of cell surface markers and spheroid colony formation assay. MKN-45; top right: MKN-74; bottom left: N-87; bottom right: MKN-28; horizontal axis: FITC-CD44; vertical axis: PE-CD24. (B): Spheroid colony formation of gastric cancer cell lines in serum-free media. Each human gastric cancer cell line was fractionated and inoculated by FACS sorting for CD44 positivity in ultra-low-attachment 96-well plates with serum-free medium (10 cells per plate) containing human epidermal growth factor (20 ng/ml) and basic fibroblast growth factor (10 ng/ml). After 3–4 weeks of culture, the number of spheroid colony-positive wells was counted. Top left: MKN-45; top right: N-87; bottom left: AGS; bottom right: KATO-III. MKN-45 and N-87 cells produced spheroid colonies, while AGS and KATO-III cells did not. (C): Spheroid colony-positive well numbers. Left: MKN-45; right: N-87. CD44-positive cells produced significantly higher numbers of spheroid colonies in low-attachment 96-well cell plates. *, p < .01. Abbreviations: FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Table 1.
Cell surface marker expression and tumorigenicity of human gastric cancer cell lines
| Cell line | CD44 | CD24 | CD133 | CD166 | SSEA-1 | SSEA-4 | Tumorigenicity |
|---|---|---|---|---|---|---|---|
| MKN-45 | ++ | + | − | − | + | + | + |
| MKN-74 | ++ | + | − | − | + | + | + |
| NCI-N87 | ± | + | − | − | ± | ± | + |
| AGS | + | − | − | − | − | − | ± |
| KATO-III | + | + | ++ | + | + | + | − |
| MKN-28 | − | + | − | + | + | + | − |
Surface marker “++” means more than 50% of the cells expressed the marker, while “+” means positive cells were between 10% and 50%, and “±” indicates that less than 10% cells are positive. Tumorigenicity “+” means the cells produced skin or gastric tumors in both immunodeficient severe combined immunodeficient mice and Rag2/gammaC double knockout mice, while tumorigenicity “±” means tumors appeared only in Rag2/gammaC double knockout mice.
Abbreviation: SSEA, stage-specific embryonic antigen.
We also performed transplantation of these cell lines into the skin and stomach of SCID mice and Rag2/gammaC mice (0.5–1.0 million cells per site), to determine whether there was any correlation between marker expression and in vivo tumorigenicity. These studies revealed that MKN-45, MKN-74, and N-87 demonstrated significant tumor formation in these mice at 8–12 weeks after injection, while KATO-III and MKN-28 did not produce any tumors. AGS cells showed tumor growth in Rag2/gammaC mice but not in SCID mice. The maximum observation period was 16 weeks for all experiments, and the results of these experiments are summarized in Table 1. Overall, the studies suggest a reasonably good correlation in this panel of cell lines between the level of CD44 expression and tumor growth in vivo.
CD44-Positive Gastric Cancer Cells Produce Spheroid Colonies in Serum-Free Medium
Several different approaches have typically been used to identify CSCs in published studies [23, 28]. One approach has been an in vitro method “spheroid colony formation” that involves culturing candidate CSCs under nonadherent conditions with serum-free media containing only EGF and bFGF. The growth of spherical colonies after several weeks is considered indicative of self-renewal ability and would be consistent with a CSC phenotype. After in vitro culture for 3–4 weeks in serum-free media under nonadherent conditions, MKN-45, MKN-74, and N-87 cells produced spheroid colonies, while the other cell lines did not (Fig. 1B). Based on these findings, we fractionated these three cell lines by FACS sorting for surface markers listed in Table 1, and we found that the CD44(+) cell fraction could generate many more spheroid colonies compared with the CD44(−) cell fraction (Fig. 1C). We examined the cell viability by staining cells with trypan blue at several time points. One week after inoculation, both the CD44(+) and CD44(−) cell fractions for MKN-45, MKN-74, and N-87 cells were not stained (i.e., all cells appeared viable); however, after 2 weeks, most of the CD44(−) cells were trypan blue positive (i.e., the cells were dead), while most of CD44(+) cells were viable. After 4 weeks, the CD44(+) spheroid colony-forming cells remained alive.
We also performed a limiting dilution assay for spheroid colony formation using CD44(+) and CD44(−) cells, respectively. As shown in supporting information Table 1, approximately 10%--20% of CD44(+) cells could produce spheroid colonies, while less than 1% of CD44(−) cells could generate spheroid colonies after 4 weeks culture. Therefore, within the CD44(+) cell fraction, we could estimate that the spheroid-forming cell population comprised a maximum of 20% of the cells.
Next, we performed spheroid colony formation assay using fractionated populations of MKN-45, MKN-74, and N-87 cells with other potential CSC markers such as CD24, CD133, CD166, SSEA-1, or SSEA-4. However, these markers did not show any differences between positive and negative fractions in spheroid colony formation.
Finally, we cultured FACS-sorted CD44(+) and CD44(−) populations of MKN-45 cells separately with the regular medium of RPMI-1640 supplemented with 10% FBS in the regular cell culture plates. After 4 weeks of culture, the original CD44(+) and CD44(−) populations were analyzed for CD44 expression, respectively. The ratio of CD44-expressing cells in the original CD44(+) populations was decreased to approximately 60%. In contrast, the original CD44(−) populations did not yield CD44(+) cells.
CD44-Positive Gastric Cancer Cells Show Tumorigenicity In Vivo
To validate our in vitro findings, we performed transplantation of FACS-sorted gastric cancer cells such as MKN-45, MKN-74, and N-87 cells into the skin and stomach of SCID mice. We found that the CD44(+) fraction (20–30 thousand cells per site injection) generated tumors in both the skin and stomach of SCID mice after 8–12 weeks, while the CD44(−) fraction (30–100 thousand cells per site) did not generate tumors even after a longer observation period of 16 weeks (Fig. 2A, 2B and Table 2). For each individual cell line, this striking difference between the CD44(+) and CD44(−) cellular fractions was identical with the results of the spheroid colony formation assay. We also tested fractionated cells sorted by other candidate markers such as CD24, CD133, CD166, SSEA-1, and SSEA-4 in SCID mice, but no difference in tumorigenicity was observed for these markers (data not shown).
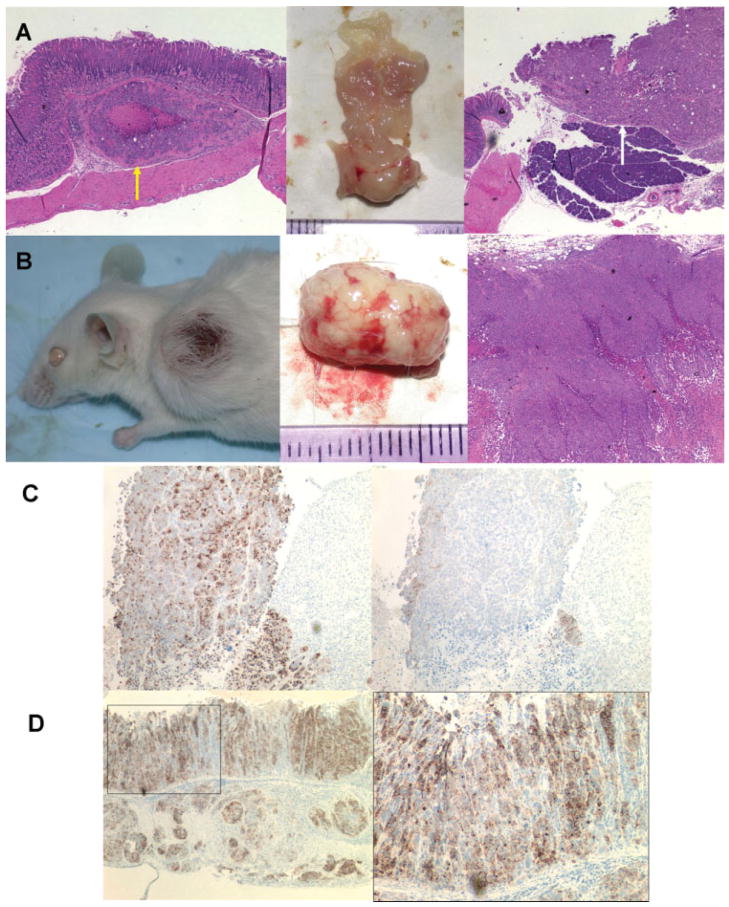
Figure 2.
Tumor formation of tumorigenic gastric cancer cell lines implanted in the stomach and skin of SCID mice. (A, B): CD44-positive fraction of MKN-74 cells implanted in the stomach and skin of SCID mice (30,000 cells per site) produced tumors in each site after 8–12 weeks. Peritoneal dissemination was observed in the mice injected with CD44(+) cells into the stomach, and some of these mice also showed tumors invaded into pancreas or liver. (A): Stomach with tumor. Yellow arrow indicates tumor in submucosal area, and white arrow indicates tumor invasion into pancreas. (B): Skin tumor. H&E images: original magnification, ×40. (C): Immunohistochemistry of tumors produced by CD44-positive MKN-45 cells implanted in the skin of SCID mice; left: immunostaining with anti-human-specific epithelial membrane antigen (EMA) antibody; right: immunostaining with anti-human-specific CD44 antibody. CD44-positive cells in skin tumor were mainly detected at the marginal area of tumor. Most of the cells were CD44 negative. (D): Immunohistochemistry of tumors produced by CD44-positive MKN-45 cells implanted in the stomach of SCID mice; left and right: both immunostaining with anti-human-specific EMA antibody. CD44-positive MKN-45 cells produced not only submucosal tumor but also human gastric glands in the SCID mouse stomach. Left: original magnification, ×60; right: higher magnification image (original magnification, ×200) of the rectangular area in the left.
Table 2.
Number of tumor-bearing mice per total SCID mice implanted with fluorescence-activated cell sorter-sorted gastric cancer cells
| Cell line | Fraction | Skin | Stomach |
|---|---|---|---|
| MKN-45 | CD44(+) | 4/4 | 4/4 |
| CD44(−) | 0/4 | 0/4 | |
| MKN-74 | CD44(+) | 4/4 | 3/4 |
| CD44(−) | 0/4 | 0/4 | |
| N-87 | CD44(+) | 4/4 | 2/4 |
| CD44(−) | 0/4 | 0/4 |
All of the mice implanted with CD44(+) gastric cancer cells produced tumors in the skin or stomach, while CD44(−) cells did not generate tumors in SCID mice after an observation period of 16 weeks. Number of implanted cells: CD44(+), 20,000–30,000 cells per site; CD44(−), 30,000–100,000 cells per site.
Abbreviation: SCID, severe combined immunodeficient.
To demonstrate that tumors which appeared in the skin and stomach of SCID mice truly originated from the injected human gastric cancer cells, we performed immunostaining of these tissues with antibodies for human-specific EMA as well as human CD44. The vast majority of cells in the skin tumor were EMA positive and thus of human origin (Fig. 2C). Interestingly, not all of the cells were CD44(+), despite the fact that all the injected cells were CD44(+), indicating that CD44(+) cells likely gave rise to CD44(−) cells. The CD44(+) cells were clearly present but localized mainly at the margins of the tumor (Fig. 2C). A similar staining pattern was observed for xenograft tumors in the stomach. Interestingly, the CD44(+) cells injected into the stomach produced not only invasive tumors but also partially differentiated human gastric glands in the mouse stomach (Fig. 2D), and the human pseudo gastric glands were strongly CD44 positive, although the staining pattern was somewhat different from that observed for skin tumors. CD44 is the receptor for HA, one of the extracellular matrix components [28], and as shown in supporting information Figure S1, CD44 expressed in MKN-45 cells actually bound to HA and the different staining patterns of CD44 might originate from different HA localization patterns. In any case, these human gastric glands did not have parietal cells or neuroendocrine cells, because immunostaining with anti-HK-ATPase beta or anti-chromogranin A antibodies was negative (data not shown). These findings indicated that the CD44(+) cells were able to achieve only limited differentiation into gastric glandular cells. The pseudo gastric glands could be obtained with MKN45 cells but not with the other two tumorigenic gastric cancer cell lines, MKN-74 and N-87 cells, and thus MKN-45 cells may have a greater differentiation capacity than the other two cancer cell lines [29].
In addition, in limiting dilution experiments, we found that 3000 and 300 cells of the CD44(+) fraction per site injection still produced gastric and skin tumors, while injection of 30 cells per site did not yield any tumor (data not shown). Therefore, we could roughly estimate that gastric cancer-initiating cells in SCID mice during a maximum observation period of 16 weeks may comprise less than 3% of the total CD44(+) fraction.
To demonstrate the self-renewal properties of CD44(+) gastric cancer cells, we performed serial transplantation of CD44(+) cells, that is, isolating these cells from one xenograft and transplanting them into the skin and stomach of the second group of SCID recipient mice. Skin tumors produced from the CD44(+) fraction of MKN-45 cells as well as MKN-74 cells were dissected and cultured with regular medium for 2–3 weeks, and then FACS sorted for CD44 surface expression. The CD44(+) and CD44(−) fractions were separately reimplanted into the skin and stomach of SCID mice. After 8–12 weeks, we found that the CD44(+) cells generated skin and gastric tumors, while CD44(−) cells did not generate tumors during the 16-week observation period (supporting information Fig. S2). We also performed an additional serial transplantation using xenograft tumor of MKN-74 cells from the second recipient mice into a third group of mice, and we confirmed that only the CD44(+) fraction in the secondary tumors could produce skin and gastric tumors in the tertiary recipient mice (data not shown). Taken together, these results demonstrate that CD44(+) cells possess the features of self-renewal and strongly support the existence of gastric cancer stem-like cells in this cellular fraction.
Chemoresistance and Radioresistance of CD44-Positive Gastric Cancer Cells
Studies in the past have suggested that CSCs in several solid tumors showed greater resistance to anticancer chemotherapy drugs as well as to irradiation compared with the non-CSC population [9, 23]. To examine this question with respect to gastric CSCs, we performed cell survival assays for the tumorigenic gastric cancer cell lines MKN-45 and MKN-74. The CD44(+) fraction from MKN-74 cells had much greater resistance to the anticancer drugs 5-FU and etoposide (Fig. 3A, 3B). The CD44(+) cells of MKN-45 also showed similar chemoresistance compared with the CD44(−) cells (data not shown). In addition, the CD44(+) fraction from MKN-45 cells exhibited stronger radioresistance compared with the CD44(−) cells (Fig. 3C, 3D). Taken together, these results are compatible with previously reported CSC phenotypes.
Figure 3.


Chemoresistance and radioresistance of CD44-positive and -negative fractions of tumorigenic human gastric cell lines MKN-45 and MKN-74. (A): FACS-sorted CD44-positive and -negative MKN-74 cells in 12-well plates (10,000 cells per well) were treated with 5-FU (10 mM), etoposide (VP-16; 200 μM), or without drugs for 8 days (media with respective drug were changed to fresh one after 4 days), and then living cell numbers were counted using Cell Counting Kit-8. Y-axis data show optical absorbance of reagent WST-8 at a wavelength of 450 nm, which represent the number of survived cells. **, p < .01. (B): Survived cells treated with 5-FU after 8 days. Left: CD44 positive; right: CD44 negative. (C): FACS-sorted CD44-positive and -negative MKN-45 cells in six-well plates (50,000 cells per well) were irradiated with 3 or 6 Gy, respectively. The number of living cells was counted using Cell Counting Kit-8 after 6 days (medium with 10% FBS was changed to fresh one after 3 days). Y-axis data show optical absorbance of reagent WST-8 at a wavelength of 450 nm, which represent the number of survived cells. *, p < .05; **, p < .01. (D): Survived cells at 6 days after irradiation. Left: CD44 positive; right: CD44 negative. Abbreviation: 5-FU, 5-fluorouracil.
CD44 Knockdown Reduced Tumorigenicity of CD44-Positive Gastric Cancer Cells
A recent study of CD44 knockdown in colon CSCs indicated that CD44 expression in CSCs may have a significant role in supporting tumorigenicity in vitro and in vivo [30]. Therefore, we performed a similar experiment using lentivirus-mediated CD44 knockdown in MKN-45 and MKN-74 cells. First, we confirmed successful knockdown, with a 45%--50% decrease in CD44 expression in treated cells compared with control cells (Fig. 4A). Using these CD44 knockdown (CD44KD) cells, we performed spheroid colony formation assay and found that CD44KD cells generated significantly fewer colonies (Fig. 4B). Next, we injected these cells subcutaneously in SCID mice, and after 4 weeks, we observed that CD44KD cells produced much smaller tumors than those from control cells (Fig. 4C). Based on these results, we also fractionated our original CD44(+) populations of MKN-45 and MKN-74 cells into two groups, high CD44-expressing cells and low CD44-expressing cells, and we analyzed their tumorigenicity by spheroid colony formation assay as well as subcutaneous injections in SCID mice. After 4 weeks, high CD44-expressing cells showed much stronger tumorigenicity than low CD44-expressing cells in vitro and in vivo (Fig. 4D). These data firmly imply that CD44 is more than just a CSC marker, and it functionally contributes to the tumorigenicity of gastric cancer cells.
Figure 4.
CD44 knockdown by lentivirus-mediated shRNA and the significant reduction of the tumorigenic ability of gastric cancer stem cells. (A): Fluorescence-activated cell sorter analysis of CD44 knockdown in MKN-45 cells by lentivirus-mediated human CD44-specific shRNA. Left: scramble shRNA as control; right: human CD44-specific shRNA. CD44 expression in MKN-45 cells was successfully knocked down. CD44 expression in MKN-74 cells was also downregulated to a similar level. (B): Spheroid colony formation of CD44 downregulated MKN-45 and MKN-74 cells. After 4 weeks of culture, CD44 shRNA-infected cells produced significantly lower number of colonies than scramble shRNA virus-infected cells. (C): Skin tumors produced by CD44 downregulated MKN-45 cells. CD44 shRNA-infected MKN-45 cells as well as scramble shRNA virus-infected cells were injected under the skin of SCID mice, respectively, and after 4 weeks, although all mice had tumors, CD44 shRNA virus-infected cells produced much smaller tumors than scramble shRNA virus-infected cells. CD44 downregulated MKN-74 cells also showed similar results. (D): Spheroid colony formation of high CD44-expressing and low CD44-expressing populations in MKN-45 and MKN-74 cells. After 4 weeks of culture, high CD44-expressing cells produced significantly larger number of colonies than low CD44-expressing cells. (E): Skin tumors produced by high CD44-expressing MKN-45 cells. High CD44-expressing as well as low CD44-expressing populations in MKN-45 cells were injected under the skin of SCID mice, respectively, and after 4 weeks, high CD44-expressing cells produced much larger tumors than low CD44-expressing cells. High CD44-expressing MKN-74 cells also showed similar results. Abbreviations: FITC, fluorescein isothiocyanate; PE, phycoerythrin; shRNA, short hairpin RNA.
Side Population and Non-Side Population Gastric Cancer Cells Are Both Tumorigenic
While the isolation of CSCs in solid tumors has in most cases been performed using cell surface markers, in some cases side-population (SP) analysis has been used for the detection of CSCs [31]. Therefore, we also investigated whether our panel of human gastric cancer cell lines contained SP cells, and whether the SP fraction contains CSCs. MKN-45 cells contain approximately 0.33% of SP cells (supporting information Fig. S3A, S3B). Other cell lines, such as N-87, KATO-III, and MKN-28, also possessed an SP fraction of 0.16%, 0.36%, and 0.25% of total cells, respectively, while AGS and MKN-74 cells did not have a measurable SP fraction (data not shown). Next, we tested whether the SP and non-SP fractions show a CSC phenotype, using both in vitro and in vivo assays. Both the SP and non-SP fractions of MKN-45 cells produced spheroid colonies as well as gastric and skin tumors in SCID mice (supporting information Fig. S3C, S3D). There was no significant difference between two groups, indicating that CSCs did not specifically reside within the SP fraction.
CD44 Expression in a Mouse Model of Gastric Cancer and Human Gastric Cancer Specimen
Based on our finding that the CD44(+) fraction in several human cell lines contains gastric CSCs, we investigated whether CD44 expression correlated with tumorigenicity in our Helicobacter-dependent mouse model of gastric cancer. Thus, we examined the expression of CD44 in our H. felis-infected INS-GAS mice, which developed invasive gastric lesions after 6–7 months of infection [25]. Highly dysplastic gastric glands, especially gastric lesions invasive into the submucosa, were strongly CD44 positive (Fig. 5A, 5B). In addition, a number of immune cells as well as stromal cells were CD44 positive. We also examined human gastric adenocarcinoma samples, and strong CD44 expression could be detected in gastric cancer cells, especially invasive cells at the leading edge of the tumor (Fig. 5C, 5D), as well as in some immune cells and stromal cells, whose staining pattern was similar to our mouse model in most respects. In contrast, occasional CD24-positive staining could be observed in our mouse or human gastric cancer tissues but only in immune cells or stromal cells and not in gastric cancer cells (supporting information Fig. S4).
Figure 5.
CD44 expression in mouse and human gastric cancer tissues. (A, B): Immunostaining with anti-CD44 antibody of hypergastrinemia (insulin-gastrin) mouse with Helicobacter felis infection (7 months postinfection). (C, D): Immunostaining with anti-CD44 antibody of human gastric adenocarcinoma (moderately differentiated type). Original magnification: (A), ×60; (B), ×200; (C), ×40; (D), ×200. Scale bar = (A, B), 200 μm; (C, D), 50 μm. Many gastric cancer cells, especially invasive front area, were strongly CD44 positive for both mouse and human gastric cancer tissues. In addition, some parts of immune cells as well as stromal cells were CD44 positive.
Discussion
A number of recent studies have demonstrated in solid tumors the presence of CSCs, which share many characteristics with tissue stem cells, such as self-renewal and differentiation, and are primarily responsible for sustaining the growth of tumors [9, 22, 23]. In this study, we show for the first time in a panel of human gastric cancer cell lines that gastric CSCs exist in the CD44-positive fraction. We initially observed that gastric cancer cell lines such as MKN-45 and MKN-74, which are highly tumorigenic in xenograft models, contain a high proportion of the CD44(+) cells, while cell lines that did not grow well in SCID mice (e.g., MKN-28 cells) possess few if any CD44(+) cells. When tumorigenic cell lines were fractionated by CD44 expression, the CD44(+) cells demonstrated the capability of spheroid colony formation under nonadherent conditions in serum-free media as well as the ability to form xenograft tumors in the stomach and skin of SCID mice, consistent with the standard definition of CSCs. Through serial passage of the CD44(+) fraction, we demonstrated that these cells had the ability to self-renew. In addition, the CD44(+) cells were clearly able to give rise to the CD44(−) cells and exhibited differentiation ability to some extent, because they were able to form gastric glands when injected into the stomachs of SCID mice. These dual abilities were consistent with earlier reports regarding CSCs [9, 22, 23]. Finally, CD44 expression correlated with the presence of dysplasia in murine models of gastric cancer as well as in gastric cancer surgical specimens from human patients. In these samples, CD44-positive cancer cells were surrounded by thick and wide stromal areas which contain many immune cells and stromal cells such as fibroblasts or myofibroblasts. A recent study reported that several factors secreted by cancer-associated stromal cells, such as TGF-beta, may be involved in the regulation of CD44 expression as well as the induction or maintenance of breast CSCs [32].
Taken together, these results firmly establish the fact that the CD44(+) fraction contains gastric CSCs. Nevertheless, CD44 is not highly specific for gastric CSCs, because CD44 positivity is also present in nontumorigenic gastric cancer cells. It will be important in the future to identify additional markers that may further narrow and define gastric CSCs, which likely account for a minority of the CSC population. Unfortunately, all of the other potential cell surface markers that we have examined to date (CD24, CD133, CD166, SSEA-1, and SSEA-4) have not shown any correlation with CSCs. We also analyzed the expression of other stem cell-related genes LGR5 (GPR49) [33], BMI-1 [34], and DCAMKL-1 [35]. However, LGR5 and BMI-1 were expressed in both tumorigenic and nontumorigenic gastric cancer cell lines at a similar level, and DCAMKL-1 was not detected in any of the cell lines examined (supporting information Fig. S5).
Presently, CD44 appears to be the most useful marker for prospective purification of gastric CSCs and has been identified as a CSC marker for a number of other solid tumors [7, 10, 15, 18, 20]. CD44 is a class I transmembrane glycoprotein, and its transcripts are subject to alternative splicing which creates more than 10 different isoforms. It can act as a ligand-binding receptor for the extracellular matrices (mainly for hyaluronic acid; see supporting information Fig. S1) and as a specialized platform for growth factors and matrix metalloproteinases [28]; it is well known as a downstream target of the Wnt/β-catenin pathway [36]. Several recent studies suggest that CD44 ablation in some types of murine (as well as human) cancers significantly reduced tumor induction [37–39]. In addition, a study of CD44 knockdown in colon CSCs indicated that CD44 may have an important functional role in the tumorigenicity of CSCs [30], and as shown in Figure 4, our data strongly support this earlier observation. In addition, CD44 positivity may reflect the ability of the cells to escape from rejection by host animals. However, CD44 is also expressed in immune cells (such as leukocytes) and stromal cells (such as fibroblasts), and administration of anti-CD44 antibodies inhibited inflammation in murine models of inflammatory bowel disease, experimental autoimmune encephalomyelitis, etc. [40]. For pilot studies, H. felis infection of CD44−/− knockout mice as well as C57BL/6 wild-type control mice showed that after 8 months infection, the infected CD44−/− knockout mice had much more severe gastritis and metaplastic changes in the gastric corpus compared with the infected control mice (supporting information Fig. S6). This suggests that CD44 may modulate the immune response to Helicobacter infection. Although CD44 knockdown may represent a potentially useful approach in invasive gastric cancer, it should be used with caution in patients with preneoplastic lesions.
The majority of CSC studies using transplantation of human cancer cells into immunodeficient mice have supported the CSC paradigm. However, there are a number of limitations to the model. A recent short report has provided evidence that in mouse-to-mouse transplantation using preB/B lymphoma cells from Eμ-myc transgenic mice, most of the cells within mouse tumors retain the CSC phenotype [41]. The authors suggest that the rarity of CSCs found in human cancers may result from species-mismatch in xenograft transplantations. Although one must be careful about overinterpreting findings regarding CSCs from transgenic mouse models, it is conceivable that the immunodeficient mouse may not provide the ideal local environment for the growth of human cancer cells [42]. Indeed, our finding that AGS cells could produce tumors in the skin and stomach of Rag2/gammaC double knockout mice but not SCID mice does indicate that the severity of immunodeficiency of the recipient mice may influence the outcome of xenograft transplantation studies. Recently a similar observation has been reported for melanoma CSCs [43]. This report described the use of the more highly immunocompromised NOD/SCID interleukin-2 receptor gamma chain null (Il2rg−/−) mice with a longer term observation period (up to 8 months), which resulted in a much higher rate (25%) of detection of cancer-initiating melanoma cells.
Another weakness of current support for the CSC hypothesis is that the markers used to purify the CSCs are not highly specific, and in most cases, the CSCs have not been completely purified. For example, as recently reported [44], CD133 may not be a true marker for colon CSC, although two earlier studies independently described CD133 as a good CSC marker for colon cancer [13, 14]. Despite these limitations, there is a growing consensus that many cancers can be viewed as differentiated tissues, and that only CSCs are able to initiate and sustain tumors, leading eventually to cancer invasion and metastasis. Importantly, the CD44(+) fraction from gastric cancer cell lines showed much greater chemo- and radiation-resistance, a property that has previously been reported for CSCs from a variety of solid organs [9, 23]. This unique survival ability of CSCs and its implications for therapy of most human cancers are without question the source of great interest in CSCs.
In most cases, the isolation of CSCs from solid tumors has been accomplished using cell surface markers, but the alternative approach of using SP cells has also been applied for the detection of CSCs in some organs [31]. The concept of a SP was first described for isolating hematopoietic stem cells from murine bone marrow [45]. Subsequently, SP sorting has been applied to the purification of stem cells from a variety of other tissues such as skeletal muscle, breast, liver, small intestine, and uterine [46–50]. With respect to a possible relationship between the SP fraction and CSCs, Hirschmann-Jax et al. described the existence of SP cells in neuroblastoma cell lines as well as in breast and lung cancers [51]. An SP fraction was also found for cancers of liver and ovary [52, 53]. However, other studies have not confirmed an association between the SP fraction and CSCs. Patrawala et al. reported that glioma cell lines which expressed ABCG2, an ATP-binding cassette half-transporter that is associated with SP cells, had a similar tumorigenicity as ABCG2-negative cells [54]. Burkert et al. also reported that among four colon cancer cell lines examined, SP and non-SP cells were similarly clonogenic in vitro and tumorigenic in vivo and displayed equivalent multipotential differentiation potential. In addition, they also showed that SP and non-SP populations are interconvertible, each giving rise to the other in culture [55]. In our study, human gastric cancer MKN-45 cells were found to have a significant SP fraction, but our data clearly indicated that SP and non-SP cells both possess tumorigenic ability in vitro and in vivo. Another gastric cancer cell line, MKN-28, also contained SP cells, and this cell line did not produce tumors in SCID mice. Therefore, in our study, we would conclude that sorting for SP cells does not result in enrichment or purification of gastric CSCs.
The origin of human gastric CSCs has yet to be elucidated, but we have previously reported that data obtained from a mouse model of Helicobacter-induced gastric cancer have implicated bone marrow-derived cells as a potential candidate source [56, 57]. These findings require further examination, especially in human patients with gastric cancer. While many stem cell markers are shared between CSCs and tissue stem cells, few studies have addressed the cellular origins of most CSCs. CD44 immunostaining of the stomach of wild-type C57BL/6 mice showed that the lower glandular cells of the gastric antrum are strongly CD44 positive, while in the corpus, CD44-positive cells are rarely detected (supporting information Fig. S7). In view of previous papers reporting that isthmus or basal antral glandular cells were potential candidates for gastric stem cells [58], our data may indicate CD44 as the marker not only for gastric CSCs but also for gastric tissue stem cells, and this might suggest that the origin of some types of gastric cancers could be the gastric antral stem cells. However, the development of better markers for both transformed and nontransformed stem cells will hopefully allow lineage tracing experiments that can further clarify these relationships.
Conclusion
For the first time we have identified a gastric CSC population with a defined surface marker, CD44, from several human gastric cancer cell lines. This finding needs to be confirmed in freshly resected human surgical specimens, and related studies are currently underway. However, confirmation of CD44 as a marker for gastric CSCs may very well facilitate the development of additional novel therapies for the treatment of gastric cancer.
Supplementary Material
Acknowledgments
We thank Kelly S. Betz, Anthony Mitchell, and Rongzhen Chen for their technical assistance. We also thank the Department of Surgery, Hyogo College of Medicine, where Y.S. previously worked, for providing paraffin sections of human surgical specimens. This work was supported by NIH Grants R01 CA120979 and R01 CA093405 (to T.C.W.), Japanese Ministry of Education, Culture, Sports, Science, and Technology Grant 19390356 (to Y.S.), and the Uehara Memorial Foundation (to T.O.).
Footnotes
Author contributions: S. Takaishi: conception and design, execution and collection of data, data analysis, manuscript writing; T.O.: experimental design, execution and collection of data; S. Tu and S.S.W.W.: execution and collection of data; W.S., R.V., S.A.K.G., and Y.S.: provision of study materials; T.C.W.: principal investigator, conception and design, laboratory facility and financial support, experimental design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest The authors indicate no potential conflicts of interest.
See www.StemCells.com for supporting information available online.
References
- 1.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Takaishi S, Okumura T, Wang TC. Gastric cancer stem cells. J Clin Oncol. 2008;26:2876–2882. doi: 10.1200/JCO.2007.15.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houghton J, Morozov A, Smirnova I, et al. Stem cells and cancer. Semin Cancer Biol. 2007;17:191–203. doi: 10.1016/j.semcancer.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 9.Lobo NA, Shimono Y, Qian D, et al. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 10.Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 11.Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 12.Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 14.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 15.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma S, Chan KW, Hu L, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Yang ZF, Ho DW, Ng MN, et al. Significance of CD90(+) cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 19.Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 22.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells—Perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 23.Vermeulen L, Sprick MR, Kemper K, et al. Cancer stem cells—Old concepts, new insights. Cell Death Differ. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- 24.Szulc J, Aebischer P. Conditional gene expression and knockdown using lentivirus vectors encoding shRNA. Methods Mol Biol. 2008;434:291–309. doi: 10.1007/978-1-60327-248-3_18. [DOI] [PubMed] [Google Scholar]
- 25.Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 26.Fenderson BA, De Miguel MP, Pyle AD, et al. Staining embryonic stem cells using monoclonal antibodies to stage-specific embryonic antigens. Methods Mol Biol. 2006;325:207–224. doi: 10.1385/1-59745-005-7:207. [DOI] [PubMed] [Google Scholar]
- 27.Ponta H, Sherman L, Herrlich PA. CD44: From adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 28.Vargo-Gogola T, Rosen JM. Modelling breast cancer: One size does not fit all. Nature Rev Cancer. 2007;7:659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- 29.Arima N, Yamashita Y, Nakata H, et al. Presence of histamine H2-receptors on human gastric carcinoma cell line MKN-45 and their increase by retinoic acid treatment. Biochem Biophys Res Commun. 1991;176:1027–1032. doi: 10.1016/0006-291x(91)90385-k. [DOI] [PubMed] [Google Scholar]
- 30.Du L, Wang H, He L, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- 31.Hadnagy A, Gaboury L, Beaulieu R, et al. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312:3701–3710. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 34.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May R, Riehl TE, Hunt C, et al. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 36.Wielenga VJ, Smits R, Korinek V, et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol. 1999;154:515–523. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin L, Hope KJ, Zhai Q, et al. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 38.Krause DS, Lazarides K, von Andrian UH, et al. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 39.Zeilstra J, Joosten SP, Dokter M, et al. Deletion of the WNT target and cancer stem cell marker CD44 in Apc(Min/+) mice attenuates intestinal tumorigenesis. Cancer Res. 2008;68:3655–3661. doi: 10.1158/0008-5472.CAN-07-2940. [DOI] [PubMed] [Google Scholar]
- 40.Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- 41.Kelly PN, Dakic A, Adams JM, et al. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 42.Marx J. Molecular biology. Cancer’s perpetual source? Science. 2007;317:1029–1031. doi: 10.1126/science.317.5841.1029. [DOI] [PubMed] [Google Scholar]
- 43.Quintana E, Shackleton M, Sabel MS, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shmelkov SV, Butler JM, Hooper AT, et al. CD133 expression is not restricted to stem cells, and both CD133 and CD133 metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asakura A, Seale P, Girgis-Gabardo A, et al. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alvi AJ, Clayton H, Joshi C, et al. Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 2003;5:R1–R8. doi: 10.1186/bcr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimano K, Satake M, Okaya A, et al. Hepatic oval cells have the side population phenotype defined by expression of ATP-binding cassette transporter ABCG2/BCRP1. Am J Pathol. 2003;163:3–9. doi: 10.1016/S0002-9440(10)63624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dekaney CM, Rodriguez JM, Graul MC, et al. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology. 2005;129:1567–1580. doi: 10.1053/j.gastro.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Ono M, Maruyama T, Masuda H, et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci USA. 2007;104:18700–18705. doi: 10.1073/pnas.0704472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirschmann-Jax C, Foster AE, Wulf GG, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiba T, Kita K, Zheng YW, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- 53.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian inhibiting substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 55.Burkert J, Otto WR, Wright NA. Side populations of gastrointestinal cancers are not enriched in stem cells. J Pathol. 2008;214:564–573. doi: 10.1002/path.2307. [DOI] [PubMed] [Google Scholar]
- 56.Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 57.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: A new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 58.Qiao XT, Ziel JW, McKimpson W, et al. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.