Abstract
Rationale
There is growing evidence of alterations in brain stress and reward circuits associated with cocaine dependence. Sex differences are also documented and sex steroid hormones have been linked to cocaine reinforcement.
Objectives
The current study therefore assessed daily fluctuations in stress and sex hormones in cocaine-dependent females compared with healthy females.
Method
Daily salivary samples of cortisol, progesterone, and estradiol were collected at waking across 28 days from 12 cocaine-dependent females receiving inpatient treatment and 10 healthy females. Participants also completed mood-rating scales each week corresponding to four phases of the menstrual cycle and cocaine craving was monitored in cocaine patients at each phase.
Results
Cocaine-dependent females in their first month of abstinence demonstrated significantly higher levels of both cortisol and progesterone across the menstrual cycle and significantly lower estradiol/progesterone (E2/P) ratios compared to healthy controls. They also showed significantly increased negative mood compared with controls, but no variation in cocaine craving across the menstrual cycle.
Conclusions
Findings indicate altered stress and sex hormones suggestive of an overactive stress system during the first month of cocaine abstinence after chronic cocaine abuse. These increased levels of cortisol and progesterone could impact both abstinence-related symptoms such as negative mood and susceptibility to drug-seeking behavior in cocaine-dependent females.
Keywords: Cortisol, Progesterone, Estradiol, Cocaine, Gender, Stress, Mood
Introduction
Gender differences have been reported across all phases of cocaine dependence, including initiation, maintenance, and treatment outcome (Quiñones-Jenab 2006). Males and females report dissimilar motives for drug use (Piko et al. 2007; Sonne et al. 2003) and experience markedly different subjective drug effects (McCance-Katz et al. 2005; Kosten et al. 1996). Females also progress to drug dependence more rapidly than men through more addictive routes (McCance-Katz et al. 1999; Sinha and Rounsaville 2002). Furthermore, gender differences in stress response have been documented in samples of both healthy and cocaine-dependent individuals (Fox et al. 2006a; Kajantie and Phillips 2006; Kirschbaum et al. 1999).
Cocaine administration is known to stimulate the hypothalamic–pituitary–adrenal (HPA) axis, causing alteration of HPA axis function (for review, see Mello and Mendelson 1997). Moreover, these changes have been documented during protracted cocaine abstinence (Mantsch et al. 2003; Zhou et al. 2003) alongside a range of symptoms comprising dysphoria, anxiety, and increased sensitivity to stress and craving (Kampman et al. 2001; Mulvaney et al. 1999). Recent laboratory studies have also shown increased sensitivity to stress-induced craving, HPA axis function, and negative emotion in recently abstinent cocaine (Fox et al. 2007a; Sinha et al. 2003) and alcohol (Fox et al. 2007b; Sinha et al. 2005) dependent individuals. Notably, these factors have been shown to be sex-specific (Fox et al. 2006a; Kirschbaum et al. 1999) and integral to relapse (Adinoff et al. 2005; Sinha et al. 2006). Given this and the vulnerability of females to poor relapse-related outcomes, it may be important to examine the complex interactions between drug abuse, stress, and hormonal status in early abstinent cocaine-dependent women.
Previous preclinical and clinical research has tended to examine these associations by assessing the impact of either drug or hormone administration on behavioral and/or hormonal response at various phases of the estrus or menstrual cycle. Studies in laboratory animals have shown that estradiol and progesterone substitutions stimulate HPA axis function in nonhuman primates (Norman et al. 1992) and acute “binge” doses of cocaine have been shown to increase progesterone levels in female rats particularly during proestrus (Quiñones-Jenab et al. 2000). Administering stimulants to female rats during estrus also induces increased behavioral activity (Sell et al. 2002), self-administration of higher cocaine doses (Lynch et al. 2000), and potentiation of drug reinstatement (Kippin et al. 2005). Conversely, in proestrus rats, high levels of progesterone have been associated with significantly less cocaine seeking (Feltenstein and See 2007).
Although human data are more limited, chronic drug abuse culminates in reproductive problems such as early menopause, amenorrhea, and luteal phase dysfunction (Mello 1988; Teoh et al. 1992). Moreover, marked subjective and cardiovascular variations have been reported in women, as well as in women compared to men, in response to stimulants at different phases of the menstrual cycle and after exogenous hormonal administration. Reports of an attenuated “high” and improvement in dysphoric mood have been documented following smoked cocaine during the luteal menstrual cycle phase when progesterone levels are typically elevated (Evans et al. 2002; Sofuoglu et al. 1999). Other research has reported that the positive subjective effects of amphetamine, such as “high”, “euphoria”, and “like drug” are potentiated during the follicular compared to the midluteal phase, when progesterone levels are typically low (Justice and de Wit 1999). Administration of exogenous progesterone during the follicular phase has also been shown to attenuate some of the subjective responses to cocaine such as “feeling the effect of last dose” (Evans and Foltin 2006; Sofuoglu et al. 2002, 2004) and blood pressure response (Sofuoglu et al. 2004) in females compared to males.
Thus, previous research indicates that there are cocaine-related effects on corticotropin-releasing factor (CRF) and the HPA axis as well as the involvement of the sex steroid hormones in cocaine reinforcement. As such, the current study aims to systematically monitor daily hormonal fluctuations of waking salivary estrogen, progesterone, and cortisol during early abstinence in cocaine-dependent females. Reliable salivary assays for estradiol, progesterone as well as cortisol are now established (Chatterton et al. 2005; Fox et al. 2006b; Gandara et al. 2007) providing a noninvasive method to address this issue. To examine the impact of these hormonal changes on self-reported craving and mood, participants also completed self-report ratings at each phase of the current menstrual cycle. We predict that the hormonal patterns observed across 28 days in abstinent cocaine-dependent women will deviate from those observed in healthy women and be accompanied by variations in reported affect.
Materials and method
Participants
Twelve treatment-seeking cocaine-dependent (CD) females and 10 healthy control (HC) females were recruited through local newspapers and advertisements. All CD females smoked crack cocaine and met DSM-IV criteria for current cocaine dependence. They also tested positive for cocaine in their urine toxicology screens upon entry into a locked inpatient treatment and research facility for 4 weeks of treatment and study participation. The Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) is a locked inpatient treatment research facility with no access to alcohol or drugs and limited and monitored access to visitors. All participant visits take place in the staffed communal area of the inpatient unit and all visitors are checked by staff on entry. Drug testing is conducted every 3 days to ensure drug abstinence.
All HC females were excluded if they met either past or current criteria for alcohol and/or drug use disorders. Exclusion criteria for CD females included dependence on any substances other than cocaine, alcohol, or nicotine and the use of any medications for medical or psychiatric conditions and oral contraceptives. All participants underwent stringent medical assessments that included electrocardiography and laboratory tests of renal, hepatic, pancreatic, hematopoietic, and thyroid function and were excluded if they were not in good general health. HC females were required to abstain from alcohol during the 4 weeks of study participation and were asked to report weekly if they had consumed any alcohol during the previous week. Alcohol consumption was minimal (one participant reported consuming one drink during week 2, and another reported consuming one drink during the initial week and two drinks during week 3). The study was approved by the Human Investigation Committee of the Yale University.
Procedures
CD females participated in data collection each morning during their 28-day inpatient stay, while the HC females collected saliva samples daily at waking and completed study assessments during four appointments once per week for 4 weeks. Upon study initiation, all subjects completed a detailed menstrual cycle history questionnaire providing self report information about the dates and regularity of their last three menstrual cycles, the length of menses each time, and the onset of menses in the current cycle. Research staff also asked controls to provide the start day of menses in the current cycle at each of the four visits, and cocaine women were required to provide the same weekly information by nurses on the inpatient unit. These data were used to determine day and phase of the menstrual cycle for each participant over the 28 days. All CD females were allowed four monitored smoke breaks per day to curb nicotine craving.
Saliva samples
Collection of saliva samples for the CD females was initiated on the first morning of inpatient stay, after the first night of abstinence. All participants were required to provide saliva samples upon morning waking to analyze levels of cortisol, estradiol, and progesterone. All participants were provided with plastic scintillation vials, (Salimetrics, State College, PA, USA) that were labeled with date and collection time. Research staff on the inpatient unit ensured that the CD females provided saliva samples at 7.30 a.m. each day. The average collection time logged for HCs across the 28 days was 7:20 a.m. To minimize assay contamination, tooth brushing before sample collection was forbidden and participants rinsed their mouths thoroughly with water 10 min before saliva collection. An unstimulated passive drool method of collection was used to best maintain the integrity of estradiol and progesterone salivary concentrations (Lewis 2006; Shirtcliff et al. 2001) and all participants were required to produce approximately 4 ml of saliva into the vials each morning at waking. The inpatient unit nurse supervised the cocaine participants as they deposited the saliva and then collected the tubes and placed them into a standard (−20) freezer. The HC females were given seven tubes at a time for each week and detailed instructions for saliva collection at waking using methods previously described. They were asked to freeze the samples immediately in their home freezer and a freezer pack was provided so they could transport the samples to our research unit weekly. Each HC female came in weekly and brought in samples at which time they also completed questionnaires and ratings.
Saliva samples of estradiol and progesterone, collected at home on a daily basis, have been shown to provide reliable assessments of menstrual cycle profiles in HC females using similar collection procedures to those documented in the current study as well as previous research (Chatterton et al. 2005; Gandara et al. 2007). Salivary cortisol assays have also been shown to reliably reflect plasma cortisol in CD individuals across various time points (Fox et al. 2006b).
All salivary samples were assayed in duplicate. Cortisol samples were assayed using salivary high sensitivity cortisol enzyme immunoassay (EIA) kits (Salimetrics LLC, State College, PA, USA). Intra-assay coefficients of variation ranged from 4.3 to 5.3%. Estradiol and progesterone were assayed using standard EIA kits (Salimetrics LLC, State College, PA, USA). Intra-assay coefficients of variation ranged from 5.0 to 7.3% (estradiol) and 4.0 to 8.4% (progesterone).
Subjective measure of mood and craving
The Profile of Mood States Bi-Polar Form (POMS-BI; McNair et al. 1971): comprises a list of 72 mood-related words (e.g. happy, angry, tense, shaky) and participants were requested to rate the extent to which they felt each emotion on a scale of 0 (not at all) to 4 (extremely). Positive items were reverse coded. The 72 items were then collapsed into eight subscales: anger, anxiety, arousal, confusion, depression, elation, fatigue, and vigor. The following subscales were then collapsed to create a positive mood subscale (vigor and elation) and a negative mood subscale (anger, anxiety, and depression).
The cocaine craving questionnaire-brief version (CCQ-brief; Tiffany et al. 1993): This is a brief version of the 45-item Tiffany cocaine craving self-report questionnaire and has demonstrated good internal consistency, reliability, and validity within CD participants (Sussner et al. 2006). All 10 items are in the form of a craving-related statement (e.g., “I want cocaine so bad I can almost taste it”) and scores for each item response range from 1 (strongly disagree) to 7 (strongly agree). Positive items are reverse scored. Total score ranges from 0 to 70.
The POMS was administered weekly to all participants and the CCQ-brief was also assessed weekly in CD females. While the daily saliva samples were synchronized with the menstrual cycle day for each participant, weekly data for craving (CCQ-brief) and mood ratings (POMS) were grouped into four MC phases: phase 1: days 1–10 (follicular); phase 2: days 11–15 (ovulation); phase 3: days 16–24 (early and midluteal); and phase 4: days 25–28 (lateluteal/premenstrual).
Design and statistical analyses
Linear mixed effect (LME; Laird and Ware 1982) models were implemented to analyze the data, using SAS, version 8 software (SAS Institute, Cary, NC, USA). To examine group differences in daily cortisol, estradiol, and progesterone levels, group (CD vs HC females) represented the between subject factor and MC day (28 days), the within subject factor. Log transformation was applied to make the distribution of all the outcome variables symmetric. Years in education was used as a covariate. For subjective data, LME model analysis was performed with group (CD vs HC females) representing the between subject factor and MC phase (1–4), the within subject factor. A series of persons product moment correlations were also conducted to assess the associations between craving, negative mood and hormonal fluctuations across each MC phase for both CD and HC females. Correlations were also performed assessing the relationship between cocaine consumption variables before intake and hormonal changes.
Results
Participants
All demographics are shown in Table 1. Self-reported length of menstrual cycle and menses for CD females was (28.9±1.0 and 4.5±0.8 days, respectively) and (29.1±1.0 and 4.7±1.2 days, respectively) for HC females. These lengths corroborated with the menstrual cycle length in the current cycle.
Table 1.
Demographics and drug use
| Variable | CD females (N=12) | HC females (N=10) | p |
|---|---|---|---|
| Age | 37.7±7.14 | 35.6±8.23 | NS |
| Race | |||
| African American | 6 (50.0%) | 4 (40.0%) | NS |
| Caucasian | 5 (41.7%) | 5 (50.0%) | |
| Hispanic | 1 (8.3%) | 0 (0.0%) | |
| Other | 0(0.0%) | 1(1%) | |
| Years of education | 12.2±1.7 | 14.7±2.2 | <0.02 |
| Years of cocaine use | 9.0±7.3 | NA | |
| Amount of cocaine use in last 30 days (g) | 56.55±44.87 | NA | |
| Years of alcohol use | 14.2±8.3 | 12.4±9.9 | NS |
| Number of drinks in last 30 days | 204.3±149.6 | 0.9±0.74 | <0.0001 |
| Number of regular smokers (>3 per day) | 10 (83.3%) | 4 (40%) | |
| Lifetime mood disorder | 2 (16.7%) | 0 | |
| Lifetime anxiety disorder (without PTSD) | 5 (41.7%) | 0 | |
| Lifetime anxiety disorder (with PTSD) | 6 (50.0%) | 0 | |
| Lifetime criteria for alcohol dependence | 8 (66.7%) | 0 | |
CD Cocaine-dependent females, HC healthy control females
Hormonal data
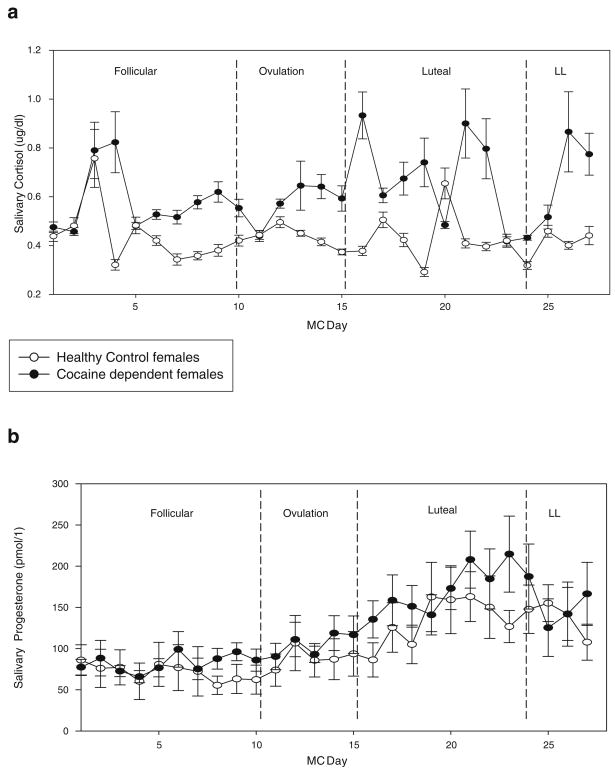
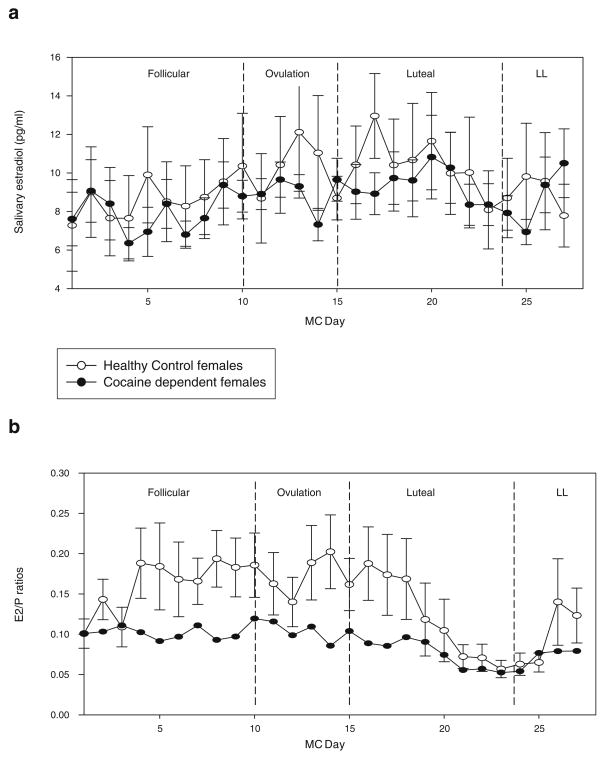
A main effect of group for cortisol [F(1,19)=9.28, p<0.007] and progesterone [F(1,20)=5.52, p<0.03] indicated that CD females demonstrated significantly higher levels of both hormones across their 28-day cycle compared with HCs (see Fig. 1a,b). For progesterone, a main effect of MC day [F(26,438)=1.64, p<0.03] was also obtained resulting from significantly increased progesterone levels during the luteal phase (phase 3: days 16–24; p=0.02). No significant main effect of group, MC day, or group X MC day interaction was observed for estradiol (see Fig. 2a).
Fig. 1.
a Daily salivary cortisol levels for CD and HC females at each MC phase. All bars represent SEM. Main effect of group (p<0.007). b Daily salivary progesterone levels for CD and HC females at each MC phase. All bars represent SEM. Main effect of group (p<0.03)
Fig. 2.
a Daily salivary estradiol levels for CD and HC females at each MC phase. All bars represent SEM. b Daily estradiol/progesterone (E2/P) ratios for CD and HC females at each MC phase. All bars represent SEM. Main effect of group (p<0.002)
Estradiol–progesterone (E2/P) ratios
A main effect of group [F(1,19)=13.34, p<0.002] showed that E2/P ratios were significantly lower in CD females compared with HC females across their cycle. A main effect of MC day [F (26,419)=1.5, p=0.05] also indicated that, as expected, E2/P ratios decreased across the luteal phase in all women (p= 0.01; see Fig. 2b). All salivary cortisol, progesterone, and estradiol levels fall with the expected range for premenopausal women (Salimetrics LLC, Sate College, PA, USA).
Subjective emotional ratings
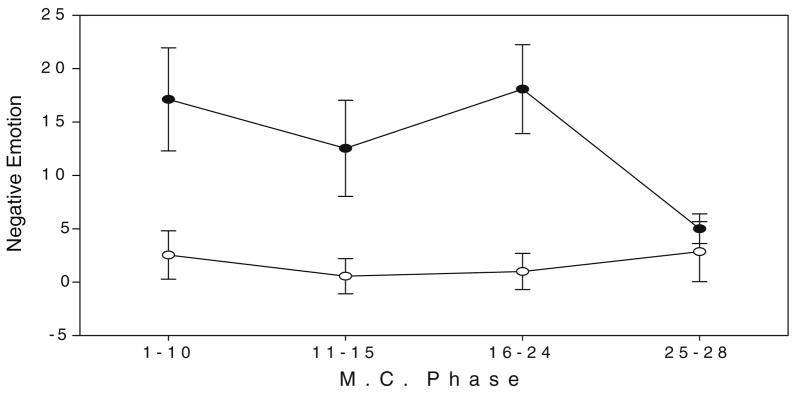
A main effect of group for negative emotions [F(1,20)=5.43, p<0.03] indicated that CD females reported significantly greater negative emotions across their cycle compared with HC females (see Fig. 3). No significant main effect of group, MC day, or group X MC day interaction was observed for positive emotions.
Fig. 3.
Negative emotion ratings (POMS) for cocaine-dependent and healthy females at each MC phase. All bars represent SEM. Main effect of group (p<0.03)
Mean scores of the CCQ-brief were assessed in the CD females only for changes by MC phase and no significant effects were observed.
Secondary analyses
Extended analyses indicated that in CD females craving was positively associated with negative mood during both the follicular (r=0.84; p=0.001) and early to midluteal (r=0.67; p=0.02) phase. Craving was also positively associated with E2/P ratios during the lateluteal (premenstrual) phase of their cycle (r=−0.81; p<.05). In HC females, negative mood was negatively associated with progesterone levels in the late luteal phase (r=−0.71; p<0.05).
No significant associations were observed between hormonal levels across each menstrual phase and cocaine consumption variables in the 3 months before inpatient treatment. These intake variables included the number of days cocaine was used and the average amount of cocaine used per occasion.
A mixed model reanalysis of the hormonal data was also performed as a function of stay on the inpatient unit (or just “study day” in the case of HC females), where “study day” was used as the fixed factor rather than “MC day”. A main effect of group was still observed in relation to cortisol levels [F(1,18)=10.4, p<0.005]; however, no effect of study day was seen for cortisol, progesterone, or E2/P ratios.
Discussion
This is the first study to assess daily fluctuations in waking cortisol, progesterone, and estradiol alongside changes in mood and craving across one complete menstrual cycle, in CD females over their first month of abstinence compared with HC females. CD females showed significantly higher levels of cortisol and progesterone, and significantly lower estradiol to progesterone (E2/P) ratios across their menstrual cycle compared with controls. Furthermore, these hormonal fluctuations were accompanied by higher ratings of negative mood in CD females compared to HCs.
Increased levels of salivary cortisol, salivary progesterone, and negative mood are consistent with human studies documenting enhanced sensitivity to stress and negative affect during early and protracted abstinence in cocaine abusers (Fox et al. 2007a; Kampman et al. 2001; Sinha et al. 2003). Findings also support extensive preclinical studies which have indicated that progesterone levels are elevated after a range of stressors in laboratory animals (Deis et al. 1989; Schaeffer and Aron 1987; Shevchenko et al. 2006) as well as human clinical studies which report increased levels of plasma and salivary cortisol and progesterone after metabolic stress (Breier and Buchanan 1992; Elman and Breier 1997; George et al. 1994), fear of rejection (Wirth and Schultheiss 2006) and emotional distress in men as well as women taking hormonal contraceptives (Wirth et al. 2007).
Such increases in subjective and hormonal stress markers across the menstrual cycle may also be indicative of hormonal adaptations in response to cocaine withdrawal and abstinence in CD females. For example, the acute effects of progesterone and its metabolite allopregnanolone have been shown to exert anxiolytic and antidepressant-like properties via GABAA receptors (for review, see Bitran et al. 1995; Brot et al. 1997; Rupprecht 2003). In animal models, allopregnanolone has been shown to be stress responsive and play a homeostatic role in restoring normal GABAergic and HPA axis function. Notably, allopregnanolone has been shown to reduce alcohol withdrawal symptoms in voluntary and chronic alcohol-drinking rats (Martin-García et al. 2007) as well as reduce prenatal stress (Zimmerberg and Blaskey 1998) and cocaine-induced kindling in mice (Leskiewicz et al. 2003). In humans, high levels of both progesterone and allopregnanolone have been observed in schizophrenia (Marx et al. 2006) anxiety and panic disorders (Brambilla et al. 2003, 2005) as well as after laboratory manipulated panic (Eser et al. 2005).
It may be the case then, that increased levels of progesterone in early abstinent CD females potentially represent a compensatory adaptation to an enhanced distressed state, characterized in the current sample, by increased levels of cortisol and enhanced negative mood. Consistent with this explanation, recent preliminary data from our laboratory indicate that high luteal-phase progesterone levels in CD females is associated with lower stress and drug cue-induced craving (Sinha et al. 2007). Moreover, previous human studies have shown that increased progesterone levels, achieved either by artificial exogenous administration or by testing women during different phases of their menstrual cycle, have attenuated subjective response to either acute administration of cocaine (Evans et al. 2002; Evans and Foltin 2006; Sofuoglu et al. 2002, 2004) or stress response (Del Rio et al. 1998). Clearly, although a more comprehensive assessment concerning the role of progesterone in modifying stress-related craving in CD females is warranted current findings do indicate that perturbations in steroid hormone levels continue after 1 month of abstinence and may be associated with relapse vulnerability.
CD females also demonstrated significantly lower E2/P ratios across their menstrual cycle compared with HCs, reflecting the significantly increased progesterone levels particularly during the follicular and ovulation phases. Although little is known regarding the specific pathophysiological consequences of low E2/P ratios, one possibility is that it may eventually culminate in progesterone deficiency and adrenal exhaustion (Studd 2006). While stress-related increases in neurosteroids such as progesterone and allopregnanolone may be adaptive at first, the longer-term consequences of these agents may have opposing effects resulting in increased anxiety (Gulinello and Smith 2003). Repeated biological adaptations to stress have also culminated in depleted basal levels of allopregnanolone as well as blunted response to stress in depressive disorders and premenstrual dysphoric disorder (PMDD; Girdler and Klatzkin 2007; Romeo et al. 1998). Importantly therefore, persistently high progesterone levels and low E2/P ratios may eventually predispose females to increased anxiety, reduced tolerance for stress, mood swings, and agitated depression (Fiad et al. 1996; Kaplan and Manuck 2004). As these stress-related behaviors are associated with abstinence symptoms and relapse susceptibility in recently abstinent CD patients (Kampman et al. 2004; Sinha et al. 2006), the current hormonal neuroadaptions may have important implications for relapse susceptibility in women.
In view of this, it is also notable that reduced E2/P ratios in the premenstrual or late luteal phase were associated with increased cocaine craving. While lower ratios were also associated with increased negative mood in the HC females, this was not observed in the CD sample. One tentative explanation for such associations during the premenstrual phase may be because of the fact that the late-luteal phase has been characterized by endogenous progesterone withdrawal in humans (Herzog 1995; Moran et al. 1998) and associated with both increased anxiety (Gallo and Smith 1993; Smith et al. 1998) and insensitivity to the potentiating effects of GABA-modulatory drugs (Gulinello et al. 2001). Hence, GABAergic neuromodulatory mechanisms specific to the late-luteal phase may enhance E2/P ratios and craving during early cocaine abstinence.
Notably, limited associations were observed between craving, negative mood, and hormonal changes across the menstrual cycle in both CD and HC females. Disparity between subjective and biological data, particularly across numerous time points, is a common issue and consistent with previous findings (Back et al. 2005; Cohen et al. 1995) and may reflect the existence of semi-independent behavioral and neurovegetative stress systems which impact different aspects of adaptation to distressing situations (Fox et al. 2006a; Sinha et al. 2003).
CD females in the current sample experienced cocaine craving at equivalent levels across all four menstrual phases. It is interesting to note that the levels of cocaine craving did co-vary with negative mood as evidenced by the strong positive correlations between mood and craving in the follicular and luteal phases. As such, increased levels of progesterone, cortisol, and negative mood may represent important hormonal and emotional adaptations underpinning the cocaine abstinence state during the initial 4 weeks of abstinence. Most importantly, elevations in morning basal cortisol may represent overactivity of the CRF-HPA axis and subsequent impaired HPA and emotional response to stressors and drug-related cues, both of which have been shown to be gender-specific (Back et al. 2005; Fox et al. 2006a) and predict relapse factors in cocaine-abusing populations (Sinha et al. 2006).
Some limitations of the current study should be noted. Compared with HC females, CD females reported a higher prevalence of lifetime alcohol dependence as well as mood and anxiety disorders. Findings may therefore reflect the combined effects of alcohol and cocaine use and psychiatric comorbidity on hormonal fluctuations. It is important to note, however, that CD females receiving any type of prescribed medication for either detoxification purposes or any current psychiatric/medical condition were excluded from the study. Another caveat pertains to the fact that while the CD females were tested in a highly controlled environment across the 28 days, this was not the case for the HC females. Although CD females stayed on a locked hospital unit and were largely protected from day-to-day hassles and stressors, there may have been some aspects of inpatient units that could also be considered stressors. It is therefore conceivable that these differences in context could have contributed to the group differences observed in neuromodulatory function. In the present study, no hormonal verification of MC day was used to corroborate the self-report data; however, our subsequent findings show that the daily pattern of progesterone reflects the normal pattern of variation observed across the menstrual cycle (Gandara et al. 2007). Current findings are also preliminary due to the small participant sample and may account for the lack of menstrual cycle effect on subjective ratings of craving and mood. Future research may also benefit from daily, rather than weekly assessment of mood to provide a more sensitive marker of hormonal modulation of affect across the menstrual cycle.
Previous human and preclinical studies have shown that both acute (Mantsch et al. 2003; Quiñones-Jenab et al. 2000) short-term (Zhou et al. 1999) and, in some cases, long-term (Sarnyai et al. 1998) cocaine use culminates in increased progesterone and HPA axis activity. As such, secondary analysis was performed in the current study to investigate more clearly the impact of both cocaine consumption and abstinence on hormonal fluctuations first, by correlating drug consumption variables in the three months before in-patient entry with hormonal changes and second, by examining hormonal changes as a function of study day rather than MC day. It is interesting to note that changes in hormonal levels in the current sample of CD females were not significantly correlated with drug consumption variables in the 3 months before in-patient entry. Moreover, no effect of study day was observed in relation to hormonal changes in cortisol, progesterone, or E2/P ratios.
It is clear therefore that further research is warranted to fully examine the nature of these neuroendocrine perturbations in early abstinence from cocaine and ascertain whether the hormonal and emotional elevations observed in the current study are reflective of excessive cocaine use or represent adaptive markers of a protracted abstinent state. Nonetheless, the current findings highlight alterations in the daily fluctuation of sex and stress steroid hormones in recently abstinent CD females compared with HC females. Most importantly, these neuroadaptations could have important implications for understanding cocaine abstinence symptomatology and relapse susceptibility in women.
Acknowledgments
We wish to thank the staff at the Clinical Neuroscience Research Unit and the Yale Center for Clinical Investigation (YCCI) at Yale University School of Medicine for their assistance in completing these studies. The authors declare that they have no competing financial interests. This study was supported in part by NIH grants P50-DA16556 (Sinha), K02-DA17232 (Sinha), and UL1 RR024139 (Yale Clinical Translational Science Award).
References
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29(7):1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180(1):169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7(3):171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Biggio G, Pisu MG, Bellodi L, Perna G, Bogdanovich-Djukic V, Purdy RH, Serra M. Neurosteroid secretion in panic disorder. Psychiatry Res. 2003;118(2):107–116. doi: 10.1016/s0165-1781(03)00077-5. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Mellado C, Alciati A, Pisu MG, Purdy RH, Zanone S, Perini G, Serra M, Biggio G. Plasma concentrations of anxiolytic neuroactive steroids in men with panic disorder. Psychiatry Res. 2005;135(3):185–190. doi: 10.1016/j.psychres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW. The effects of metabolic stress on plasma progesterone in healthy volunteers and schizophrenic patients. Life Sci. 1992;51(19):1527–1534. doi: 10.1016/0024-3205(92)90563-5. [DOI] [PubMed] [Google Scholar]
- Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol. 1997;325 (1):1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Jr, Mateo ET, Hou N, Rademaker AW, Acharya S, Jordan VC, Morrow M. Characteristics of salivary profiles of oestradiol and progesterone in premenopausal women. J Endocrinol. 2005;186(1):77–84. doi: 10.1677/joe.1.06025. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Gordon LU. Strategies for measuring stress in studies of psychiatric and physical disorders. In: Cohen S, Kessler RC, Gordon LU, editors. Measuring stress: a guide for health and social scientists. Oxford University Press; New York: 1995. pp. 3–26. [Google Scholar]
- Deis RP, Leguizamon E, Jahn GA. Feedback regulation by progesterone of stress-induced prolactin release in rats. J Endocrinol. 1989;20(1):37–43. doi: 10.1677/joe.0.1200037. [DOI] [PubMed] [Google Scholar]
- Del Rio G, Velardo A, Menozzi R, Zizzo G, Tavernari V, Venneri MG, Marrama P, Petraglia F. Acute estradiol and progesterone administration reduced cardiovascular and catecholamine responses to mental stress in menopausal women. Neuroendocrinology. 1998;67(4):269–274. doi: 10.1159/000054322. [DOI] [PubMed] [Google Scholar]
- Elman I, Breier A. Effects of acute metabolic stress on plasma progesterone and testosterone in male subjects: relationship to pituitary–adrenocortical axis activation. Life Sci. 1997;61(17):1705–1712. doi: 10.1016/s0024-3205(97)00776-5. [DOI] [PubMed] [Google Scholar]
- Eser D, di Michele F, Zwanzger P, Pasini A, Baghai TC, Schule C, Rupprecht R, Romeo E. Panic induction with cholecystokinin-tetrapeptide (CCK-4) Increases plasma concentrations of the neuroactive steroid 3alpha, 5alpha tetrahydrodeoxycorticosterone (3alpha, 5alpha-THDOC) in healthy volunteers. Neuropsychopharmacology. 2005;30(1):192–195. doi: 10.1038/sj.npp.1300572. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31(3):659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychoparmacology (Berlin) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89(2–3):183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiad TM, Cunningham SK, McKenna TJ. Role of progesterone deficiency in the development of luteinizing hormone and androgen abnormalities in polycystic ovary syndrome. Eur J Endocrinol. 1996;135(3):335–339. doi: 10.1530/eje.0.1350335. [DOI] [PubMed] [Google Scholar]
- Fox HC, Garcia M, Kemp K, Milivojevic V, Kreek MJ, Sinha R. Gender differences in cardiovascular and corticoadrenal response to stress and drug-cue in cocaine dependent individuals. Psychopharmacology. 2006a;185(3):348–357. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- Fox HC, Wilker EH, Kreek MJ, Sinha R. Reliability of salivary cortisol assessments in cocaine dependent individuals. J Psychopharmacol. 2006b;20(5):650–655. doi: 10.1177/0269881106063474. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced emotional and physiological sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to socially drinking controls. Neuropsychopharmacology. 2007a doi: 10.1038/sj.npp.1301470. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Berquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcohol Clin Exp Res. 2007b;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Gallo MA, Smith SS. Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav. 1993;46(4):897–904. doi: 10.1016/0091-3057(93)90219-j. [DOI] [PubMed] [Google Scholar]
- Gandara BK, Leresche L, Mancl L. Patterns of salivary estradiol and progesterone across the menstrual cycle. Ann N Y Acad Sci. 2007;1098:446–450. doi: 10.1196/annals.1384.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Lindquist T, Alim T, Flood M, Eckardt MJ, Linnoila M. Abstinent alcoholics exhibit an exaggerated stress response to 2-deoxy-D-glucose challenge. Alcohol Clin Exp Res. 1994;18(3):685–691. doi: 10.1111/j.1530-0277.1994.tb00931.x. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. 2007 doi: 10.1016/j.pharmthera.2007.05.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305(2):541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases alpha4 GABA(A) receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910(1–2):55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45 (9):1660–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H. Acute doses of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Psychopharmacology. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, Mulholland EM, Jawad AF, Parikh GA, Mulvaney FD, Weinrieb RM, O’Brien CP. Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychol Addict Behav. 2001;15(1):52–59. doi: 10.1037/0893-164x.15.1.52. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Volpicelli JR, Oslin DM, Lipkin C, Sparkman T, O’Brien CP. Cocaine dependence severity predicts outcome in outpatient detoxification from cocaine and alcohol. Am J Addict. 2004;13(1):74–82. doi: 10.1080/10550490490265389. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR J. 2004;45(2):89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 2005;182(2):245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39(2):147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Leskiewicz M, Budziszewska B, Jaworska-Feil L, Kubera M, Basta-Kaim A, Lason W. Inhibitory effect of some neuroactive steroids on cocaine-induced kindling in mice. Pol J Pharmacol. 2003;55 (6):1131–1136. [PubMed] [Google Scholar]
- Lewis JG. Steroid analysis in saliva: an overview. Clin Biochem Rev. 2006;27:139–146. [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000;152(2):132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use. Psychoneuroendocrinology. 2003;28(7):836–862. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Martin-García E, Darbra S, Pallarès M. Intrahippocampal allopregnanolone decreases voluntary chronic alcohol consumption in non-selected rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(4):823–831. doi: 10.1016/j.pnpbp.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31(6):1249–1263. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- McCance-Katz E, Sevarino K, Gottschalk PC, Kosten T. Pharmacotherapy of stimulant dependence: one of Japan’s greatest public health challenges. Nihon Shinkei Seishin Yakurigaku Zasshi. 1999;9(4):159–186. [PubMed] [Google Scholar]
- McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst Use Misuse. 2005;40(4):511–528. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Mello NK. Effects of alcohol abuse on reproductive function in women. Recent Dev Alcohol. 1988;6:253–276. doi: 10.1007/978-1-4615-7718-8_14. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Cocaine’s effects on neuroendocrine systems: clinical and preclinical studies. Pharmacol Biochem Behav. 1997;57(3):571–599. doi: 10.1016/s0091-3057(96)00433-9. [DOI] [PubMed] [Google Scholar]
- Moran MH, Goldberg M, Smith SS. Progesterone withdrawal. II: Insensitivity to the sedative effects of a benzodiazepine. Brain Res. 1998;807(1–2):91–100. doi: 10.1016/s0006-8993(98)00781-1. [DOI] [PubMed] [Google Scholar]
- Mulvaney FD, Alterman AI, Boardman CR, Kampman K. Cocaine abstinence symptomatology and treatment attrition. J Subst Abuse Treat. 1999;16(2):129–135. doi: 10.1016/s0740-5472(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Norman RL, Smith CJ, Pappas JD, Hall J. Exposure to ovarian steroids elicits a female pattern of cortisol levels in castrated males macaques. Steroids. 1992;57:37–43. doi: 10.1016/0039-128x(92)90094-p. [DOI] [PubMed] [Google Scholar]
- Piko BF, Wills TA, Walker C. Motives for smoking and drinking: Country and gender differences in samples of Hungarian and US high school students. Addict Behav. 2007 doi: 10.1016/j.addbeh.2007.01.013. in press. [DOI] [PubMed] [Google Scholar]
- Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155(7):910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V. Why are women from Venus and men from Mars when they abuse cocaine? Brain Res. 2006;1126(1):200–203. doi: 10.1016/j.brainres.2006.08.109. [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Perrotti LI, Ho A, Jenab S, Schlussman SD, Franck J, Kreek MJ. Cocaine affects progesterone plasma levels in female rats. Pharmacol Biochem Behav. 2000;66 (2):449–453. doi: 10.1016/s0091-3057(00)00213-6. [DOI] [PubMed] [Google Scholar]
- Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28(2):139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Dhabhar FS, McEwen BS, Kreek MJ. Neuroendocrine-related effects of long-term, ‘binge’ cocaine administration: diminished individual differences in stress-induced corticosterone response. Neuroendocrinology. 1998;68(5):334–344. doi: 10.1159/000054382. [DOI] [PubMed] [Google Scholar]
- Schaeffer C, Aron C. Stress-related effects on the secretion of progesterone by the adrenals in castrated male rats presented to stimulus males. Involvement of oestrogen. Acta Endocrinol (Copenh) 1987;114(3):440–445. doi: 10.1530/acta.0.1140440. [DOI] [PubMed] [Google Scholar]
- Shevchenko YA, Yakovleva TV, Makarova EN, Bazhan NM. Stress-hyperresponsive period in postnatal development of hypothalamic-hypophyseal-adrenal system in mice. Ross Fiziol Zh Im I M Sechenova. 2006;92(11):1351–1357. [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26(2):165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67:281–290. doi: 10.1016/s0376-8716(02)00085-6. [DOI] [PubMed] [Google Scholar]
- Sinha R, Rounsaville BJ. Sex differences in depressed substance abusers. J Clin Psychiatry. 2002;63(7):616–627. doi: 10.4088/jcp.v63n0715. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic–pituitary–adrenal axis and sympatho-adrenomedullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Kemp K, Krystal J, O’Malley SS. Alcohol craving and subjective emotional state during stress and alcohol cue exposure in alcoholics and social drinkers. Alcohol Clin Exp Res. 2005;29(5):150A. [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63(3):324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Paliwal P, Hong KA, Morgan PT, Bergquist KL. Sex steroid hormones, stress response and drug craving in cocaine dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol. 2007 doi: 10.1037/1064-1297.15.5.445. in press. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3alpha-OH-5alpha-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor alpha4 subunit in association with increased anxiety. J Neurosci. 1998;18(14):5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7(3):274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72(1–2):431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78(4):699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sonne SC, Back SE, Diaz Zuniga C, Randall CL, Brady KT. Gender differences in individuals with comorbid alcohol dependence and post-traumatic stress disorder. Am J Addict. 2003;12 (5):412–423. [PubMed] [Google Scholar]
- Studd J. Ovariotomy for menstrual madness and premenstrual syndrome—19th century history and lessons for current practice. Gynecol Endocrinol. 2006;22(8):411–415. doi: 10.1080/09513590600881503. [DOI] [PubMed] [Google Scholar]
- Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83(3):233–237. doi: 10.1016/j.drugalcdep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Teoh SK, Lex BW, Mendelson JH, Mello NK, Cochin J. Hyperprolactinemia and macrocytosis in women with alcohol and polysubstance dependence. J Stud Alcohol. 1992;53(2):176–182. doi: 10.15288/jsa.1992.53.176. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of the cocaine craving questionnaire. Drug Alcohol Depend. 1993;34(1):19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Schultheiss OC. Effects of affiliation arousal (hope of closeness) and affiliation stress (fear of rejection) on progesterone and cortisol. Horm Behav. 2006;50(5):786–795. doi: 10.1016/j.yhbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Meier EA, Fredrickson BL, Schultheiss OC. Relationship between salivary cortisol and progesterone levels in humans. Biol Psychol. 2007;74(1):104–107. doi: 10.1016/j.biopsycho.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Schlussman SD, Ho A, Spangler R, Fienberg AA, Greengard P, Kreek MJ. Effects of chronic ‘Binge’ cocaine administration on plasma ACTH and corticosterone levels in mice deficient in DARPP-32. Neuroendocrinology. 1999;70(3):196–199. doi: 10.1159/000054476. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Ho A, Kreek MJ. Increased CRH mRNA levels in the rat amygdala during short-term withdrawal from chronic ‘binge’ cocaine. Brain Res Mol Brain Res. 2003;114(1):73–79. doi: 10.1016/s0169-328x(03)00139-6. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Blaskey LG. Prenatal stress effects are partially ameliorated by prenatal administration of the neurosteroid allopregnanolone. Pharmacol Biochem Behav. 1998;59(4):819–827. doi: 10.1016/s0091-3057(97)00540-6. [DOI] [PubMed] [Google Scholar]