Abstract
Transitional cells represent a crucial step in the differentiation and selection of the mature B cell compartment. Human transitional B cells have previously been variably identified based on the high level of expression of CD10, CD24, and CD38 relative to mature B cell populations and are expanded in the peripheral blood following rituximab induced B cell depletion at reconstitution. Here, we take advantage of the gradual acquisition of the ABCB1 transporter during B cell maturation to delineate refined subsets of transitional B cells including a late transitional B cell subset with a phenotype intermediate between T2 and mature naïve. This late transitional subset appears temporally following the T1 and T2 populations in the peripheral compartment after rituximab-induced B cell reconstitution (and is thus termed T3) and is more abundant in normal peripheral blood than T1 and T2 cells. The identity of this subset as a developmental intermediate between early transitional and mature naïve B cells was further supported by its ability to differentiate to naïve during in vitro culture. Later transitional B cells, including T2 and T3, are found at comparatively increased frequencies in cord blood and spleen but were relatively rare in bone marrow. Additional studies demonstrate that transitional B cells mature across a developmental continuum with gradual upregulation of mature markers, concomitant loss of immature markers, and increased responsiveness to B cell receptor cross-linking in terms of proliferation, calcium flux, and survival. The characterization of multiple transitional B cell sub-populations provides important insights into human B cell development.
Keywords: B cells, autoimmunity, tolerance, rituximab
INTRODUCTION
Transitional B cells represent a critical link between the initial stochastic B cell compartment formed in the bone marrow and the mature peripheral B cell repertoire that is selected into either the follicular or marginal zone compartments at least in part on the basis of antigen specificity and strength of BCR signaling (1). Given the fact that only a small fraction of immature B cells survive the transition to the mature naïve stage, the transitional B cell compartment is widely believed to represent a key negative selection checkpoint for autoreactive B cells (2, 3). Consistent with this view, mouse T1 B cells readily undergo apoptosis upon B cell receptor cross-linking (3, 4). Moreover, recent human studies have suggested that different autoimmune diseases may share a relaxation of the censoring of transitional cells thereby leading to increased autoreactivity in the mature naïve compartment (5, 6). Interestingly, expansions of circulating transitional cells have been reported both in patients with SLE and in patients with immunodeficiency (7, 8). Therefore, understanding the biology of transitional cells has important implications for immune tolerance, autoimmunity, and immune competence. Such understanding rests heavily on the appropriate characterization of transitional subpopulations and their differentiation pathways.
Much of our knowledge of transitional B cell biology has derived from murine studies (3, 9, 10). As in the mouse, human transitional cells can be found in the bone marrow, peripheral blood, and spleen. However, in contrast to the nuanced models proposed in the mouse, thus far human studies have, by and large, described a rather homogenous population of transitional B cells (T1/T2) defined by the expression of high levels of CD24, C38 and CD10 (7, 8, 11). However, the presumption of a homogenous surface phenotype is counterintuitive when the significant heterogeneity described for mouse transitional B cells is taken into consideration. Thus, discreet stages of differentiation are observed in murine transitional cells (T1, T2 and T3) (3, 9). The ability to discriminate distinct subsets has led to the identification of refined transitional fractions containing marginal zone precursors, proposed by some to be represented by a fraction of T2 cells characterized by high expression of CD21 and CD23, and to a better definition of the biology of this important population (10). It has also generated controversy regarding the actual significance of T3 cells and whether they represent late transitional cells or anergic mature naïve B cells generated upon encountering self antigen (12).
Following the mouse model, the necessary maturation of transitional cells in different anatomical locales, and their presumed divergent differentiation potential (into either follicular or marginal zone B cells), we initially hypothesized the existence of additional phenotypic heterogeneity within the human transitional compartment. In order to elucidate such heterogeneity we have taken advantage of our previous observation that transitional cells represent a large fraction of all peripheral blood B cells for remarkably long periods of time after rituximab treatment (13, 14). We have taken advantage of this opportunity and the discriminatory power afforded by polychromatic flow cytometry to further define the characteristics of human transitional B cells. The data presented clearly demonstrate the presence of a new B cell population that represents a phenotypic and functional continuum between conventionally defined T1/T2 and mature naïve B cells. This previously unrecognized late transitional B cell population is phenotypically indistinguishable from mature naïve B cells by most conventional markers but revealed by the lack of expression of the ABCB1 transporter and represents the largest transitional fraction in normal peripheral blood.
MATERIALS AND METHODS
Sample procurement and cell isolation
Detailed written informed consent was obtained from all patients and healthy donors, in accordance with protocols approved by the Human Subjects Institutional Review Board (IRB) of the University of Rochester Medical Center (URMC). Peripheral blood lymphocytes (PBMCs) from rituximab treated patients (lupus, rheumatoid arthritis, or lymphoma) and healthy controls were isolated from heparinized blood by Ficoll-Hypaque density gradient centrifugation (Pharmacia Biotech, Uppsala, Sweden). Patients were treated as part of routine clinical care with 1000 mg/m2 rituximab × 2 (every other week) or 375 mg/m2 × 4 (weekly) or as part of previously described clinical trials (15, 16). Tonsil samples were obtained from normal subjects undergoing routine tonsillectomy. Cord blood, bone marrow, and spleen samples were obtained from pathology specimens at URMC per IRB approved protocols.
Flow cytometry and cell sorting
Immunofluorescence staining for flow cytometric analysis was performed by incubating PBMCs with excess mAb in PBS/1% BSA on ice for 20 min after blocking with 10 μg human IgG for 20 minutes. Cells were washed in PBS/BSA and in some cases incubated with streptavidin-conjugate. Cells were then washed and fixed in PBS/1% paraformaldehyde before analysis on a dual-laser FACSCalibur (Becton-Dickinson, Mountain View, CA) or three-laser 11-color LSRII (Becton-Dickinson). For some experiments B cells were purified using RosetteSep (Stem Cell Technology, Vancouver, BC, Canada) and enriched for naïve and transitional B cells by CD27 cell depletion, using CD27 microbeads and magnetic sorting (Miltenyi Biotech, Auburn, CA). For purification of distinct transitional cell populations RosetteSep enriched B cells were sorted on a FACSAria as further defined in the Figure legends.
B cells were identified based on CD19 expression and additionally classified by multi-parameter flow cytometry along a developmental pathway based on the expression of defined surface markers as follows: immature (CD38hiCD24hiCD10+IgD−IgM+CD27−), transitional (CD38hiCD24hiIgD+CD27−CD10+/−), naïve (CD38intCD24intIgD+CD27−CD10−), pre-germinal center (CD38hiCD24loIgD+CD27+/−), nonswitched memory (CD27+IgD+), and switched memory (CD27+IgD−) (Table 1) (7, 8, 17, 18). Distinct transitional cells within the IgD+CD27− compartment were defined based on R123 or mitotracker retention (further described below) and thresholds of expression of CD24 and CD38. FMO controls were used to define negative cell populations. Thresholds of CD24/CD38 expression were defined as follows: bone marrow precursor populations including T1 (CD10+) have the highest expression (+++), T2 cells (also CD10+ and R123+) have intermediate expression (++), and naïve cells (R123 negative) the lowest expression (+). Thus, T1 (R123+CD38+++CD24+++CD10+IgD+CD27−), T2 (R123+CD38++CD24++CD10+IgD+CD27−), and T3 (R123+CD38+CD24+IgD+CD27−) cells were discriminated. In addition to examining a bone marrow control with each experiment, a normal PBMC control was also included in order to further define the CD38/CD24 expression threshold on mature naïve B cells. Transitional cells from multiple tissue sources were additionally examined for the expression of a variety of developmentally regulated and other markers (7, 8, 19).
Table I.
Surface marker expression phenotype of human B cell subsets
| Subset | CD38 | CD24 | IgM | IgD | CD10 | CD27 | ABCB1 |
|---|---|---|---|---|---|---|---|
| Immature | +++a | +++ | ++ | − | + | − | − |
| Transitional 1 | +++ | +++ | +++ | + | + | − | − |
| Transitional 2 | ++ | ++ | ++ | ++ | + | − | − |
| Transitional 3 | + | +c | + | ++ | +/−b | − | − |
| Mature naïve | + | + | + | ++ | − | − | + |
| Pre-GC (Bm2′tonsil) | ++ | +/− | + | + | −/+b | −/+ | − |
| GC (Bm3/4) | ++ | − | − | − | + | + | − |
| Memory | + | ++c | + or −d | + or −d | − | + | − |
represents the level of positive expression
gradual loss (+/−) or gain (−/+) of expression during development
T3 cells have a shift toward higher expression of CD24 compared to mature naïve cells, as do memory B cells
Memory B cells are heterogeneous and include unswitched IgD+IgM+ cells, switched IgD−IgM− cells, and a small population of CD27−IgD− cells
Analysis of B cell proliferation and function
To determine proliferation, 1 × 105 B cells or purified subsets were labeled with carboxyfluorescein succimidyl ester (CFSE; Molecular Probes) and cultured for 1–4 days in medium supplemented with various stimuli: F(ab′)2 anti-IgM (Jackson Immunoresearch) (10 μg/ml for all conditions except in combination with CpG where 2.5 μg/ml is used) +/− CpG ODN 2006 (2.5 μg/ml) (Oligos Etc., Wilsonville, OR.), CD40L (1 μg/ml) (Fitzgerald Industries, Concord, MA.), or BAFF (1.5 μg/ml) (Fitzgerald Industries, Concord, MA.). Survival of B cell subsets was determined by annexin V-FITC or PE staining per manufacturer’s protocol (Southern Biotech., Birmingham, AL.).
For analysis of calcium signaling, B cells were purified by negative selection, stained with CD38/CD24 +/− mitotracker (for distinction between naïve and T3), resuspended in Hank’s buffered salt solution (HBSS) containing 1 mM Ca and Mg and 1% FCS at a concentration of 2 × 106 cells/ml, and loaded with Indo-1 AM (Molecular Probes, Eugene, OR) (2 μM final) for 30 min. at 37° in the presence of pluronic F127 (Molecular Probes). CD2/3/14/16/27 staining was utilized to gate out remaining non-B cells and memory B cells at the time of the calcium measurements. Calcium responses were measured on a Becton-Dickinson FACS Vantage SE with UV excitation. Data was collected and displayed as the relative ratio of intensities of Indo fluorescence (Ca-bound Indo violet emission 405 nm/free Indo blue emission 485 nm) for each cell over time and analyzed with FlowJo software (Tree Star Inc., San Carlos, CA). Samples were analyzed for a 30–60 second baseline at 37 degrees followed by the addition of 20 μg/ml F(ab′)2 goat anti-human IgM or anti-IgD.
ABC transporter activity
Naïve B cells were distinguished from transitional cells and memory B cells by the expression of ABCB1 transporter activity and rhodamine (R123) or mitotracker dye extrusion as described (19). Cells were stained in culture medium at 37° C with Rhodamine 123 (R123) at 6 μM for 10 min. and chased for 3 h prior to flow cytometry analysis (19). Dose ranging studies were performed to determine the optimal loading concentrations and timing for extrusion. Alternatively, cells were stained and chased for 30 min at 37° C with mitotracker. Equal loading of different B cell subsets from a variety of tissues and peripheral blood B cells from multiple normal controls and B cell reconstituting patients was demonstrated in control experiments (data not shown). B cells were gated as described above and dye extrusion examined. Specifically, gated memory B cells (CD27+) do not express the ABCB1 transporter and thus fully retain R123. In contrast, gated naive B cells (CD38intCD24intIgD+CD27−CD10−) effectively extrude R123, with the MFI for R123 on normal naïve peripheral blood B cells (n=7 donors) significantly different from the MFI for R123 on normal memory B cells (p=3.0E-5). Transitional B cells within the gated naïve population were distinguished by the intermediate/high expression of R123.
Statistical analysis
Statistical significance of comparisons of mean values was assessed by two-tailed Student’s t-test or non-parametric Mann-Whitney U test using XLSTAT Excel software. P values ≤0.05 were considered significant.
RESULTS
Developmental kinetics of human transitional B cells and demonstration of a late transitional subset
We have previously reported an expansion of transitional B cells in diverse patient populations reconstituting after B cell depletion therapy, including lymphoma and autoimmune disease (lupus and rheumatoid arthritis) (13, 15). Here we sought to better delineate human transitional B cell subsets and the time course of B cell development through defined transitional B cell stages during B cell reconstitution. Circulating transitional B cells were initially analyzed based on surface phenotype: IgD+CD27−CD38hiCD24hi (Figure 1A) (7). High expression of CD38 and CD24 was defined based on their level on the CD10+ peripheral blood B cells, a well accepted marker expressed by most transitional cells (5). In normal peripheral blood transitional B cells are detected at low frequency (Figure 1A and Table 2). In order to better discriminate distinct transitional B cell subsets, we compared B cells from bone marrow and cord blood which contain increased proportions of less mature B cells (20). In the bone marrow, there is a large population of cells very high for expression of CD38 and CD24 (+++). Further characterization of these cells resolves them into IgD−IgM− pre/pro B cells, IgD−IgM+ immature B cells, and IgD+IgM+ T1 cells (Figure 1B). There is also a population of cells intermediate for expression of CD38 and CD24 (+), which represent mature re-circulating B cells, but a relative paucity of cells with moderately high expression (++) (T2). In contrast, in peripheral blood CD38+++CD24+++ T1 cells are sparse and CD38++CD24++ T2 cells more abundant. Cord blood also contains a high proportion of T2 cells with levels of expression of CD38 and CD24 in between the position of the T1 and mature B cells (Fig. 2A).
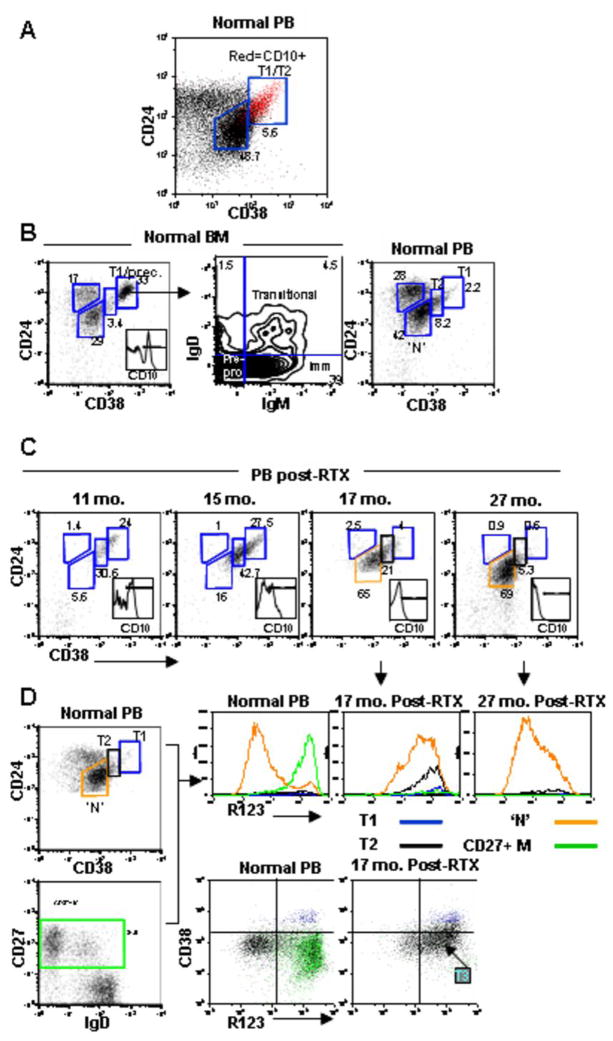
Figure 1. Reconstitution of peripheral blood B cell subsets after rituximab and revelation of a late transitional B cell population.
(A) CD19+ gated peripheral blood B cells were examined by flow cytometry for the expression of CD38, CD24, and CD10 in a normal control in order to define the CD24 and CD38 expression level on CD10+ transitional B cells.
(B) Further resolution of transitional B cells into T1 and T2 subsets. CD38 and CD24 levels on FSC- and SSC- gated CD19+ B cells in bone marrow (BM) was used to define the CD24/CD38 expression level on the T1 subset. BM is enriched for CD10+ precursor cells (inset histogram) which have uniformly high expression of CD24 and CD38 (+++) and based on additional analysis of IgD and IgM correspond to pre/pro, immature, and T1 B cells. The corresponding T1 population is depicted in PB with the T2 subset intermediate for CD24/CD38 (++) compared to mature naïve (+).
(C) Immune reconstitution post-rituximab reveals an expansion of CD24hiCD38hi transitional B cells with progression from T1 to T2 to mature naïve over time.
(D) Cells from normal and reconstituting PB were examined for R123 dye extrusion, which is reflective of expression of functional ABCB1 transporter by naïve B cells. The gating strategy on the CD24 v CD38 and CD27 v IgD dot plots is shown in color coded gates, with the respective R123 expression depicted in the histograms. The relatively inefficient R123 extrusion of the gated ‘N’ population in the reconstituting subject compared to the normal naïve population suggests an intermediate phenotype between T2 and mature naïve. This population is also revealed in the CD38 vs. R123 dot plots, with the gated T1 subset in blue and the CD27+ M in green.
Table II.
Transitional B cell fractions in different lymphoid compartments
| Naive | T3 | T2 | T1 | M/‘N’ MFI | |
|---|---|---|---|---|---|
| Normal PB (mean+/−s.d.) | 59.1±12.3 | 11.2±2.2 | 4.2±1.6 | 0.9±0.7 | 7.2±2.2 |
| Post-RTX PB | 31.8±13.1 | 41.2±12.9b | 26.6±13.3a | 10.3±11.8c | 2.5±0.9b |
| Bone marrow | 39±26 | 5.3±3.7 | 2.6±1.2 | 6.3±0.9b | 7.1±3.3 |
| Cord blood | 19.2±8.6 | 31.8±11.1d | 28.2±7.7b | 20.8±9.5a | 2.0±0.5e |
| Spleen | 27.7±12.1 | 15.3±6.1 | 5.4±0.9 | 1.8±0.7 | 2.2±0.7f |
The fraction of each subset relative to the entire B cell population is shown (normal PB n=7, post-RTX PB n=8, bone marrow n=4, cord blood n=5, spleen n=4). The post-RTX subjects include lymphoma (n=6), RA (n=1), and SLE (n=1) patients and were studied a mean of 16.5 months after treatment. For analysis of T1 and T2 subsets post-RTX an additional 6 lymphoma and SLE patients were available and included in the analysis. T1 cells were defined based on high expression of both CD24 and CD38 within the IgD+CD27− population, whereas T2 cells have intermediate expression of both these markers. The gated ‘naïve’ (‘N’) B cell population (CD24intCD38intIgD+CD27−) actually contains both a R123 low true naïve (N) population and a R123 high late transitional (T3) population. The larger the T3 fraction of the gated ‘N’ population (yielding a higher R123 MFI), the lower the M/’N’ R123 MFI.
p=0.0002 compared to normal PB
p<0.0001 compared to normal PB
p=0.05 compared to normal PB
p=0.0007 compared to normal PB
p=0.005 compared to normal PB
p=0.002 compared to normal PB
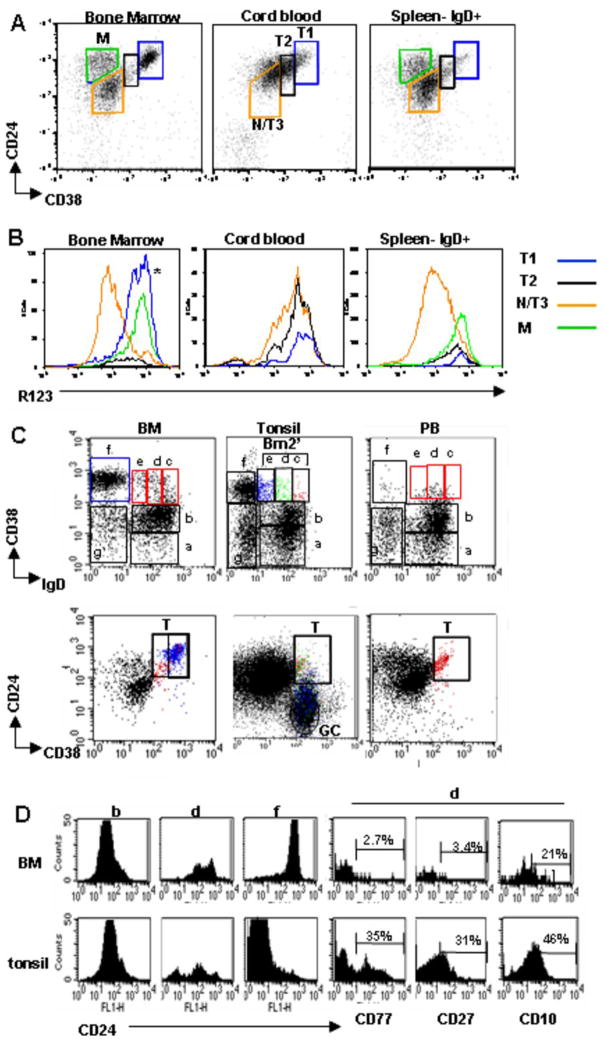
Figure 2.
Resolution of discrete transitional B cell subsets in different lymphoid compartments (A) CD19+ B cells from bone marrow (BM), cord blood (CB), or spleen were examined for CD38, IgD and CD24 in order to define the N/T3, T2, and T1 cell populations. For spleen, the cells are gated on IgD in addition to CD19 in order to largely exclude the MZ B cells. For BM, the CD24/CD38+++ high cells (*) contain T1, immature, and pre-pro subsets as described in the prior figure. (B) R123 extrusion was examined in BM, CB, and spleen in the respective gated populations defined above. Compared to PB (Fig. 1) and BM N/T3 B cells (CD24intCD38int) from CB and spleen are shifted toward inefficient R123 extrusion, consistent with a less mature phenotype. (C) Position of the Bm2′ population (CD38hiIgD+ on CD38 vs. IgD plot) (red gate in BM, PB) in CD24 vs. CD38 dot plots in tonsil B cells differs from BM and PB. The Bm2′ subset is divided further (into populations c, d, e). In tonsil these are color gated (red, green, blue) to reveal that the majority of the Bm2′ subset falls outside of the transitional B cell gate in the CD24 vs. CD38 plot and instead represent CD24 low pre-GC cells. In the BM the blue gate delineates the Bm3 population (‘f’) (representing immature and precursor cells here). (D) The expression of CD24 on the gated populations b, d, and f from (C) is examined in BM vs. tonsil. In the BM the B cell populations from ‘b’ to ‘f’ display increased CD24 expression, whereas in the tonsil this progression to the GC population ‘f’ decreases CD24 expression, again indicating that the Bm2′ population in tonsil contains mainly pre-GC cells. Population ‘d’ from the Bm2′ population in the tonsil expresses CD77 and CD27, in contrast to the respective BM population. Results are representative of 4 bone marrow aspirates, 5 cord blood samples, 4 spleens, 5 tonsil specimens, and 7 peripheral blood samples.
The developmental relationships between the T1, T2, and mature B cell populations as defined above were further substantiated by the developmental kinetics during B cell reconstitution after rituximab. Notably, even more than a year after rituximab-induced B cell depletion, nearly all of the B cells in the peripheral blood display a transitional phenotype (Figure 1C). As reconstitution continues, the peripheral blood B cells gradually down-regulate CD24 and CD38 and lose CD10 expression, shifting to an apparently naïve phenotype with decreased fractions of transitional cells (15). A gradual progression from T1 to T2 to mature naïve is evident (Figure 1C).
In order to additionally distinguish transitional from mature naïve B cells, we examined the activity of the ATP-binding cassette transporter, ABCB1. Wirth and Lanzavecchia have described this transporter as being upregulated during the final differentiation from transitional B cells to mature naïve and irreversibly lost on memory cell differentiation (19). We have shown that the ABCB1 is also lost in CD27 negative memory cells (21). When peripheral blood B cells are labeled with the vital dye R123 and then chased at 37°C for 3 hours, a heterogeneous staining pattern is observed because some cells do not extrude the dye at all (memory and transitional B cells) and others extrude efficiently (naïve cells). The R123 expression for each gated B cell population is depicted in the histograms in Fig. 1D. In this analysis it is apparent that a fraction of the gated ‘naïve’ B cells (CD24intCD38intIgD+CD27−) do not extrude R123 efficiently, suggestive of a late transitional B cell phenotype that has down-regulated CD38 and CD24.
The identification of late transitional cells is best illustrated by the examination of B cells during the reconstitution phase in patients treated with rituximab. Such analysis readily detects an enrichment of cells with inefficient rhodamine extrusion within the CD24intCD38intIgD+CD27− gate (Fig. 1D). We postulate that these cells are in transition between T2 and mature naïve, representing a population akin to the T3 subset described in mice (9). This developmental relationship is supported by the appearance of the T3 subset after T1 and T2 in diverse patient populations (lymphoma, SLE, and RA) reconstituting after rituximab (Table 2) and its gradual replacement by mature naïve B cells. It is further notable that the peripheral blood is enriched for this T3 population compared to normal adult peripheral blood even years after treatment.
Late transitional B cells are enriched in cord blood and spleen but not tonsil
Next we examined different lymphoid tissue for late transitional B cells by combining surface markers (CD38, CD24, CD27, IgD) with dye extrusion (Fig. 2A). The naïve surface phenotype (IgD+CD27−CD24intCD38int) cell population contains both naïve and T3 cells (denoted ‘N/T3’). An increase in the proportion of the T3 subset is then revealed based on a higher R123 MFI for the N/T3 gated population (Fig. 2B and Table 2) (an alternative gating strategy for the T3 subset is described in the next section). T1, T2, and T3 cells are equally dominant in cord blood (T1 20.8+9.5; T2 28.2+7.7; T3 31.8+11.1, p=0.0002, <0.0001, and =0.0007 for T1, T2, T3 respectively, compared to PB), as revealed by the gated T1 and T2 populations (Fig. 2A) and the shift in R123 expression in the N/T3 population (Fig. 2B) (Table 2: lower M/N MFI). Based on this analysis cord blood contains few if any mature naïve B cells. Splenic B cells also display a shift towards poor R123 extrusion with enrichment for T2 and late transitional B cells relative to mature naïve (Fig. 2B, Table 2), suggesting that this is the site of their development at least under physiological circumstances.
There has been some confusion in the literature regarding the distinction between pre-germinal center (pre-GC) cells and transitional B cells. This is an important distinction since the expansion of one or the other subset in the PB may not only reflect different immunological processes but also have separate biological consequences (22). To clarify this issue, we asked whether the CD38hi Bm2′ (pre-GC) population in tonsil (based on the Bm1-5 designation via CD38 and IgD expression) (17) overlaps with the CD38hiCD24hi subset that we are identifying as transitional B cells. In fact, it does not (Fig. 2C) given that the tonsil Bm2′ population has decreasing expression of CD24 (Fig. 2D). Consistent with the identity of these cells as predominantly pre-GC we found increasing expression of CD27 and CD77 (markers not found on transitional cells) (Fig. 2D). In contrast, the Bm2′ compartment in PB and BM overlaps with the CD38hiCD24hi transitional B cell population. As described in the last section, amongst transitional B cells T1 predominates in BM and T2 in PB, with both populations expressing IgD.
Phenotypic characterization of distinct transitional B cell subsets
We used 7–10 color flow cytometry to analyze simultaneously multiple surface markers in order to better define discrete human transitional B cell subsets. Figure 3 depicts the gating strategy which examines dye extrusion in the IgD+CD27− population, with the dye negative population representing the naïve B cells and the dye positive population further divided into T1, T2, and T3 based on the level of CD38/CD24 expression. By excluding the double negative (IgD−CD27−) population, we avoid contamination with memory B cells which predominate in this minor subset in PB (21). Of note, the T3 subset represents the dominant transitional B cell population in PB (Fig. 3A). Overall, as cells mature from the T1 to mature naïve B cell stage there is an increase in the expression of mature markers (CD22, CD44, CD21, and CD23; data shown for latter two in Fig. 3 for PB and CB) and a gradual decrease in CD10, CD5, and IgM (Fig. 3B, C). In this analysis, T1 and T2 B cells are clearly distinguishable with the latter expressing lower levels of CD10, CD5, and IgM and higher levels of CD21. It is notable that most T3 cells do not express CD10, making this an imperfect marker for the delineation of all human transitional B cells, despite its wide use for that purpose. T3 cells are best distinguished from mature naïve by higher expression of IgM and lower expression of CD23, especially pronounced in cord blood (Fig. 3C) but also evident in PB (Fig. 3B). In BM, T1 cells similarly express high levels of CD10, CD5, and IgM and low levels of CD21 and CD23 compared to mature naïve recirculating cells, although IgM expression overall was lower on BM T1 cells than on CB and PB T1 cells (data not shown). Notably, BM B cell precursor populations expressed the highest levels of CD10 but were distinguished from transitional cells by the lack of CD5 expression (data not shown).
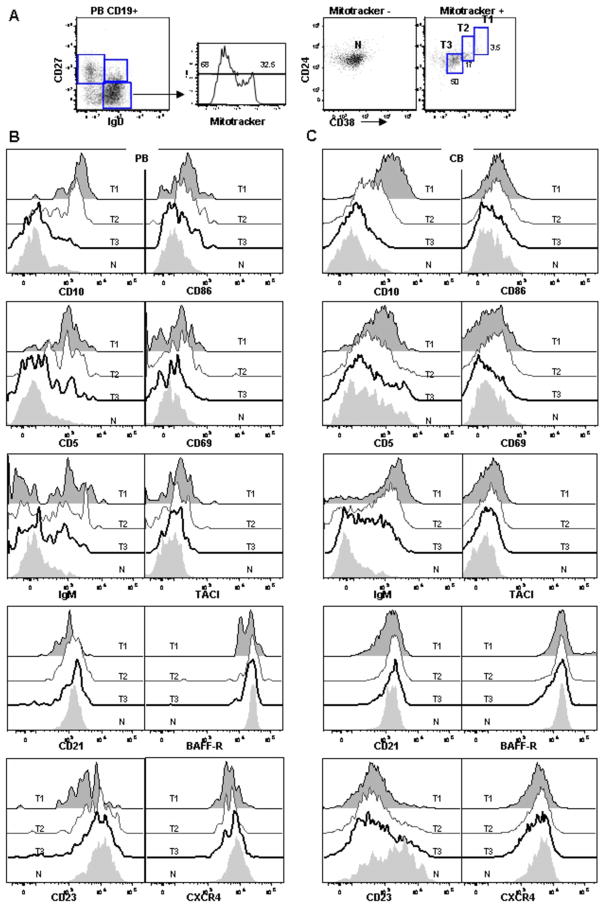
Figure 3. Phenotypic characterization of transitional B cells.
(A) CD19+ B cells from normal PB were examined for CD27, IgD, CD24, CD38, and mitotracker extrusion to define the N, T3, T2, and T1 fractions shown. (B) Expression of discrete markers on the four populations defined above in PB. (C) Expression of discrete markers on the four populations in CB. Representative of more than 5 independent experiments.
To exclude the possibility that T3 cells represent an activated B cell intermediate (either activated T2 or naïve), activation markers were examined. In PB and CB, CD86, CD69 (Fig. 3B, C), and CD80 (data not shown) are clearly not up-regulated on T3 cells compared to other transitional and mature naïve B cells. CD95 also was not differentially expressed (data not shown). Given the role of BAFF in murine transitional B cell homeostasis we also examined the expression of two BAFF receptors, BAFF-R and TACI. Overall, expression of TACI was low on all subsets examined although higher on early transitional B cells. In contrast, BAFF-R was expressed at high levels on all transitional and mature B cells subsets (data not shown for memory B cells). In the BM, BAFF-R was also expressed at high levels by T1 and mature naïve re-circulating cells but not by B cell precursors (data not shown).
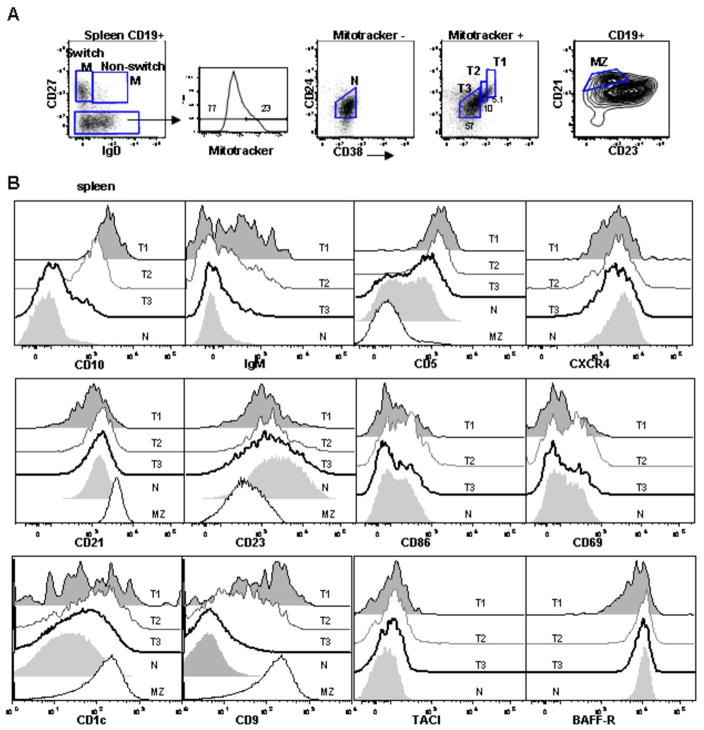
In spleen, transitional B cells similarly up-regulate mature markers with concomitant down-regulation of immature markers as they progress across the developmental continuum. In notable contrast to PB and CB, however, CD5 displays heterogeneous expression on the T3 and mature naïve subsets in the spleen (Figure 4). This suggests additional heterogeneity in these populations and, indeed, human spleen is more complicated than PB and CB with a sizable contribution to the B cell compartment by the marginal zone subset. To exclude the possibility of a MZ contaminate in the T3 and naïve populations as an explanation for the heterogeneous CD5 expression, we examined the CD21HiCD23Lo MZ for CD5 but its expression is low (Fig. 4B). In contrast, MZ B cells express CD1c and CD9, markers previously described on the MZ (23). The expression of CD1c by transitional B cells suggests the inclusion of a MZP population within these subsets. We also conclude that the spleen contains a unique expansion of CD5+ B cells compared to other lymphoid compartments, possibly analogous to murine B1 cells (23). Another distinction in the spleen compared to PB and CB is the up-regulation of activation markers on a fraction of all B cell subsets, although most pronounced on the T1 and T2 cells (Fig. 4B). This suggests that the spleen may be the site of activation and differentiation of early transitional B cells.
Figure 4. Characterization of transitional B cells in spleen.
(A) B cells from human spleen were examined for CD27, IgD, CD24, CD38, and mitotracker extrusion. CD21hiCD23lo cells were defined as the MZ. (B) Expression of discrete markers on the T1, T2, T3, and N populations defined above. For certain markers the expression on the MZ population is shown for comparison.
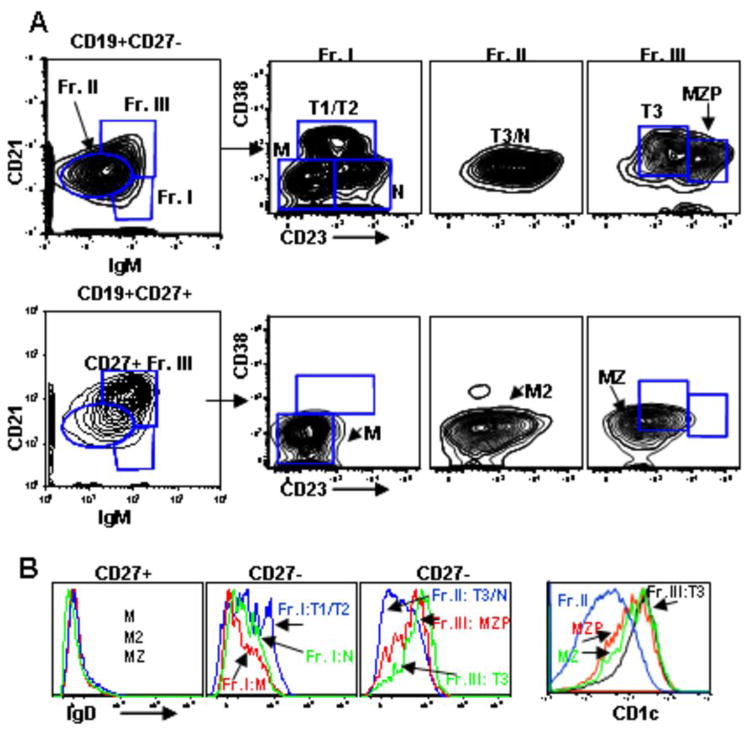
Given the critical role of the spleen in transitional B cell maturation and the apparent complexity of human B cell subsets here, the heterogeneity of splenic B cell populations was further examined using multi-parameter flow cytometry to analyze simultaneously the surface markers used in the present study and those used in recent murine studies (Figure 5) (3, 9, 10, 24). We compared the distribution of human splenic CD27+ and CD27− B cells based on CD21 and IgM expression, using initial gates to define three fractions (Frs. I–III) similar to Allman and colleagues recent description of murine splenocytes (10). Focusing first on the CD27– B cells, these three populations were further subdivided based on differential CD23 and CD38 expression, with CD38 utilized instead of AA4.1 (which in contrast to murine immature/transitional B cells is not expressed by human B cells, data not shown). Similar to Fr. I in murine spleen, human Fr. I (CD27−) contains immature B cells but also includes CD38−CD23− memory B cells (IgD−CD27−MTG+) and a subset of CD38intCD23+ naïve cells (IgD+MTG−) (data not shown for MTG). Fr. II (CD27−) includes both CD23 higher follicular naïve and CD23 lower T3 B cells. Approximately 50% of the cells within Fr. III (CD27−) are CD21hiCD23+ corresponding to the position of the recently defined murine MZ precursor population (10). Consistent with this phenotype is the high expression of IgD and CD1c on this population (Fig. 5B). The remainder of Fr. III contains CD23intIgD+ B cells phenotypically similar to T3 cells with inefficient MTG extrusion and high CD5 expression (data not shown). The majority of the CD27+ B cells fall in the CD21hiIgMhi Fr. III compartment and represent MZ B cells, although memory B cells are also represented within Fr. II. In conclusion, this flow cytometric scheme resolves at least 9 subpopulations of splenic B cells, including T1, T2, T3, and MZP subsets.
Figure 5. Additional phenotypic characterization of B cells in human spleen.
(A) CD19+ splenocytes were examined for expression of CD21 and IgM to define three fractions in both the CD27− and CD27+ compartments. CD23 and CD38 levels were examined on these fractions. M=memory; MZP=marginal zone precursor; MZ=marginal zone. M2 indicates a second memory population in the CD27− fraction with different phenotypic characteristics, appearing in Fr. II. (B) IgD and CD1c levels on the populations defined in C.
T3 cells are nonproliferative in vivo
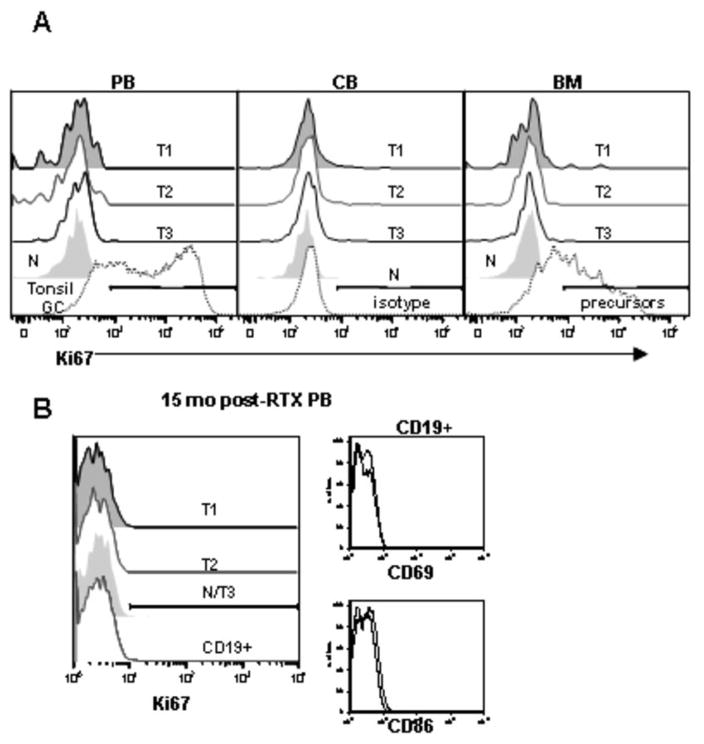
Since B cell depletion is associated with compensatory increases in BAFF levels and certain subsets of murine B cells are known to undergo homeostatic proliferation in response to BAFF (24), we examined whether transitional B cells express the proliferation antigen Ki67. None of the transitional B cell subsets express significant Ki67 in PB, CB, or BM (Fig. 6A). In contrast, positive controls including tonsil germinal center B cells and BM precursor B cells express significant Ki67. Transitional B cells arising during B cell reconstitution after rituximab also do not express Ki67, nor activation markers (Fig. 6B).
Figure 6.
The T3 subset is non-proliferative in vivo and lacks activation marker expression. (A) B cells from PB, CB, and BM were examined for CD27, IgD, CD24, CD38, and mitotracker extrusion in order to define the T1, T2, T3, and N populations as described above. The intracellular expression of the proliferation antigen Ki67 was examined. In the CB graphs comparison to isotype control is shown, whereas in the PB and BM graphs positive controls depict the high Ki67 expression in tonsil GC cells and BM precursors.
(B) Ki67 expression on discrete B cell populations and activation marker expression (CD69, CD86) on CD19+ B cells in a reconstituting subject after rituximab.
Transitional B cells have reduced proliferative capacity
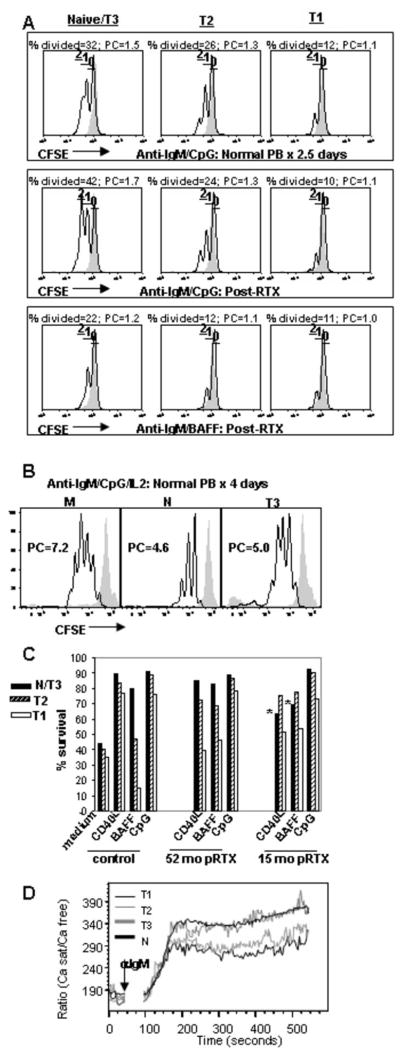
B cell functional maturity can be assessed by examining the in vitro proliferative responses to BCR cross-linking (8, 9). Thus, the proliferative capacity of CD38hiCD24hi B cells was compared to CD24intCD38int B cells by measuring the CFSE dilution in the corresponding subsets from reconstituting rituximab treated patients and normal controls (PB) during in vitro culture. B cells did not proliferate when unstimulated or in response to anti-Ig alone, but with additional co-stimulation up to 2 rounds of division were induced at 2–3 days (CpG>BAFF>CD40L). The proliferative capacity of mature naïve B cells was significantly greater than T2 cells (p=0.05 for T2 compared to naïve) which was greater than the T1 cells (p=0.034 for T1 compared to T2) (Figure 7A).
Figure 7.
Transitional cells display reduced survival, proliferative capacity, and calcium flux compared to mature B cells. (A) B cells were purified by negative selection and depleted of CD27+ memory B cells from a representative normal control vs. reconstituting rituximab patient, loaded with CFSE, and cultured for 2.5 days with anti-IgM + CpG, BAFF, or CD40L as described in the Methods. Naïve/T3, T2, and T1 B cells were gated based on CD24 and CD38 expression as in Figure 1. The majority of CD24+CD38+ (naïve) B cells undergo 1 or 2 rounds of division, whereas the CD24++CD38++ (T2) B cells proliferate less efficiently and the CD24+++CD38+++ (T1) B cells even less so. This is reflected in the lower %divided (responder frequency) and proliferative capacities (PC) calculated from the plots by the method of Wells et al. (25). The gray filled line graph depicts non-proliferating cells cultured in medium alone.
(B) Response of sorted CFSE-labeled memory (M) (CD27+), naïve (N), and T3 B cells to BCR-cross-linking (anti-IgM) + CpG + IL2 after 4 days of culture. B cell populations were sorted as described in Figure 3 and Figure 8 to >90% purity. Plots show resulting CFSE levels on live CD19+ cells at day 4 of culture with unstimulated cells in the gray filled line graphs. Proliferative capacities (PC) calculated from the plots by the method of Wells et al. (25) are shown. All CD19+ populations displayed 100% responder frequencies at day 4 of culture under these conditions.
(C) B cells purified and gated as in A were cultured for 2.5 days in the presence of the indicated stimuli. For normal controls, survival of the N/T3 subset is greater than the T2 subset which is greater than the T1 subset. For early reconstituting subjects, the survival of the N/T3 B cells is significantly lower than T2 (*).
(D) Calcium responses in normal peripheral blood B cells after anti-IgD or anti-IgM stimulation. B cells were purified by negative selection and stained with CD2, CD27, CD3, CD14, and CD16 antibodies (to gate out non-B cells and memory B cells). The expression of CD27, CD24, CD38, and mitotracker extrusion was used in order to define the T1, T2, T3, and N populations as described in Figure 3. The median responses are depicted for the gated B cell populations after the addition of 20μg/ml of Fab′2 anti-IgM. Data are representative of at least 3 independent experiments for (A)–(C).
In order to define the proliferative capacity of the late transitional B cell population, we can compare the proliferation of IgD+CD27− CD24intCD38int peripheral B cells from normal controls and reconstituting rituximab patients. As described above, the latter group at early reconstitution time points has essentially all late transitional B cells (T3) within the phenotypically defined mature naïve (N) compartment. Despite this, the proliferation of the N/T3 population was similar between controls and rituximab treated subjects, suggesting that T3 cells proliferate at least as efficiently as naïve cells. This result was confirmed by sorting naïve and T3 subsets from normal PB and examining the proliferation profile of the cultured CFSE-labeled populations. Figure 7B shows that the responder frequencies (100% at day 4) and proliferative capacities (PC) calculated from these CFSE profiles are similar (25).
Transitional B cell subsets display reduced survival and calcium signaling
When viability was assessed after 3 days of in vitro culture in the absence of any exogenous stimuli, survival was poor (Fig. 7C). Supplementing the cultures with anti-Ig and stimulatory CpG DNA, CD40L, or BAFF improved survival, with CpG providing the strongest survival stimulus. However, the survival of the T1 subset was still lower than the T2 subset which was lower than the mature naïve subset (Figure 7C). The survival effects of BAFF were more pronounced in the later transitional (T2, T3) and naïve B cell subsets (T2, T3), consistent with the higher expression of BAFF-R by these populations (Fig. 3 and 4). When we compared IgD+CD27− CD24intCD38int B cells from normal controls and post-rituximab subjects, the T3-dominated N/T3 population from the latter group displayed significantly reduced survival that was proportional to the degree of T3 expansion (Fig. 7C). In fact, during early reconstitution when the N/T3 gated subset contains predominantly T3 cells, the apoptosis of this population is even greater than the T2 subset (* in Fig. 7C). That the T3 subset as a population has increased apoptosis with BCR engagement is important as this complements the assertions based on phenotypic markers and R123 staining to define this novel subset. The enhanced apoptosis of the T3 population was confirmed with sort purified and cultured naïve, T3, and early transitional B cell subsets (data not shown).
The ability of CD24HiCD38Hi B cells to flux calcium in response to BCR cross-linking was also significantly reduced compared to naïve B cells (Figure 7D). Of note, this was true for signaling through both IgD and IgM, despite the higher expression of IgM on the transitional B cells (data shown for the latter). This suggests an overall hypo-responsiveness of transitional B cells to cross-linking of the B cell receptor. Upon further discrimination of mature naïve, T3, T2, and T1 subsets, early transitional B cells (T1 and T2) displayed reduced calcium flux compared to both naïve and T3 B cells (Figure 7D).
Developmental kinetics of transitional B cells
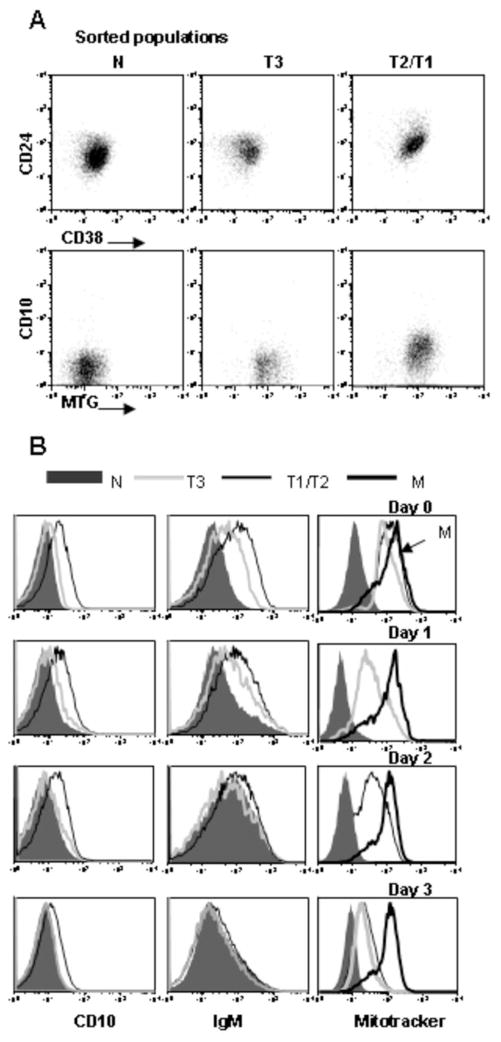
The finding that the T3 population is prominent during immunologic reconstitution and is gradually replaced by R123/mitotracker extruding naïve B cells, first suggested to us that it represents a developmental intermediate. However, in order to further substantiate the developmental relationships between the transitional B cell subsets we sort purified T1/T2 and T3 cells (Fig. 8A) and cultured them in vitro (Fig. 8B). T3 cells displayed higher expression of CD24 and IgM relative to mature naïve B cells at baseline, as expected (Fig. 8A and B). Notably, T3 B cells gave rise to mitotracker extruding naïve B cells with progressive down-regulation of CD24 and IgM during culture (Figure 8). Given that T1 and T2 B cells represent precursors of mature naïve B cells, we reasoned that these cells should also give rise to a mature naïve phenotype upon culture. Furthermore, if the T3 population is an intermediate in mature naïve B cell development, then the T1/T2 cells should pass through this stage as they differentiate to mature naïve. At baseline and day 1 of culture the T1/T2 cells had significantly higher expression of CD10, CD24, CD38, IgM, and mitotracker compared to T3 and naïve cells. By day 3, the T1/T2 cells had differentiated to IgMlowCD10−, overlapping in expression with the mature naïve population. At earlier time points, the cells displayed an intermediate phenotype consistent with transit through a T3 stage en route to the mature naïve B cell stage (Fig. 8B).
Figure 8.
T3 B cells differentiate into mature naïve in vitro. (A) B cell subsets were sorted from PB on a FACSAria using the surface markers (CD27, IgD, CD24, and CD38) and sorting strategy (MTG) defined in Figure 3. The sort purity is depicted based on the expression of CD24 vs. CD38 and CD10 vs. MTG and is >90%. (B) Sort purified populations were cultured with anti-IgM/CpG/IL2, harvested at the indicated time points, stained with the indicated antibodies, and dye retention examined to define the MTG− naïve B cell population.
DISCUSSION
Our data reveal several novel aspects of human peripheral B cell development. We show that human transitional B cells can be subdivided into multiple populations based on CD24/CD38 expression, a gradation of immature and mature surface marker expression, dye extrusion (reflecting the expression and functionality of the ABCB1 transporter), and functional responses. Notably, we identify a late transitional B cell population intermediate between T2 and mature naïve stages that is a normal B cell developmental intermediate, the dominant peripheral transitional B cell population in healthy adults, and substantially increased in the peripheral compartment of patients reconstituting after B cell depletion therapy. Overall, our results indicate that human transitional cells exist along a phenotypic and functional continuum between immature and mature, both confirming and substantially extending the prior characterization of human transitional B cells (7, 8). Similar to the mouse, we suggest that immune competence is gradually acquired as immature B cells transit through the transitional cell stage toward mature naïve (1). Thus, mature surface markers are gradually acquired, the ABCB1 transporter is expressed, and proliferation and survival begin to be favored over apoptosis in response to BCR stimulation.
There have been a number of recent reports defining human transitional B cells (7, 8, 11). These studies have demonstrated a population of CD24hiCD38hi B cells with distinct phenotype and function compared to mature naïve B cells. However, discrete developmental subsets have not been well characterized. Reduced proliferation, survival, differentiation, and chemotaxis of CD24hiCD38hi human transitional B cells have been demonstrated in vitro, but notably there are several discrepancies in the literature regarding the nature of the signals that mediate the survival and proliferation of distinct transitional B cell subsets. For example, one publication has proposed that human T1 cells have marked CD40L enhanced proliferation but little response to BAFF stimulation (26). Other studies have demonstrated more of a gradation of response, with mature subsets simply having more proliferation (8) (or entry into cell cycle) in response to CD40L/anti-Ig than their transitional cell counterparts. Similarly, Sims et al. found BAFF to be a relatively inefficient inducer of cell cycle entry in human B cells, but still with a trend toward greater induction in mature than transitional B cell populations (7). Our data are in accord with the view of mature human B cells having greater proliferative responses to a range of stimuli compared to transitional B cells. Thus, based on CFSE dilution, proliferation of the T1 subset is clearly induced by anti-Ig + either CpG or BAFF, although to a lesser degree than in the T2 and mature naïve subsets. It is of additional note in our study that CpG was the most highly effective stimulus for the proliferation and survival of human transitional cells, in accord with a recent publication (27), and raising the question of how autoreactive transitional cells escape such stimulation in vivo. We also demonstrate for the first time that human transitional B cells (T1/T2) have reduced calcium signaling compared to mature naïve B cells.
Of note, even within the murine literature there is some disagreement as to the precise delineation of transitional B cell subsets. Thus, Loder et al. first proposed the partition of transitional B cells into two subpopulations based on CD21, CD23, and IgD: T1 and T2, with similar phenotypic properties to that described here with the exception of IgD expression on human T1 cells (3). A notable parallel between this classification scheme and our characterization of human transitional B cells is the increasing expression of CD21 on maturation from T1 to T2. However, Allman et al. have described an alternate classification scheme using the developmental marker AA4 and variable expression of IgM and CD23 to delineate three transitional populations T1-T3. Similar to this analysis, we find higher expression of CD23 on human transitional B cells upon maturation from T1 to T2 and decreasing IgM expression. The T2 populations defined by these different murine classification schemes are likely distinct, with the CD21high/CD23+ T2 population of Loder et al. recently postulated to be a marginal zone precursor (10). This is interesting as other data indicates that B cells transferred into lymphopenic murine hosts, a situation possibly recapitulated after B cell depletion therapy, undergo rapid activation and differentiation into cells phenotypically indistinguishable from purported MZ precursors and MZ B cells (10, 28). Our detailed characterization of human spleen defines for the first time an analogous MZ precursor population, although notably MZ B cells are not expanded in the peripheral blood following BCD or in the context of the peripheral blood lymphopenia associated with SLE (29).
As with the human T3 population characterized for the first time here, murine T3 cells would be included within the mature naïve compartment by conventional gating strategies. Their presence in human peripheral blood is revealed here by the inability to efficiently extrude R123/mitotracker, a higher CD24 and IgM expression, and a lower CD23 expression compared to conventional mature naïve B cells, functional immaturity based on increased susceptibility to apoptosis, and a dramatic expansion during immunologic reconstitution after B cell depletion therapy. The delineation of a T3 population in humans is an important departure from recent studies of human transitional B cells, where it has been suggested that human B cells may comprise only a single transitional stage or contiguous T1 and T2 stages. Regardless, it is clear that further identification of markers differentially expressed by subsets of human transitional B cells would greatly facilitate their resolvability. An additional layer of complexity has recently been proposed by Cambier and colleagues. Their results demonstrate that non-transgenic mature naïve B cells can develop into anergic cells with a T3 phenotype in response to chronic antigenic stimulation (12). Along these lines is important to bear in mind that activated naïve B cells may also lose expression of the ABCB1 transporter and become unable to extrude rhodamine, although this generally parallels the acquisition of CD27 expression (19). Hence, one possible alternative explanation for our results would be to postulate that peripheral blood B cells with a T3 phenotype represent mature anergic cells that have developed in response to repeated antigenic stimulation presumably by self antigens. These interpretations are not mutually exclusive as it is possible that T3 cells could indeed contain a fraction of mature or immature anergic B cells. However, we believe that the dominance of a T3 phenotype within cord blood as well as the ability of these cells to differentiate to mature naïve in vitro makes it unlikely that the majority of these cells would represent anergized mature cells rather than transitional cells.
The discrimination of multiple human transitional B cells is meaningful in that it provides important insight into the developmental process followed by human B cells. Thus, it should be noted that in mice immature T1 cells home to the spleen which has been purported be the main or only environment for transitional cells differentiation (9). On the other hand, Lindsley et al. have recently described a later murine transitional B cell subset that develops in the BM in parallel with peripheral splenic T2 development (30). The detection of abundant T2 and later transitional B cells in human peripheral blood post-rituximab indicates either substantially increased differentiation in and release from the bone marrow or greatly increased generation in and recirculation from the spleen. Based on the fact that in normal subjects the bone marrow contains mainly the T1 subset of transitional cells and the spleen is enriched for later T3 subsets and contains activated T1/T2 cells, we would suggest increased BM generation of T1 cells and differentiation in the spleen with subsequent recirculation in the peripheral blood. Extra-splenic maturation of B cells may also take place given the expansion of T2 and T3 subsets in cord blood as well. Of note, the finding of late transitional B cells in human spleen and cord blood has been documented here for the first time.
The factors that govern the survival and selection of human transitional cells are important to further delineate, particularly given that such processes shape the mature B cell repertoire, deviations from normal regulation may precipitate autoimmunity, and the potential restoration of proper regulation will determine the long-term outcome of B cell depletion therapy. There has been consensus in the mouse literature that T1 B cells are a primary target of negative selection (4, 31). However, the factors governing selection in the later transitional stages (T2 and T3) in the periphery are more controversial (24). A general principle has existed that mature B cells are activated upon BCR stimulation, whereas the same signals on developing B cells lead to unresponsiveness or cell death. Critically, however, exactly where in the transitional cell spectrum this change in responsiveness occurs is unclear. Although it has been suggested that murine T2 B cells, when compared to T1 B cells, acquire responsiveness to T-cell help signals (32), other data supports a more gradual acquisition of immune competence within the transitional B cell compartment (1). T2 and T3 subsets may also undergo negative selection vs. positive selection depending on the combination of antigen-receptor mediated and non-antigen-receptor mediated signals, the provision of T cell help, and cytokine stimulation (including BAFF) (24). Later transitional B cells may be particularly responsive to BAFF (24) and thus it is interesting to speculate whether the enhanced BAFF characteristic of a B cell depleted state (33) may contribute to the prominent expansion of T3 cells during rituximab induced B cell depletion and reconstitution. Further definition of the signals that mediate survival of human transitional B cells will elucidate our understanding of normal tolerance mechanisms and biological events underlying autoimmunity. Moreover, an improved understanding of the progressive maturation stage of human B cells will greatly enhance our ability to elucidate the precise checkpoints and mechanisms involved in the enforcement of B cell tolerance.
Acknowledgments
We are grateful to Sunil Kemshetti, Emily Cushing, and Jennifer Kelly for assistance with patient recruitment and Eric Milner for critical review of the manuscript. We thank Jonathan Friedberg, MD and Richard Fisher, MD for their collaborative efforts with rituximab treated lymphoma patients. We appreciate Craig Jordan’s help in obtaining cord blood samples and Eun-Hyung Lee’s assistance in obtaining bone marrow and spleen samples.
Supported in part by grants to JHA from NIH K08AR048303, R01AI077674-01A1, the Lupus Foundation of America, the Alliance for Lupus Research, and the Lupus Research Institute; the Lupus Clinical Research Consortium to RJL; and R01 AI049660-01A1 and U19 Autoimmunity Center of Excellence AI56390 to IS.
References
- 1.Chung JB, Silverman M, Monroe JG. Transitional B cells: step by step towards immune competence. Trends in Immunology. 2003;24:342–348. doi: 10.1016/s1471-4906(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 2.Carsetti R, Kohler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su TT, Rawlings DJ. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J Immunol. 2002;168:2101–2110. doi: 10.4049/jimmunol.168.5.2101. [DOI] [PubMed] [Google Scholar]
- 5.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant Autoantibody Production by Early Human B Cell Precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 6.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuss AK, Avery DT, Cannons JL, Yu LJ, Nichols KE, Shaw PJ, Tangye SG. Expansion of Functionally Immature Transitional B Cells Is Associated with Human-Immunodeficient States Characterized by Impaired Humoral Immunity. J Immunol. 2006;176:1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 9.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava B, Quinn WJ, 3rd, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J Exp Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 12.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of Anergic B Cells within a Wild-Type Repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 13.Anolik J, Barnard J, Owen T, Zheng B, Kemshett S, Looney J, Sanz I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis & Rheumatism. 2007;56:3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 14.Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:2377–2386. doi: 10.1002/art.22019. [DOI] [PubMed] [Google Scholar]
- 15.Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E, Kelly J, Milner ECB, Fisher RI, Sanz I. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clinical Immunology. 2007;122:139–145. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis & Rheumatism. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 17.Pascual V, Liu YJ, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. Journal of Experimental Medicine. 1994;180:329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bornhorst J, Bjorgan M, Thoen J, Natvig J, Thompson K. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary sjogren’s syndrome. J Immunology. 2001;167:3610–3618. doi: 10.4049/jimmunol.167.7.3610. [DOI] [PubMed] [Google Scholar]
- 19.Wirth S, Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol. 2005;35:3433–3441. doi: 10.1002/eji.200535364. [DOI] [PubMed] [Google Scholar]
- 20.Loken MR, V, Shah O, Dattilio KL, Civin CI. Flow cytometric analysis of human bone marrow. II. Normal B lymphocyte development. Blood. 1987;70:1316–1324. [PubMed] [Google Scholar]
- 21.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, Milner ECB, Sanz I. A New Population of Cells Lacking Expression of CD27 Represents a Notable Component of the B Cell Memory Compartment in Systemic Lupus Erythematosus. J Immunol. 2007;178:6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 22.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 23.Dono M, Zupo S, Colombo M, Massara R, Gaidano G, Taborelli G, Ceppa P, Burgio VL, Chiorazzi N, Ferrarini M. The human marginal zone B cell. Ann N Y Acad Sci. 2003;987:117–124. doi: 10.1111/j.1749-6632.2003.tb06039.x. [DOI] [PubMed] [Google Scholar]
- 24.Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med. 2008;205:155–168. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malaspina A, Moir S, Ho J, Wang W, Howell ML, O’Shea MA, Roby GA, Rehm CA, Mican JM, Chun TW, Fauci AS. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci U S A. 2006;103:2262–2267. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, Seganti G, Zuntini R, Ferrari S, Cagliuso M, Quinti I, Carsetti R. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 28.Cabatingan MS, Schmidt MR, Sen R, Woodland RT. Naive B lymphocytes undergo homeostatic proliferation in response to B cell deficit. J Immunol. 2002;169:6795–6805. doi: 10.4049/jimmunol.169.12.6795. [DOI] [PubMed] [Google Scholar]
- 29.Sanz I, Wei C, Lee FEH, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Seminars in Immunology. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 31.Carsetti R, Kohler G, Lamers M. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181:2129. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung JB, Sater R, Fields ML, Erikson J, Monroe JG. CD23 defines two distinct subsets of immature B cells which differ in their responses to T cell help signals. Int Immunol. 2002;14:157–166. doi: 10.1093/intimm/14.2.157. [DOI] [PubMed] [Google Scholar]
- 33.Lavie F, Miceli-Richard C, Ittah M, Sellam J, Gottenberg JE, Mariette X. Increase of B cell-activating factor of the TNF family (BAFF) after rituximab treatment: insights into a new regulating system of BAFF production. Ann Rheum Dis. 2007;66:700–703. doi: 10.1136/ard.2006.060772. [DOI] [PMC free article] [PubMed] [Google Scholar]