Abstract
Successful tissue regeneration requires that biomaterials have optimal bioactivity and mechanical properties. Heparin-containing hydrogels that can be crosslinked in situ were designed to contain tunable amounts of biological components (e.g. heparin, arginine–glycine–aspartate (RGD)) as well as to exhibit controlled mechanical properties (e.g. shear modulus). These gel parameters can also be tuned to provide controlled delivery of proteins, such as growth factors, for regulating cellular behavior. Maleimide-functionalized low-molecular-weight heparin (LWMH) was conjugated to a poly(ethylene glycol) (PEG) hydrogel. The elastic shear modulus, as assessed via oscillatory rheology experiments, could be tuned by the concentration of polymer in the hydrogel, and by the end group functionality of PEG. Hydrogels of two different moduli (2.8 and 0.4 kPa) were used to study differences in the response of human aortic adventitial fibroblasts (AoAF) in two-dimensional cell culture experiments. These experiments indicated that the AoAFs show improved adhesion to materials with the higher modulus. Evaluation of cell responses to hydrogels with RGD linked to the hydrogels via conjugation to PEG or to LMWH indicated improved cellular responses to these materials when the bioactive ligands were chemically attached through linkage to the PEG rather than to the LMWH. These results highlight important design considerations in the tailoring of these materials for cardiovascular tissue engineering applications.
Keywords: Cell binding, Heparin, Cell response, Hydrogel
1. Introduction
Controlling cell-mediated remodeling of cardiovascular implants using biomaterials is a concept with great appeal. Biological vessels often become occluded due to maladaptive cellular responses elicited by the tissue injury and hemodynamic changes associated with surgical or catheter-based procedures [1]. Autologous vein grafts used in leg artery bypass surgery, for example, fail in the first few years at a rate approaching 50% primarily due to inappropriate graft remodeling [2]. The development of biomaterials that beneficially influence cell responses and vessel remodeling would have significant clinical impact.
Blood vessel remodeling depends to a great degree on the activation of adventitial fibroblasts (AFs) [3,4], which populate the outermost layer of blood vessels. AFs are the major cell type in the adventitia and critical contributors to the structural integrity, growth and remodeling of blood vessels in vivo [4–6]. AFs not only play a significant role in extracellular matrix production during adventitial remodeling but also help recruit and organize the microvascular blood supply necessary to feed the cells within the vessel [7]. The remodeling and organizing functions of AFs are largely determined by physical and chemical cues from the extracellular matrix, and AFs undergo phenotypic conversion in response to changes in their local physical and humoral environment [6,8]. Biomaterials that can be placed easily within or immediately surrounding the adventitia of at-risk blood vessels and that are capable of controlling AF function would be useful in attenuating maladaptive vessel remodeling and encouraging the recruitment of microvasculature to improve graft survival.
Hydrogels offer unique opportunities to control the distribution and function of cells in engineered or biological tissues, and several characteristics recommend advanced hydrogels for cardiovascular applications. Hydrogel components are injectable, and gels can be formed rapidly in situ using catheter-based injection methods applied to the target blood vessel at the time of the clinical procedure or subsequently. Since the elastic shear modulus of hydrogels can be easily controlled, gels of relatively low modulus could be designed to optimize cellular responses without adversely affecting intrinsic vessel stiffness. Cellular components (e.g. AFs and microvascular endothelial cells) could be readily encapsulated within gel matrices to help drive the formation of healthy adventitia and would remain localized at the therapeutic target site during the gel erosion process. Precisely designed hydrogels with controlled local mechanical properties and the inclusion of critical growth factors could direct formation of the target vessel’s vascular supply (i.e. vaso vasorum) while controlling maladaptive remodeling. Thus, understanding how the chemical and physical characteristics of hydrogels influence AFs is an important goal in the development of hydrogels for cardiovascular applications.
It has been well documented that variations in the mechanical properties of matrices result in significant differences in cell behavior [9]. Matrix stiffness affects cell adhesion, proliferation, and differentiation [10,11]. The actin-myosin cytoskeleton, whose contractile forces are transmitted through transcellular structures, may play an important role in transferring information about the mechanical properties of matrices to cell nuclei. In previous work, primate aortic AFs (AoAFs) and smooth muscle cells (SMCs) were found to mediate matrix contraction of collagen-hyaluronan (HA) gels. In particular, the results showed that HA-promoted contraction of collagen gels by AFs and SMCs leading to changes in collagen organization and cell shape [12].
The further development of the above and other hydrogel materials, which have water content and mechanical properties that are often comparable to that of soft tissue, has remained an area of great research interest owing to their potential clinical application in drug delivery, tissue augmentation, and tissue repair and regeneration [9,13]. Strategies to integrate synthetic and biomolecular materials into delivery vehicles have remained an important goal. The cell-adhesive character of hydrogels can be readily manipulated via the inclusion of integrin-binding ligands, such as arginine–glycine–aspartate (RGD). It is well recognized that the presentation of RGD alone, within intact cell-adhesion proteins or within matrices containing multiple adhesion sequences, can alter the effectiveness of RGD-binding by cells [14–17]. The evaluation of these events in a given material of interest is necessary for implementation. The use of polysaccharides, particularly glycosaminoglycans such as heparin, offers opportunities to tailor the hydrophilicity of the gel, to modulate mechanical properties via noncovalent interactions with proteins and peptides, and to sequester and protect growth factors [18,19]. Accordingly, heparin has been incorporated covalently into numerous diverse drug delivery vehicles in which its interactions with proteins have permitted the controlled release of growth factors [20–22]. Our group and others have been investigating the noncovalent assembly, in addition to or instead of covalent crosslinking, of heparinized materials as a route to responsive, reversible and injectable drug delivery systems, with main interests in protein delivery and the production of extracellular matrix (ECM)-mimetic materials [19,23–26]. We have demonstrated multiple methods for producing noncovalently assembled hydrogel materials that are capable of either passive [19,24,27,28] or targeted delivery of growth factors in response to the VEGFR-2 receptor [25], and have developed covalent strategies to expand the versatility of these materials [19]. We have investigated methods to reliably functionalize heparin with chemically reactive groups at controlled degrees of substitution, and have studied the rapid in situ crosslinking between maleimide-functionalized heparin with thiol-functionalized poly(ethylene glycol) (PEG)-based polymers of various molecular weights and structures to produce hydrogels with controlled growth factor delivery profiles [19].
In our work here, we report modifications of hydrogel synthesis to increase its compositional flexibility, to reduce the necessary chemical functionalization of heparin, to permit secondary noncovalent crosslinking by interactions with heparin-binding molecules, to allow for growth factor sequestration and delivery, and to render the resulting hydrogels more cell-adhesive for potential use in cardiovascular applications. Our current approaches utilize hydrogels comprising mainly star PEGs (both maleimide-and thiol-functionalized); such strategies permit increased independence in the tuning of modulus and composition of the hydrogels. A variety of hydrogels have been formed with the star PEGs and maleimide-functionalized heparin, thiol-functionalized RGD peptide and/or fibronectin (FN); presumably via reaction of thiol groups [10]). Oscillatory rheology indicates the flexibility in tuning of the hydrogel elastic shear modulus. The development of these new functionalization strategies also broadens the flexibility of the hydrogel synthesis and provides facile routes to tune the cell-adhesion to the hydrogels; these materials can be applied as substrates for endothelial cells and fibroblasts in the design of therapeutic materials in cardiovascular applications. In particular, here we show that human aortic adventitial fibroblasts (AoAFs) exhibit altered adhesion and cell spreading in response to changes in the chemical and mechanical formulation of the hydrogel substrata. Our data suggest straightforward strategies to tune these materials to obtain desired cellular responses.
2. Materials and methods
2.1. Design of the hydrogel
Four-arm maleimide-functionalized PEG (f = 4, Mn 10,000 g mol−1; JenKem Technology USA Inc., Allen, TX), and four-arm thiol-functionalized PEG (f = 4, Mn 10,000 g mol−1; Creative PEGWorks, Winston-Salem, NC) were employed to form gel networks with appropriately modified heparins [19]. Maleimide-functionalized heparin (f=2, average Mw 3,000 g mol−1) was used to react with thiol-functionalized PEG. The thiol-functionalized peptide, AcGCGYRGDSPG, was prepared for reaction with maleimide-functionalized heparin or maleimide-functionalized PEG. FN was also incorporated into hydrogels via reaction with maleimide-functionalized PEG to provide cell-adhesive materials.
2.2. Material synthesis
2.2.1. Synthesis of maleimide-functionalized LMWH
The synthesis of maleimide-functionalized heparin has been described previously [19]. Briefly, low-molecular-weight heparin (LMWH, Mw 3,000 g mol−1; Celsus, Cincinnati, OH) was reacted with N-(2-aminoethyl)maleimide, trifluoroacetate salt (AEM) in the presence of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl) and 1-hydroxybenzotriazole hydrate (HOBT) dissolved in 2-(N-morpholino)ethanesulfonic acid buffer. The resultant product was dialyzed (MWCO 1000, Spectropor) against 1 M NaCl solution and the water, and lyophilized to yield the resultant product. 1H nuclear magnetic resonance (NMR) characterization of the product indicated a degree of functionalization of 2.3. 1H NMR (400 MHz, D2O):δ = 7.84 (CO–CH=CH–CO, m), δ = 3.90–3.50 (CH2–CH2–NH, m), δ = 5.43–3.38 (heparin, m).
2.2.2. Synthesis of peptide AcGCGYRGDSPG
The desired peptide was prepared via standard Fmoc solid-phase peptide protocols on a PS3 Solid-Phase Peptide Synthesizer (Protein Technologies, Inc., Tucson, AZ). The peptide was cleaved from the resin with TFA/water/TIS/EDT (94:2.5:2.5:1) and precipitated in diethyl ether. Peptide purification was performed via reverse-phase chromatography on a Delta600 high-performance liquid chromatograph (Waters Delta 600, Waters Corporation, Milford, MA) equipped with a preparative Symmetry300 C18 column (Waters; 5μm particle size, 3.9 × 150 mm). The purity of the peptide was generally greater than 80%, as determined by high-performance liquid chromatography. ESI: M+ 1066 (theoretical); 1066 (observed).
2.2.3. PEG–LMWH hydrogel formation
A typical procedure for the preparation of high modulus gels is as follows. The hydrogel was made of 4 wt.% PEG, 0.1 mM LMWH, 0.1 mM RGD-(SH) or 10−4 mM FN. First, PEG-(maleimide)4 (2 mM) was mixed with RGD-(SH) (0.1 mM) or FN (10−4 mM) in Tris buffer (pH 6) for attachment of the bioactive groups to the PEG prior to hydrogel formation. PEG-(SH)4 (2 mM) was mixed with maleimide-functionalized LMWH (0.1 mM) in Tris buffer. These two solutions were then mixed together; changes in viscoelasticity were immediately apparent. Basic fibroblast growth factor (bFGF; 6 ×10−5 mM) was added during gel formation for Cell Titer Blue experiments. The resulting hydrogels were stored at 4 °C overnight prior to further use. They were then swelled for an additional 30 min in medium before cell culture experiments. Hydrogels with two different moduli (2.8 and 0.4 kPa) were prepared by slightly changing the concentration of PEG-(SH)4 (1 and 2 mM, respectively) while keeping the volume of the hydrogels constant between samples. The concentrations of RGD peptide and FN were also kept constant for all of the gels.
2.2.4. PEG hydrogel formation
For PEG hydrogel formation, 2 mM PEG-(maleimide)4, 2 mM PEG-(SH)4 and 0.1 mM RGD-(SH) were mixed together in Tris buffer. The hydrogels were stored and swelled in medium as described above.
2.3. 1H NMR spectroscopy
A Bruker DRX-400 NMR spectrometer (Bruker Daltonics, Billerica, MA) was used to collect all NMR spectra under standard quantitative conditions. CDCl3 or deuterium oxide was used as the NMR solvent and TMS or DSS as the reference.
2.4. Rheological characterization of hydrogels
Rheology samples were prepared in a similar manner. Hydrogel formation was initiated on the rheometer stage by mixing the solutions of maleimide- and thiol-functionalized PEG. The evolution of elasticity and the time for onset of gelation were determined with the use of an AR-2000 rheometer (TA Instruments, New Castle, DE) at a constant temperature of 25 °C in a constant (0.02%) strain mode. In the time-sweep experiments, the moduli were measured at a constant frequency of 5 rad s−1 for 3 h.
2.5. Cell culture
Human AoAFs (Lonza, East Rutherford, NJ) were cultured at 37 °C in a 5% CO2 atmosphere using Stromal Cell Basal medium with 10% fetal bovine serum and 10 ng ml−1 bFGF as recommended by the supplier unless otherwise noted in the text. Cells were used between passages 5 and 8.
2.6. Cell adhesion experiments
Hydrogels with the compositions described in Section 3.1 were synthesized according to the methods outlined in Section 2.2. Gels of 12 mm diameter were prepared on glass cover slips and placed in 24-well tissue culture plates. The hydrogels were then incubated in cell culture medium (stromal cell basal medium) for 30 min prior to the introduction of cells. AoAFs were seeded on the surface of the gel materials at a density of 20,000 per gel (i.e. per 1.1 cm2 of nominal surface area) and incubated at 37°C for 8 h prior to analysis via Cell Titer Blue and staining assays. For the control experiment (AoAFs on tissue culture polystyrene (TCPS)), in order to obtain greater similarity in the resulting adherent cell density on all of the materials, a decreased cell number (2000 cells/1.1 cm2 surface area), was seeded on the TCPS.
2.6.1. Staining and image analysis with fluorescence microscopy
Nuclear staining by ethidium homodimer (EtHD) was used to determine the degree of cell death/necrosis. Briefly, prior to fixation, cultures were stained with EtHD (red fluorescence), which accumulates in the DNA of dead cells. Samples were then rinsed to remove unbound EtHD, fixed with 2% paraformaldehyde in PBS and stained to detect nuclei using bisbenzimide (Hoechst 33258; blue fluorescence). A necrosis index was calculated as the proportion of EtHD-positive nuclei per total nuclei present. To estimate the degree of cell spreading, filamentous actin was visualized using Alexa-488 conjugated phalloidin (green fluorescence) and the area of phalloidin staining per cell was determined. Images of stained samples were acquired using an Evolution QEi, 12-bit, cooled CCD camera (Media Cybernetics, Silver Spring, MD) mounted on an Olympus model BX-60 epifluorescence microscope operated using Image Pro Plus software (Media Cybernetics).
2.6.2. Cell Titer Blue assay
Adhesion was determined based on the accumulation of viable cells over the first 8 h of culture using Cell Titer Blue assays (Promega, Madison, WI) to estimate the cell number. Cell Titer Blue assays are based on the reduction of resazurin to resorufin by living cells. An adhesion index was calculated based on resorufin fluorescence normalized to that found in control TCPS-based cultures and expressed as a percent.
2.7. Data analysis
Statistical analysis was performed using one-way analysis of variance with Tukey’s HSD post hoc tests; p values less than 0.05 were considered statistically significant.
3. Results and discussion
3.1. Hydrogel design
In this work we have continued to focus on PEG-based hydrogels owing to their hydrophilicity, biocompatibility and chemical flexibility. Heparins were employed as a component in our materials owing to their ability to sequester growth factors and the opportunities to modify the mechanical properties of the hydrogel networks via noncovalent interactions that are responsive to the biological environment [19]. In the present studies, we also included either an RGD-containing peptide (AcGCGYRGDSPG) or FN to promote cell adhesion to the surfaces of these materials. The choice of the AcGCGYRGDSPG peptide was motivated by the observation that RGDSP peptides promote cell adhesion via the α5β1 integrin, thereby playing an important role in vascularization [29]. In addition, this sequence has also been shown to bind multiple types of endothelial cells, smooth muscle cells and cardiac fibroblasts [30], indicating its general use as an adhesive domain. The inclusion of integrin-binding domains should also increase the effectiveness of any immobilized growth factors during application, given the anchorage-increased sensitivity of cells to growth factors [29].
In contrast to our previous studies, in which we employed HMWH (functionalized with multiple maleimide groups) and linear PEGs in the formation of covalently crosslinked PEG–HWMH hydrogels [19], we employed maleimide-functionalized low-molecular-weight heparin (LMWH) in these investigations. The use of the four-arm star PEGs in these hydrogels permitted the facile incorporation of LMWH, minimized the extent of chemical modification of the LMWH and expanded our ability to tune the amount of LMWH in the resulting hydrogels. We employed star PEGs with termini carrying either maleimide or thiol groups as the main component in these materials in order to permit rapid crosslinking of the matrix, with the concomitant incorporation of the bioactive LMWH. This chemical strategy also permitted the incorporation of peptides in proximity to either the PEG or LMWH chains, and allowed us to investigate the impact of this change on the response of cells to these materials. The utility of these hydrogels in a variety of potential applications would be facilitated by the rapid crosslinking of the thiol–maleimide reaction, which would enable facile three-dimensional (3-D) encapsulation of cells and the injection of in-situ forming hydrogels. Indeed, preliminary data from related PEG-HMWH hydrogels (not shown) showed that human umbilical vein endothelial cells (HUVECs) can be readily encapsulated with excellent cell viability and 3-D distribution (with no cell precipitation).
As illustrated in Fig. 1, several different hydrogel compositions were designed to study the effects of heparin, RGD–peptide conjugation to the gel, and RGD vs. FN incorporation on cell adhesion. The effects of heparin on cell adhesion to these materials were studied by comparison of AoAF response to a PEG–RGD-based hydrogel (in which the RGD-containing peptide was conjugated to the star PEG prior to gelation), and their response to PEG(RGD)–LMWH gels (in which the RGD-containing peptide was conjugated to PEG prior to gelation). To study the effect of the positioning of the RGD-containing peptide (e.g. chemically attached to PEG or to LMWH) on AoAF response, comparisons were made of AoAF responses to PEG(RGD)–LMWH gels vs. responses to PEG–LMWH(RGD) gels (in which the RGD-containing peptide was conjugated to LMWH prior to gelation). Finally, comparisons of the impact of the RGD-containing peptide vs. the impact of FN in the gels were made by comparisons of PEG(RGD)–LMWH gels with PEG(FN)–LMWH gels (in which FN was conjugated to PEG prior to hydrogel formation).
Fig. 1.
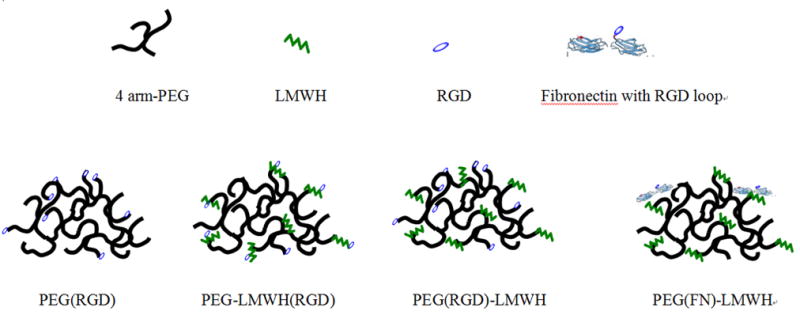
Schematic of hydrogels designed for study of the effects of heparin and RGD position on cell adhesion.
3.2. Hydrogel formation and rheological characterization
As in our earlier studies, the heparin intended for hydrogel formation was modified with maleimide groups via the coupling of N-(2-aminoethyl)maleimide, trifluoroacetate salt (AEM) to carboxylates of the HMWH with activation by EDC and HOBT [19]. The selectivity of the maleimide group near neutral pH for sulfhydryl reagents over amines (~1000-fold difference in reaction rates) [31] offers advantages in the conjugation of maleimide-functionalized heparins to a variety of peptides and polymers. An advantage of these chemical strategies is their rapid reaction times and selectivity, which permits the incorporation of additional peptides and proteins with minimal reaction of free amines on the protein. The efficiency of the reaction also minimizes the presence of any toxic unreacted functional groups.
The pH conditions employed for the modification of heparin with maleimide groups were optimized to minimize ring-opening of the AEM, as previously described [19]. In contrast to our previous investigations, however, we employed LMWH functionalized with maleimide at low degrees of substitution (f = 2). This LMWH was then pre-reacted with thiol-functionalized four-arm star PEG, and hydrogel formation was initiated by mixing this molecule with maleimide-functionalized four-arm star PEGs. Thiol-functionalized RGD-containing peptide and FN could also be easily incorporated into the hydrogels by their reaction with maleimide-functionalized PEG prior to hydrogel formation.
We investigated, via oscillatory rheometry, the formation and properties of hydrogels of various compositions. Solutions of LMWH-modified thiol-functionalized star PEG and of RGD- or FN-modified maleimide-functionalized star PEG were co-injected into the plate gap of the rheometer, and both G′ and G″ were measured as a function of time at constant frequency (ω= 5 rad s−1). Hydrogels with elastic moduli ranging from 0.4 to 2.8 kPa formed immediately, as expected (Fig. 1a). Determination of the gel formation kinetics of the materials showed that the gel formed essentially immediately with storage moduli (G′) were greater than loss moduli (G″) (Fig. 1b). Variations in PEG-(SH)4 concentration (1–2 mM) yielded hydrogels with storage moduli, G′, ranging from approximately 0.4 to 2.8 kPa. FN-modified hydrogels of these two different moduli (0.4 or 2.8 kPa) were used to study the impact of variations in hydrogel mechanical properties (see below). The choice of these moduli was motivated by multiple factors. First, the lower moduli were employed owing to their relevance for in situ injection of materials as soft tissue substitutes [32]. Secondly, in previous studies of the adhesion of HUVECs on related HMWH-PEG hydrogels, we observed that hydrogels with a lower storage modulus (~0.4 kPa) exhibited improved cell adhesion over that observed on hydrogels with greater storage moduli (~2.8 kPa) (unpublished observations). Finally, previous reports of differences in the adhesion of human dermal fibroblasts to HA/FN hydrogels with similar moduli suggested that differences in the behavior of AoAFs, relevant to the ultimate uses of these PEG–LMWH materials in cardiovascular applications, might be observed [10].
As shown in Fig. 1b, the pre-loading of the peptide AcGCGYRGDSPG on maleimide-functionalized star PEG decreased the ultimate storage moduli of the RGD-modified hydrogels compared with the FN-containing materials. This is almost certainly because of its comparably high concentration in the hydrogel (e.g. 0.1 mM RGD peptide), which would react with a greater number of termini of the star PEG and reduce the extent of crosslinking in the resulting hydrogel. Nevertheless, the homogeneity and known chemical conjugation mechanism of RGD to these materials motivated our use of this domain. Given the lower activity of RGD in promoting cell adhesion to polysaccharide hydrogel materials [16,33], such an increase in concentration was necessary, and the impact of its incorporation on the mechanical properties of these materials must be considered in hydrogel design. Given that the amount of FN employed in the hydrogels is 1000-fold lower than the concentration of RGD (0.1 vs. 10−4 mM), it is extremely unlikely that the reaction of any FN affected the modulus significantly.
3.3. Adhesion of AoAFs on PEG–LMWH hydrogels
The assessment of early cell – material interactions is complicated by the potential presence of encapsulated but non-adherent cells within 3-D matrices. Accordingly, we employed 2-D cell adhesion assays as an initial and facile assay format to evaluate the responses of AoAFs to the PEG–LMWH materials. Calculated cell areas and images from the adhesion assays (Fig. 3) indicated that the presence of heparin and RGD in combination differentially affected cell adhesion. The cell number data from Cell Titer Blue assays are presented in Fig. 4. The projected area and images for the FN-containing gels of different moduli are given in Fig. 5, with Cell Titer Blue assay results presented in Fig. 6.
Fig. 3.
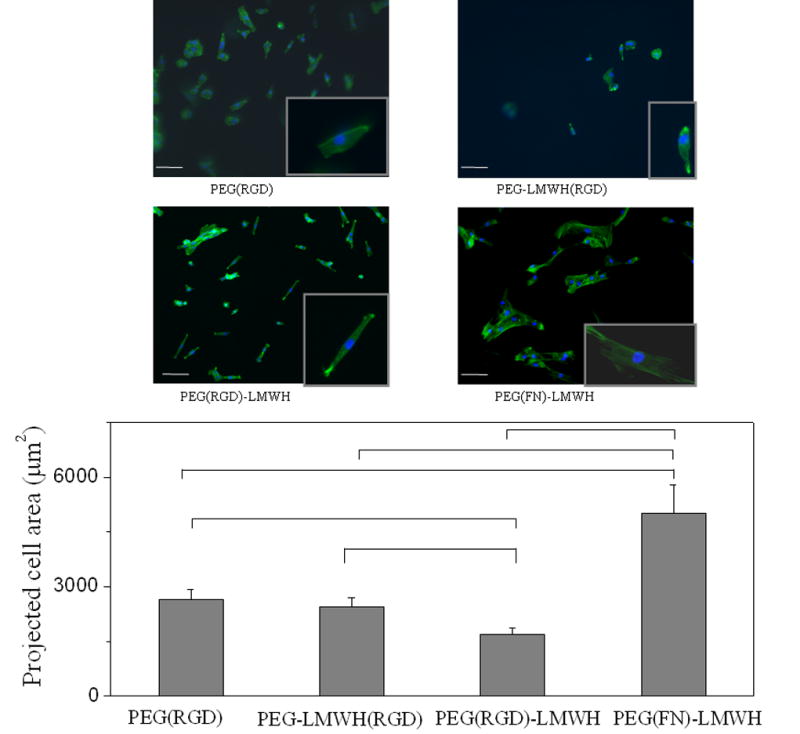
AoAF cell spreading results illustrating the effect of LMWH and RGD on cell adhesion. Scale bar = 100 μm (n = 4, p < 0.05 for indicated hydrogels).
Fig. 4.
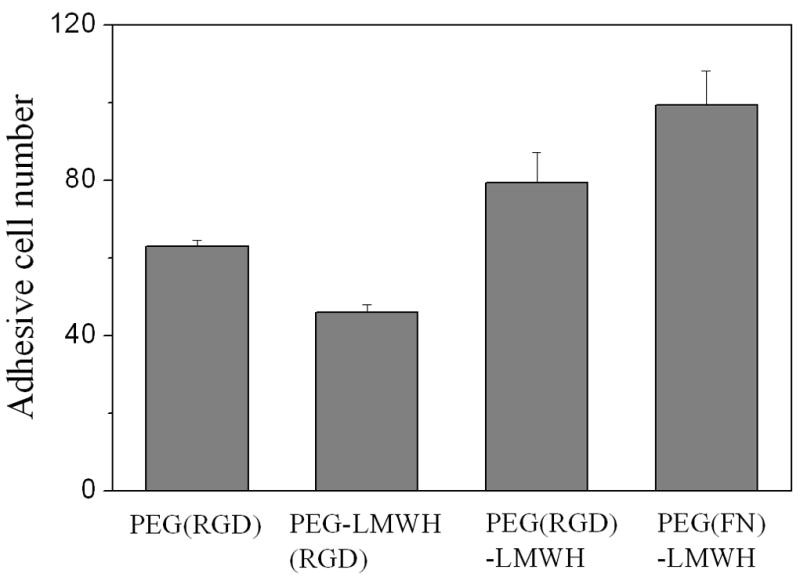
Cell Titer Blue results for AoAF adhesion on hydrogel surfaces in the presence of bFGF. TCPS denotes tissue culture polystyrene. bFGF (6 × 10−5 mM) was loaded in gels during gel formation (n = 4, p < 0.05).
Fig. 5.
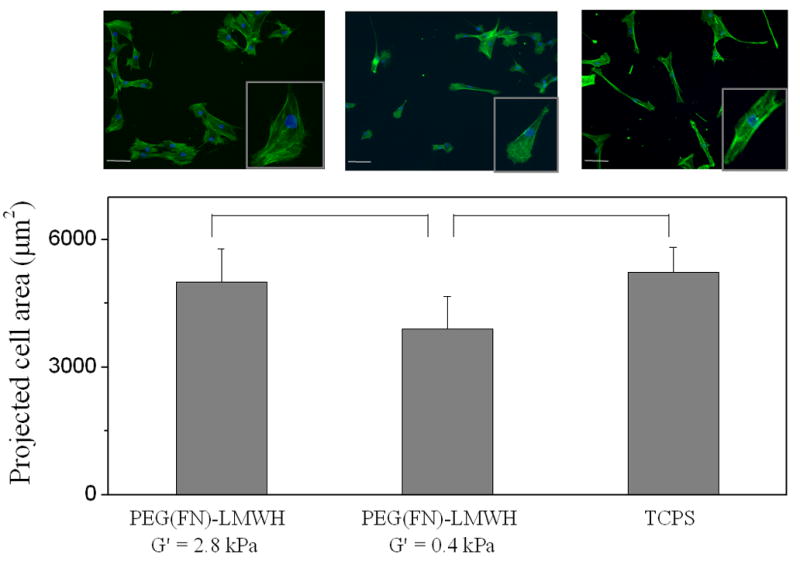
AoAF cell spreading on PEG(FN)–LMWH hydrogels with moduli of 0.4 and 2.8 kPa. Scale bar = 100 μm (n = 4, p < 0.05 for indicated hydrogels).
Fig. 6.
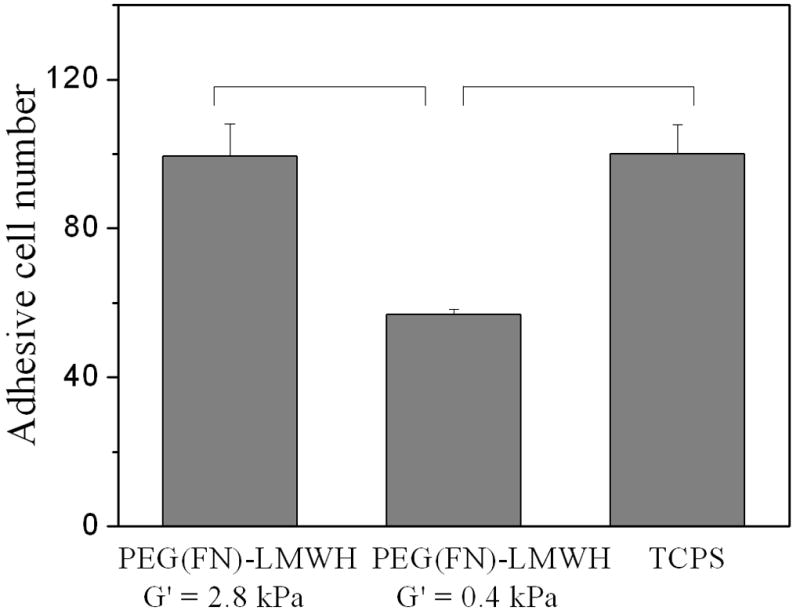
Cell Titer Blue results for AoAF adhesion of AoAFs on PEG(FN)–LMWH hydrogels with moduli of 0.4 and 2.8 kPa. bFGF (6 × 10−5 mM) was loaded in gels (n = 4, p < 0.05 between the gels of different moduli).
3.3.1. Effect of heparin
The comparisons of the PEG(RGD), PEG–LMWH(RGD), PEG(RGD)–LMWH and PEG(FN)–LMWH gels (G′ ~ 2.8kPa) in Fig. 3 illustrate the impact of heparin on the adhesion of AoAFs to these materials. As shown in the figure, the greatest number of adherent cells are observed on the PEG(RGD) hydrogels. These gels also show the greatest projected cell area (~2800 μm2). In contrast, the PEG–LMWH(RGD) and (PEG(RGD)–LMWH gels, which contain 0.1 mM heparin, show fewer cells or lower projected cell areas, indicating that the heparin interferes with the adhesion of AoAFs on these surfaces. Although the adherent cell number is similar on the PEG(RGD) and PEG(RGD)–LMWH gels, the greatest spreading areas are observed on the PEG(RGD) gels, indicating improvements in the response of the cells to the PEG(RGD) vs. the PEG(RGD)–LMWH (~2500 and ~1800 μm2 respectively); the cells adhering to the PEG(RGD)–LMWH gels adopted a spindle-like shape that produced a lower projected area. A similar cell shape has also been observed for embryonic fibroblasts grown in collagen gels [34]. Although it is difficult to make conclusions about cell adhesion solely on the basis of cell morphology or to discern whether the shape of cells on different hydrogels is due directly to cell–matrix interactions or secondarily to alterations in cell function induced by the materials, the matrix binding, shape and function are inter-related cellular properties [35–37], and the specific physical–chemical properties of different hydrogels could be expected to affect all three parameters. The results also suggest that the LMWH in these gels does not significantly bind free adhesive proteins in medium. If so, the presence of LMWH should aid cell binding, based on our results in which FN is included at low concentrations (and cell adhesion is improved). In contrast, the inclusion of heparin in the gels in the absence of FN reduces cell binding.
The observed effects of heparin in our gels are perhaps not unexpected, as previous studies have shown that glycosaminoglycans (GAGs) can inhibit some cell-substratum adhesions [38,39] and that GAG chains such as hyaluronic acid and heparin are usually cell non-adhesive molecules [16,40]. Indeed, in other heparinized materials with higher heparin concentrations (about 10 mM, compared to the 0.1 mM in our work) the incorporation of fibrinogen was necessary to support cell adhesion and proliferation [41]. Furthermore, it has been shown that heparin can interfere with the binding of fibroblasts to ECM proteins [42] and block growth stimulatory signals in SMCs [43]; thus, heparin in the hydrogels may modulate cell adhesion and proliferation via similar mechanisms. Finally, it is also possible that the heparin in the hydrogels sequestered cytokines necessary for robust cell adhesion. Although our investigations do not provide sufficient evidence to determine whether bulk negative charge and/or specific interactions between heparin and AoAFs are the cause of our observations, they point to the need for evaluation of the impact of heparin and its possible origins on specific cell types during the development of these gels for specific applications.
The impact of heparin on cell adhesion in gels preloaded with basic fibroblast growth factor was also tested. bFGF is a well-known mediator of cell proliferation and AF cell migration [44], and has been associated with vascular remodeling in response to changes in arterial blood flow [45]. The infusion of bFGF onto the adventitia of rat common carotid artery increases the capillary density of the vaso vasorum [46], and bFGF has been shown to stabilize fibroblasts and inhibit the transition to a myofibroblastic phenotype. Thus, bFGF is a potentially useful mediator of AF activity in remodeling blood vessels. In these experiments, bFGF was loaded into the hydrogels during gel formation (see Materials and methods), and may therefore be at least partially covalently coupled to the hydrogels [22].
With the bFGF incorporation, a significantly larger number of AoAFs adhered to all the hydrogel compositions (while the cell spreading area did not significantly change), making Cell Titer Blue assays, which present a more quantitative picture of cell adhesion than microscopy images, possible. The Cell Titer Blue assay results illustrated the differences in the numbers of attached cells, which provided additional evidence about the impact of gel composition on cell adhesion. Fig. 4 presents cell adhesion data for the PEG(RGD), PEG–LMWH(RGD) and PEG(RGD)–LMWH gels. Although the PEG–LMWH(RGD) gels still showed less cell adhesion than the PEG(RGD) gels, the PEG(RGD)–LMWH gel showed greater cell adhesion than did the PEG(RGD) gel. These results illustrate that the negative impact of LMWH on cell response can be attenuated in the presence of growth factor with proper presentation of cell-adhesive domains, suggesting the opportunity for these materials when formulated with growth factors (GFs).
Given that LMWH (0.1 mM) is in large excess relative to either bFGF (6 × 10−5 mM) or FN (10−4 mM), bFGFs loaded in the gels and the GFs from the medium would be expected to sequester in the hydrogels, where they would stimulate cell activity and thus aid in cell adhesion [47], perhaps overcoming any negative impact of the inclusion of heparin in the hydrogel. Previous work on human microvascular endothelial cells and primary endothelial progenitor cells, seeded onto disks of either control or bFGF-treated, heparin-coated, decellularized vascular tissue, demonstrated increased numbers of cells for bFGF-treated samples [48]. Indeed, a positive impact of heparin on cell response in the presence of growth factors, and also on the effectiveness of the growth factors (which is well accepted), has been demonstrated recently for human mesenchymal stem cells encapsulated in bFGF-containing PEG-heparin gels [21], although in that work lower heparin concentrations (0.01–0.02 mM compared with 0.1 mM in our work) were employed in the PEG gels, with higher bFGF concentration (750 ng ml−1 compared with 100 ng ml−1 in our work). In addition, it may be possible that there is local blocking of heparin by the bFGF that improves cell adhesion, but that effect is likely not significant, given that the bFGF is loaded in the gel at a 10,000-fold lower concentration than LMWH. In addition, given the transient nature of the binding of bFGF to LMWH in the gel, the bFGF should have significant mobility, and thus would not permanently block the heparin in any given site. Moreover, the size of a cell is several thousand-fold larger than the potential bFGF “sites”, so the net effect of any bFGF in ameliorating the negative effects of heparin is not likely to be significant on the cellular length scale. Thus, it is most likely that the improved adhesion of the cells in the presence of bFGF alters their response to the PEG–LMWH gels. These results further illustrate the need for evaluation of the responses of specific cell types with specific gel compositions.
3.3.2. Effect of cell adhesion molecules
Previous studies have indicated the importance of the position of the RGD peptide, a major integrin binding site in FN, in promoting cell adhesion [17], and RGD-functionalized PEG gels can enhance cell attachment, differentiation, proliferation and migration [49,50]. We thus anticipated that the attachment of RGD to either the PEG chain or the LMWH chain may impact cell adhesion to our PEG–LMWH materials. The images and projected areas in Fig. 3 illustrate the impact of differences in the conjugation of RGD on the response of AoAFs to these materials. Specifically, comparison of the cell adhesion on the PEG–LMWH(RGD) gels, relative to that on the PEG(RGD)–LMWH gels, indicates differences in cell adhesion. In the case where the RGD-containing peptide is attached directly to the LMWH chain, only a few cells attach and spread on the substrate. The number of adherent cells is significantly greater when the RGD–peptide is conjugated directly to the PEG chain, although, as mentioned above, the bipolar-spindle morphology of these cells differs from that seen on the other hydrogels evaluated in the present study. Furthermore, as shown in Fig. 4, the PEG–LMWH(RGD) gel does not show increased adhesion in the presence of bFGF, where increased cell attachment is observed in the PEG(RGD)–LMWH gels. There are three possible explanations for the effect of RGD placement on cell adhesion to these materials. First, the physical interaction between the RGD in the material and the cellular integrin may be blocked by the nearby heparin. Secondly, since LMWH–RGD interactions are determined by the conformation of the RGD peptide [51], conformational constraints imparted by the LMWH–RGD linkage or by the charged environment surrounding the heparin may account for decreased integrin binding to the LMWH–RGD. Thirdly, heparin may modulate the RGD–integrin interaction; studies with unfractionated heparin have shown that heparin can interact directly with integrins to alter cell adhesion [52,53]. Although LMWH was found to be less potent in this regard, the close proximity of the heparin in our materials may force a negative interaction with integrins attempting to partner with the neighboring RGD.
Finally, comparisons of the responses of AoAFs to the RGD-containing gels relative to those to the FN-containing gels (Fig. 3) clearly illustrate that FN significantly increased cell adhesion, even though the synthetic RGD peptide is present at a 1000-fold greater molar concentration than the native RGD domains of FN in the gels. While the moduli of these hydrogels are slightly different, our data indicate that the effect of FN is more significant than the effect of the modulus, as AoAFs showed better adhesion, as assessed via projected cell area, on the low modulus FN-containing hydrogel (0.4 kPa; Fig. 6 below) than on a higher modulus RGD-containing hydrogel (1.8 kPa; Fig. 3), and the best adhesion was observed for the high modulus, FN-containing hydrogel. These observations are most likely due to the fact that the RGD motifs reside on the FN chain, which places them further away from the heparin, as well as the fact that the FN chains carry heparin-binding and other domains that improve cell adhesion and sequestration of additional cytokines [54,55].
3.3.3. Effect of hydrogel moduli
The adhesion of AoAFs was evaluated on PEG(FN)–LMWH gels of two different moduli, 0.4 and 2.8 kPa. The resulting data are presented in Figs. 5 and 6. As shown in Fig. 5, the average projected area of AoAFs on hydrogels with higher moduli was greater than those on hydrogels of lower moduli, suggesting a higher degree of adhesion. This is consistent with the number of cells counted on each hydrogel, shown in Fig. 6, which is also higher on the higher modulus gels; it is interesting to note that the cell adhesion to the high modulus gels in the presence of bFGF is very similar to that observed for the TCPS (identical numbers of cells were seeded on the two different surfaces in these experiments). These results are similar to those found for adult human dermal fibroblasts (ADHF) on hyaluronan hydrogels of similar modulus [10], suggesting the importance of mechanical cues in the adhesion of fibroblasts to polysaccharide-containing hydrogel matrices. Our ongoing studies have also preliminarily shown that different cell types (fibroblasts (AoAF), endothelial cells (HUVEC) and smooth muscle cells (T/G HA-VSMC)) behave differently on the surfaces of PEG–LMWH hydrogels with identical biochemical composition but different mechanical properties in the range of those described here. Interestingly, although the elastic shear modulus of blood vessels differs markedly from that of the described hydrogels, applications designed to alter the remodeling of existing vessels may benefit from a material that does not interfere with the extant mechanical characteristics of the target blood vessel but rather functions to locally deliver cells and factors. The combination of these studies and previous literature underscores the need for evaluation of specific cell types on materials of interest for specific applications; such studies are particularly needed for investigations aimed at generating physiologically relevant phenotypes of cells in complex multicomponent and multicellular devices.
4. Conclusions
These studies illustrate the versatility and flexibility of thiol–maleimide chemistry in the design and production of cell-adhesive, heparinized hydrogels for potential use in controlling adventitial remodeling of blood vessels. The use of four-arm star PEGs with appropriate chemical reactivity has permitted assessments of the impact of heparin, RGD position and hydrogel modulus on the adhesion of AoAFs to a set of PEG–LMWH-based, covalently cross-linked materials. Our studies highlight the need to optimize the biochemical and mechanical properties of the matrix for a given cell type, and suggest that such tailoring is possible via simple chemical strategies that allow the composition of the matrix to be controlled; for the PEG–LMWH gels here, the inclusion of FN and bFGF maximized cell adhesion under the conditions investigated. Coupled with our additional observations indicating differences in responses of cardiovascular cell types based on differences in moduli of these materials, our studies also suggest that the requirements of multiple cardiovascular cell types colocalized in tissue replacement scaffolds may differ significantly, and that composite materials with spatially resolved mechanical properties may be necessary for the application of these heparinized materials.
Fig. 2.
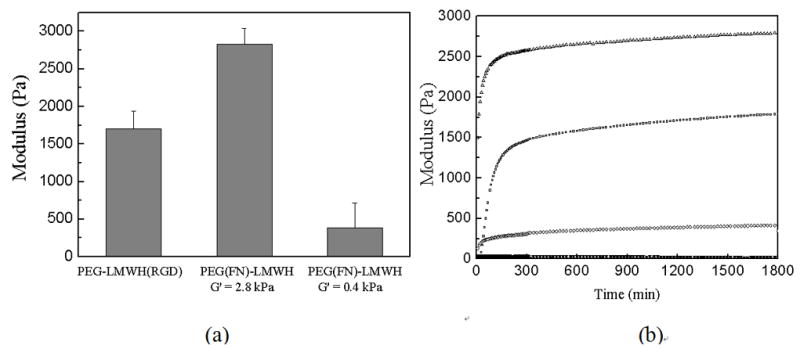
Oscillatory rheology results illustrating (a) typical shear elastic moduli of hydrogels prepared with star PEGs and LMWH; (b) gel formation kinetics, where storage (G′, open symbol) and loss (G″, solid symbol at bottom) moduli are given as a function of gelation time.
Acknowledgments
This work was supported by grants from the National Institutes of Health (1RO1 EB00317201), the National Center for Research at NIH (RPG#1P20-RR020173-01 to REA), and the Nemours Foundation (REA). Terry McLaughlin, Karyn Robinson, Sung Hye Kim, Aaron Baldwin and Danielle N. Rockwood are thanked for their helpful discussions and experimental assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strauss BH, Rabinovitch M. Adventitial fibroblasts: defining a role in vessel wall remodeling. Am J Respir Cell Mol Biol. 2000;22(1):1–3. doi: 10.1165/ajrcmb.22.1.f172. [DOI] [PubMed] [Google Scholar]
- 2.Conte MS. Molecular engineering of vein bypass grafts. J Vasc Surg. 2007;45(6 Supplement 1):A74–A81. doi: 10.1016/j.jvs.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Cai W-J, et al. Remodeling of the adventitia during coronary arteriogenesis. Am J Physiol Heart Circ Physiol. 2003;284(1):H31–40. doi: 10.1152/ajpheart.00478.2002. [DOI] [PubMed] [Google Scholar]
- 4.Sartore S, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89(12):1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 5.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8(11):1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Z. TGF-β- and CTGF-mediated fibroblast recruitment influences early outward vein graft remodeling. Am J Physiol Heart Circ Physiol. 2007;293(1):H482–488. doi: 10.1152/ajpheart.01372.2006. [DOI] [PubMed] [Google Scholar]
- 7.Davie NJ, Gerasimovskaya EV, Hofmeister SE, Richman AP, Jones PL, Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin-1. Am J Pathol. 2006;168(6):1793–807. doi: 10.2353/ajpath.2006.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol Cell Physiol. 2004;286(2):416–425. doi: 10.1152/ajpcell.00169.2003. [DOI] [PubMed] [Google Scholar]
- 9.Kong HJ, Boontheekul T, Mooney DJ. Quantifying the relation between adhesion ligand–receptor bond formation and cell phenotype. Proc Natl Acad Sci. 2006;103(49):18534–18539. doi: 10.1073/pnas.0605960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh K, et al. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28(4):671–679. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong JY, Leach JB, Brown XQ. Balance of chemistry, topography, and mechanics at the cell–biomaterial interface: issues and challenges for assessing the role of substrate mechanics on cell response. Surf Sci. 2004;570(1–2):119–133. [Google Scholar]
- 12.Travis JHMG, Wong JM, Wagner WD, Geary RL. Hyaluronan enhances contraction of collagen by smooth muscle cells and adventitial fibroblasts: role of CD44 and implications for constrictive remodeling. Circ Res. 2001;88(1):77–83. doi: 10.1161/01.res.88.1.77. [DOI] [PubMed] [Google Scholar]
- 13.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 14.Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. Proc Natl Acad Sci. 2002;99(19):12025–12030. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 16.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 17.Salinas CN, Anseth KS. The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J Tissue Eng Regen Med. 2008;2(5):296–304. doi: 10.1002/term.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65(3):389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 19.Nie T, Baldwin A, Yamaguchi N, Kiick KL. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J Control Release. 2007;122(3):287–296. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linhardt RJ, Murugesan S, Xie J. Immobilization of heparin: approaches and applications. Curr Top Med Chem. 2008;8:80–100. doi: 10.2174/156802608783378891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benoit DSW, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005;1(4):461–470. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Cai SS, Liu YC, Shu XZ, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26(30):6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Seal BL, Panitch A. Physical polymer matrices based on affinity interactions between peptides and polysaccharides. Biomacromolecules. 2003;4(6):1572–1582. doi: 10.1021/bm0342032. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi N, Chae BS, Zhang L, Kiick KL, Furst EM. Rheological characterization of polysaccharide–poly(ethylene glycol) star copolymer hydrogels. Biomacromolecules. 2005;6(4):1931–1940. doi: 10.1021/bm0500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi N, Zhang L, Chae BS, Palla CS, Furst EM, Kiick KL. Growth factor mediated assembly of cell receptor-responsive hydrogels. J Am Chem Soc. 2007;129(11):3040–3041. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seal BL, Panitch A. Viscoelastic Behavior of environmentally sensitive biomimetic polymer matrices. Macromolecules. 2006;39(6):2268–2274. [Google Scholar]
- 27.Yamaguchi N, Kiick KL. Polysaccharide–poly(ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules. 2005;6(4):1921–1930. doi: 10.1021/bm050003+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Furst EM, Kiick KL. Manipulation of hydrogel assembly and growth factor delivery via the use of peptide–polysaccharide interactions. J Control Release. 2006;114(2):130–142. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosso F, Marino G, Giordano A, Barbarisi M, Parmeggiani D, Barbarisi A. Smart materials as scaffolds for tissue engineering. J Cell Physiol. 2005;203(3):465–470. doi: 10.1002/jcp.20270. [DOI] [PubMed] [Google Scholar]
- 30.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 31.Hermanson GT. Bioconjugate Techniques. Academic Press; New York: 1996. [Google Scholar]
- 32.Dahl SL, Rhim C, Song YC, Niklason LE. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng. 2007;35(3):348–55. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh K, Ren X-D, Shu XZ, Prestwich GD, Clark RAF. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 2006;12(3):601–613. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- 34.Tomasek JJ, Hay ED, Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: distribution of actin, α-actinin, and myosin. Dev Biol. 1982;92(1):107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- 35.Berrier AL, Yamada KM. Cell–matrix adhesion. J Cell Physiol. 2007;213(3):565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 36.Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol. 2008;97(2–3):163–179. doi: 10.1016/j.pbiomolbio.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20(5):551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutishauser U, Hoffman S, Edelman GM. Binding properties of a cell adhesion molecule from neural tissue. Proc Natl Acad Sci. 1982;79(2):685–689. doi: 10.1073/pnas.79.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole GGL. A heparin-binding domain from N-CAM is involved in neural cell-substratum adhesion. J Cell Biol. 1986;102(2):403–412. doi: 10.1083/jcb.102.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shu XZ, Ghosh K, Liu Y, Palumbo FS, Luo Y, Clark RA, Prestwich GD. Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J Biomed Mater Res A. 2004;68(2):365–375. doi: 10.1002/jbm.a.20002. [DOI] [PubMed] [Google Scholar]
- 41.Tae G, Kim YJ, Choi WI, Kim M, Stayton PS, Hoffman AS. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules. 2007;8(6):1979–1986. doi: 10.1021/bm0701189. [DOI] [PubMed] [Google Scholar]
- 42.San Antonio JD, Lander AD, Wright TC, Karnovsky MJ. Heparin inhibits the attachment and growth of Balb/c-3T3 fibroblasts on collagen substrata. J Cell Physiol. 1992;150:8 –16. doi: 10.1002/jcp.1041500103. [DOI] [PubMed] [Google Scholar]
- 43.Pukac LA, Carter JE, Ottlinger ME, Karnovsky MJ. Mechanisms of inhibition by heparin of PDGF stimulated MAP kinase activation in vascular smooth muscle cells. J Cell Physiol. 1997;172:69–78. doi: 10.1002/(SICI)1097-4652(199707)172:1<69::AID-JCP8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 44.Liu G, Eskin SG, Mikos AG. Integrin αvβ3 is involved in stimulated migration of vascular adventitial fibroblasts by basic fibroblast growth factor but not platelet-derived growth factor. J Cell Biochem. 2001;83(1):129–135. doi: 10.1002/jcb.1208. [DOI] [PubMed] [Google Scholar]
- 45.Bryant SR, Bjercke RJ, Erichsen DA, Rege A, Lindner V. Vascular remodeling in response to altered blood flow is mediated by fibroblast growth factor-2. Circ Res. 1999;84:323–328. doi: 10.1161/01.res.84.3.323. [DOI] [PubMed] [Google Scholar]
- 46.Cuevas P, Gonzalez AM, Carceller F, Baird A. Vascular response to basic fibroblast growth factor when infused onto the normal adventitia or into the injured media of the rat carotid artery. Circ Res. 1991;69:360–369. doi: 10.1161/01.res.69.2.360. [DOI] [PubMed] [Google Scholar]
- 47.Ornitz D, Itoh N. Fibroblast growth factors. Genome Biology. 2001;2(3):3005.3001–3005.3012. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conklin B, Wu H, Lin PH, Lumsden AB, Chen CY. Basic fibroblast growth factor coating and endothelial cell seeding of a decellularized heparin-coated vascular graft. Artif Organs. 2004;28(7):668–675. doi: 10.1111/j.1525-1594.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- 49.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113(10):1677–1686. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 50.Fittkau MH, et al. The selective modulation of endothelial cell mobility on RGD peptide containing surfaces by YIGSR peptides. Biomaterials. 2005;26(2):167–174. doi: 10.1016/j.biomaterials.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Lanza P, Felding-Habermann B, Ruggeri ZM, Zanetti M, Billetta R. Selective interaction of a conformationally-constrained Arg-Gly-Asp (RGD) motif with the Integrin receptor αvβ3 expressed on human tumor Cells. Blood Cells Mol Dis. 1997;23(2):230–241. doi: 10.1006/bcmd.1997.0140. [DOI] [PubMed] [Google Scholar]
- 52.Sobel M, Fish WR, Toma N, Luo S, Bird K, Mori K, Kusumoto S, Blystone SD, Suda Y. Heparin modulates integrin function in human platelets. J Vasc Surg. 2001;33(3):587–594. doi: 10.1067/mva.2001.112696. [DOI] [PubMed] [Google Scholar]
- 53.Da Silva MS, et al. Heparin modulates integrin-mediated cellular adhesion: specificity of interactions with α and β integrin subunits. Cell Commun Adhes. 2003;10(2):59–67. [PubMed] [Google Scholar]
- 54.Obara M, Kang MS, Yamada KM. Site-directed mutagenesis of the cell-binding domain of human fibronectin: separable, synergistic sites mediate adhesive function. Cell. 1988;53(4):649–657. doi: 10.1016/0092-8674(88)90580-6. [DOI] [PubMed] [Google Scholar]
- 55.Koivunen E, Wang B, Dickinson CD, Ruoslahti E. Peptides in cell adhesion research. Methods Enzymol. 1994;245:346–369. doi: 10.1016/0076-6879(94)45019-6. [DOI] [PubMed] [Google Scholar]


