Abstract
The objective of the study was to identify predictors of obesity. One hundred eleven nonobese and 48 obese HIV-1 seropositive patients provided information on medical history and other characteristics. They were then asked to detect the passage of 2-s time intervals while the contingent negative variation (CNV) was recorded. Obese patients were healthier, more likely to be receiving Highly Active Antiretroviral Therapy, and less likely to be substance dependent. Obese patients also exhibited a greater CNV slope and responded prematurely. A path model suggested that CD4+count and protease inhibitor use directly predicted obesity. Depression had no direct effect. However, when incorporated into a hypothetical construct, “mood dysregulation,” that also included childhood conduct problems and stimulant dependence, the shared variance among the indicators did predict obesity. This relationship was mediated through premature response preparation (anterior scalp CNV amplitude) and its hypothesized association with impatience/impulsivity.
Keywords: Obesity, HIV-1, Contingent negative variation, Impulsivity
Loss of weight and lean muscle mass is a common feature of HIV/AIDS (Tang, Jacobson, Spiegelman, Knox, & Wanke, 2005). If severe, it can be a sign of a poor prognosis, including a more rapid progression to death (Shor-Posner et al., 2000; Tang et al., 2002). The risk for severe weight loss and wasting varies with specific patient characteristics. For example, wasting occurs more commonly among patients with higher plasma viral loads and lower CD4+lymphocyte counts than among patients with less severe disease (Batterham, Garsia, & Greenop, 2002; Dworkin & Williamson, 2003; Lyles et al., 1999; Malvy, Thiebaut, Marimoutou, & Dabis, 2001; Wanke et al., 2003; Welch & Morse, 2002). Another vulnerable group consists of patients with comorbid substance use disorders (Campa et al., 2005). Their increased risk may result directly from the anorectic effects of alcohol, cocaine, or nicotine (Chen et al., 2005; Forrester, Tucker, & Gorbach, 2005; Levine, Harris, & Morgan, 2000; Pacy, Preedy, Peters, Read, & Halliday, 1991; Saarni, Silventoinen, Rissanen, Sarlio-Lahteenkorva, & Kaprio, 2004) or indirectly from an effect of substance abuse on disease severity (Bagby, Zhang, Purcell, Didier, & Nelson, 2006; Molina et al., 2006; Poonia et al., 2006; Potula et al., 2006) and/or antiretroviral treatment compliance (Chander, Lau, & Moore, 2006; Hinkin et al., 2007; Mohammed et al., 2004; Pence, Miller, Gaynes, & Eron, 2007; Rodriguez-Arenas et al., 2006; Sharpe, Lee, Nakashima, Elam-Evans, & Fleming, 2004).
Although weight loss remains problematic for many patients, its incidence has declined dramatically in recent years. In the U.S. Multicenter AIDS Cohort Study, for example, the incidence of wasting declined from a peak of 22.1 per 1000 person-years in 1994–1995 to 13.4 in 1996–1999 (Smit et al., 2002). Similarly, Hodgson et al. (2001) reported a 77% decline in the prevalence of wasting as an AIDS-defining event from a survey of 162 HIV/AIDS cases studied between 1992 and 2001 in the United Kingdom. Other surveys have yielded similar results (Dworkin & Williamson, 2003; Mocroft et al., 1999). The declining incidence of wasting coincides with the increased availability and prescription of Highly Active Antiretroviral Therapy (HAART) and is often attributed to that cause. Across multiple studies, protease-inhibitor-based HAART has been associated with many factors that either promote or accompany weight gain, including insulin resistance (Walli et al., 1998), hyperglycemia (Dever, Oruwari, Figueroa, O'Donovan, & Eng, 2000; Tsiodras, Mantzoros, Hammer, & Samore, 2000), new onset Type 2 diabetes mellitus (Justman et al., 2003; Ledergerber et al., 2007), hyperlipidemia (Mulligan et al., 2005; Rimland et al., 2006), lipodystrophy (McDermott et al., 2001), and the metabolic syndrome (Jacobson et al., 2006; Jerico et al., 2005). Indeed, as a result of the success of HAART, health care providers now face an unexpected challenge—managing the growing population of HIV/AIDS patients who are obese (Amorosa et al., 2005; Engelson et al., 2006; Hendricks, Willis, Houser, & Jones, 2006; Karmon et al., 2005; Kruzich, Marquis, Wilson, & Stephensen, 2004; Silva et al., 1998).
As the prevalence of obesity increases among patients with HIV/AIDS, the cumulative risk for cardiovascular disease deriving from combined effects of obesity (Desai et al., 2004; Isozumi, 2004), HIV/AIDS (Cole et al., 2004; Gorczyca, Stanek, Podlasin, Furmanek, & Pniewski, 2005; Tipping, de Villiers, Wainwright, Candy, & Bryer, 2007; Triant, Lee, Hadigan, & Grinspoon, 2007), and antiretroviral treatment (Barbaro & Barbarini, 2006; d'Arminio et al., 2004; Friis-Moller et al., 2007; Vaughn & Detels, 2007) is also likely to increase. More information is therefore needed about obesity predictors in this population such that high-risk cases can be identified. One goal of the present study was to evaluate several candidate predictors of obesity, including disease severity, protease-inhibitor-based HA-ART, and substance abuse. Admittedly, information concerning these variables already appears in the literature. Therefore, the first goal is an attempt to replicate previously reported findings in a diverse sample of patients.
A second and more innovative goal was to incorporate psychological and neurophysiological factors in the analysis. Such factors are typically not acknowledged in studies of obesity among HIV-1 seropositive patients. Yet, they are commonly considered in studies of weight gain in other populations. Psychological factors are obviously key to the motivations that drive eating habits and may interact with other factors, including disease severity, HAART compliance, and substance abuse, to promote obesity as the outcome. Among the most significant psychological factors contributing to obesity is a dysregulation of mood (Cugini et al., 1999; McIntyre, Konarski, Wilkins, Soczynska, & Kennedy, 2006) and behavior, which may be variably manifest in depression or anxiety (Heo, Pietrobelli, Fontaine, Sirey, & Faith, 2006; McIntyre et al., 2006; Moreira, Marca, Appolinario, & Coutinho, 2007), a history of conduct problems (Pine, Cohen, Brook, & Coplan, 1997; Sawyer et al., 2006; Specker, de Zwaan, Raymond, & Mitchell, 1994), and/or substance dependence (Ehlers & Wilhelmsen, 2007; Hill, Shen, Locke Wellman, Rickin, & Lowers, 2005; Levine et al., 2001).
Another hypothetical factor worthy of consideration is an impaired ability of obese patients to await the passage of a fixed interval of time (Braet, Claus, Verbeken, & Van Vlierberghe, 2007; Faulkner & Duecker, 1989; Gardner, Reyes, Brake, & Salaz, 1984; Nederkoorn, Jansen, Mulkens, & Jansen, 2007; Rodin, 1975; Yeomans, Leitch, & Mobini, 2008), that is, impatience. Impatience is relevant to obesity when interpreted as an impaired ability to delay gratification coupled with a heightened reactivity to salient stimuli, for example, rewards (Leon & Roth, 1977; Pliner, 1976; Schachter & Gross, 1968). Yet, it may be a more basic characteristic also expressed in settings where no reward is imminent (i.e., impulsivity; Nasser, Gluck, & Geliebter, 2004; Nederkoorn et al., 2007). Rodin (1975) and Gardner et al. (1984) reasoned that the tendency of obese patients to overestimate time passage is one manifestation of more general impairment in the ability to detect and act upon internal cues (Larsen, van Strien, Eisinga, & Engels, 2006), including cues that signal time passage, satiety, or mood.
The present study was unique in employing a simple time estimation task, similar to that employed by Bauer (2001), to provide an objective measure of patience in non-obese (BMI<30) and obese (BMI ≥ 30) patients. Across 50 trials of the task, patients were asked to press a response key exactly 2 s after the onset of a visual cue. The latency of the key press response provided a behavioral measure of time estimation accuracy. In addition, electroencephalographic (EEG) activity was recorded throughout the time estimation interval to provide a measure of covert preparation: a slowly developing, frontally dominant (Gomez, Flores, & Ledesma, 2007; Rosahl & Knight, 1995) negative voltage known as the Contingent Negative Variation (CNV). The CNV emerges during periods of time in which subjects are anticipating future events of personal or instructed significance (Brunia & van Boxtel, 2001; Elbert, Ulrich, Rockstroh, & Lutzenberger, 1991; Hiraku & Sakuma, 1996; Ruchkin, McCalley, & Glaser, 1977; Timsit-Berthier, 1984). Healthy subjects with accurate time estimation ability have been shown to exhibit CNVs of a smaller amplitude and slower rise time than healthy subjects with poor time estimation ability (Brown, Fenwick, & Howard, 1989; Ladanyi & Dubrovsky, 1985).
The present study evaluated obesity predictors in HIV-1 seropositive patients using two different approaches. The first approach was broad inasmuch as it tested the simple effects of obesity, using t test and χ2 methods, across a long list of potentially relevant dependent measures. The list included demographic characteristics, IQ, medical characteristics, and psychiatric symptoms and disorders. The second approach was more constrained and respectful of the limited statistical power afforded by a sample size of 159 patients. It employed structural equation methods to test multivariate models. It included factors that have previously been associated with obesity (illness severity, protease inhibitor use, nicotine/stimulant dependence, conduct problems) as well as the CNVas an objective neurophysiological indicator of impatience or impulsivity. Unfortunately, some potentially relevant variables (anxiety) were not measured. Others (e.g., age, gender, Bipolar Disorder, Borderline Personality Disorder) could not be included because power was insufficient or the frequency was too low. The models were, therefore, incomplete. Yet, they may prove valuable for informing the direction of future investigations.
Method
Patients
One hundred fifty-nine HIV-1 seropositive patients, aged 25–55 years, were recruited via advertisements posted within outpatient Infectious Disease Clinics in the greater Hartford, Connecticut, region. Interested individuals were invited to telephone a member of the research staff for eligibility screening. The telephone interview included questions about demographic characteristics, general medical status, substance use, and psychiatric symptoms. Individuals who passed the initial telephone screen were invited to visit the Health Center on a subsequent day, during which an IRB-approved consent form and a medical records release were signed. Additional eligibility screening and laboratory evaluations were performed on that day.
Collection of Medical and Psychological History and Clinical Laboratory Data
After completing the informed consent and medical release documents, all patients were asked to provide a blood sample for laboratory confirmation of HIV serostatus. The clinical laboratory evaluation also included CBC with differential, HIV RNA viral load, CD4 lymphocyte count and percent, VDRL, HBV screen, HCV, toxoplasmosis and cytomegalovirus antibody titers, renal and liver function, serum protein, albumin, and G-6-PD. Toxicological analyses for cocaine, opiates, amphetamine, and marijuana were performed on urine samples (Ontrak, Varian Inc., Palo Alto, CA) and a breathalyzer was used to detect recent alcohol use. In addition, an Optec 2000 Vision Tester was used to confirm normal color vision and acuity (with correction).
A highly structured, computer-driven psychiatric interview, namely, the CDIS-IV (American Psychiatric Association, 1994; Robins et al., 2002), used for detecting selected DSM-IV Axis I and II disorders, was then administered by a research assistant formally trained in its administration and with 11 years of relevant experience. Patients also completed questionnaires or brief interviews assessing medical history, medication use, demographics, psychiatric symptoms, alcohol and drug use, and cognitive status. The assessments included the Addiction Severity Index (ASI; McLellan, Luborsky, Woody, & O'Brien, 1980), Michigan Alcoholism Screening Test (MAST; Selzer, 1971), Drug Abuse Screening Test (DAST-10; Skinner, 1982), and Beck Depression Inventory Version II (BDI-II; Beck, Steer, & Brown, 1996). In addition, the Kaufman Brief Intelligence Test (KBIT; Kaufman & Kaufman, 1990) was administered to derive an estimate of IQ.
Exclusion criteria included recent (past year) pregnancy, seizures, mental retardation, dementia, neurosurgery, and a history of head injury with loss of consciousness for greater than 10 min. In addition, participants were required to have no acute illness, an IQ score greater than 70, and no major neurological or medical (i.e., chronic obstructive pulmonary disease, Type 1 or Type 2 diabetes, cirrhosis, hepatic encephalopathy, ocular disorders, etc.) disorders unrelated to HIV-1. Positive urine toxicology or breathalyzer tests or recent (past year) dependence upon alcohol, cocaine, or opiates were also exclusions. However, current use of methadone was not. Subjects were likewise excluded if they met the DSM-IV criterion for a diagnosis of schizophrenia or bipolar disorder. Major depressive disorder was not an exclusion.
Collection of Time Estimation Task Data
For the collection of EEG data during the time estimation task, tin electrodes were applied to 31 scalp sites positioned by an electrode cap (ElectroCap International, Eaton, Ohio). A reference electrode was taped over the bridge of the nose. The ground electrode was applied to the middle of the forehead. Interelectrode impedance was maintained below 5 kΩ.
After electrode application was complete, the subject was escorted into a sound-shielded chamber and seated in a comfortable chair. The chair faced a 14-in. computer monitor used for the presentation of visual stimuli. A response key was incorporated in a plastic box that the subject held in his/her lap.
The subject was then instructed that he/she should press a response key to designate the passage of a 2-s interval following the onset of each of 50 cue stimuli. The cue stimulus was the letter X presented in the middle of a computer display for 50 ms. A small fixation spot was presented in the center of the monitor at all other times. The computer was programmed to present either a 500 or 2000-Hz tone 3 s after response execution. The 500-Hz tone was presented if response latency was within a ±250-ms window of the designated 2-s target. A 2000-Hz tone was presented if the response latency was outside of this range. The next trial commenced 5–10 s later. Subjects were allowed to practice the task for 5 to 10 trials to verify their comprehension of the instructions.
The electroencephalogram was recorded throughout the task. For the detection of eyeblink and eye movement artifacts, a pair of electrodes was placed diagonally above and below the left eye. The 31 channels of the EEG and 1 channel of eye movement (EOG) activity were appropriately amplified (EEG gain = 20K, EOG gain = 2K) and filtered (bandpass = 0.01–12 Hz) using a SA Instrumentation Company amplification system. Along with markers indicating stimulus and response onsets, the EEG and EOG channels were routed to an A/D converter and sampled at a rate of 200 Hz for 50 ms preceding and 1950 ms following the onset of each stimulus. During off-line computations, single-trial data were sorted by electrode. Before averaging, trials containing an eye movement deviation greater than 50 μV were deleted. Trials with A/D converter overflow and omitted responses were also deleted.
Time-point averaged waveforms were then created from a minimum of 20 accepted trials. The amplitude of the CNV was estimated by the area under the curve between 1100 and 1900 ms following stimulus onset. The area under the curve between 350 and 1000 ms was then subtracted from this value. As a result, CNV amplitude was adjusted for individual differences in the starting point for the CNV.
To reduce the number of analyses and the attendant risk of Type I error, a factor analysis was performed on CNVarea across the 31 electrode sites to find topographic regions of homogeneity that could be reduced to a single score. A principal components analysis followed by varimax rotation yielded two factors that explained most of the variance across the 31 sites. The anterior sites exhibited greater (>.5) loadings on the first factor. The sites around and posterior to the central sulcus exhibited greater (>.5) loadings on the second factor. Average CNV amplitudes within these two scalp regions were significantly different, F(1,157) = 36.4, p<.001. We therefore chose to calculate average area scores representing CNV amplitude within anterior (FP1, FP2, AF1, AF2, F3, F4, F5, F7, FZ, FC2, FC4, FC5, FC6) and posterior (C3, C4, CZ, CP1, CP2, CP5, CP6, P3, P4, PZ, T7, T8, P7, P8, PO1, PO2, O1, O2) regions. These regional amplitudes were analyzed separately.
Data Analysis
Analysis plan
The first stage of the analysis was designed to identify variables that differentiated obese and non-obese groups of patients. Fisher's Exact Test was used to evaluate differences on categorical variables. A t test for independent samples served the same purpose for continuous variables. The variables examined in the analysis were patient demographics, IQ, disease severity and treatment, substance use and other psychiatric characteristics, and behavioral performance (median reaction time) and anterior and posterior region CNV amplitude during the time estimation task. The results of these analyses are presented in Tables 1 and 2.
Table 1.
Demographic, Medical, and Psychological Characteristics of Study Groups
| Non-obese (n=111) |
Obese (n=48) |
t(157) or χ2a | P= | |
|---|---|---|---|---|
| Demographics and IQ | ||||
| Age (SE) | 39.7 (0.6) | 37.7 (1.0) | 1.6 | .10 |
| % female | 65.8 | 68.8 | 0.13 | .71 |
| % caucasian | 25.2 | 31.3 | 0.61 | .43 |
| Years education | 12.0 (0.3) | 12.0 (0.3) | −0.02 | .98 |
| KBIT standard IQ | 92.3 (1.5) | 91.6 (1.7) | 0.33 | .73 |
| Medical characteristics | ||||
| CD4 count (cells/μl) | 606.4 (36) | 824.2 (65) | −2.9 | .005* |
| % receiving protease inhibitors | 26.1 | 56.2 | 4.9 | .0005* |
| % receiving psychoactive medication | 20.8 | 29.1 | 1.1 | .30 |
| Substance use characteristics | ||||
| Lifetime no. of alcohol problems (MAST) | 4.4 (0.6) | 3.3 (0.7) | 1.2 | .22 |
| Lifetime no. of drug problems (DAST-10) | 3.2 (0.3) | 3.1 (0.5) | 0.14 | .88 |
| % alcohol dependent (lifetime) | 34.2 | 16.7 | 5.0 | .03* |
| % cocaine dependent (lifetime) | 52.3 | 47.9 | 0.25 | .73 |
| % heroin dependent (lifetime) | 38.7 | 35.4 | 0.15 | .72 |
| % on methadone maintenance | 20.7 | 18.7 | 0.04 | .83 |
| % nicotine or cocaine dependent (lifetime) | 73.8 | 56.2 | 3.8 | .05* |
| Other psychiatric characteristics | ||||
| No. of current depression symptoms (BDI-II) | 13.8 (1.0) | 13.2 (1.5) | 0.31 | .75 |
| % Major Depressive Disorder–recurrent | 26.1 | 20.8 | 1.4 | .55 |
| No. of childhood Conduct Disorder symptoms | 2.7 (0.24) | 2.7 (0.39) | 0.12 | .90 |
| No. of adult Antisocial Personality Disorder symptoms | 2.5 (0.19) | 2.5 (0.27) | 0.05 | .96 |
Fisher's Exact Test.
p<.05.
Table 2.
Time Estimation Task CNV Amplitude and Performance
| Non-obese (n=111) |
Obese (n=48) |
t(157) or χ2a |
P= | |
|---|---|---|---|---|
| Anterior region CNVarea (SE) | −2044 (1154) |
−9677 (1649) |
2.41 | .02* |
| Posterior region CNVarea | −241 (1004) |
−5227 (1220) |
1.97 | .06 |
| % with median reaction time<2 s |
50.4 | 68.7 | 3.77 | .05a |
Fisher's Exact Test.
p<.05.
The second analysis stage was an attempt to discern the relationships among obesity predictors selected from the variables listed in Tables 1 and 2. It employed structural equation modeling (SEM; Kline, 2004) as the analytic method. Structural equation modeling is an elaboration of an older method—path analysis—that permits simultaneous testing of multiple paths toward the outcome(s). The conceptual model tested by SEM may include single or multiple indicators of a construct (e.g., mood dysregulation, disease severity), mediating and moderating factors, as well as other interactions (e.g., correlated error terms). SEM is typically used as a confirmatory procedure. Yet, it can also be used to develop a conceptual model through an iterative process of comparing changes in the goodness-of-fit indices associated with changes in the path relationships, indicators, or hypothetical constructs.
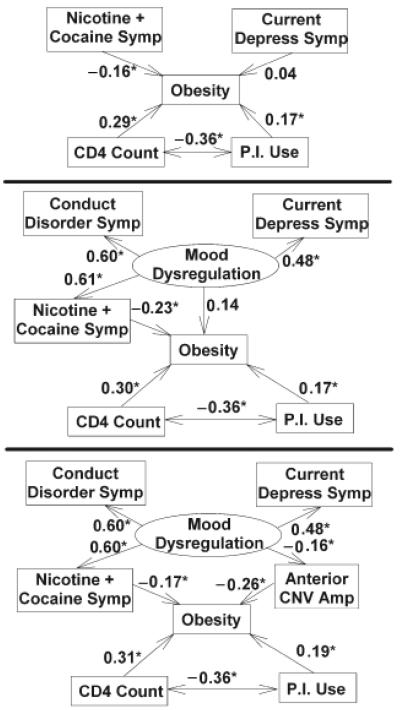
Three models were compared (see Figure 2, below). Model 1 was the simplest and included current depression symptoms from the BDI-II, stimulant dependence symptoms, disease severity (CD4+cell count), and use of protease inhibitors as predictors. Model 2 was similar. However, it combined depression symptoms and stimulant dependence symptoms with one additional variable, childhood conduct disorder problems, to represent a single unifying hypothetical construct—mood dysregulation. Model 3 was identical to Model 2 but added anterior region CNV amplitude (a neurophysiological indicator of “impatience”) as a factor through which the effects of mood dysregulation were mediated. For each of the three models, the discrepancy χ2 index for model fit was evaluated. In addition, the adequacy of each model fit was evaluated by the Root Mean Square Error of Approximation (RMSEA) and Adjusted Global Fit Indices (AGFI), which are less distorted than the discrepancy index by a moderate sample size and the complexity of the model.
Figure 2.
Structural Equation Models 1, 2, and 3. Measured variables are included in boxes. The hypothetical construct of mood dysregulation, reflecting variance shared by two or more measured variables, is included in an ellipse. Statistically significant standardized path coefficients appear with asterisks. The negative coefficients relating CNV amplitude to the other predictors indicate that a more negative (i.e., larger) CNV is related to obesity and mood dysregulation. Note in Model 3 that nicotine/cocaine dependence symptoms and premature response preparation (anterior CNVamplitude) are the final common pathways through which mood dysregulation effects obesity.
Path relationships were evaluated within each model. The critical ratios of path coefficients linking indicators and/or constructs were tested for statistical significance against a criterion z score of 1.96. Standardized coefficients are reported in the Figure 2, below.
Screening of variables
Before entry into either of the two analysis stages, the data were inspected for outliers and errors. The distributional properties of continuous variables were examined to confirm univariate normality, that is, skew<2 and kurtosis<8. Variables that did not meet this criterion were transformed to a normal distribution prior to analysis.
Two issues in the data analysis were specific to the second analysis stage. The first issue pertains to the inherently nonnormal distribution of categorical variables, for example, obesity. In fact, until recently, this distributional problem precluded the entry of categorical variables into structural equation models (Kupek, 2006). However, the version of the analysis program employed here does implement a solution. To provide reassurance, the structural equation models presented in Figure 2, below, were also tested with a continuous variable, BMI, substituted for the categorical variable, obesity. The findings from these alternative analyses were equivalent to the findings from the analyses of obesity. Therefore, only the results from the obesity analyses are reported.
The second issue pertinent to the second analysis stage is the threat resulting from high collinearity among measures. Prior to the analyses, simple pairwise correlations were computed between all candidate measures. An examination of the results revealed high correlations between alcohol and tobacco dependence symptoms (r=.81) and between reaction time and CNV amplitude (r= −.76). Accordingly, alcohol dependence symptoms and reaction time were not included in the the SEM analyses.
Results
T test and χ2 analyses of differences between obese and non-obese patients revealed several findings. For example, obese patients, in comparison to their non-obese peers, presented with a higher CD41lymphocyte count, t(157)= −2.9, p=.005) and were more likely to be the recipients of protease-inhibitor-based HAART (χ2=4.9, p=.0005). They were also more likely to meet DSM-IV criteria for alcohol (χ2=5.0, p=.03) and stimulant, that is, nicotine or cocaine (χ2=3.8, p=.05), dependence over their lifetime. The groups did not differ significantly in demographic or other psychiatric characteristics, use of psychoactive medications, or IQ.
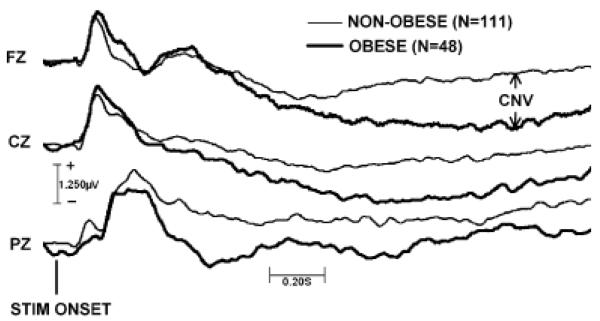
An intriguing finding was the significant difference between the groups in the amplitude of the CNV (anterior region CNV: t[157]=2.4, p=.02; posterior region CNV: t[157]=1.9, p=.06) recorded while patients awaited the passage of a 2-s time estimation interval. Group-averaged event-related potential wave-forms from three representative midline electrode sites are shown in Figure 1. As shown in the figure, obese patients exhibited a greater (more negative) rise in CNV amplitude, particularly over the frontal scalp, toward the conclusion of the interval. Analyses of median reaction times during the time estimation task yielded a result consistent with the CNV result: A greater percentage of patients in the obese group overestimated the passage of the 2-s time estimation interval and responded prematurely (χ2=3.8, p=.05).
Figure 1.
Group-averaged event related potential waveforms at three representative electrode sites during the 2-s time estimation interval. The waveforms span an epoch of 50 ms preceding to 1950 ms following onset of the visual cue, marking the beginning of the interval.
The results of analyzing the fit of the three candidate conceptual models yielded other intriguing results. Model 1 did not fit the data well. It explained 11% of the variance in obesity. The poor fit of the model was indicated by discrepancy, χ2(5)=15.3, p=.009, and RMSEA, RMSEA=.114, pclose=.046, indices that were statistically significant and an AGFI of 0.89, which is less than the desired minimum of 0.90. Importantly, an examination of the structural paths within Model 1 indicated no significant association between depression symptoms and obesity (β=.04, p=.64). Obesity was, however, significantly associated with CD4+cell count, protease inhibitor use, and the number of symptoms of stimulant dependence (Figure 2).
In contrast to Model 1, Models 2 and 3 fit the data adequately. The adequacy of Model 2 was indicated by nonsignificant discrepancy, χ2(7)=2.83, p=.90, and RMSEA, RMSEA=0.0, pclose=.96, indices and an AGFI=.98. For Model 3, adequacy of fit was indicated by χ2(12)=4.67, p=.96, RMSEA=0.0, pclose=.99, and AGFI=.98.
An examination of the path coefficients within each of the latter models revealed the same strong associations between obesity, CD4+cell count, and protease inhibitor use shown in Model 1. But, in these models, a stronger association was found with depression symptoms and obesity, when depression symptoms were incorporated into a larger construct that we arbitrarily label as “mood dysregulation.” Across Models 2 and 3, the significant associations of current depression symptoms with this construct were indicated by standardized regression coefficients, β, of .48 (p<.05). The construct was also strongly and significantly (βs ≥ .6, ps<.05) associated with the number of childhood conduct problems and stimulant dependence symptoms in both models.
Models 2 and 3 differed in the explained amount of variance in obesity: 12% versus 18%, respectively. As illustrated in Figure 2, the two models also differed in the route by which the construct of mood dysregulation (i.e., the variance shared by depression symptoms, conduct problems, and stimulant dependence symptoms) was linked to obesity. In Model 2, the path was direct, but not statistically significant (β=.14, p=.38). In Model 3, the path was indirect and significant: The effects of mood dysregulation were mediated (β= −.16, p=.05) through anterior CNVamplitude, which was, in turn, related (β= −.26, p<.001) to obesity.
Discussion
The present study was founded on the assertion that obesity is an emerging health problem among patients living with HIV/AIDS. The results of the study support the assertion. Obesity was a prevalent condition, affecting 30.1% of the sample. This rate is minimally greater than the 9%–28% prevalence rates reported in other studies (Amorosa et al., 2005; Hodgson et al., 2001; Jacobson et al., 2006; Shor-Posner et al., 2000). Although remarkably high, 30.1% may be an understimate of the true population prevalence, because obesity was defined by the presenting BMI versus the peak BMI since seroconversion.
Given the paucity of literature on the topic of HIV-1-associated obesity, in comparison to the extensive literature on wasting, one might view our prevalence estimate as an artifact, originating from an experimental design in which patients free of acute illness were recruited from ambulatory settings. Yet, before dismissing the finding as an oddity with limited generalizability, one should recognize that the majority of patients now living with HIV/AIDS in the United States could be similarly described. In addition, there may be factors beyond those examined that will increase the prevalence beyond 30.1%. Unfortunately, the sample size of 159 patients simply does not provide adequate power for assessing these additional factors. Among the candidates relevant to impatience, impulsivity, or obesity and therefore worthy of future study are gender (Amorosa et al., 2005), age (Baum, 2007), medication use, and Bipolar (Garcia-Portilla et al., 2007; McIntyre et al., 2006; Wildes, Marcus, & Fagiolini, 2006), Anxiety (Kasen, Cohen, Chen, & Must, 2007; Scott, McGee, Wells, & Oakley Browne, 2008), or Borderline Personality (Frankenburg & Zanarini, 2006; Sansone, Sansone, & Morris, 1996) Disorders.
The present study did demonstrate associations between obesity and a selected set of medical, psychological, and neurophysiological variables. Significant positive associations with CD4+cell count (Batterham et al., 2002; Dworkin & Williamson, 2003; Lyles et al., 1999; Malvy et al., 2001; Wanke et al., 2003; Welch & Morse, 2002) and protease inhibitor use (Dever et al., 2000; Jacobson et al., 2006; Jerico et al., 2005; McDermott et al., 2001; Mulligan et al., 2005; Rimland et al., 2006; Silva et al., 1998; Tsiodras et al., 2000; Walli et al., 1998) were expected from findings previously reported by other investigators and were replicated presently. The negative association between stimulant dependence symptoms and obesity was also expected (Forrester et al., 2005; Saarni et al., 2004). Because the analysis controlled for the potential confounding effects of substance abuse-related variability in disease severity (Bagby et al., 2006; Molina et al., 2006; Poonia et al., 2006; Potula et al., 2006) and antiviral medication use (Hinkin, Castellon, Hardy, Granholm, & Siegle, 1999; Mohammed et al., 2004; Sharpe et al., 2004), the association between stimulant use and obesity can be more compellingly ascribed, in comparison to other studies, to the known pharmacological effects of stimulants on appetite and/or energy expenditure.
An intriguing result of the study was the interplay of psychological and neurophysiological measures in predicting obesity. As shown in Model 3 and mentioned above, stimulant dependence symptoms were negatively associated with obesity via a simple direct path. But another component of the variance in stimulant dependence symptoms was subsumed within the variance it shares with conduct problems, depression symptoms, and impatience (i.e., CNVamplitude) and associated with obesity via an alternate indirect path. The connection of obesity to stimulant dependence and to a known risk factor for stimulant and other substance dependence (i.e., conduct problems; Hodgins, Tiihonen, & Ross, 2005; Modestin, Matutat, & Wurmle, 2001; Robins, 1991; Rowe, Sullivan, Mulder, & Joyce, 1996) via this other path is consistent with recent hypotheses proposed by Volkow and Wise (2005) and Ehlers and Wilhelmsen (2007) wherein substance dependence and obesity are viewed as outcomes of a similar genetic diathesis.
A second intriguing result was the absence of a simple association between depression symptoms and obesity (see Model 1)—a finding that challenges the common view (Dragan & Akhtar-Danesh, 2007; Moreira et al., 2007). Instead, effects of depression were only apparent in the context of the variance it shares with conduct problems, stimulant dependence, and CNV amplitude. One possible interpretation suggested by the analyses is that depressed mood is an insufficient predictor of obesity. Instead, it is the combination of depressed mood with a dysregulated behavioral control and monitoring system (Alciati et al., 2007; Cugini et al., 1999; McIntyre et al., 2006) that appears essential. In keeping with this interpretation, we chose to assign a label of “mood dysregulation” to the variance shared by these indicators.
Admittedly, another label, for example, impulsivity, might better describe the “mood dysregulation” factor. A more complete assessment of psychiatric symptoms and disorders (i.e., beyond those listed in Table 1) and a larger sample would be useful for defining the boundaries of the underlying construct. A secondary benefit of the larger sample would be the opportunity to perform analyses on a subsample uncomplicated by the effects of prior alcohol/drug use, active medication use, and other potential confounds.
The final and most intriguing result was the association with CNVamplitude recorded while patients estimated the passage of a 2-s interval. As predicted, obese patients, in comparison to their non-obese peers, emitted large (more negative) cortical readiness potentials well before the termination of the interval (Figure 1) and also emitted their behavioral responses prematurely. In simple terms, obese patients were impatient. Including CNV amplitude as an indicator of impatience within the model added value to the model: It improved the variance explained in obesity from 12% (Model 2) to 18% (Model 3), a 50% increase. As shown in Figure 2, impatience also appeared to be a pathway through which mood dysregulation promoted obesity, because in the absence of this intervening step (Model 2), mood dysregulation did not predict it (β = .14, p = .38).
The present demonstration of an association between impatience (or impulsivity) and obesity is not a new idea. In 1971, Schachter incorporated impatience as an element of his psychological theory of obesity. The theory viewed the obese as dominated by a hyperreactivity to cues in the external environment at the cost of accurate processing of internal cues, including cues that signal time passage (Gardner et al., 1984; Rodin, 1975) and satiety. Schachter's theory was subject to many tests in its time. It was subsequently abandoned when investigators could not consistently demonstrate that the obese were more easily distracted by external cues. Unfortunately, the remaining and more reliable predictions of his theory were then also forgotten.
Recently, economists have revived these notions and reemphasized the importance of impatience as a contributor to obesity (Komlos, Smith, & Bogin, 2004). The modern theory of impatience applied to obesity is not dissimilar to some modern economic theories of drug and alcohol dependence, inasmuch as it emphasizes a description of reinforcers and choice behavior over molecular or neurophysiological explanations. In this view, the preference of the obese (or substance-dependent) patient for shorter time intervals is a simple manifestation of delay discounting wherein greater value is assigned to immediate versus delayed reinforcers.
Acknowledgments
This research was supported by PHS grant R01MH61346 funded jointly by NIMH and NIDA. Aditional support was provided by grants P50AA03510 and M01RR06192 funded by NIAAA and NCRR, respectively.
REFERENCES
- Alciati A, D'Ambrosio A, Foschi D, Corsi F, Mellado C, Angst J. Bipolar spectrum disorders in severely obese patients seeking surgical treatment. Journal of Affective Disorders. 2007;101:131. doi: 10.1016/j.jad.2006.11.008. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed Author; Washington, DC: 1994. [Google Scholar]
- Amorosa V, Synnestvedt M, Gross R, Friedman H, MacGregor RR, Gudonis D, et al. A tale of 2 epidemics: The intersection between obesity and HIV infection in Philadelphia. Journal of Acquired Immune Deficiency Syndromes. 2005;39:557–561. [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcoholism, Clinical and Experimental Research. 2006;30:1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Barbaro G, Barbarini G. Highly active antiretroviral therapy-associated metabolic syndrome and cardiovascular risk. Chemotherapy. 2006;52:161–165. doi: 10.1159/000093034. [DOI] [PubMed] [Google Scholar]
- Batterham MJ, Garsia R, Greenop P. Prevalence and predictors of HIV-associated weight loss in the era of highly active antiretroviral therapy. International Journal of STD & AIDS. 2002;13:744–747. doi: 10.1258/095646202320753682. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Antisocial personality disorder and cocaine dependence: Their effects on behavioral and electroencephalographic measures of time estimation. Drug and Acohol Dependence. 2001;63:87–95. doi: 10.1016/s0376-8716(00)00195-2. [DOI] [PubMed] [Google Scholar]
- Baum CL. The effects of race, ethnicity, and age on obesity. Journal of Population Economics. 2007;20:687. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory, Version II Manual. Psychological Corporation/Harcourt Brace; San Antonio, TX: 1996. [Google Scholar]
- Braet C, Claus L, Verbeken S, Van Vlierberghe L. Impulsivity in overweight children. European Child & Adolescent Psychiatry. 2007;16:473–483. doi: 10.1007/s00787-007-0623-2. [DOI] [PubMed] [Google Scholar]
- Brown D, Fenwick P, Howard R. The contingent negative variation in a Go/No Go avoidance task: Relationships with personality and subjective state. International Journal of Psychophysiology. 1989;7:35–45. doi: 10.1016/0167-8760(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Brunia CH, van Boxtel GJ. Wait and see. International Journal of Psychophysiology. 2001;43:59–75. doi: 10.1016/s0167-8760(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Campa A, Yang Z, Lai S, Xue L, Phillips JC, Sales S, et al. HIV-related wasting in HIV-infected drug users in the era of highly active antiretroviral therapy. Clinical Infectious Diseases. 2005;41:1179–1185. doi: 10.1086/444499. [DOI] [PubMed] [Google Scholar]
- Chander G, Lau B, Moore RD. Hazardous alcohol use: A risk factor for non-adherence and lack of suppression in HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2006;43:411–417. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Vlahos R, Bozinovski S, Jones J, Anderson GP, Morris MJ. Effect of short-term cigarette smoke exposure on body weight, appetite and brain neuropeptide Y in mice. Neuropsychopharmacology. 2005;30:713–719. doi: 10.1038/sj.npp.1300597. [DOI] [PubMed] [Google Scholar]
- Cole JW, Pinto AN, Hebel JR, Buchholz DW, Earley CJ, Johnson CJ, et al. Acquired immunodeficiency syndrome and the risk of stroke. Stroke. 2004;35:51–56. doi: 10.1161/01.STR.0000105393.57853.11. [DOI] [PubMed] [Google Scholar]
- Cugini P, Cilli M, Salandri A, Ceccotti P, Di Marzo A, Rodio A, et al. Anxiety, depression, hunger and body composition: III. Their relationships in obese patients. Eating and Weight Disorders. 1999;4:115–120. doi: 10.1007/BF03339726. [DOI] [PubMed] [Google Scholar]
- d'Arminio A, Sabin CA, Phillips AN, Reiss P, Weber R, Kirk O, et al. Cardio- and cerebrovascular events in HIV-infected persons. AIDS. 2004;18:1811–1817. doi: 10.1097/00002030-200409030-00010. [DOI] [PubMed] [Google Scholar]
- Desai MY, Nasir K, Braunstein JB, Rumberger JA, Post WS, Budoff MJ, et al. Underlying risk factors incrementally add to the standard risk estimate in detecting subclinical atherosclerosis in low- and intermediate-risk middle-aged asymptomatic individuals. American Heart Journal. 2004;148:871–877. doi: 10.1016/j.ahj.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Dever LL, Oruwari PA, Figueroa WE, O'Donovan CA, Eng RH. Hyperglycemia associated with protease inhibitors in an urban HIV-infected minority patient population. Annals of Pharmacotherapy. 2000;34:580–584. doi: 10.1345/aph.19231. [DOI] [PubMed] [Google Scholar]
- Dragan A, Akhtar-Danesh N. Relation between body mass index and depression: A structural equation modeling approach. BMC Medical Research Methodology. 7. 2007:17. doi: 10.1186/1471-2288-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin MS, Williamson JM. AIDS wasting syndrome: Trends, influence on opportunistic infections, and survival. Journal of Acquired Immune Deficiency Syndromes. 2003;33:267–273. doi: 10.1097/00126334-200306010-00024. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wilhelmsen KC. Genomic screen for substance dependence and body mass index in southwest California Indians. Genes, Brain, and Behavior. 2007;6:184–191. doi: 10.1111/j.1601-183X.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Elbert T, Ulrich R, Rockstroh B, Lutzenberger W. The processing of temporal intervals reflected by CNV-like brain potentials. Psychophysiology. 1991;28:648–655. doi: 10.1111/j.1469-8986.1991.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Engelson ES, Agin D, Kenya S, Werber-Zion G, Luty B, Albu JB, et al. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism. 2006;55:1327–1336. doi: 10.1016/j.metabol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Faulkner KK, Duecker SJ. Stress, time distortion, and failure to recover among obese individuals: Implications for weight gain and dieting. International Journal of Eating Disorders. 1989;8:247. [Google Scholar]
- Forrester JE, Tucker KL, Gorbach SL. The effect of drug abuse on body mass index in Hispanics with and without HIV infection. Public Health Nutrition. 2005;8:61–68. doi: 10.1079/phn2005667. [DOI] [PubMed] [Google Scholar]
- Frankenburg FR, Zanarini MC. Obesity and obesity-related illnesses in borderline patients. Journal of Personality Disorders. 2006;20:71–80. doi: 10.1521/pedi.2006.20.1.71. [DOI] [PubMed] [Google Scholar]
- Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, ElSadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. New England Journal of Medicine. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- Garcia-Portilla MP, Saiz PA, Benabarre A, Sierra P, Perez J, Rodriguez A, et al. The prevalence of metabolic syndrome in patients with bipolar disorder. Journal of Affective Disorders. 2007;106:197–210. doi: 10.1016/j.jad.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Gardner RM, Reyes B, Brake SJ, Salaz VE. Reproduction and discrimination of time in obese subjects. Personality and Social Psychology Bulletin. 1984;10:564. [Google Scholar]
- Gomez CM, Flores A, Ledesma A. Fronto-parietal networks activation during the contingent negative variation period. Brain Research Bulletin. 2007;73:40–47. doi: 10.1016/j.brainresbull.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Gorczyca I, Stanek M, Podlasin B, Furmanek M, Pniewski J. Recurrent cerebral infarcts as the first manifestation of infection with the HIV virus. Folia Neuropathologica. 2005;43:45–49. [PubMed] [Google Scholar]
- Hendricks KM, Willis K, Houser R, Jones CY. Obesity in HIV-infection: Dietary correlates. Journal of the American College of Nutrition. 2006;25:321–331. doi: 10.1080/07315724.2006.10719542. [DOI] [PubMed] [Google Scholar]
- Heo M, Pietrobelli A, Fontaine KR, Sirey JA, Faith MS. Depressive mood and obesity in US adults: Comparison and moderation by sex, age, and race. International Journal of Obesity. 2006;30:513–519. doi: 10.1038/sj.ijo.0803122. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke Wellman J, Rickin E, Lowers L. Offspring from families at high risk for alcohol dependence: Increased body mass index in association with prenatal exposure to cigarettes but not alcohol. Psychiatry Research. 2005;135:203–216. doi: 10.1016/j.psychres.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS and Behavior. 2007;11:185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Hardy DJ, Granholm E, Siegle G. Computerized and traditional Stroop task dysfunction in HIV-1 infection. Neuropsychology. 1999;13:306–316. doi: 10.1037//0894-4105.13.2.306. [DOI] [PubMed] [Google Scholar]
- Hiraku S, Sakuma H. Effects on contingent negative variation of set created by anticipating variable foreperiods. Perceptual and Motor Skills. 1996;83:1163–1169. doi: 10.2466/pms.1996.83.3f.1163. [DOI] [PubMed] [Google Scholar]
- Hodgins S, Tiihonen J, Ross D. The consequences of Conduct Disorder for males who develop schizophrenia: Associations with criminality, aggressive behavior, substance use, and psychiatric services. Schizophrenia Research. 2005;78:323–335. doi: 10.1016/j.schres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Hodgson LM, Ghattas H, Pritchitt H, Schwenk A, Payne L, Macallan DC. Wasting and obesity in HIV outpatients. AIDS. 2001;15:2341–2342. doi: 10.1097/00002030-200111230-00024. [DOI] [PubMed] [Google Scholar]
- Isozumi K. Obesity as a risk factor for cerebrovascular disease. The Keio Journal of Medicine. 2004;53:7–11. doi: 10.2302/kjm.53.7. [DOI] [PubMed] [Google Scholar]
- Jacobson DL, Tang AM, Spiegelman D, Thomas AM, Skinner S, Gorbach SL, et al. Incidence of metabolic syndrome in a cohort of HIV-infected adults and prevalence relative to the US population (National Health and Nutrition Examination Survey) Journal of Acquired Immune Deficiency Syndromes. 2006;43:458–466. doi: 10.1097/01.qai.0000243093.34652.41. [DOI] [PubMed] [Google Scholar]
- Jerico C, Knobel H, Montero M, Ordonez-Llanos J, Guelar A, Gimeno JL, et al. Metabolic syndrome among HIV-infected patients: Prevalence, characteristics, and related factors. Diabetes Care. 2005;28:132–137. doi: 10.2337/diacare.28.1.132. [DOI] [PubMed] [Google Scholar]
- Justman JE, Benning L, Danoff A, Minkoff H, Levine A, Greenblatt RM, et al. Protease inhibitor use and the incidence of diabetes mellitus in a large cohort of HIV-infected women. Journal of Acquired Immune Deficiency Syndromes. 2003;32:298–302. doi: 10.1097/00126334-200303010-00009. [DOI] [PubMed] [Google Scholar]
- Karmon SL, Moore RD, Dobs AS, Keruly J, Barnett S, Cofrancesco J., Jr. Body shape and composition in HIV-infected women: an urban cohort. HIV Medicine. 2005;6:245–252. doi: 10.1111/j.1468-1293.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- Kasen S, Cohen P, Chen H, Must A. Obesity and psychopathology in women: A three decade prospective study. International Journal of Obesity. 2007;32:558–566. doi: 10.1038/sj.ijo.0803736. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. American Guidance Services; Circle Pines, MN: 1990. [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. Guilford Press; New York: 2004. [Google Scholar]
- Komlos J, Smith PK, Bogin B. Obesity and the rate of time preference: Is there a connection? Journal of Biosocial Science. 2004;36:209–219. doi: 10.1017/s0021932003006205. [DOI] [PubMed] [Google Scholar]
- Kruzich LA, Marquis GS, Wilson CM, Stephensen CB. HIV-infected US youth are at high risk of obesity and poor diet quality: A challenge for improving short- and long-term health outcomes. Journal of the American Dietetic Association. 2004;104:1554–1560. doi: 10.1016/j.jada.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Kupek E. Beyond logistic regression: Structural equations modelling for binary variables and its application to investigating unobserved confounders. BMC Medical Research Methodology. 2006;6:13. doi: 10.1186/1471-2288-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladanyi M, Dubrovsky B. CNV and time estimation. International Journal of Neuroscience. 1985;26:253–257. doi: 10.3109/00207458508985622. [DOI] [PubMed] [Google Scholar]
- Larsen JK, van Strien T, Eisinga R, Engels RC. Gender differences in the association between alexithymia and emotional eating in obese individuals. Journal of Psychosomatic Research. 2006;60:237–243. doi: 10.1016/j.jpsychores.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Ledergerber B, Furrer H, Rickenbach M, Lehmann R, Elzi L, Hirschel B, et al. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clinical Infectious Diseases. 2007;45:111–119. doi: 10.1086/518619. [DOI] [PubMed] [Google Scholar]
- Leon GR, Roth L. Obesity: Psychological causes, correlations, and speculations. Psychology Bulletin. 1977;84:117–139. [PubMed] [Google Scholar]
- Levine J, Chengappa KN, Patel A, Vagnucci A, John V, Brar JS, et al. Obesity and medical illnesses in psychiatric patients admitted to a long-term psychiatric facility. Journal of Psychiatric Practice. 2001;7:432–439. doi: 10.1097/00131746-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Levine JA, Harris MM, Morgan MY. Energy expenditure in chronic alcohol abuse. European Journal of Clinical Investigation. 2000;30:779–786. doi: 10.1046/j.1365-2362.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- Lyles RH, Tang AM, Smit E, Mellors JW, Margolick JB, Visscher BR, et al. Virologic, immunologic, and immune activation markers as predictors of HIV-associated weight loss prior to AIDS. Multicenter AIDS Cohort Study. Journal of Acquired Immune Deficiency Syndromes. 1999;22:386–394. doi: 10.1097/00126334-199912010-00010. [DOI] [PubMed] [Google Scholar]
- Malvy E, Thiebaut R, Marimoutou C, Dabis F. Weight loss and body mass index as predictors of HIV disease progression to AIDS in adults. Aquitaine cohort, France, 1985–1997. Journal of the American College of Nutrition. 2001;20:609–615. doi: 10.1080/07315724.2001.10719065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott AY, Shevitz A, Knox T, Roubenoff R, Kehayias J, Gorbach S. Effect of highly active antiretroviral therapy on fat, lean, and bone mass in HIV-seropositive men and women. American Journal of Clinical Nutrition. 2001;74:679–686. doi: 10.1093/ajcn/74.5.679. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Konarski JZ, Wilkins K, Soczynska JK, Kennedy SH. Obesity in bipolar disorder and major depressive disorder: Results from a national community health survey on mental health and well-being. Canadian Journal of Psychiatry. 2006;51:274–280. doi: 10.1177/070674370605100502. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Sabin CA, Youle M, Madge S, Tyrer M, Devereux H, et al. Changes in AIDS-defining illnesses in a London Clinic, 1987–1998. Journal of Acquired Immune Deficiency Syndromes. 1999;21:401–407. [PubMed] [Google Scholar]
- Modestin J, Matutat B, Wurmle O. Antecedents of opioid dependence and personality disorder: Attention-deficit/hyperactivity disorder and conduct disorder. European Archives of Psychiatry and Clinical Neuroscience. 2001;251:42–47. doi: 10.1007/s004060170067. [DOI] [PubMed] [Google Scholar]
- Mohammed H, Kieltyka L, Richardson-Alston G, Magnus M, Fawal H, Vermund SH, et al. Adherence to HAART among HIV-infected persons in rural Louisiana. AIDS Patient Care and STDs. 2004;18:289–296. doi: 10.1089/108729104323076025. [DOI] [PubMed] [Google Scholar]
- Molina PE, McNurlan M, Rathmacher J, Lang CH, Zambell KL, Purcell J, et al. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcoholism, Clinical and Experimental Research. 2006;30:2065–2078. doi: 10.1111/j.1530-0277.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- Moreira RO, Marca KF, Appolinario JC, Coutinho WF. Increased waist circumference is associated with an increased prevalence of mood disorders and depressive symptoms in obese women. Eating and Weight Disorders. 2007;12:35–40. doi: 10.1007/BF03327770. [DOI] [PubMed] [Google Scholar]
- Mulligan K, Anastos K, Justman J, Freeman R, Wichienkuer P, Robison E, et al. Fat distribution in HIV-infected women in the United States: DEXA substudy in the Women's Interagency HIV Study. Journal of Acquired Immune Deficiency Syndromes. 2005;38:18–22. doi: 10.1097/00126334-200501010-00004. [DOI] [PubMed] [Google Scholar]
- Nasser JA, Gluck ME, Geliebter A. Impulsivity and test meal intake in obese binge eating women. Appetite. 2004;43:303–307. doi: 10.1016/j.appet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behaviour Research and Therapy. 2007;45:1071–1075. doi: 10.1016/j.brat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Pacy PJ, Preedy VR, Peters TJ, Read M, Halliday D. The effect of chronic alcohol ingestion on whole body and muscle protein synthesis—A stable isotope study. Alcohol and Alcoholism. 1991;26:505–513. doi: 10.1093/oxfordjournals.alcalc.a045152. [DOI] [PubMed] [Google Scholar]
- Pence BW, Miller WC, Gaynes BN, Eron JJ., Jr. Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2007;44:159–166. doi: 10.1097/QAI.0b013e31802c2f51. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Brook J, Coplan JD. Psychiatric symptoms in adolescence as predictors of obesity in early adulthood: A longitudinal study. American Journal of Public Health. 1997;87:1303–1310. doi: 10.2105/ajph.87.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliner P. External responsiveness in the obese. Addictive Behaviors. 1976;1:169. [Google Scholar]
- Poonia B, Nelson S, Bagby GJ, Zhang P, Quniton L, Veazey RS. Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS Research and Human Retroviruses. 2006;22:589–594. doi: 10.1089/aid.2006.22.589. [DOI] [PubMed] [Google Scholar]
- Potula R, Haorah J, Knipe B, Leibhart J, Chrastil J, Heilman D, et al. Alcohol abuse enhances neuroinflammation and impairs immune responses in an animal model of human immunodeficiency virus-1 encephalitis. American Journal of Pathology. 2006;168:1335–1344. doi: 10.2353/ajpath.2006.051181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimland D, Guest JL, Hernandez-Ramos I, Del Rio C, Le NA, Brown WV. Antiretroviral therapy in HIV-positive women is associated with increased apolipoproteins and total cholesterol. Journal of Acquired Immune Deficiency Syndromes. 2006;42:307–313. doi: 10.1097/01.qai.0000220164.72113.12. [DOI] [PubMed] [Google Scholar]
- Robins LN. Conduct disorder. Journal of Child Psychology and Psychiatry. 1991;32:193–212. doi: 10.1111/j.1469-7610.1991.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) Washington University; St. Louis, MO: 2002. [Google Scholar]
- Rodin J. Causes and consequences of time perception differences in overweight and normal weight people. Journal of Personality and Social Psychology. 1975;31:898–904. doi: 10.1037/h0076866. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arenas MA, Jarrin I, del Amo J, Iribarren JA, Moreno S, Viciana P, et al. Delay in the initiation of HAART, poorer virological response, and higher mortality among HIV-infected injecting drug users in Spain. AIDS Research and Human Retroviruses. 2006;22:715–723. doi: 10.1089/aid.2006.22.715. [DOI] [PubMed] [Google Scholar]
- Rosahl SK, Knight RT. Role of prefrontal cortex in generation of the contingent negative variation. Cerebral Cortex. 1995;5:123–134. doi: 10.1093/cercor/5.2.123. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Sullivan PF, Mulder RT, Joyce PR. The effect of a history of conduct disorder in adult major depression. Journal of Affective Disorders. 1996;37:51–63. doi: 10.1016/0165-0327(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, McCalley MG, Glaser EM. Event related potentials and time estimation. Psychophysiology. 1977;14:451–455. doi: 10.1111/j.1469-8986.1977.tb01311.x. [DOI] [PubMed] [Google Scholar]
- Saarni SE, Silventoinen K, Rissanen A, Sarlio-Lahteenkorva S, Kaprio J. Intentional weight loss and smoking in young adults. International Journal of Obesity and Related Metabolic Disorders. 2004;28:796–802. doi: 10.1038/sj.ijo.0802627. [DOI] [PubMed] [Google Scholar]
- Sansone RA, Sansone LA, Morris DW. Prevalence of borderline personality symptoms in two groups of obese subjects. American Journal of Psychiatry. 1996;153:117–118. doi: 10.1176/ajp.153.1.117. [DOI] [PubMed] [Google Scholar]
- Sawyer MG, Miller-Lewis L, Guy S, Wake M, Canterford L, Carlin JB. Is there a relationship between overweight and obesity and mental health problems in 4- to 5-year-old Australian children? Ambulatory Pediatrics. 2006;6:306–311. doi: 10.1016/j.ambp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Schachter S. Some extraordinary facts about obese humans and rats. The American Psychologist. 1971;26:129. doi: 10.1037/h0030817. [DOI] [PubMed] [Google Scholar]
- Schachter S, Gross LP. Manipulated time and eating behavior. Journal of Personality and Social Psychology. 1968;10:98. doi: 10.1037/h0026285. [DOI] [PubMed] [Google Scholar]
- Scott KM, McGee MA, Wells JE, Oakley Browne MA. Obesity and mental disorders in the adult general population. Journal of Psychosomatic Research. 2008;64:97–105. doi: 10.1016/j.jpsychores.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. American Journal of Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. Journal of Community Health. 2004;29:117–127. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- Shor-Posner G, Campa A, Zhang G, Persaud N, Miguez-Burbano MJ, Quesada J, et al. When obesity is desirable: A longitudinal study of the Miami HIV-1-infected drug abusers (MIDAS) cohort. Journal of Acquired Immune Deficiency Syndromes. 2000;23:81–88. doi: 10.1097/00126334-200001010-00011. [DOI] [PubMed] [Google Scholar]
- Silva M, Skolnik PR, Gorbach SL, Spiegelman D, Wilson IB, Fernandez-DiFranco MG, et al. The effect of protease inhibitors on weight and body composition in HIV-infected patients. AIDS. 1998;12:1645–1651. doi: 10.1097/00002030-199813000-00012. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Smit E, Skolasky RL, Dobs AS, Calhoun BC, Visscher BR, Palella FJ, et al. Changes in the incidence and predictors of wasting syndrome related to human immunodeficiency virus infection, 1987–1999. American Journal of Epidemiology. 2002;156:211–218. doi: 10.1093/aje/kwf039. [DOI] [PubMed] [Google Scholar]
- Specker S, de Zwaan M, Raymond N, Mitchell J. Psychopathology in subgroups of obese women with and without binge eating disorder. Comprehensive Psychiatry. 1994;35:185–190. doi: 10.1016/0010-440x(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Tang AM, Forrester J, Spiegelman D, Knox TA, Tchetgen E, Gorbach SL. Weight loss and survival in HIV-positive patients in the era of highly active antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2002;31:230–236. doi: 10.1097/00126334-200210010-00014. [DOI] [PubMed] [Google Scholar]
- Tang AM, Jacobson DL, Spiegelman D, Knox TA, Wanke C. Increasing risk of 5% or greater unintentional weight loss in a cohort of HIV-infected patients, 1995 to 2003. Journal of Acquired Immune Deficiency Syndromes. 2005;40:70–76. doi: 10.1097/01.qai.0000159627.54149.2e. [DOI] [PubMed] [Google Scholar]
- Timsit-Berthier M. [Contingent negative variation and endogenous components of the evoked potential] Revue d'Electroencephalographie et Neurophysiologie Clinique. 1984;14:77–96. doi: 10.1016/s0370-4475(84)80012-x. [DOI] [PubMed] [Google Scholar]
- Tipping B, de Villiers L, Wainwright H, Candy S, Bryer A. Stroke in patients with human immunodeficiency virus infection. Journal of Neurology, Neurosurgery, and Psychiatry. 78. 2007:1320–1324. doi: 10.1136/jnnp.2007.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. Journal of Clinical Endocrinology and Metabolism. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Archives of Internal Medicine. 2000;160:2050–2056. doi: 10.1001/archinte.160.13.2050. [DOI] [PubMed] [Google Scholar]
- Vaughn G, Detels R. Protease inhibitors and cardiovascular disease: Analysis of the Los Angeles County adult spectrum of disease cohort. AIDS Care. 2007;19:492–499. doi: 10.1080/09540120701203329. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nature Neuroscience. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Walli R, Herfort O, Michl GM, Demant T, Jager H, Dieterle C, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12:F167–173. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- Wanke CA, Silva M, Ganda A, Fauntleroy J, Spiegelman D, Knox TA, et al. Role of acquired immune deficiency syndrome-defining conditions in human immunodeficiency virus-associated wasting. Clinical Infectious Diseases. 2003;37(Suppl 2):S81–84. doi: 10.1086/375894. [DOI] [PubMed] [Google Scholar]
- Welch K, Morse A. The clinical profile of end-stage AIDS in the era of highly active antiretroviral therapy. AIDS Patient Care and STDs. 2002;16:75–81. doi: 10.1089/10872910252806126. [DOI] [PubMed] [Google Scholar]
- Wildes JE, Marcus MD, Fagiolini A. Obesity in patients with bipolar disorder: A biopsychosocial-behavioral model. Journal of Clinical Psychiatry. 2006;67:904–915. doi: 10.4088/jcp.v67n0607. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Leitch M, Mobini S. Impulsivity is associated with the disinhibition but not restraint factor from the Three Factor Eating Questionnaire. Appetite. 2008;50:469–476. doi: 10.1016/j.appet.2007.10.002. [DOI] [PubMed] [Google Scholar]