Abstract
Working memory mediates the short-term maintenance of information. Virtually all empirical research on working memory involves investigations of working memory for verbal and visual information. Whereas aging is typically associated with a deficit in working memory for these types of information, recent findings suggestive of relatively well-preserved long-term memory for emotional information in older adults raise questions about working memory for emotional material. This study examined age differences in working memory for emotional versus visual information. Findings demonstrate that, despite an age-related deficit for the latter, working memory for emotion was unimpaired. Further, older adults exhibited superior performance on positive relative to negative emotion trials, whereas their younger counterparts exhibited the opposite pattern.
Keywords: emotion, working memory, affect, cognition, positivity effect
In recent years, research on aging has begun to challenge long-standing assumptions about ubiquitous decline and paint a more nuanced characterization of psychological functioning in later life. To be clear, we do note that decline is well documented, notably so in cognitive processing capacity, also known as fluid intelligence (see Craik & Salthouse, 2000). However, decline is less evident in other types of cognitive processes, for instance, those that require procedural memory, world knowledge, or cultural knowledge, also known as crystallized intelligence (see Schaie, 2005). Research also suggests that emotional functioning is largely spared from aging decline (see Carstensen, Mikels, & Mather, in press). For example, few age differences are apparent on measures of subjective reports and observations of facial expressions during controlled laboratory tasks (e.g., Levenson, Carstensen, Friesen, & Ekman, 1991; Tsai, Levenson, & Carstensen, 2000) and even in autonomic responding when stimuli are highly age relevant (Kunzmann & Grühn, 2005). Moreover, studies of everyday emotional experience suggest that a relatively positive emotional balance is associated with age (Carstensen, Pasupathi, Mayr, & Nesselroade, 2000; Labouvie-Vief & Medler, 2002; Mroczek & Kolarz, 1998). Memory for emotional material is relatively good in old age (Charles, Mather, & Carstensen, 2003; Kensinger, Brierley, Medford, Growdon, & Corkin, 2002), and although there are some documented age deficits on experimental measures of emotion that place significant demands on deliberative processing (Labouvie-Vief & Diehl, 2000), measures of emotional aging that are less cognitively demanding reliably follow a positive trajectory.
Given such observations, questions follow about the ways diverging trajectories interact. In this report, we consider working memory for emotional material. On the one hand, there is considerable evidence that working memory declines with age. On the other hand, emotional processing and emotional long-term memory are relatively preserved. If working memory for emotional material is well maintained, the finding may hold relevance for ways to present information such that older adults process it more efficiently.
Working memory is the multicomponential cognitive system involved in the maintenance and manipulation of information in the service of goals (Baddeley, 1986; Baddeley & Hitch, 1974). The functions of working memory include not only the short-term maintenance or rote storage of information but also the manipulation, recoding, or processing of briefly stored information. Researchers have used a variety of tasks to investigate different aspects of working memory, from simple (e.g., Miller, 1956) to complex span tasks (e.g., Daneman & Carpenter, 1980; Turner & Engle, 1989). Whereas simple span tasks require the maintenance of information, complex span tasks require the maintenance of information with additional processing (see Miyake, 2001). Another type of working memory task, the delayed-response task, has been widely used to investigate the neural correlates of working memory in both animals and humans (D'Esposito et al., 1998; Goldman-Rakic, 1987; Sternberg, 1966). The use of these different tasks has allowed for the examination of subsidiary processes. In addition to examination of the different processes involved in working memory, much work has examined different types of material maintained in working memory. Many different types of information can be held in working memory, including verbal information (Baddeley, 1986), visual information (Logie, 1995), object representations (Courtney, Petit, Maisog, Ungerleider, & Haxby, 1998; Smith et al., 1995), semantic information (Potter, 1993; Shivde, 2002), and emotion (Gooding & Tallent, 2003; Luciana, Burgund, Berman, & Hanson, 2001; Mikels, Reuter-Lorenz, Beyer, & Fredrickson, 2005).
Researchers have studied extensively the effects of normal aging on working memory (see Reuter-Lorenz & Sylvester, 2005, for a review). Some of the initial work examining age differences in working memory suggested that age differences are slight on simple span tasks that require only storage but relatively large for complex span tasks that require storage and processing (for a review, see Baddeley, 1986; e.g., Dobbs & Rule, 1989). However, it now appears that although age differences on tasks that require both storage and processing are especially pronounced, smaller but reliable age differences are found even on tasks that primarily emphasize storage (Babcock & Salthouse, 1990; Verhaeghen, Marcoen, & Goossens, 1993). Moreover, age differences have been documented in working memory for an array of stimuli, including verbal information (Park et al., 2002; Park, Smith, Lautenschlager, & Earles, 1996), visual images (Park et al., 2002), objects (Hartley, Speer, Jonides, Reuter-Lorenz, & Smith, 2001), spatial locations (Myerson, Hale, Rhee, & Jenkins, 1999; Salthouse, 1995), and faces (Grady et al., 1995, 1998). Not only do both the storage and the processing components of working memory appear to be negatively impacted by age, these changes are notable across numerous content domains. However, researchers have not yet examined changes in working memory for emotion in different age groups. Ostensibly, given consistent decline in working memory with age, one might expect deficits in working memory for emotion as well. However, there is also reason to expect otherwise.
Research on socioemotional aging indicates considerable preservation of emotional processing. When solving highly emotional everyday problems that require deliberate and effortful processes, older adults consider the emotional factors more than do younger adults (Blanchard-Fields, Jahnke, & Camp, 1995). Not only do older adults disproportionately consider emotional information, but they also evidence superior long-term memory for emotional relative to nonemotional information, which indicates that the emotional memory enhancement effect may be intact across the life span (Charles et al., 2003; Denburg, Buchanan, Tranel, & Adolphs, 2003; Fung & Carstensen, 2003; Kensinger et al., 2002). Finally, this preservation of emotional long-term memory may be valence specific. There is growing evidence that older adults better attend to and remember positively valenced relative to negatively valenced emotional information (Charles et al., 2003; Mather & Carstensen, 2003). The developmental trend toward increasingly preferential attention to and better memory for positive information has been termed the positivity effect (Carstensen & Mikels, 2005). It is important to note, though, that the positivity effect does not have unqualified support. A handful of studies have not found positivity effects in long-term memory (Denburg et al., 2003; Kensinger et al., 2002). Although closer examination is needed, it appears that positivity effects may be missed if statistical power is low and also may not appear when older participants display relatively low executive functioning. See Carstensen et al. (in press) for a more nuanced discussion.
Socioemotional selectivity theory (Carstensen, 1995; Carstensen, Isaacowitz, & Charles, 1999) asserts that changes in the life of older adults result from motivational changes. The pursuit of knowledge-related goals in youth is surpassed by the pursuit of emotion-related goals in later years. According to the theory, time horizons determine goal structures. When people perceive time as expansive, they prioritize goals related to knowledge acquisition. In contrast, when they perceive time as limited, they prioritize goals related to emotional meaning. Because of the positive association between chronological age and mortality, older adults tend to perceive the future as shorter, and consequently they are motivated by emotion-related goals and emotion regulation. The theory asserts that, in the service of emotional goals and emotion regulation, attention and memory function preferentially for emotional information in general and for positive relative to negative emotions in particular (Carstensen & Mikels, 2005). Further, the theory predicts that, given younger adults' focus on information acquisition, they may be more willing than older adults to endure and focus on negative emotions, given these emotions' information value. The notion that younger adults are more attuned to negative relative to positive emotions is fully substantiated in the literature and has been termed the negativity bias (Baumeister, Bratslavsky, Finkenauer, & Vohs, 2001; Cacioppo, Gardner, & Berntson, 1999; Rozin & Royzman, 2001). For instance, people spend more time attending to, thinking about, and reasoning about negative versus positive events and emotions and better remember negative relative to positive information. Researchers reason that being highly motivated to attend to and remember negative emotions is adaptive and helps to ensure survival—we contend, especially at young ages. Reasoning from the influence of motivation on long-term memory and attention, we predicted that older adults would show relatively spared performance on a test of working memory for emotional information.
To investigate age-related differences in working memory for emotion, we used a modified delayed-response emotion maintenance task. The task was designed to test emotion maintenance and was modeled after standard delayed-response tasks used to test working memory (D'Esposito et al., 1998; Goldman-Rakic, 1987). The task required that participants experience a negative or positive feeling elicited by a visual image from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1999), maintain that feeling during a delay when the image was no longer present, and compare it with a feeling from a second image. We chose emotional intensity as the element to remember because, unlike valence, it is not easily verbally coded and, in contrast to arousal, it is a psychological dimension of emotion rather than a physiological dimension (Feldman Barrett & Russell, 1999; Frijda, Ortony, Sonnemans, & Clore, 1992; Reisenzein, 1994). Research also shows that older adults do not differ from younger adults in self-reports of emotional intensity (Carstensen et al., 2000; Levenson et al., 1991; Malatesta, Izard, Culver, & Nicolich, 1987; Tsai et al., 2000). We also used an analogous brightness task in the current project to achieve the additional objective of comparing older and younger adults on a nonemotional delayed-response task, for which we expected to observe an age-related deficit in performance. The brightness maintenance task also required the maintenance of a subjective impression from visual images, similar to the requirements of the emotion maintenance task. Thus, the current report examines age-related changes in the maintenance of brightness and emotion intensity in working memory, in addition to comparing the effects of valence on emotional working memory.
Given the relative preservation of emotional processing in older age, we predicted that we would see intact performance in the older adults on the emotion maintenance task and a deficit in performance on the brightness maintenance task. Additionally, given the negativity bias in memory and attention associated with younger ages (Baumeister et al., 2001; Cacioppo et al., 1999; Rozin & Royzman, 2001), we expected younger adults to show superior performance on negative relative to positive trials. Finally, relative to this negativity bias in younger adults, we predicted that older adults would show the opposite pattern of performance, consistent with the positivity effect evident in our earlier work.
Method
Participants
Twenty older adults between 64 and 80 years of age and 20 younger adults between 18 and 28 years of age were recruited from the San Francisco Bay area by a survey research firm and were paid for their participation. The characteristics for these two groups of participants are presented in Table 1. All participants were community dwelling and were free of major neurological and psychiatric problems. As can be seen in Table 1, the participant groups did not differ in years of education, t(38) = 0.58, p > .5; scaled income, t(38) = 0.56, p > .5; or self-reported health, t(38) = 1.41, p > .15. With respect to neuropsychological tests and consistent with typical profiles in the literature, the older adults did not differ from the young on a measure of world knowledge (the Vocabulary test from the Wechsler Adult Intelligence Scale—Revised [WAIS–R]; Wechsler, 1981), t(38) = 0.66, p > .5, but the older group performed more poorly than the younger group on the measures of speed of processing (the Digit-Symbol Coding test from the WAIS–R), t(38) = 6.67, p < .001, and working memory (the Digit Span test from the WAIS–R), t(38) = 2.36, p < .05.
Table 1. Participant Characteristics by Age Group.
| Younger | Older | |||
|---|---|---|---|---|
| Characteristic | M | SD | M | SD |
| Age (in years) | 22.35 | 2.98 | 72.50 | 5.81 |
| Sex | 50% F, 50% M | 50% F, 50% M | ||
| Ethnicity | 50% AA, 50% EA | 50% AA, 50% EA | ||
| Education (in years) | 14.16 | 1.57 | 13.85 | 1.76 |
| Scaled income | 5.65 | 3.67 | 4.94 | 4.11 |
| Self-reported health (Wahler) | 39.55 | 20.17 | 30.95 | 18.25 |
| Vocabulary (WAIS–R) | 43.10 | 16.31 | 46.10 | 11.99 |
| Digit-Symbol Coding (WAIS–R) | 59.21 | 10.58 | 37.35 | 9.90 |
| Digit Span (WAIS–R) | 15.45 | 3.97 | 12.55 | 3.80 |
Note. Sex: F = female, M = male; Ethnicity: AA = African American, EA = European American; Scaled income: on a scale of 1–16 at intervals of $10,000 total household income for the last year; Self-reported health (Wahler, 1973): rating of 42 different symptoms on a scale of 05, maximum score = 210; Vocabulary from the WAIS–R (Wechsler, 1981): maximum score = 66; Digit-Symbol Coding from the WAIS–R: maximum score = 93; Digit Span from the WAIS–R: maximum score = 28.
Apparatus
We used a Macintosh G4 computer with PsyScope software (Cohen, MacWhinney, Flatt, & Provost, 1993) for stimulus presentation and data acquisition.
Development and Description of Stimulus Materials
Our experimental delayed-response task required participants to compare the emotional or brightness intensity of two visual images; thus, we needed to develop image pairs that differed in their emotional or brightness intensity. We used emotional and brightness intensity data that ranged from 1 (none at all) to 7 (a great amount).1 To make the task sufficiently difficult to avoid ceiling effects, we constructed approximately half of the image pairs to differ by less than 1 point and the other half of the pairs to differ by 1–2 points. We created 40 neutral image pairs differing in brightness intensity along with 40 negative image pairs and 40 positive image pairs differing in emotional intensity. For details about the image pairs, see Appendixes A, B, and C. The average difference between images for the negative pairs was 1.00 (SD = 0.47) in emotional intensity, the average difference between the positive pairs was 1.00 (SD = 0.46) in emotional intensity, and the average difference between images for the neutral brightness pairs was 1.00 (SD = 0.45) in brightness intensity. We used emotion-eliciting images in the emotion maintenance task and neutral images in the brightness maintenance task because of the emotional influences on verbal and visual working memory (see Gray, 2001). We segregated the stimuli in this way to minimize the influence of the emotional images on the performance of the brightness maintenance task. Using the emotional images in the brightness maintenance task would allow for a stimulus-controlled comparison; however, using nonemotional stimuli allows for a process-controlled comparison. In other words, using nonemotional images in the brightness task guaranteed that brightness processes were kept independent of emotional processes. Accuracy on the tasks was measured by participants' agreement with the relative intensity assignments (i.e., the high vs. low member of a pair).
Appendix A. Positive Image Pairs.
| Pic1 | Title | Intensity | Pic2 | Title | Intensity | Difference |
|---|---|---|---|---|---|---|
| 60204 | City | 3.98 | 8185 | Skydivers | 4.25 | 0.27 |
| 60018 | Waterfront | 3.98 | 5629 | Hiker | 4.33 | 0.35 |
| 8501 | Money | 4.03 | 4599 | Romance | 4.55 | 0.52 |
| 1650 | Jaguar | 3.88 | 8180 | Cliff Divers | 4.68 | 0.80 |
| 60112 | Lake | 3.73 | 5594 | Sky | 4.53 | 0.80 |
| 93052 | Pie | 2.95 | 2050 | Baby | 3.78 | 0.83 |
| 5830 | Sunset | 3.53 | 2070 | Baby | 4.55 | 1.02 |
| 1942 | Turtles | 3.50 | 5910 | Fireworks | 4.63 | 1.13 |
| 2345 | Children | 3.28 | 8490 | Roller Coaster | 4.43 | 1.15 |
| 60134 | Fort | 3.05 | 60267 | Rainbow | 4.30 | 1.25 |
| 8497 | Carnival Ride | 2.98 | 5621 | Sky-divers | 4.63 | 1.65 |
| 5991 | Sky | 3.00 | 5950 | Lightning | 4.73 | 1.73 |
| 5982 | Sky | 3.38 | 4598 | Couple | 5.13 | 1.75 |
| 93060 | Cheesecake | 2.88 | 2260 | Baby | 3.15 | 0.27 |
| 1340 | Women | 3.40 | 60273 | Houses | 3.75 | 0.35 |
| 8034 | Skier | 3.63 | 8117 | Hockey | 4.15 | 0.52 |
| 7195 | Teeth | 3.13 | 1810 | Hippo | 3.88 | 0.75 |
| 60262 | Sand | 3.20 | 1850 | Camels | 4.00 | 0.80 |
| 1500 | Dog | 3.00 | 2550 | Couple | 3.83 | 0.83 |
| 60084 | Sunset | 3.08 | 5890 | Earth | 4.10 | 1.02 |
| 8220 | Runners | 3.35 | 8116 | Football | 4.48 | 1.13 |
| 60152 | Fort | 3.05 | 60288 | Waterfall | 4.20 | 1.15 |
| 2560 | Picnic | 2.80 | 2311 | Mother | 4.05 | 1.25 |
| 8600 | Mascot | 2.65 | 1811 | Monkeys | 4.30 | 1.65 |
| 5201 | Nature | 2.30 | 2540 | Mother | 4.03 | 1.73 |
| 60025 | Car | 2.55 | 8030 | Skier | 4.30 | 1.75 |
| 2040 | Baby | 3.28 | 8130 | Pole Vaulter | 3.53 | 0.25 |
| 1660 | Gorilla | 3.43 | 4601 | Romance | 3.78 | 0.35 |
| 60099 | Plaza | 3.23 | 8503 | Money | 3.75 | 0.52 |
| 2240 | Child | 3.10 | 60224 | Boat | 3.83 | 0.73 |
| 8250 | Motorcyclist | 3.20 | 2209 | Bride | 3.95 | 0.75 |
| 8170 | Sailboat | 3.20 | 2170 | Mother | 4.00 | 0.80 |
| 8041 | Diver | 3.15 | 5831 | Seagulls | 3.98 | 0.83 |
| 8460 | Runner | 3.18 | 8300 | Pilot | 4.20 | 1.02 |
| 60316 | Poppies | 3.05 | 1721 | Lion | 4.18 | 1.13 |
| 1610 | Rabbit | 3.08 | 1440 | Seal | 4.23 | 1.15 |
| 1590 | Horse | 2.55 | 8280 | Diver | 3.80 | 1.25 |
| 1540 | Cat | 2.98 | 1710 | Puppies | 4.23 | 1.25 |
| 1604 | Butterfly | 2.48 | 2057 | Father | 4.13 | 1.65 |
| 60220 | Umbrella Monks | 2.53 | 1460 | Kitten | 4.28 | 1.75 |
Appendix B. Negative Image Pairs.
| Pic1 | Title | Intensity | Pic2 | Title | Intensity | Difference |
|---|---|---|---|---|---|---|
| 1030 | Snake | 2.98 | 9594 | Injection | 3.25 | 0.27 |
| 9582 | Dental Exam | 2.55 | 1274 | Roaches | 2.88 | 0.33 |
| 9584 | Dental Exam | 2.73 | 1051 | Snake | 3.30 | 0.57 |
| 1220 | Spider | 3.08 | 9180 | Seal | 3.83 | 0.75 |
| 1080 | Snake | 3.10 | 9560 | Duck In Oil | 3.85 | 0.75 |
| 2810 | Boy | 3.18 | 1300 | Pitbull | 4.05 | 0.87 |
| 1070 | Snake | 3.60 | 3160 | Eye Disease | 4.68 | 1.08 |
| 1090 | Snake | 3.08 | 9620 | Shipwreck | 4.20 | 1.12 |
| 1270 | Roach | 2.58 | 1111 | Snakes | 3.78 | 1.20 |
| 1201 | Spider | 3.70 | 9571 | Cat | 4.95 | 1.25 |
| 9101 | Cocaine | 3.00 | 6360 | Attack | 4.65 | 1.65 |
| 1230 | Spider | 2.73 | 9181 | Dead cows | 4.45 | 1.72 |
| 1390 | Bees | 2.43 | 1019 | Snake | 4.20 | 1.77 |
| 1110 | Snake | 3.35 | 5972 | Tornado | 3.58 | 0.23 |
| 1302 | Dog | 3.23 | 1052 | Snake | 3.58 | 0.35 |
| 7380 | Roach On Pizza | 4.25 | 9300 | Dirty | 4.80 | 0.55 |
| 9830 | Cigarettes | 2.80 | 7360 | Flies On Pie | 3.55 | 0.75 |
| 1945 | Turtle | 3.33 | 7361 | Meat Slicer | 4.13 | 0.80 |
| 3220 | Hospital | 3.88 | 9050 | Plane Crash | 4.70 | 0.82 |
| 2700 | Woman | 3.35 | 3300 | Disabled Child | 4.38 | 1.03 |
| 2271 | Woman | 2.93 | 2141 | Grieving Fem | 4.05 | 1.12 |
| 3280 | Dental Exam | 2.53 | 1113 | Snake | 3.73 | 1.20 |
| 9290 | Garbage | 2.78 | 8230 | Boxer | 4.00 | 1.22 |
| 2490 | Man | 2.95 | 9561 | Sick Kitty | 4.65 | 1.70 |
| 9331 | Bag man | 2.70 | 3230 | Dying Man | 4.43 | 1.73 |
| 9373 | Garbage | 3.30 | 3250 | Open Chest | 5.03 | 1.73 |
| 9110 | Puddle | 2.68 | 6840 | Police | 4.43 | 1.75 |
| 2120 | Angry Face | 2.70 | 9230 | Oil Fire | 4.43 | 1.73 |
| 6200 | Aimed Gun | 3.00 | 6213 | Terrorist | 4.25 | 1.25 |
| 6010 | Jail | 3.33 | 6260 | Aimed Gun | 4.58 | 1.25 |
| 9440 | Skulls | 3.63 | 3500 | Attack | 4.78 | 1.15 |
| 2682 | Police | 2.70 | 5971 | Tornado | 3.83 | 1.13 |
| 8231 | Boxer | 3.23 | 6370 | Attack | 4.25 | 1.02 |
| 3022 | Scream | 3.78 | 5940 | Lava | 4.03 | 0.25 |
| 6410 | Aimed Gun | 2.63 | 3210 | Surgery | 2.93 | 0.30 |
| 2692 | Bomb | 3.35 | 6300 | Knife | 3.88 | 0.53 |
| 6930 | Missiles | 3.00 | 9160 | Soldier | 3.73 | 0.73 |
| 9280 | Smoke | 3.35 | 9480 | Skull | 4.10 | 0.75 |
| 6210 | Aimed Gun | 3.75 | 6211 | Attack | 4.55 | 0.80 |
| 9404 | Soldiers | 3.50 | 9622 | Jet | 4.35 | 0.85 |
Appendix C. Brightness Image Pairs.
| Pic1 | Title | Intensity | Pic2 | Title | Intensity | Difference |
|---|---|---|---|---|---|---|
| 1121 | Lizard | 4.60 | 2575 | Propeller | 3.73 | 0.88 |
| 1560 | Hawk | 4.60 | 7351 | Pizza | 3.58 | 1.03 |
| 1670 | Cow | 4.73 | 5532 | Mushrooms | 3.63 | 1.10 |
| 2020 | Adult | 4.85 | 2480 | Elderly Man | 3.70 | 1.15 |
| 2220 | Male Face | 4.88 | 5533 | Mushrooms | 3.38 | 1.50 |
| 2514 | Woman | 4.95 | 1313 | Frog | 3.55 | 1.40 |
| 2516 | Elderly Woman | 4.58 | 7034 | Hammer | 4.05 | 0.53 |
| 2620 | Woman | 4.80 | 5940 | Lava | 3.63 | 1.18 |
| 2702 | Binge Eating | 4.50 | 5510 | Mushroom | 3.90 | 0.60 |
| 2840 | Chess | 4.78 | 5530 | Mushroom | 3.68 | 1.10 |
| 2850 | Tourist | 5.00 | 5950 | Lightning | 3.38 | 1.63 |
| 4571 | Attractive Man | 4.13 | 7080 | Fork | 3.83 | 0.30 |
| 4610 | Romance | 4.68 | 2487 | Musician | 4.15 | 0.53 |
| 5000 | Flower | 4.78 | 5410 | Violinist | 3.78 | 1.00 |
| 5120 | Pine Needles | 4.43 | 7320 | Desserts | 3.80 | 0.63 |
| 5130 | Rocks | 4.45 | 7829 | Agate | 3.95 | 0.50 |
| 5220 | Nature | 4.48 | 7190 | Clock | 3.98 | 0.50 |
| 5520 | Mushroom | 4.53 | 7030 | Iron | 3.83 | 0.70 |
| 5534 | Mushrooms | 4.88 | 7004 | Spoon | 3.73 | 1.15 |
| 5731 | Flowers | 5.05 | 2485 | Man | 3.50 | 1.55 |
| 5779 | Courtyard | 4.58 | 60060 | Clock | 4.05 | 0.53 |
| 5800 | Leaves | 4.38 | 7560 | Freeway | 4.13 | 0.25 |
| 6150 | Outlet | 4.20 | 2810 | Boy | 3.73 | 0.48 |
| 7002 | Towel | 4.58 | 7234 | Ironing Board | 3.90 | 0.68 |
| 7035 | Mug | 4.55 | 2600 | Beer | 3.78 | 0.78 |
| 7140 | Bus | 4.85 | 2681 | Police | 3.75 | 1.10 |
| 7150 | Umbrella | 4.43 | 2890 | Twins | 2.93 | 1.50 |
| 7160 | Fabric | 4.55 | 7090 | Book | 4.05 | 0.50 |
| 7170 | Light Bulb | 4.55 | 5740 | Plant | 4.03 | 0.52 |
| 7402 | Pastry | 4.83 | 2130 | Woman | 3.63 | 1.20 |
| 7490 | Window | 4.85 | 2383 | Secretary | 4.15 | 0.70 |
| 7705 | Cabinet | 4.70 | 7283 | Fruit | 3.63 | 1.08 |
| 7830 | Agate | 4.50 | 9070 | Boy | 3.38 | 1.13 |
| 8311 | Golfer | 5.25 | 5920 | Volcano | 3.38 | 1.88 |
| 60323 | Guards | 4.78 | 7620 | Jet | 3.65 | 1.13 |
| 93012 | Food | 4.68 | 7175 | Lamp | 3.18 | 1.50 |
| 93025 | Hamburger | 5.15 | 2385 | Girl | 3.43 | 1.73 |
| 93049 | Sorbet | 5.00 | 9700 | Worker Trash | 3.38 | 1.63 |
| 93073 | Corkscrew | 4.98 | 7920 | Car Crash | 4.10 | 0.88 |
| 93079 | Pot | 5.15 | 2580 | Chess | 3.28 | 1.88 |
For the negatively and positively valenced pairs, specific emotion category was not an explicit part of the task but was used for control purposes in stimulus pair construction via the data of Mikels et al. (in press). In two studies, Mikels et al. (in press) had participants (overall mean age = 18.75; overall 50% female) rate either positive or negative subsets of IAPS images on discrete emotional categories. For the positive subset, participants rated each image on four emotion categories: amusement, awe, contentment, and excitement. For the negative subset, they rated each image on four emotions: disgust, fear, sadness, and anger. The images were then grouped into three metacategories separately for the negative and positive subsets: single discrete emotion, blended emotion, and undifferentiated emotion. The single discrete emotion category consisted of images rated as eliciting one discrete emotion, whereas the blended emotion category elicited a mixture of two or more emotions (e.g., disgust and sadness as rated higher than fear and anger), and the undifferentiated emotion category elicited negative or positive affect without categorical specificity (i.e., images for which no emotion label was rated as higher than any other). The 40 negative image pairs used in the current study consisted of the following categories: 13 single emotion pairs (5 disgust, 3 fear, 5 sadness), 13 blended emotion pairs, and 14 undifferentiated emotion pairs. The 40 positive image pairs were from the following categories: 13 single emotion pairs (3 amusement, 3 awe, 4 contentment, 3 excitement), 13 blended emotion pairs, and 14 undifferentiated emotion pairs.
Design and Procedure
Task parameters were identical for all tasks and conditions: An image was presented for 5 s (target), immediately followed by a retention interval (3 s), a second image for 5 s (probe), and a green cross. For a schematic of representative trials, see Figure 1. For the positive and negative emotion maintenance task conditions, we told participants that in each trial they would view an emotion-eliciting image and that they should let their feelings occur naturally. After the image disappeared, we instructed them to maintain their gaze on a fixation cross and to sustain the feeling at the same intensity that they felt while viewing the image. After the delay, participants viewed a second image and were to experience the emotions elicited by it. After viewing the second image, a green cross appeared, which signaled participants to indicate whether their feelings from the second image had a higher or lower emotional intensity compared with their feelings from the first image in the pair. For the brightness maintenance task, the instructions were the same, except that we instructed participants to assess, hold in mind, and compare the brightness intensity they perceived in the two images.
Figure 1.
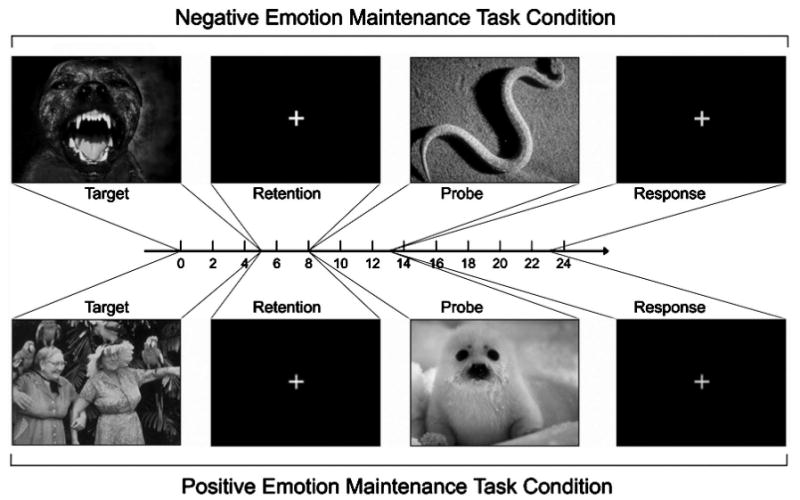
Task schematic for the emotion maintenance task. An image was presented for 5 s, followed by a 3-s retention interval and then a second image for 5 s. After the second image, an intertrial interval followed, during which participants made a higher versus lower intensity judgment.
For half of the trials in each subset (20 pairs), the second image presented was higher in the task's respective emotional or brightness intensity than the first, and for the remaining pairs (the other 20 pairs), the second image was lower. The emotional or brightness intensity order of the images in a pair was counterbalanced across participants. Finally, to make the tasks sufficiently difficult, for each task and valence condition, we divided the pairs by their intensity similarity: highly similar pairs (emotional intensity difference of 0.87 or less for the negative trials and 0.83 or less for the positive trials; brightness intensity difference of 0.88 or less for the brightness trials; i.e., difficult trials) and highly dissimilar pairs (emotional intensity difference of 1.02 or more for the negative trials and for the positive trials; brightness intensity difference of 1.00 or more for the brightness trials; i.e., easy trials). This manipulation was intended to verify that our performance measurements were consistent with expectations regarding difficulty. For these two variables (order and similarity), we expected to observe no age differences. Thus, overall, there were two within-subject factors, intensity similarity (highly similar or highly dissimilar) and intensity order (second image higher or lower). In the emotion maintenance task, there was the additional within-subject factor of valence (negative or positive).
Emotion maintenance task trials were completed with positive and negative trials randomly intermixed in two blocks that were independent from one block of brightness maintenance trials. Pairs were presented randomly within each block, and each block was followed by a short break before the next block. The ordering of these blocks was counterbalanced across participants.
After performing the maintenance tasks, participants completed the neuropsychological test battery and then completed emotion and brightness ratings for each image. In both rating tasks, images viewed during the maintenance tasks were presented again with the emotional and neutral images in separate blocks. For the emotion block, participants were instructed to provide a rating on a scale of 1–7 as to the intensity of the emotion they experienced from the image. For the neutral block, participants were instructed to provide a rating on a scale of 1–7 as to the intensity of the brightness they perceived in the image.
Results
Preliminary Analyses
The intensity ratings collected after the working memory tasks served two purposes. First, we used them to determine whether the older and younger groups differed in their ratings of either emotion or brightness intensity. This was important because the image pairs used in this experiment were based on ratings obtained from an independent sample of younger adults. Second, we used these ratings normatively in additional analyses that served as a parity check to determine whether the image pairs created on the basis of previously obtained norms agreed with the relative intensity ratings generated by the present sample of participants (see Primary Analyses section).
To determine whether the emotion or brightness intensity ratings of the older adults differed from those obtained from the younger adults, we computed independent-sample t tests on the ratings for each subset of images (positive, negative, brightness). These tests revealed that ratings generated by the older adults were equivalent to those generated by the young. For means, standard deviations, and ranges, see Table 2. To further explore the variability and reliability of these emotion and brightness ratings, we examined intraindividual variability as a function of age. We first calculated the standard deviation for each individual's mean emotional intensity rating for the positive and negative images and the standard deviation for each individual's mean brightness intensity rating for the neutral images. We then computed independent-sample t tests on these standard deviations for each subset (positive, negative, brightness) to examine whether older adults individually were more variable than the younger adults. These tests revealed that the intraindividual variability was equivalent for both older and younger adults. Thus, with reasonable confidence, we can assume that the emotional and brightness intensity ratings were equivalent for the younger and older adults.
Table 2. Intensity Ratings Broken Down by Age Group and Task Domain.
| Negative | Positive | Brightness | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | M | SD | Range | |
| Young | 4.80 | 0.80 | 3.24–6.74 | 3.50 | 1.22 | 1.14–6.38 | 4.33 | 0.45 | 3.58–5.31 |
| Old | 4.33 | 1.11 | 2.25–6.21 | 3.39 | 1.01 | 1.80–5.49 | 4.55 | 0.79 | 3.13–5.98 |
| Overall | 4.57 | 3.45 | 4.44 | ||||||
Primary Analyses
We computed repeated measures analyses of variance (ANOVAs) to examine effects of age and condition. The principal dependent variable was concordance with the same ratings that provided the basis for constructing the image pairs. That is, accuracy was based on participants' agreement with relative intensity assignments made by an independent sample of raters (i.e., the high vs. low member of a pair).2
As the two valence conditions of the emotion maintenance task had no equivalent in the brightness maintenance task, we analyzed the concordance scores using two separate repeated measures ANOVAs: The first compared emotion and brightness maintenance, and the second compared positive versus negative conditions in the emotion maintenance task. We computed both ANOVAs with the one between-subjects factor of age group (young, older). For the first ANOVA, there were also the three within-subject factors of task domain (emotion, brightness), intensity similarity (highly similar, highly dissimilar), and intensity order (second image lower, second image higher). For the second ANOVA, there were the three within-subject factors of valence (positive, negative), intensity similarity (highly similar, highly dissimilar), and intensity order (second image lower, second image higher).3
With respect to emotion and brightness maintenance, main effects for intensity similarity, F(1, 38) = 29.29, p < .001, and order, F(1, 38) = 5.52, p < .05, showed that performance was superior for both groups on highly dissimilar pairs (69.82%) compared with highly similar pairs (59.89%) and that performance was superior when the second image was higher (67.40%) versus lower (62.32%). Additionally, a main effect of group emerged, F(1, 38) = 6.15, p < .05, indicating that, overall, older adults (61.34% concordance) performed more poorly than younger adults (68.38% concordance). It is important to note that the effect was qualified by an interaction of group and task domain, F(1, 38) = 5.60, p < .05, showing that the deficit in performance was driven by the brightness maintenance task. That is, whereas the older adults displayed significantly poorer performance on the brightness maintenance task relative to the younger adults, t(38) = 2.68, p < .05, these groups performed equivalently on the emotion maintenance task, t(38) = 0.17, p > .85. For means and standard deviations, see Table 3.
Table 3. Concordance Means and Standard Deviations Broken Down by Group and Task Domain.
| Brightness | Emotion | Overall | ||||
|---|---|---|---|---|---|---|
| M (%) | SD | M (%) | SD | M (%) | ||
| Young | 73.53 | 12.70 | 63.23 | 7.70 | 68.38 | |
| Old | 59.85 | 18.93 | 62.82 | 7.60 | 61.34 | |
| Overall | 66.69 | 63.03 | ||||
The second ANOVA for emotion maintenance alone revealed a main effect for intensity similarity, F(1, 38) = 29.21, p < .001, indicating that both groups performed better on trials in which the pairs were highly dissimilar (67.97%) relative to highly similar (58.08%) in their emotional intensity. This effect was qualified with an Intensity Similarity × Valence interaction, F(1, 38) = 9.00, p < .01; the intensity similarity effect was stronger for the negative emotion trials, t(39) = 5.54, p < .001, than for the positive emotion trials, t(19) = 3.10, p < .005 (see Table 4 for means and standard deviations). It is interesting to note that an intensity order main effect did not emerge, which may be explained by a marginally significant Group × Order interaction, F(1, 38) = 3.75, p = .06. In the emotion maintenance task, performance was superior for the younger adults when the second image was higher versus lower than the first, t(19) = 2.18, p < .05, but not significantly better for the older adults, t(19) = 0.24, p > .8. Although the younger adults performed significantly better than chance when the second image was higher (p < .001), they performed at chance levels when the second image was lower than the first (p > .05). For means and standard deviations, see Table 5. The key result to emerge from the analysis of the emotion maintenance task alone was a Group × Valence interaction, F(1, 38) = 9.37, p < .005; whereas younger adults showed superior working memory for negative relative to positive emotional trials, t(19) = 2.15, p < .05, older adults exhibited superior working memory for positive relative to negative emotional trials, t(19) = 2.25, p < .05. Moreover, on the positive trials, the older adults actually performed better than the younger adults, t(38) = 3.38, p < .05, one-tailed. For a visual depiction of this interaction, see Figure 2.
Table 4. Concordance Means and Standard Deviations Broken Down by Valence and Intensity Similarity.
| Highly Dissimilar | Highly Similar | Overall | |||
|---|---|---|---|---|---|
| M (%) | SD | M (%) | SD | M (%) | |
| Negative | 69.42 | 15.09 | 55.58 | 9.19 | 66.69 |
| Positive | 66.53 | 12.08 | 60.58 | 9.99 | 63.03 |
| Overall | 67.98 | 58.08 | |||
Table 5. Concordance Means and Standard Deviations Broken Down by Group and Intensity Order.
| 2nd Higher | 2nd Lower | Overall | |||
|---|---|---|---|---|---|
| M (%) | SD | M (%) | SD | M (%) | |
| Young | 69.15 | 12.49 | 57.32 | 16.06 | 63.24 |
| Old | 62.38 | 11.41 | 63.26 | 10.98 | 62.82 |
| Overall | 65.77 | 60.29 | |||
Figure 2.
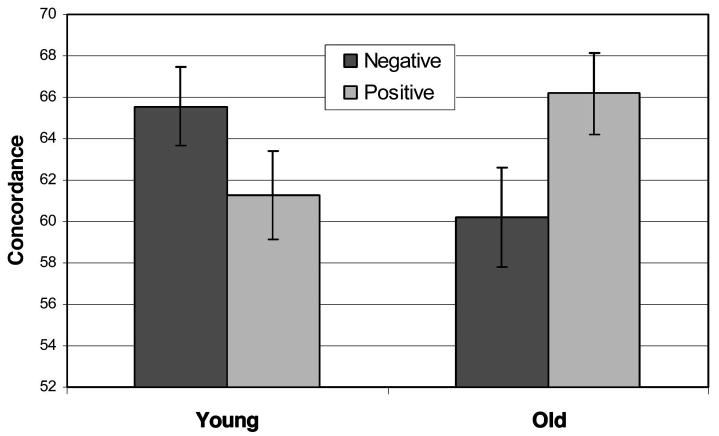
A graph depicting emotion maintenance performance as a function of age and valence. Older adults showed superior performance for positive relative to negative emotional stimuli, whereas younger adults showed the opposite pattern.
Finally, to examine whether performance on either maintenance task was related to another measure of working memory, we conducted correlations with digit span performance. Performance on the brightness maintenance task correlated with digit span performance (r = .390, p < .05), whereas performance on the emotion maintenance task did not (r = .204, p > .20). These correlational results suggest that our brightness maintenance task relies on working memory processes that are related to the cognitive working memory processes underlying the digit span task and are potentially dissociable from the processes underlying the emotion maintenance task.
Discussion
The current study examines age differences in performance on a working memory task in which participants were required to maintain a representation of emotional intensity while they made judgments about pairs of images. We hypothesized that working memory for emotional material would be relatively well preserved in older age, given prior findings that indicate that older adults remember emotional material better than nonemotional information. We also hypothesized—on the basis of prior findings—that older people would perform best on trials involving positive images.
Our findings document the expected age deficit on a working memory maintenance task in which participants made brightness judgments. However, the findings also reveal that the age deficit was eliminated on a working memory maintenance task in which the elements to be remembered were emotional and that the effect was most dramatic when the emotional content was positively valenced as opposed to negatively valenced.
The striking interaction of age with valence provides support for the positivity effect, namely, the developmental trend in which the ratio of positive to negative material becomes more positive over time in attention and memory (Carstensen & Mikels, 2005). In younger adults, negative emotions and events appear to have a stronger impact than positive emotions in physiological reactivity, attentional focus, reasoning, and long-term memory (see Baumeister et al., 2001; Cacioppo et al., 1999; Rozin & Royzman, 2001). In contrast, several studies have shown that older adults exhibit preferential attention to and superior memory for stimuli that are positively valenced (for a review, see Carstensen & Mikels, 2005). In the context of socioemotional selectivity theory, older adults' focus on the positive represents a form of emotion regulation.
With respect to several other effects, we found that, on both maintenance tasks, performance was superior when the intensity similarity between the images compared was dissimilar versus similar. Additionally, we found that performance was better on trials in which the second image was higher rather than lower than the first image, which indicates a bias to respond “higher” more often than “lower.” In other words, the participants exhibited a bias to judge the current state as higher than the one held in memory, which we could consider an immediate dominance effect, suggesting that the power of the current state overrides previous states. It is interesting, though, that when we examined only the emotion maintenance task, the intensity order effect did not emerge. However, a marginally significant Intensity Order × Group interaction did surface, indicating that, for emotion maintenance, younger adults did exhibit a significant immediate dominance effect, whereas older adults did not. Given that older adults report a greater ability to regulate their emotions, perhaps older adults are able to overcome the dominance of the current emotional state in a regulatory fashion. Alternatively, though, the bias of younger adults to judge the second state as higher could reflect that their emotional reactions have longer duration than those of older adults; perhaps their emotional reactions to the first image spilled over to the second. This explanation suggests that older adults are less affected by spillover effects, which could be a result of age differences in the duration, differentiation, or complexity of emotional experiences (Carstensen et al., 2000; Charles, 2005; Labouvie-Vief, DeVoe, & Bulka, 1989; Labouvie-Vief & Medler, 2002). Teasing apart these hypotheses represents an exciting direction for future research.
The current findings elucidate age-related changes on a task that required the maintenance and comparison of feelings. It remains unclear to what extent the results are due to maintenance processes versus comparison processes. Given that older adults appear to regulate emotions even better than younger adults (see Carstensen, Fung, & Charles, 2003), the executive processes associated with working memory for emotion may be unimpaired with age, despite the consistent age-related deficits on tests that measure cognitive executive or frontal lobe functions (for reviews, see Moscovitch & Winocur, 1995; Rhodes, 2004; West, 1996; Winocur, 1998). However, there is not necessarily reason to assume that emotion maintenance processes are impaired with advanced age. Thus, future research is needed to disentangle the relation of age to maintenance versus executive processes associated with working memory for emotion. With respect to different aspects of emotional experience, are there age differences in the ability of older adults to maintain other aspects of emotion, such as representations of facial affect or physiological arousal? Finally, certain discrete emotions may be more age relevant, such as sadness (Kunzmann & Grühn, 2005), whereas older adults may experience other discrete emotions to a lesser degree, such as anger (Lawton, Kleban, & Dean, 1993; Malatesta-Magai, 1999). Are there age differences in the ability to maintain different discrete emotional states, such as sadness versus anger? Clearly, much future work is needed to more fully understand the changes in working memory for emotion with age; however, the current study suggests that such investigations may yield promising new insights.
Hypotheses tested in the current study were motivated by socioemotional selectivity theory and provide support for a motivational interpretation. However, the findings do not rule out alternative explanations, including the possibility that distinct neural changes associated with aging (Raz et al., 1997) and age-related changes in different brain regions contributed to this pattern of findings. It appears that aging may be associated with differential atrophy of prefrontal subregions; whereas the dorsolateral prefrontal cortex is marked by substantial atrophy, the ventromedial prefrontal cortex may be less affected by age-related deterioration (Haug et al., 1983). Further, tasks that require dorsolateral prefrontal executive functions show age-related deficits, whereas tasks that rely on ventromedial and orbitofrontal social and emotional processes show age invariance (MacPherson, Phillips, & Della Sala, 2002). Thus, perhaps the ventromedial and orbitofrontal regions of the prefrontal cortex underlie emotion maintenance and affective executive function. As such, the current findings may be a result of different prefrontal brain atrophy. These rival explanations are not necessarily mutually exclusive; however, the results of the current project do not favor either interpretation.
The observation that the maintenance of brightness intensity was more susceptible to age-related decrements than was the maintenance of emotional intensity is consistent with the possibility that different and dissociable working memory processes underlie these tasks. Moreover, the fact that valence influenced performance on the emotion maintenance task suggests that the processes mediating this task were indeed affective in nature.
Regardless of the underlying mechanisms, these findings underscore the importance of emotion–cognition interactions in understanding the aging mind. These findings also have important implications for improving the psychological functioning of older adults in various domains. For instance, everyday reasoning and problem solving require the maintenance of multiple mental representations in working memory, such as the goals; the rules; and the actual stimuli, facts, and details (Carpenter, Just, & Shell, 1990). Everyday problem solving often also requires the processing and maintenance of other types of mental representations, including the emotional valence, intensity, or reward value of a stimulus or event (Blanchard-Fields et al., 1995; Damasio, 1994; Davidson & Irwin, 1999). Given the preservation of working memory maintenance for emotional intensity in older age, if older adults encoded information emotionally and used these affective representations to a greater extent than cognitive representations, would they make better decisions? Given the positivity effect in affective working memory with age, if health care messages were presented to older adults in a positive manner, might these individuals be better able to process the messages? These questions represent a few implications of the current project and suggest that the preserved and upward trajectory of emotional functioning may help to counteract cognitive deterioration in certain domains.
Acknowledgments
This project was supported by National Institute on Aging Research Grant AG08816 to Laura L. Carstensen, National Institute on Aging Research Grant AG18286 to Patricia A. Reuter-Lorenz, and Ruth L. Kirschstein National Research Service Award AG022264 to Joseph A. Mikels. We thank Sarah Sullivan, Gina Schiel, and Sam Maglio for assistance with data collection and analysis.
Footnotes
In three studies, Mikels et al. (2005) collected emotional intensity data on (a) 203 negatively valenced IAPS images and (b) 238 positively valenced IAPS images as well as (c) brightness intensity data on 199 neutral IAPS images from 120 participants total (overall mean age = 19.20; overall 51% female). They ran 40 participants in each study (negative, positive, brightness). In the studies with negative and positive images, participants rated the intensity of their emotional experience on a 7-point scale ranging from 1 (none at all) to 7 (a great amount). In the study with neutral images, participants rated the subjective intensity of their perception of brightness, also on a 7-point scale.
As we have noted, to establish that our sample of older adults did not rate the intensity of stimuli differently from the independent sample on which image pairs were based, we repeated the analyses using only those image pairs whose relative intensity ratings from the current sample corresponded with those obtained from the previous norming study. This resulted in the exclusion of 16 trials for the older adults and 10 trials for the younger adults, so power was compromised. Despite this reduction in power, these analyses reproduced all of the same effects with one-tailed t tests based on our a priori predictions, so we describe the following results including all pairs.
Analyses for specific emotional category revealed no additional significant effects and are therefore not discussed.
Contributor Information
Joseph A. Mikels, Department of Psychology, Stanford University
Gregory R. Larkin, Department of Psychology, Stanford University
Patricia A. Reuter-Lorenz, Department of Psychology, University of Michigan
Laura L. Carstensen, Department of Psychology, Stanford University
References
- Babcock RL, Salthouse TA. Effects of increased processing demands on age differences in working memory. Psychology and Aging. 1990;5:421–428. doi: 10.1037//0882-7974.5.3.421. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford, England: Clarendon Press/Oxford University Press; 1986. [Google Scholar]
- Baddeley AD, Hitch G. Working memory. In: Bower G, editor. The psychology of learning and motivation. Vol. 8. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. [Google Scholar]
- Blanchard-Fields F, Jahnke HC, Camp C. Age differences in problem-solving style: The role of emotional salience. Psychology and Aging. 1995;10:173–180. doi: 10.1037//0882-7974.10.2.173. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. The affect system has parallel and integrative processing components: Form follows function. Journal of Personality and Social Psychology. 1999;76:839–855. [Google Scholar]
- Carpenter PA, Just MA, Shell P. What one intelligence test measures: A theoretical account of the processing in the Raven Progressive Matrices Test. Psychological Review. 1990;97:404–431. [PubMed] [Google Scholar]
- Carstensen LL. Evidence for a life-span theory of socioemotional selectivity. Current Directions in Psychological Science. 1995;4(5):151–156. doi: 10.1177/09637214211011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Fung HH, Charles ST. Socioemotional selectivity theory and the regulation of emotion in the second half of life. Motivation & Emotion. 2003;27:103–123. [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA. At the intersection of emotion and cognition: Aging and the positivity effect. Current Directions in Psychological Science. 2005;14:117–121. [Google Scholar]
- Carstensen LL, Mikels JA, Mather M. Aging and the intersection of cognition, motivation and emotion. In: Birren J, Schaie KW, editors. Handbook of the psychology of aging. 6th. San Diego, CA: Academic Press; in press. [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–655. [PubMed] [Google Scholar]
- Charles ST. Viewing injustice: Greater emotion heterogeneity with age. Psychology and Aging. 2005;20:159–164. doi: 10.1037/0882-7974.20.1.159. [DOI] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavior Research Methods, Instruments & Computers. 1993;25:257–271. [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998 February 27;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2nd. Mahwah, NJ: Erlbaum; 2000. [Google Scholar]
- Damasio AR. Descartes' error: Emotion, reason, and the human brain. New York: Avon Books; 1994. [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning & Verbal Behavior. 1980;19:450–466. [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Cognitive Brain Research. 1998;7(1):1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal older persons. Emotion. 2003;3:239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychology and Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Feldman Barrett L, Russell JA. The structure of current affect: Controversies and emerging consensus. Current Directions in Psychological Science. 1999;8(1):10–14. [Google Scholar]
- Frijda NH, Ortony A, Sonnemans J, Clore GL. The complexity of intensity: Issues concerning the structure of emotion intensity. In: Clark MS, editor. Review of personality and social psychology: Vol 13 Emotion. Thousand Oaks, CA: Sage; 1992. pp. 60–89. [Google Scholar]
- Fung HH, Carstensen LL. Sending memorable messages to the old: Age differences in preferences and memory for advertisements. Journal of Personality and Social Psychology. 2003;85:163–178. doi: 10.1037/0022-3514.85.1.163. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, editor. Handbook of physiology: The nervous system. Bethesda, MD: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- Gooding DC, Tallent KA. Spatial, object, and affective working memory in social anhedonia: An exploratory study. Schizophrenia Research. 2003;63:247–260. doi: 10.1016/s0920-9964(02)00326-2. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Bookstein F, Horwitz B, Rapoport SI, Haxby JV. Age-related changes in regional cerebral blood flow during working memory for faces. NeuroImage. 1998;8:409–425. doi: 10.1006/nimg.1998.0376. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, et al. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995 July 14;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: Approach–withdrawal states double-dissociate spatial from verbal two-back task performance. Journal of Experimental Psychology: General. 2001;130:436–452. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Hartley AA, Speer NK, Jonides J, Reuter-Lorenz PA, Smith EE. Is the dissociability of working memory systems for name identity, visual-object identity, and spatial location maintained in old age? Neuropsychology. 2001;15(1):3–17. doi: 10.1037//0894-4105.15.1.3. [DOI] [PubMed] [Google Scholar]
- Haug H, Barmwater U, Eggers R, Fischer D, Kuhl S, Sass NL. Anatomical changes in aging brain: Morphometric analysis of the human prosencephalon. In: Cervos-Navarro JS, Sarkander HI, editors. Brain aging: Neuropathology and neuropharmacology. New York: Raven Press; 1983. pp. 1–12. [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. Effects of normal aging and Alzheimer's disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Kunzmann U, Grühn D. Age differences in emotional reactivity: The sample case of sadness. Psychology and Aging. 2005;20:47–59. doi: 10.1037/0882-7974.20.1.47. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G, DeVoe M, Bulka D. Speaking about feelings: Conceptions of emotion across the life span. Psychology and Aging. 1989;4:425–437. doi: 10.1037//0882-7974.4.4.425. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G, Diehl M. Cognitive complexity and cognitive-affective integration: Related or separate domains of adult development? Psychology and Aging. 2000;15:490–504. doi: 10.1037/0882-7974.15.3.490. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G, Medler M. Affect optimization and affect complexity: Modes and styles of regulation in adulthood. Psychology and Aging. 2002;17:571–587. doi: 10.1037//0882-7974.17.4.571. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical manual and affective ratings. Gainsville: Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Lawton MP, Kleban MH, Dean J. Affect and age: Cross-sectional comparisons of structure and prevalence. Psychology and Aging. 1993;8:165–175. doi: 10.1037//0882-7974.8.2.165. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Friesen WV, Ekman P. Emotion, physiology, and expression in old age. Psychology and Aging. 1991;6:28–35. doi: 10.1037//0882-7974.6.1.28. [DOI] [PubMed] [Google Scholar]
- Logie RH. Visuo-spatial working memory. Hove, England: Erlbaum; 1995. [DOI] [PubMed] [Google Scholar]
- Luciana M, Burgund ED, Berman M, Hanson KL. Effects of tryptophan loading on verbal, spatial and affective working memory functions in healthy adults. Journal of Psychopharmacology. 2001;15:219–230. doi: 10.1177/026988110101500410. [DOI] [PubMed] [Google Scholar]
- MacPherson SE, Phillips LH, Della Sala S. Age, executive function and social decision making: A dorsolateral prefrontal theory of cognitive aging. Psychology and Aging. 2002;17:598–609. [PubMed] [Google Scholar]
- Malatesta CZ, Izard CE, Culver C, Nicolich M. Emotion communication skills in young, middle-aged, and older women. Psychology and Aging. 1987;2:193–203. doi: 10.1037//0882-7974.2.2.193. [DOI] [PubMed] [Google Scholar]
- Malatesta-Magai C. Personality change in adulthood: Loci of change and the role of interpersonal processes. International Journal of Aging & Human Development. 1999;49:339–352. doi: 10.2190/5RDT-W7CV-YQ41-TDTE. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14:409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mikels JA, Fredrickson BL, Larkin GR, Lindberg CM, Maglio SJ, Reuter-Lorenz PA. Emotional category data on images from the International Affective Picture System. Behavior Research Methods, Instruments, & Computers. doi: 10.3758/bf03192732. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels JA, Reuter-Lorenz PA, Beyer J, Fredrickson BL. Working memory for emotion? Investigations into the viability of affective working memory. 2005 Manuscript submitted for publication. [Google Scholar]
- Miller GA. The magical number seven plus or minus two: Some limits on our capacity for processing information. Psychological Review. 1956;63:81–97. [PubMed] [Google Scholar]
- Miyake A. Individual differences in working memory: Introduction to the special section. Journal of Experimental Psychology: General. 2001;130:163–168. doi: 10.1037//0096-3445.130.2.163. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. Frontal lobes, memory, and aging. New York: New York Academy of Sciences; 1995. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: A developmental perspective on happiness. Journal of Personality and Social Psychology. 1998;75:1333–1349. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- Myerson J, Hale S, Rhee SH, Jenkins L. Selective interference with verbal and spatial working memory in young and older adults. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1999;54:P161–P164. doi: 10.1093/geronb/54b.3.p161. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Smith AD, Lautenschlager G, Earles JL. Mediators of long-term memory performance across the life span. Psychology and Aging. 1996;11:621–637. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- Potter MC. Very short-term conceptual memory. Memory & Cognition. 1993;21:156–161. doi: 10.3758/bf03202727. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head DP, Dupuis JH, McQuain JM, Briggs SD, et al. Selective aging of human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Reisenzein R. Pleasure-arousal theory and the intensity of emotions. Journal of Personality and Social Psychology. 1994;67:525–539. [Google Scholar]
- Reuter-Lorenz PA, Sylvester CYC. The cognitive neuroscience of working memory and aging. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. London: Oxford University Press; 2005. pp. 186–217. [Google Scholar]
- Rhodes MG. Age-related differences in performance on the Wisconsin Card Sorting Test: A meta-analytic review. Psychology and Aging. 2004;19:482–494. doi: 10.1037/0882-7974.19.3.482. [DOI] [PubMed] [Google Scholar]
- Rozin P, Royzman E. Negativity bias, Negativity dominance, and cognition. Personality & Social Psychology Review. 2001;5:296–320. [Google Scholar]
- Salthouse TA. Differential age-related influences on memory for verbal-symbolic information and visual-spatial information? Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1995;50:P193–P201. doi: 10.1093/geronb/50b.4.p193. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Developmental influences on adult intelligence: The Seattle Longitudinal Study. London: Oxford University Press; 2005. [Google Scholar]
- Shivde GS. Semantic working memory: Evidence for a separate system that maintains meaning. Dissertation Abstracts International: Section B: The Sciences & Engineering. 2002;62(8B):3824. [Google Scholar]
- Smith EE, Jonides J, Koeppe RA, Awh E, Schumacher EH, Minoshima S. Spatial versus object working memory: PET investigations. Journal of Cognitive Neuroscience. 1995;7:337–356. doi: 10.1162/jocn.1995.7.3.337. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966 August 5;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Tsai JL, Levenson RW, Carstensen LL. Autonomic, subjective, and expressive responses to emotional films in older and younger Chinese Americans and European Americans. Psychology and Aging. 2000;15:684–693. doi: 10.1037//0882-7974.15.4.684. [DOI] [PubMed] [Google Scholar]
- Turner ML, Engle RW. Is working memory capacity task dependent? Journal of Memory & Language. 1989;28(2):127–154. [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: A quantitative integration of research findings. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1993;48:P157–P171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- Wahler HJ. Wahler Physical Symptoms Inventory. Los Angeles: Western Psychological Services; 1973. [Google Scholar]
- Wechsler D. WAIS-R administration and scoring manual. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Winocur G. Environmental influences on cognitive decline in aged rats. Neurobiology of Aging. 1998;19:589–597. doi: 10.1016/s0197-4580(98)00107-9. [DOI] [PubMed] [Google Scholar]


