Abstract
Objectives:
To summarize and evaluate the patient-based outcome measures (PBOMs) that have been used to study women with abnormal uterine bleeding (AUB)
Design:
Systematic review
Setting:
Original articles that used at least one PBOM and were conducted within a population of women with AUB
Patients:
Women with AUB
Interventions:
The titles, abstracts, and studies were systematically reviewed for eligibility. PBOMs used in eligible studies were summarized. Essential psychometric properties were identified and a list of criteria for each property was generated.
Main Outcome Measures:
“Quality” of individual PBOMs as determined using the listed criteria for psychometric properties.
Results:
Nine hundred eighty three studies referenced AUB and patient reported outcomes. Of these, eighty studies met the eligibility criteria. Fifty different instruments were used to evaluate amount of bleeding, bleeding related symptoms, or menstrual bleeding-specific quality of life. The “quality” of each of these instruments was evaluated on eight psychometric properties. The majority of instruments had no documentation of reliability, precision, or feasibility. There was not satisfactory evidence that any one instrument completely addressed all eight psychometric properties.
Conclusions:
Studies of women with AUB are increasingly utilizing PBOMs. Many different PBOMs were used; however no single instrument completely addressed eight important measurement properties
Keywords: Patient-based outcome measures, abnormal uterine bleeding, questionnaires, menstrual bleeding
INTRODUCTION
Abnormal uterine bleeding (AUB) is defined as any alteration in the pattern or volume of menstrual blood flow. Two main categories of AUB are heavy menstrual bleeding or irregular menstrual bleeding. Menstrual disorders are the most prevalent gynecologic health problems in the United States and heavy menstrual bleeding (HMB) affects up to 30% of women at some time during their reproductive years (1-3). The societal and personal burden of AUB lies in its major impact on quality of life, productivity, and healthcare utilization and costs (4-6).
Traditionally, research on AUB objectively measured menstrual blood loss as the main study outcome. In 1964, Hallberg and Nilsson described a method to objectively measure mean menstrual blood loss (MBL) from sanitary products, a method that is still used in many studies evaluating women who report heavy menstrual bleeding (7). Other methods have been proposed for the objective quantification of menstrual blood loss, including determining menstrual fluid volume (8). In these studies heavy menstrual bleeding, or menorrhagia, is often defined as at least 80 mls MBL per cycle. However, less than half of women seeking treatment for heavy menstrual periods have MBL greater than this defined 80 ml cut-off and almost half of all women reporting heavy menstrual periods have less than 40 mls MBL (9-11). This means that something other than the objectively measured amount of bleeding, such as bleeding pattern, patient perception of bleeding, or quality of life, is leading women to seek medical attention.
In clinical practice, the diagnosis and evaluation of AUB is based on a woman's personal assessment of her blood loss and its impact upon her quality of life. However, this instrumental outcome has not been measured in a consistent manner. Recent research in this area recognized the importance of the “patient experience” as an outcome that should be measured (12). Thus, patient-based outcome measures (PBOM) have been developed and utilized for clinical research in this area.
Patient-based outcome measures can be generic or disease-specific and include questionnaires, standardized interviews, and other varied methods that assess health and illness from the patient's perspective. They have been applied to research in the area of AUB to evaluate patient-determined blood loss (through pictorial blood loss assessment scales, such as the PBAC) (13), disease-specific symptoms (through menstrual questionnaires), and quality of life (through quality of life (QOL) instruments). The quality of PBOMs varies, however. To be the “standard of care” for evaluation of a condition or disease, a PBOM should be reliable, valid, responsive, precise, interpretable, acceptable, and feasible (14). In 2002, a systematic review evaluating the “quality” of all QOL instruments (both generic and disease-specific) used in studies on heavy menstrual bleeding found that although the QOL instruments were of good quality in terms of “measurement properties”; the validity of the instruments was not well established (12).
As PBOMs are increasingly being utilized in clinical research, it is important that investigators constantly evaluate the quality of these PBOMs. For this study, our objectives were to (1) Summarize all patient-based outcomes measures that have been applied to research in the area of AUB over the past 20 years; and (2) Evaluate whether or not PBOMs developed specifically for the population of women with AUB demonstrated eight psychometric properties experts consider important for PBOMs: appropriateness, reliability, validity, responsiveness, preciseness, interpretability, acceptability, and feasibility (14).
MATERIALS AND METHODS
Study identification
A search of the PubMed electronic database was performed. PubMed provides access to bibliographic information that includes MEDLINE, OLDMEDLINE, citations that precede the date that a journal was selected for MEDLINE indexing, and in-process citations. This database was chosen because of its comprehensive cataloguing of high-impact gynecologic and women's health journals and its universal accessibility by physicians and researchers. The search was limited to the years 1987-2007, English language, human, and female.
The search terms used to define and describe the population of interest were “menstrual bleeding, menorrhagia, menometrorrhagia, dysfunctional uterine bleeding, DUB, AUB, abnormal uterine bleeding, heavy periods, heavy menses, anovulatory bleeding, or irregular bleeding”. To identify studies that measured patient-based outcomes, the following search terms were used: “patient based outcome measure, patient reported outcome, patient based outcome, quality of life, scale, chart, diary, questionnaire, or survey”.
Inclusion and Exclusion Criteria
Our intent was to review patient-based outcome measurement instruments that were specific for AUB, but not specific to any individual systemic or structural etiology, such as uterine leiomyomata. The inclusion criteria for this study were: (1) The population of interest was women with AUB; and (2) At least one patient based outcome measure was used in the study. Studies were excluded if: (1) The population was only women with AUB attributed to a specific etiology (such as fibroids or bleeding disorders) (2) The study was a review article and (3) The study only measured “satisfaction with treatment” as its patient-based outcome measure.
Process of systematic review of articles and PBOMs
The titles, abstracts, and studies identified through the PubMed search were sequentially reviewed for eligibility by the first author (KAM). Additional studies were included if they were referenced in the “methods” section of eligible studies. The studies that met all inclusion and exclusion criteria were described in terms of study design, the study's independent variable, and number of instruments utilized. PBOMs were then described in terms of what types of outcomes they measured including amount of bleeding, menstrual symptoms, quality of life, depression, anxiety, body image, sexual functioning, or a combination of these listed outcomes.
Instruments that evaluated amount of menstrual bleeding, menstrual symptoms, or AUB-specific quality of life were evaluated for “quality”. The method of PBOM quality evaluation is described in detail in the following paragraph. General instruments (general health related quality of life instruments, psychiatric instruments, sexual functioning instruments) were reviewed and described. Previous studies have evaluated the quality of general instruments that have been used in a population of women with AUB and determined the need for a “condition-specific” instrument (12). We, therefore, evaluated the “quality” of all “condition-specific” instruments but reviewed and described all general instruments that were used to assess women with AUB.
Method of PBOM quality evaluation
We identified important instrument properties using existing methodologic publications and other studies that reviewed the quality of PBOMs (12, 14). Eight psychometric properties were identified as essential: appropriateness, reliability, validity, responsiveness, precision, interpretability, acceptability, and feasibility. We developed three criteria for each property. These properties and criteria are displayed in Table 1. Psychometric properties were assessed using the available information in the eligible study. If the eligible study provided additional references for the PBOM, the referenced study was reviewed to more comprehensively evaluate the PBOM's psychometric properties.
Table 1.
Evaluation of “quality” of patient based outcome measures: Description of criteria for psychometric properties
| Psychometric Property |
Criteria |
|---|---|
| Appropriateness | (1) Outcomes are justified: evidence that aspects of patients' lives that they are known to value are being measured;(2) Outcomes have been shown as important in previous studies; (3) Outcome appears important/appropriate to the purpose of the trial |
| Reliability | (1) More than one item is used to measure a construct; (2) Calculated Cronbach's alpha, compared item responses to the scale as a whole, or test-retest performed; (3) Any mention of “reliability” of the outcome |
| Validity | (1) Criterion validity: how this “new” measure correlates with some other accepted measure was described; (2) Content validity or face validity was described: Did individuals with AUB or individuals with health status methodology expertise participate in the preparation of the measurement tool?; (3) Construct validity: Was the relationships between sets of variables within the measure described? (For example: the relationship between menstrual symptoms and quality of life OR the relationship between menstrual symptoms and subjectively recorded amount of bleeding) |
| Responsiveness | (1) Any evidence that this was addressed? Requires evidence that changes in measurement over time are seen when there is good reason to think that changes have occurred that are of importance to patients; (2) Scores of the instrument converted into categorical data and the sensitivity or specificity of the categories were tested OR sensitivity or specificity plotted as ROCs; (3) Change scores: changes in different measured variables correlated |
| Precision | (1)Precision of response categories or numeric values: At least 5 response categories used to generate scores(if seven used, give an extra point) OR evidence that the instrument used metrics that were capable of reflecting changes; (2) Any evidence that the instrument was able to measure items across the full range of experience (the sickest and the healthiest, the most affected and the least affected); (3) Evidence that items in the instrument measured only one dimension (for example one question aiming to address depression did not address both depression and physical functioning) or that scale bias was addressed |
| Interpretability | (1) Were the instruments used considered somewhat “familiar” to a clinician-researcher?; (2) Is the “score” obtained meaningful to a clinician-researcher?; (3) Is it possible to determine at which point of a scale or what difference seen in a scale would identify a clinically important finding? |
| Acceptability | (1) Any evidence that the acceptability of the instrument to patients was addressed; (2) Response rates reported; (3) Time to complete the forms were reported |
| Feasibility | (1) Time or resources required to collect the measure were reported; (2)Time or resources required to process the measure were reported; (3)Time or resources required to analyze the measure were reported |
If the study met: None of the criteria, it got a “0” (no evidence that the property was addressed), one of the criteria, it got a “1” (Some evidence that the property was addressed), two or three of the criteria, it got a “2” (Complete evidence that the property was addressed). Criteria based on “Evaluating patient-based outcome measures for use in clinical trials”, by Fitzpatrick et al. (14)
Data collection and synthesis
A list of PBOMs that were AUB-specific and either quantified amount of menstrual bleeding, evaluated bleeding and menstrual symptoms, or assessed AUB-specific quality of life was generated by the first author (KAM). AUB-specific PBOMs were then systematically reviewed and rated, using the criteria listed in Table 1, separately by two independent reviewers (KAM, LAB).
For each psychometric property: (1) If the article provided information that 2 or more criteria were met, the instrument was given a score of “2” and we considered there to be “complete evidence” that the psychometric property was addressed; (2) If the article provided information that one criterion was met, the instrument was given a score of “1” and we considered there to be “some evidence” that the property was addressed; (3) If the article contained no information that any of the criteria were met, the instrument was given a score of “0” and we considered there to be “no evidence” that the property was addressed. Each instrument could receive a maximum of 16 points. Disagreements between raters were discussed, and consensus was reached after re-review of the instrument and associated article(s).
This project was exempt from review by the Institutional Review Board. Conflicts of interest are listed for both Dr. Boardman and Dr. Munro. Dr. Boardman is on the speakers' bureau for Merck. Dr. Munro is a consultant for Boston Scientific Inc, Ethicon Inc, Covidien INc, Gynesonics Inc, Karl Storz Endoscopy America, and AMAG Pharmaceuticals. He is a shareholder in Gynesonics Inc and Impres Medical.
RESULTS
Overview of articles reviewed
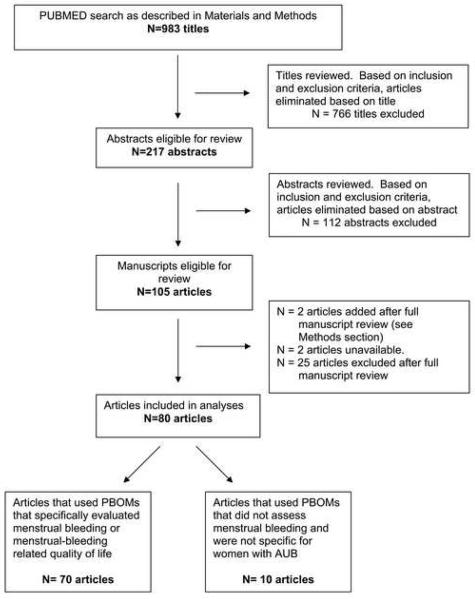
The PUBMED search as described in the “Materials and Methods” section generated 983 articles. Figure 1 outlines the study selection process.
Figure 1.
The PUBMED search as described in the “Materials and Methods” section generated 983 articles. Figure 1 outlines the study selection process.
Based on the inclusion and exclusion criteria, 80 articles were available and relevant for analyses. Of these studies, sixteen developed, tested, or validated at least one PBOM, 26 were descriptive or cohort studies, and 38 were randomized clinical trials. The following interventions were evaluated in the 64 descriptive or randomized clinical studies: Medical therapies [excluding the intrauterine device (IUD)] (n=7), endometrial ablation or resection (n=40), the IUD (n=9), other interventions including dilation and curettage, hysterectomy, and decision aids (n=16).
Of the 80 articles reviewed, 70 used PBOMs that were specifically designed to evaluate menstrual bleeding or AUB related quality of life. Thirty-eight articles used only PBOMs that were specifically designed to evaluate women with AUB. Twenty-three articles used both generic QOL instruments and AUB-specific instruments to measure outcomes. Several articles documented that the AUB-specific multidimensional PBOM contained items from previously tested generic QOL questionnaires (15-20). Nine articles used only generic PBOMs that were not specifically designed for women with AUB. A median of 2 PBOMs (minimum=1, maximum=8) was employed by each article.
Overview of the PBOMs
Fifty identified PBOMs were specifically designed to evaluate women with AUB and were subsequently evaluated for quality. These PBOMs are listed in Table 2. Of these instruments, 7 quantified the amount of bleeding and 23 evaluated menstrual or gynecologic symptoms.
Table 2.
Summary of the PBOMS that were reviewed for overall quality: Instruments that quantified bleeding, evaluated menstrual and gynecological symptoms, or evaluated AUB-related quality of life
| Name of the Instrument | Background studies*, instrument development, or instrument testing |
Studies which utilized the instrument to measure study outcome |
|---|---|---|
| Instruments that quantified bleeding (n=7) | ||
| Barr pictoral chart | -------- | (21) |
| Higham PBAC | (13) | (18, 19, 22-38) |
| Janssen pictoral | (13, 39) | (40, 41) |
| Mansfield-Voda-Jorgensen Menstrual bleeding scale | (42) | -------- |
| Menstrual pictogram (paper and computerized) | (37, 38) | -------- |
| Pad and tampon count | -------- | (43, 44) |
| Unspecified Pictoral bleeding | -------- | (45) |
| Menstrual and Gynecological symptoms (n= 23) | ||
| Bleeding Diary and bleeding patterns | (46) | (18) |
| Categorical bleeding questionnaire | -------- | (47) |
| Clinical Questionnaire | -------- | (48-54) |
| Detailed menstrual questionnaire | -------- | (55) |
| Follow-up menstrual bleeding and gynecologic questionnaire |
-------- | (29) |
| Gynecologic symptoms questionnaire | -------- | (56) |
| Individual subjective change in menstrual loss | -------- | (23) |
| Menorrhagia questionnaire | -------- | (57, 58) |
| Menstrual and gynecologic questionnaire | -------- | (59) |
| Menstrual bleeding questionnaire | -------- | (60) |
| Menstrual pattern chart and questionnaire | -------- | (61) |
| Menstrual pattern questionnaire | -------- | (62) |
| Menstrual symptoms and bleeding days | -------- | (44) |
| Menstrual symptoms and gynecologic questionnaire | -------- | (30) |
| Menstrual symptoms questionnaire | -------- | (63) |
| Menstrual symptoms questionnaire | -------- | (64) |
| Menstrual symptoms questionnaire | -------- | (65) |
| Menstrual symptoms questionnaire | -------- | (32) |
| Menstrual symptoms score | -------- | (66) |
| Menstrual variations questionnaire | -------- | (36) |
| Numeric scale rating of bleeding and symptoms | -------- | (67) |
| Questionnaire including menstrual symptoms | -------- | (45) |
| Structured clinical history questionnaire for menorrhagia |
-------- | (68) |
| Multidimensional questionnaires (both menstrual symptoms and quality of life) (n=20) | ||
| Aberdeen Menorrhagia Severity Scale | (69) | (40, 41, 69-71) |
| Dartmouth COOP QOL questionnaire supplemented with questions about menstrual bleeding |
(72) | (19) |
| Follow-up questionnaire that addressed menstrual symptoms, daily activities, and sexual functioning |
-------- | (73, 74) |
| Follow-up questionnaire that assessed gynecologic symptoms, satisfaction, anxiety, and depression, sexual activity |
(75) | (15) |
| General questionnaire (patterns of menstrual bleeding, gynecologic and unrelated symptoms, time to resume sexual activity, return to normal activity |
-------- | (76) |
| Menstrual and quality of life questionnaire | -------- | (43) |
| Menstrual bleeding and quality of life | -------- | (77) |
| Menstrual bleeding and Quality of life questionnaire | -------- | (78) |
| Menstrual Bleeding-related quality of life | (79) | (20) |
| Menstrual Experience Questionnaire (MEQ) | (10) | (10, 80) |
| Menstrual symptoms and associated quality of life | (81) | (16) |
| Menstrual symptoms and perceived inconvenience | (82) | (83) |
| Menstrual symptoms and quality of life | -------- | (31) |
| MS questionnaire | (81, 84-99) | (99, 100) |
| Multiattribute utility assessment | (101) | (60, 101) |
| Patient Generated Index | (102) | -------- |
| Post-operative menorrhagia outcomes questionnaire | (103, 104) | -------- |
| Social impact score | -------- | (66) |
| Subjective change in menstrual bleeding, quality of life, and sexual functioning |
(87, 88, 97, 105, 106) |
(18) |
| VAS menstrual symptoms and quality of life | -------- | (28) |
“Background information”: Many questionnaires incorporated validated scales or portions of validated questionnaires. References containing background information for questionnaire development or information on the original validated scales or questionnaires are included in this part of the table.
Twenty instruments were multidimensional questionnaires that assessed both menstrual symptoms and AUB-related quality of life.
Twenty-seven PBOMs, not specifically designed to evaluate women with AUB, were used in the included studies. These instruments are listed in Table 3. Twelve instruments measured health-related quality of life and eight instruments measured anxiety and depression. Of the remaining instruments, five evaluated sexual functioning, one assessed body image, and one evaluated sleep.
Table 3.
Summary of PBOMs that did not evaluate symptoms or quality of life specific to the condition of interest, AUB. Either portions of these instruments or the full instrument was used in the studies reviewed.
| Name of the Instrument | Background studies*, instrument development, or instrument testing |
Studies which utilized the instrument to measure study outcome |
|---|---|---|
| Instruments that evaluate Quality of life and Health-Related Quality of Life (n =12) | ||
| A General health questionnaire | ---------- | (37, 38) |
| EQ5D | (95, 107) | (30, 45, 60, 70, 77, 108-110) |
| General Quality of Life | ---------- | (26) |
| Health Distress Scale | (90) | (99, 100) |
| Scale of overall health | (84-91, 95, 107) | (99, 100) |
| The Activity Index | (111) | (16, 17) |
| The General Health Index | (112, 113) | (16, 17) |
| The General Health Questionnaire | (114) | (76) |
| The Medical Outcomes Study (MOS) Short- Form-36 (SF36) |
(84-91,107, 115) | (1, 24, 27, 35, 38, 48- 53, 65, 66, 68-70, 77, 83, 99, 100, 102, 108-110, 116-120) |
| The MOS Short From -12 (SF12) | (121) | (25, 30, 45) |
| The Rotterdam symptoms checklist | (122) | (68) |
| The VAS for perceived health | ---------- | (83) |
| Instruments that evaluate Depression or Anxiety ( n= 8 ) | ||
| Beck's Depression Inventory | (123) | (108-110) |
| Hospital Anxiety and Depression Scale HADS |
(124) | (15, 35, 48-53, 116) |
| Mental Health Index | (105) | (17, 99, 100) |
| The Finnish Psychosomatic questionnaire | (125) | (83) |
| The modified social adjustment scale | (126) | (76) |
| The outpatient mood scale | (127) | (76) |
| The self-rating depression scale | (128, 129) | (68) |
| The State-Trait Anxiety Inventory | (130) | (68, 83, 108-110) |
| Instruments that evaluate sexual functioning, body image, or sleep (n=7) | ||
| Body Image scale (adapted from Body Attitudes Questionnaire) |
(92) | (99, 100) |
| McCoy sex scale | (131, 132) | (83, 108-110) |
| Psychosexual function | ----------- | (133) |
| Sabbatsberg Sexual rating scale | (94) | (116) |
| Sexual activity questionnaire | (135) | (30, 45) |
| Sexual functioning scale | (93, 94, 96-98) | (99, 100) |
| Sleep Problems Scale | (89) | (99, 100) |
“Background information”: Many questionnaires incorporated validated scales or portions of validated questionnaires. References containing background information for questionnaire development or information on the original validated scales or questionnaires are included in this part of the table
PBOMs specific for AUB
Instruments that quantified menstrual bleeding
Table 2 lists the 7 instruments which quantified the amount of menstrual bleeding by using a diary, chart, or questionnaire. Of these instruments, the Pictorial Bleeding Assessment Chart (PBAC) (13), by Higham et al, was used most frequently (n=19 studies).
Instruments that evaluated bleeding and menstrual symptoms
Twenty three instruments evaluated menstrual and gynecologic symptoms. (Table 2) The most frequently used questionnaire, a clinical questionnaire first utilized by Pinion et al (54), was used in 7 separate studies generated from the same institution. A menorrhagia questionnaire was used by two separate studies by Fernandez et al (57, 58) and all other instruments that evaluated menstrual and gynecological symptoms were used by only one study.
Instruments that evaluated both menstrual bleeding and AUB-related quality of life
Twenty multidimensional questionnaires evaluated both menstrual bleeding and AUB-related quality of life. (Table 2) The Aberdeen Menorrhagia Severity Scale, by Ruta et al (69), was used by five studies. All other multidimensional questionnaires were used to measure outcomes in only one or two studies.
PBOMs not specific for AUB
Instruments that evaluated generic health-related quality of life
Twelve general and health-related quality of life instruments were used by the eligible studies. These are listed in Table 3. The Medical Outcomes Study (MOS) Short-Form-36 (SF-36) (84-86, 107, 116) (used by 29 studies), the EQ5D (95, 107) (used by 9 studies), and the MOS Short-Form-12 (SF-12) (121) (used by 3 studies), were the most frequently used instruments. All other instruments were used by one or two studies.
Instruments that evaluated depression and anxiety
Eight instruments specifically evaluated depression or anxiety. The Hospital Anxiety and Depression Scale (HADS) (124) was used by 9 studies, the State-Trait Anxiety Inventory (STAI) (130) was used by 5 studies, and the Beck's Depression Inventory (124) and the Mental Health Index (105) were each used by 3 studies. The remaining four instruments were each used by one study.
Instruments that evaluated sexual functioning, body image, or sleep
Five instruments evaluated sexual functioning, one instrument evaluated body image, and one instrument evaluated sleep problems. Of these, the McCoy Sex Scale (131, 132), used in four studies, was the most frequently used. All others instruments were used by one or two studies.
Quality assessment of the PBOMs specifically designed to evaluate women with AUB
Table 4 summarizes the available evidence about the psychometric properties of the PBOMs. “Complete evidence” that the PBOM satisfied the criteria for appropriateness was available for 92% (46/50) of PBOMs. In contrast, “complete evidence” that the PBOM was feasible was available for only 2% (1/50) of PBOMs. For over half of the PBOMs, “no evidence” was provided that the instrument was reliable (32/48, 64%), precise (25/50, 50%), or feasible (48/50, 96%).
Table 4.
Summary of available evidence that the specified psychometric properties were addressed.
| Psychometric Property |
Complete Evidencea n ( row %) |
Some evidenceb n ( row %) |
No evidencec n (row %) |
|---|---|---|---|
| Appropriateness | 46 (92.0) | 4 (8.0) | 0 (0.0) |
| Reliability | 6 (12.0) | 12 (24.0) | 32 (64.0) |
| Validity | 13 (26.0) | 18 (36.0) | 19 (38.0) |
| Responsiveness | 6 (12.0) | 32 (64.0) | 12 (24.0) |
| Precision | 8 (16.0) | 17 (34.0) | 25 (50.0) |
| Interpretability | 8 (16.0) | 37 (74.0) | 5 (10.0) |
| Acceptability | 4 (8.0) | 36 (72.0) | 10 (20.0) |
| Feasibility | 1 (2.0) | 1 (2.0) | 48 (96.0) |
“Complete evidence” = a score of “2” for the property
“Some evidence” = a score of “1” for the property
“No evidence” = a score of “0” for the property
The “scores” for quality were broken down by the type of PBOM and are displayed in Table 5. A total of 16 points translates into “complete evidence” that the instrument met all psychometric properties (appropriateness, reliability, validity, responsiveness, precision, interpretability, acceptability, and feasibility), and was therefore highest quality. No instrument had “complete evidence” available that all psychometric properties were addressed. Bleeding quantification instruments, menstrual and gynecological symptoms questionnaires, and multidimensional instruments received 56%, 31%, and 44%, respectively, of possible points for instrument quality.
Table 5.
Summary of overall “quality” scores for individual PBOMs, by type of PBOM
| Instrument | Number of instruments |
Median Score ( 95% CI) |
Min Score |
Max Score |
|---|---|---|---|---|
| All instruments | 50 | 6 (5,7) | 2 | 15 |
| Bleeding quantification | 7 | 9 (4,12) | 4 | 12 |
| Menstrual/gynecologic symptoms | 23 | 5 (4,5) | 2 | 11 |
| Multidimensional instrument | 20 | 7 (6,10) | 5 | 15 |
DISCUSSION
Abnormal Uterine Bleeding is a major health problem that adversely affects the lives of women. The clinical management of women with AUB aims to improve the patient's symptoms and quality of life. Because PBOMs allow clinicians and researchers to assess health and illness from the patient's perspective, they are increasingly being used to measure clinical outcomes. Researchers in the area of AUB have used PBOMs to evaluate patient-determined blood loss (through pictorial blood loss assessment scales), disease-specific symptoms (through menstrual questionnaires), and health related quality of life (through QOL instruments). We found that although some instruments were used more frequently than others, there was no one instrument that was considered the “standard of care” for evaluating women with AUB. Additionally, there was wide variation in the “quality” of PBOMs used to assess women with AUB.
Although heavy menstrual bleeding is clinically defined as greater than 80mls MBL per cycle, less than half of women seeking medical attention for their bleeding lose more than this defined amount (9, 10). This means that something other than the amount of bleeding is driving women to seek medical attention. Quality of life (QOL) is likely one factor influencing women with AUB to request treatment. Health related quality of life (HRQOL) represents an individual's perceived physical and mental health and how it affects his or her day-to-day activities. HRQOL, often used by physicians and researchers to measure the effects of illness in patients, can be measured using general HRQOL instruments, such as the MOS SF36, or disease-specific instruments.
Disease-specific QOL instruments should be considered for the evaluation of women reporting AUB because symptoms are not generally constant and symptoms are disturbing but not necessarily life-threatening (12). Our review identified 20 different “AUB-specific instruments” that evaluated both menstrual bleeding and quality of life. Although the Aberdeen Menorrhagia Severity Scale, by Ruta et al (69), was the most frequently utilized multidimensional disease-specific questionnaire, it was used in only 5 of the 80 studies reviewed. No one instrument was used consistently as the “standard of care” for the evaluation of AUB. .
The SF-36, a general HRQOL instrument which was used in 29 of the 80 articles that we reviewed, is a 36-item questionnaire that generates an overall “score” for HRQOL based on scores across 8 domains (Physical Functioning, Role Limitations due to physical health problems, Bodily Pain, Social Functioning, General Mental Health, Role Limitations due to emotional problems, Vitality, and General Health Perceptions). Although the SF-36 has been shown to be both reliable and responsive in women with AUB, women with AUB have reported difficulty answering some of its questions because of the intermittent nature of AUB and how that may affect perceptions of general health (117-120). Therefore, to evaluate outcomes in research, investigators have suggested either the use of disease-specific QOL instruments or the use of disease-specific instruments together with generic QOL instruments (12). Twenty three studies that we reviewed used generic instruments in combination with disease-specific instruments
For a PBOM to become the “standard of care” for evaluating a condition, it should be psychometrically sound. To be psychometrically sound, an instrument should be appropriate, reliable, valid, responsive, precise, interpretable, acceptable, and feasible. In our review of all instruments used to evaluate women with AUB, including bleeding quantification questionnaires, symptom questionnaires, and multidimensional questionnaires, we found that no instrument completely met all criteria for psychometric properties. Additionally, the majority of instruments lacked evidence that they were precise, reliable, or feasible.
For this study, we reviewed 50 different PBOMs specific for the evaluation of women with AUB. Although no one instrument was used consistently as the “standard of care” and no instrument addressed all important psychometric properties, we identified several good quality instruments. Of the instruments that quantified bleeding, both the Higham PBAC (13) and the Janssen pictorial scale (18, 39) were good quality based on meeting the criteria for addressing eight psychometric properties. A limitation to using these instruments, however, is that they estimate only one dimension of AUB, the quantity of menstrual blood lost. Of the 20 multidimensional instruments reviewed, the AMSS (69), the MS questionnaire (99, 100), the Patient Generated Index (102), and the Post-operative Menorrhagia Outcomes Questionnaire were the best quality (103, 104).
This article has several strengths. First, it is a comprehensive review of PBOMs used to evaluate women with AUB in studies published over the past 20 years. The expansive list of articles reviewed was generated using broad search terms in widely used databases to maximize the number of articles captured. Second, two investigators independently reviewed each eligible article and PBOM for information on the psychometric properties to determine the “quality” of the instrument. One limitation of this study is that the exact list of criteria (Table 1) we used to assess the quality of PBOMs has not been validated. However, it was developed by experts in the field and was based carefully upon previous literature (12, 14). An additional limitation of this study is that the assessment of the quality of the PBOMs was based on the information provided by the studies in which they were used. It is possible that instruments may have been psychometrically evaluated but it was not discussed or referenced in the article. We would urge researchers to mention whether or not these psychometric properties were addressed or reference appropriate articles in which the properties were addressed. This would allow readers and other researchers to evaluate for themselves the validity of outcomes that the PBOMs generated. If researchers and clinicians are to consider altering their clinical practice based upon study results, they should be able to evaluate the quality with which the study results were obtained.
The quality of research on AUB is adversely affected by the lack of an accepted, validated, high quality patient-based outcome measure. Future research is necessary to develop a PBOM that satisfies the eight psychometric properties; Such a PBOM is currently being designed by the authors of this paper. We plan to develop and test a comprehensive PBOM for women with AUB which will become the standard of care for evaluating women with AUB. The diagnosis and evaluation of AUB is largely based upon “patient experience”, the woman's personal assessment of her blood loss and its impact upon her quality of life. Many AUB-specific PBOMs have been used over the past 20 years to evaluate women reporting AUB; However, our ability to perform research on AUB could be greatly improved with the development and utilization of a high quality standardized PBOM that provides a global assessment of the “patient experience” for women reporting AUB.
Acknowledgments
Financial Support: This research is made possible through the National Institutes of Health-funded K12 - HD050108-02 WIH/Brown Women's Reproductive Health Research (WRHR) Grant (PI: Donald R. Coustan, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Conflicts of Interest: Matteson: none Boardman: Merck Munro: Ethicon Women's Health, Covidien, Gynesonics Inc., Karl Storz Endoscopy, AMAG Pharmaceuticals, Impres Medical Clark: none
Presentation at medical meeting: This study was presented as an oral presentation at the First International Meeting on Abnormal Uterine Bleeding, The American Association of Gynecologic Laparoscopists and the Society for Endoscopic Gynecologists of Italy, Palermo, Italy, June 2007
REFERENCES
- 1.Barnard K, Frayne SM, Skinner KM, Sullivan LM. Health status among women with menstrual symptoms. J Womens Health. 2003;12:911–9. doi: 10.1089/154099903770948140. [DOI] [PubMed] [Google Scholar]
- 2.Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health. 1996;86:195–9. doi: 10.2105/ajph.86.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MORI Women's Health in 1990. Market Opinion and Research International (Research study conducted on behalf of Parke-Davis Laboratories) 1990 [Google Scholar]
- 4.Cote I, Jacobs P, Cumming D. Work loss associated with increased menstrual loss in the United States. Obstet Gynecol. 2002;100:683–7. doi: 10.1016/s0029-7844(02)02094-x. [DOI] [PubMed] [Google Scholar]
- 5.Cote I, Jacobs P, Cumming DC. Use of health services associated with increased menstrual loss in the United States. Am J of Obstet Gynecol. 2003;188:343–8. doi: 10.1067/mob.2003.92. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health care costs and utilization in abnormal uterine bleeding. Value in Health, Published on behalf of the International Society for Pharmacoeconomics and Outcomes Research. 2007;10:173–82. doi: 10.1111/j.1524-4733.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 7.Hallberg L, Nilsson L. Determination of Menstrual Blood Loss. Scand J Clin Lab Invest. 1964;16:244–8. [PubMed] [Google Scholar]
- 8.Fraser IS, Warner P, Marantos PA. Estimating menstrual blood loss in women with normal and excessive menstrual fluid volume. Obstet Gynecol. 2001;98:806–14. doi: 10.1016/s0029-7844(01)01581-2. [DOI] [PubMed] [Google Scholar]
- 9.Haynes P, Hodgson H, Anderson AB, Turnbull AC. Measurement of menstrual blood loss in patients complaining of menorrhagia. Br J Obstet Gynaecol. 1977;84:763–8. doi: 10.1111/j.1471-0528.1977.tb12490.x. [DOI] [PubMed] [Google Scholar]
- 10.Warner PE, Critchley HO, Lumsden MA, Campbell-Brown M, Douglas A, Murray GD. Menorrhagia I: measured blood loss, clinical features, and outcome in women with heavy periods: a survey with follow-up data. Am J of Obstet Gynecol. 2004;190:1216–23. doi: 10.1016/j.ajog.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Hallberg L, Hogdahl AM, Nilsson L, Rybo G. Menstrual blood loss--a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand. 1966;45:320–51. doi: 10.3109/00016346609158455. [DOI] [PubMed] [Google Scholar]
- 12.Clark TJ, Khan KS, Foon R, Pattison H, Bryan S, Gupta JK. Quality of life instruments in studies of menorrhagia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;104:96–104. doi: 10.1016/s0301-2115(02)00076-3. [DOI] [PubMed] [Google Scholar]
- 13.Higham JM, O'Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97:734–9. doi: 10.1111/j.1471-0528.1990.tb16249.x. [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess (Winch Eng) 1998;2:i–iv. [PubMed] [Google Scholar]
- 15.Aberdeen Endometrial Ablation Trials Group A randomised trial of endometrial ablation versus hysterectomy for the treatment of dysfunctional uterine bleeding: outcome at four years. Aberdeen Endometrial Ablation Trials Group. Br J Obstet Gynaecol. 1999;106:360–6. doi: 10.1111/j.1471-0528.1999.tb08275.x. [DOI] [PubMed] [Google Scholar]
- 16.Carlson KJ, Miller BA, Fowler FJ., Jr The Maine Women's Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566–72. doi: 10.1097/00006250-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Carlson KJ, Miller BA, Fowler FJ., Jr The Maine Women's Health Study: I. Outcomes of hysterectomy. Obstet Gynecol. 1994;83:556–65. doi: 10.1097/00006250-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Davis A, Godwin A, Lippman J, Olson W, Kafrissen M. Triphasic norgestimateethinyl estradiol for treating dysfunctional uterine bleeding. Obstet Gynecol. 2000;96:913–20. doi: 10.1016/s0029-7844(00)01029-2. [DOI] [PubMed] [Google Scholar]
- 19.Duleba AJ, Heppard MC, Soderstrom RM, Townsend DE. A randomized study comparing endometrial cryoablation and rollerball electroablation for treatment of dysfunctional uterine bleeding. J Am Assoc Gynecol Laparosc. 2003;10:17–26. doi: 10.1016/s1074-3804(05)60229-0. [DOI] [PubMed] [Google Scholar]
- 20.Winkler UH. The effect of tranexamic acid on the quality of life of women with heavy menstrual bleeding. Eur J Obstet Gynecol Reprod Biol. 2001;99:238–43. doi: 10.1016/s0301-2115(01)00414-6. [DOI] [PubMed] [Google Scholar]
- 21.Barr F, Brabin L, Agbaje O. A pictorial chart for managing common menstrual disorders in Nigerian adolescents. Int J Gynaecol Obstet. 1999;66:51–3. doi: 10.1016/s0020-7292(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 22.Barrington JW, Arunkalaivanan AS, Abdel-Fattah M. Comparison between the levonorgestrel intrauterine system (LNG-IUS) and thermal balloon ablation in the treatment of menorrhagia. Eur J Obstet Gynecol Reprod Biol. 2003;108:72–4. doi: 10.1016/s0301-2115(02)00408-6. [DOI] [PubMed] [Google Scholar]
- 23.Barrington JW, Bowen-Simpkins P. The levonorgestrel intrauterine system in the management of menorrhagia. Br J Obstet Gynaecol. 1997;104:614–6. doi: 10.1111/j.1471-0528.1997.tb11542.x. [DOI] [PubMed] [Google Scholar]
- 24.Busfield RA, Farquhar CM, Sowter MC, Lethaby A, Sprecher M, Yu Y, et al. A randomised trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. Br J Obstet Gynaecol. 2006;113:257–63. doi: 10.1111/j.1471-0528.2006.00863.x. [DOI] [PubMed] [Google Scholar]
- 25.Cooper J, Gimpelson R, Laberge P, Galen D, Garza-Leal JG, Scott J, et al. A randomized, multicenter trial of safety and efficacy of the NovaSure system in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:418–28. doi: 10.1016/s1074-3804(05)60513-0. [DOI] [PubMed] [Google Scholar]
- 26.Corson SL, Brill AI, Brooks PG, Cooper JM, Indman PD, Liu JH, et al. One-year results of the vesta system for endometrial ablation. J Am Assoc Gynecol Laparosc. 2000;7:489–97. doi: 10.1016/s1074-3804(05)60361-1. [DOI] [PubMed] [Google Scholar]
- 27.Crosignani PG, Vercellini P, Mosconi P, Oldani S, Cortesi I, De Giorgi O. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstet Gynecol. 1997;90:257–63. doi: 10.1016/S0029-7844(97)00226-3. [DOI] [PubMed] [Google Scholar]
- 28.Deeny M, Davis JA. Assessment of menstrual blood loss in women referred for endometrial ablation. Eur J Obstet Gynecol Reprod Biol. 1994;57:179–80. doi: 10.1016/0028-2243(94)90297-6. [DOI] [PubMed] [Google Scholar]
- 29.Gallinat A. NovaSure impedance controlled system for endometrial ablation: three-year follow-up on 107 patients. American Journal of Obstet Gynecol. 2004;191:1585–9. doi: 10.1016/j.ajog.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Hawe J, Abbott J, Hunter D, Phillips G, Garry R. A randomised controlled trial comparing the Cavaterm endometrial ablation system with the Nd:YAG laser for the treatment of dysfunctional uterine bleeding. Br J Obstet Gynaecol. 2003;110:350–7. [PubMed] [Google Scholar]
- 31.Loffer FD. Three-year comparison of thermal balloon and rollerball ablation in treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2001;8:48–54. doi: 10.1016/s1074-3804(05)60548-8. [DOI] [PubMed] [Google Scholar]
- 32.Rauramo I, Elo I, Istre O. Long-term treatment of menorrhagia with levonorgestrel intrauterine system versus endometrial resection. Obstet Gynecol. 2004;104:1314–21. doi: 10.1097/01.AOG.0000143824.16435.91. [DOI] [PubMed] [Google Scholar]
- 33.Reid PC, Coker A, Coltart R. Assessment of menstrual blood loss using a pictorial chart: a validation study. Br J Obstet Gynaecol. 2000;107:320–2. doi: 10.1111/j.1471-0528.2000.tb13225.x. [DOI] [PubMed] [Google Scholar]
- 34.Reid PC, Virtanen-Kari S. Randomised comparative trial of the levonorgestrel intrauterine system and mefenamic acid for the treatment of idiopathic menorrhagia: a multiple analysis using total menstrual fluid loss, menstrual blood loss and pictorial blood loss assessment charts. Br J Obstet Gynaecol. 2005;112:1121–5. doi: 10.1111/j.1471-0528.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 35.Soysal M, Soysal S, Ozer S. A randomized controlled trial of levonorgestrel releasing IUD and thermal balloon ablation in the treatment of menorrhagia. Zentralbl Gynakol. 2002;124:213–9. doi: 10.1055/s-2002-32434. [DOI] [PubMed] [Google Scholar]
- 36.Vercellini P, Oldani S, De Giorgi O, Milesi M, Merlo D, Crosignani PG. Endometrial ablation with a vaporizing electrode. II. Clinical outcome of a pilot study. Acta Obstet Gynecol Scand. 1998;77:688–93. doi: 10.1034/j.1600-0412.1998.770619.x. [DOI] [PubMed] [Google Scholar]
- 37.Wyatt KM, Dimmock PW, Hayes-Gill B, Crowe J, O'Brien PM. Menstrual symptometrics: a simple computer-aided method to quantify menstrual cycle disorders. Fertil Steril. 2002;78:96–101. doi: 10.1016/s0015-0282(02)03161-8. [DOI] [PubMed] [Google Scholar]
- 38.Wyatt KM, Dimmock PW, Walker TJ, O'Brien PM. Determination of total menstrual blood loss. Fertil Steril. 2001;76:125–31. doi: 10.1016/s0015-0282(01)01847-7. [DOI] [PubMed] [Google Scholar]
- 39.Janssen CA, Scholten PC, Heintz AP. A simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Obstet Gynecol. 1995;85:977–82. doi: 10.1016/0029-7844(95)00062-V. [DOI] [PubMed] [Google Scholar]
- 40.Corson SL. A multicenter evaluation of endometrial ablation by Hydro ThermAblator and rollerball for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2001;8:359–67. doi: 10.1016/s1074-3804(05)60331-3. [DOI] [PubMed] [Google Scholar]
- 41.Ulmsten U, Carstensen H, Falconer C, Holm L, Lanner L, Nilsson S, et al. The safety and efficacy of MenoTreat, a new balloon device for thermal endometrial ablation. Acta Obstet Gynecol Scand. 2001;80:52–7. doi: 10.1034/j.1600-0412.2001.800110.x. [DOI] [PubMed] [Google Scholar]
- 42.Mansfield PK, Voda A, Allison G. Validating a pencil-and-paper measure of perimenopausal menstrual blood loss. Womens Health Issues. 2004;14:242–7. doi: 10.1016/j.whi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Feitoza SS, Gebhart JB, Gostout BS, Wilson TO, Cliby WA. Efficacy of thermal balloon ablation in patients with abnormal uterine bleeding. Am J Obstet Gynecol. 2003;189:453–7. doi: 10.1067/s0002-9378(03)00403-4. [DOI] [PubMed] [Google Scholar]
- 44.Munro MG, Mainor N, Basu R, Brisinger M, Barreda L. Oral medroxyprogesterone acetate and combination oral contraceptives for acute uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2006 Oct;108:924–9. doi: 10.1097/01.AOG.0000238343.62063.22. [DOI] [PubMed] [Google Scholar]
- 45.Abbott J, Hawe J, Hunter D, Garry R. A double-blind randomized trial comparing the Cavaterm and the NovaSure endometrial ablation systems for the treatment of dysfunctional uterine bleeding. Fertil Steril. 2003;80:203–8. doi: 10.1016/s0015-0282(03)00549-1. [DOI] [PubMed] [Google Scholar]
- 46.Belsey EM, Machin D, d'Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986;34:253–60. doi: 10.1016/0010-7824(86)90006-5. [DOI] [PubMed] [Google Scholar]
- 47.Romer T, Muller J. A simple method of coagulating endometrium in patients with therapy-resistant, recurring hypermenorrhea. J Am Assoc Gynecol Laparosc. 1999;6:265–8. doi: 10.1016/s1074-3804(99)80058-9. [DOI] [PubMed] [Google Scholar]
- 48.Bain C, Cooper KG, Parkin DE. Microwave endometrial ablation versus endometrial resection: a randomized controlled trial. Obstet Gynecol. 2002;99:983–7. doi: 10.1016/s0029-7844(02)01663-0. [DOI] [PubMed] [Google Scholar]
- 49.Cooper KG, Bain C, Lawrie L, Parkin DE. A randomised comparison of microwave endometrial ablation with transcervical resection of the endometrium; follow up at a minimum of five years. Br J Obstet Gynaecol. 2005;112:470–5. doi: 10.1111/j.1471-0528.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 50.Cooper KG, Bain C, Parkin DE. Comparison of microwave endometrial ablation and transcervical resection of the endometrium for treatment of heavy menstrual loss: a randomised trial. Lancet. 1999;354:1859–63. doi: 10.1016/S0140-6736(99)04101-X. [DOI] [PubMed] [Google Scholar]
- 51.Cooper KG, Jack SA, Parkin DE, Grant AM. Five-year follow up of women randomised to medical management or transcervical resection of the endometrium for heavy menstrual loss: clinical and quality of life outcomes. Br J Obstet Gynaecol. 2001;108:1222–8. doi: 10.1111/j.1471-0528.2001.00275.x. see comment. [DOI] [PubMed] [Google Scholar]
- 52.Cooper KG, Parkin DE, Garratt AM, Grant AM. A randomised comparison of medical and hysteroscopic management in women consulting a gynaecologist for treatment of heavy menstrual loss. Br J Obstet Gynaecol. 1997;104:1360–6. doi: 10.1111/j.1471-0528.1997.tb11004.x. [DOI] [PubMed] [Google Scholar]
- 53.Cooper KG, Parkin DE, Garratt AM, Grant AM. Two-year follow up of women randomised to medical management or transcervical resection of the endometrium for heavy menstrual loss: clinical and quality of life outcomes. Br J Obstet Gynaecol. 1999;106:258–65. doi: 10.1111/j.1471-0528.1999.tb08240.x. [DOI] [PubMed] [Google Scholar]
- 54.Pinion SB, Parkin DE, Abramovich DR, Naji A, Alexander DA, Russell IT, et al. Randomised trial of hysterectomy, endometrial laser ablation, and transcervical endometrial resection for dysfunctional uterine bleeding. BMJ. 309:979–83. doi: 10.1136/bmj.309.6960.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chullapram T, Song JY, Fraser IS. Medium-term follow-up of women with menorrhagia treated by rollerball endometrial ablation. Obstet Gynecol. 1996;88:71–6. doi: 10.1016/0029-7844(96)00085-3. [DOI] [PubMed] [Google Scholar]
- 56.Mints M, Radestad A, Rylander E. Follow up of hysteroscopic surgery for menorrhagia. Acta Obstet Gynecol Scand. 1998;77:435–8. [PubMed] [Google Scholar]
- 57.Fernandez H, Capella S, Audibert F. Uterine thermal balloon therapy under local anaesthesia for the treatment of menorrhagia: a pilot study. Hum Reprod. 1997;12:2511–4. doi: 10.1093/humrep/12.11.2511. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez H, Kobelt G, Gervaise A. Economic evaluation of three surgical interventions for menorrhagia. Hum Reprod. 2003;18:583–7. doi: 10.1093/humrep/deg141. [DOI] [PubMed] [Google Scholar]
- 59.O'Connor H, Magos A. Endometrial resection for the treatment of menorrhagia. N Engl J Med. 1996;335:151–6. doi: 10.1056/NEJM199607183350302. [DOI] [PubMed] [Google Scholar]
- 60.Clark TJ, Gupta JK. Outpatient thermal balloon ablation of the endometrium. Fertil Steril. 2004;82:1395–401. doi: 10.1016/j.fertnstert.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 61.Radesic B, Sharma A. Levonorgestrel-releasing intrauterine system for treating menstrual disorders: a patient satisfaction questionnaire. Aust N Z J Obstet Gynaecol. 2004;44:247–51. doi: 10.1111/j.1479-828X.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 62.Tam WH, Yuen PM, Shan Ng DP, Leung PL, Lok IH, Rogers MS. Health status function after treatment with thermal balloon endometrial ablation and levonorgestrel intrauterine system for idiopathic menorrhagia: a randomized study. Gynecol Obstet Invest. 2006;62:84–8. doi: 10.1159/000092660. [DOI] [PubMed] [Google Scholar]
- 63.Boe Engelsen I, Woie K, Hordnes K. Transcervical endometrial resection: long-term results of 390 procedures. Acta Obstet Gynecol Scand. 2006;85:82–7. doi: 10.1080/00016340500424314. [DOI] [PubMed] [Google Scholar]
- 64.Degen AF, Gabrecht T, Mosimann L, Fehr MK, Hornung R, Schwarz VA, et al. Photodynamic endometrial ablation for the treatment of dysfunctional uterine bleeding: a preliminary report. Lasers Surg Med. 2004;34:1–4. doi: 10.1002/lsm.10244. [DOI] [PubMed] [Google Scholar]
- 65.Henshaw R, Coyle C, Low S, Barry C. A retrospective cohort study comparing microwave endometrial ablation with levonorgestrel-releasing intrauterine device in the management of heavy menstrual bleeding. Aust N Z J Obstet Gynaecol. 2002;42:205–9. doi: 10.1111/j.0004-8666.2002.00205.x. [DOI] [PubMed] [Google Scholar]
- 66.Coulter A, Peto V, Jenkinson C. Quality of life and patient satisfaction following treatment for menorrhagia. Fam Pract. 1994;11:394–401. doi: 10.1093/fampra/11.4.394. [DOI] [PubMed] [Google Scholar]
- 67.Martyn P, Allan B. Long-term follow-up of endometrial ablation. J Am Assoc Gynecol Laparosc. 1998;5:115–8. doi: 10.1016/s1074-3804(98)80075-3. [DOI] [PubMed] [Google Scholar]
- 68.Bongers MY, Bourdrez P, Heintz AP, Brolmann HA, Mol BW. Bipolar radio frequency endometrial ablation compared with balloon endometrial ablation in dysfunctional uterine bleeding: impact on patients' health-related quality of life. Fertil Steril. 2005;83:724–34. doi: 10.1016/j.fertnstert.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 69.Ruta DA, Garratt AM, Chadha YC, Flett GM, Hall MH, Russell IT. Assessment of patients with menorrhagia: how valid is a structured clinical history as a measure of health status? Qual Life Res. 1995;4:33–40. doi: 10.1007/BF00434381. [DOI] [PubMed] [Google Scholar]
- 70.Dickersin K, Munro M, Langenberg P, Scherer R, Frick KD, Weber AM, et al. Surgical Treatments Outcomes Project for Dysfunctional Uterine Bleeding (STOP-DUB): design and methods. Control Clin Trials. 2003;24:591–609. doi: 10.1016/s0197-2456(03)00023-0. [DOI] [PubMed] [Google Scholar]
- 71.Rosenbaum SP, Fried M, Munro MG. Endometrial hydrothermablation: a comparison of short-term clinical effectiveness in patients with normal endometrial cavities and those with intracavitary pathology. Journal of Minimally Invasive Gynecology. 2005;12:144–9. doi: 10.1016/j.jmig.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 72.Nelson E, Wasson J, Kirk J, Keller A, Clark D, Dietrich A, et al. Assessment of function in routine clinical practice: description of the COOP Chart method and preliminary findings. Journal of chronic diseases. 1987;40(Suppl 1):55S–69S. doi: 10.1016/s0021-9681(87)80033-4. [DOI] [PubMed] [Google Scholar]
- 73.el Senoun GS, Mousa HA, Mahmood TA. Medium-term follow-up of women with menorrhagia treated by rollerball endometrial ablation. Acta Obstet Gynecol Scand. 2000;79:879–83. [PubMed] [Google Scholar]
- 74.Mousa HA, Abou El Senoun GM, Mahmood TA. Medium-term clinical outcome of women with menorrhagia treated by rollerball endometrial ablation versus abdominal hysterectomy with conservation of at least one ovary. Acta Obstet Gynecol Scand. 2001;80:442–6. [PubMed] [Google Scholar]
- 75.Derogatis LR. The psychosocial adjustment to illness scale (PAIS) J Psychosom Res. 1986;30:77–91. doi: 10.1016/0022-3999(86)90069-3. [DOI] [PubMed] [Google Scholar]
- 76.O'Connor H, Broadbent JA, Magos AL, McPherson K. Medical Research Council randomised trial of endometrial resection versus hysterectomy in management of menorrhagia. Lancet. 1997;349:897–901. doi: 10.1016/S0140-6736(96)07285-6. [DOI] [PubMed] [Google Scholar]
- 77.Sculpher MJ, Dwyer N, Byford S, Stirrat GM. Randomised trial comparing hysterectomy and transcervical endometrial resection: effect on health related quality of life and costs two years after surgery. Br J Obstet Gynaecol. 1996;103:142–9. doi: 10.1111/j.1471-0528.1996.tb09666.x. [DOI] [PubMed] [Google Scholar]
- 78.Meyer WR, Walsh BW, Grainger DA, Peacock LM, Loffer FD, Steege JF. Thermal balloon and rollerball ablation to treat menorrhagia: a multicenter comparison. Obstet Gynecol. 1998;92:98–103. doi: 10.1016/s0029-7844(98)00141-0. see comment. [DOI] [PubMed] [Google Scholar]
- 79.Edlund M, Magnusson C, Von Schoultz B. Quality of life - a Swedish Survey of 2200 women; Key Paper Conferences: The Royal Society of Medicine Press Limited.1997. [Google Scholar]
- 80.Warner PE, Critchley HO, Lumsden MA, Campbell-Brown M, Douglas A, Murray GD. Menorrhagia II: is the 80-mL blood loss criterion useful in management of complaint of menorrhagia? Am J Obstet Gynecol. 2004;190:1224–9. doi: 10.1016/j.ajog.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 81.Andrews FM, Withey SB. Social indicators of well-being. Plenum; New York: 1976. [Google Scholar]
- 82.Vuorma S, Teperi J, Hurskainen R, Aalto AM, Rissanen P, Kujansuu E. Correlates of women's preferences for treatment of heavy menstrual bleeding. Patient education and counseling. 2003;49:125–32. doi: 10.1016/s0738-3991(02)00069-1. [DOI] [PubMed] [Google Scholar]
- 83.Vuorma S, Teperi J, Aalto AM, Hurskainen R, Kujansuu E, Rissanen P. A randomized trial among women with heavy menstruation -- impact of a decision aid on treatment outcomes and costs. Health Expectations. 2004;7:327–37. doi: 10.1111/j.1369-7625.2004.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McHorney CA, Ware JE, Jr., Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med care. 1994 Jan;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 85.McHorney CA, Ware JE, Jr., Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med care. 1993 Mar;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Stewart AL, Greenfield S, Hays RD, Wells K, Rogers WH, Berry SD, et al. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA. 1989;262:907–13. [PubMed] [Google Scholar]
- 87.Ware JE, Jr., Kosinski M. SF-36 Physical and mental health summary scales: a manual for users of version 1. QualityMetric; Lincoln, RI: 2001. [Google Scholar]
- 88.Ware JE, Jr., Snow KK, Kosinski M, Gondek G. SF-36 health survey: manual and interpretative guide. The Health Institute, New England Medical Center; Boston, MA: 1994. [Google Scholar]
- 89.Hays RD, Stewart AL. Sleep measures. In: Stewart AL, Ware JE Jr., editors. Measuring functioning and well-being: the medical outcomes study approach. Duke University Press; Durham, NC: 1992. pp. 235–9. [Google Scholar]
- 90.Lorig K, Sterwart AL, Ritter P, et al. Measures for health education and other health care interventions. Sage Publications; Thousand Oaks: 1996. [Google Scholar]
- 91.Stewart AL, Ware JE, Jr., Sherbourne CD. Psychological distress/well-being and cognitive functioning measures. The Duke University Press; Durham, NC: 1992. [Google Scholar]
- 92.Ben-Tovim DI, Walker MK. The influence of age and weight on women's body attitudes as measured by the Body Attitudes Questionnaire (BAQ) J Psychosom Res. 1994;38:477–81. doi: 10.1016/0022-3999(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 93.Farrell SA, Kieser K. Sexuality after hysterectomy. Obstet Gynecol. 2000;95:1045–51. doi: 10.1016/s0029-7844(00)00784-5. [DOI] [PubMed] [Google Scholar]
- 94.Garratt AM, Torgerson DJ, Wyness J, Hall MH, Reid DM. Measuring sexual functioning in premenopausal women. Br J Obstet Gynaecol. 1995;102:311–6. doi: 10.1111/j.1471-0528.1995.tb09138.x. [DOI] [PubMed] [Google Scholar]
- 95.Group TE. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 96.Rhodes JC, Kjerulff KH, Langenberg PW, Guzinski GM. Hysterectomy and sexual functioning. JAMA. 1999;282:1934–41. doi: 10.1001/jama.282.20.1934. [DOI] [PubMed] [Google Scholar]
- 97.Sherbourne CD. Social functioning: sexual problems measure. The Duke University Press; Durham, NC: 1992. [Google Scholar]
- 98.Taylor JF, Rosen RC, Leiblum SR. Self-report assessment of female sexual function: psychometric evaluation of the Brief Index of Sexual Functioning for Women. Arch Sex Behav. 1994;23:627–43. doi: 10.1007/BF01541816. [DOI] [PubMed] [Google Scholar]
- 99.Varner RE, Ireland CC, Summitt RL, Jr., Richter HE, Learman LA, Vittinghoff E, et al. Medicine or Surgery (Ms): a randomized clinical trial comparing hysterectomy and medical treatment in premenopausal women with abnormal uterine bleeding. Control Clin Trials. 2004;25:104–18. doi: 10.1016/j.cct.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 100.Kuppermann M, Varner RE, Summitt RL, Jr., Learman LA, Ireland C, Vittinghoff E, et al. Effect of hysterectomy vs medical treatment on health-related quality of life and sexual functioning: the medicine or surgery (Ms) randomized trial. JAMA. 2004;291:1447–55. doi: 10.1001/jama.291.12.1447. see comment. [DOI] [PubMed] [Google Scholar]
- 101.Shaw RW, Brickley MR, Evans L, Edwards MJ. Perceptions of women on the impact of menorrhagia on their health using multi-attribute utility assessment. Br J Obstet Gynaecol. 1998;105:1155–9. doi: 10.1111/j.1471-0528.1998.tb09968.x. [DOI] [PubMed] [Google Scholar]
- 102.Ruta DA, Garratt AM, Russell IT. Patient centred assessment of quality of life for patients with four common conditions. Quality in Health Care. 1999;8:22–9. doi: 10.1136/qshc.8.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lamping DL, Rowe P. Users' Manual for Purchasers and Providers: Menorrhagia Outcomes Questionnaire (Short Form) Health Services Research Unit, London School of Hygiene and Tropical Medicine; London: 1995. [Google Scholar]
- 104.Lamping DL, Rowe P, Clarke A, Black N, Lessof L. Development and validation of the Menorrhagia Outcomes Questionnaire. Br J Obstet Gynaecol. 1998;105:766–79. doi: 10.1111/j.1471-0528.1998.tb10209.x. [DOI] [PubMed] [Google Scholar]
- 105.Ware JE, Jr., Davies A. Scoring the short-form Mental Health Inventory (MHI-5) The RAND Corporation; Santa Monica, CA: 1983. [Google Scholar]
- 106.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med care. 1992;30:473–83. [PubMed] [Google Scholar]
- 107.Brooks R. EuroQol: the current state of play. Health policy (Amsterdam, Netherlands) 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 108.Hurskainen R, Teperi J, Aalto AM, Grenman S, Kivela A, Kujansuu E, et al. Levonorgestrel-releasing intrauterine system or hysterectomy in the treatment of essential menorrhagia: predictors of outcome. Acta Obstet Gynecol Scand. 2004;83:401–3. doi: 10.1111/j.0001-6349.2004.00440.x. [DOI] [PubMed] [Google Scholar]
- 109.Hurskainen R, Teperi J, Rissanen P, Aalto AM, Grenman S, Kivela A, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273–7. doi: 10.1016/S0140-6736(00)03615-1. see comment. [DOI] [PubMed] [Google Scholar]
- 110.Hurskainen R, Teperi J, Rissanen P, Aalto AM, Grenman S, Kivela A, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA. 2004;291:1456–63. doi: 10.1001/jama.291.12.1456. see comment. [DOI] [PubMed] [Google Scholar]
- 111.Fowler FJ, Jr., Wennberg JE, Timothy RP, Barry MJ, Mulley AG, Jr., Hanley D. Symptom status and quality of life following prostatectomy. JAMA. 1988 May 27;259:3018–22. [PubMed] [Google Scholar]
- 112.Veit CT, Ware JE., Jr The structure of psychological distress and well-being in general populations. J Consult Clin Psychol. 1983;51:730–42. doi: 10.1037//0022-006x.51.5.730. [DOI] [PubMed] [Google Scholar]
- 113.Brook RH, Ware JEJ, Davies-Avery A. Conceptualization and measurement of health for adults in the Health Insurance Study. The Rand Corporation; Santa Monica, CA: 1970. ea. [Google Scholar]
- 114.Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychological medicine. 1979;9:139–45. doi: 10.1017/s0033291700021644. [DOI] [PubMed] [Google Scholar]
- 115.Stewart AL, Ware JE., Jr . Measuring Functioning and Well-being: The Medical Outcomes Study Approach. Duke University Press; Durham, NC: 1992. [Google Scholar]
- 116.Crosignani PG, Vercellini P, Apolone G, De Giorgi O, Cortesi I, Meschia M. Endometrial resection versus vaginal hysterectomy for menorrhagia: long-term clinical and quality-of-life outcomes. Am J Obstet Gynecol. 1997;177:95–101. doi: 10.1016/s0002-9378(97)70445-9. [DOI] [PubMed] [Google Scholar]
- 117.Garratt AM, Ruta DA, Abdalla MI, Buckingham JK, Russell IT. The SF36 health survey questionnaire: an outcome measure suitable for routine use within the NHS? BMJ. 1993;306:1440–4. doi: 10.1136/bmj.306.6890.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garratt AM, Ruta DA, Abdalla MI, Russell IT. SF 36 health survey questionnaire: II. Responsiveness to changes in health status in four common clinical conditions. Quality in Health Care. 1994;3:186–92. doi: 10.1136/qshc.3.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jenkinson C, Peto V, Coulter A. Measuring change over time: a comparison of results from a global single item of health status and the multi-dimensional SF-36 health status survey questionnaire in patients presenting with menorrhagia. Qual Life Res. 1994;3:317–21. doi: 10.1007/BF00451723. [DOI] [PubMed] [Google Scholar]
- 120.Jenkinson C, Peto V, Coulter A. Making sense of ambiguity: evaluation in internal reliability and face validity of the SF 36 questionnaire in women presenting with menorrhagia. Quality in Health Care. 1996;5:9–12. doi: 10.1136/qshc.5.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 122.De Haes JCJM, Olschewski M, Fayers P, Visser MRM, Cull A, Hoppwood Pea. Measuring the quality of life of cancer patients with the Rotterdam Symptoms Checklist: a manual. Northern Centre for Healthcare Research; Gronigen: 1996. [Google Scholar]
- 123.Beck AT, Rial WY, Rickels K. Short form of depression inventory: cross-validation. Psychol Rep. 1974;34:1184–6. [PubMed] [Google Scholar]
- 124.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 125.METELI . Ocupational status, working environment, and morbidity among employees of metal industry factories. Gummerus; Jyvaskyla: 1977. [Google Scholar]
- 126.Cooper P, Osborn M, Gath D, Feggetter G. Evaluation of a modified self-report measure of social adjustment. Br J Psychiatry. 1982l;141:68–75. doi: 10.1192/bjp.141.1.68. [DOI] [PubMed] [Google Scholar]
- 127.McNair DM, Lorr M. An Analysis of Mood in Neurotics. J Abnorm Psychol. 1964;69:620–7. doi: 10.1037/h0040902. [DOI] [PubMed] [Google Scholar]
- 128.Zung WW. From art to science. The diagnosis and treatment of depression. Arch Gen Psychiatry. 1973;29:328–37. doi: 10.1001/archpsyc.1973.04200030026004. [DOI] [PubMed] [Google Scholar]
- 129.Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry. 1965;13:508–15. doi: 10.1001/archpsyc.1965.01730060026004. [DOI] [PubMed] [Google Scholar]
- 130.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1980. [Google Scholar]
- 131.McCoy NL, Davidson JM. A longitudinal study of the effects of menopause on sexuality. Maturitas. 1985;7:203–10. doi: 10.1016/0378-5122(85)90041-6. [DOI] [PubMed] [Google Scholar]
- 132.Wiklund I, Karlberg J, Mattsson LA. Quality of life of postmenopausal women on a regimen of transdermal estradiol therapy: a double-blind placebo-controlled study. Am J Obstet Gynecol. 1993;168(3 Pt 1):824–30. doi: 10.1016/s0002-9378(12)90828-5. [DOI] [PubMed] [Google Scholar]
- 133.McPherson K, Herbert A, Judge A, Clarke A, Bridgman S, Maresh M, et al. Psychosexual health 5 years after hysterectomy: population-based comparison with endometrial ablation for dysfunctional uterine bleeding. Health Expectations. 2005;8:234–43. doi: 10.1111/j.1369-7625.2005.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thirlaway K, Fallowfield L, Cuzick J. The Sexual Activity Questionnaire: a measure of women's sexual functioning. Qual Life Res. 1996;5:81–90. doi: 10.1007/BF00435972. [DOI] [PubMed] [Google Scholar]
- 135.Abbott JA, Hawe J, Garry R. Quality of life should be considered the primary outcome for measuring success of endometrial ablation. J Am Assoc Gynecol Laparosc. 2003;10:491–5. doi: 10.1016/s1074-3804(05)60153-3. [DOI] [PubMed] [Google Scholar]
- 136.Cooper GS, Sandler DP, Whelan EA, Smith KR. Association of physical and behavioral characteristics with menstrual cycle patterns in women age 29-31 years. Epidemiology. 1996;7:624–8. doi: 10.1097/00001648-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 137.Hurskainen R, Aalto AM, Teperi J, Grenman S, Kivela A, Kujansuu E, et al. Psychosocial and other characteristics of women complaining of menorrhagia, with and without actual increased menstrual blood loss. Br J Obstet Gynaecol. 2001;108:281–5. doi: 10.1111/j.1471-0528.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 138.Johannes CB, Crawford SL, Woods J, Goldstein RB, Tran D, Mehrotra S, et al. An electronic menstrual cycle calendar: comparison of data quality with a paper version. Menopause. 2000;7:200–8. doi: 10.1097/00042192-200007030-00011. [DOI] [PubMed] [Google Scholar]
- 139.Ruta DA, Abdalla MI, Garratt AM, Coutts A, Russell IT. SF 36 health survey questionnaire: I. Reliability in two patient based studies. Quality in Health Care. 1994;3:180–5. doi: 10.1136/qshc.3.4.180. [DOI] [PMC free article] [PubMed] [Google Scholar]