Abstract
Earlier, we reported that there was an increase in angiotensin II AT2 receptor expression in the renal proximal tubule, and selective activation of the AT2 receptor by AT2 agonist inhibits Na,KATPase activity in the proximal tubules and increases urinary Na excretion in obese Zucker rats. We hypothesized that AT2 receptor has a protective role against blood pressure increase in obese Zucker rats. To test this hypothesis, we treated obese Zucker rats with the AT2 receptor antagonist PD123319 (30 μg/kg/min) using osmotic pumps. Age-matched lean rats and vehicle treated obese Zucker rats served as controls. On day 15 of the treatment with PD, arterial blood pressure was measured by cannulation of the left carotid artery under anesthesia. Control obese rats exhibited higher mean arterial pressure (MAP, mmHg) (122±3.4) compared to lean control rats (97±4.8). The PD123319-treatement of obese rats raised MAP further by 13 mmHg. The plasma renin activity (PRA) was significantly increased in the PD-treated obese compared with control-obese or lean rats. Western blot analysis revealed that the PD-treatment in obese rats caused approximately 3-fold increase in the renin expression in the kidney cortex, but had no effect on the expression of the cortical AT1 and AT2 receptors. The present study suggests that the renal AT2 receptors provide a protective role against blood pressure increase in obese Zucker rats, and this protective effect, in part, could be due to the ability of the AT2 receptors to keep the kidney renin expression low in obese rats.
Keywords: angiotensin II receptors, renin, kidney, obesity, hypertension
INTRODUCTION
Of the Angiotensin (Ang II) receptors, the AT1 receptor is known to mediate most of the Ang II actions, including vasoconstriction, antinatriuresis, aldosterone secretion, increased sympathetic outflow and cellular growth/proliferation.1 Abnormal regulation and function of AT1 receptor contributes to development and maintenance of various forms of hypertension.2-6 On the other hand, the AT2 receptor is suggested as a functional antagonist of AT1 receptors.7 However, the AT2 receptor has been implicated in cardiovascular functions such as vasodilatation, depressor effect on blood pressure or cardio-protection.8 The AT2 receptor is involved in blood pressure regulation in various animal models such as the renal wrap hypertension model, AT2 knock out mice and diet-induced hypertension.9-12 After the discovery of the AT2 receptor in various parts of the kidney, including in tubules,13,14 attempts have been made to establish a link between the renal AT2 receptor, renal Na excretion and blood pressure regulation. The AT2 receptor null mouse develops hypertension associated with an inhibition in pressure natriuresis.15 Rats with selective intra-renal reduction of the AT2 receptors produced by antisense oligonucleotides exhibit increased blood pressure.16 However, these studies involved either genetic manipulations of the AT2 receptor or blockade of the AT1 receptor and subsequent infusion of an AT2 agonist or antagonist to produce acute changes in the arterial blood pressure of these animal models. Although these studies suggest a role for AT2 receptors in blood pressure regulation, there exists a gap in our understanding the role of AT2 receptors in long-term blood pressure control.
Recently, we have reported an increase in the AT2 receptor expression in the kidney cortex, which upon activation inhibits the Na,K-ATPase activity in the proximal tubules of obese Zucker rats.17,18 Obese Zucker rat is a model of insulin resistance and develops hypertension.19 An impaired pressure natriuresis is believed to be a cause of hypertension in obese Zucker rats and other animal models of obesity.20-22 Hypertension in obese Zucker rats is associated with an enhanced renal AT1 receptor function.6,23-25 We hypothesized that while enhanced AT1 receptor function may contribute to increased renal Na retention and hypertension, AT2 receptor-mediated inhibition of NKA and enhanced renal Na excretion 17,18 may be beneficial by limiting the blood pressure increase in obese rats. Therefore, to test this hypothesis, we measured blood pressure in obese Zucker rats after 2-weeks of treatment with selective AT2 receptor antagonist.
MATERIALS AND METHODS
Animal models and drug treatment
Male obese and lean Zucker rats (10-11 weeks of age) were purchased from Harlan, Indianapolis, IN. Animals were housed in the University of Houston animal care facility. Food and water were supplied ad libitum and their daily consumption was recorded. The Institutional Animal Use and Care Committee approved the animal experimental protocols. Body weight of the animals was recorded at the start and the end of the drug treatments. For drug treatment, the obese rats were divided into 2-sub groups (n=6-7 per group) i.e., obese-control rat group and obese-PD rat group. Obese-control group was treated with normal saline as vehicle, and obese-PD group was treated with PD123319 (30 μg/kg/min)), an AT2R antagonist, for 2 weeks using Alzet osmotic pumps, implanted subcutaneously (model 2ML-2, Alza, Palo Alto, CA). Lean Zucker rats (n=6) served as normal control. Due to limited supply of PD123319, only obese rats were treated to investigate the effect of AT2 blockade on the blood pressure.
General parameters
During the course of treatment, daily food and water intake was recorded. On day 12, blood glucose was measured using a Glucometer (AccuChek- Compact, Roche diagnostics, Indianapolis, IN) after 6-hr of fasting. On day 13, the rats were placed in metabolic cages for 48-hrs. After initial 24 hrs of acclimatization, urine was collected over the next 24-hr period. Urinary sodium was measured using a flame photometer (Cole Parmer, Model 2655-10, Vernon Hills, Illinois).
Blood pressure and heart rate measurements
On day 15 of the treatment period, the rats were anesthetized using Inactin (100 to 150 mg/kg IP) for measuring blood pressure. After tracheotomy, right carotid artery was cannulated with PE10 and attached to data acquisition system (PolyView, Grass Ins), via Grass pressure transducer PT300. Heart rate and blood pressure were continuously monitored. After 30-45 min of stabilization period, the systolic and diastolic blood pressure and heart rate were recorded. At the end of the blood pressure measurement, blood sample was collected for plasma renin activity (PRA). Kidneys were excised, patted dry to weigh and stored frozen at -80°C for measuring the expression of AT1 receptors, AT2 receptors and renin in the kidney cortex.
Western blotting
The expression of AT1 receptor, AT2 receptor and renin in the kidney cortex of various rat groups was determined by western blotting. For this purpose, the kidney cortices were homogenized in the buffer containing (in mM) Tris 50, EDTA 10, PMSF 1, cocktail of protease inhibitors (aprotinin, calpain inhibitors, leupeptin, pepstatin and trypsin inhibitor). Proteins in the homogenates were determined by BCA method using a kit (Pierce, Rockford, IL). Equal amounts of protein, 30 μg for AT1, 60 μg for AT2 receptors, 30 μg for renin, from various rat groups were subjected to SDS-PAGE and electroblotting onto immobilon P (blot). The blot was incubated with primary polyclonal antibodies for the AT1, AT2 receptor or renin. Following the incubation with the primary antibodies, the blots were incubated with HRP-conjugated antirabbit IgGs. The signal was detected by ECL system, recorded and analysed by FluorChem 8800 (Alpha Innotech Imaging System, San Leandro, CA) for the densitometry of the bands. For loading control, the blots were stripped, and re-probed with either β-actin antibody or GAPDH antibody.
Plasma renin activity (PRA) Assay
The PRA was assayed by radioimmunoassay (GammaCoat 125I-PRA Radioimmunoassay Kit, cat # CA-1533, DiaSorin, Stillwater, MN) as per the manufacturer's instructions. The plasma samples were subjected to the Ang I generation reaction in tubes coated with rabbit anti-Ang I. After the reaction was terminated, the tubes were washed and decanted. The 125I radioactivity in the tubes was counted using gamma counter (LKB Wallace Model 1282).
Chemicals
PD123319 was a generous gift of Pfizer Inc. Polyclonal antibodies for AT1 receptor and renin were purchased from Santa Cruz Biotechnology, (Santa Cruz, CA). Polyclonal AT2 receptor antibody, HRP-coupled anti-IgG and enhanced chemiluminescence substrates were obtained from Alpha Diagnostics Intl (San Antonio, TX). All other chemicals used in the study were purchased from Sigma Aldrich (St. Louis, MO).
Statistical analysis
Results are expressed as mean±SEM. All the data were subjected to statistical analyses using GraphPad Prism 4, San Diego, CA. One-way analysis of variance (ANOVA) and Student's t-tests were performed to determine the significance of differences between different groups. Statistical significance was set at P < 0.05.
RESULTS
General parameters
Compared with lean Zucker rats, obese Zucker rats had greater body weight and consumed more food and water. The kidney weight of obese rats was higher comparing with those from lean rats. The PD123319 treatment did not affect the food intake in obese animals. Plasma glucose was greater in obese than in lean rats, and the PD treatment modestly, but statistically insignificant, increased fasting blood glucose in obese rats. Compared with control obese rats, PD-treated obese rats exhibited higher plasma renin activity (Table 1). The urinary volume (UV) and urinary Na (UNaV) excretion over 24-hour period in obese rats was greater than in lean rats. The PD treatment caused a significant increase in UV, but had no effect on UNaV in obese rats. It should be noted that the extent of increase in UV was similar to the extent of increase in water-intake in PD-treated obese rats (Table 1).
Table.
General Parameters
| Parameter/Rat Group | Lean | Obese (Vehicle) | Obese (PD-123319) |
|---|---|---|---|
| Food, g/d | 23±1 | 37±2* | 35±1* |
| Water, mL/d | 26±3 | 52±2* | 61±3*† |
| Body weight, g | 300±9 | 562±8* | 580±8* |
| Kidney weight, g | 2.9±0.08 | 3.5±0.05* | 3.5±0.07* |
| Urine volume, mL/day | 18±1 | 28±3* | 39±5*† |
| UNAV, mmol/day | 2.7±0.4 | 4.6±0.4* | 4.9±0.7* |
| Fasting glucose, mg/dL | 104±7 | 123±5* | 130±5* |
| PRA, ng/mL per h | 1.69±0.3 | 1.83±0.3 | 2.5±0.2*† |
Data were analyzed using 1-way ANOVA followed by Neuman-Keuls multiple comparison test (P<0.05 considered as significant. n=6 lean, 6 obese-vehicle, 6 obese-PD).
Significantly different from lean rats.
Significantly different from obese vehicle rats.
Effects of PD123319 on blood pressure
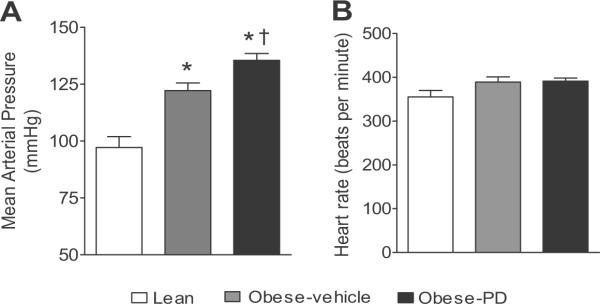
Compared to lean rats, obese Zucker rats exhibit higher systolic and diastolic blood pressure (lean: 114±5/88±5 vs obese: 135±5/105±6 mmHg, p<0.05). The 2 week treatment of obese Zucker rats with PD 123319 caused a significant increase by 13 mm Hg in mean arterial blood pressure (MAP) (Fig 1A). The heart rates were similar in lean and obese rats and were not affected by the PD-treatment (control obese: 389±12 bpm vs. PD-obese 391±7 bpm) (Fig. 1B).
Fig. 1.
(A) Mean arterial blood pressure and (B) heart rate of lean, obese vehicle and obese Zucker rats treated with PD-123319 for two weeks. *significantly different compared with lean rats, † significantly different compared with obese control. (One-way ANOVA followed by Neuman-Keuls test, p<0.05, n=5-6 rats in each group).
Effect of PD123319 on the expression of AT1 and AT2 receptor and renin in kidney cortex
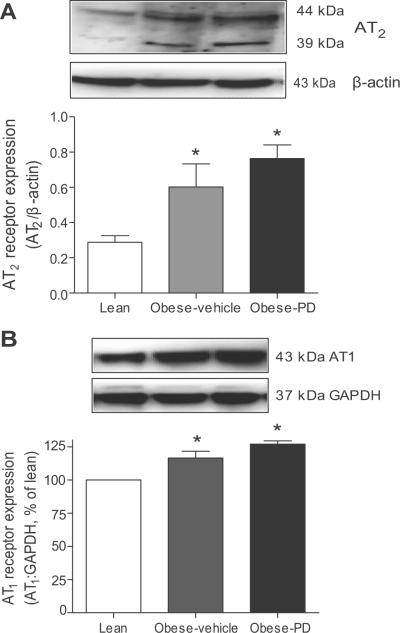
Western blot shows the presence of AT2 receptor in multiple bands (44 and 39 kDa) in the renal cortex. These multiple bands of the AT2 receptor are likely due to various degree of glycosylation, as reported earlier.18 Densitometric analysis of the AT2 bands revealed that the cortical AT2 receptor expression was significantly elevated in obese compared with lean rats, as reported earlier.18 The PD-treatment did not affect either the AT2 receptor (Fig 2A) or the AT1 receptor (Fig 2B) expression in the kidney cortex of obese rats. The cortical AT1 receptor expression was modestly but significantly greater in obese rat compared to lean Zucker rats (Fig. 2B). These AT1 receptor expression data is consistent to earlier reports.24-26
Fig. 2.
(A) AT2 receptor expression and (B) AT1 receptor expression in the kidney cortex of lean, obese vehicle and PD 123319-treated obese rats. Upper panel: Representative western blots for AT2 and AT1 receptor expression. Lower panel: Bar graphs represent the densities of AT2 and AT1 receptor bands normalized with β-actin and GAPDH respectively as loading controls. Values are represented as mean ± SEM, *significantly different from lean rats. (One way ANOVA, p<0.05 n=5 in each group).
Western blot demonstrates the presence of renin as approximately 41 kDa band in the renal cortex. Densitometric analysis of the bands suggests that the cortical renin expression in obese rats was significantly lower compared with lean rats which is consistent with earlier study. 27 The PD-treatment of obese rats caused a significant increase in the cortical renin expression (Fig 3).
Fig. 3.
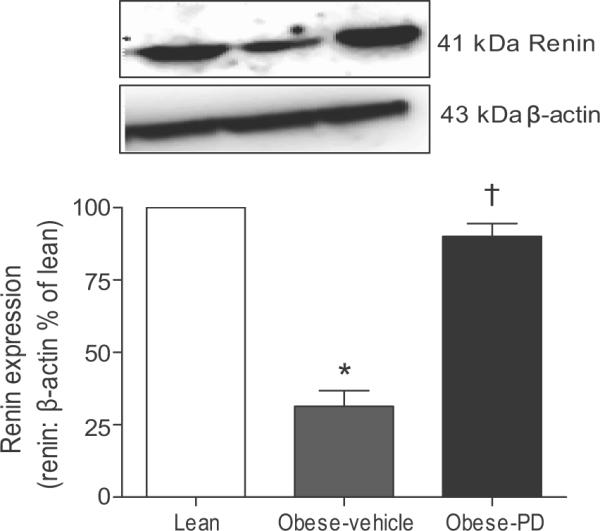
Expression of renin in the kidney cortex of lean, obese vehicle and PD 123319-treated obese Zucker rats. Upper panel: Representative western blot of renin and β-actin. Lower panel: Bar graphs with values of renin band density normalized with β-actin as loading control. *significantly different compared with lean rats, † significantly different compared with obese control rats. (One way ANOVA, p<0.05, n=5 in each group).
DISCUSSION
In the present study, we demonstrate the protective role of the AT2 receptor in long-term blood pressure regulation in obese Zucker rats. Treatment of obese Zucker rats with the AT2 receptor antagonist PD123319 for 2-weeks caused a significant elevation in blood pressure. The PD treatment did not affect the expression of the AT2 receptor or the AT1 receptor in the kidney cortex. However, the PD-treatment of obese rats caused an increase in the PRA and the kidney renin expression.
Earlier we have shown that the AT2 receptor expression in the proximal tubules and renal cortical membranes is increased in obese compared with lean Zucker rats.17 A selective activation of the AT2 receptors promotes natriuresis via tubular mechanism, possibly by inhibiting Na,K-ATPase activity.17,18 Obese Zucker rat is a model of insulin resistance that exhibits hyperglycemia and high blood pressure.19 Impaired renal function and consequently, abnormal renal Na handling is believed to be a factor that contributes to the development of hypertension in this animal model.6,20 Based on the enhanced renal AT2 receptor expression and natriuretic function in obese rats, we predicted a compensatory and protective role of the AT2 receptor against blood pressure increase in obese Zucker rats. Earlier studies using a genetic manipulation of the AT2 receptor, including selective renal AT2 receptor knockout approach 16 have suggested the role of the AT2 receptor in blood pressure regulation. In our study, we used a direct pharmacological approach to test the role of AT2 receptor in the long-term blood pressure control in a disease animal model. The 2-weeks treatment with AT2 antagonist clearly elevated systolic and diastolic blood pressure in obese Zucker rats, suggesting a protective effect.
Since treatment with PD123319 was systemic, the precise mechanism responsible for BP increase by PD treatment is not known. It could be the global blockade of AT2 receptors as well as the un-opposed action of AT1 receptors in the central and peripheral organs/tissues that contributed to the BP increase by PD-treatment. The BP increase in PD-treated rats was also associated with water intake and proportional urinary excretion. Although the increase in water intake in the present study may be related to the blockade of the central AT2 receptor, the role of the central AT2 receptors in thirst is not clear. Some studies have suggested that AT2 receptors are not involved in thirst regulation 28-30 while other studies reported that AT2 receptor may have a role in thirst in response to water-deprivation.31-34 Higher blood pressure could be responsible for increase in diuresis in the PD-treated obese rats. Another plausible explanation of the increase in water-intake and urinary volume could be related to a modest increase in blood glucose by PD-treatment of obese rats, which already are hyperglycemic compared with lean rats. While the increase in fasting blood glucose did not reach statistical significance in PD-treated obese rats compared with control obese, the non-fasting blood glucose (measured on day 15 of the treatment) was significantly higher 160±10 mg/dl in PD-treated compared with 136±4 mg/dl in control obese rats. The positive relationship among hyperglycemia, water consumption and polyuria is well known. However, interesting observation is that the blockade of AT2 receptors contributed to hyperglycemia in obese rats. This could be due to enhanced action of AT1 receptors and/or inaction of AT2 receptors affecting insulin sensitivity in PD-treated rats. There is evidence implicating AT1 receptors in the development of insulin resistance in obese rat models and in humans. 35,36 On the other hand, AT2 receptors have been shown to stimulate peroxisome-proliferation-activator receptor-γ (PPARγ) in PC12 cells.37 The PPARγ is a transcription factor known to enhance insulin sensitivity. 38 If AT2 receptors are linked to PPARγ stimulation in insulin-dependent tissues, such as muscles and adipose tissue, the AT2 receptor antagonism potentially would affect insulin-sensitivity. Therefore, it is possible that blocking of the AT2 receptor and un-opposed action of the AT1 receptors by PD-treatment contributed to insulin resistance further elevating blood glucose, which in turn induces thirst and increase in the urinary excretion. This notion warrants further systematic study. Earlier, it has been shown that AT2 receptor knock-out in mice causes a shift in pressure-natriuresis and an increase in blood pressure.15 Whether BP elevation by long-term pharmacological blockade of the AT2 receptor, as seen in our present study, is a consequence of a shift in pressure natriuresis is yet to be investigated. We found that PD-treatment caused a modest increase in PRA but a profound increase in kidney renin expression. Higher Ang II in the circulation and in the kidney and subsequent un-opposed function of AT1 receptor could have a differential role in the regulating blood pressure in obese animals. However, the enhanced contractile response mediated by AT1 receptor in obese rats might have been compensated by higher endothelial AT1 receptor and eNOS expression as shown in earlier studies.39 Therefore, it is unlikely that the vascular AT1 receptor might have contributed to the blood pressure increase in PD-treated obese rats. Higher kidney renin expression and unopposed action of tubular AT1 receptor function might have contributed to the BP increase in PD-treated rats. This notion is consistent with other studies suggesting abnormal tubular handling of Na as the cause of hypertension in this and other animal model of obesity. The AT2 receptors have been implicated in inhibiting the renin synthesis.40 Increase in the cortical renin expression in PD-treated obese rats is a remarkable observation suggesting that the AT2 receptor maintains kidney renin expression at lower level, and provides a protective and compensatory role in limiting blood pressure increase in obese rats. Since the dose of PD123319 used in the present study, when given acutely does not affect BP17 it further supports the notion that long-term blocking of the AT2 receptor may reset the blood pressure raising mechanisms. Since this study does not include experimental protocols to determine the renal function parameters such as GFR, blood flow and fraction of sodium excretion (FENa), a definite role of the kidneys in the blood pressure increase by PD-treatment of obese rats can not established. Some investigators have reported a decrease in the renal expression of AT2 receptors in diabetic animal models.41,42 Contrary to these reports, we found enhanced renal AT2 receptor expression as reported earlier18,43 and in the present study. Our findings are supported by other reports showing that AT2 receptor expression is increased in human diabetic kidney, 44 and in diabetic rat aorta.45 Reasons for the discrepancy in AT2 receptor expression could be due to the difference in renal preparations, strain of rats and the methods of AT2 receptor measurements. In our studies, we measured AT2 receptor expression by western blotting (and RT-PCR, unpublished data in proximal tubules) in the isolated proximal tubules, and purified basolateral and brush-border membranes prepared from the kidneys of either Zucker rats or streptozotocin (STZ)-treated Sprague Dawley rats. Additionally, the increase in AT2 receptor expression was supported by the enhanced AT2 receptor functions, in terms of Na,K-ATPase inhibition in the proximal tubules, urinary sodium excretion and vascular tone in these animals models.17,43,45 On the other hand, Wehbi et al42 measured AT2 receptors by western blotting in glomeruli and by immunostaining in the kidney from STZ-treated rats. Bonnet et al41 measured AT2 receptors by RT-PCR, immunostaining and autoradiography in STZ-treated spontaneously hypertensive rats and Wistar-Kyoto rats. These investigators did not extend the studies to demonstrate whether reduction in AT2 receptor expression was associated with a reduction in AT2 receptor-mediated functions in their animal models.
Protective effects of the AT2 receptor against various patho-physiological conditions have been documented. For example, treatment with AT2 receptor antagonist caused an increase in blood pressure in the renal-wrap hypertensive rats.9 In another study, cardiac-specific over-expression of AT2 receptor provides protection against AT1 receptor-mediated pressure and chronotropic effects.7 The AT2 receptor provides protection against L-NAME-induced cardiac hypertrophy and fibrosis.7 In brief, our study demonstrates that long-term blockade of the AT2 receptor results in an increase in blood pressure in obese Zucker rats, suggesting a protective and possibly compensatory role of AT2 receptor in blood pressure increase in this animal model. This AT2 receptor role could be important in light of the reports showing that the various hormonereceptors regulating renal sodium homeostasis are impaired. For example, natriuretic function of dopamine D1 receptors 46,47 and ANP 48 is reduced, while antinatriuretic function of Ang II AT1 receptor is enhanced in obese Zucker rats.17,25 However, the mechanism of the protective role of the renal AT2 receptor in blood pressure increase remains to be determined.
Perspective
Present study demonstrates a beneficial role of the AT2 receptor in long-term blood pressure control in obese Zucker rats. The AT1 receptor blockers (ARBs) and angiotensin converting enzyme inhibitors (ACEIs) are used for improving renal function in diabetes and treating hypertension. The preference one over the other or combination of both is based on that ARBs selectively block AT1 receptors and leave AT2 receptors unopposed to function, whereas ACEIs reduces Ang II production, leading to attenuation of function of both AT1 and AT2 receptor. The present study supports the notion that beneficial role of AT2 receptors in protecting against blood pressure elevation should be a part of modalities for treating hypertension.
Acknowledgments
Sources of Funding The study is supported by NIH grant R01-DK61578 and ARP Higher Education Coordinating Board, State of Texas. PD123319 was a kind gift from Pfizer Inc.
Footnotes
Disclosures: None
References
- 1.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger TH. International Union of Pharmacology XXIII: the angiotensin receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 2.Thomas D, Harris PJ, Morgan TO. Altered responsiveness of proximal tubule fluid reabsorption of peritubular angiotensin II in spontaneously hypertensive rats. J Hypertens. 1990;8:407–410. doi: 10.1097/00004872-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Navar LG, Von Thun AM, Zou L, el-Dahr SS, Mitchell KD. Enhancement of intrarenal angiotensin II levels in 2 kidney 1 clip and angiotensin II induced hypertension. Blood Press Suppl. 1995;2:88–92. [PubMed] [Google Scholar]
- 4.Cheng HF, Wang JL, Vinson GP, Harris RC. Young SHR express increased type 1 angiotensin II receptors in renal proximal tubule. Am J Physiol. 1998;274:F10–F17. doi: 10.1152/ajprenal.1998.274.1.F10. [DOI] [PubMed] [Google Scholar]
- 5.Bonnardeaux A, Davies E, Jeunemaitre X, Féry I, Charru A, Clauser E, Tiret L, Cambien F, Corvol P, Soubrier F. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension. 1994;24:63–69. doi: 10.1161/01.hyp.24.1.63. [DOI] [PubMed] [Google Scholar]
- 6.Alonso-Galacia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension. 1996;28:1047–1054. doi: 10.1161/01.hyp.28.6.1047. [DOI] [PubMed] [Google Scholar]
- 7.Masaki H, Kurihara T, Yamaki A, Inomata N, Nozawa Y, Mori Y, Murasawa S, Kizima K, Maruyama K, Horiuchi M, Dzau VJ, Takahashi H, Iwasaka T, Inada M, Matsubara H. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J Clin Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey RM. Angiotensin type-2 receptors and cardiovascular function: are angiotensin type-2 receptors protective? Curr Opin Cardiol. 2005;20:264–269. doi: 10.1097/01.hco.0000166596.44711.b4. [DOI] [PubMed] [Google Scholar]
- 9.Siragy HM, Carey RM. Protective Role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–1242. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- 10.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci USA. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Nimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 12.Tamura M, Takagi T, Howard EF, Landon EJ, Steimle A, Tanner M, Myers PR. Induction of angiotensin II subtype 2 receptor-mediated blood pressure regulation in synthetic diet-fed rats. J Hypertens. 2000;18:1239–1246. doi: 10.1097/00004872-200018090-00010. [DOI] [PubMed] [Google Scholar]
- 13.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 14.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 15.Gross V, Schunck WH, Honeck H, Milia AF, Kargel E, Walther T, Bader M, Inagami T, Schneider W, Luft FC. Inhibition of pressure natriuresis in mice lacking the AT2 receptor. Kidney Int. 2000;57:191–202. doi: 10.1046/j.1523-1755.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- 16.Moore AF, Heiderstadt NT, Hunag E, Howell NL, Wang ZQ, Siragy HM, Carey RM. Selective inhibition of the renal angiotensin type 2 receptor increases blood pressure in conscious rats. Hypertension. 2001;37:1285–1291. doi: 10.1161/01.hyp.37.5.1285. [DOI] [PubMed] [Google Scholar]
- 17.Hakam AC, Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension. 2005;45:270–275. doi: 10.1161/01.HYP.0000151622.47814.6f. [DOI] [PubMed] [Google Scholar]
- 18.Hakam AC, Hussain T. Angiotensin II type 2 receptor agonist directly inhibits proximal tubule sodium pump activity in obese but not in lean Zucker rats. Hypertension. 2006;47:1117–1124. doi: 10.1161/01.HYP.0000220112.91724.fc. [DOI] [PubMed] [Google Scholar]
- 19.Kurtz TW, Morris RC, Pershad Singh HA. The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension. 1989;13:896–901. doi: 10.1161/01.hyp.13.6.896. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara K, Hayashi K, Matsuda H, Kubota E, Honda M, Ozawa Y, Saruta T. Altered pressure-natriuresis in obese Zucker rats. Hypertension. 1999;33:1470–1475. doi: 10.1161/01.hyp.33.6.1470. [DOI] [PubMed] [Google Scholar]
- 21.Granger JP, West D, Scott J. Abnormal pressure natriuresis in the dog model of obesity-induced hypertension. Hypertension. 1994;23:I8–I11. doi: 10.1161/01.hyp.23.1_suppl.i8. [DOI] [PubMed] [Google Scholar]
- 22.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 23.Becker M, Umrani D, Lokhandwala MF, Hussain T. Increased renal angiotensin II AT1 receptor function in obese Zucker rat. Clin Exp Hypertens. 2003;25:35–47. doi: 10.1081/ceh-120017739. [DOI] [PubMed] [Google Scholar]
- 24.Shah S, Hussain T. Enhanced angiotensin II-induced activation of Na+,K+-ATPase in the proximal tubules of obese Zucker rats. Clin Exp Hypertens. 2006;28:29–40. doi: 10.1080/10641960500386650. [DOI] [PubMed] [Google Scholar]
- 25.Tallam LS, Jandhyala BS. Significance of exaggerated natriuresis after angiotensin AT1 receptor blockade or angiotensin-converting enzyme inhibition in obese Zucker rats. Clin Exp Pharmacol Physiol. 2001;28:433–440. doi: 10.1046/j.1440-1681.2001.03457.x. [DOI] [PubMed] [Google Scholar]
- 26.Xu ZG, Lanting L, Vaziri ND, Li Z, Sepassi L, Rodriguez-Iturbe B, Natarajan R. Upregulation of angiotensin II type 1 receptor, inflammatory mediators, and enzymes of arachidonate metabolism in obese Zucker rat kidney: reversal by angiotensin II type 1 receptor blockade. Circulation. 2005;111:1962–1969. doi: 10.1161/01.CIR.0000161831.07637.63. [DOI] [PubMed] [Google Scholar]
- 27.Harker CT, O'Donnel MP, Kasiske BL, Keane WF, Katz SA. The renin-angiotensin system in the type II diabetic obese Zucker rat. J Am Soc Nephrol. 1993;4:1354–1361. doi: 10.1681/ASN.V461354. [DOI] [PubMed] [Google Scholar]
- 28.Beresford MJ, Fitzsimons JT. Intracerebroventricular angiotensin II-induced thirst and sodium appetite in rat are blocked by the AT1 receptor antagonist, Losartan (DuP 753), but not by the AT2 antagonist, CGP 42112B. Exp Physiol. 1992;77:761–764. doi: 10.1113/expphysiol.1992.sp003643. [DOI] [PubMed] [Google Scholar]
- 29.Weisinger RS, Blair-West JR, Denton DA, Tarjan E. Role of brain angiotensin II in thirst and sodium appetite of sheep. Am J Physiol. 1997;273:R187–R196. doi: 10.1152/ajpregu.1997.273.1.R187. [DOI] [PubMed] [Google Scholar]
- 30.Cooney AS, Fitzsimons JT. The effect of the putative AT2 agonist, p-aminophenylalanine6 angiotensin II, on thirst and sodium appetite in rats. Exp Physiol. 1993;78:767–774. doi: 10.1113/expphysiol.1993.sp003724. [DOI] [PubMed] [Google Scholar]
- 31.Abrão Saad W, Antonio De Arruda Camargo L, Sérgio Cerri P, Simões S, Abrão Saad W, Garcia G, Izabel Gutierrez L, Guarda I, Saad Guarda R. Influence of arginine vasopressin receptors and angiotensin receptor subtypes on the water intake and arterial blood pressure induced by vasopressin injected into the lateral septal area of the rat. Auton Neurosci. 2004;111:66–70. doi: 10.1016/j.autneu.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Lee WJ, Kim KS, Yang EK, Lee JH, Lee EJ, Park JS, Kim HJ. Effect of brain angiotensin II AT1, AT2, and cholinergic receptor antagonism on drinking in water-deprived rats. Regul Pept. 1996;66:41–46. doi: 10.1016/0167-0115(96)00063-8. [DOI] [PubMed] [Google Scholar]
- 33.Widdop RE, Gardiner SM, Bennett T. Effects of angiotensin II AT1 or AT2-receptor antagonists on drinking evoked by angiotensin II or water deprivation in rats. Brain Res. 1994;648:46–52. doi: 10.1016/0006-8993(94)91903-8. [DOI] [PubMed] [Google Scholar]
- 34.Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 35.Cefalu WT. Insulin resistance: cellular and clinical concepts. Exp Biol Med. 2001;226:13–26. doi: 10.1177/153537020122600103. [DOI] [PubMed] [Google Scholar]
- 36.Tobli JE, Munoz MC, Cao G, Mella J, Preyra L, Mastai R. ACE inhibition and AT1 receptor blockade prevent fatty liver and fibrosis in obese Zucker rats. Obesity (Silver Spring) 2008;16:770–776. doi: 10.1038/oby.2007.114. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Foryst-Ludwig A, Bruemmer D, Culman J, Bader M, Unger T, Kintscher U. Angiotensin II induces peroxisome proliferator-activated receptor gamma in PC12W cells via angiotensin type 2 receptor activation. J Neurochem. 2005;94:1395–1401. doi: 10.1111/j.1471-4159.2005.03275.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim MK, Chae YN, Son MH, Kim SH, Kim JK, Moon HS, Park CS, Bae MH, Kim E, Han T, Choi HH, Shin YA, Ahn BN, Lee CH, Lim JI, Shin CY. PAR-5359, a well balanced PPARα/γ dual agonist, exhibits equivalent antidiabetic and hypolipidemic activities in vitro and in vivo. Eur J Pharmacol. 2008;595:119–125. doi: 10.1016/j.ejphar.2008.07.066. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqui AH, Hussain T. Enhanced AT1 receptor-mediated vasocontractile response to ANG II in endothelium-denuded aorta of obese Zucker rats. Am J Physiol Heart Circ Physiol. 2007;292:H1722–H1727. doi: 10.1152/ajpheart.00612.2006. [DOI] [PubMed] [Google Scholar]
- 40.Siragy HM, Xue C, Abadir P, Carey RM. Angiotensin subtype-2 receptors inhibit renin biosynthesis and angiotensin II formation. Hypertension. 2005;45:133–137. doi: 10.1161/01.HYP.0000149105.75125.2a. [DOI] [PubMed] [Google Scholar]
- 41.Bonnet F, Candido R, Carey RM, Casley D, Russo LM, Osicka TM, Cooper ME, Cao Z. Renal expression of angiotensin receptors in long-term diabetes and the effects of angiotensin type 1 receptor blockade. J Hypertens. 2002;20:1615–1624. doi: 10.1097/00004872-200208000-00025. [DOI] [PubMed] [Google Scholar]
- 42.Wehbi GJ, Zimpelmann J, Carey RM, Levine DZ, Burns KD. Early streptozotocin-diabetes mellitus downregulates rat kidney AT2 receptors. Am J Physiol Renal Physiol. 2001;280:F254–F265. doi: 10.1152/ajprenal.2001.280.2.F254. [DOI] [PubMed] [Google Scholar]
- 43.Hakam AC, Siddiqui AH, Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2006;290:F503–508. doi: 10.1152/ajprenal.00092.2005. [DOI] [PubMed] [Google Scholar]
- 44.Mezzano S, Droguett A, Burgos ME, Ardiles LG, Flores CA, Carosi I, Vio CP, Ruiz-Ortega, Egido J. Renin-angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int. 2003;64:S64–S70. doi: 10.1046/j.1523-1755.64.s86.12.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee JH, Xia S, Ragolia L. Up-regulation of AT2 Receptor and iNOS impairs angiotensin II-induced contraction without endothelium influence in young normotensive diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R144–R154. doi: 10.1152/ajpregu.00191.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marwaha A, Lokhandwala MF. Diminished natriuretic response to dopamine D1 receptor agonist, SKF-38393 in obese Zucker rats. Clin Exp Hypertens. 2003;25:509–15. doi: 10.1081/ceh-120025334. [DOI] [PubMed] [Google Scholar]
- 47.Hussain T, Beheray SA, Lokhandwala MF. Defective dopamine receptor function in proximal tubules of obese Zucker rats. Hypertension. 1999;34:1091–1096. doi: 10.1161/01.hyp.34.5.1091. [DOI] [PubMed] [Google Scholar]
- 48.Zeigler DW, Patel KP. Reduced renal responses to an acute saline load in obese Zucker rats. Am J Physiol. 1991;261:R712–R718. doi: 10.1152/ajpregu.1991.261.3.R712. [DOI] [PubMed] [Google Scholar]