Abstract
Aims
We examined the relationship between plasma selenium levels at enrollment and all-cause mortality over a 6-year period among participants in the InCHIANTI study.
Methods
1042 men and women ≥65 years from the InCHIANTI study, a population-based study of older adults living in the Chianti region of Tuscany, a population-based cohort in Tuscany, Italy. Plasma selenium was measured at enrollment (1998–2000), and vital status was ascertained until May 2006.
Results
During follow-up, 237 participants (22.7%) died. At enrollment, mean (SD) plasma selenium concentrations among participants who survived or died were 0.96 (0.14) and 0.87 (0.18) µmol/L (p<0.0001), respectively. The proportion of participants who died, from lowest to highest quartile of selenium, was 41.3, 27.0, 18.1 and 13.5% (p<0.0001 by Mantel-Haenszel chi-square). After adjusting for age, sex, education, and chronic diseases, adults in the lowest quartile of plasma selenium at enrollment had higher mortality compared with those in the highest quartile (Hazard Ratio (HR) 1.60, 95% Confidence Interval (CI) 1.04–2.47, p=0.034).
Conclusion
Low plasma selenium may be an independent predictor of mortality among older adults living in the community.
Keywords: Aging, mortality, selenium
INTRODUCTION
Selenium is an essential trace element and a normal constituent of the diet. It is a component of selenoproteins, including selenoenzymes such as glutathione peroxidase, selenoprotein-P and thioredoxin reductase (1). Serum selenium concentrations seem to decrease with age (2, 3) and are lower in persons with cancer and chronic diseases (3). Low serum/plasma selenium concentrations have been associated with increased mortality from cancer (4, 5) and increased all-cause mortality among community-dwelling older men and women in France (6) and women in the USA (7).
Selenium function is best characterized by the role it plays in the glutathione peroxidase enzyme system, a major antioxidant defense system that uses reduced glutathione to decrease oxidative stress by breaking down hydrogen peroxide and lipid peroxides (1, 8). Low activity of glutathione peroxidase has been associated with increased risk of cardiovascular events among adults with suspected coronary artery disease (9, 10).
We hypothesized that low plasma selenium concentrations are associated with a higher risk of mortality among older adults. To address this hypothesis, we examined the relationship between plasma selenium levels at enrollment and mortality among participants in the InCHIANTI study, a population-based study of older adults living in the Chianti region of Tuscany, Italy.
METHODS
The study participants consisted of men and women, aged 65 and older, who participated in the Invecchiare in Chianti, “Aging in the Chianti Area” (InCHIANTI) study, conducted in two small towns in Tuscany, Italy. Study rationale, design and data collection have been described elsewhere (11). Briefly, in August 1998, 1270 people aged 65 years and older were randomly selected from the population registry of Greve in Chianti (population 11,709) and Bagno a Ripoli (pop. 4704) and, of 1256 eligible subjects, 1155 (90.1%) agreed to participate. Of the 1155 participants, 1055 (91.3%) gave blood samples and 1042 had complete data to be included into the study. Participants received an extensive description of the study aims and participated after giving their written informed consent. Participants were evaluated again for three-year follow-up visits from 2001–2003 (n=926) and six-year follow-up visits from 2004–2006 (n=844), at which time they underwent repeated phlebotomy and laboratory testing and assessment of physical performance. The study protocol complied with the Declaration of Helsinki and was approved by the Italian Institute of Research and Care on Aging Ethical Committee. At the end of field data collection, we collected mortality data of the original InCHIANTI cohort, with data from the General Mortality Registry of the Tuscany Region and death certificates, which are deposited immediately after death at the municipality of residence.
Demographic information and information on smoking were collected using standardized questionnaires. Smoking history was determined from self-reports and dichotomized in the analysis as “current smoker” vs “ever smoked” and “never smoked”. Education was recorded as years of schooling. Average daily intakes of energy (kcal), total protein and fish were estimated with the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire, validated in the InCHIANTI population (12). All participants were examined by a trained geriatrician, and diseases were ascertained according to standard, pre-established criteria and algorithms based upon those used in the Women’s Health and Aging Study for coronary heart disease, chronic heart failure, peripheral artery disease, stroke, Parkinson’s Diseases, diabetes mellitus, chronic obstructive pulmonary disease, and cancer (13). Weight was measured on a high-precision mechanical scale. Standing height was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight/height2 (kg/m2).
Blood samples were collected in the morning after a 12-h fast. Aliquots of serum and plasma were immediately obtained and stored at −80°C. Aliquots of plasma were shipped on dry ice to Dr. Semba’s laboratory for measurements of plasma selenium. Plasma selenium was measured by graphite furnace atomic absorption spectrometry in a Perkin Elmer AAnalyst 600 with Zeeman background correction. Samples were diluted 1:4 with a Triton-X (Sigma Chemical, St. Louis, MO) and nitric acid solution (Fisher Scientific, Pittsburgh, PA), and the matrix modifier was a palladium and magnesium nitrate solution (both Perkin Elmer, Norwalk, CT). The instrument was calibrated daily to known plasma selenium standards (UTAK Laboratories, Inc., Valencia, CA). Within-run and between-run coefficients of variation, respectively, were 3.1% and 7.1%.
Variables are reported as means (standard deviations) for normally distributed parameters or as percentages. The characteristics of subjects according to their vital status were compared by the t-test, and percentages were compared by chi-square tests. Plasma selenium was analyzed both as a continuous variable and as quartiles, in which the quartiles for selenium were defined as <0.839, 0.839–0.934, 0.935–1.037 and >1.037 µmol/L. Survival analysis (Kaplan Meyer) and Cox proportional hazards models, adjusted for age, sex, education, BMI, total energy intake, congestive heart failure, peripheral artery disease, stroke, Parkinson’s disease and chronic obstructive pulmonary disease (COPD), were used to examine the relationship between plasma selenium and mortality. Covariates for adjustment were selected by univariate logistic regression analysis. Kaplan-Meyer survival curves were compared by the log-rank test. All analyses were performed by SAS (v. 8.2, SAS Institute, Inc., Cary, NC), with statistical significance level at p<0.05.
RESULTS
From the 1042 (90.2%) participants who had plasma selenium concentrations available for this analysis at baseline, after six years of follow-up, 237 participants (22.7%) had died. At enrollment, the concentration of the mean (SD) plasma selenium of all participants was 0.94 (0.16), and among participants who had survived or died 0.96 (0.14) and 0.87 (0.18) µmol/L (p<0.0001), respectively. Subjects who gave blood samples were generally older and had greater comorbidity than those who died. The characteristics of the study population at enrollment are listed in Table 1. Participants who died during follow-up were significantly older, more likely to be female, with lower education, lower total energy intake, and more likely to have chronic obstructive pulmonary disease and congestive heart failure than those who survived during follow-up. Body mass index was significantly lower between those who died and survived. The proportion of participants with peripheral artery disease was higher among those who died (p=0.06). There were no significant differences in current smoking, diabetes mellitus, cardiovascular disease or cancer between those who died and those who survived.
Table 1.
Characteristics of study population at enrollment.
| Characteristic | All Participants | Survived | Died | p2 |
|---|---|---|---|---|
| (n=1042) | (n=805) | (n=237) | ||
| Age (yrs) | 75.6 (7.4) | 73.6 (6.2) | 82.2 (7.5) | <0.001 |
| Sex (% female) | 56.7 | 59.1 | 48.5 | 0.004 |
| Education (yrs)1 | 5.3 (3.3) | 5.6 (3.3) | 4.4 (3.1) | <0.001 |
| Current smokers (%) | 16.6 | 13.5 | 14.2 | 0.507 |
| Body mass index (kg/m2) | ||||
| <18.5 | 7.5 | 2.7 | 23.6 | <0.001 |
| 18.5–24.9 | 26.3 | 26.7 | 24.9 | |
| 25.0–29.9 | 42.9 | 45.2 | 35.0 | |
| ≥30 | 23.3 | 25.3 | 16.5 | |
| Plasma selenium (µmol/L)1 | 0.94 (0.16) | 0.96 (0.14) | 0.87 (0.18) | <0.001 |
| Total energy intake (kcal/day)1 | 1905 (565) | 1940 (570) | 1786 (530) | <0.001 |
| Coronary heart disease (%) | 4.5 | 4.2 | 5.5 | 0.411 |
| Congestive heart failure (%) | 5.4 | 2.7 | 14.4 | <0.001 |
| Peripheral artery disease (%) | 6.1 | 3.7 | 14.3 | 0.058 |
| Diabetes mellitus (%) | 10.9 | 10.1 | 13.9 | 0.139 |
| Chronic obstructive pulmonary disease (%) | 8.2 | 6.0 | 15.6 | 0.003 |
| Cancer (%) | 6.1 | 5.7 | 7.2 | 0.407 |
| Stroke | 5.4 | 3.3 | 11.8 | <0.001 |
| Parkinson’s Diseases | 1.3 | 0.9 | 2.35 | 0.03 |
Mean (SD) for continuous variables or percentages as noted
Test for trend, where analyzed, expresses selenium quartiles as dummy variables.
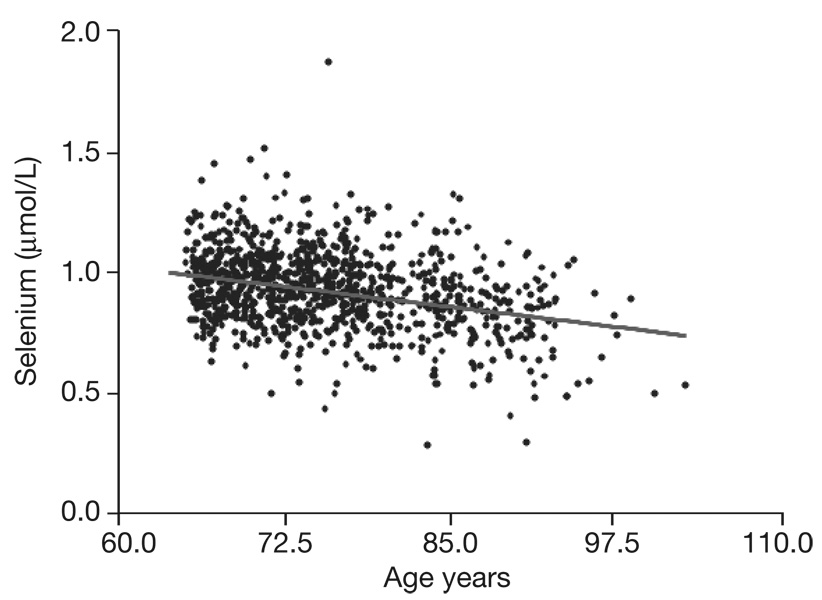
After adjusting for age, at baseline we found a significant association between plasma selenium and intakes of energy (kcal) (r=0.07; p=0.03), total protein (r=0.11; p=0.001) and fish (r=0.08; p=0.01), COPD (r=−0.06; p=0.05) and Parkinson’s Disease (r=−0.06; p=0.05). We found no difference between mean selenium values in men or women (men: 0.933 µmol/L±0.158; women: 0.940 µmol/L±0.158; p=0.51). Figure 1 shows the distributions of selenium levels in the whole population with aging. The selenium level progressively falls with increasing age (B: −0.007, SD: 0.0006; p=<0.0001).
Fig. 1.
Distribution of selenium level in whole population with aging.
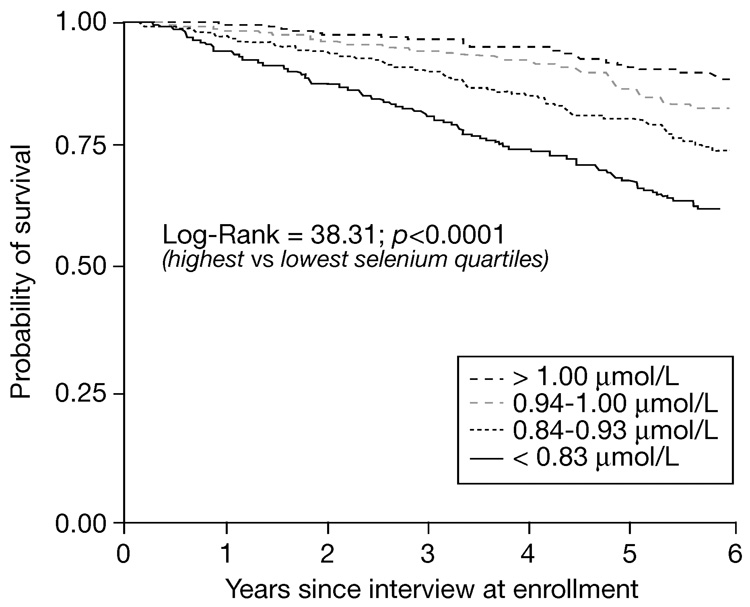
In those participants who died, the proportions of selenium from the lowest to highest quartile were 41.3, 27.0, 18.1 and 13.5% (p<0.0001 by Mantel-Haenszel chi-square). The survival of participants by quartile of plasma selenium at baseline is shown in Figure 2. Univariate and multivariate Cox proportional hazards models were used to examine the relationship between plasma selenium concentrations at baseline and mortality (Table 2 and Table 3). In a univariate analysis (model 1), multivariate analyses adjusted for age and sex (model 2), and adjusted for age, sex, education, BMI and chronic diseases, participants in the lowest quartile of plasma selenium were at increased risk of death (HR 1.60, 95% CI 1.04–2.47, p=0.034). In an alternative model, in which selenium was examined as a continuous variable, after adjusting for the same covariates as in model 3, baseline plasma selenium (µmo/L) was associated with mortality (HR 0.32, 95% CI 0.13–0.79, p=0.014).
Fig. 2.
Kaplan-Meyer survival curves of older adults by quartile of plasma selenium at enrollment (Log-Rank: 38.31, p<0.0001 by log-rank test from highest to lowest quartile of selenium).
Table 2.
Univariate relationship between selenium and other risk factors with all-cause mortality in older adults.
| Characteristic | HR | 95% CI | p |
|---|---|---|---|
| Plasma selenium | p1 for trend | <0.001 | |
| Quartile 1 | 3.82 | 2.56–5.69 | <0.001 |
| Quartile 2 | 2.26 | 1.48–3.46 | <0.001 |
| Quartile 3 | 1.40 | 0.89–2.21 | 0.149 |
| Quartile 4 | 1.00 | – | – |
| Age (yrs) | 1.19 | 1.16–1.22 | <0.001 |
| Sex (female) | 0.69 | 0.52–0.92 | 0.01 |
| Education (yrs) | 0.87 | 0.83–0.93 | <0.001 |
| Body mass index (kg/m2) | 0.97 | 0.93–1.01 | 0.12 |
| Total energy intake (kcal/day) | 0.99 | 1.99–1.00 | <0.001 |
| Current smoking | 1.01 | 0.83–1.23 | 0.92 |
| Congestive heart failure | 1.66 | 1.41–1.96 | <0.001 |
| Coronary heart disease | 1.33 | 0.69–2.52 | 0.39 |
| Peripheral artery disease | 1.25 | 0.99–1.56 | 0.05 |
| Chronic obstructive pulmonary disease | 1.43 | 1.08–1.90 | 0.01 |
| Cancer | 1.15 | 0.65–2.05 | 0.63 |
| Stroke | 1.93 | 1.49–2.49 | <0.0001 |
| Parkinson’s Diseases | 1.82 | 1.03–3.23 | 0.04 |
p for trend obtained by considering selenium quartile level as ordinal variable.
Table 3.
Multivariate relationship between selenium and other risk factors with all-cause mortality in older adults.
| Characteristic | HR | 95% CI | p |
|---|---|---|---|
| Model 1 | |||
| Plasma selenium | p1 for trend | 0.001 | |
| Quartile 1 | 1.82 | 1.20–2.75 | 0.005 |
| Quartile 2 | 1.48 | 0.96–2.29 | 0.073 |
| Quartile 3 | 1.14 | 0.72–1.81 | 0.567 |
| Quartile 4 | 1.00 | – | – |
| Age (yrs) | 1.14 | 1.12–1.16 | <0.001 |
| Sex (female) | 0.55 | 0.43–0.72 | <0.001 |
| Model 2 | |||
| Plasma selenium | p1 for trend | 0.004 | |
| Quartile 1 | 1.62 | 1.06–2.43 | 0.024 |
| Quartile 2 | 1.42 | 0.94–2.18 | 0.092 |
| Quartile 3 | 1.09 | 0.68–1.74 | 0.729 |
| Quartile 4 | 1.00 | – | – |
| Age (yrs) | 1.11 | 1.09–1.14 | <0.001 |
| Sex (female) | 0.53 | 0.39–0.70 | <0.001 |
| Education (yrs) | 0.97 | 0.93–1.02 | 0.122 |
| Body mass index (kg/m2) | |||
| <18.5 | 2.10 | 1.39–3.17 | 0.0004 |
| 18.5–24.9 | 1.00 | – | – |
| 25.0–29.9 | 1.23 | 0.84–1.57 | 0.475 |
| ≥30 | 1.16 | 0.78–1.72 | 0.458 |
| Total energy intake (kcal/day) | 1.00 | 1.00–1.00 | 0.368 |
| Congestive heart failure | 1.38 | 1.20–1.59 | <0.001 |
| Peripheral artery disease | 1.10 | 0.90–1.35 | 0.339 |
| Chronic obstructive pulmonary disease | 1.23 | 0.94–1.64 | 0.122 |
| Stroke | 1.25 | 1.04–1.49 | 0.015 |
| Parkinson’s Diseases | 1.35 | 0.87–2.09 | 0.185 |
p for trend obtained by considering selenium quartile level as ordinal variable.
After removing participants with a history of cancer, cardiovascular disease or chronic heart failure at baseline (n=108), participants in the lowest quartile at baseline were at higher risk of dying compared with those in the highest quartile (HR 1.79, 95% CI 1.14–2.82).
DISCUSSION
This study shows that, in a representative sample of the Italian elderly population, participants with low plasma selenium levels are at higher risk of death. These results are consistent with observations from two other cohort studies, the Etude du Vieillissement Arteriel (EVA) study, involving 1389 adults aged 59–71 years in Nantes, France (6) and among 632 women aged 70–79 in the Women’s Health and Aging Studies I and II (WHAS) in Baltimore, Maryland (7). In the EVA study, mean baseline plasma selenium concentrations were 1.01 µmol/L among participants who died, compared with 1.10 µmol/L among those who survived during 9 years of follow-up (6). In the WHAS, mean baseline serum selenium concentrations were 1.53 µmol/L and 1.67 µmol/L among women who died and survived during 5 years of follow-up (7). The present study and the two previous ones are remarkably consistent in showing a relationship between plasma/serum selenium levels and mortality among community-dwelling adults in three distinct geographic locations.
A limitation of the present study is that the specific causes of death among the 237 participants who died have not yet been ascertained, and that this study reports only all-cause mortality. Among the 89 women who died in WHAS, the major causes of death were heart disease, cancer, stroke, infection, and chronic obstructive pulmonary disease (7). Although specific causes of death were known in the InCHIANTI study, the power to detect a relationship between plasma selenium levels and specific causes of mortality may currently be limited because of small sample size.
Selenium intake or levels have been linked with various neoplasms, such as prostate (14), colorectal (15) and lung (16). In an area of China with low serum selenium concentrations, low serum selenium was associated with death from esophageal cancer and stomach cancer (17). The relationship between selenium and cardiovascular disease is still unclear. In the Health Professionals Follow-Up Study, selenium levels were inversely associated with risk of non-fatal myocardial infarction but not fatal coronary heart disease (18). A secondary analysis of the Nutritional Prevention of Cancer Trial showed that selenium supplementation (200 µg/day) was not associated with cardiovascular disease outcomes (19). The heterogeneity among studies may be due to the differences in selenium levels in the populations studied, as the selenium content of plant and animal foods is usually related to the local selenium content of the soil.
The exact biological mechanisms by which low selenium levels contribute to an increased risk of mortality may be related to the role that selenium plays in the age-related pro-inflammatory state (20). Higher selenium levels may potentially protect against oxidative stress and reduce redox-related upregulation of IL-6 (21). Another mechanism may be modulation of arachidonic acid metabolism (22). In the Uppsala Longitudinal Study of Adult Men, high serum selenium levels were predictive of lower urinary F2 isoprostane concentrations, a biomarker of lipid peroxidation and oxidative stress (23). Low selenium may also compromise health by having an adverse effect on the synthesis and activity of deiodinase, the enzyme that transforms thyroxine into the biologically active tri-iodothyronine (24).
The plasma selenium concentrations in our population, which is representative of the Italian elderly population, are consistent with previous reports from Italy. In the Veneto region, mean plasma selenium concentrations were 0.82 µmol/L among adults (25), and 1.12 and 0.86 among adults aged 65–89 years and ≥90 years, respectively, from Bologna (26). Mean serum selenium concentrations in Italian adults vary between 1.09 and 1.17 µmol/L, decreasing values being observed in adults over age 60 (27). The average daily intake of selenium in the Italian population is about 51 µg/day, which compares with the RDA of 55 µg/day for adult men and women (28) - a value which may be even lower than the recommended optimal plasma selenium concentration for GSHPx activity or for cancer protection (29).
CONCLUSIONS
Low plasma selenium concentrations were associated with an increased risk of dying among older adults living in the community. Whether selenium status is simply a marker of health or is implicated in specific mechanisms that enhance the individual’s defenses against diseases remains unknown, and should be tested in a specifically designed clinical trial of selenium supplementation in older persons.
ACKNOWLEDGEMENTS
Financial Disclosure: The authors received no financial support in relation to this manuscript and declare that they have no conflict of interest concern this manuscript.
This work was supported by National Institute on Aging Contracts N01-AG-916413, N01-AG-821336, N01-AG-5-0002 and NIA Grant R01 AG027012. This research was partly supported by the Intramural Research Program, National Institute on Aging, NIH.
Sponsors’ Role: None.
REFERENCES
- 1.Klein EA. Selenium: epidemiology and basic science. J Urol. 2004;171:S50–S53. doi: 10.1097/01.ju.0000107837.66277.e9. [DOI] [PubMed] [Google Scholar]
- 2.Savarino L, Granchi D, Ciapetti G, et al. Serum concentrations of zinc and selenium in elderly people: results in healthy nonagenarians/centenarians. Exp Gerontol. 2001;36:327–339. doi: 10.1016/s0531-5565(00)00218-7. [DOI] [PubMed] [Google Scholar]
- 3.Bates CJ, Thane CW, Prentice A, et al. Selenium status and its correlates in a British national diet and nutrition survey: people aged 65 years and over. J Trace Elem Med Biol. 2002;16:1–8. doi: 10.1016/s0946-672x(02)80002-5. [DOI] [PubMed] [Google Scholar]
- 4.Kok FJ, de Bruijn AM, Vermeeren R, et al. Serum selenium, vitamin antioxidants, and cardiovascular mortality: a 9-year follow-up study in the Netherlands. Am J Clin Nut. 1987;45:462–468. doi: 10.1093/ajcn/45.2.462. [DOI] [PubMed] [Google Scholar]
- 5.Kornitzer M, Valente F, De Bacquer D, et al. Serum selenium and cancer mortality: a nested case-control study within an age- and sex-stratified sample of the Belgian adult population. Eur J Clin Nutr. 2004;58:98–104. doi: 10.1038/sj.ejcn.1601754. [DOI] [PubMed] [Google Scholar]
- 6.Akbaraly NT, Arnaud J, Hininger-Favier I, et al. Selenium and mortality in the elderly: results from the EVA study. Clin Chem. 2005;51:2117–2123. doi: 10.1373/clinchem.2005.055301. [DOI] [PubMed] [Google Scholar]
- 7.Ray AL, Semba RD, Walston J, et al. Low serum selenium and total carotenoids predict mortality among older women living in the community: the Women’s Health and Aging Studies. J Nutr. 2006;136:172–176. doi: 10.1093/jn/136.1.172. [DOI] [PubMed] [Google Scholar]
- 8.Burk RF, Levander OA. Selenium. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 312–325. [Google Scholar]
- 9.Blankenberg S, Rupprecht HJ, Bickel C, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 10.Schnabel R, Lackner KJ, Rupprecht HJ, et al. Glutathione peroxidase-1 and homocysteine for cardiovascular risk prediction: results from the AtheroGene study. J Am Coll Cardiol. 2005;45:1631–1637. doi: 10.1016/j.jacc.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 11.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the In CHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 12.Pisani P, Faggiano F, Krogh V, et al. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26 Suppl 1:S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 13.Guralnik JM, Fried LP, Simonsick EM, et al. Bethesda, MD: National Institute on Aging; The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. 1995 NIH Publication No. 95-4009.
- 14.Etminan M, Fitzgerald JM, Cleave M, et al. Intake of selenium in the prevention of prostate cancer: a systematic review and meta-analysis. Cancer Causes Control. 2005;16:1125–1131. doi: 10.1007/s10552-005-0334-2. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs ET, Jiang R, Alberts DS, et al. Selenium and colorectal adenoma: results of a pooled analysis. J Natl Cancer Inst. 2004;96:1669–1675. doi: 10.1093/jnci/djh310. [DOI] [PubMed] [Google Scholar]
- 16.Zhuo H, Smith AH, Steinmaus C. Selenium and lung cancer: a quantitative analysis of heterogeneity in the current epidemiological literature. Cancer Epidemiol Biomarkers Prev. 2004;13:771–778. [PubMed] [Google Scholar]
- 17.Wei WQ, Abnet CC, Qiao YL, et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2004;79:80–85. doi: 10.1093/ajcn/79.1.80. [DOI] [PubMed] [Google Scholar]
- 18.Yoshizawa K, Ascherio A, Morris JS, et al. Prospective study of selenium levels in toenails and risk of coronary artery disease in men. Am J Epidemiol. 2003;158:852–860. doi: 10.1093/aje/kwg052. [DOI] [PubMed] [Google Scholar]
- 19.Stranges S, Marshall JR, Trevisan M, et al. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. Am J Epidemiol. 2006;163:694–699. doi: 10.1093/aje/kwj097. [DOI] [PubMed] [Google Scholar]
- 20.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walston JD, Xue QL, Semba RD, et al. Serum antioxidants, inflammation, and mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 22.Alissa EM, Bahijri SM, Ferns GA. The controversy surrounding selenium and cardiovascular disease: a review of the evidence. Med Sci Monit. 2003;9:RA9–RA18. [PubMed] [Google Scholar]
- 23.Helmersson J, Ärnlöv J, Vessby B, et al. Serum selenium predicts levels of F2-isoprostanes and prostaglandin F2; in a 27 year follow-up study of Swedish men. Free Radic Res. 2005;39:763–770. doi: 10.1080/10715760500108513. [DOI] [PubMed] [Google Scholar]
- 24.Beckett GJ, Arthur JR. Selenium and endocrine systems. J Endocrinol. 2005;184:455–465. doi: 10.1677/joe.1.05971. [DOI] [PubMed] [Google Scholar]
- 25.Bellisola G, Perona G, Galassini S, et al. Plasma selenium and glutathione peroxidase activities in individuals living in the Veneto region of Italy. J Trace Elem Electrolytes Health Dis. 1993;7:242–247. [PubMed] [Google Scholar]
- 26.Ravaglia G, Forti P, Maioli F, et al. Blood micronutrient and thyroid hormone concentrations in the oldest-old. J Clin Endocrinol Metab. 2000;85:2260–2265. doi: 10.1210/jcem.85.6.6627. [DOI] [PubMed] [Google Scholar]
- 27.Morisi G, Patriarca M, Marano G, et al. Age and sex specific reference serum selenium levels estimated for the Italian population. Ann Ist Super Sanita. 1989;25:393–403. [PubMed] [Google Scholar]
- 28.Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, D.C.: National Academy Press; 2000. Food and Nutrition Board, Institute of Medicine. [PubMed] [Google Scholar]
- 29.Fleet JC. Dietary selenium repletion may reduce cancer incidence in people at high risk who live in areas with low soil selenium. Nutr Rev. 1997;55:277–279. doi: 10.1111/j.1753-4887.1997.tb01617.x. [DOI] [PubMed] [Google Scholar]