Abstract
This study examined the frequency of personality, language, and social-behavioral characteristics believed to comprise the broad autism phenotype (BAP), across families differing in genetic liability to autism. We hypothesized that within this unique sample comprised of multiple-incidence autism families (MIAF), single-incidence autism families (SIAF), and control Down syndrome families (DWNS), a graded expression would be observed for the principal characteristics conferring genetic susceptibility to autism, in which such features would express most profoundly among parents from MIAFs, less strongly among SIAFs, and least of all among comparison parents from DWNS families, who should display population base rates. Analyses detected linear expression of traits in line with hypotheses, and further suggested differential intrafamilial expression across family types. In the vast majority of MIAFs both parents displayed BAP characteristics, whereas within SIAFs, it was equally likely that one, both, or neither parent show BAP features. The significance of these findings is discussed in relation to etiologic mechanisms in autism and relevance to molecular genetic studies.
Keywords: autism, genetic, broad autism phenotype
INTRODUCTION
Autism is a complex neurodevelopmental disorder involving disruptions in language and social-emotional functioning, and markedly restricted interests and activities [American Psychiatric Association, 1994]. Strong evidence supports the role of genetic factors in the etiology of autism. Indeed, with 5–8% recurrence rate within families [Szatmari et al., 1998], and concordance of 60% and 3–5% in monozygotic (MZ) and dizygotic (DZ) twins, respectively [Bailey et al., 1995], autism is among the most heritable of complex psychiatric disorders.
Identification of autism susceptibility loci, however, has been hampered by the clinical and etiologic complexity of the disorder, along with the suspected presence of gene–gene and gene–environment interactions [Pickles et al., 1995; Risch et al., 1999; Szatmari, 1999]. Following a promising approach increasingly employed in studies of complex disorders [e.g., schizophrenia, among others [Weinberger et al., 2001; Egan and Goldberg, 2003; Gottesman and Goulde, 2003; Bramon et al., 2005], the study of endophenotypes, or “intermediate phenotypes”as they have been referred to in the literature more recently [see Carlson et al., 2004] can offer a means of overcoming the obstacles to gene detection imposed by genetically complex psychiatric disorders. Intermediate phenotypes are heritable sub-clinical markers of disease (behavioral, physiological, neuropsychological, etc.) present among both affected and unaffected individuals [Gershon and Goldin, 1986; Almasy and Blangero, 2001; Gottesman and Goulde, 2003]. As they are thought to represent more basic components of complex disorders, intermediate phenotypes are hypothesized to hold more straightforward ties to underlying biological pathways and also show increased penetrance [Leboyer et al., 1998]. Intermediate phenotype-based approaches may thus avail genetic studies by pinpointing characteristics which are measurable in larger samples of both affected and unaffected individuals, and which are hypothesized to be more proximally related to underlying etiology.
Evidence of an intermediate phenotype in autism was first reported in the landmark twin study of Folstein and Rutter [1977]. Examining the cognitive and language abilities of MZ twin pairs wherein only one child was autistic, investigators detected increased rates of language and cognitive deficits similar to those seen in autism (e.g., language delay, mental retardation). Comparisons of MZ and DZ twins indicated that whereas the concordance rate for autism was 36% and 0%, respectively, concordance for the presence of more broadly defined phenotype, including autism or language/ cognitive impairments, was strikingly higher (MZ = 82% vs. DZ = 10%). Several case-control family studies (reviewed in subsequent sections) later confirmed and extended these findings by documenting a constellation of subtle language, cognitive, social and personality characteristics that parallel the defining features of autism, now commonly referred to as the “Broad Autism Phenotype” (BAP) [Piven et al., 1990; Bolton et al., 1994; Le et al., 1996; Piven et al., 1997; Murphy et al., 2000; Pickles et al., 2000; Szatmari et al., 2000; Goldberg et al., 2005].
The existence of behavioral markers of vulnerability to autism is now widely acknowledged; however, those characteristics most strongly reflecting genetic liability have not been definitively identified. The present study is an attempt to define specific traits sensitive to genetic liability to autism and document their patterns of segregation within families through examination of a broad range of personality and language characteristics across family types hypothesized to differ in genetic liability to autism. Building on the Iowa Family Study, which compared the parents of multiple children with autism with the parents of children with Down syndrome across various personality, social-behavioral, language, and cognitive features [Piven et al., 1997], this study includes a newly ascertained sample of single-incidence families for comparisons with these previously studied groups.
Because families with multiple incidences of autism are likely to have higher genetic loading than single-incidence cases [Folstein and Piven, 1991], we hypothesized that within this unique sample, graded expression would be observed for the principal characteristics conferring genetic susceptibility to autism, in which such features would express most profoundly among parents from multiple-incidence families, less strongly among single-incidence parents, and least of all among comparison parents from Down syndrome families, who should display population base rates. Using the family history informant method, Szatmari et al. [2000] previously reported such a pattern in communication, repetitive, and social domains and, using parent reports Constantino et al. [2006] detected a linear expression of mild autistic traits across such family types. We aim to build on these findings by employing direct assessment measures and examining a broader range of potential liability markers than has been previously studied in a single sample.
Furthermore, we examine interrelations among traits to investigate patterns of co-occurrence that might help to define specific intermediate phenotypes and inform models of genetic transmission. Whereas several twin and family studies suggest that these features may reflect common underlying genetics [Le Couteur et al., 1996; Constantino and Todd, 2003; Sung et al., 2005], recent twin studies from a large population-based sample indicate that while the social, communicative, and ritualistic behavioral features associated with autism are highly heritable, they appear largely independent, with relatively little phenotypic or genetic overlap [Ronald et al., 2005, 2006]. This result is consistent with earlier family studies reporting independent segregation of the BAP among relatives of individuals with autism [Piven et al., 1997; Pickles et al., 2000]. In an attempt to contribute to this literature, we employ factor analysis to reduce our behavioral measures into key domains, and examine their association within individuals and families to explore the separability or potential co-segregation of the various components of the BAP. Such analyses could help to determine whether features of the BAP represent distinct intermediate phenotypes, or rather, variable expression of common underlying etiologic factors. In what follows, we present brief rationale for the range of features analyzed as potential autism intermediate phenotypes.
Personality and Social Behavior
Converging evidence from a number of case-control studies indicates that certain personality traits and social behaviors are observed more commonly among autism relatives than control relatives of individuals with Down syndrome [Piven et al., 1990; Bolton et al., 1994; Le et al., 1996; Piven et al., 1997; Murphy et al., 2000; Pickles et al., 2000; Szatmari et al., 2000; Lainhart et al., 2002]. Both family history and direct assessment studies have reported elevated rates of socially reticent, or aloof personalities among autism parents, as well as untactful behavior, and fewer high quality (i.e., emotionally reciprocal) friendships. Autism relatives have also been reported to more commonly display rigid personalities, showing relatively little interest in novelty or difficulty in adjusting to change in environment and activities, as well as perfectionistic or overly conscientious, detail-oriented traits. Finally, anxiety-related features (e.g., anxious and hypersensitive personalities, increased rates of anxiety disorders, and elevated scores on the neuroticism/anxiety-fearfulness domain of the NEO-PI) also appear more common among parents of individuals with autism [Bolton et al., 1994; Le et al., 1996; Piven et al., 1997; Murphy et al., 2000; Pickles et al., 2000; Micali et al., 2004]. These characteristics closely correspond to the social impairments, ritualistic/repetitive, and anxious behaviors observed in autism, making them good candidates as autism intermediate phenotypes. The present study examined these features using direct assessment with the Modified Personality Assessment Schedule, Revised and the Friendship Interview, and self-report with the NEO Personality Inventory [Costa and McCrae, 1995].
Language
Impaired language is a defining feature of autism. Family studies examining parents and relatives of individuals with autism have detected increased rates of developmental language-related delays [Folstein and Rutter, 1977; August et al., 1981; Steffenburg et al., 1989; Szatmari et al., 2000], impaired pragmatic language use [Landa et al., 1991, 1992; Piven et al., 1997], and difficulties on standardized tests of verbal fluency and reading [Smalley and Asarnow, 1990; Piven et al., 1997, though see Bishop and Norbury, 2002; Folstein et al., 1999; Hughes et al., 1999; Pilowsky et al., 2003]. Recent findings from genetic linkage analyses also point toward language impairment as a genetically significant feature of autism. In particular, subsetting families based on history of early language-related delays in children with autism and their parents, the Collaborative Linkage Study of Autism [CLSA, 2001] reported peak findings on regions of interest on 7q and 13q that were nearly entirely attributable to the subgroup of families in which both children and parents showed histories of language delay. Subsequent studies incorporating language information have provided further support for a language-related peak on chromosome 7q [Alarcon et al., 2002, 2005]. Based on these findings, which together suggest that language-related abnormalities constitute a genetically meaningful feature of autism, we evaluated parents’ pragmatic language abilities. Problematic pragmatic language use is universally observed in autism spectrum disorders, and as previously noted, has been repeatedly documented among relatives, making this feature a good candidate intermediate phenotype within the language domain.
To detect from among these possible intermediate phenotypes those principal characteristics conferring genetic susceptibility to autism, each was examined for the presence of linear trends consistent with the hypothesized gradation of expression across family types (MIAF > SIAF > DWNS). Factor analysis was employed to examine underlying interrelationships among key variables and trace their expression within families. That is, BAP features evident in both parents could be suggestive of bilineal transmission of genes relevant to autism, whereas unilineal transmission could be suggested by cases in which only a single parent showed features of the BAP.
METHODOLOGY
Participants
Participants included an epidemiologic sample of 25 multiple-incidence autism families (MIAF), 40 single-incidence autism families (SIAF), and 30 Down syndrome families (DWNS). As earlier noted, data from multiple-incidence and Down syndrome families has been previously analyzed and reported [Piven et al., 1997]. Ascertainment strategies for each group are described below.
Multiple-incidence autism families
A systematic search for all families with at least two children with autism within Iowa and two tertiary autism clinics in the Midwest was conducted to identify this sample. Families were eligible for this study if (1) two children (aged 4–30 years) showed evidence of autism on the basis of prior clinical diagnosis or school screening evaluation; and (2) review of medical records revealed no evidence of a co-morbid medical condition that might cause autistic symptoms (e.g., tuberous sclerosis) [see Piven et al., 1997 for a more detailed description of ascertainment strategies for this sample]. In addition, five MIAF families were recruited through colleagues at the University of Chicago. The total MIAF sample comprised 25 mothers and 23 fathers. Two fathers were excluded, as they were parents of only one child with autism (i.e., mothers had children with two different fathers).
Single-incidence autism families
Families with one child with autism were identified through a registry of 1,200 individuals diagnosed with autism, living within a 150 miles radius of the University of Iowa Hospitals and Clinics. Two hundred eleven introductory letters describing the study were mailed. Of the 211 contacted, 109 did not respond and a further 58 were excluded for the following: moved outside the 150-mile radius (N = 34); no autism diagnosis (N = 7); adoption of individuals with autism (N = 6); confirmed chromosomal anomalies (N = 4); evidence of neurological insult (N = 2); exceeded age limits (N = 2); diagnosis of Rett’s Syndrome (N = 1); positive testing for Fragile-X (N = 1); documented history of significant brain atrophy (N = 1). Additionally, three families dropped out of the study during the course of data collection, and one individual failed to meet diagnostic criteria for autism on direct assessment. The resulting sample included 40 families ascertained through one child with autism. Thirty-five of these families included both a child with autism and non-autistic siblings, making it possible to definitively assign single-incidence status. Five of these families, however, had only a single child with autism, and no siblings. Because we could not rule out the possibility that these latter families may have gone on to have an additional child with autism, these families were excluded from comparisons across family types.
Down syndrome families
Thirty families of a child with DWNS due to non-dysjunction of chromosome 21 were included as a comparison group to control for the stress of caring for a child with a developmental disorder. These families were randomly recruited from a list of newborns living within 150 miles of the University of Iowa. Further details of recruitment strategies are described in Piven et al. [1997].
PROCEDURES
Diagnostic Evaluations
All autism families had one or more children meeting criteria for autistic disorder based on DSM-IV criteria and met algorithm cut-offs on the Autism Diagnostic Interview-Revised [Le Couteur et al., 1989] and Autism Diagnostic Observation Schedule [Lord et al., 1989]. All children were screened for medical conditions that might explain autistic symptoms (e.g., tuberous sclerosis, fragile X syndrome) and evidence of gross central nervous system injury and/or severe perinatal events (aside from those obstetric features of interest).
All individuals with autism or DWNS had IQ estimates above 30.Wheremultiple IQ tests had been performed, the test closest to age 12 was used for estimating performance IQ. The following IQ tests were considered adequate for estimation of performance IQ (listed in order of priority assigned): Wechsler Intelligence Scale for children, Revised [1974], Wechsler Intelligence Scale for Children-III [1991], Wechsler Adult Intelligence Scale, Revised [1981], Leiter International Performance Scale [Arthur, 1949], and Merrill-Palmer Scale of Mental Tests [Stutsman, 1948]. Children’s IQ distributions were comparable across MIAF, SIAF, and DWNS groups.
Evaluation of Parents
Personality and social behavior
Modified personality assessment schedule-revised
The MPAS-R is a semi-structured interview for rating personality characteristics that was adapted from the Personality Assessment Schedule (PAS) [Tyrer, 1988; Piven et al., 1994] to assess parents of individuals with autism and DWNS in the Baltimore Family Study [Piven et al., 1994]. Based on the experience and results from that study, the MPAS was further revised and currently assesses six personality characteristics: aloof, anxious, hypersensitive, overly conscientious, rigid, and untactful [see Piven et al., 1997 for further description of this instrument]. Participants are led through a series of questions about themselves and an informant (usually the spouse) is asked similar questions in separate interviews. Personality characteristics are rated by combining information from the subject and informant interviews according to specified rules. Ratings are based on behavioral examples given by the subject and/or informants in response to a number of probes. Characteristics are rated either as present (2) or absent (0, 1). If the rater believes a trait is present but no behavioral example is elicited from either the subject or informant, it is scored as 1 and considered as absent for the purposes of this study.
The friendship interview
This interview was developed for the Baltimore Family Study and provides an objective gauge of interest in developing and maintaining supportive and intimate friendships which has shown excellent discrimination between the family members of cases and controls. Interviewers ask participants to identify three friends outside their immediate family. The degree of mutual support and confiding is determined for each friend identified, producing a score ranging from 0 to 15.
The NEO personality inventory [NEO-PI; Costa and McCrae, 1995]
The NEO-PI is based on the five-factor model of personality and was developed to assess quantitative dimensions of normal personality traits. The reliability and validity of the NEO are well established [Costa and McCrae, 1997]. Although only the neuroticism domain will be included in analyses, as this characteristic has proven to distinguish relatives of individuals with autism from controls [Piven et al., 1997], the entire questionnaire was administered to maintain the integrity of the instrument.
Language
Pragmatic rating scale [PRS; Landa et al., 1992]
The PRS includes 19 items tapping pragmatic language skills (e.g., maintaining discourse topics, providing adequate background information), and six items measuring prosodic and grammatical speech errors. Interviewers rate these items 0, 1, or 2 based on a conversation (“ The Chat”) that is incorporated into the MPAS-R and friendship interview. Interviewers are trained to guide the conversation in such a way that there are opportunities to observe all the behaviors to be rated. For example, the interviewer will, if necessary, feign confusion to see whether the subject can adequately revise a statement in different terms.
All characteristics were rated from videotaped interviews by two independent raters who were blind to group status. To ensure comparability in ratings over time, tapes from each group were interspersed and identifying information (e.g., references to children’s diagnoses) omitted from tapes. Inter-rater reliability assessments for each of the characteristics of the MPAS-R, friendship interview, and pragmatic rating scale yielded Kappa coefficients ranging from 0.67 to 1.0.
Statistical Analyses
A series of one-way analyses of variance were conducted to examine differences between parent groups. Polynomial trend analyses were used to assess the hypothesis that a gradation of expression would be observed across family types (MIAF>SIAF>DWNS). Dichotomous variables were analyzed using Mantel-Haenszel χ2 test for linear association. Follow-up planned comparisons contrasted the DWNS group against the SIAF group, and SIAF parents against MIAF parents. Comparisons between DWNS and MIAF groups have been previously published, and are reported again here for clarity.
RESULTS
Groups were comparable in age (mean age for all groups 39–40 years), gender distribution (1:1 for all groups), and SES (P-values ranged from 0.29 to 0.72) as measured by the British Manual of the Classification of Occupations [1980]. Groups were also comparable in the mean number of unaffected siblings per family: MIAF = 1.8, SIAF = 1.7, DWNS = 1.9, suggesting that any “ stoppage” effects present were comparable across groups. Although all parents had IQs well within the normal range, the DWNS parent group displayed a somewhat higher mean IQ (109.8) than either the MIAF and SIAF parent groups (109.8 vs. 103.7 and 103.8, respectively), P < 0.05.
Personality Characteristics
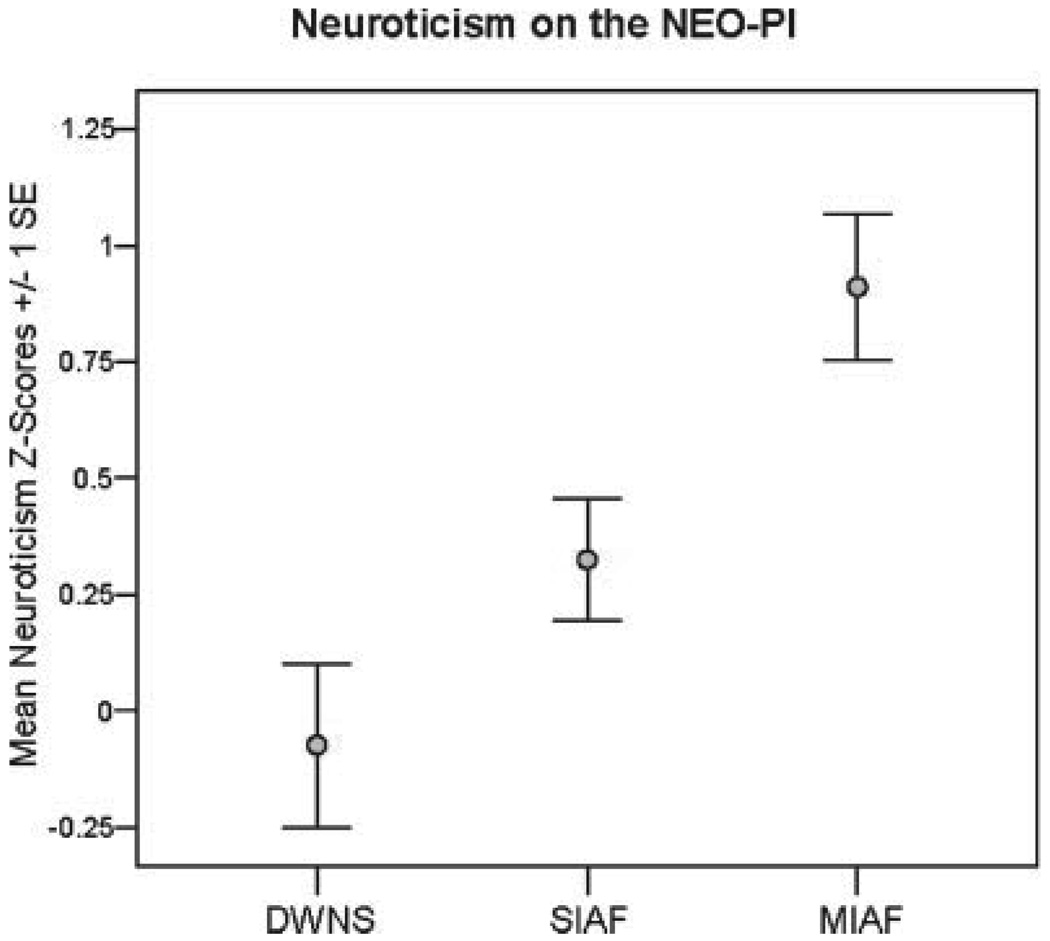
Significant linear associations across the three parent groups were detected for all personality characteristics assessed through the MPAS-R (see Table I). Although specific group contrasts did not reach significance in all cases, observing the frequency of each characteristic (i.e., the proportion of parents displaying each trait) across family types indicated a graded pattern of expression across family types (MIAF>SIAF>DWNS) in all traits except overly conscientious, where MIAF and SIAF parents displayed similarly high rates. Analysis of neuroticism scores on the NEO also revealed a significant linear trend across groups (F (1, 161) = 16.05, P < 0.0005), with SIAF parents showing significantly higher neuroticism scores than DWNS parents (t (51) = 3.77, P < 0.0005) and higher scores observed among MIAF parents than SIAF parents (t (84) = 2.56, P < 0.05) (see Fig. 1).
Table I.
Tests for Trends in Personality Characteristics
| DWNS (%) N = 60 |
SIAF (%) N = 78 |
MIAF (%) N = 48 |
Mantel-Haenszel test for linear association χ2(df), P (follow-up χ2) |
|
|---|---|---|---|---|
| MPAS-R personality characteristics | ||||
| Aloof | 3 | 17 | 29 | 13.60 (2), P < 0.0005 |
| DWNS versus SIAF | (6.10 (1), P < 0.01) | |||
| DWNS versus MIAF | (9.06 (1), P < 0.005) | |||
| MIAF versus SIAF | (10.75 (1), P < 0.05) | |||
| Anxious | 5 | 12 | 31 | 14.16 (2), P < 0.0005 |
| DWNS versus SIAF | (6.17 (1), P < 0.05) | |||
| DWNS versus MIAF | (8.41 (1), P < 005) | |||
| MIAF versus SIAF | (7.68 (1), P < 0.01) | |||
| Hypersensitive | 3 | 13 | 29 | 8.20 (2), P < 0.005 |
| DWNS versus SIAF | (3.75 (1), P = 0.05) | |||
| DWNS versus MIAF | (12.63 (1), P < 0.0005) | |||
| MIAF versus SIAF | (5.31 (1), P < 0.05) | |||
| Overly conscientious | 12 | 30 | 27 | 4.02 (2), P < 0.05 |
| DWNS versus SIAF | (6.13 (1), P < 0.05) | |||
| DWNS versus MIAF | (3.89 (1), P < 0.05) | |||
| MIAF versus SIAF | (5.47 (1), P = 0.24) | |||
| Rigid | 10 | 23 | 48 | 19.75 (2), P < 0.0005 |
| DWNS versus SIAF | (3.90 (1), P < 0.05) | |||
| DWNS versus MIAF | (20.43 (1), | |||
| P < 0.00005) | ||||
| MIAF versus SIAF | (8.63 (1), P < 0.005) | |||
| Untactful | 7 | 18 | 29 | 8.95 (2), P < 0.005 |
| DWNS versus SIAF | (3.04 (1), P = 0.08) | |||
| DWNS versus MIAF | (6.56 (1), P < 0.05) | |||
| MIAF versus SIAF | (10.34 (1), P < 0.05) |
Fig. 1.
Neuroticism on the NEO.
Mothers and fathers displayed similar profiles across all personality traits with the exception of untactful on the MPASR. MIAF fathers more often showed this characteristic than mothers .
Friendships
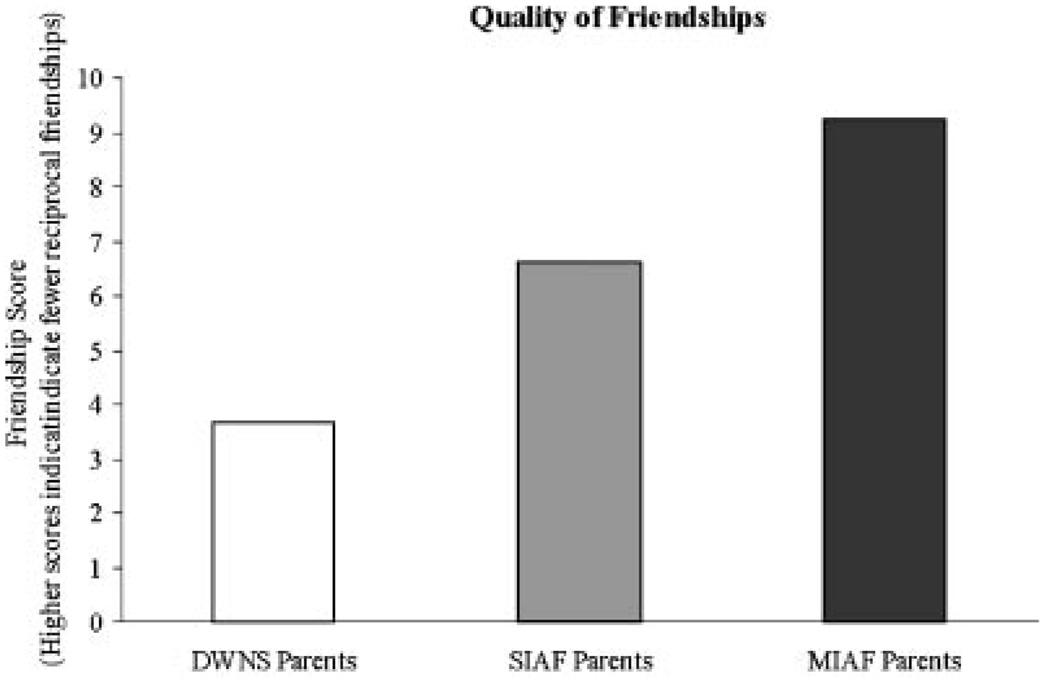
In line with findings for personality variables, the quality of friendships assessed through the friendship interview also showed a linear trend across groups (F (1, 187) = 30.20, P < 0.0005), with MIAF parents reporting lower quality friendships than SIAF parents (t (97) = 2.58, P < 0.05), and SIAF parents’ friendships lower in quality than those of DWNS parents (t (127) = 3.27, P < 0.005) (see Fig. 2). Additionally, 23% of MIAF parents and 11% of SIAF parents reported having no friendships, in contrast to only 3% of DWNS parents. Fathers scored significantly higher on the FI (i.e., fewer reciprocal friendships) than mothers in both SIAF (t (70) = 2.78, P < 0.01) and MIAF (t (45) = 1.58, P = 0.08) groups. No differences between mothers and fathers were detected in DWNS families.
Fig. 2.
Friendship quality.
Language
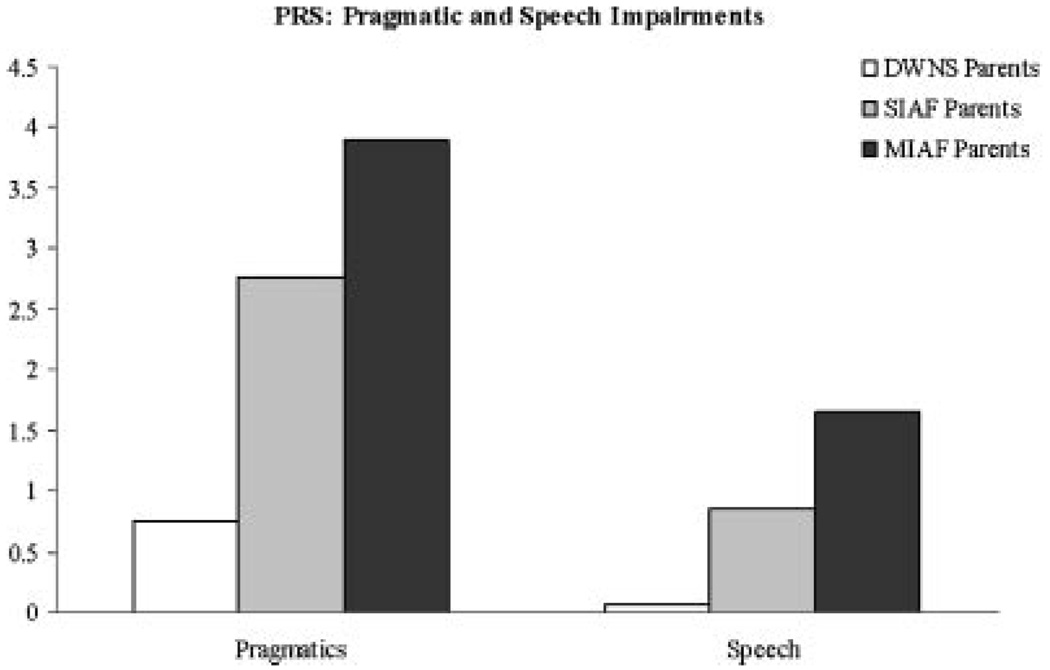
Significant linear trends were detected in the frequency of pragmatic language violations (F (1, 187) = 4.58, P < 0.0005) and speech errors (F (1, 187) = 18.09, P < 0.0005). As illustrated in Figure 3, although not all comparisons reached significance, MIAF parents committed more pragmatic (t (127) = 1.24, P = 0.20) and speech errors (t (127) = 1.85 (127), P = 0.06) than SIAF parents, who in turn committed significantly more pragmatic violations (t (139) = 3.33, P < 0.005) and speech errors (t (139) = 3.57, P < 0.005) than DWNS parents.
Fig. 3.
Pragmatic language.
Exploring underlying factor structure of BAP features
Having investigated this range of potential endophenotypic markers for linear expression across family types, we next examined relationships among variables within families of individuals with autism using exploratory factor analysis (EFA). Neuroticism scores on the NEO were not included as scores were available for fewer than half of SIAF parents (N = 39). Furthermore, because similar patterns were observed in MIAF and SIAF parents, and in mothers and fathers (i.e., both autism parent groups showed similar differences across measures relative to control DWNS parents and very few gender differences emerged), family types and genders were combined to increase sample size.
Using the inter-item correlation matrix from the nine behavioral markers, an EFA was conducted using the maximum likelihood discrepancy function in comprehensive exploratory factor analysis [CEFA; Browne et al., 2002] with oblique quartimax rotation. Oblique rotation was used because it seemed likely that the variables would be correlated. Fabriger et al. [1999] noted that other advantages of oblique rotation (over orthogonal rotation) include better simple structure and provision of estimates of correlation among common factors. These advantages have led many researchers to favor oblique rotation when assessing human traits that are likely to be correlated with one another [Costello and Osborne, 2005;Fabriger et al., 1999; Floyd and Widamin, 1995].
The number of factors to retain was guided by: (a) the scree plot method [Cattell, 1966], (b) eigenvalues above 1.0, (c) goodness-of-fit as estimated by Root Mean Square Error of Approximation [RMSEA; Browne and Cudeck, 1992], and (d) interpretability. Solutions between two and four factors were evaluated using these criteria. Items were adopted as loading on a given factor if (a) they loaded 0.40 or higher on that factor and (b) this loading was at least 0.10 higher than the loading on any other factor. The four-factor solution was chosen as it showed the best fit and interpretability. The RMSEA point estimate for this four-factor solution was 0.10, which is considered a mediocre fit [Browne and Cudeck, 1992]. We named these factors: (I) “Language,” (II) “Rigidity,” (III) “Anxiety,” and (IV) “Sociability” (Table II). This solution has a simple structure in that the high factor loadings were high and the other loadings were low. Correlations among factors were relatively low (see Table III), with the highest correlation between Factors I (“Language”) and IV (“Sociability”).
Table II.
Factor Structure of Key Behavioral Markers
| Behavioral markers | Factor I “Language” |
Factor II “Rigidity” |
Factor III “Anxiety” |
Factor IV “Sociability” |
|---|---|---|---|---|
| Overly conscientious | −0.12 | 0.56 | 0.14 | −0.02 |
| Rigid | 0.15 | 0.91 | −0.16 | 0.21 |
| Aloof | 0.24 | 0.06 | 0.21 | 0.53 |
| Anxious | −0.05 | −0.03 | 0.57 | 0.26 |
| Hypersensitive | 0.16 | 0.11 | 0.50 | −0.15 |
| Untactful | 0.40 | 0.27 | −0.02 | 0.09 |
| Pragmatic language | 0.98 | −0.02 | 0.13 | −0.13 |
| Speech | 0.61 | −0.12 | −0.01 | 0.21 |
| Friendships | 0.02 | 0.10 | 0.01 | 0.49 |
The values in bold represents significant factor loadings.
Table III.
Correlations Between Factors
| Factor I “Language” |
Factor II “Rigidity” |
Factor III “Anxiety” |
Factor IV “Sociability” |
|
|---|---|---|---|---|
| Factor I “Language” | 1.0 | |||
| Factor II “Rigidity” | 0.21 | 1.0 | ||
| Factor III “Anxiety” | 0.27 | 0.28 | 1.0 | |
| Factor IV “Sociability” | 0.35 | 0.29 | 0.172 | 1.0 |
Exploring BAP expression within individuals and families
Using results from this EFA as a guide, we next investigated patterns of BAP features within individuals and families. For these analyses, individuals were considered “positive” for a domain if they rated on one of the two characteristics in each factor (i.e., either overly conscientious or rigid trait would qualify as “Rigid Positive”). Categorical cut-offs for the continuous measures (FI and PRS) were set at 1.5 standard deviations above the mean for the DWNS group (scores ≥ 10 on the FI and ≥ to 4 on the pragmatic and speech items of the PRS combined). For instance, an individual was considered positive for the “Sociability” factor if they were rated as aloof or received a score of 10 or greater on the FI (strongly indicating diminished quality friendships). Note that pragmatic and speech items of the PRS were combined, as the range of scores on “speech” was insufficient for setting a cut-off score according to the scheme above.
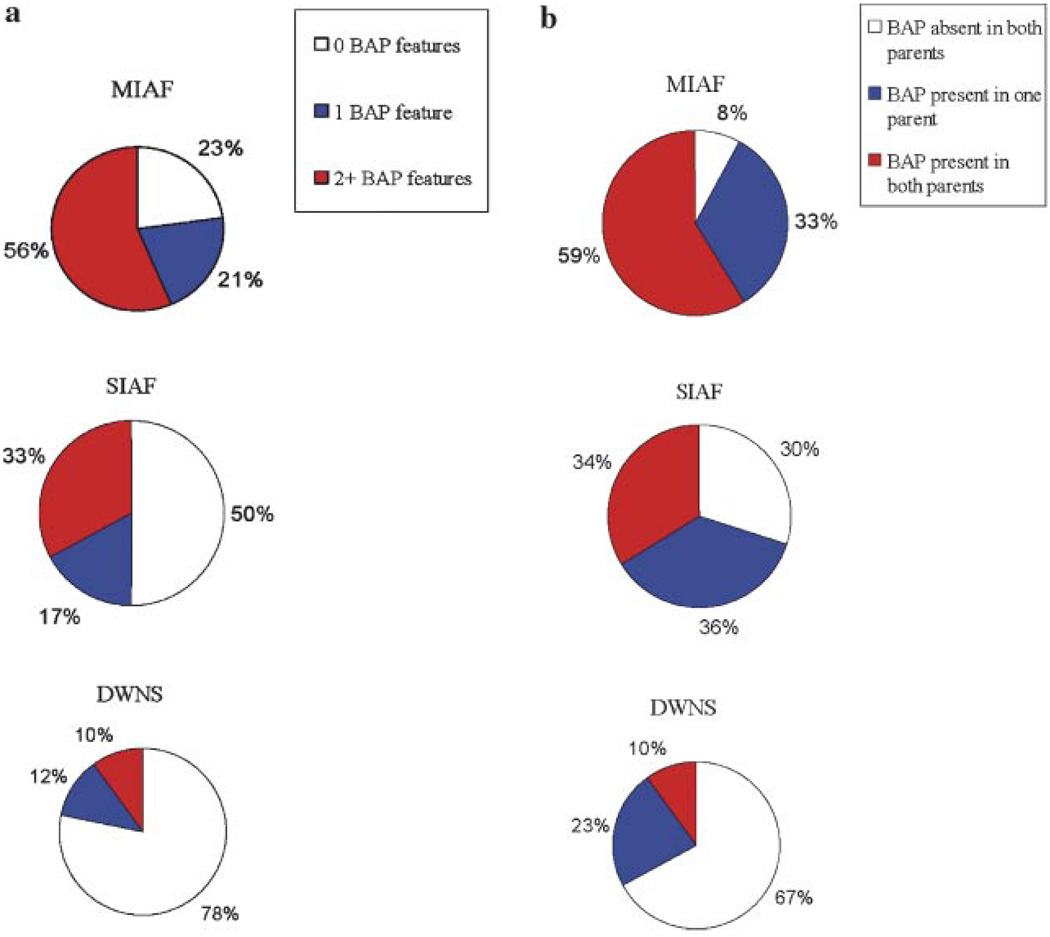
We examined the frequency of individuals within each group positive for zero BAP factors, one BAP factor, or two or more BAP factors (i.e., “Rigid”, “Language”, “Social”, or “Anxious”). Odds ratios were conducted examining the likelihood of displaying heightened BAP expression (defined as showing ≥2 factors). Results indicate stronger odds for such elevated expression within MIAFs (OR = 4.2, 95% CI = 2.1–8.4), and around chance levels for SIAFs (OR = 1.21, 95% CI = 0.6–2.3). As would be expected from group frequency analyses reported earlier, “Rigidity” was the most common factor in all groups, followed by “Language”, “Anxiety”, and “Sociability”.
We next turned to analyses of BAP factors within families, in search of evidence for patterns of genetic transmission. Presented in Table IV, odds ratios assessing rates of BAP features within families suggest that among MIAFs, BAP expression in both parents was most likely, whereas SIAFs displayed equal chance of BAP expression in one, both, or neither parent, and a protective effect was observed among families of individuals with DWNS. Figure 4b shows the proportions of families showing each level of parental BAP expression.
Table IV.
Frequency of the BAP With
| Both parents BAP+ | One parent BAP+ | Neither parent BAP+ | |
|---|---|---|---|
| MIAF (N = 23a) | OR = 4.6 (1.7–12.8) | OR = 1.2 (0.4–3.1) | OR = 0.11 (0.02–0.5) |
| SIAF (N = 33) | OR = 1.1 (0.4–2.8) | OR = 1.6 (0.6–3.9) | OR = .59 (0.2–1.5) |
Within-family analyses did not include two mothers whose spouses were excluded (see subject description for further details).
Fig. 4.
a: BAP features within individuals. b: BAP features within families. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
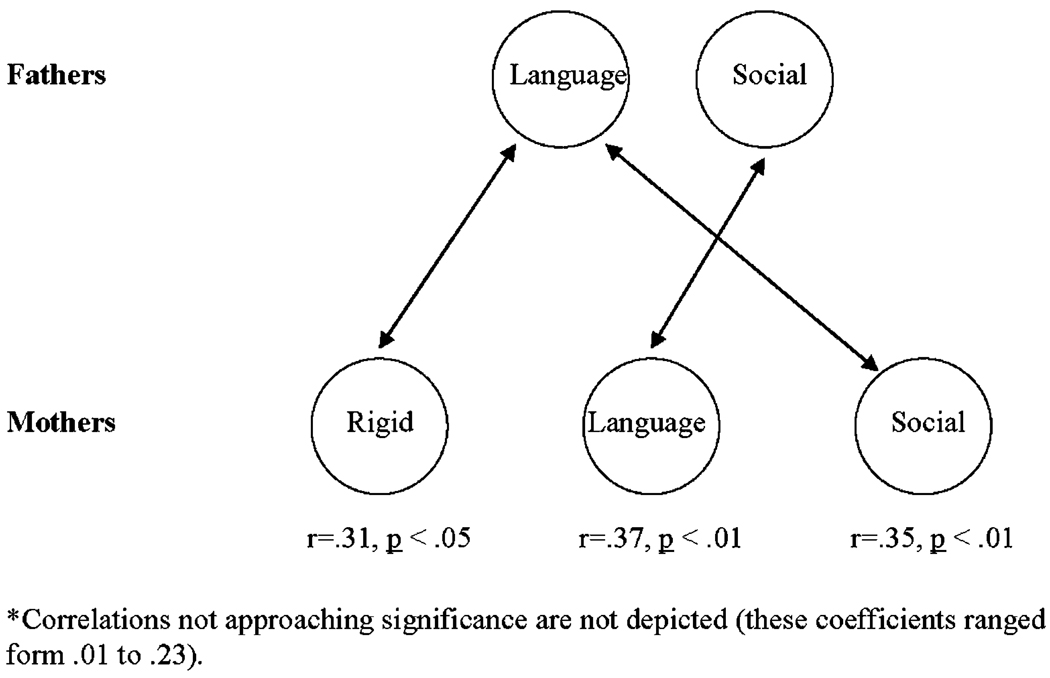
Finally, within the autism parent group the co-occurrence of specific BAP factors was examined within families, across mothers and fathers. Spearman correlation coefficients detected several significant associations between BAP features within families (see Fig. 5). Namely, correlations suggest that fathers positive for the “Language” factor were more commonly married to mothers positive for “Rigid” and “Social” factors, and those fathers who were positive on the “Social” factor were more often married to mothers positive on the “Language” factor. No significant within-family associations were detected with “Rigidity” in mothers, or with “Anxiety” in either parent.
Fig. 5.
Interrelations of BAP factors within families.
DISCUSSION
This study aimed to identify autism intermediate phenotypes by examining the frequency and co-occurrence of features believed to constitute the BAP in families differing in genetic liability to autism. We first examined a battery of personality, language, and social-behavioral measures, hypothesizing that genetically meaningful features would be expressed in linear fashion across family types consistent with increasing genetic liability to autism (MIAF>SIAF>DWNS). We then conducted an exploratory factor analysis to investigate interrelationships among these features such that we might reduce them to discrete factors and trace their patterns of expression within individuals and families.
BAP Expression Across Groups: Evidence for Autism Intermediate Phenotypes
Consistent with predictions, analyses revealed linear trends of expression across family types on measures of personality (all but overly conscientious), language (i.e., pragmatics), and social behavior (i.e., friendship quality). Of the personality features studied, it is of note that rigidity was the most common. Given that this trait was also most common among control DWNS parents, it could be that this personality style is most reflective of the demands of caring for a child with a disability, where adherence to routines is commonly necessary. Additionally, that no linear trend was observed for in overly conscientiousness could suggest that this feature does not reflect genetic liability to autism.
Results from our exploratory factor analysis suggest four separate factors underlying these features, which we describe in their correspondence to domains associated with autism: “Rigidity”, “Language”, “Sociability” and “Anxiety” Consistent with evidence that the social and non-social features of autism may arise in part from separate genetic effects [Ronald et al., 2005], these findings may suggest the presence of distinctly separable intermediate phenotypes in unaffected relatives. However, it should be noted that the four-factor analysis was limited by the fact that there were only nine behavioral markers to enter into the model; this resulted in three of the four factors consisting of only two items each. Given that a general “rule-of-thumb” of EFA is that a factor consists of at least three items, our results cannot be used to make broad statements about the underlying latent structure of behavioral characteristics in unaffected relatives. While these results should therefore be interpreted with caution, they nonetheless provided an empirical approach for reducing this broad array of behavioral data into meaningful component features for subsequent analyses of within-individual and within-family patterns of expression.
Together, results discussed thus far have two important implications for genetic studies of autism. First, they suggest that these personality social-behavioral and language features are potential markers of genetic susceptibility to autism. The identification of such subclinical markers may help to distill the clinical and genetic heterogeneity of autism to identify more etiologically homogeneous subgroups [e.g., Leboyer, 2003]. In this same vein, the finding that those families most densely affected with autism (i.e., MIAFs) also showed the greatest expression of BAP traits in parents provides further evidence of higher genetic loading among MIAFs, indicating that these families would be highly informative to include in molecular genetic studies.
BAP Expression Within Individuals and Families: Evidence for Bilineal Transmission in MIAFs
Having identified a range of features which may be to be good candidates as autism intermediate phenotypes, we then examined their patterns of expression within individuals and families. Findings could suggest different modes of transmission across the two autism family types. Within MIAFs, it was likely for both parents to display multiple BAP features, whereas SIAFs were equally likely to have both, one, or neither parent “affected”, and half of the SIAF group showed no evidence of the BAP whatsoever. As expected, DWNS families very rarely had either parent showing evidence of the BAP. These patterns could indicate higher genetic loading among MIAFs, as well as increased rates of bilineal transmission, but not unilineal. That is, whereas for both MIAF and SIAF families it was equally likely that only one parent would show BAP traits, MIAF families had increased rates of both parents showing the BAP. This latter finding is important in that it suggests that the difference observed in bilineal transmission rates cannot simply be explained by increased rates of BAP features among MIAFs in general. Furthermore, such a pattern could suggest assortative mating in the MIAF group. That ~ 1/3 of parents from the SIAF group showed no evidence of the BAP (vs. only 8% of MIAF parents without BAP characteristics) could suggest that SIAFs may be an important group to examine for the evidence of alternative etiologic mechanisms. Of course the possibility that variable expression of common underlying genes may account for these patterns cannot be ruled out. Nor can the possibility that the stress for caring for two children with autism may impact the personality and language features we have studied, and perhaps contribute to the “dosage” effects observed.
Finally, within-family correlations indicated complementary pairing of BAP features between mothers and fathers, and could also reflect patterns of assortative mating. That is, those fathers showing language difficulties tended to pair with mothers positive for the Rigid and Sociability factors, and fathers positive for Sociability were more likely to pair with mothers showing language difficulties. Considered in the context of a threshold liability model of complex disorders [Falconer, 1981], and assuming that autism is polygenic in nature, such findings may suggest potentially distinct genetic contributions from each parent, which acting alone produce only the BAP (or components of the BAP), but when acting together in co-dominant fashion produce autism. If this is the case, it would imply a simpler genetic basis to the BAP than autism per se, and support the use of such intermediate phenotypes as we have identified in molecular genetic studies. Of course this scenario is but one of several possible etiologic mechanisms consistent with these data and further work is needed to explain these patterns and examine environmental factors potentially also at play.
Conclusions and Limitations
This study identified a range of features which show promise as autism intermediate phenotypes—these personality, social-behavioral, and language characteristics are qualitatively similar to the core features of autism, were more common among relatives of individuals with autism than controls, and expressed in linear fashion across groups believed to vary in genetic liability to autism, suggesting they could likely reflect genetic effects relevant to autism. These findings extend prior reports of such patterns [Szatmari et al., 2000; Constantino et al., 2006] in that they were observed along a more extensive battery of phenotypic characteristics than had been previously examined in a single sample, and were derived from direct assessments and ratings from videotape by raters blind to group status. It will be important for future work to replicate these results in extended families varying in density of autism cases (including additional family members, which would provide an opportunity to assess more broadly intrafamilial patterns of expression of hypothesized intermediate phenotypes) and in comparison to additional control groups, in order to refine the results of this study and assess the specificity of these characteristics to autism [see Yirmiya and Shaked, 2005 on the importance of including different comparison groups varying in liability to autism in studies of the BAP]. Moreover, the inclusion of an additional comparison group consisting of parents of two developmentally disabled children would arguably better control for the effects of parenting multiple children with autism in the MIAF group. Such a design would help to tease out potential environmental factors, which could contribute to differences observed between the SIAF and MIAF groups.
A further limitation of this study concerns our relatively small sample size, which may have limited our power to detect group differences, particularly when partitioning groups for within-family analyses of BAP traits. Finally, while we believe that the labor-intensive clinical ratings employed in this study (and those preceding it) provide valid and highly informative data, genetic studies will benefit from the development of more efficient means of reliably measuring autism endophenotypes, and employing quantitative scales measurable in both affected and unaffected individuals. Such efforts are underway by a number of groups [e.g., Constantino et al., 2006; Dawson et al., 2007], including our own development of a self and informant report measure of the BAP [i.e., the BAP Questionnaire, Hurley et al., 2006], and are likely to offer a highly promising approach for building on these findings in larger scale family and molecular genetic studies of autism and autism intermediate phenotypes.
ACKNOWLEDGMENTS
We would like to thank all the families who participated in this study and acknowledge the support of NIMH grants U54 MH66418 (JP) and K12 HD052191 (ML).
Grant sponsor: NIMH; Grant numbers: U54 MH66418, K12 HD052191.
REFERENCES
- Alarcon M, Cantor RM, Liu J, Gilliam TC, Geschwind DH The Autism Genetic Resource Exchange Consortium. Evidence for a language quantitative trait locus on chromosome 7q in multiplex autism families. Am J Hum Genet. 2002;70:60–71. doi: 10.1086/338241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon M, Yonan AL, Gilliam TC, Cantor RM, Geschwind DH. Quantitative genome scan and Ordered-Subsets Analysis of autism endophenotypes support language. QTLs Mol Psychiatry. 2005;10(8):747–757. doi: 10.1038/sj.mp.4001666. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Endophenotypes as quantitative risk factors for psychiatric disease: Rationale and study design. Am J Med Genet. 2001;105:42–44. [PubMed] [Google Scholar]
- Arthur G. The Arthur Adaptation of the Leiter International Performance Scale. J Clin Psychol. 1949;5:345–349. doi: 10.1002/1097-4679(194910)5:4<345::aid-jclp2270050402>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- August G, Stewart M, Tsai L. The incidence of cognitive disabilities in the siblings of autistic children. Br J Psychiatry. 1981;138:416–422. doi: 10.1192/bjp.138.5.416. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Norbury CF. Exploring the borderlands of autistic disorder and specific language impairment: A study using standardised diagnostic instruments. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2002;43(7):917–929. doi: 10.1111/1469-7610.00114. [DOI] [PubMed] [Google Scholar]
- Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, Bailey A, Rutter M. A case-control family history study of autism. J Child Psychol Psychiatr. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Bramon E, McDonald C, Croft RJ, Landau S, Filbey F, Gruzelier JH, et al. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage. 2005;27:960–968. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociol Methods Res. 1992;21:230–258. [Google Scholar]
- Browne MW, Cudeck R, Tatenini K, Mels G. CEFA: Comprehensive exploratory factor analysis. Authors. (Computer program); 2002. [Google Scholar]
- Carlson C, Eberle M, Kruglyak L, Nickerson D. Mapping complex disease loci in whole-genome association studies. Nature. 2004;429:446–452. doi: 10.1038/nature02623. [DOI] [PubMed] [Google Scholar]
- Cattell RB. Chicago: Rand McNally; 1966. Handbook of multivariate experimental psychology. [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: A twin study. Arch Gen Psychiat. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, et al. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006;163:294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Domains and facets: Hierarchical personality assessment using the revised NEO personality inventory. J Pers Assess. 1995;64:21–50. doi: 10.1207/s15327752jpa6401_2. [DOI] [PubMed] [Google Scholar]
- Costa P, McCrae R. Stability and change in personality assessment: The revised NEO Personality Inventory in the Year 2000. J Pers Assess. 1997;68:86–94. doi: 10.1207/s15327752jpa6801_7. [DOI] [PubMed] [Google Scholar]
- Costello AB, Osborne JW. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Practical Assess Res Eval. 2005;10:1–9. [Google Scholar]
- Dawson G, Estes A, Munson J, Schellenberg G, Bernier R, Abbott R. Quantitative assessment of autism symptom-related traits in probands and parents: Broader phenotype autism symptom scale. J Autism Dev Disord. 2007;37(3):523–536. doi: 10.1007/s10803-006-0182-2. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Egan MF, Goldberg TE. Intermediate cognitive phenotypes associated with schizophrenia. Methods Mol Med. 2003;77:163–197. doi: 10.1385/1-59259-348-8:163. [DOI] [PubMed] [Google Scholar]
- Fabrigar LR, Wegener DT, MacCallum RC, Strahan EJ. Evaluating the use of exploratory factor analysis in psychological research. Psychol Meth. 1999;4:272–299. [Google Scholar]
- Falconer DS. An introduction to quantitative genetics. 2nd edition. New York: Longman; 1981. [Google Scholar]
- Floyd FJ, Widaman KF. Factor analysis in the development and refinement of clinical assessment instruments. Psychol Assess. 1995;7:286–299. [Google Scholar]
- Folstein SE, Piven J. Etiology of autism: Genetic influences. Pediatrics. 1991;87:767–773. [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile autism: A genetic study of 21 twin pairs. J Child Psychol Psychiat. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Santangelo SL, Gilman SE, Piven J, Landa R, Lainhart J, Hein J, Wzorek M. Predictors of cognitive test patterns in autism families. J Child Psychol Psychiatry. 1999;40:1117–1128. [PubMed] [Google Scholar]
- Gershon ES, Goldin LR. Clinical methods in psychiatric genetics. I. Robustness of genetic marker investigative strategies. Acta Psychiatr Scand. 1986;74:113–118. doi: 10.1111/j.1600-0447.1986.tb10594.x. [DOI] [PubMed] [Google Scholar]
- Goldberg WA, Jarvis KL, Osann K, Laulhere TM, Straub C, Thomas E, et al. Brief report: Early social communication behaviors in the younger siblings of children with autism. J Autism Dev Disord. 2005;35:657–664. doi: 10.1007/s10803-005-0009-6. [DOI] [PubMed] [Google Scholar]
- Gottesman I, Goulde T. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hughes C, Plumet MH, Leboyer M. Towards a cognitive phenotype for autism: Increased prevalence of executive dysfunction and superior spatial span amongst siblings of children with autism. J Child Psychol Psychiatry. 1999;40(5):705–718. [PubMed] [Google Scholar]
- Hurley RS, Losh M, Parlier M, Reznick JS, Piven J. The Broad Autism Phenotype Questionnaire. J Autism Dev Disord. 2006 doi: 10.1007/s10803-006-0299-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Ozonoff S, Coon H, Krasny L, Dinh E, Nice J, et al. Autism, regression, and the broader autism phenotype. AmJ Med Genet. 2002;113:231–237. doi: 10.1002/ajmg.10615. [DOI] [PubMed] [Google Scholar]
- Landa R, Folstein S, Isaacs C. Spontaneous narrative discourse performance of parents of autistic individuals. J Speech Hearing Res. 1991;34:1339–1345. doi: 10.1044/jshr.3406.1339. [DOI] [PubMed] [Google Scholar]
- Landa R, Piven J, Wzorek M, Gayle J, Chase G, Folstein S. Social Language use in parents of autistic individuals. Psychol Med. 1992;22:245–254. doi: 10.1017/s0033291700032918. [DOI] [PubMed] [Google Scholar]
- Leboyer M. Searching for alternative phenotypes in psychiatric genetics. Meth Mol Med. 2003;77:145–161. doi: 10.1385/1-59259-348-8:145. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Bellivier F, Nosten-Bertrand M, Jouvent R, Pauls D, Mallet J. Psychiatric genetics: Search for phenotypes. Trends Neurosci. 1998;21:102–105. doi: 10.1016/s0166-2236(97)01187-9. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C. Autism diagnostic interview: A standardized investigator-based instrument. J Autism Dev Disord. 1989;19(3):363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Bailey A, Goode S, Pickes A, Robertson S, Gottesman I, Rutter M. A broader phenotype of autism: The clinical spectrum in twins. J Child Psychol Psychiat. 1996;7:785–801. doi: 10.1111/j.1469-7610.1996.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Le CA, Bailey A, Goode S, Pickles A, Robertson S, Gottesman I, et al. A broader phenotype of autism: The clinical spectrum in twins. J Child Psychol Psychiatry. 1996;37:785–801. doi: 10.1111/j.1469-7610.1996.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19(2):185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Micali N, Chakrabarti S, Fombonne E. The broad autism phenotype: Findings from an epidemiological survey. Autism. 2004;8:21–37. doi: 10.1177/1362361304040636. [DOI] [PubMed] [Google Scholar]
- Murphy M, Bolton P, Pickles A, Fombonne E, Piven J, Rutter M. Personality traits of the relatives of autistic probands. Psychol Med. 2000;30:1411–1424. doi: 10.1017/s0033291799002949. [DOI] [PubMed] [Google Scholar]
- Pickles A, Bolton P, Macdonald H, Bailey A, Le CA, Sim CH, et al. Latent-class analysis of recurrence risks for complex phenotypes with selection and measurement error: A twin and family history study of autism. Am J Hum Genet. 1995;57:717–726. [PMC free article] [PubMed] [Google Scholar]
- Pickles A, Starr E, Kazak S, Bolton P, Papanikolaou K, Bailey A, Goodman R, Rutter M. Variable expression of the autism broader phenotype: Findings from extended pedigrees. J Child Psychol Psychiatry. 2000;41:491–502. [PubMed] [Google Scholar]
- Pilowsky T, Yirmiya N, Shalev RS, Gross-Tsur V. Language abilities of siblings of children with autism. J Child Psychol Psychiat. 2003;44:914–925. doi: 10.1111/1469-7610.00175. [DOI] [PubMed] [Google Scholar]
- Piven J, Gayle J, Chase GA, Fink B, Landa R, Wzorek MM, et al. A family history study of neuropsychiatric disorders in the adult siblings of autistic. J Am Acad Child Adolesc Psychiatry. 1990;29:177–183. doi: 10.1097/00004583-199003000-00004. [DOI] [PubMed] [Google Scholar]
- Piven J, Wzorek M, Landa R, Lainhart J, Bolton P, Chase G, et al. Personality characteristics of the parents of individuals with autism. Psychol Med. 1994;24:783–795. doi: 10.1017/s0033291700027938. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P, Landa R, Santangelo S, Jacobi D, Childress D. Personality and language characteristics in parents from multiple-incidence autism families. Am J Med Genet. 1997;74:398–411. [PubMed] [Google Scholar]
- Risch N, Spiker D, Lotspeich L, Nouri N, Hinds D, Hallmayer J, et al. A genomic screen of autism: Evidence for a multilocus etiology. Am J Hum Genet. 1999;65:493–507. doi: 10.1086/302497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Happe R, Plomin R. The genetic relationship between individual differences in social and nonsocial behaviours characteristic of autism. Dev Sci. 2005;8:444–458. doi: 10.1111/j.1467-7687.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- Ronald A, Happe F, Thomas P, Baron-Cohen S, Plomin R. Phenotypic and genetic overlap between autistic traits at the extremes of the general population. J Am Acad Child Adolesc Psychiatry. 2006;45:1206–1214. doi: 10.1097/01.chi.0000230165.54117.41. [DOI] [PubMed] [Google Scholar]
- Smalley SL, Asarnow RF. Cognitive subclinical markers in autism. J Autism Dev Disord. 1990;20:271–278. doi: 10.1007/BF02284724. [DOI] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiat. 1989;30:405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Stutsman R. Preprints of Part III, Mental Measurement of Pre-school Children. Chicago, Ill: Stoelting Co; 1948. Merrill-Palmer Scale of Mental Tests. [Google Scholar]
- Sung JU, Dawson G, Munson J, Estes A, Schellenberg J, Wijsman EM. Genetic investigation of quantitative traits related to autism: Use of multivariate polygenic models with ascertainment adjustment. Am J Hum Genet. 2005;76:68–81. doi: 10.1086/426951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P. Heterogeneity and the genetics of autism. J Psychiatry Neurosci. 1999;24:159–165. [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Jones M, Zwaigenbaum L, Maclean J. Genetics of autism: Overview and new directions. J Autism Dev Disord. 1998;28:351–368. doi: 10.1023/a:1026096203946. [DOI] [PubMed] [Google Scholar]
- Szatmari P, MacLean JE, Jones MB, Bryson SE, Zwaigenbaum L, Bartolucci G, et al. The familial aggregation of the lesser variant in biological and nonbiological relatives of PDD probands: A family history study. J Child Psychol Psychiatry. 2000;41:579–586. doi: 10.1111/1469-7610.00644. [DOI] [PubMed] [Google Scholar]
- Tyrer P. Personality assessment schedule. In: Pddma, editor. Course. London: Butterworth and Company; 1988. [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Shaked M. Psychiatric disorders in parents of children with autism: A meta-analysis. J Child Psychol Psychiat. 2005;46(1):69–83. doi: 10.1111/j.1469-7610.2004.00334.x. [DOI] [PubMed] [Google Scholar]