Abstract
In this study, doxorubicin (DOX) was physically incorporated into pH sensitive micelles made from a mixture of poly(l--Histidine)-b-poly(ethylene glycol)/ poly(l--Lactide)-b-poly(ethylene glycol) (75/25, wt.%). The DOX-loaded mixed micelles were formulated using dialysis methods and optimal DOX incorporation was achieved at a drug/polymer feed ratio of 0.2 (wt./wt.) when proper amount of aqueous phase (0.2, v./v.) was added into the common solvent (DMSO) solution, followed by dialysis at 4°C. Based on the results obtained from dynamic light scattering, UV-Vis absorption, and fluorescence experiments, it was demonstrated that the encapsulated drugs were mainly located inside the hydrophobic micelle cores, well protected and inaccessible to exterior molecules. Under in vitro conditions, although the microstructure of the micelles was altered below pH 8.0 by the encapsulated drugs, the drug-loaded micelles still exhibited desirable ability to control the drug release in response to tumor extracellular pH.
Keywords: polymeric mixed micelles, poly(l--histidine), pH sensitive, anti-cancer drug delivery, doxorubicin, drug incorporation, in vitro kinetics
INTRODUCTION
In recent years, significant efforts have been devoted to developing biodegradable nanoparticles for drug delivery, since they can be used to provide targeted delivery of drugs, to improve oral bioavailability, to sustain drugs in target tissues, and to improve the stability of therapeutic agents against enzymatic degradation [1, 2]. One such particulate system that satisfies the characteristics mentioned above is a multimolecular assembly of block copolymers with a core-shell architecture, so-called polymeric micelles [3-6], where a segregated core embedded in the hydrophilic palisade can serve as a reservoir for a variety of hydrophobic drugs. Nanosized polymeric micelles as vehicles for anticancer drugs have a passive targeting capability due to the enhanced permeability and retention (EPR) effect, resulting from leaky vasculature and a lack of lymphatic drainage in tumor tissues. Moreover, the innovative idea of “intelligent delivery” has motivated the development of nanocarriers with a triggered release mechanism which enables the carriers to release their cargo in response to specific external or internal stimuli [7-9]. Among these stimuli, changes in acidity are particularly useful for treating solid-tumor cancers, since the relatively acidic extracellular pH (pHe: 5.7-7.2) is a distinguishing phenotype of solid tumors from surrounding normal tissues [10] and more acidic conditions (pH: 5.5-6.0) are also encountered in endosomes once the micelle enters cells via endocytosis pathways. Quite a few polymeric micellar nanocarriers endowed with pH responsive properties have been exploited to date [11-18].
Our group recently developed a novel polymeric micellar platform for targeting cytotoxic agents to tumors based on the block copolymer poly(l--Histidine)-b-poly(ethylene glycol) (PH-PEG) [19-23]. Mixed micelles formed by PH (Mn~5K Da)-PEG (Mn: 2K Da) and another block copolymer, poly(l--Lactic acid) (Mn~3K Da)-b-poly(ethylene glycol) (Mn: 2K Da) (PLLA-PEG) at the weight ratio of 75/25 showed desirable pH responses to extracellular tumor pH [19]. Further studies on the empty mixed micelles revealed they were very stable above pH 7.4 but destabilized as the pH decreased below 6.8, which was attributed to increased electrostatic repulsions arising from the progressive protonation of the imidazole rings on the poly(l--Histidine) blocks [21].
Although the pH sensitivity of a micellar system itself can be considered to be a crucial factor in the development of a pH responsive delivery system, there are several other important issues that should not be neglected from a practical point of view, such as the efficient incorporation of drugs, stability and storage of drug-loaded micelles, and more importantly the effect of the encapsulated drugs on the microstructure and pH sensitivity of the carriers, because these factors could greatly affect the in vivo behavior of the drug-loaded vehicles and even the overall therapeutic efficacy of the drug. However few such studies have been performed on pH sensitive micelle formulations thus far. In this work, a widely-used anticancer drug, i.e. doxorubicin (DOX) was physically loaded into mixed micelles made from PH-PEG/PLLA-PEG (75/25, wt.%). The drug incorporation procedure was optimized by varying the drug/polymer feed ratio and initial buffer content as well as the dialysis temperature. Physicochemical properties of the DOX-loaded micelles were examined by a variety of experimental techniques. Stability of the drug-loaded micelles when stored in solution and as a freeze dried solid was evaluated respectively. In vitro pH dependent kinetics of the drug-loaded micelles was further investigated. In the discussion section, a detailed analysis of the drug incorporation process was performed to rationalize the experimentally obtained optimal conditions for efficient drug loading. Finally, the mechanism causing the change of properties of the carrier following drug encapsulation was elucidated.
MATERIALS AND METHODS
Materials
Z-His(Bzl)-OH, isopropylamine, triethylamine, PEG (Mn: 2K Da), (DEAE) Sephadex A-25, potassium tetraborate, ammonium bicarbonate, N-hydroxysuccinimide, N,N’-dicyclohexylcarbodiimide, anhydrous dimethylformamide, anhydrous 1,4-dioxane and dimethylsulfoxide (DMSO) were purchased from Sigma Co and. Thionyl chloride was purchased from Fluka Co. Potassium tert-butoxide and ethyl bromoacetate were purchased from Acros Organics. Doxorubicin·HCl and Dulbecco’s Phosphate Buffered Saline (DPBS, without MgCl2 and CaCl2) were purchased from Sigma Chemical Co. and used as received.
Polymer synthesis and characterization
(1) Poly(l--Histidine)-b-poly(ethylene glycol)
Synthesis of PH-PEG was described in detail elsewhere [21, 24]. Briefly, poly(Bzl-Histidine) was first synthesized by a ring open reaction of N-carboxyanhydride of Z-His(Bzl)-OH using isopropylamine as an initiator and was then coupled with PEG monoacid (MW: 2000 Da) via an amide bond. Subsequently the protecting (benzyl) group of His was removed and the obtained PH-PEG was purified by a dialysis method followed by lyophilization.
(2) Poly(l--Lactic acid)-b-poly(ethylene glycol)
PLLA-PEG diblock copolymer was synthesized by ring opening polymerization of l--Lactide initiated by the hydroxy group of PEG monoacid (MW: 2000 Da) in the presence of stannous octoate as a catalyst [25].
(3) Polymer characterization
1H NMR spectra of the polymers in deuterated DMSO solutions were recorded by a Varian 400 MHz NMR system. Gel permeation chromatography (GPC) was carried out at 30°C using DMF as the eluent on an Aligent 1100 series HPLC instrument equipped with an Aligent Plgel Mixed-C column and a refractive index detector using polystyrene standards for calibration. The molecular weights and PDI (Mw/Mn) of the polymers are given in Table 1. The chemical structures of the two polymers are showed in Figure 1.
Table 1.
Characterization of the block copolymers.
| Polymer | Mn (1H NMR) | Mn (GPC) | Mw/Mn (GPC) |
|---|---|---|---|
| PH-PEG | 7200 | 10050 | 1.3 |
| PLLA-PEG | 4860 | 4600 | 1.2 |
Figure 1.
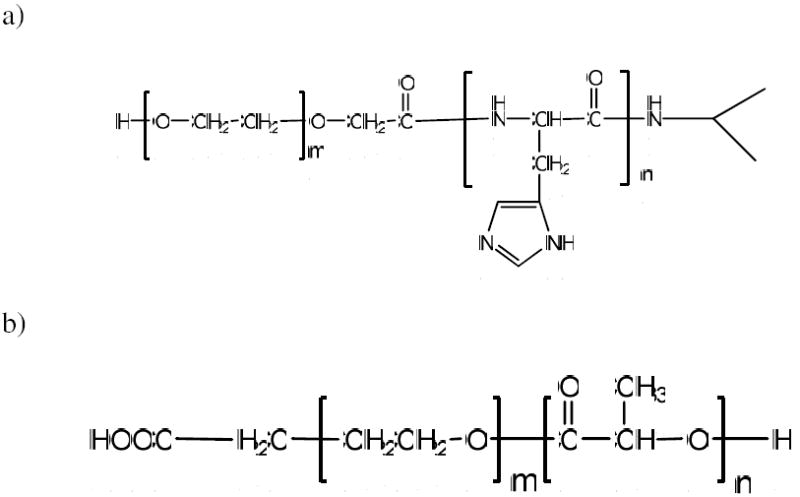
Chemical structures of poly(l--Histidine)-b-poly(ethylene glycol) a) and poly(l--Lactic acid)-b-poly(ethylene glycol) b).
Preparation of DOX-loaded micelles
DOX·HCl was dissolved with 2 molar ratio of triethylamine in DMSO and stirred overnight. The DOX base solution was blended with PH-PEG/PLLA-PEG mixtures (75/25 wt.%) at a predetermined feed ratio. A predetermined amount of 10mM borate buffer (pH 9.0) was added dropwise into the DMSO solution under vigorous stirring. The buffer content (X0) was calculated using the following equation:
| (1) |
A varied value from 0 to 0.4 for X0 was applied in the experiments. The resulting solution was continuously stirred for another 6 h before it was transferred into a pre-swollen dialysis membrane (SPECTRA/POR, MWCO: 15000) and dialyzed against 1 L 10 mM pH 9.0 borate buffer. The outer aqueous phase was replaced with fresh buffer solution at 1, 2, 4, 6, and 12 h. After 24 h, samples of the drug-loaded micelles were recovered from the membrane and filtered through a 0.8 μm membrane filter to remove any unloaded drug. The drug loading content (LC%) and loading efficiency (LE%) were calculated using the following equations:
| (2) |
| (3) |
where the amount of encapsulated DOX was determined according to the following spectrophotometric method.
Quantitative determination of encapsulated doxorubicin
2 mg DOX.HCl was dissolved in 50 ml DMSO in a volumetric flask. Standard DOX solutions with varied concentrations (1-100 μg.mL-1) were prepared by dilution from this stock solution. The absorbance of the standard solutions was measured by a SPECTRAmax M2 UV-Vis spectrophotometer at wavelength of 480 nm using blank DMSO as a reference. Using the Lambert-Beer law, a linear regression equation relating the absorbance (A) to the DOX concentration (c) was obtained.
| (4) |
where r is the correlation coefficient. 100 μl of the DOX-loaded micelle formulation was lyophilized and the freeze dried sample was re-dissolved in 3 ml DMSO. The absorbance of the resulting solution was measured at 480 nm and the drug concentration was determined from the regression equation.
Dynamic light scattering
Dynamic light scattering (DLS) experiments were carried out using a Brookhaven Instruments Corp. system consisting of a BI-200SM goniometer and a BI-9000AT autocorrelator. The samples were filtered prior to measurement using a 0.80 μm disposable membrane filter. The field correlation function was measured at 90° and analyzed by the constrained regularized CONTIN method [26] to yield information on the distribution of the characteristic line width (Г). The normalized distribution function of the characteristic line width <Г> can be used to determine an average apparent diffusion coefficient.
| (5) |
where q = 4πn sin(θ / 2) / λ is the magnitude of the scattering wave vector. The apparent hydrodynamic diameter dh,app is related to Dapp via the Stocks-Einstein equation:
| (6) |
where k is the Boltzmann constant and η is the viscosity of water at temperature T. The value of the relative variance (u2 / <Г>2) obtained from the size distribution plot is considered the size polydispersity (PI). All measurements were performed at 25°C unless specified.
Doxorubicin fluorescence experiment
All fluorescence measurements were performed using a SHIMADZU RF-5301PC spectrofluorometer. Excitation and emission were set at 480 nm and 555 nm respectively with bandwidths of 5 nm. Samples were aqueous solutions of free DOX and DOX-loaded mixed micelles. A water-soluble quenching agent, KI, was used for the fluorescence quenching study. The level of KI ranged from 0.0 to 0.6 M and the ionic strength of the solution was kept constant by the addition of NaCl. Na2S2O4 (10-5 M) was added to prevent the oxidation of iodide.
Drug stability study
The stability of DOX in aqueous solution over time was monitored by tracking the change in the drug amount as determined from the absorbance at 480 nm as described before. For drug-loaded micelles, a dialysis procedure was applied for 6 h with the outside borate buffer (10 mM, pH 9.0) replaced every 2 h to remove free drugs before each measurement. It should be mentioned that this method does not provide any information regarding the mechanism of any reactions taking place during the decomposition of DOX. Since we did not intend to quantify the amount of degraded products of DOX in this study, the absorption method was used to provide semi-quantitive results on the degree of DOX degradation [27, 28].
In vitro kinetics of DOX-loaded micelles
2 ml of pre-filtered DOX-loaded micelle formulation was transferred into a dialysis membrane (MWCO: 15000). Subsequently, the membrane was immersed into a beaker containing 1 L DPBS buffer adjusted to pre-determined pH under mechanic shaking (40 rpm) in a 37°C water bath. The outer buffer phase was replaced periodically to maintain a sink condition. At pre-determined time intervals, the solution inside the dialysis membrane was withdrawn for measurements and immediately put back thereafter. Changes in the micelle size were tracked by DLS and the amount of retained DOX was measured by the absorption method.
RESULTS
Drug incorporation and fabrication of drug-loaded micelles
Since the polymer mixtures were not readily dissolved in water, a dialysis method was employed to fabricate drug-loaded micelles. Maintaining a fixed feed ratio of drug/polymer (0.2, wt./wt.), two variables were considered for this process, namely initial buffer content (X0) and dialysis temperature (T). Specifically the polymers and drugs were first dissolved in DMSO, followed by addition of predetermined amounts of pH 9.0 buffer under vigorous stirring. The resulting solution was continuously stirred for another 6h and was then dialyzed against aqueous buffer at a temperature of 25°C and 4°C respectively. The size (hydrodynamic diameter) and drug loading efficiency of the obtained drug-loaded micelles were measured and the results are shown in Figure 2.a and 2.b respectively. At a dialysis temperature of 25°C, the prepared drug-loaded micelles without buffer addition had a size of 260±20 nm. Micelle size decreased noticeably upon buffer addition. As the initial buffer content (X0) increased to 0.2 (v./v.), micelle size dropped to 174±13 nm and remained virtually unchanged with further X0 increase. The drug loading efficiency (LE%) did not follow the same tendency. LE% initially increased with X0 until a maximum value of 77±5 % at X0 of 0.2, but it began to decrease with further increases of X0. A similar trend was noted as the dialysis temperature was changed from 25°C to 4°C, but a smaller size and higher LE% were obtained at the same value of X0. It was determined that for this system a minimal size value of 153±15 nm and a maximal LE% value of 90±5 % can be achieved at X0 of 0.2. The optimal conditions for the two factors i.e. X0=0.2 and T=4°C were employed for preparation of all the drug-loaded micelle formulations.
Figure 2.
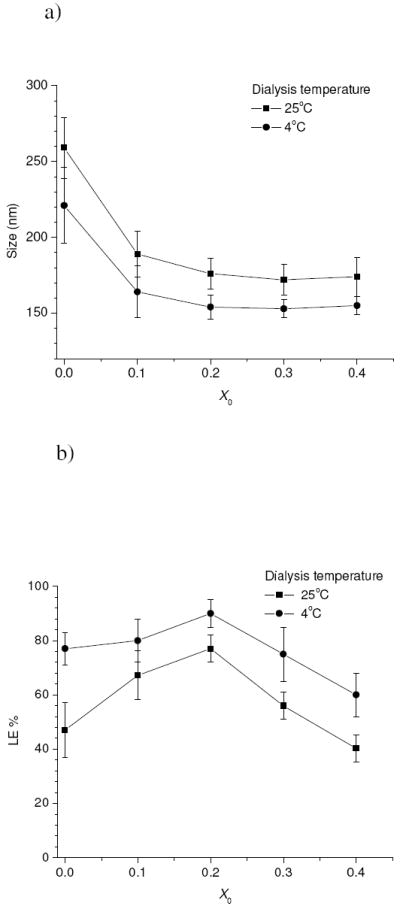
Variations in size a) and drug loading efficiency b) for the drug-loaded micelles using the drug/polymer feed ratio of 0.2 (wt./wt.) as a function of initial buffer fraction (X0) and dialysis temperature.
Figure 3.a shows the changes in drug loading content (LC%) and loading efficiency (LE%) as a function of the DOX/polymer feed ratio (wt./wt.), and the corresponding changes in size and size polydispersity index (PI) for the obtained drug-loaded micelles are plotted in Figure 3.b. LC% was found to increase almost linearly with the feed ratio up to 0.2 with a high value of LE% (~90%) and no significant change in the size. However, as the feed ratio increased above 0.2, LE% began to sharply drop and a significant increase in the size was also observed. Therefore the optimal drug/polymer feed ratio where a relatively small size and high loading efficiency could both be fulfilled was 0.2. Taken all together, an optimal drug-loaded micelle formulation could be obtained by preparing a mixture using a drug/polymer feed ratio of 0.2, initial buffer content of 0.2 and dialysis temperature of 4°C, producing micelles with an LC% of 18±1 % and a size of 153±15 nm, which were then utilized for all further studies.
Figure 3.
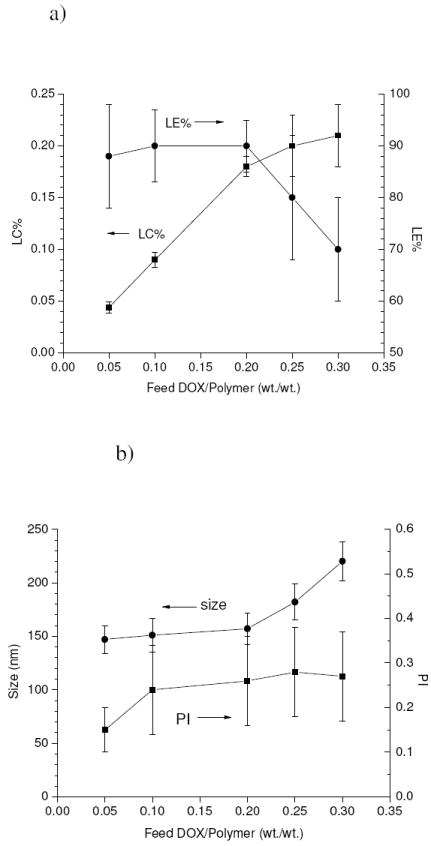
Variations in loading content (LC%) and loading efficiency (LE%) a), and micelle size and size polydispersity index (PI) b) for the drug-loaded micelles as a function of drug/polymer feed ratio.
Physicochemical characterizations of drug-loaded micelles
It is known that the DOX molecule bears a chromophore composed of three aromatic hydroxyanthraquinonic rings which can be used to monitor its interactions with other molecules. The visible absorption spectrum of encapsulated DOX showed only slight differences from free DOX in aqueous solution, as illustrated in Figure 4.a; however, the presence of a red-shift in the absorption peak is a sign of increased local concentrations and also of DOX-DOX interactions, due to π-π stacking [29, 30]. More clear evidence came from the fluorescence spectrum (Figure 4.b), where a remarkable decrease in the fluorescence intensity was observed for the encapsulated drugs compared to equivalent free drug concentrations. This is a typical effect of self quenching behavior arising from increased local concentrations of the fluorescent molecules, indicating that the encapsulated DOX was confined inside the polymeric micelles.
Figure 4.
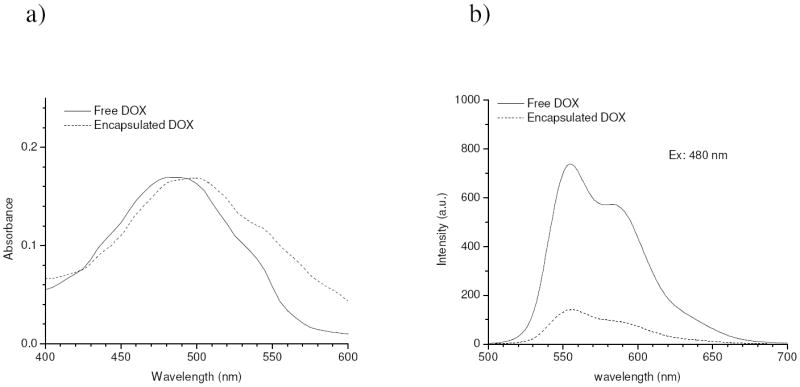
Visible absorption a) and fluorescence spectra b) of free DOX (10 ug/ml) and encapsulated DOX (eq. 10 ug/ml).
The hydrophobic character of encapsulation was further revealed by fluorescent quenching studies. Quenching activity of free and encapsulated DOX molecules was evaluated using hydrated iodide ions and the collisional quenching constant Ksv, which reflects the degree of DOX exposure to I- ions, was estimated from the Stern-Volmer plot of fluorescent quenching data (Figure 5), using
Figure 5.
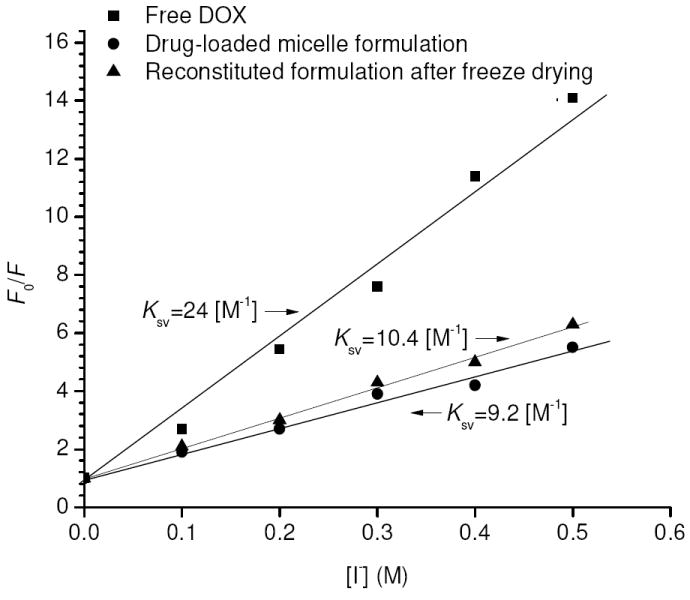
Stern-Volmer plots of free DOX (10 ug/ml), freshly prepared drug-loaded micelles and reconstituted formulation after freeze drying (e.q. DOX 10 ug/ml), respectively at 25°C and pH 9.0. Solid lines indicate the best linear fit.
| (7) |
where F and F0 are the total fluorescence intensities of DOX with and without I-, respectively. Free DOX had a Ksv of 24 M-1, whereas DOX-loaded micelles had a Ksv of 9.2 M-1. The lower value of Ksv for the latter case indicates the drugs were not readily accessible to I- [31], which is consistent with hydrophobic trapping of drugs inside the micelle cores. It is worth noting that the encapsulated DOX was still slightly quenched by I-, which may suggest either some degree of accessibility of I- in the hydrophobic domains of the micelles or the presence of some drugs in the palisade region where partial hydration may exist.
Storage and stability of drug-loaded micelles
The fabricated drug-loaded micelles exhibited good stability at 4°C over time and no significant change in the micelle size was detected following storage in the dark for 30 days (Figure 6.a). It is known that the stability of DOX in aqueous solution can be affected by many factors such as pH, temperature, light etc. The kinetics of drug stability changes at 4°C for free and encapsulated DOX was tracked over a period of 30 days in the absence of light, and the results are shown in Figure 6.b. It was found that free DOX was subject to rapid decomposition, which is probably attributed to accelerated auto-oxidation or hydrolysis rates at pH 9.0 [32, 33]. Nevertheless a notably slower degradation rate was observed for the encapsulated DOX and 78±5 wt.% of DOX still remained intact inside the micelles after 30 days, probably due to the fact that the encapsulated drugs were largely protected by the micellar carriers from contact with outside water molecules and the drug release rate was considerably low at 4°C.
Figure 6.
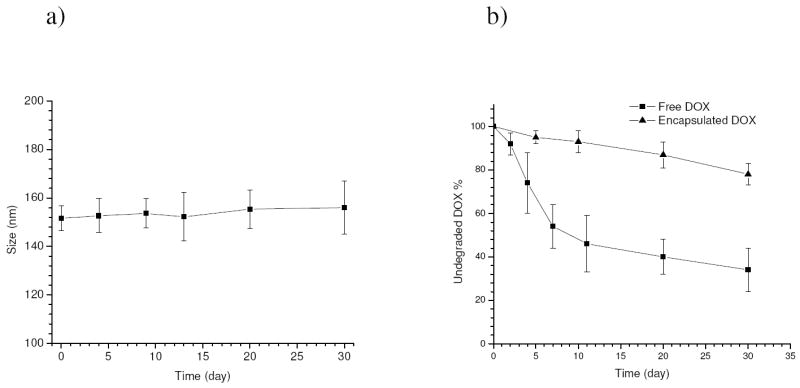
Changes in size of the drug-loaded micelles a), and fraction of undegradated drugs for free DOX (10 ug/ml) and encapsulated DOX (eq. 10 ug/ml) b) when stored at 4°C and pH 9.0 over a period of 30 days (all the measurements were performed at 25°C).
Freezing drying is another method of conserving drug-loaded micelles. However one problem that may arise in this situation is the micelles might be disrupted as water molecules are removed. Therefore, the sample was re-dissolved in water after freeze drying to check its integrity. Compared to a sample without freeze drying, there was only a slight increase in size for the immediately reconstituted sample and also for a sample reconstituted following 30 days of storage (Figure 7), indicating the micelles remained virtually undisturbed during the process of freeze drying and they can be recovered nearly intact following reconstitution. In addition to micelle integrity, it is also important to determine whether or not DOX is still present inside the micelle cores after the reconstitution. To this end, fluorescent quenching studies were performed for the reconstituted samples and a collisional quenching constant (Ksv) of 10.4 M-1 was obtained (Figure 5), very close to that before freeze drying (9.2 M-1). Therefore it can be considered that the majority of the drugs were still retained inside the micelles upon reconstitution. Moreover, the encapsulated drugs in the freeze dried sample showed significantly better stability when stored at room temperature than the micelles kept in solution which was subject to gradual drug release. It was found following 30 days of storage at 25°C less than 30 wt.% of DOX was retained in the drug-loaded micelles kept in solution whereas 83±6 wt.% of DOX was still incorporated inside the micelles reconstituted from the freeze dried sample. Hence freeze drying could be a desirable method of preserving drug-loaded micelles especially when long-time storage at room temperature is required.
Figure 7.
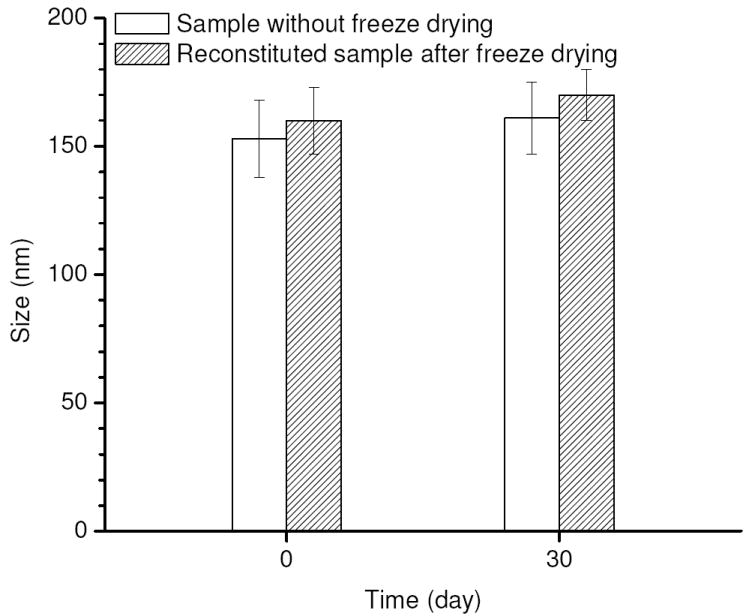
Changes in size of drug-loaded micelles for the sample without freeze drying and the reconstituted sample after freeze drying following 30 days of storage at 25°C.
In vitro pH-dependent kinetics of drug-loaded micelles
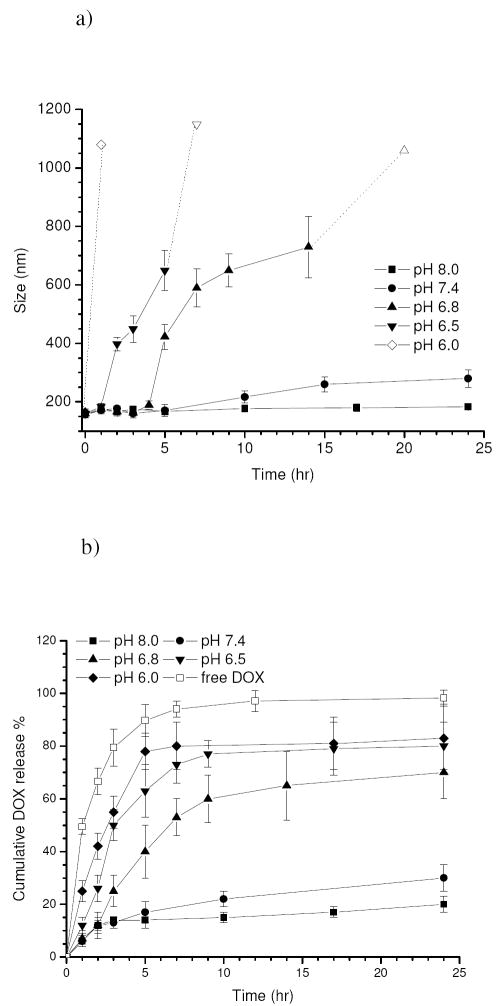
Figure 8.a shows the change in size of the drug-loaded micelles as a function of pH over a period of 24 hr under in vitro conditions, and the corresponding DOX release profiles are plotted in Figure 8.b. In addition, a release profile of free DOX with the same equivalent concentration is included as a control. It can be noted that 94±3 wt.% of the free DOX was released following 5 hr of incubation due to a fast diffusion rate of the drug molecules under the sink condition. For drug-loaded micelles, the size remained almost constant (155±8 nm) within 24 hr at pH 8.0 while a burst release of 12±2 wt.% of DOX was observed in the initial 2 hr followed by a delayed release up to 24 hr. As pH decreased to 7.4, a slight increase in size took place after 5 hr and the size gradually increased up to 183±10 nm over 24 hr. Following the initial burst release, the sample showed a slightly faster drug release rate at pH 7.4 than at pH 8.0. Nevertheless, the majority of the encapsulated drugs (ca. 70 wt.%) was still incorporated after 24 hr. A dramatically different situation occurred when the pH dropped to 6.8. Although the micelle remained relatively stable in the first 4 hr, a remarkable increase in the size from 189±15 nm to 423±43 nm occurred after 5 hr. Thereafter, the size continuously went up and it increased above 1 μm after 14 hr which was beyond the upper detection limit of the instrument. An accelerated release of DOX was also observed over time with about 70 wt.% of the drugs released after 24 hr. A similar situation was found at pH 6.5, although size increase and drug release rate were faster compared to pH 6.8. As the pH further decreased to 6.0, micron-sized particles were detected within the first hour and most of the encapsulated drugs (78±7 wt.%) were released within 5 hr.
Figure 8.
Changes in size a) and corresponding cumulative DOX release b) for the drug-loaded micelles (eq. 100 ug/ml) as a function of pH under in vitro conditions at 37°C over a period of 24 hr, including a release profile of free DOX (100 ug/ml) as a control. The hollow data points in Figure 8.a indicate the values were not precisely determined since they were beyond the measurement limit of the instrument. The release of free DOX was measured at pH 6.0.
DISCUSSION
Efficient and controlled drug incorporation into polymeric micelles
Since the mixture of PH-PEG/PLLA-PEG (75/25, wt.%) is known to have a relatively high glass transition temperature (Tg=131°C) [21], complete polymeric micelles formed in aqueous solution will have no ability to incorporate drugs into their glassy cores. Therefore a dialysis method was employed to incorporate drugs into the core, starting from a common solvent (DMSO) solution where both the polymers and drugs were molecularly dissolved. In addition, borate buffer with a pH value of 9.0 was utilized as the aqueous phase to ensure the hydrophobic character of encapsulation, since protonation of the imidazole groups on the PH block takes place below pH 8.0 [23]. During the dialysis process, intermediate micelle-like structures having cores swollen by common solvent-water mixtures will be formed. The liquid-like character of the cores would make it feasible for the drugs to enter and interact with hydrophobic segments of the polymers [34]. As micelle formation progresses with the water gradually replacing DMSO, the micelle core will become more and more solid-like until finally all entries for free drugs are completely blocked. However, using the situation where the DMSO solution is directly dialyzed against water, the content of the aqueous phase inside the dialysis membrane may change very quickly with time and would also be difficult to control. DOX could not be incorporated at high efficiencies under these conditions, probably due to the very short time period that the suitably swollen core existed [34]. Instead, pre-adding proper amounts of aqueous buffer into the DMSO solution followed by stirring for quite a period of time can provide controllable and suitably swollen cores as well as a sufficient time frame for drugs to incorporate, therefore leading to a higher drug loading efficiency. Nevertheless, excess buffer addition can also cause the cores to be “frozen” and block the entry of free drugs, which would explain why there is a maximum drug loading efficiency with increasing buffer content. As for the dialysis temperature, it is not difficult to imagine that a lower temperature will cause decreased diffusion rate of the drugs. In addition, the PEG corona hydration shell of the micelles is thought to become thicker as temperature decreases [35]. Both these effects tend to prevent encapsulated drugs from leaking outside. One should also note that compared to the blank micelles [21], a larger size was adopted for the drug-loaded micelles. This may suggest the micelles were swollen by the incorporated drugs as normally happened for micellar carriers. On the other hand, since the PH-PEG/PLLA-PEG mixtures were revealed to be secondary aggregates associated by individual micelles [21], the state of aggregation in one secondary micelle may be altered following drug incorporation, which could also contribute to the observed size change.
Considering the finite space of the micelle cores, the degree of drug solubilization into the micelles should be limited to a certain level to maintain an optimal balance among the drug content, loading efficiency and micelle stability. Although excess loading of the drugs was still possible using higher feed ratios of drug/polymer in our experiments, stability of the micelles was jeopardized in this case as indicated by an obvious increase in size coupled with decreased loading efficiency. Thus taking the above factors together, optimal conditions for efficient and controlled drug incorporation could be achieved by selecting proper feed drug/polymer ratios, adjusting the aqueous content in common solvent, and fabricating (dialyzing) at a low temperature.
Effect of the encapsulated drugs on pH dependency of micellar carriers
Although the empty polymeric micelles exhibited desirable pH sensitivity in response to extracellular tumor pH [21], it should be realized that pH dependency of the micellar carriers could be altered after drug encapsulation, especially in this case the drug (DOX) itself is a weak base. It is this concern that motivated the study of in vitro pH-dependent kinetics of drug-loaded micelles. At pH 8.0, the drug-loaded micelles exhibited good kinetic stability and minimal drug leakage was detected over the time period studied, indicating the structure of the carriers was probably hardly affected by the drugs. As pH decreased to 7.4, a slight increase in size and higher amount of drug release were observed with time. Note the fact that the structure of the empty micelles was still undisturbed in this case [21] and DOX has a higher pKa (8.3) [36] than that of imidazole groups (~7.0) [23], so the swelling of the drug-loaded micelles was probably caused by protonation of the encapsulated drugs. Nevertheless, retention of 70 wt.% of the drugs after 24 h suggests that the micellar carriers still effectively did their job by preventing most of the encapsulated drugs from releasing. This situation was dramatically changed at pH 6.8. A remarkable increase in carrier size took place after 5 h of incubation, accompanied by an accelerated release of DOX. Referring to the study on empty mixed micelles [21], this abrupt increase in size can be mainly attributed to destabilization of the micelle cores arising from protonation of the imidazole rings on the PH-PEG polymers and undoubtedly this tendency could be further promoted by protonation of the drugs. As a result, the hydrophobic barrier of the micelle cores that once securely held the drugs was disrupted, resulting in enhanced release of DOX. However, the size increase caused by the destabilization of the micelle cores should have been limited [21], which does not correlate to the continuous increase in size over time that was observed. Given enough time under a sink condition, the released drugs could have been completely removed out of the dialysis membrane through diffusion. Nevertheless, when a large amount of DOX was released in a short period of time as the case for the system at pH 6.8, there would be a temporary increase of local DOX concentration around the micelles. Considering the fact that the micellar core became swollen due to increasing electrostatic repulsions, the relatively short PEG blocks (Mw: 2000) may no longer provide sufficient shielding against aggregation for the hydrophobic cores. Moreover, micelle aggregation could be facilitated by the tendency of adjacent free DOX to form aggregates by π-π stacking [18]. As more drugs were released over time, the micelle aggregation could be further promoted and finally reached micron sizes. It is not hard to imagine this situation will become more remarkable at lower pH. As the pH was decreased to 6.0, large aggregates were observed within the first hour of incubation, which probably reflected a large amount of released DOX and rapid micelle swelling/aggregation within this short time. Based on the above discussion, it can be considered that although the microstructure of the micellar carriers of PH-PEG/PLLA-PEG (75/25, wt.%) was affected by the encapsulated drugs below pH 8.0, the drug-loaded micelles sill maintained considerable stability at pH 7.4 while offering the ability of controlled drug release in response to relatively acidic conditions (extracellular tumor pH).
CONCLUSIONS
The pH sensitive mixed micelles of PH-PEG/PLLA-PEG (75/25, wt.%) were able to attain high drug content and loading efficiency of DOX encapsulation via a controllable drug loading process. The DOX-loaded micelle formulation possessed desirable physiochemical properties and pH sensitivity that will provide some advantages for targeted anticancer drug delivery. Moreover, the structure and kinetic behavior of the micelles could be altered by the encapsulated drugs under in vitro conditions and the influence may be pH dependent. We hope this study will also shed some light on studying the in vivo fate of drug-loaded micelles which still remains as a challenge in this field.
Acknowledgments
This work was supported by the NIH grant CA101850. The first author is grateful to Deepa Mishra (University of Utah) for her editorial aid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 2.Oh KT, Yin HQ, Lee ES, Bae YH. Polymeric nanovehicles for anticancer drugs with triggering release mechanisms. J Mater Chem. 2007;17:3987–4001. [Google Scholar]
- 3.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 4.Kataoka K. Targetable polymeric drugs. In: Park K, editor. Controlled Drug Delivery Challenges and Strategies. American Chemical Society; Washington, DC: 1997. pp. 49–71. [Google Scholar]
- 5.Jones M, Leroux J. Polymeric micelles - a new generation of colloidal drug carriers. Eur J Pharm Biopharm. 1999;48:101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 6.Kwon GS. Polymeric micelles for delivery of poorly water-soluble compounds. Crit Rev Ther Drug Carrier Syst. 2003;20:357–403. doi: 10.1615/critrevtherdrugcarriersyst.v20.i5.20. [DOI] [PubMed] [Google Scholar]
- 7.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 8.Breunig M, Bauer S, Goepferich A. Polymers and nanoparticles: intelligent tools for intracellular targeting? Eur J Pharm Biopharm. 2008;68:112–128. doi: 10.1016/j.ejpb.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–4384. [PubMed] [Google Scholar]
- 11.Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed Engl. 2003;42:4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 12.Heller J, Barr J, Ng SY, Abdellauoi KS, Gurny R. Poly(ortho esters): synthesis, characterization, properties and uses. Adv Drug Deliv Rev. 2002;54:1015–1039. doi: 10.1016/s0169-409x(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 13.Leroux J, Roux E, Le Garrec D, Hong K, Drummond DC. N-isopropylacrylamide copolymers for the preparation of pH-sensitive liposomes and polymeric micelles. J Control Release. 2001;72:71–84. doi: 10.1016/s0168-3659(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 14.Licciardi M, Giammona G, Du J, Armes SP, Tang Y, Lewis AL. New folate-functionalized biocompatible block copolymer micelles as potential anti-cancer drug delivery systems. Polymer. 2006;47:2946–2955. [Google Scholar]
- 15.Ko J, Park K, Kim YS, Kim MS, Han JK, Kim K, Park RW, Kim IS, Song HK, Lee DS, Kwon IC. Tumoral acidic extracellular pH targeting of pH-responsive MPEG-poly(beta-amino ester) block copolymer micelles for cancer therapy. J Control Release. 2007;123:109–115. doi: 10.1016/j.jconrel.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka K, Matsumoto T, Yokoyama M, Okano T, Sakurai Y, Fukushima S, Okamoto K, Kwon GS. Doxorubicin-loaded poly(ethylene glycol)-poly(beta-benzyl-L-aspartate) copolymer micelles: their pharmaceutical characteristics and biological significance. J Control Release. 2000;64:143–153. doi: 10.1016/s0168-3659(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 17.Hruby M, Konak C, Ulbrich K. Polymeric micellar pH-sensitive drug delivery system for doxorubicin. J Control Release. 2005;103:137–148. doi: 10.1016/j.jconrel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Gillies ER, Frechet JM. pH-Responsive copolymer assemblies for controlled release of doxorubicin. Bioconjug Chem. 2005;16:361–368. doi: 10.1021/bc049851c. [DOI] [PubMed] [Google Scholar]
- 19.Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J Control Release. 2003;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J Control Release. 2005;103:405–418. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Yin HQ, Lee ES, Kim D, Lee KH, Oh KT, Bae YH. Physicochemical characteristics of pH-sensitive poly(L-Histidine)-b-poly(ethylene glycol)/poly(L-Lactide)-b-poly(ethylene glycol) mixed micelles. J Control Release. 2008;126:130–138. doi: 10.1016/j.jconrel.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao ZG, Lee DH, Kim DI, Bae YH. Doxorubicin loaded pH-sensitive micelle targeting acidic extracellular pH of human ovarian A2780 tumor in mice. J Drug Target. 2005;13:391–397. doi: 10.1080/10611860500376741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J Contorl Release. 2003;90:363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 24.Kim GM, Bae YH, Jo WH. pH-induced micelle formation of poly(histidine-co-phenylalanine)-block-poly(ethylene glycol) in aqueous media. Macromolecular Bioscience. 2005;5:1118–1124. doi: 10.1002/mabi.200500121. [DOI] [PubMed] [Google Scholar]
- 25.Han SK, Na K, Bae YH. Sulfonamide based pH-sensitive polymeric micelles: physicochemical characteristics and pH-dependent aggregation, Colloids Surf. A Physicochem Eng Aspects. 2003;214:49–59. [Google Scholar]
- 26.Provencher SW. A Fourier method for the analysis of exponential decay curves. Biophys J. 1976;16:27–41. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandak S, Ramu A, Barenholz Y, Gabizon A. Reduced UV-induced degradation of doxorubicin encapsulated in polyethyleneglycol-coated liposomes. Pharm Res. 1999;16:841–846. doi: 10.1023/a:1018869818282. [DOI] [PubMed] [Google Scholar]
- 28.Prokopowicz M, Lukasiak J, Przyjazny A. Utilization of a sol-gel method for encapsulation of doxorubicin. J Biomater Sci Polym Ed. 2004;15:343–356. doi: 10.1163/156856204322977229. [DOI] [PubMed] [Google Scholar]
- 29.Porumb H. The solution spectroscopy of drugs and the drug-nucleic acid interactions. Prog Biophys Molec Biol. 1978;34:175–195. doi: 10.1016/0079-6107(79)90017-8. [DOI] [PubMed] [Google Scholar]
- 30.Missirlis D, Kawamura R, Tirelli N, Hubbella JA. Doxorubicin encapsulation and diffusional release from stable, polymeric, hydrogel nanoparticles. EUR J PHARM SCI. 2006;29:120–129. doi: 10.1016/j.ejps.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Kwon G, Naito M, Yokoyama M, Okano T, Sakurai Y, Kataoka K. block copolymer micelles for drug delivery: loading and release of doxorubicin. J Control Release. 1997;48:195–201. [Google Scholar]
- 32.Janssen MJH, Crommelin DJA, Storm G, Hulshoff A. Doxorubicin decomposition on storage. Effect of pH, type of buffer and liposome encapsulation. Int J Pharm. 1985;23:1–11. [Google Scholar]
- 33.Beijnen JH, van der Houwen OAGJ, Underberg WJM. Aspects of the degradation kinetics of doxorubicin in aqueous solution. Int J Pharm. 1986;32:123–131. [Google Scholar]
- 34.Kohori F, Yokoyama M, Sakai K, Okano T. Process design for efficient and controlled drug incorporation into polymeric micelle carrier systems. J Control Release. 2002;78:155–163. doi: 10.1016/s0168-3659(01)00492-8. [DOI] [PubMed] [Google Scholar]
- 35.Branca C, Magazu S, Maisano G, Migliardo F, Migliardo P, Romeo G. Hydration Study of PEG/Water Mixtures by Quasi Elastic Light Scattering, Acoustic and Rheological Measurements. J Phys Chem B. 2002;106:10272–10276. [Google Scholar]
- 36.Adams DJ. The impact of tumor physiology on camptothecin-based drug development. Curr Med Chem Anticancer Agents. 2005;5:1–13. doi: 10.2174/1568011053352596. [DOI] [PubMed] [Google Scholar]