Abstract
Experiments were designed to test the hypothesis that antioxidant treatment would increase the antihypertensive actions of endogenous kinins during angiotensin converting enzyme (ACE) inhibition. Four groups of rats, all given angiotensin II (Ang II) for 2 weeks, were studied: 1) control, 2) enalapril, 3) tempol or 4) both tempol and enalapril. Ang II significantly increased systolic blood pressure (BP) when compared with the baseline (170± 8 vs. 128± 4 mm Hg, P<0.05). Neither enalapril nor tempol alone was able to attenuate the elevation in BP (165± 7 and 164± 6 mm Hg, respectively). In contrast, combined administration of tempol and enalapril prevented the increase in BP (137± 5 mmHg). Plasma 8-isoprostane increased in Ang II infused rats when compared with control untreated rats (69± 14 vs. 23± 0.5 pg/ml, P<0.05). Tempol alone or tempol plus enalapril significantly attenuated the increase in plasma 8-isoprostane (29± 6 and 34± 7 pg/ml, respectively). In additional experiments, we used the bradykinin B2 antagonist, icatibant to determine if increased B2 receptor contributes to the antihypertensive effect of combined tempol and enalapril in Ang II infused rats. Icatibant decreased the ability of this combination to lower arterial pressure. Additionally, a significant increase in B1 receptor protein expression in renal cortex of Ang II infused rats was observed compared to control suggesting that the bradykinin receptors activation could account for the effect of enalapril to enhance the actions of tempol. These data support the hypothesis that combined reduction of superoxide along with enhanced endogenous kinins may facilitate blood pressure lowering in Ang II hypertension.
Keywords: superoxide, oxidative stress, angiotensin converting enzyme inhibitors, 8-isoprostane, arterial pressure, rats
Introduction
Accumulating evidence has indicated that angiotensin II (Ang II) may contribute to vascular hypertrophy and hypertension via the stimulation of the NADPH oxidase system to increase reactive oxygen species (ROS) in vascular cells (Griendling et al., 1994; Rajagopalan et al., 1996; Nickenig and Harrison, 2002). The increase in ROS production may play a role in the elevation of blood pressure by a direct vasoconstrictor effect or indirectly by reducing the activity of vasodilators such as NO and arachidonic acid metabolites (Reckelhoff and Romero, 2003). Studies have also shown that treatment with liposome-encapsulated superoxide dismutase (SOD) or the membrane-permeable SOD mimetic, tempol, markedly blunts the increase in blood pressure caused by chronic, low-dose Ang II administration, suggesting that stimulation of ROS formation is critically involved in the blood pressure response to Ang II (Laursen et al., 1997; Nishiyama et al., 2001; Ortiz et al., 2001; Kawada et al., 2002).
Some of the most convincing evidence that Ang II plays an important role in the pathogenesis of cardiovascular disease comes from The Heart Outcomes Prevention Evaluation (HOPE) trial showing that angiotensin converting enzyme (ACE) inhibitor treatment caused a remarkable decrease in cardiovascular morbidity and mortality in individuals at high risk (Yusuf et al., 2000). It is important to remember, however, that ACE also serves to degrade kinins so it is possible that some of the beneficial effects of ACE inhibition are by inhibiting kininase activity. Recently, our laboratory has shown that the ACE inhibitor, enalapril, but not angiotensin receptor blockade, attenuated the increase in blood pressure produced by acute endothelin-1 infusion (Elmarakby et al., 2003). Since a bradykinin receptor antagonist reversed the effect of enalapril, we concluded that the influence of ACE inhibition is attributed to the increase in kinin survival (Elmarakby et al., 2003). Bradykinin produces vasodilatory effect via the stimulation of the bradykinin B1 and B2 receptors. The B2 receptor is widely expressed under most physiological conditions and the B1 is only expressed in some pathophysiological conditions (Marceau et al., 1998; Mclean el al., 2000; Duka et al., 2001). Chronic Ang II infusion is associated with increased ET-1 levels (Alexander et al., 2001; Sasser et al., 2002), so it is possible that kinins could influence blood pressure regulation in this model. Therefore, the present study was designed to determine the influence of enhancing endogenous kinins on blood pressure and oxidative stress in Ang II dependent hypertension. More specifically, we determined the influence of the ACE inhibitor, enalapril, and SOD mimetic, tempol, on blood pressure and oxidative stress during chronic Ang II infusion in rats.
Methods
Blood pressure measurements and metabolic cage studies
Experiments were conducted using male Sprague-Dawley rats with an initial body weight of 200-250 g (Harlan Laboratories, Indianapolis, IN). Animal protocols were in accordance with National Institutes of Health guidelines and approved and monitored by the Medical College of Georgia Institutional Animal Care and Use Committee. Rats were housed under conditions of constant temperature and humidity in a 12:12-h light-dark cycle and fed normal rat chow diet (Harlan Teklad Global diets, Wilmington, DE) throughout the experiment. Rats were allowed to adapt to these conditions for several days before starting any experimental procedure. At the beginning of each experiment, rats were placed in metabolic cages to allow for a baseline 24-hour urine collection. Rats were then anesthetized with isofluorane and osmotic minipumps (Alzet model 2002) were implanted (s.c.) to deliver Ang II at a dose of 65 ng/min (Phoenix Pharmaceutical Inc., Belmont, CA) for 14 days as previously described (Sasser et al., 2002). Rats were then divided into 4 groups (n=11 per group) and allowed to drink tap water or water containing the ACE inhibitor, enalapril (10 mg/kg/day), the SOD mimetic, tempol (2 mM), or both tempol and enalapril simultaneously with Ang II infusion until the end of experiment (both from Sigma Chemical Co., St. Louis, MO). Drinking solutions were prepared fresh every 1-2 days and shielded from light. Rats were again placed in metabolic cages during days 13 and 14 of Ang II infusion. At the end of the experiment, rats were anesthetized using sodium pentobarbital (65 mg/kg i.p., Abbott Laboratories, North Chicago, IL). A midline incision was used to isolate the abdominal aorta and a 5-8 ml blood sample was quickly drawn. Blood was immediately centrifuged and plasma was separated and frozen at -80°C for later analysis. The thoracic aorta was isolated for determination of superoxide production as described below. Urine samples were also centrifuged to remove any particulate matter and then stored at -80°C for later analysis. Plasma, urine and thoracic aorta were also taken from a separate group of untreated male Sprague-Dawley rats (n=6) and used for determination of 8-isoprostane, urinary peroxide, and lucigenin chemiluminescence, respectively.
Systolic blood pressure was measured during baseline and after one and two weeks of Ang II infusion (n=11/group) using the tail cuff pressure method as previously described (Pollock and Rekito, 1998). A subset of rats from each group (n=3 in each group) were also implanted with telemetry transmitters at least one week prior to beginning the protocol to allow measurement of arterial pressure as previously described (Pollock and Pollock, 2001). Telemetry transmitters (Data Sciences, Inc., St. Paul, MN) were implanted according to manufacturer's specifications while under sodium pentobarbital anesthesia (65 mg/kg, i.p.). Rats were allowed to recover for at least one week. Arterial pressure waveforms were continuously recorded throughout the protocol. After 3-4 days of baseline blood pressure measurements, osmotic minipumps were implanted to deliver Ang II and treatments were given as described above.
To explore role of bradykinin B2 receptor in Ang II hypertension, another group of rats were implanted with telemetry transmitters and received Ang II minipumps as described above. Rats were then divided into 3 groups (n=6-7 per group). The first two groups were allowed to drink tap water with or without enalapril plus tempol. The third group was allowed to drink water containing enalapril plus tempol and osmotic minipumps were implanted (s.c.) to deliver icatibant (a kind gift from Jerini AG, Berlin) at a dose of (500 μg/kg/day) for 14 days simultaneous with Ang II infusion. This dose has been previously shown to effectively block bradykinin B2 receptor in-vivo (Xu et al., 2005).
Urine and plasma analysis
Plasma 8-isoprostane concentrations were measured by enzyme immunoassay (Cayman chemical, Ann Arbor, MI). Urinary hydrogen peroxide was determined using Amplex Red Hydrogen Peroxide/Peroxidase assay kit (Molecular Probes, Eugene, OR).
Chemiluminescent detection of superoxide
Aortic superoxide production was determined as described before (Elmarakby et al., 2005). Following isolation, the thoracic aorta was immediately placed in ice-cold physiological saline solution (PSS) of the following concentrations (mM): NaCl 130, KCl 4.7, KH2PO4 1.8, MgSO4·7H2O 1.17, NaHCO3 14.9, dextrose 5.5, EDTA 0.26, CaCl2 1.6. The aorta was cleaned of visible adventitial tissue and cut into ring segments approximately 2 mm in length. Superoxide was measured by lucigenin chemiluminescence as previously described (Loomis et al., 2005).
Dihydroethidium (DHE) staining
DHE (Molecular Probes, Eugene, OR) is a lipophilic cell-permeable dye that has been used as a tool for specifically measuring superoxide (Somers et al., 2000). Superoxide was also detected by dihydroethidine staining using frozen aortic section as previously described (Elmarakby et al., 2005).
Western blotting for bradykinin B1 receptor
Renal cortex was isolated from separate groups of untreated control and Ang II infused rats (n=3-4) as above and immediately frozen in liquid nitrogen for determination of bradykinin B1 protein expression. Tissues were placed in 2 ml ice cold homogenization buffer (50 mmol/L Tris · HCl, pH 7.4, 0.1 mmol/L EDTA, and 0.1 mmol/L EGTA, 250 mmol/L sucrose, 10% glycerol) in the presence of protease inhibitors (1 mmol/L PMSF, 1 μmol/L pepstatin A, 2 μmol/L leupeptin, and 0.1% aprotinin) and homogenized on ice in the presence of fresh protease inhibitors with a glass-glass homogenizer for 10 strokes. The homogenate was centrifuged at 4°C at 100,000g for 30 minutes to separate into cytosolic and particulate fractions. The particulate fraction was then re-suspended in half original volume of homogenization buffer. Protein concentrations were determined by the colorimetric bicinchoninic acid (BCA) protein assay kit (Pierce Biotechnology, Inc., Rockford, IL) using albumin as the standard. Western blotting protocol was performed as previously described (30) using 100 μg protein for loading gels. The primary antibody was a goat polyclonal anti-B1 receptor M-19 (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and the secondary antibody was horseradish peroxidase-conjugated donkey anti-goat antibody (1:5000; Santa Cruz Biotechnology, Inc.). A second primary antibody to β-actin (1:10000, Sigma) was applied followed by a secondary antibody to goat (1:10000; Santa Cruz Biotechnology, Inc.). Equal protein loading was calculated based on protein concentrations from the BCA assay and verified by β-actin. Specific bands were detected with enhanced chemiluminescence (Supersignal Chemiluminescent Substrate, Pierce Biotechnology, Inc., Rockford, IL). Densitometry was performed using a digital imaging system (Alpha Innotech Corporation).
Statistical analysis
ANOVA for repeated measures and post-hoc contrasts were used for statistical evaluation of systolic blood pressure data (Super ANOVA, Abacus Concepts, Berkeley, CA). Statistical differences among plasma 8-isoprostane and aortic lucigenin measurements were determined by ANOVA and Fisher's protected least significant difference test (StatView, Abacus Concepts, Berkeley, CA). The B1 receptor protein expression data were analyzed by unpaired t-test. Values are reported as mean ± SE with P<0.05 being considered significant.
Results
Infusion of Ang II significantly increased systolic blood pressure when compared with the baseline values (Figure 1A). Neither enalapril nor tempol treatments alone were able to attenuate the elevation in systolic blood pressure. In contrast, combined administration of tempol and enalapril prevented the increase in systolic blood pressure. In a subgroup of rats, telemetry was used to measure mean arterial pressure (MAP) in Ang II infused rats (Figure 1B). Consistent with the tail cuff pressure data, Ang II infusion significantly increased MAP (169±8 mmHg on day 12 vs. 110±4 baseline, P<0.05) while tempol or enalapril treatment alone had little effect on this increase. However, combined administration of tempol and enalapril significantly attenuated the elevation of MAP in Ang II infused rats (128±6 mmHg on day 12).
Figure 1.
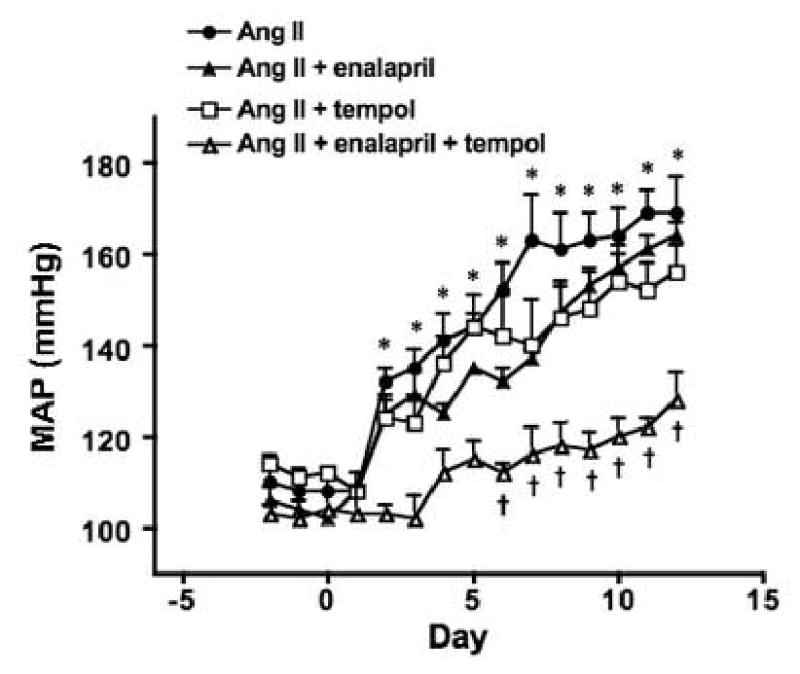
(A) Tail cuff pressure (TCP) used as a measure of systolic arterial pressure in rats infused with Ang II for two weeks with or without tempol, enalapril, or both in the drinking water (n=11/group) (B) Average 24 hours MAP (telemetry) in similarly treated rats (n=3). *P<0.05 vs. day 0 in groups given Ang II, Ang II + tempol, and Ang II + enalapril, †P< 0.05 vs. Ang II group.
Figure 2A shows plasma 8-isoprostane levels in each group of rats. Plasma 8-isoprostane concentrations were significantly higher in Ang II infused rats when compared with measurements from a separate group of untreated Sprague-Dawley rats (69±14 vs. 23± 0.5 pg/ml, P<0.05). Enalapril had no effect on the Ang II induced increase in plasma 8-isoprostane (65±15 pg/ml) while tempol treatment alone or tempol plus enalapril significantly attenuated the increase in plasma 8-isoprostane (29±6 and 34±7 pg/ml, respectively).
Figure 2.
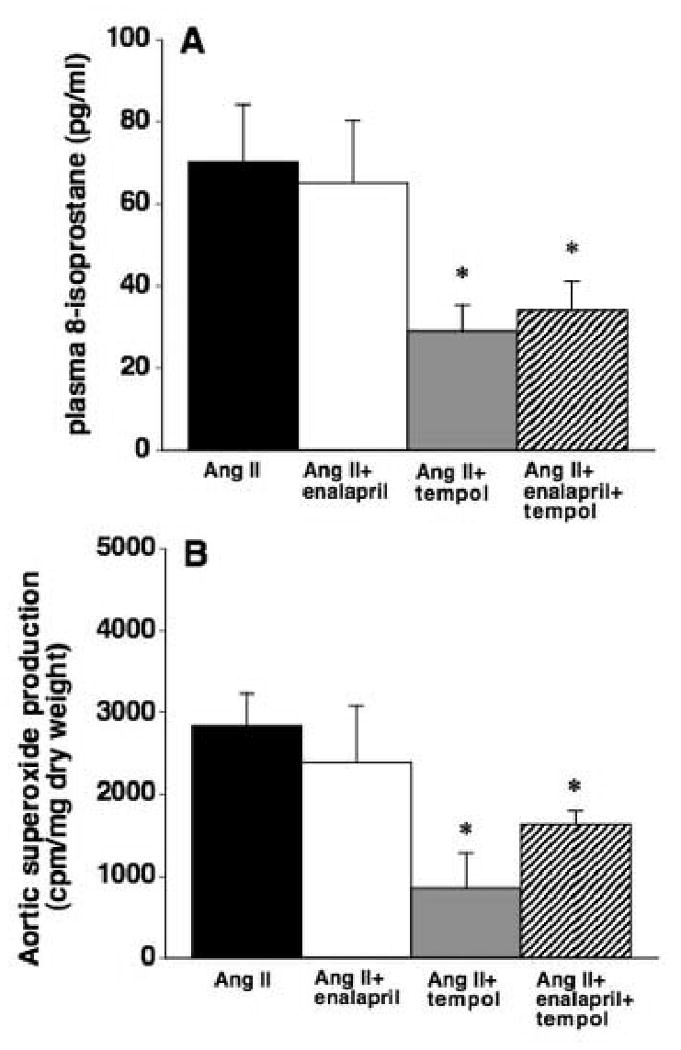
(A) Plasma 8-isoprostane levels in rats infused with Ang II for two weeks with or without enalapril, tempol, or both in the drinking water (n=10-15). (B) Aortic superoxide production in rats infused with Ang II for two weeks with or without enalapril, tempol, or both in the drinking water (n=8-10). *P< 0.05 vs. Ang II group.
Aortic superoxide production was determined using the lucigenin chemiluminescence method. As shown in Figure 2B, Ang II infusion significantly increased aortic superoxide production compared with measurements from a separate group of untreated Sprague-Dawley rats (2842±384 vs. 1691±90 cpm/mg dry wt, P<0.05). Following a similar pattern as 8-isoprostane data, enalapril treatment failed to attenuate the elevation in aortic superoxide produced by Ang II (2392±690 cpm/mg dry wt) yet tempol alone or in combination with enalapril significantly abrogated the increase (847±433 and 1634±164 cpm/mg dry wt, respectively).
DHE staining was also used as a qualitative method for detection of superoxide in frozen aortic sections (Figure 3). Similar to previous observations, considerable staining intensity was evident in the aorta of Ang II treated rats. Consistent with 8-isoprostane and lucigenin measurements, enalapril appeared to have no effect on DHE staining while tempol treatment, with or without enalapril, clearly reduced staining intensity.
Figure 3.
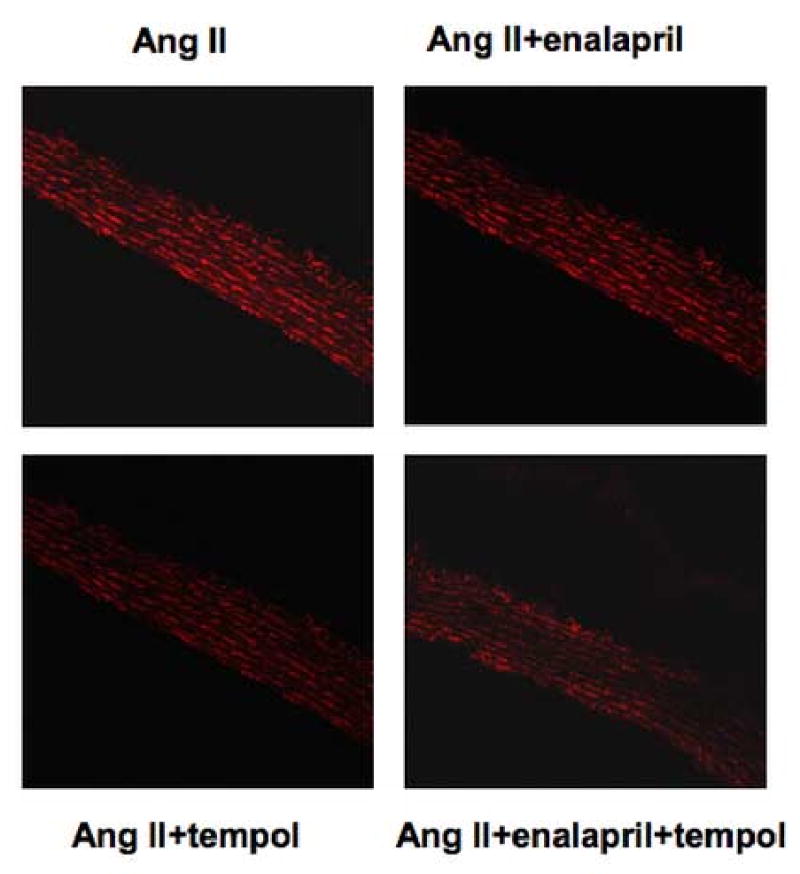
Superoxide detection using the DHE staining in rat thoracic aortic sections from rats infused with Ang II for two weeks with or without enalapril, tempol, or both (n=5-6).
Urinary hydrogen peroxide excretion was significantly greater in rats receiving chronic Ang II compared to values obtained from a separate group of untreated Sprague-Dawley rats (29±11 vs. 6±1 nmol/day, P<0.05). Enalapril treatment had no significant effect on the increase in hydrogen peroxide excretion in Ang II hypertensive rats (49±17 nmol/day, Figure 4). Hydrogen peroxide excretion was significantly increased in the tempol treated group, but this effect was attenuated by the combined administration of enalapril and tempol (66±24 and 22±10 nmol/day, respectively).
Figure 4.
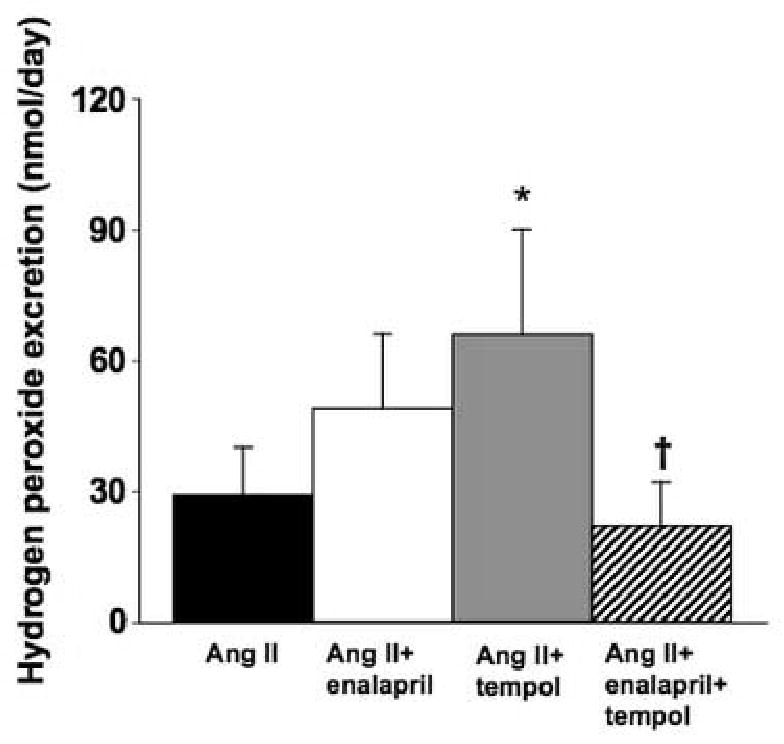
Urinary hydrogen peroxide excretion in Ang II infused rats given either tap water or water containing enalapril, tempol, or both to drink for 2 weeks (n=6-7). *P< 0.05 vs. Ang II and †P< 0.05 vs. Ang II plus tempol.
A separate series of rats were treated with or without the bradykinin B2 receptor antagonist, icatibant, to investigate the role of the bradykinin in the actions of combined enalapril and tempol administration in Ang II infused rats. Consistent with our initial data, Ang II infusion increased MAP (155±8 mmHg on day 12) and combined administration of tempol plus enalapril prevented this effect (129±9 mmHg on day 12, P<0.05 vs. Ang II only). Concurrent administration of icatibant reduced the ability of tempol plus enalapril to lower MAP in Ang II infused rats (147±10 mmHg on day 12; Figure 5A). However, icatibant had no effect on the ability of combined tempol and enalapril administration to decrease plasma 8-isoprostane (Figure 5B).
Figure 5.
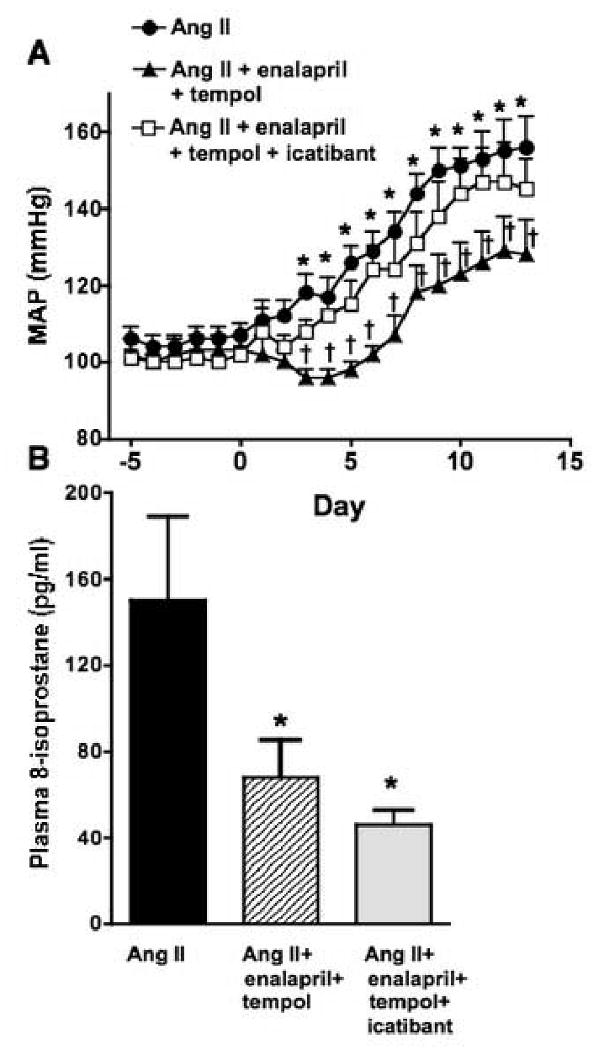
(A) Average 24 hours MAP (telemetry) in Ang II rats that were drinking tap water, water containing tempol plus enalapril, or water containing tempol plus enalapril in addition to icatibant infusion (s.c.) for 2 weeks (n=6-7 per group). *P< 0.05 vs. day 0 and †P< 0.05 vs. Ang II group. (B) Plasma 8-isoprostane levels in Ang II infused rats with or without enalapril + tempol, or tempol + enalapril + icatibant for 2 weeks (n=3-4 animals per group; *P< 0.05 vs. Ang II group).
To explore the possibility that B1 receptors may be up regulated in Ang II hypertensive model, B1 receptor expression was measured in the renal cortex of control and Ang II infused rats. Densitometric analysis (normalized to β-actin expression) revealed a significant increase in bradykinin B1 receptor protein expression in the kidneys of Ang II hypertensive rats compared to untreated control rats (Figure 6).
Figure 6.
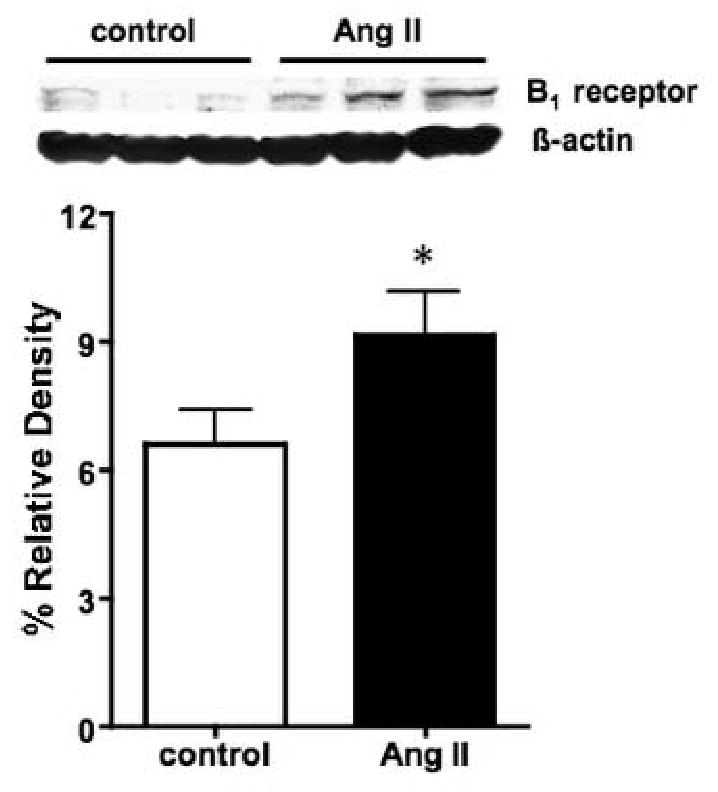
Representative Western blot measuring bradykinin B1 receptor protein expression in renal cortex isolated from Ang II and untreated control rats. A total of 100 μg of protein was loaded per lane. Relative densitometry normalized to β-actin is also shown in the bar graph (n=3-4). *P< 0.05 vs. control.
Discussion
Consistent with previous findings, results from the present study demonstrate that chronic Ang II infusion significantly increased blood pressure and oxidative stress. Enalapril had no effect on chronic Ang II hypertension. Despite reducing 3 distinct measure of oxidative stress, tempol also had no effect on the blood pressure increase in this model. Combined administration of enalapril and tempol attenuated the elevation in blood pressure in addition to reducing measures of vascular oxidative stress. In contrast to previous reports using solubilized SOD (Laursen et al., 1997), or lower doses of Ang II (Ortiz el al., 2001), our findings indicate that reducing aortic superoxide alone is not sufficient to reverse chronic Ang II hypertension. Our results also provide evidence that increasing kinin survival enhances the blood pressure lowering effects of the SOD mimetic.
We have previously shown that the ACE inhibitor, enalapril, but not angiotensin receptor blockade, attenuated the increase in blood pressure produced by acute endothelin-1 infusion via increased kinin survival (Elmarakby et al., 2003). Additionally, we and others have also demonstrated that chronic Ang II infusion is associated with increased ET-1 levels (Alexander et al., 2001; Sasser et al., 2002). Thus, we used the chronic Ang II model, as an example of high ET-1 model to examine the influence of kinins via kininase inhibition since Ang II levels are already high and inhibition of the angiotensin converting enzyme aspect of enalapril would be irrelevant. Enhancing endogenous kinin levels with enalapril had no effect on the elevation in MAP produced by Ang II indicating that the increase in kinin survival alone does not attenuate chronic Ang II hypertension. These results are similar to those of Pasquié et al. showing that chronic administration of exogenous bradykinin can attenuate chronic Ang II induced increases in renal vascular resistance, but has no effect on blood pressure (Pasquié et al., 2001). However, the current study suggests that the combination of increasing endogenous bradykinin and reducing superoxide is sufficient to overcome the hypertension produced by chronic Ang II. In agreement with our data, a recent study has shown that enalapril plus tempol reduced blood pressure in Dahl salt-sensitive rats although the authors attributed this effect to the decrease in Ang II levels in this model (Bayroh et al., 2006).
A somewhat surprising aspect of our experiments was that the membrane-permeable SOD mimetic, tempol, did not have any effect on the blood pressure elevation in Ang II rats when given alone. Several studies have shown that acute administration of tempol can inhibit or reduce the hemodynamic actions of acute or chronic Ang II (Nishiyama el al., 2001; Zhang et al., 2004; Lopez et al., 2003). Using long-term tempol treatment, Ortiz et al. found that tempol at 1 mM in the drinking water blocked Ang II induced hypertension and oxidative stress (Ortiz et al., 2001). However, the ability of tempol to lower arterial pressure in their studies may be related to the very low dose of Ang II (5 ng/kg/min) compared to the current study (approximately 200 ng/kg/min). In the mouse, Kawada et al. observed that tempol could inhibit Ang II hypertension using a dose of 400 ng/kg/min whereas our dose was approximately 300 ng/kg/min (Kawada et al., 2002). However, Ang II in the mouse produces a far less potent hypertensive response compared to the rat. The “effective” dose in their study would appear to be far less since Ang II does not increase blood pressure in control mice at this dose until after the 9th day of treatment. Therefore, it appears as though the anti-hypertensive actions of tempol are limited to a modest degree of Ang II dependent hypertension. It is not clear whether there are other factors that limit tempol's activity since Laursen et al. have shown that liposome-encapsulated SOD is effective at preventing the hypertension produced by a high dose of Ang II in the rat (Laursen et al., 1997). In other models of hypertension where Ang II activity is not expected to be very high such as DOCA-salt, Dahl salt-sensitive rat, and SHR, oral administration of 1-2 mM concentration of tempol in the drinking water significantly attenuated the elevation in blood pressure and oxidative stress (Schnackenberg and Wilcox 1999; Beswick et al., 2001; Hoagland et al., 2003). Tempol may have non-specific actions independent of reducing superoxide, but this possibility has not been confirmed (Xu et al., 2004).
Consistent with observations in the current study, Bell et al. have provided evidence for a synergistic action of ACE inhibition and antioxidant treatment. These investigators have shown that pressure overload-induced left ventricular hypertrophy of guinea pigs is associated with endothelial dysfunction, elevated plasma Ang II and ET-1 levels, and increased tissue NADPH dependent superoxide production (Bell et al., 2001). These changes were inhibited by treatment with the ACE inhibitor, quinapril, and vitamin C, either alone or in combination, but did not lower arterial pressure. However, combined administration of quinapril and vitamin C provided a greater effect than either treatment alone in reducing superoxide production. Taken together with the current study, these observations suggest that reduction of Ang II activity either directly or through enhancement of kinin activity may improve the anti-hypertensive actions of antioxidant treatment. One possible explanation for the synergism between tempol and enalapril may be that Ang II–induced release of vascular superoxide inactivates endothelial-derived vasodilators, such as NO and arachidonic acid metabolites, which can be compensated for by increasing kinin activity. Previous studies have reported that increases in vascular superoxide production are associated with an impaired vascular relaxation response to acetylcholine, nitroglycerin, and nitroprusside in Ang II–infused hypertensive rats (Rajagopalan et al., 1996; Laursen et al., 1997). Further studies are needed to discern the mechanisms of interaction between the actions of enalapril and tempol.
Generation of hydrogen peroxide may be an explanation as to how tempol could lower superoxide and not reduce blood pressure. Makino et al. have recently shown that direct infusion of hydrogen peroxide into the renal medulla increased MAP (Makino et al., 2003). They also showed that tempol increased renal hydrogen peroxide levels. Our laboratory has also observed that chronic tempol treatment in rats increases urinary hydrogen peroxide excretion during hypertension associated with high salt and endothelin B receptor blockade, but not in normotensive controls (Williams et al., 2004). In agreement with these findings, we observed that urinary hydrogen peroxide excretion was significantly elevated in tempol treated rats compared to those receiving Ang II alone. Therefore, it is possible that the antihypertensive actions of tempol are limited in chronic studies because of elevations in renal hydrogen peroxide levels. Although the mechanism is yet unknown, it is interesting that enalapril treatment significantly inhibited the tempol-induced increase in urinary hydrogen peroxide. It is possible that the inhibitory effect of enalapril on tempol-induced increases in hydrogen peroxide may account for the effectiveness of combined drug treatment to lower arterial pressure although this will need to be investigated.
One potential explanation for the synergistic actions of enalapril plus tempol in the Ang II infused rat is through increased B2 receptor activation, an established NO-dependent vasodilator. To explore this possibility, we used the bradykinin B2 receptor antagonist, icatibant, to determine if B2 receptor blockade would restore the hypertension during enalapril plus tempol administration. Indeed, icatibant decreased the anti-hypertensive actions of enalapril plus tempol. Previous studies have shown that bradykinin B2 receptor activation contributes to the antihypertensive and antifibrotic effects of ACE inhibitors in Ang II-dependent hypertension (Rossi et al., 2002; Seccia et al., 2006). In agreement with these studies, our data suggest that increased kinin survival along with B2 receptor activation is an important mechanism that allows enalapril to enhance the effect of tempol in Ang II infused rats.
The B2 receptor is widely expressed and mediates the vasodilator and antihypertensive actions of kinins under most physiological conditions (Marceau et al., 1998). In contrast, the B1 receptor functions similar to the B2 receptor but typically is not expressed except during certain pathological conditions such as ischemia, cardiovascular diseases, or exposure to inflammatory cytokines (Linz et al., 1995; McLean et al., 2000; Duka el al., 2001). Therefore, it is possible that increased B1 receptor activity in Ang II infused rats given enalapril may enhance the blood pressure lowering effects of tempol. Unfortunately, B1 specific receptor antagonists are not readily available for chronic in vivo studies and so we could not directly determine the degree of B1 receptor involvement. Nonetheless, we observed increased B1 receptor expression in the renal cortex of Ang II infused rats and so it is possible that enhanced B1 receptor activation could account for the enhanced anti-hypertensive effects of enalapril plus tempol. Furthermore, previous cell culture studies have demonstrated that enalapril in nanomolar concentrations can directly activate the human or bovine B1 receptor at a different extracellular domain than the native peptide (Ignjatovic el al., 2002). There is also evidence that ACE inhibition can induce expression of the B1 receptor in rodent kidney, heart, and vasculature and that B1 receptor stimulation plays a role in the hypotensive effect of ACE inhibitors (Marin-Castano et al., 2002). Thus, activation of the B1 receptor by enalapril could account for the enhanced antihypertensive effect during tempol treatment during Ang II induced hypertension. Another possibility that will need to be explored in future studies is whether the synergistic actions of enalapril plus tempol is through an increase in Ang-(1-7) activity. Ang-(1-7) is a major metabolite of Ang II that increases during ACE inhibition and can have antihypertensive actions (Tom et al., 2003).
In summary, results from the current study support the hypothesis that reducing vascular superoxide is not sufficient to prevent hypertension produced by chronic Ang II infusion. Our results also suggest that enhancement of endogenous kinin activity in combination with reducing superoxide facilitates blood pressure lowering in this model. The mechanism of the synergistic effects of tempol and enalapril appears to be via the increase in B2 receptor. However, a role for the B1 receptor or other undefined actions of these drugs remain possible.
Acknowledgments
The authors wish to express their appreciation for the expert technical assistance provided by Mr. Hiram Ocasio and Ms. Janet Hobbs.
These studies were supported by grants from the National Heart Lung and Blood Institute (HL64776, HL74167, HL60653), American Heart Association Established Investigator Awards to D. Pollock and J. Pollock, and Pre- and post-doctoral Fellowships from Southeast Affiliate of the American Heart Association awarded to A. Elmarakby.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander BT, Cockrell KL, Rinewalt AN, Herrington JN, Granger JP. Enhanced renal expression of preproendothelin mRNA during chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1388–R1392. doi: 10.1152/ajpregu.2001.280.5.R1388. [DOI] [PubMed] [Google Scholar]
- Bayorh MA, Mann G, Walton M, Eatman D. Effects of enalapril, tempol, and eplerenone on salt-induced hypertension in Dahl salt-sensitive rats. Clin Exp Hypertens. 2006;28:121–32. doi: 10.1080/10641960500468276. [DOI] [PubMed] [Google Scholar]
- Bell JP, Mosfer SI, Lang D, Donaldson F, Lewis MJ. Vitamin C and quinapril abrogate LVH and endothelial dysfunction in aortic-banded guinea pigs. Am J Physiol Heart Circ Physiol. 2001;281:H1704–H1710. doi: 10.1152/ajpheart.2001.281.4.H1704. [DOI] [PubMed] [Google Scholar]
- Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension. 2001;37:781–786. doi: 10.1161/01.hyp.37.2.781. [DOI] [PubMed] [Google Scholar]
- Duka I, Kintsurashvili E, Gavras I, Johns C, Bresnahan M, Gavras H. Vasoactive potential of the B1 bradykinin receptor in normotension and hypertension. Circ Res. 2001;88:275–281. doi: 10.1161/01.res.88.3.275. [DOI] [PubMed] [Google Scholar]
- Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension. 2005;45:283–287. doi: 10.1161/01.HYP.0000153051.56460.6a. [DOI] [PubMed] [Google Scholar]
- Elmarakby AA, Morsing P, Pollock DM. Regulation of cardiovascular signaling by kinins and products of similar converting-enzyme systems: enalapril attenuates endothelin-1-induced hypertension via increased kinin survival. Am J Physiol Heart Circ Physiol. 2003;284:H1899–H1903. doi: 10.1152/ajpheart.00027.2003. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Hoagland KM, Maier KG, Roman RJ. Contributions of 20-HETE to the antihypertensive effects of Tempol in Dahl salt-sensitive rats. Hypertension. 2003;41:697–702. doi: 10.1161/01.HYP.0000047881.15426.DC. [DOI] [PubMed] [Google Scholar]
- Ignjatovic T, Tan F, Brovkovych V, Skidgel RA, Erdos EG. Novel mode of action of angiotensin I converting enzyme inhibitors: direct activation of bradykinin B1 receptor. J Biol Chem. 2002;277:16847–16852. doi: 10.1074/jbc.M200355200. [DOI] [PubMed] [Google Scholar]
- Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002;13:2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- Linz W, Wiemer G, Gohlke P, Unger T, Scholkens BA. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled NOS in the rat aorta. J Pharmacol Exp Ther. 2005;315:1058–1064. doi: 10.1124/jpet.105.091728. [DOI] [PubMed] [Google Scholar]
- Lopez B, Salom MG, Arregui B, Valero F, Fenoy FJ. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension. 2003;42:1150–1156. doi: 10.1161/01.HYP.0000101968.09376.79. [DOI] [PubMed] [Google Scholar]
- Makino A, Skelton MM, Zou AP, Cowley AW., Jr Increased renal medullary H2O2 leads to hypertension. Hypertension. 2003;42:25–30. doi: 10.1161/01.HYP.0000074903.96928.91. [DOI] [PubMed] [Google Scholar]
- Marceau F, Hess JF, Bachvarov DR. The B1 receptors for kinins. Pharmacol Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- Marin-Castano ME, Schanstra JP, Neau E, Praddaude F, Pecher C, Ader JL, Girolami JP, Bascands JL. Induction of functional bradykinin B1 receptors in normotensive rats and mice under chronic angiotensin-converting enzyme inhibitor treatment. Circulation. 2002;105:627–632. doi: 10.1161/hc0502.102965. [DOI] [PubMed] [Google Scholar]
- McLean PG, Perretti M, Ahluwalia A. Kinin B1 receptors and the cardiovascular system: regulation of expression and function. Cardiovasc Res. 2000;48:194–210. doi: 10.1016/s0008-6363(00)00184-x. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Harrison DG. The AT1-type angiotensin receptor in oxidative stress and atherogenesis: Part II: AT1 receptor regulation. Circulation. 2002;105:530–536. doi: 10.1161/hc0402.102619. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, Abe Y. Systemic and regional hemodynamic responses to tempol in angiotensin II-Infused hypertensive rats. Hypertension. 2001;37:77–83. doi: 10.1161/01.hyp.37.1.77. [DOI] [PubMed] [Google Scholar]
- Ortiz MC, Manriquez MC, Romero JC, Juncos LA. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension. 2001;38:655–659. doi: 10.1161/01.hyp.38.3.655. [DOI] [PubMed] [Google Scholar]
- Pasquie JL, Herizi A, Jover B, Mimran A. Chronic bradykinin infusion and receptor blockade in angiotensin II hypertension in rats. Hypertension. 1999;33:830–834. doi: 10.1161/01.hyp.33.3.830. [DOI] [PubMed] [Google Scholar]
- Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol. 2001;281:F144–F150. doi: 10.1152/ajprenal.2001.281.1.F144. [DOI] [PubMed] [Google Scholar]
- Pollock DM, Rekito A. Hypertensive response to chronic NO synthase inhibition is different in Sprague-Dawley rats from two suppliers. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1719–R1723. doi: 10.1152/ajpregu.1998.275.5.R1719. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:R893–R912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- Rossi GP, Cavallin M, Rizzoni D, Bova S, Mazzocchi G, Agabiti-Rosei E, Nussdorfer GG, Pessina AC. Dual ACE and NEP inhibitor MDL-100, 240 prevents and regresses severe angiotensin II-dependent hypertension partially through bradykinin type 2 receptor. J Hypertens. 2002;20(7):1451–9. doi: 10.1097/00004872-200207000-00034. [DOI] [PubMed] [Google Scholar]
- Sasser JM, Pollock JS, Pollock DM. Renal endothelin in chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol. 2002;283:R243–R248. doi: 10.1152/ajpregu.00086.2002. [DOI] [PubMed] [Google Scholar]
- Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension. 1999;33:424–428. doi: 10.1161/01.hyp.33.1.424. [DOI] [PubMed] [Google Scholar]
- Seccia TM, Belloni AS, Guidolin D, Sticchi D, Nussdorfer GG, Pessina AC, Rossi GP. The renal antifibrotic effects of angiotensin-converting enzyme inhibition involve bradykinin B2 receptor activation in angiotensin II-dependent hypertension. J Hypertens. 2006;24(7):1419–27. doi: 10.1097/01.hjh.0000234124.94013.ac. [DOI] [PubMed] [Google Scholar]
- Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–1728. doi: 10.1161/01.cir.101.14.1722. [DOI] [PubMed] [Google Scholar]
- Sullivan JC, Pollock DM, Pollock JS. Altered nitric oxide synthase 3 distribution in mesenteric arteries of hypertensive rats. Hypertension. 2002;39:597–602. doi: 10.1161/hy0202.103286. [DOI] [PubMed] [Google Scholar]
- Tom B, Dendorfer A, Jan Danser AH. Bradykinin, angiotensin-(1-7), and ACE inhibitors: how do they interact? The international Journal of Biochemistry & Cell Biology. 2003;35:792–801. doi: 10.1016/s1357-2725(02)00273-x. [DOI] [PubMed] [Google Scholar]
- Williams JM, Pollock JS, Pollock DM. Arterial pressure response to the antioxidant tempol and ETB receptor blockade in rats on a high-salt diet. Hypertension. 2004;44:770–775. doi: 10.1161/01.HYP.0000144073.42537.06. [DOI] [PubMed] [Google Scholar]
- Xu J, Carretero OA, Sun Y, Shesely EG, Rhaleb NE, Liu YH, Liao TD, Yang JJ, Bader M, Yang XP. Role of the B1 kinin receptor in the regulation of cardiac function and remodeling after myocardial infarction. Hypertension. 2005;45:747–753. doi: 10.1161/01.HYP.0000153322.04859.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- Xu H, Fink GD, Galligan JJ. Tempol lowers blood pressure and sympathetic nerve activity but not vascular O2- in DOCA-salt rats. Hypertension. 2004;43:329–334. doi: 10.1161/01.HYP.0000112304.26158.5c. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Abe Y. ROS during the acute phase of Ang II hypertension participates in cardiovascular MAPK activation but not vasoconstriction. Hypertension. 2004;43:117–124. doi: 10.1161/01.HYP.0000105110.12667.F8. [DOI] [PubMed] [Google Scholar]


