Figure 5. Recombinant ERAAP Trims the Final QL9 as well as Its X6[QL9] Precursor In Vitro, and Ld MHC Can Protect QL9 Peptide.
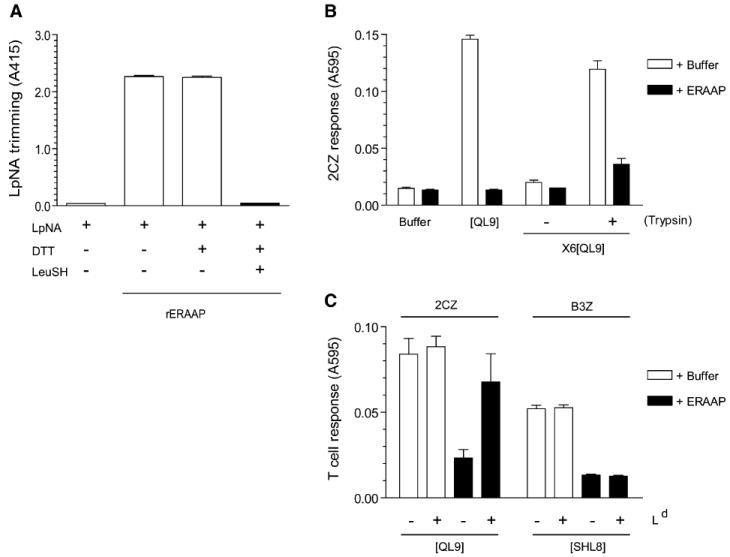
(A) Purified recombinant ERAAP (rERAAP) is active in trimming the model LpNA substrate, and leucinethiol inhibits this activity. The rERAAP purified from baculovirus-infected insect cells was incubated with Leucine pnitroanilide (LpNA) with or without leucinethiol (LeuSH) and DTT, which is required to keep LeuSH in its reduced and active form. The LpNA hydrolysis was measured as light absorbance at 415 nm.
(B) The indicated synthetic peptides were incubated with either buffer alone or with rERAAP. The peptides were assayed by their ability to stimulate 2CZ T cells in the presence of Ld-L cells as APCs. To detect the generation of QL9 peptide from its N-terminally extended X6[QL9] precursor, the peptides were either assayed as such or after trypsin treatment.
(C) The Ld MHC protects QL9 but not SHL8 peptide from degradation by rERAAP. Synthetic QL9 or SHL8 peptides were incubated with buffer or rERAAP in the presence or absence of recombinant Ld-Ig fusion protein. The QL9 and SHL8 peptides were assayed with 2CZ or B3Z T cells and Ld or Kb-L cells as APCs, respectively. The graph shows the mean values with SD from three independent experiments (A–C).
