Abstract
Recent demonstrations that positive modulators of AMPA-type glutamate receptors (ampakines) increase neuronal brain-derived neurotrophic factor (BDNF) expression have suggested a novel strategy for treating neurodegenerative diseases. However, reports that AMPA and BDNF receptors are down-regulated by prolonged activation raise concerns about the extent to which activity-induced increases in BDNF levels can be sustained without compromising glutamate receptor function. The present study constitutes an initial test of whether ampakines can cause enduring increases in BDNF content and signaling without affecting AMPA receptor (AMPAR) expression. Prolonged (12–24 h) treatment with the ampakine CX614 reduced AMPAR subunit (GluR1-3) mRNA and protein levels in cultured hippocampal slices whereas treatment with AMPAR antagonists had the opposite effects. The cholinergic agonist carbachol also depressed GluR1-3 mRNA levels, suggesting that AMPAR down-regulation is a global response to extended periods of elevated neuronal activity. Analyses of time courses and thresholds indicated that BDNF expression is influenced by lower doses of, and shorter treatments with, the ampakine than is AMPAR expression. Accordingly, daily 3 h infusions of CX614 chronically elevated BDNF content with no effect on GluR1-3 concentrations. Restorative deconvolution microscopy provided the first evidence that chronic up-regulation of BDNF is accompanied by increased activation of the neurotrophin’s TrkB receptor at spine synapses. These results show that changes in BDNF and AMPAR expression are dissociable and that up-regulation of the former leads to enhanced trophic signaling at excitatory synapses. These findings are thus encouraging with regard to the feasibility of using ampakines to tonically enhance BDNF-dependent functions in adult brain.
Keywords: AMPA receptor modulator, synaptic scaling, gene expression, TrkB, histone deacetylase, neurotrophin
Introduction
Brain-derived neurotrophic factor (BDNF) promotes synaptic plasticity (Kang and Schuman, 1995, Kramár et al., 2004, Kuipers and Bramham, 2006) and is protective in animal models of brain injury and of neurodegenerative diseases. Accordingly, there is an intense and ongoing effort to find physiologically plausible means for increasing BDNF supply in adult brain, either through delivery of exogenous factor or by amplifying synthesis. The discovery that BDNF expression is positively regulated by neuronal activity (Zafra et al., 1990, Isackson et al., 1991, Gall and Lauterborn, 2000) pointed to the possibility of using positive modulation of excitatory transmission for the latter purpose and tests of this idea were successful. Ampakines, a family of compounds that slow the deactivation and desensitization of AMPA-type glutamate receptors and thereby enhance fast excitatory transmission (Staubli et al., 1994a, Lynch and Gall, 2006, Arai and Kessler, 2007), markedly increase expression of BDNF and nerve growth factor (Lauterborn et al., 2000). Up-regulation is triggered by structurally distinct families of positive modulators (Legutko et al., 2001, Dicou et al., 2003) and occurs in vivo after peripheral administration (Lauterborn et al., 2000, Mackowiak et al., 2002, Dicou et al., 2003). There is also evidence that in vivo ampakine treatment reduces neuronal death in animal models of Parkinson’s disease (O’Neill et al., 2004a) and excitotoxic brain damage (Bahr et al., 2002, Dicou et al., 2003), and can reverse deficits in hippocampal long-term potentiation (LTP) (Rex et al., 2006, Simmons et al., 2008); in the latter instances, improved survival and function were associated with elevated BDNF (Dicou et al., 2003, Rex et al., 2006, Simmons et al., 2008).
However, other studies have identified counterbalancing, homeostatic changes that return synaptic strength to normal levels in the face of chronic increases or decreases in neuronal activity. Such bidirectional processes of synaptic scaling have been demonstrated for AMPAR-mediated transmission after prolonged exposure to TTX or GABA receptor antagonists, to cite two examples (O’Brien et al., 1998, Turrigiano et al., 1998). Although the observed effects could in principle have a pre- or post-synaptic locus, altered responses to applied glutamate as well as changes in dendritic protein levels (O’Brien et al., 1998, Turrigiano et al., 1998) suggest that synaptic scaling is predominantly postsynaptic (Wierenga et al., 2005). There is evidence that prolonged exposure to AMPAR modulators elicits compensatory changes in the postsynaptic machinery as well. Continuous incubation of cultured hippocampal slices with the ampakine CX614 rapidly increased BDNF mRNA (over 3–12 h) but this was followed by a gradual decrease to control values over the next 36 hours (Lauterborn et al., 2000). A similar time course was described for a structurally distinct AMPAR modulator (LY392098) in studies using dissociated hippocampal neurons (Legutko et al., 2001). Further analysis of slice cultures showed that the fall in BDNF expression was accompanied by decreases in AMPAR (GluR1, GluR2) mRNA and protein levels (Lauterborn et al., 2003, Jourdi et al., 2005) suggesting that the decline in the ampakine response resulted, in part, from a loss of target receptors.
These findings raise the possibility that negative scaling of AMPAR-mediated transmission constitutes a limit on the use of ampakines for generating chronic increases in neurotrophin levels. Evaluation of the issue requires information on whether and to what degree the drug’s effects on BDNF and AMPAR gene expression are dissociable with different treatment regimens. Also of critical importance is the largely unexplored question of whether BDNF signaling at synapses is actually enhanced by elevating brain concentrations of the neurotrophin and, if so, does the effect undergo time-dependent compensatory changes. The present studies addressed the first of these points by asking if near-threshold conditions for increasing BDNF expression have detectable effects on AMPAR concentrations and then determining if predicted treatment regimens could sustain BDNF protein levels for days without altering AMPAR protein levels. Restorative deconvolution microscopy and double immunofluorescence labeling were then used to test if the up-regulation of BDNF content increases activation of the neurotrophin’s TrkB receptors at excitatory synapses in hippocampal field CA1. Results of these studies indicate that appropriate ampakine treatment regimens produce marked and sustained increases in BDNF protein, and BDNF signaling at glutamatergic synapses, without detectably affecting AMPAR protein levels.
Experimental Procedures
Preparation of Cultured Hippocampal Slices
Cultured hippocampal-entorhinal slices were prepared from Sprague-Dawley rat pups (9–11 d postnatal; Simonsen Labs, Gilroy, CA) (n = 40) as previously described (Lauterborn et al., 2000). The cultured slices included hippocampus, entorhinal cortex and portions of the adjacent neocortex. For each rat pup, slices from both hippocampi were explanted onto 4 Millicel-CM biomembrane inserts (Millipore, St. Louis, MO) with 4 slices/insert and maintained with medium containing 20% horse serum (pH 7.2); unless otherwise stated, reagents were obtained from Sigma (St. Louis, MO). The slices were maintained in an interface configuration in a humidified incubator at 37°C in 5% CO2 and medium was changed every other day; experiments used explants after 10–14 days in vitro (DIV). All experiments were performed in accordance with NIH R&D Systems, guidelines and protocols were approved by the Institutional Animal Care and Use Committee with care to minimize distress to the animals.
Drug Treatments
The positive AMPAR modulator CX614 (a.k.a., LiD37, BDP-37) (Arai et al., 2000, Lauterborn et al., 2000) was generously provided by Cortex Pharmaceuticals, Inc. (Irvine, CA). CX614, GYKI-52466 (1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride] (GYKI), and trichostatin A (TSA) were dissolved in 100% dimethyl sulfoxide (DMSO); carbachol, AMPA and the TrkB ligand scavenger TrkB-Fc (R&D Systems, Minneapolis, MN) were dissolved in serum-free medium. All drug stocks were stored at −20°C. Treatment schedules and drug concentrations are presented in the Results section with each experiment. In all cases, control cultures were treated with equivalent concentrations of vehicle and received the same schedule of medium changes as paired, drug-treated cultures. In preliminary studies, DMSO diluted 1:2000 (the highest concentration used in the present studies) was found to have no effect on mRNA levels relative to naive-control cultures at all time points examined.
Treatments were terminated by tissue fixation (4% paraformaldehyde) for in situ hybridization and immunofluoresence analyses or by freezing for western blots and BDNF immunoassays.
In Situ Hybridization
Fixed hippocampal slices were cryoprotected, and sectioned (20 μm) parallel to the broad explant surface using a freezing microtome. Sections were mounted onto Superfrost Plus slides (Fisher Scientific, Tustin, CA) and processed for in situ hybridization as described (Lauterborn et al., 2000), with the hybridization incubation at 60°C for 16–20 h and the 35S-labeled cRNA probes at 1×107 cpm/ml. The GluR1, GluR2 and GluR3 cRNAs were transcribed from BglI, BamHI, and SalI digests of p59/2, pRB14, and pRB312, respectively (Gold et al., 1997): the cDNAs are complementary to 895, 900, and 720 bases, respectively, of the 3′-ends including non-coding regions. Previous work showed there is no cross-hybridization between these subunit cRNAs and the heterotypic GluR mRNAs (Gold et al., 1997). After the final post-hybridization wash, the tissue was air-dried and processed for Biomax film (Eastman Kodak, Rochester, NY) autoradiography with exposure times of 1–2 days. In situ hybridization labeling densities were measured from film autoradiograms, and calibrated relative to film images of commercial 14C-labeled standards (American Radiochemicals Inc., St. Louis, MO) using the AIS imaging system (Imaging Research Inc., St. Catherines, Ontario) as described (Lauterborn et al., 2000). Hybridization densities were measured for the internal blade of dentate gyrus stratum granulosum, CA1b-c and CA3a-b stratum pyramidale, and, as an estimate of background, the internal blade of the dentate molecular layer.
Western Blot Analyses
Homogenates were prepared from previously frozen hippocampal slice cultures by tip sonication in 0.32 M sucrose, 1 mM EDTA, 0.1 M Tris (pH 7.4) and protease inhibitor cocktail (PIC Complete™, Amersham Pharmacia Biotech.). Sample protein levels were adjusted to normalize μg/ml protein content. Samples were then diluted 1:1 by volume with 2× sample buffer (10% glycerol, 0.5% sodium dodecylsulfate, 62.5 mM Tris (pH 6.9), 5% 2-mercaptoethanol, and 0.1% bromophenol blue), boiled 10 min, separated by 7.5% PAGE (30 μg/lane) and transferred onto polyvinylidene difluoride membranes (Hybond-P™, Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were blocked in 5% non-fat dry milk, 1% bovine serum albumin in Tris-buffered saline for 1 h at room temperature and then incubated overnight with primary antibodies in Tris-buffered saline containing 5% bovine serum albumin at 4°C. Blots were probed with rabbit polyclonal antisera to GluR1 and GluR2 (AB1504 and AB1768, respectively; Chemicon, Temecula, CA) at 1:1000 dilution with either monoclonal anti-β actin at 1:200,000 (clone AC-15; Sigma) or polyclonal anti-β tubulin at 1:400,000 (T4026; Sigma) as a loading control. Immunoreactive bands were visualized by enhanced chemiluminescence using the ECL Plus kit and reagents (Amersham Pharmacia Biotech). Band densities were quantified from film using the AIS imaging system (Imaging Research).
BDNF Immunoassay
Cultures were collected into 100 μl of cold lysis buffer (137mM NaCl, 20mM Tris, 10% glycerol, 1mM PMSF, 0.5mM sodium vanadate, 1% NP-40) containing 1 × PIC Complete™. The 4 cultured slices within a treatment well were pooled for each sample assayed. Tissue was tip sonicated in lysis buffer and acidified to pH 2.5 with 1 N HCl (20 min); the pH was neutralized to pH 8.0 with 1 N NaOH and samples were stored at −70°C until assayed. Total BDNF protein content was measured using the BDNF Emax Immunoassay System (Promega, Madison, WI).
Phosphorylated TrkB Immunocytochemistry
Hippocampal slice cultures were treated with 50 μM CX614 or vehicle for two days (3 h/day) and then (i) harvested without further treatment, (ii) treated with 50 μM AMPA for 30 min prior to harvest, or (iii) treated with 1 μg/ml TrkB-Fc (Shelton et al., 1995, Rex et al., 2007) beginning 2 h before harvest. Slices were fixed overnight, subsectioned and slide mounted as for in situ hybridization (above). The tissue was processed for double-immunolabeling using published techniques (Chen et al., 2007) with incubation in a primary antisera cocktail of mouse anti-PSD95 (1:1000; #MA1-045, Affinity Bioreagents) and rabbit anti-phospho (p)-Trk Y490 (1:200; Cell Signaling, #9141) for 24 h at room temperature followed by incubation in a secondary antisera cocktail including Alexafluor anti-rabbit 594 and Alexaflour anti-mouse 488 (Molecular Probes) for 1 h at room temperature. With these procedures, pTrk immunoreactivity (ir) is labeled red and PSD95-ir is labeled green. Control sections were processed through all procedures with individual primary antisera omitted from the first incubation. The Trk antisera (Danzer et al., 2004) detects the autophosphorylation site conserved across Trks A,B, and C but, in the field of analysis (CA1b-c) should primarily (if not only) detect increases in dendritic spine pTrkB because TrkA is not expressed by resident neurons and the ligand for TrkC (NT3) is not expressed by afferents or resident neurons within this aspect of CA1 in vivo (Lauterborn et al., 1995; Vigers et al., 2000) or in cultured hippocampal slices (Gall et al., 2003); i.e., although NT3 expression has been reported for the most medial CA1 region (Ernfors et al., 1990; Phillips et al., 1990; Patterson et al., 1992), the area of expression described in those studies lies medial to the field evaluated here, and appears to correspond to the ‘fasciola cinerea’ as opposed to CA1 stratum pyramidale per se. In contrast, both BDNF and its TrkB receptor are expressed by afferents and resident neurons (Lauterborn et al., 1995) within field CA1 and are upregulated by the ampakine treatments employed (Lauterborn et al., 2000).
Immunostained sections were examined using a Leica DM6000 epifluorescence microscope. Three tissue sections were evaluated from each cultured slice. For each tissue section, a z-stack of digital images were collected at 63× with 0.2 μm z-steps and through a depth of 3 μm; the field of analysis covered an area of 42,000 μm3. Images were used to construct a 3-dimensional montage that was processed for iterative deconvolution (Volocity 4.0 Restorative Deconvolution software, Improvision) to sharpen images of individual immunolabeled spines. Automated systems were then used to count numbers of pTrk-ir spines (i.e., doubly pTrk- and PSD95-ir spine-like puncta) for individual sections as described elsewhere (Chen et al., 2007). Values for sections from each explant were averaged to obtain a mean count of doubly immunoreactive puncta for the sample field in each cultured slice.
Statistical Analyses
The significance of effects of treatments were determined by one or two way analysis of variance (ANOVA) followed by Student-Newman-Keuls (SNK) post hoc test or Student’s t test for paired comparisons. In circumstances where standard deviations were significantly different between the groups (as determined by Bartlett’s test for homogeneity of variances), significance was determined using the Kruskal-Wallis nonparametric ANOVA followed by the Mann-Whitney U test for paired comparisons. Statistical analyses were conducted using Prism software (V3; GraphPad Software, Inc., San Diego, CA) and the 95% confidence level was considered significant. Unless otherwise stated, statistical results presented in the text are for comparison to control values.
RESULTS
Prolonged ampakine treatment decreases AMPA receptor subunit expression
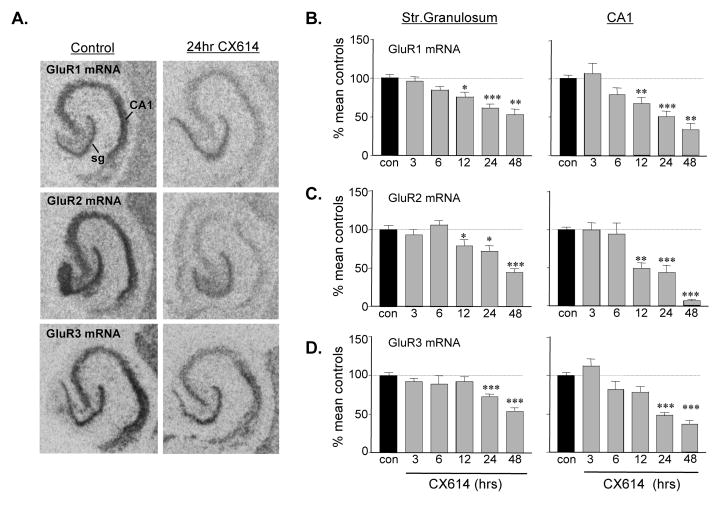
Previous work showed that prolonged treatment with the ampakine CX614 reduces expression of the AMPAR subunits GluR1,2 (Lauterborn et al., 2003, Jourdi et al., 2005) but threshold conditions for eliciting changes in mRNA and protein content were not identified. To this end, the first set of experiments were designed to identify more precisely the time to decline in mRNA and protein content, to extend the analysis to GluR3 gene expression, and to identify potential differences in responses within hippocampus proper and the dentate gyrus. As shown in figure 1A, in situ hybridization labeling of the GluR1-3 transcripts is dense in stratum (str.) granulosum and CA1 str. pyramidale of untreated, control slice cultures and markedly reduced after 24 h treatment with 50 μM CX614. Hybridization densities for groups of slices treated with CX614 for various intervals are shown in figures 1B–D. Decreases in GluR1 and GluR2 mRNAs were significant by 12 h and somewhat greater after 24 h with labeling in experimental slices being reduced to 40–60% of control values. For GluR3 mRNA, hybridization densities were first significantly reduced at 24 h. Forty-eight hour incubations produced further reductions, particularly for the GluR2 transcript (Fig. 1C). There did not appear to be prominent differences in subfield responses with the possible exception of the very dramatic reduction in GluR2 mRNA in the pyramidal cells from 24 to 48 h of CX614 treatment.
Figure 1.
Prolonged CX614 treatment down-regulates AMPAR subunit mRNA levels. A) Film autoradiograms show isotopic in situ hybridization labeling of GluR1-3 mRNAs in hippocampal explants fixed without treatment (Control) or after 24 h of 50 μM CX614 treatment: As shown, CX614 decreased of all three transcript levels in the neuronal layers including stratum (str.) granulosum (sg) and CA1 str. pyramidale (CA1). B–D) Bar graphs show quantification of hybridization densities presented as percent control values for GluR1 (B), GluR2 (C) and GluR3 (D) mRNAs in str. granulosum and CA1 str. pyramidale for groups of slices fixed without treatment (con) or after 3 to 48 h of 50 μM CX614 exposure (n ≥ 7 to 50 slices/group; bars show group means ± S.E.M; *p < 0.05; **p < 0.01; ***p < 0.001 compared to control, SNK).
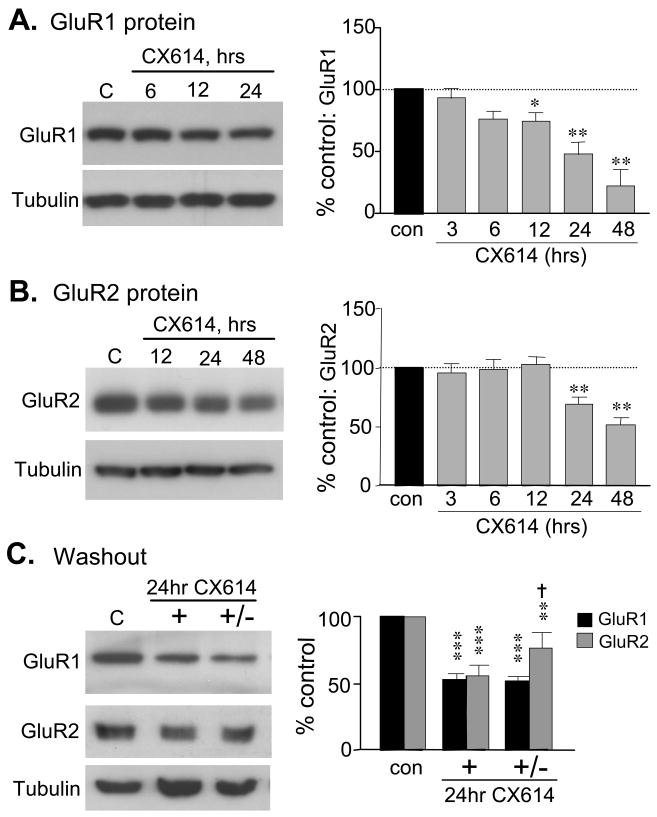
Western blot analyses demonstrated that AMPAR subunit protein levels generally followed the same time course as described above. GluR1-immunoreactivity (ir) was modestly reduced by 6–12 h and substantially depressed by 24 h and 48 h (Fig. 2A). Reductions in GluR2 protein were not evident through 12 h (Fig. 2B) despite the significant decrease in GluR2 mRNA at that time point (see Fig. 1C). These results suggest boundaries on the delay from the onset of reduced gene expression to reductions in AMPAR protein levels. For GluR1, the first trend toward decreased mRNA levels was detected 6 h prior to a significant decrease in protein content; for GluR2 changes in mRNA content preceded decreased protein levels by 12 h. Reduced AMPAR protein levels did not appear to be due to a general pathological response in that actin levels were not detectably changed during 24 or 48 h exposures to CX614 (Figs. 2A & 2B).
Figure 2.
Prolonged CX614 treatment decreases GluR protein levels. A and B) Representative western blots (left) and quantification of western blot band densities (right) show effects of CX614 treatment on total GluR1 (A) and GluR2 (B) protein levels in cultured slices harvested without treatment (con) or after 3 to 48 h of 50 μM CX614 exposure (group means ± S.E.M. for n ≥ 6/group; GluR band densities normalized to sample actin levels and presented as % paired control values). A) CX614 treatment caused a progressive reduction in GluR1-ir that was first significantly different from control at 12 h and continued to fall through 48 h (*p < 0.05; **p< 0.01, vs. con values, SNK). B) CX614 treatment decreased GluR2-ir by 24 h (**p< 0.01 versus control, SNK). C) Representative western blots (left) and bar graphs (right) show the effect of drug washout on total GluR1 and GluR2 levels: Slices were harvested without drug treatment (C, con), after 24 h exposure to CX614 (+) or after 24 h exposure to CX614 followed by 24 h ‘washout’ in normal medium (+/−) (group mean values normalized as in A; n ≥ 9/group). Both GluR1 and GluR2 levels were reduced by 24 h of 50 μM CX614 treatment; GluR2 levels were partially restored by washout (†p < 0.05 versus 24 h CX614 group; **p < 0.01 and ***p< 0.001 versus respective control group, SNK). Levels of tubulin-ir, shown at the bottom of each panel and used as a loading control, were unaffected by treatment (panels A–C; p > 0.05 for each treatment group vs control).
GluR2-ir recovered more quickly than did GluR1-ir with ampakine washout. In the experiment illustrated in figure 2C, slices were treated with CX614 for 24 h and were then harvested or transferred to drug-free medium for 24 or 48 h before harvest. Western blot analysis indicated that both GluR1 and GluR2 protein levels were reduced by about 50% at the end of ampakine treatment and that levels of GluR2, but not GluR1, partially recovered after one day of washout. Partial recovery of GluR1 protein levels was observed with 48 h of washout (data not shown).
Effects of GYKI 52466 on AMPA receptor subunit expression
The effects of CX614 on GluR subunit expression could be a consequence of excessive excitatory drive or instead could reflect normal mechanisms of homeostatic scaling of synaptic strength (O’Brien et al., 1998, Watt et al., 2000). From the latter perspective one would anticipate that (i) reducing glutamatergic activity through treatment with an AMPAR antagonist would increase GluR mRNA levels and (ii) increasing excitatory drive using any number of pharmacological strategies would decrease AMPAR subunit gene expression. We tested the first of these predictions by examining the effect of 24 h incubation with the specific AMPAR channel blocker GYKI-52466. GYKI increased GluR1 mRNA content: Measures of in situ hybridization labeling of GluR1 mRNA in control and 24 h treatment groups were 1.59 ± 0.07 and 3.84 ± 0.64 μCi/g, respectively (p = 0.029, Mann Whitney U) for field CA1, and 2.18 ± 0.22 and 4.28 ± 0.57 μCi/g, respectively (p = 0.015, Student’s t test) for field CA3. GluR2 cRNA levels in control and 24 h treatment groups were 4.54 ± 0.86 and 12.74 ± 3.56 μCi/g, respectively (p = 0.029) for CA1 and 5.09 ± 0.96 and 10.68 ± 2.32 μCi/g, respectively, (p = 0.029) for CA3. Thus, GYKI treatment caused a 2- to 3-fold increase in GluR1 and GluR2 transcript levels in str. pyramidale over 24 h. Together the effects of ampakine and GYKI treatment indicate that levels of AMPAR-mediated synaptic activity provide tonic, bidirectional regulation of AMPAR subunit gene expression, such that positive or negative changes in baseline activity, if sustained for several hours, will be reflected in opposing changes in AMPAR subunit mRNA levels.
Cholinergic stimulation down-regulates expression of AMPA receptor subunits
The above results could be due to the direct action of the tested compounds on AMPARs or to the accompanying changes in overall neuronal activity. We tested effects of the cholinergic agonist carbachol to distinguish between these possibilities. Previous work showed that carbachol increases neuronal activity and up-regulates BDNF in a manner that does not depend on glutamate receptors (i.e., increases are unaffected by co-treatment with AMPA or NMDA receptor antagonists) (Lauterborn et al., 2003).
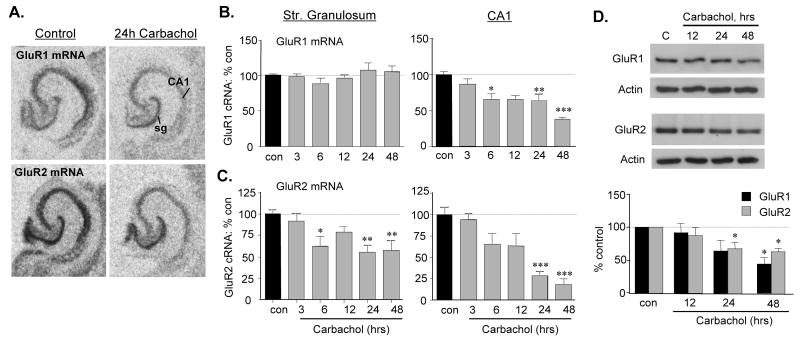
Hippocampal slices were treated with 50 μM carbachol for 3 to 48 h and effects on GluR1 and GluR2 mRNA levels were assessed by in situ hybridization. As shown for 24 h treatment groups in figure 3A, carbachol markedly reduced GluR1 and 2 cRNA labeling in CA1 with lesser effects in the granule cell layer. Densitometric measures of slices harvested at a range of treatment intervals confirmed these impressions (Figs. 3B and 3C). Carbachol-induced decreases in AMPAR subunit mRNA levels began at about the same time point (6 h) as was observed with CX614 and, for the pyramidal cells at 24 to 48 h, reached about the same magnitude. Western blot analyses confirmed that carbachol reduced total GluR1 and GluR2 protein levels over the same time frame. Whereas 12 h treatment had no reliable effect on AMPAR protein levels, 24 and 48 h applications produced substantial reductions in GluR1 and GluR2 immunoreactivities (Fig. 3D). The delays between reductions in GluR mRNA and protein content were comparable to those obtained with ampakine treatment (i.e., 6 to 12 h). These results indicate that down-regulation of AMPAR expression can be elicited with increased excitatory drive and is not specific to physical manipulations of the AMPA receptors themselves.
Figure 3.
GluR mRNA and protein levels are reduced by carbachol treatment. A) In situ hybridization labeling of GluR1 and GluR2 mRNAs in control and carbachol-treated (50 μM, 24 h) hippocampal slices: carbachol decreased both mRNAs with effects being greatest in CA1 str. pyramidale (abbreviations: sg, str. granulosum; CA1, field CA1 str. pyramidale). B and C) Bar graphs show quantification of hybridization densities to GluR1 (B) and GluR2 (C) mRNAs in str. granulosum and CA1 str. pyramidale after 3 to 48 h treatments (mean ± SEM values presented as percent paired control; *p < 0.05, **p < 0.01, ***p< 0.001 vs. con, SNK; n ≥ 10–28 and 7–10 slices/group for GluR1 and GluR2 mRNAs, respectively). D) Representative western blots and quantification of band densities (normalized to same-sample actin-ir, presented as percent paired control values) show total GluR1 and GluR2 protein levels in cultured hippocampal slices harvested without treatment (C, con), or after 12 to 48 h 50 μM carbachol treatment (*p < 0.05 versus control, SNK; n ≥ 4/group). Actin-ir was unaffected by carbachol treatment (D).
CX614 effects on AMPAR and BDNF gene expression have different thresholds and signaling mechanisms
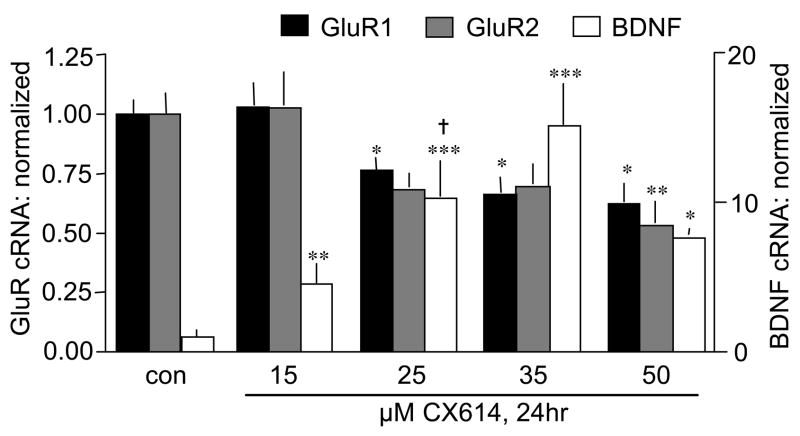
The above results show that the delay to onset of changes in gene expression is much greater for the AMPAR subunits than for BDNF (< 3h) (Lauterborn et al., 2000) but do not speak to the question of whether the two responses are tightly linked. Dose ranging studies were conducted to test the point. Cultured hippocampal slices were treated with CX614 at 15 to 50 μM for 24 h. As shown in Figure 4, GluR1 and GluR2 mRNA levels in stratum granulosum were unchanged by 15 μM CX614 but were reduced by 25 μM and more reliably so with 50 μM. The dose-response effects were comparable for GluR1 and GluR2. In contrast, granule cell BDNF mRNA levels were increased almost 5-fold by 15 μM CX614 (p < 0.01, Welch’s test, 1 tail). Still larger effects were obtained with 25 μM (10-fold) and 35 μM (15-fold) treatments (Fig. 4). BDNF mRNA levels were still elevated in slices treated with 50 μM CX614 but the magnitude of the effect was less than that obtained with 35 μM (p< 0.01). Similar responses were observed in CA3 and CA1 str. pyramidale including significant increases in BDNF mRNA (p<0.05 versus control, for each subfield) and no change in GluR mRNA levels with 15 μM CX614 treatment (data not shown). These results show that the threshold for changing BDNF gene expression with ampakines is substantially lower than that for modifying AMPAR subunit expression.
Figure 4.
Differences in the dose-response for CX614 effects on BDNF and GluR transcripts. Bar graph shows quantification of in situ hybridization labeling of GluR1, GluR2, and BDNF mRNAs in str. granulosum after treatment of cultured hippocampal slices with different doses of CX614 for 24 h. Bars show group mean ± S.E.M. values (measures from experimental slices normalized to paired controls; n ≥ 8 for GluR2 and BDNF; n ≥ 4 for GluR1). GluR and BDNF cRNA measures are plotted relative to the left and right y-axes, respectively. As shown, GluR1 and GluR2 mRNA levels were not affected by 15 μM CX614 treatment but were reduced by 25 to 50 μM doses. For BDNF, hybridization densities were increased by 15 μM CX614 and more greatly increased by higher doses. (*p < 0.05, **p < 0.01, ***p < 0.001, vs. respective control group; †p < 0.05 for comparison to 15 μM group; SNK).
It has been suggested that histone deacetylation regulates the expression of both the AMPAR subunits and BDNF (Huang et al., 2002, Aid et al., 2007). Trichostatin A (TSA), a selective inhibitor of histone deacetylase, prevents (i) pilocarpine-induced deacetylation of histone H4 associated with the GluR2 promoter and (ii) the down-regulation of hippocampal GluR2 expression associated with status epilepticus (Huang et al., 2002). To test if histone deacetylation mediates ampakine effects on GluR2 gene expression, cultured slices were treated with 50 μM CX614 in combination with 3 μM TSA for 5 h followed by a change to fresh medium containing only TSA for an additional 19 h. Other cultures were similarly treated with CX614 alone followed by washout and 19 h in drug-free medium. As shown in figure 5A, TSA prevented CX614-induced decreases in GluR2 mRNA levels in both CA3-CA1 str. pyramidale and stratum granulosum. Quantification of hybridization densities in CA1 str. pyramidale confirmed these findings (p<0.05 for the CX614 group versus the CX614+TSA group, Fig. 5C); treatment with TSA alone had no significant effect (Fig. 5C).
Figure 5.
Trichostatin A (TSA) blocks GluR2 down-regulation, but not BDNF up-regulation, by ampakine treatment. A) Photomicrographs of film autoradiograms show in situ hybridization to GluR2 mRNA in cultured hippocampal slices treated with vehicle (Control), CX614 (50 μM, 5 h pulse); or CX614 + 3 μM TSA (5 h pulse) followed by TSA alone; harvest 24 hr after treatment onset. CX614 alone reduced GluR2 mRNA levels throughout hippocampus (middle panel) whereas co-treatment with TSA blocked this down-regulation (right panel). B) Film autoradiograms show in situ hybridization to BDNF mRNA in cultured hippocampal slices treated with vehicle (Control), CX614 (50 μM, 12 h treatment); or CX614 + 3 μM TSA (12 h). CX614 alone increased BDNF mRNA levels throughout hippocampus (middle panel) and this effect was not influenced by co-treatment with TSA (right panel). C) Bar graph shows quantification of in situ hybridization to GluR2 (black bars) and BDNF (white bars) mRNAs in CA1 str. pyramidale after CX614 treatment (time points selected to best detect CX614 effects on each transcript) with and without TSA present; bars show mean ± S.E.M. labeling densities expressed as % paired control values. As shown, TSA blocked the reduction in GluR2 mRNA, but did not influence changes in BDNF mRNA, induced by ampakine treatment (*p<0.05, ***p < 0.001 vs. respective untreated-control, †p<0.05 vs CX614-treatment only, SNK).
Twelve hour treatments with CX614 (50 μM) produce a near-maximal increase in BDNF mRNA levels in cultured hippocampal slices (Lauterborn et al., 2000). Therefore, to test if histone deacetylation contributes to ampakine-induced BDNF gene expression, cultures were treated with 50 μM CX614 alone or in combination with 3 μM TSA for 12 h. TSA had no effect on BDNF mRNA levels in CA1 when applied alone or with CX614 (Fig. 5B,C); similar results were obtained for CA3 stratum pyramidale and stratum granulosum (Fig. 5B). These results further differentiate the effects of the drugs on BDNF and AMPAR expression.
CX614 can sustain increases in BDNF protein without AMPAR downregulation
The above results demonstrate that ampakine effects on AMPAR and BDNF expression are dissociable but leave open the critical question of whether the compounds can be used to sustain elevated BDNF protein levels without altering GluR expression. This issue was addressed by first determining how long a given concentration of CX614 must be present before AMPAR subunit expression is reduced. Hippocampal slices were treated with CX614 at 25 or 50 μM for 1–6 h and harvested for in situ hybridization analysis 24 h after treatment onset (Fig. 6A). As shown in figure 6B, GluR1 mRNA levels were not significantly different from control values with 1, 2 or 3 h treatments. Levels were slightly depressed after 4 h exposure to the higher ampakine dose, but this did not reach significance. However, 5 h exposures to CX614 (50 μM) significantly reduced GluR1 mRNA levels in str. granulosum and CA1 str. pyramidale (p < 0.001 and p < 0.01 versus control, respectively). By contrast, GluR1 transcript levels were not reduced by 6 h treatment with 25 μM CX614.
Figure 6.
Spaced ampakine treatments sustain elevated BDNF protein content without down-regulating AMPAR expression. A and B) As schematized in A, hippocampal slices were treated with CX614 for varied intervals (1 – 6 hr) and harvested 24 h after treatment onset. B) Bar graphs show group mean ± S.E.M. in situ hybridization labeling densities for GluR1 mRNA in str. granulosum and CA1 str. pyramidale. With CX614 at 50 μM (light bars), labeling was unaffected through 3 h but was lower than control values after 5 h treatments (*p < 0.05, **p < 0.01, ***p < 0.001 vs. con, SNK). CX614 at 25 μM (dark bars) did not affect GluR1 mRNA levels. C–E) As schematized in C, slices were treated with 50 μM CX614 for 3 h on four successive days; slices were collected daily (i) at the end of treatment for GluR1 mRNA analysis (D) or (ii) at the end of the 24 h period for ELISA measures of BDNF protein (E). D) Bar graph shows that GluR1 mRNA levels in str. granulosum (SG) remained at control values through treatment day 4. E) Plot of protein measures shows that total slice BDNF protein content was elevated at the end of the first day of treatment and remained elevated through treatment day 4 (**p < 0.01 vs. Con group, SNK) whereas GluR2 protein levels were unchanged throughout treatment. Mean ± S.E.M. values shown for n ≥ 8/group.
Slices were then treated for 3 h per day for 4 days. Groups of slices were collected daily at the end of the drug treatment period and GluR1 mRNA levels measured (see Fig. 6C for paradigm). Daily 3 h treatments had no effect on GluR1 mRNA levels in str. granulosum or str. pyramidale (Fig. 6D). Increases in BDNF and GluR protein lag behind increases in mRNA content by several hours (see above), and for BDNF elevated protein levels persist for at least 12 to 24 hours (Nawa et al., 1995, Lauterborn et al., 2000, Lauterborn et al., 2003). Accordingly, to assess treatment effects on protein content slices were harvested 24 h after the onset of the 3 h pulse ampakine treatment on the preceding day and processed for measures of BDNF and GluR protein content (Fig. 6E). Western blots indicated that total GluR1 and GluR2 protein levels did not across groups (i.e., from control through Day 4 of daily ampakine treatments; n ≥ 8/group, p > 0.05, one way ANOVA for both). However, total BDNF protein levels were significantly increased after the first ampakine treatment (Day 1) and elevated levels were maintained for 4 days (Fig. 6E).
Increases in BDNF expression are associated with increases in synaptic TrkB signaling
The relationship between BDNF concentrations and synaptic BDNF signaling has not been established and it cannot be assumed that the chronic mRNA and protein increases produced by ampakines translate into enhanced neurotrophin effects at excitatory synapses. Tests of this were carried out using immunolabeling with antisera that detects the activated (autophosphorylated) form of the TrkB receptor that is known to be critical for BDNF’s effects on cell survival and synaptic plasticity (Lu et al., 2005). Cultured hippocampal slices were pretreated for 3 h/day for 2 days with 50 μM CX614 or vehicle; as shown, this regimen increases BDNF protein content approximately 3-fold as assessed one day after the last ampakine treatment (Fig. 6). Slices were harvested into fixative and processed for the immunocytochemical localization of (i) pTrk (Y490) and (ii) PSD95 as a marker for glutamatergic synapses. To resolve spine immunostaining, 3 μm deep digital image z-stacks were collected from CA1 str. radiatum using 63× objective magnification and 0.2 μm z-steps. The images were then processed through iterative deconvolution (Volocity 4.0 software) to create a 3-dimensional montage of the sample field.
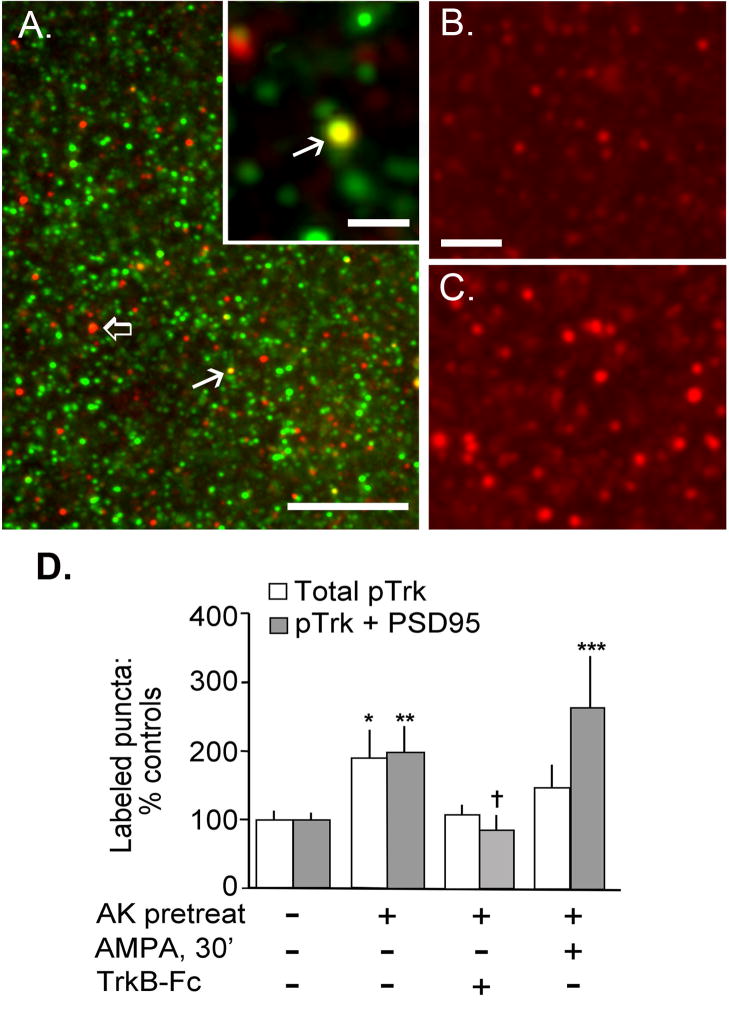
Figure 7A shows a representative field of immunostaining illustrating well-defined puncta double-labeled for pTrk-ir and PSD95-ir (e.g., at white arrows) and more numerous profiles single-labeled for each antigen (e.g., open arrow). Pretreatment with CX614 lead to a striking increase in numbers of pTrk-ir puncta (Fig. 7B,C). An automated system was used to count numbers of single-labeled (pTrk) and double-labeled (pTrk + PSD95) puncta as described in detail elsewhere (Rex et al., 2006, Chen et al., 2007); after counting, the image files were reviewed to verify that the double-labeled profiles identified fell within the size range of spines and post-synaptic densities (Rex et al., 2006). Quantification of labeled profiles determined that ampakine treatment increased both the total number of pTrk-ir puncta (single- and double-labeled; p=0.020 vs control; Fig. 7D) and numbers of PSD95+/pTrkB+ doubly immunoreactive profiles (hereafter referred to as pTrk-ir synapses). The latter results confirmed our experimental prediction and demonstrated that there were twice as many pTrk-ir synapses in field CA1 of slices collected 21 h after the last CX614 pretreatment as compared to numbers in control tissue (p=0.008) (Fig. 7D).
Figure 7.
Increases in BDNF expression are associated with elevated synaptic TrkB activation. Cultured hippocampal slices were pretreated with CX614 (“AK-pretreat”; 50 μM; 3 h/day for 2 days) or with vehicle in normal media and on day 3 were treated with 50 μM AMPA (or vehicle) for 30 min until harvest. For some CX614 pretreated slices, TrkB-Fc was added 2 h before harvest. A) Immunofluorescent double-labeling for PSD95 (green) and pTrk (red) immunoreactivities show that the two antigens are localized to scattered puncta in CA1 stratum radiatum; double labeled puncta appear yellow (bar, 20 μm). Both single-labeled (open arrow) and double-labeled (arrow) puncta can be seen. Inset shows at higher magnification a representative double-labeled element (arrow) surrounded by puncta that are singly labeled (bar, 2 μm). B–C) Photomicrographs show pTrk-ir puncta in the CA1 field in a control hippocampal slice (B) and one pretreated with CX614 (C) (bar, 5 μm). D) Bar graph shows counts of total pTrk-ir puncta and pTrk+/PSD95+ double-labeled, puncta in the CA1 sample field. Individual slice measures were normalized to mean values from paired control slices; the normalized individual-slice values were used to generate the group mean ± SEM values shown. As shown, total pTrk-positive puncta and double-labeled pTrk-ir spines were more numerous in slices given CX614 pretreatment (without or without acute AMPA treatment) than in control tissue (*p<0.05, **p<0.01 and ***p<0.001 vs. respective control group, 2-tailed t-test). AMPA treatment did not significantly increase these values (beyond effects of ampakine pretreatment alone). TrkB-Fc blocked increases in numbers of labeled puncta elicited by CX614-pretreatment ( p<0.05 vs “AK-pretreat”; a similar trend was seen for total pTrk-ir puncta).
Recent work has shown that TrkB can be transactivated by local Src activity in the absence of ligand (Lee and Chao, 2001, Berghuis et al., 2005). To test if the increases in synaptic pTrk-ir in ampakine-pretreated slices are dependent upon extracellular ligand, and to reinforce the interpretation that the activated receptor is likely to be TrkB, the ligand scavenger protein TrkB-Fc was added to slices 2 h before harvest. TrkB-Fc sequesters extracellular BDNF (and the far less abundant NT4/5) and therefore blocks effects of constitutive and activity-induced release of BDNF (Shelton et al., 1995, Rex et al., 2007). As shown in figure 7D, TrkB-Fc, eliminated the increase in numbers of pTrk-ir synapses otherwise seen in CX614 pretreated slices (p=0.028 for numbers of doubled-labeled puncta in ampakine vs. ampakine + TrkB-Fc groups). The TrkB-Fc treatment caused a similar reduction in total numbers of pTrk-ir puncta although this effect did not attain statistical significance.
Finally, an additional group of pretreated slices was given a 30 minute infusion of AMPA (50 μM) prior to harvest in an attempt to increase synaptic activity and activity-dependent BDNF release (Balkowiec and Katz, 2000, Gartner and Staiger, 2002, Lessmann et al., 2003). The acute exposure did not result in a statistically reliable elevation in numbers of double-labeled synapses above values obtained with the ampakine pretreatment alone (p>0.2, Fig. 7D).
Discussion
The discovery that BDNF gene expression is regulated by neuronal activity raised speculation about the possibility of using drugs (Castren, 2004, O’Neill et al., 2004b, Lynch et al., 2008) or behavioral manipulations (Cotman and Berchtold, 2002, Mattson et al., 2004, Gomez-Pinilla, 2008) to increase brain levels of the neurotrophin with the goal of stimulating trophic signaling and enhancing plasticity. Ideas relating to this necessarily face the problem of selectivity: Is the proposed manipulation for up-regulating BDNF sufficiently discrete in its actions to be a plausible therapeutic? One of the goals of the work described here was to investigate this issue with regard to ampakines, a broad family of small molecules that enhance excitatory drive on neurons and thereby increase BDNF transcription. Preclinical studies have shown that the compounds at dosages that enhance memory (Staubli et al., 1994b, Hampson et al., 1998, Lynch, 2006, Lynch and Gall, 2006) and reverse age-related deficits in LTP (Rex et al., 2006) do not produce evident physiological or behavioral side-effects. But work on synaptic scaling has raised the possibility that protracted changes in excitatory drive on cortical neurons will inevitably activate homeostatic mechanisms that return transmission, and thus BDNF gene expression, to baseline levels. Indeed, a process of this kind pertinent to an ampakine strategy has already been identified: prolonged exposure to the drugs reduces expression of AMPA-type glutamate receptors (Lauterborn et al., 2003, Jourdi et al., 2005) and thereby presumably restores a normal level of excitatory transmission. However, surprisingly little is known about either the tightness of coupling between the amount of excitatory transmission (size and frequency of EPSPs) and glutamate receptor concentrations or about the mechanisms that mediate this relationship. Similarly, while there is evidence that BDNF infusion can down-regulate the BDNF TrkB receptors (Frank et al., 1996, Knusel et al., 1997), there are no data describing how prolonged increases in the endogenous neurotrophin affects BDNF signaling at synapses. The results described here address these broad issues.
Extended treatments with the AMPAR antagonist GYKI-52466 caused a 2–3 fold increase in the expression of the Glur1 and 2 subunits. These findings, together with demonstrations that prolonged (24 h) infusions of ampakine compounds down-regulate the AMPAR subunits, accord well with the idea that increases or decreases in the size of synaptic responses are counteracted by adjustments to the glutamate receptor pool. However, it is also possible that the compensatory effect is not due to the direct effects of the test compounds on AMPARs but instead is secondary to the depolarization and network activity arising from those effects. Results obtained with a cholinergic agonist favor this latter idea. Carbachol increases rhythmic neuronal firing in hippocampal slices (Colgin et al., 2003, Konopacki et al., 2006) and induces BDNF via AMPAR-independent mechanisms (Lauterborn et al., 2003). Carbachol down-regulated GluR1 and GluR2 mRNA and protein levels in field CA1 with the same magnitude and time course as did the ampakine CX614.
That modifications of neuronal activity trigger compensatory responses is in agreement with previous work on dissociated neurons. Thus, reducing activity with TTX or AMPAR antagonists increases mEPSC amplitude and responses to applied glutamate, whereas increasing activity through GABA receptor inhibition reduces these measures (O’Brien et al., 1998, Turrigiano et al., 1998). Parallel changes in total and synaptic GluR levels, but not in numbers of synapses (O’Brien et al., 1998, Wierenga et al., 2005), support the view that cell wide alterations in AMPAR levels, and synaptic currents, contribute to homeostatic corrections in synaptic strength. Although changes in AMPAR gene expression have been proposed as a mechanism for synaptic scaling (Burrone and Murthy, 2003), the point has not been extensively studied. O’Brien et al. (1998) found that chronic TTX treatment nearly doubled the half-life of GluR1 in spinal cord neurons without affecting GluR1 mRNA content. The present results describe a somewhat different picture for the more complicated environment in hippocampus ---- in this case, nuclear transcriptional regulation of AMPARs makes a primary contribution to the scaling of glutamatergic synapses.
While synaptic scaling is clearly present in hippocampus, its engagement requires conditions far in excess of those needed for up-regulation of BDNF. Twenty-four hour treatments with a moderate concentration of an ampakine had no evident effects on AMPAR expression while causing a several-fold increase in BDNF mRNA levels. It thus appears that scaling events occur in response to changes in activity that are well outside the normal range. A similar conclusion holds for the time period over which activity is enhanced: a 3 h treatment with CX614 left GluR1 mRNA unchanged while producing a robust increase in BDNF expression. The low threshold for BDNF induction seen in these studies is consistent with past in vivo work (Gall and Lauterborn, 2000) and is encouraging with regard to the idea of elevating production of the neurotrophin for the treatment of various disorders. Also relevant to this are results indicating that transient increases in BDNF gene expression elevate protein levels for days (Nawa et al., 1995, Lauterborn et al., 2003). Collectively, these results point to the possibility of using relatively brief daily treatments with an ampakine to chronically elevate BDNF levels without activating compensatory responses by AMPARs. Tests of this were positive: daily 3 h treatments with CX614 increased BDNF protein content ~3-fold but had no effect on GluR mRNA or protein content through 4 days of testing.
The very different requirements for modifying AMPAR vs. BDNF expression point to the possibility that different signaling cascades are involved. Multiple lines of evidence suggest that GluR2 transcription is regulated by histone acetylation. Seizures increase the expression (Palm et al., 1998) and GluR2-promotor binding of the Repressor Element Silencing Transcription factor (REST) which, in turn, recruits histone deacetylase (Huang et al., 2002). This is followed by chromatin condensation and a reduction in GluR2 expression (Huang et al., 2002). Consonant with these findings, blocking deacetylation with TSA completely eliminated ampakine-induced down-regulation of GluR2 in the present studies. However, while there are reports of BDNF-associated histone acetylation after depolarization (Chen et al., 2003) and seizures (Huang et al., 2002), TSA failed to influence the BDNF up-regulation generated by CX614. Pertinent to this, TSA is also reported to be without effect on seizure-induced BDNF expression, possibly indicating that acetylation sufficient to prevent chromatin condensation is still present after infusion of the inhibitor (Huang et al., 2002). In any event, the TSA experiments point to critical differences in the regulation of BDNF and GluR expression and thus help explain why activity driven changes in expression of the two proteins have such different thresholds.
Elevated BDNF levels are associated with increased TrkB activation at excitatory synapses
BDNF is released in an activity-dependent fashion, from which it might be expected that increases in its protein content would be accompanied by increased release and signaling. However, while it is known that the neurotrophin is preferentially anterogradely transported (Zhou and Rush, 1996, Altar et al., 1997, Conner et al., 1998, Kokaia et al., 1998), localized to dense core vesicles (Fawcett et al., 1997, Wu et al., 2004, Buldyrev et al., 2006, Salio et al., 2007) and secreted with high frequency synaptic activity (Balkowiec and Katz, 2000, Balkowiec and Katz, 2002, Gartner and Staiger, 2002), it is not known if the concentration of BDNF is reflected in its synaptic actions. Nor, for that matter, has it been established that the TrkB receptor, which mediates neuroprotective and plasticity effects of BDNF, is reliably activated with synaptic BDNF release.
Tests of whether changing BDNF levels affects TrkB signaling are complicated by uncertainties about the percentage of the total receptor pool that is located at excitatory synapses in adult brain. However, the development of iterative deconvolution microscopy and quantitative double immunoflourescence labeling has made it possible to measure the size and number of glutamatergic synapses associated with dense deposits of specific proteins (Chen et al., 2007). A first application of these methods to ampakine-treated slices confirmed two experimental predictions: (i) the number of post-synaptic densities (labeled with anti-PSD95) co-localized with activated Trk was substantially increased above that found in untreated slices and (ii) this effect was eliminated by a 2 h infusion of TrkB-Fc, a compound that scavenges extracellular (released) BDNF (Shelton et al., 1995, Rex et al., 2007). Combined these results strongly suggest that elevation of BDNF levels increases extracellular concentrations of the neurotrophin and thereby tonically promotes TrkB activation. Attempts to test if the effects of up-regulation are amplified by heightened levels of network activity were inconclusive.
Evidence that TrkB activation was elevated by ampakine pretreatment argues against the possibility that prolonged periods of increased BDNF levels will initiate compensatory responses by the receptors. The issue was raised by studies showing that BDNF infusions down regulate TrkB (Knusel et al., 1997); opposite effects in the present experiments suggest that enhanced concentrations of endogenous neurotrophin produce a more regulated stimulation of the receptors.
The above results provide the first demonstration that increases in endogenous BDNF lead to increased signaling through TrkB at glutamatergic synapses. Elevated concentrations of pTrkB-ir are also observed in the hippocampal mossy fiber terminal field in association with increases in BDNF-ir in the granule cells and their axons following seizures (Binder et al., 1999, He et al., 2002). These earlier studies did not determine if increases in pTrk were pre- or post-synaptic, but the interpretation was that higher levels of axonal BDNF lead to greater spine TrkB activation. However, more recent work has shown that seizure-induced increases in p-Trk in the mossy fiber terminal field reflect transactivation of the TrkB receptor (through effects of zinc and postsynaptic Src signaling) as opposed to activation by endogenous BDNF (Huang et al., 2008). In another study, increases in BDNF expression accomplished by genetic manipulation of noradrenergic neurons were associated with reserpine-induced increases in cortical pTrk (Fawcett et al., 1998); in this case the interpretation was that presynaptic secretion of BDNF increased trophic signaling in neocortex but the locus of elevated pTrk was not identified.
The present finding that spaced ampakine treatments can sustain increases in BDNF content and synaptic TrkB signaling, without downregulating AMPAR expression, suggests that these treatment regimens might be exploited to increase BDNF trophic support for neuronal viability and synaptic plasticity. It is noteworthy that the episodic ampakine treatment regimen used here mimics the pattern of functional ampakine treatments likely to obtain in vivo. Several ampakines shown to upregulate BDNF (CX614, CX929, and CX546) (Lauterborn et al., 2000, Lauterborn et al., 2003), have estimated in vivo half-lives of under one hour (Hess et al., 2003, Rex et al., 2006). As a consequence, spaced systemic treatments would give rise to pulses of increased AMPAR drive predicted, from the present work, to be effective for sustaining selective increases in BDNF protein content and signaling. In agreement with this, we recently demonstrated that twice-daily intraperitoneal injections with an ampakine increased hippocampal BDNF content by >40% in middle-aged rats through 4 days of treatment and reversed age-related deficits in hippocampal LTP in a BDNF-dependent fashion (Rex et al., 2006). These results suggest that spaced treatments with a short half-life ampakine may be a viable therapeutic strategy for indications (impaired synaptic plasticity, neurotoxic insult, depression) that would benefit from sustained increases in BDNF trophic support.
Acknowledgments
The authors thank Jihua Liu and Sammie Cheng for technical assistance and Cortex Pharmaceuticals Inc. (Irvine, CA) for providing CX614. This research was supported in part by grants NS45260 from NINDS, CP30783 and CP40459 from Cortex Pharmaceuticals, S99-42 and bio05-10538 from the University of California Star Biotech/Discovery Programs. L. Y. C. is supported by NIMH predoctoral fellowship MH83396.
Abbreviations
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
AMPA receptor
- BDNF
brain-derived neurotrophic factor
- DMSO
dimethyl sulfoxide
- GluR
glutamate receptor subunit
- Ir
immunoreactivity
- REST
Repressor Element Silencing Transcription factor
- SNK
Student-Newman-Keuls
- sg
stratum granulosum
- str
stratum
- TSA
trichostatin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- Arai AC, Kessler M, Rogers G, Lynch G. Effects of the potent ampakine CX614 on hippocampal and recombinant AMPA receptors: interactions with cyclothiazide and GYKI 52466. Mol Pharmacol. 2000;58:802–813. doi: 10.1124/mol.58.4.802. [DOI] [PubMed] [Google Scholar]
- Bahr B, Bendiske J, Brown Q, Munirathinam S, Caba E, Rudin M, Urwyler S, Sauter A, Rogers G. Survival signaling and selective neuroprotection through glutamatergic transmission. Exp Neurol. 2002;174:37–47. doi: 10.1006/exnr.2001.7852. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, Schulte G, Ernfors P, Mackie K, Paratcha G, Hurd YL, Harkany T. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci U S A. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Routbort MJ, McNamara JO. Immunohistochemical evidence of seizure-induced activation of trk receptors in the mossy fiber pathway of adult rat hippocampus. J Neurosci. 1999;19:4616–4626. doi: 10.1523/JNEUROSCI.19-11-04616.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buldyrev I, Tanner NM, Hsieh HY, Dodd EG, Nguyen LT, Balkowiec A. Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem. 2006;99:1338–1350. doi: 10.1111/j.1471-4159.2006.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13:560–567. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Castren E. Neurotrophins as mediators of drug effects on mood, addiction, and neuroprotection. Mol Neurobiol. 2004;29:289–302. doi: 10.1385/MN:29:3:289. [DOI] [PubMed] [Google Scholar]
- Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Kubota D, Lynch G. Cholinergic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2003;100:2872–2877. doi: 10.1073/pnas.0530289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Gall CM. Anterograde transport of neurotrophin proteins in the CNS--a reassessment of the neurotrophic hypothesis. Rev Neurosci. 1998;9:91–103. doi: 10.1515/revneuro.1998.9.2.91. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Danzer SC, He X, McNamara JO. Ontogeny of seizure-induced increases in BDNF immunoreactivity and TrkB receptor activation in rat hippocampus. Hippocampus. 2004;14:345–355. doi: 10.1002/hipo.10190. [DOI] [PubMed] [Google Scholar]
- Dicou E, Rangon C-M, Guimiot F, Spedding M, Gressens P. Positive allosteric modulators of AMPA receptors are neuroprotective against lesions induced by an NMDA agonist in neonatal mouse brain. Brain Res. 2003;970:221–225. doi: 10.1016/s0006-8993(03)02357-6. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Fawcett JP, Aloyz R, McLean JH, Pareek S, Miller FD, McPherson PS, Murphy RA. Detection of brain-derived neurotrophic factor in a vesicular fraction of brain synaptosomes. J Biol Chem. 1997;272:8837–8840. doi: 10.1074/jbc.272.14.8837. [DOI] [PubMed] [Google Scholar]
- Fawcett JP, Bamji SX, Causing CG, Aloyz R, Ase AR, Reader TA, McLean JH, Miller FD. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci. 1998;18:2808–2821. doi: 10.1523/JNEUROSCI.18-08-02808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L, Ventimiglia R, Anderson K, Lindsay RM, Rudge JS. BDNF down-regulated neurotrophin responsiveness, trkB protein and TrkB mRNA levels in cultured rat hippocampal neurons. Eur J Neurosci. 1996;8:1220–1230. doi: 10.1111/j.1460-9568.1996.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Gall CM, Lauterborn JC. Regulation of BDNF Expression: Multifaceted, region-specific control of a neuronal survival factor in the adult CNS. In: Mocchetti I, editor. Neurobiology of the Neurotrophins. FP Graham Publishing Co.; Johnson City, TN: 2000. pp. 541–579. [Google Scholar]
- Gall CM, Pinkstaff JK, Lauterborn JC, Xie Y, Lynch G. Integrins regulate neuronal neurotrophin gene expression through effects on voltage-sensitive calcium channels. Neuroscience. 2003;118:925–940. doi: 10.1016/s0306-4522(02)00990-9. [DOI] [PubMed] [Google Scholar]
- Gartner A, Staiger V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc Natl Acad Sci U S A. 2002;99:6386–6391. doi: 10.1073/pnas.092129699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold SJ, Ambros-Ingerson J, Horowitz JR, Lynch G, Gall CM. Stoiciometries of AMPA receptor subunit mRNAs in rat brain fall into discrete categories. J Comp Neurol. 1997;385:491–502. doi: 10.1002/(sici)1096-9861(19970908)385:4<491::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. The influences of diet and exercise on mental health through hormesis. Ageing Res Rev. 2008;7:49–62. doi: 10.1016/j.arr.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson R, Rogers G, Lynch G, Deadwyler S. Facilitative effects of the ampakine CX516 on short-term memory in rats: correlations with hippocampal neuronal activity. J Neurosci. 1998;18:2748–2763. doi: 10.1523/JNEUROSCI.18-07-02748.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XP, Minichiello L, Klein R, McNamara JO. Immunohistochemical evidence of seizure-induced activation of trkB receptors in the mossy fiber pathway of adult mouse hippocampus. J Neurosci. 2002;22:7502–7508. doi: 10.1523/JNEUROSCI.22-17-07502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess U, Whalen S, Sandoval L, Lynch G, Gall C. Ampakines reduce methamphetamine-driven rotation and activate neocortex in a regionally selective fashion. Neuroscience. 2003;121:509–521. doi: 10.1016/s0306-4522(03)00423-8. [DOI] [PubMed] [Google Scholar]
- Huang Y, Doherty J, Dingledine R. Altered histone acetylation at glutamate receptor 2 and brain derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci. 2002;22:8422–8428. doi: 10.1523/JNEUROSCI.22-19-08422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Pan E, Xiong ZQ, McNamara JO. Zinc-Mediated Transactivation of TrkB Potentiates the Hippocampal Mossy Fiber-CA3 Pyramid Synapse. Neuron. 2008;57:546–558. doi: 10.1016/j.neuron.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Murray K, Huntsman M, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Jourdi H, Lu Yanagihara T, Lauterborn J, Bi X, Gall C, Baudry M. Prolonged positive modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors induces calpain-mediated PSD-95/Dlg/ZO-1 protein degradation and AMPA receptor down-regulation in cultured hippocampal slices. J Pharmacol Exp Ther. 2005;314:16–26. doi: 10.1124/jpet.105.083873. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Knusel B, Gao H, Okazaki T, Yoshida T, Mori N, Hefti F, Kaplan DR. Ligand-induced down-regulation of Trk messenger RNA, protein, and tyrosine phosphorylation in rat cortical neurons. Neuroscience. 1997;78:851–862. doi: 10.1016/s0306-4522(96)00616-1. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Andsberg G, Yan Q, Lindvall O. Rapid alterations of BDNF protein levels in the rat brain after focal ischemia: evidence for increased synthesis and anterograde axonal transport. Exp Neurol. 1998;154:289–301. doi: 10.1006/exnr.1998.6888. [DOI] [PubMed] [Google Scholar]
- Konopacki J, Eckersdorf B, Kowalczyk T, Golebiewski H. Firing cell repertoire during carbachol-induced theta rhythm in rat hippocampal formation slices. Eur J Neurosci. 2006;23:1811–1818. doi: 10.1111/j.1460-9568.2006.04679.x. [DOI] [PubMed] [Google Scholar]
- Kramár E, Lin B, Lin C, Arai A, Gall C, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Devel. 2006;9:580–586. [PubMed] [Google Scholar]
- Lauterborn JC, Berschauer R, Gall CM. Cell-specific modulation of basal and seizure-induced neurotrophin expression by adrenalectomy. Neuroscience. 1995;68:363–378. doi: 10.1016/0306-4522(95)00150-h. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Troung G, Baudry M, Bi X, Lynch G, Gall C. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther. 2003;307:297–305. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci U S A. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legutko B, Li X, Skolnick P. Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator. Neuropharm. 2001;40:1019–1027. doi: 10.1016/s0028-3908(01)00006-5. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Lynch G. Glutamate-based therapeutic approaches: ampakines. Curr Opin Pharmacol. 2006;6:82–88. doi: 10.1016/j.coph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lynch G, Gall CM. Ampakines and the threefold path to cognitive enhancement. Trends Neurosci. 2006;29:554–562. doi: 10.1016/j.tins.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: defects, treatments, and enhancement. Eur J Pharmacol. 2008;585:2–13. doi: 10.1016/j.ejphar.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak M, O’Neill MJ, Hicks CA, Bleakman D, Skolnick P. An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology. 2002;43:1–10. doi: 10.1016/s0028-3908(02)00066-7. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Nawa H, Carnahan J, Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: Partial disagreement with mRNA levels. Eur J Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- O’Neill M, Murray T, Whalley K, Ward M, Hicks C, Woodhouse S, Osborne D, Skolnick P. Neurotrophic actions of the novel AMPA receptor potentiator, LY404187, in rodent models of Parkinson’s disease. Eur J Pharmacol. 2004a;486:163–174. doi: 10.1016/j.ejphar.2003.12.023. [DOI] [PubMed] [Google Scholar]
- O’Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. AMPA receptor potentiators for the treatment of CNS disorders. Curr Drug Targets CNS Neurol Disord. 2004b;3:181–194. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- Palm K, Belluardo N, Metsis M, Timmusk T. Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J Neurosci. 1998;18:1280–1296. doi: 10.1523/JNEUROSCI.18-04-01280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–8. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Laramee GR, Rosenthal A, Winslow JW. Widespread expression of BDNF but not NT3 by targeted areas of basal forebrain cholinergic neurons. Science. 1990;250:290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramar EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lin CY, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio C, Averill S, Priestley JV, Merighi A. Costorage of BDNF and neuropeptides within individual dense-core vesicles in central and peripheral neurons. Dev Neurobiol. 2007;67:326–338. doi: 10.1002/dneu.20358. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll L, Spencer SD, Levinson AD. Human Trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesions. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Rex CS, Palmer L, Pandyarajan V, Fedulov V, Gall CM, Lynch G. Up-regulating BDNF with ampakines rescues synaptic plasticity and Memory in a mouse model of Huntington’s Disease. Proc Natl Acad Sci, USA. 2008 doi: 10.1073/pnas.0811228106. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Perez Y, Xu F, Rogers G, Ingvar M, Stone-Elander S, Lynch G. Centrally active modulators of glutamate (AMPA) receptors facilitate the induction of LTP in vivo. Proc Natl Acad Sci USA. 1994a;91:11158–11162. doi: 10.1073/pnas.91.23.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci USA. 1994b;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Vigers AJ, Baquet ZC, Jones KR. Expression of neurotrophin-3 in the mouse forebrain: insights from a targeted LacZ reporter. J Comp Neurol. 2000;416:398–415. doi: 10.1002/(sici)1096-9861(20000117)416:3<398::aid-cne10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26:659–670. doi: 10.1016/s0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YJ, Kruttgen A, Moller JC, Shine D, Chan JR, Shooter EM, Cosgaya JM. Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 are sorted to dense-core vesicles and released via the regulated pathway in primary rat cortical neurons. J Neurosci Res. 2004;75:825–834. doi: 10.1002/jnr.20048. [DOI] [PubMed] [Google Scholar]
- Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XF, Rush RA. Endogenous brain-derived neurotrophic factor is anterogradely transported in primary sensory neurons. Neuroscience. 1996;74:945–951. doi: 10.1016/0306-4522(96)00237-0. [DOI] [PubMed] [Google Scholar]