Abstract
The peripheral apelin system plays a significant role in cardiovascular homeostasis and in the pathophysiology of cardiovascular diseases. However, the central effect of this neurohormonal system in neural control of cardiovascular function remains poorly understood. Thus, this study was undertaken to evaluate the effect of apelin in the rostral ventrolateral medulla (RVLM) on blood pressure (BP), cardiac function and sympathetic nerve activity (SNA). Apelin mRNA and protein levels were detected with real time RT-PCR and Western blots, respectively. Expression of apelin was significantly enhanced in the RVLM of spontaneous hypertensive rat (SHR) compared with normotensive Wistar-Kyoto (WKY) rats. To study the functional consequence of upregulated apelin expression, apelin was overexpressed by bilateral microinjection of the AAV2-Apelin viral vector into the RVLM of WKY rats. Immunofluorescence staining and Western blots demonstrated that microinjection of AAV2-Apelin into the RVLM resulted in a significant increase in apelin expression, which was associated with a chronic elevation in BP and cardiac hypertrophy. In addition, direct microinjection of exogenous apelin-13 (200 pmol in 50 nl) into the RVLM caused a 20 mmHg elevation in BP and a 24% increase in SNA. The present study is the first to show that apelin expression is enhanced in the RVLM of SHR versus WKY rats and that overexpression of this gene in the RVLM results in chronic BP elevation and cardiac hypertrophy in normotensive rats. Thus, the apelin system in the RVLM may play a very important role in central blood pressure regulation and in the pathogenesis of hypertension.
Keywords: Blood pressure, Hypertension, Hypertrophy, Brain, Nervous system, sympathetic
Introduction
Apelin is a peptide recently isolated from bovine stomach extracts (1). It has been identified as the endogenous ligand for the orphan G-protein-coupled receptor, APJ (1, 2), which is comprised of seven transmembrane domains and shares a 31% amino acid sequence identity to the angiotensin II type 1 (AT1) receptor (3). Despite the degree of homology between these receptors, angiotensin II (Ang II) does not bind the APJ receptor, and apelin is the only known ligand for the APJ receptor (3). Multiple apelin peptides appear to be derived from a 77 amino acid precursor peptide, including preproapelin apelin-36 (42-77), apelin-17 (61-77), and apelin-13 (65-77). Apelin and APJ are widely distributed in various tissues and are thought to be involved in cardiovascular regulation (4, 5), heart contractility, body fluid homeostasis (6), control of appetite and, possibly, immune functions (7). The role of apelin and APJ in the cardiovascular system is currently the best documented. The accumulated evidence indicates that apelin and its APJ receptor are expressed throughout the cardiovascular system. Both pressor and depressor responses have been described in response to peripheral administration of apelin (1, 5, 8, 9, 10). Genetic studies in humans also demonstrate that disruption of the endogenous apelin/APJ system may have functional relevance in human heart failure. In these patients, the presence of a polymorphism of the APJ receptor, 212A, was associated with slower progression of heart failure (11). In addition, a single nucleotide polymorphism (SNP) in the APJ gene is associated with increased susceptibility to brain infarction (12). Thus, the emerging evidence indicates that the apelin/APJR system plays a significant role in cardiovascular homeostasis and in the pathophysiology of cardiovascular diseases. However, the involvement of this neurohormonal system in neural control of cardiovascular function remains poorly understood.
In the brain, apelin-immunoreactive cell bodies and fibers, and mRNA for apelin and the APJ receptor are distributed predominantly in neurons of the hypothalamus and brainstem, including cardiovascular regulatory regions such as the paraventricular nucleus (PVN), supraoptic nucleus (SON), circumventricular organs, nucleus tractus solitarius (NTS) and rostral ventrolateral medulla (RVLM) (2, 13, 14, 15). The RVLM is a key integrative site within the medulla that participates in the tonic and baroreflex regulation of blood pressure via sympathetic nerve activity. “Pacemaker cells” within this area provide the major excitatory input to sympathetic preganglionic neurons in the spinal cord that innervate sympathetic ganglia and the adrenal medulla (16, 17). In addition, the RVLM receives extensive inputs from other cardiovascular nuclei, including tonic excitatory input from the PVN and NTS and inhibitory input from the caudal ventrolateral medulla (CVLM) (18, 19). Thus, the RVLM is considered the major relay point for the transmission of sympathetic nerve activity. In addition, both altered RVLM function and elevated sympathetic nerve activity have been implicated in the pathogenesis of hypertension in several hypertensive animal models (20, 21, 22, 23).
The objectives of the present study were two fold: 1) to compare apelin expression in the RVLM between spontaneously hypertensive rats (SHR) and normotensive Wistar-Kyoto (WKY) rats; and 2) to determine the effects of endogenous and exogenous apelin in the RVLM on blood pressure and sympathetic nerve activity in vivo. Our results indicate that apelin expression is markedly enhanced in the RVLM of SHR compared with WKY rats; moreover, overexpression of apelin in the RVLM induces chronic elevation of blood pressure and significant cardiac hypertrophy in normotensive WKY rats. We also demonstrate that exogenous apelin-13, microinjected into the RVLM, causes an acute increase in blood pressure and an elevation in renal sympathetic nerve activity.
Materials and Methods
Animals
Adult male spontaneously hypertension rats (SHR) and Wistar-Kyoto (WKY) rats (9-10 wk old) were obtained from Charles River Farms (Wilmington, MA). Rats were housed individually and kept on a 12:12-hour light/dark cycle in a climate controlled room. Rat chow (Harlan Tekland, Madison, WI) and water were provided ad libitum. All experimental procedures were approved by the North Dakota State University Institutional Animal Care and Use Committee (protocol A0743).
Real-time RT-PCR
Apelin mRNA levels in the RVLM of SHR and WKY rats were determined by real time RT-PCR, as described in our previous study (24). TaqMan probes specific for rat apelin were purchased from Applied Biosytems, Inc. (Foster City, CA). Real time RT-PCR was performed in an Applied Biosystems PRISM 7000 sequence detection system according to the protocol from the manufacturer. Data were normalized to 18S RNA. In each experiment, samples were analyzed in triplicate.
Western Blot Analysis
Apelin protein levels in the RVLM, NTS, and area postrema (AP) of SHR and WKY rats were assessed by Western blot analysis, as described previously (24) with a primary antibody, rabbit anti-apelin antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, CA; dilution 1:500), and a secondary antibody, anti-rabbit peroxidase-conjugated antibody (Bio-Rad, Hercules, CA; dilution, 1:15,000). The primary rabbit anti-apelin antibody is raised against animo acids 38-77 at the C-terminus of apelin. Thus, it can identify preproapelin (apelin-36), apelin-28, and apelin-13. Immunoreactivity was detected by enhanced chemiluminescence autoradiography (ECL Western blotting detection kit, Amersham Pharmacia Biotechnology), and film was analyzed using Quantity One Software (Bio-Rad, Hercules, CA).
Immunofluorescence Staining
The immunofluorescence staining of brain RVLM sections was performed as we have described previously (24). In brief, the sections were incubated with primary antibodies (rabbit anti-apelin antibody and anti-NeuN monoclonal antibody, dilution 1:500) overnight at 4°C. On the next day, the sections were washed and incubated with secondary antibody (Alexa Fluor 488 goat anti-rabbit IgG 1:1000 and Alexa Fluor 594 goat anti-mouse IgG 1:1000, Molecular Probe, Carlsbad, CA) for 2 hours at room temperature. The sections were then washed with PBS/Tween, and mounted with anti-bleaching medium on a glass cover slip. The fluorescence staining was detected using an Olympus fluorescence microscope equipped with a color digital camera (Infinity 2), which was connected to a computer to capture and analyze images using Infinity Capture and Analyze Software.
RVLM Microinjection and Apelin Gene Transfer into the RVLM
Male WKY rats (9-10 wk old) were anesthetized with a mixture of O2 (1L/min) and isoflurane (3%) delivered through a nose cone. The rats were then placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Skin overlying the midline of the skull was incised, and a small hole was drilled bilaterally on the dorsal surface of the cranium according to the following coordinates: 1.9 mm lateral to the midline, 3.0 mm posterior to the lambdoid suture, 10 mm below the skull. Bilateral RVLM microinjections (50 nl) of either apelin-13 or saline were performed to examine the effect of exogenous apelin. In addition, an Adeno-Associated Virus type 2 (AAV2) containing the rat apelin gene (AAV2-Apelin, with AAV2-GFP as the control virus), driven by a chicken β-actin promoter (CBA) with a human cytomegalovirus (CMV) enhancer, were used to induce endogenous apelin expression in the RVLM. AAV2-GFP and AAV2-Apelin plasmids were constructed and prepared as described previously (25). The AAV2-Apelin or AAV2-GFP (all in 1 × 109 gc in 50 nl) was microinjected bilaterally into the RVLM over a 25-min period with a microinjection device. The RVLM location of the microinjection site was verified by the pressor response to L-glutamate microinjection as described in our previous publication (26).
Abdominal Aorta Cannulation
Animals were anesthetized with inhaled isoflurane as described above. Radiotelemetric pressure transducers (Data Sciences Int., St. Paul, MN) consisting of a fluid-filled catheter attached to a PA-C40 transmitter were implanted into the abdominal aorta as described previously (27). Before implantation, the aorta was clamped proximally and the catheter inserted and secured with medical adhesive. After BP transducer implantation, rats were left to recover for a week. BP and heart rate (HR) were recorded in conscious rats from 10:00 to 11:00 AM, once a day, using Dataqest IV software (Data Sciences Int., St. Paul, MN). The basal BP and HR were recorded for three days prior to RVLM microinjection of AAV2-Apelin or AAV2-GFP as described above.
Assessment of Cardiac Hypertrophy
Fourteen days after RVLM gene transfer, the animals were anesthetized with sodium pentobarbital and the hearts were removed. After the heart weight and cardiac morphology were examined, hearts were fixed in 10% formalin/PBS and embedded in paraffin. Heart sections (10 μm) were stained with hematoxylin and eosin to evaluate morphology and cellular dimensions.
Assessment of Sympathetic Nerve Activity and BP
Male WKY rats (9-10 wk old) were anesthetized with isoflurane as described above and PE-10 catheters, fused to PE-50 catheters pre-filled with heparinized saline (100 IU/ml), were placed in the right femoral artery for acute recording of BP and HR. The left kidney was exposed via a left flank incision and one of the renal nerves was dissected, hooked to a pair of silver electrodes and glued together with SilGel 604 (Wacker, Munich, Germany). The electrodes were connected to an EX1 Differential Amplifier. BP and HR were recorded using a pressure transducer, which was connected to a Bridge Amplifier (AD Instrument, Colorado Springs, CO). Both BP and renal sympathetic nerve activity (RSNA) data were collected and analyzed with PowerLab software (AD Instruments, Colorado Springs, CO).
Statistical Analyses
All data are presented as mean±SE. Statistical significance was evaluated by 1- or 2-way ANOVA, as appropriate, followed by either a Newman-Keuhls or Bonferroni posthoc analysis where appropriate. Differences were considered significant at P<0.05, and individual P values are noted in the figure legends.
Results
Expression of apelin in the RVLM of SHR vs. WKY rat
Apelin protein levels in the RVLM of SHR and WKY rats were determined by Western blot analysis and revealed that apelin protein levels were 40% higher in the RVLM of SHR as compared with WKY rats (Figures 1A and 1C, n=4 in each strain). As a control, we also determined apelin protein levels in other cardiovascular regulatory regions in the brainstem of SHR and WKY rats, including NTS and AP, using Western blot analysis. Apelin protein levels in these two brain areas were comparable between SHR and WKY rats (Figure 1B and 1C, n=4 in each strain). In addition, apelin mRNA levels were determined in micropunches of RVLM from SHR and WKY rats using real-time RT-PCR. Data in Figure 1D demonstrate that apelin is expressed in the RVLM of both SHR and WKY rats and that the mRNA levels of apelin are enhanced in the RVLM of SHR as compared with WKY rats (n=4 in each strain). Taken together, these observations demonstrate that apelin expression is enhanced in the RVLM of SHR as compared with WKY rats.
Figure 1. Expression of apelin is enhanced in the RVLM of SHR rats.
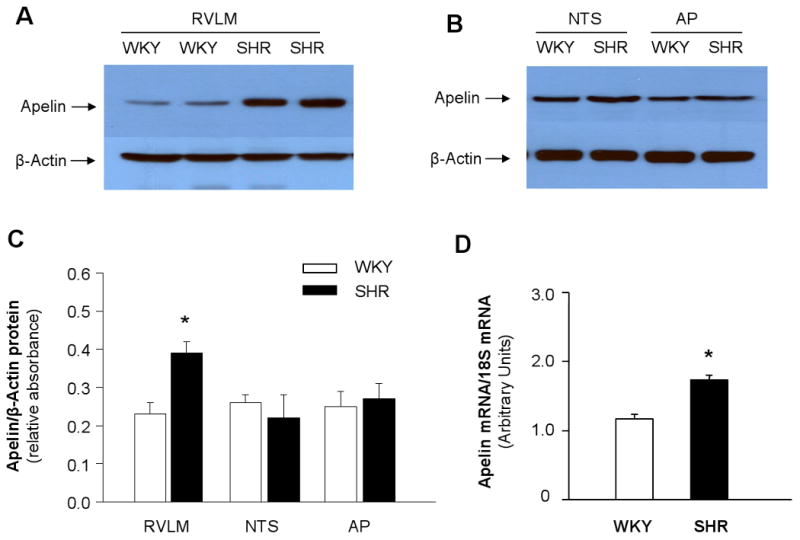
A) Representative autoradiogram of apelin protein levels in the RVLM: Western blot analysis was used to measure apelin levels from 20 μg of total cell lysate isolated from RVLM micropunches of SHR and WKY rats. Data are normalized using β-actin. B) Representative autoradiogram of apelin protein levels in the NTS and AP of SRH and WKY rats as described in (A). C) Bar graphs summarizing the quantitation of the apelin protein bands. Data are mean ± SE of apelin vs. β-actin protein in the RVLM, NTS, or AP of SHR or WKY rats (n=4 in each strain). *P<0.05 compared with apelin protein levels in the RVLM of WKY rats. D) Bar graphs showing the mRNA levels of apelin in the RVLM of SHR and WKY rats. The apelin mRNA levels were detected using real-time RT-PCR. Data are mean ± SE fromSHR and WKY rats (n=4 in each strain). *P<0.05 compared with apelin mRNA levels in WKY rats.
Overexpression of apelin in the RVLM by gene transfer
In order to study the functional consequence of enhanced apelin expression in the SHR RVLM on blood pressure regulation, the apelin gene was overexpressed using AAV2 viral vector-mediated gene transfer in the RVLM of normotensive WKY rats. Figure 2 shows immunofluorescence of apelin expression in the RVLM on the seventh day after microinjection of AAV2-Apelin. The top panels (Figures 2A-C) show a high magnification view indicating that apelin expression is localized to neurons of the RVLM. The lower panels (Figures 2D and E) demonstrate a strong immunoreactive apelin signal in the RVLM after transfection.
Figure 2. Overexpression of apelin in the RVLM of WKY rats.
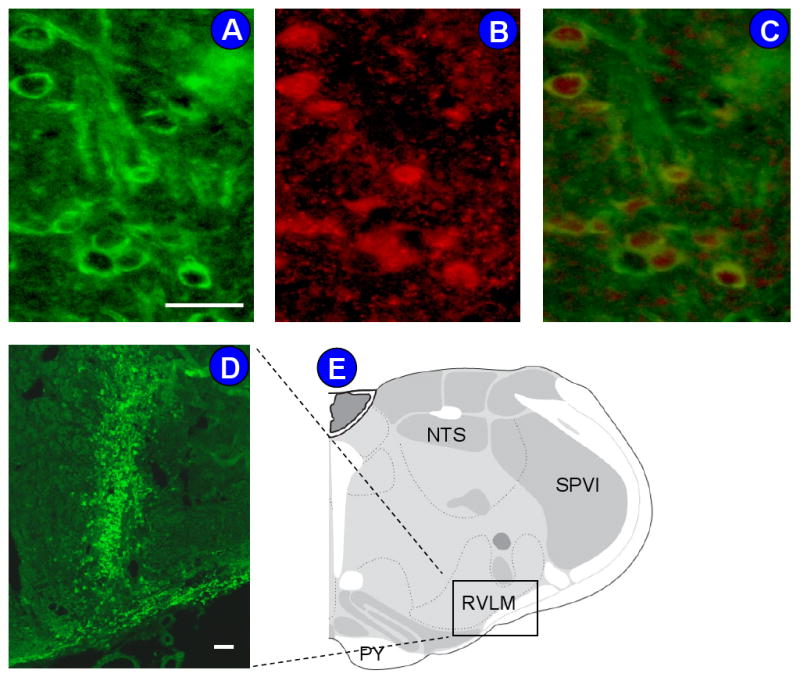
A-D, Immunofluorescence images showing overexpression of apelin protein in the RVLM of rats after seven days of apelin-gene transfer. A) Fluorescence micrograph (X 40 magnification) demonstrating localization of apelin in RVLM cells, immunostained with anti-apelin antibody (green). B) Same field of cells as in panel A, immunostained with anti-NeuN antibodies (red). C) Overlap of A and B, showing the green fluorescence is neuronally located. D) Fluorescence micrograph (× 10 magnification) demonstrating localization of apelin within the RVLM, indicated by the square in panel E, seven days after injection of AAV2-Apelin (50 nl of 1 × 109 gc). E) Location of the stained RVLM brain sections shown in A-D, based on the rat brain atlas of Swanson (43). NTS, nucleus tractus solitarii; SPVI, spinal tract of the trigeminal interpolar part; PY, pyramid. Scale bars = 50 μm
The time course of apelin protein expression in the RVLM after AAV2-Apelin or AAV2-GFP transfection is shown in Figure 3A and 3B. A representative Western blot showing apelin protein levels in the RVLM at various time points after gene transfer is shown in the top panel (Figure 3A) and bar-graph summaries of the quantified group data are shown in Figure 3B. Compared with the control rats, AAV2-mediated apelin gene transfer induced a significant increase in apelin protein expression within 24 hours (ratio of apelin:β-actin: 0.15±0.02 in control and 0.38±0.05 at 24 hours after gene transfer, n=4 rats, P<0.01). On day 2 after gene transfer, apelin protein expression reached its peak (0.68±0.05) and was sustained for at least 14 days (0.79±0.06). However, microinjection of AAV2-GFP control vector into the RVLM did not alter apelin protein levels (0.15±0.02 in control rats and 0.16±0.04 at 14 day after gene transfer, n=4 rats, P>0.05).
Figure 3. Apelin levels in the RVLM after apelin gene transfer and effect of overexpression of apelin in the RVLM on blood pressure and heart rate.
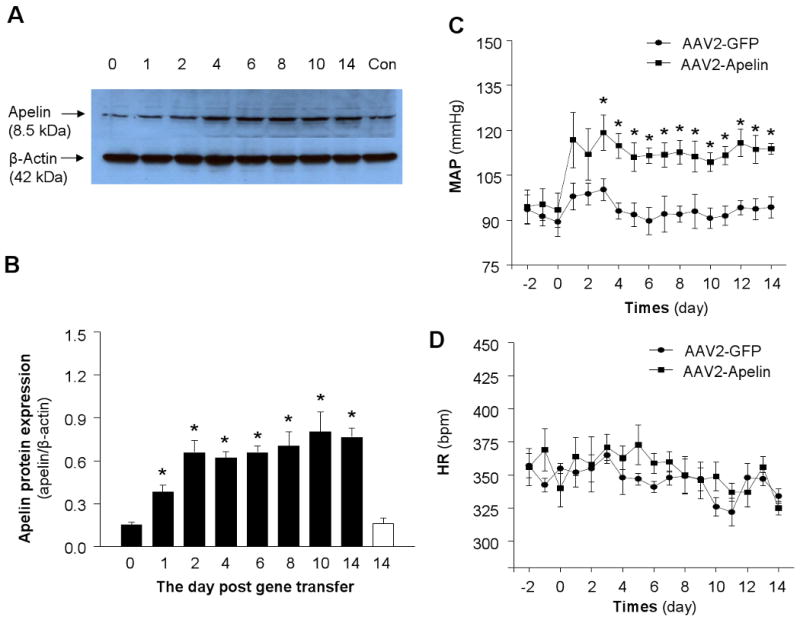
A) Representative autoradiogram of apelin levels in the RVLM. Western blot analysis was used to measure apelin protein levels in RVLM micropunches from brain sections of WKY rats at the time points indicated in the figure after AAV2-Apelin or AAV2-GFP control injection. The β-actin protein levels in each sample are shown in the lower panel of the figure. B) Bar graphs summarizing the apelin levels in the RVLM at the day indicated in the figure after bilateral microinjection of either AAV2-Apelin (black bar) or AAV2-GFP control (open bar). The apelin levels were measured in rat RVLM micropunches using Western blots. Data are normalized to β-actin protein. Data are means ±SE (n=4 rats for each group). *P<0.05 compared with control group. C-D) AAV2-Apelin or AAV2-GFP was injected bilaterally into the RVLM of WKY rats and MAP (C) and HR (D) were recorded in a conscious state using radiotelemetry. Blood pressure was elevated after apelin gene transfer into the RVLM of conscious rats. Data are means ±SE from 7 rats (AAV2-Apelin group) and 6 rats (AAV2-GFP group). *P<0.05 vs. corresponding time point in the AAV2-GFP group.
Effect of gene transfer on arterial pressure and cardiac hypertrophy
Based on the above apelin expression data, we next examined whether increased expression of apelin in the RVLM of normotensive rats would alter basal blood pressure. Nine-week-old WKY rats were fitted with telemetry pressure transducers, followed one week later by microinjection of either AAV2-Apelin (1 × 109 gc in 50 nl) or AAV2-GFP (1 × 109 gc in 50 nl) bilaterally into the RVLM. Mean arterial pressure (MAP) and heart rate (HR) were monitored via telemetry prior to AAV2 vector injections into the RVLM, and for 14 days after viral vector injections (Figure 3C). Prior to the RVLM microinjections, there was no difference in basal MAP between the AAV2-Apelin and AAV2-GFP-treated WKY rats (93.2±5.1 mm Hg in AAV2-Apelin group and 88±3.9 mmHg in AAV2-GFP group, n=7 and 6, P>0.05). After RVLM microinjection of AAV2-Apelin, the MAP increased steadily, reaching a peak level of 121±5.4 mmHg 3 days after microinjection, and remained elevated throughout the 14-day recording period. In contrast, microinjection of AAV2-GFP into the RVLM of WKY rats did not alter the basal MAP. The effect of AAV2-Apelin and AAV2-GFP microinjected into the RVLM on HR is shown in Figure 3D, indicating that neither AAV2-Aelin nor AAV2-GFP had any significant effect on HR in WKY rats. In addition, cardiac morphology was compared between the rats receiving microinjection of AAV2-GFP and AAV2-Apelin into the RVLM. The body weight was not significantly different between the two groups of rats (395±11 g in AAV2-GFP group and 395±6 g in AAV2-Apelin group). However, the heart weight to body weight ratio (HW/BW) and the myocyte cross sectional area were significantly increased by microinjection of AAV2-Apelin into the RVLM (Figure 4). Taken together, these results indicate that overexpression of apelin in the RVLM results in a chronic elevation in BP and a remarkable cardiac hypertrophy, without altering heart rate.
Figure 4. Overexpression of apelin in the RVLM results in cardiac hypertrophy.
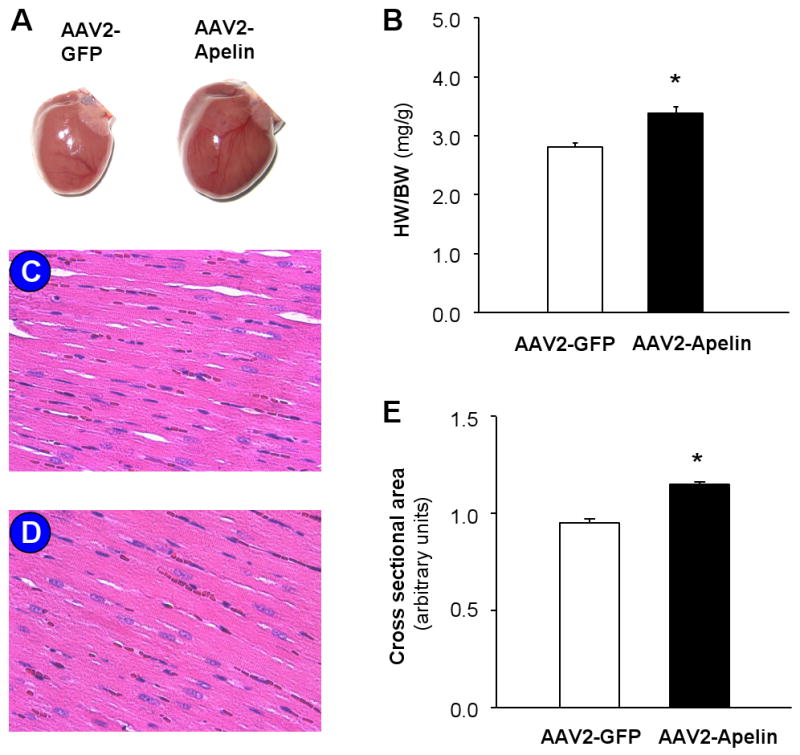
Cardiac morphological and histological characteristics were examined two weeks after microinjection of AAV2-Apelin or AAV2-GFP into the RVLM as described in the Methods. A, Representative images of the heart gross morphology of rats receiving RVLM microinjection of AAV2-GFP or AAV2-Apelin. B, Bar graphs showing heart-to-body weight ratios of rats after gene transfer (n=4 per group, *P<0.05). C and D, Micrographs (X 40 magnification) showing representative heart sections stained with hematoxylin and eosin in rats receiving RVLM microinjection of AAV2-GFP (panel C) or AAV2-Apelin (panel D) respectively. E, Average myocyte cross-sectional areas from transverse cardiac sections. Cross-sectional areas of 100 cells per rats were measured in random fields in 4 rats per group. *P<0.05 vs AAV2-GFP control rats.
Effects of exogenous apelin-13 microinjected into the RVLM on BP and RSNA
In order to determine whether elevated apelin-13 levels in the RVLM cause BP elevation and sympathetic nervous system activation, exogenous apelin-13 was microinjected into the RVLM of WKY rats. The MAP and renal sympathetic nerve activity (RSNA) were recorded before and after apelin-13 microinjection into the RVLM. The baseline MAP and HR was similar between the apelin group and saline group (Figure 5B). Microinjection of apelin-13 (200 pmol in 50 nl) into the RVLM induced a significant MAP elevation (from 95.9±1.1 mmHg to 115.5±2.5 mmHg, n=7, P<0.01, Figure 5A and B). The time-dependent pressor effect of apelin microinjection started at 2 min, peaked at 4-10 min, and lasted about 14 min after apelin injection (Figure 5B). However, RVLM microinjection of saline (50 nl) control did not alter arterial pressure (Figure 5B). In addition, apelin-13 microinjected into RVLM also resulted in a significant increase in HR (from 339±5 bpm to 378±5 bpm, n=7, P<0.01, Figure 5C). In contrast, RVLM microinjection of saline (50 nl) control had no effect on the basal HR (Figure 5C).
Figure 5. Effect of apelin-13 injected into the RVLM on blood pressure, heart rate, and renal sympathetic nerve activity.
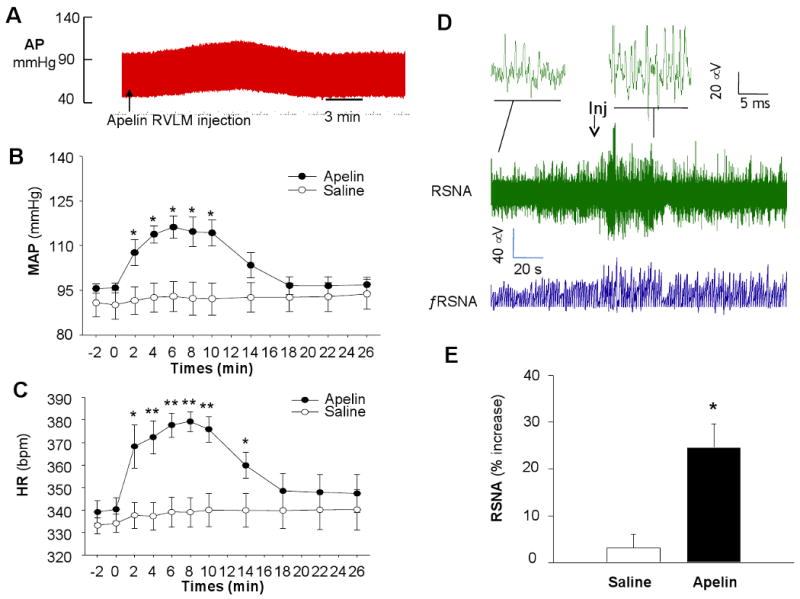
A) A representative tracing showing an arterial blood pressure recording before and after microinjection of apelin-13 into the RVLM of WKY rats. Arrow indicates the microinjection of apelin-13. B) Time course showing the effect of apelin-13 (200 pmol, 50 nl) or saline control (50 nl) microinjected into the RVLM on MAP. Data are means ± SE (n= 5 or 7 rats in each group) of MAP recorded every minute before and after RVLM injection of apelin-13 or saline control. *P<0.05 vs. saline control group. C) Time course of heart rate change before and after RVLM microinjection of apelin-13 (200 pmol, 50 nl) or saline control (50 nl). Data are means± SE (n= 5 to 7 rats in each group). *P<0.05 vs. saline control group, **P<0.01 vs. saline control group. D), A representative recording of renal sympathetic nerve activity (RSNA) and integrated RSNA (ƒRSNA) in response to exogenous apelin-13 (200 pmol in 50 nl) injected into the RVLM of WKY rats. Arrow indicates the microinjection of apelin-13. E) Bar graphs summarizing the effect of apelin -13 (200 pmol in 50 nl) or saline control (50 nl) microinjected into the RVLM on RSNA in WKY rats. Data are mean ± SE (n=5 to 7 rats). *P<0.05 compared with saline control group.
To determine whether the pressor effect of apelin-13 in the RVLM is associated with an alteration of sympathetic nerve activation, RSNA was recorded during apelin microinjection. Microinjection of apelin-13 (200 pmol in 50 nl) into the RVLM increased RSNA by 24±6% in seven WKY rats (Figure 5D and 5E), however, injection of saline control (50 nl) into the RVLM did not significantly alter the basal activity of sympathetic outflow (Figure 5D and E).
Discussion
The present studies present the first evidence that apelin expression is increased in the RVLM of SHR and that viral vector-mediated overexpression of apelin in the RVLM induces a chronic elevation in blood pressure in normotensive WKY rats. These results, coupled with the observation that exogenous microinjection of apelin-13 into the RVLM increases blood pressure and renal sympathetic nerve activity, strongly suggest that the apelin/APJ system is important in centrally-mediated neural control of the cardiovascular system.
It is well documented that apelin is distributed throughout the medulla oblongata, including the RVLM (15), but the functional role of the peptide has not yet been clarified. In the present study, we compared apelin expression in the RVLM of SHR and WKY rats and demonstrated that both mRNA and protein levels of apelin are markedly increased in the RVLM of hypertensive rats. To determine whether the upregulated expression of apelin observed in the RVLM of SHR is able to drive blood pressure to a higher level, we transferred the apelin gene into the RVLM of normotensive WKY rats using an AAV2 vector. Microinjection of AAV2-Apelin into the RVLM successfully induced a chronic overexpression of apelin. Moreover, in conscious WKY rats, overexpression of apelin by the AAV2 vector-mediated gene transfer system resulted in a significant and sustained increase in mean arterial blood pressure, as well as cardiac hypertrophy. Under these chronic conditions, heart rate was not significantly altered by AAV2-Apelin gene transfer. The mechanism of this phenomenon is unknown and this could be mediated by activation of a neuronal circuit or signaling pathways, which attenuate the apelin-induced tachycardia observed in the acute experiments of the present study.
It has been reported by Seyedabadi et al that microinjection of apelin-13 into the RVLM increases arterial pressure and phrenic nerve discharge amplitude (29). Consistent with these observations, the present study demonstrated that acute microinjection of exogenous apelin-13 into the RVLM increases blood pressure and that the pressor effect of apelin-13 injected into the RVLM is associated with an elevation in renal sympathetic nerve activity. Considerable evidence indicates that increased sympathetic nervous systemactivity appears to be a critical mechanism, not only in hypertension, but also in the development of target organ pathologies such as cardiac and vascular hypertrophy, and renal failure (30, 31, 32). For example, morbidity and mortality in cardiac failure are linked to neurohumoral excitation and increased sympathetic drive (33). Sympathetic hyperactivity is also implicated in several metabolic disorders, such as diabetes and obesity, which are well-known risk factors for cardiovascular diseases (34, 35). Thus, the excitatory effect of apelin on sympathetic outflow suggests that the apelin/APJ system may play a very important role in cardiovascular diseases such as hypertension, cardiac hypertrophy, and heart failure.
One question raised in this study centers on the cellular mechanism(s) underlying apelin-induced stimulation of sympathetic nerve activity. The effect of apelin on neuronal activity in the RVLM is still not clear. However, a recent study indicates that apelin-13 stimulates magnocellular neurons in the hypothalamic supraoptic nuclei (36). This direct stimulatory effect of apelin on neurons is supported by our preliminary study demonstrating that superfusion of neurons cultured from neonatal rat brainstem with apelin-13 resulted in a two-fold increase in neuronal firing rate (37). The second messenger signaling systems involved in the apelin-APJ stimulatory effect in neurons appear to be dependent on cell type. For example, apelin-13 is negatively coupled to cyclic AMP production, possibly via Gi, in APJ transfected Chinese hamster ovary cells (6, 38), whereas the response to apelin in the neuronal cell line 293 is mediated by the elevation of intracellular calcium (39). It has also been reported that apelin stimulates several kinases, such as ERK-1/2 and AKT (40, 41, 42). Thus, additional experiments will be necessary to unravel the signaling pathway(s) involved in the excitatory action of apelin in neurons.
One concern in this study is the identity of the specific apelin peptides that are enhanced in the RVLM of SHR. These apelin peptides are derived from a 77 amino acid precursor peptide, including preproapelin apelin-36 (42-77), apelin-17 (61-77), and apelin-13 (65-77) and they all share the C-terminus sequence. In the current studies, we used an antibody raised against amino acids 38-77 mapping at the C-terminus of preproapelin in western blots, and a real time RT-PCR probe was designed based on the cDNA coding this area. Thus, we can speculate that the preproapelin levels are enhanced and lead to increases in other apelin peptides, including apelin13, and apelin-12. This conclusion is supported by another experiment provided in the online supplemental data, which shows the enhanced expression of apelin in the RVLM of SHR versus WKY rat using an apelin-12 enzyme immunoassay (Online Figure I). Thus, this study raises another question as to which apelin peptide is responsible for the BP elevation observed in the overexpression experiments. Recent studies indicate that the shorter apelin isoforms exhibit greater binding affinity and biological potency than the full-length peptide. Apelin-13 possesses a pyroglutamate substitution at the N-terminus, a common post-translational modification that preserves biological activity by rendering the peptide more resistant to enzymatic cleavage. Thus, the pyroglutamated form of apelin-13 may be the most potent active biological ligand (8, 29, 40). This hypothesis is supported by the current observations that microinjection of apelin-13 into the RVLM induces a significant pressor effect and stimulation of renal sympathetic nerve activity. However, the effect of other apelin peptides in the RVLM on blood pressure and renal sympathetic nerve activity are in need of further investigation.
In conclusion, this study provides the first evidence that the expression of apelin is enhanced in the RVLM of SHR compared with normotensive WKY rats, and that overexpression of the apelin gene in this brain cardiovascular regulatory region results in a chronic elevation in blood pressure. Moreover, microinjection of an apelin-neutralizing antibody into the RVLM significantly lowers BP in SHR (Online Figure III). In addition, microinjection of this peptide into the RVLM induces an increase in blood pressure and heart rate, which are associated with stimulation of peripheral sympathetic nerve activity. Taken together, these results suggest that the apelin system in the RVLM may play a very important role in central blood pressure regulation and the pathogenesis of hypertension.
Acknowledgments
Sources of Funding: The project described is supported by Grant Number R21NS55008 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH).
Footnotes
Disclosures: None
References
- 1.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 2.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O'Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 3.O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–60. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 4.Lee DK, Saldivia VR, Nguyen T, Cheng R, George SR, O'Dowd BF. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 2005;146:231. doi: 10.1210/en.2004-0359. [DOI] [PubMed] [Google Scholar]
- 5.Cheng X, Cheng XS, Pang CC. Venous dilator effect of apelin, an endogenous peptide ligand for the orphan APJ receptor, in conscious rats. Eur J Pharmacol. 2003;470:171–5. doi: 10.1016/s0014-2999(03)01821-1. [DOI] [PubMed] [Google Scholar]
- 6.Reaux A, De Mota N, Skultetyova I, Lenkei Z, El Messari S, Gallatz K. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001;77:1085–96. doi: 10.1046/j.1471-4159.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- 7.Masri B, Knibiehler B, Audigier Y. Apelin signalling: a promising pathway from cloning to pharmacology. Cell Signal. 2005;17:415–26. doi: 10.1016/j.cellsig.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 2001;99:87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 9.Kagiyama S, Fukuhara M, Matsumura K, Lin Y, Fujii K, Iida M. Central and peripheral cardiovascular actions of apelin in conscious rats. Regul Pept. 2005;125:55–9. doi: 10.1016/j.regpep.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Charles CJ, Rademaker MT, Richards AM. Apelin-13 induces a biphasic haemodynamic response and hormonal activation in normal conscious sheep. J Endocrinol. 2006;189:701–10. doi: 10.1677/joe.1.06804. [DOI] [PubMed] [Google Scholar]
- 11.Sarzani R, Forleo C, Pietrucci F, Capestro A, Soura E, Guida P. The 212A variant of the APJ receptor gene for the endogenous inotrope apelin is associated with slower heart failure progression in idiopathic dilated cardiomyopathy. J Card Fail. 2007;13:521–9. doi: 10.1016/j.cardfail.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Hata J, Matsuda K, Ninomiya T, Yonemoto K, Matsushita T, Ohnishi Y. Functional SNP in an Sp1-binding site of AGTRL1 gene is associated with susceptibility to brain infarction. Hum Mol Genet. 2007;16:630–9. doi: 10.1093/hmg/ddm005. [DOI] [PubMed] [Google Scholar]
- 13.De Mota N, Lenkei Z, Llorens-Cortès C. Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology. 2000;72:400–7. doi: 10.1159/000054609. [DOI] [PubMed] [Google Scholar]
- 14.O'Carroll AM, Selby TL, Palkovits M, Lolait SJ. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim Biophys Acta. 2000;1492:72–80. doi: 10.1016/s0167-4781(00)00072-5. [DOI] [PubMed] [Google Scholar]
- 15.Reaux A, Gallatz K, Palkovits M, Llorens-Cortes C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience. 2002;113:653–62. doi: 10.1016/s0306-4522(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 16.Sun MK. Central neural organization and control of sympathetic nervous system in mammals. Prog Neurobiol. 1995;47:157–233. doi: 10.1016/0301-0082(95)00026-8. [DOI] [PubMed] [Google Scholar]
- 17.Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand. 2003;177:209–18. doi: 10.1046/j.1365-201X.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- 18.Dampney RA, Coleman MJ, Fontes MA, Hirooka Y, Horiuchi J, Li YW. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol. 2002;29:261–8. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- 19.Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol. 2002;29:514–21. doi: 10.1046/j.1440-1681.2002.03665.x. [DOI] [PubMed] [Google Scholar]
- 20.Colombari E, Sato MA, Cravo SL, Bergamaschi CT, Campos RR, Jr, Lopes OU. Role of the medulla oblongata in hypertension. Hypertension. 2001;38:549–54. doi: 10.1161/01.hyp.38.3.549. [DOI] [PubMed] [Google Scholar]
- 21.Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- 22.Mancia G, Grassi G, Parati G, Zanchetti A. The sympathetic nervous system in human hypertension. Rev Port Cardiol. 2000;19 2:II15–9. [PubMed] [Google Scholar]
- 23.de Wardener HE. The hypothalamus and hypertension. Physiol Rev. 2001;81:1599–658. doi: 10.1152/physrev.2001.81.4.1599. [DOI] [PubMed] [Google Scholar]
- 24.Yao F, Sumners C, O'Rourke ST, Sun C. Angiotensin II increases GABAB receptor expression in nucleus tractus solitarii of rats. Am J Physiol Heart Circ Physiol. 2008;294:H2712–20. doi: 10.1152/ajpheart.00729.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun C, Li H, Leng L, Raizada MK, Bucala R, Sumners C. Macrophage migration inhibitory factor: an intracellular inhibitor of angiotensin II-induced increases in neuronal activity. J Neurosci. 2004;24:9944–52. doi: 10.1523/JNEUROSCI.2856-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49:926–31. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- 27.Sellers KW, Sun C, Diez-Freire C, Waki H, Morisseau C, Falck JR, Hammock BD, Paton JF, Raizada MK. Novel mechanism of brain soluble epoxide hydrolase-mediated blood pressure regulation in the spontaneously hypertensive rat. FASEB J. 2005;19:626–8. doi: 10.1096/fj.04-3128fje. [DOI] [PubMed] [Google Scholar]
- 28.Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta. 2001;1538:162–71. doi: 10.1016/s0167-4889(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 29.Seyedabadi M, Goodchild AK, Pilowsky PM. Site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respiration. Auton Neurosci. 2002;101:32–8. doi: 10.1016/s1566-0702(02)00178-9. [DOI] [PubMed] [Google Scholar]
- 30.Leenen FH. Cardiovascular consequences of sympathetic hyperactivity. Can J Cardiol. 1999;15 A:2A–7A. [PubMed] [Google Scholar]
- 31.Campese VM, Krol E. Neurogenic factors in renal hypertension. Curr Hypertens Rep. 2002;4:256–60. doi: 10.1007/s11906-002-0016-3. [DOI] [PubMed] [Google Scholar]
- 32.Palatini P. Sympathetic overactivity in hypertension: a risk factor for cardiovascular disease. Curr Hypertens Rep. 2001;3 Suppl 1:S3–9. doi: 10.1007/s11906-001-0065-z. [DOI] [PubMed] [Google Scholar]
- 33.Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK. Heart failure and the brain: new perspectives. Am J Physiol Regul Integr Comp Physiol. 2003;284:R259–76. doi: 10.1152/ajpregu.00317.2002. [DOI] [PubMed] [Google Scholar]
- 34.Stas SN, El-Atat FA, Sowers JR. Pathogenesis of hypertension in diabetes. Rev Endocr Metab Disord. 2004;5:221–5. doi: 10.1023/B:REMD.0000032410.75638.da. [DOI] [PubMed] [Google Scholar]
- 35.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 36.Tobin VA, Bull PM, Arunachalam S, O'Carroll AM, Ueta Y, Ludwig M. The effects of apelin on the electrical activity of hypothalamic magnocellular vasopressin and oxytocin neurons and somato/dendritic peptide release. Endocrinology. 2008;149:6138–45. doi: 10.1210/en.2008-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun C, Shan Z, Raizada MK. Enhanced apelin/APJ system in the cardiovascular regulatory brain regions of spontaneously hypertensive rats. Hypertension. 2007;50:e151. abstract LB14. [Google Scholar]
- 38.Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem. 2000;275:21061–7. doi: 10.1074/jbc.M908417199. [DOI] [PubMed] [Google Scholar]
- 39.Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem. 2003;84:1162–72. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 40.Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. Apelin (65-77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J. 2004;18:1909–11. doi: 10.1096/fj.04-1930fje. [DOI] [PubMed] [Google Scholar]
- 41.Masri B, Lahlou H, Mazarguil H, Knibiehler B, Audigier Y. Apelin (65-77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem Biophys Res Commun. 2002;290:539–45. doi: 10.1006/bbrc.2001.6230. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto Y, Ishida J, Yamamoto R, Fujiwara K, Asada S, Kasuya Y, Mochizuki N, Fukamizu A. G protein-coupled APJ receptor signaling induces focal adhesion formation and cell motility. Int J Mol Med. 2005;16:787–92. [PubMed] [Google Scholar]
- 43.Swanson LW. Brain Maps: Structure of the Rat Brain. 3rd. San Diego, CA: Elsevier; 2004. p. 153. [Google Scholar]


