Abstract
Introduction
Although prostate cancer is prevalent, little information is available on how it affects couples’ quality of life (QOL) according to their age cohort. The purpose of this study was to examine how quality of life, self-efficacy and appraisal of the illness experience vary among men with prostate cancer and their partners according to age cohort: middle age (50–64); young-old (65–74); and old-old (75–84). Using an Adult Developmental and Family Stress framework, this study focuses on how normative (developmental stage) and non-normative stressors (prostate cancer) may affect a couple’s ability to adapt.
Methods
A descriptive, comparative design was used to examine age-related differences in quality of life and selected psychosocial variables in 69 men with prostate cancer and their spouses. Cross-sectional data were obtained using standardized instruments with adequate reliability and validity. ANCOVA and MANCOVA were used to determine differences among age groups.
Results
Findings indicated that patients who were ages 65–74 had better QOL and higher self-efficacy than patients ages 50–64 and less negative appraisal of illness than the other two groups. Spouses ages 50–64 reported the most distress related to sexual changes in their husbands. Spouses in both the middle age and old-old group had more bother related to hormone therapy than the young-old spouses.
Implications for Cancer Survivors
Findings suggest that interventions should be tailored to dyads’ developmental life stage. Younger and older prostate cancer survivors and their partners may benefit from tailored interventions designed to improve their quality of life and confidence in managing their treatment outcomes during the survivorship period.
Keywords: Prostate Cancer, Spouses, Developmental Life Stage, Quality of Life, Aging, Family, Caregivers, Couples, Self-Efficacy
Introduction
Prostate cancer is typically a disease of older men, with the incidence increasing dramatically in men over 75. While prostate cancer is the second leading cause of cancer deaths in men, the number of men living with the disease and with the outcomes of treatment is increasing as our population continues to age [1]. As adults age, they face normative developmental changes in their daily lives that can cause stress. The diagnosis of prostate cancer requires that they simultaneously adjust to the changes brought about by cancer as well as to other normative changes specific to their age. Often in the research literature, aging couples are viewed as a group without consideration of possible group differences in perception of illness and the ability to deal with an illness such as prostate cancer. The purpose of this study was to examine: 1) physical and mental quality of life and 2) pertinent psychosocial factors among couples with prostate cancer according to age cohort: middle age (50–64); young-old (65–74); and old-old (75–84).
Theoretical Perspective
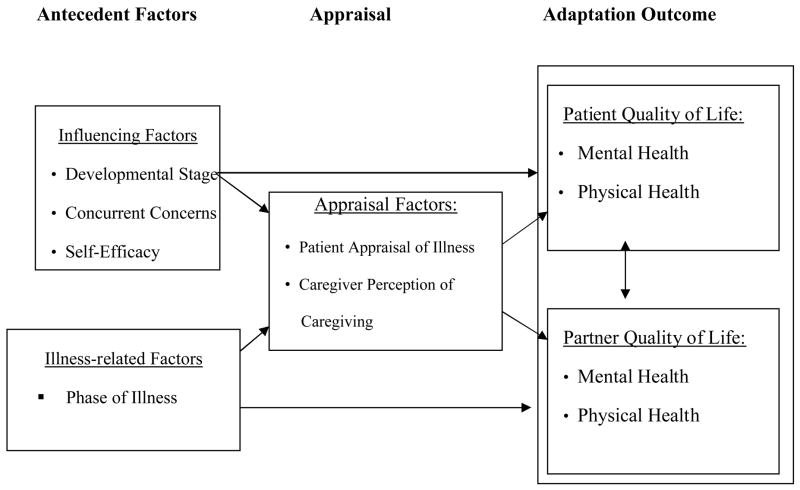
In this study, responses to the illness experience were examined using an adult developmental framework in combination with a family stress framework to guide the selection of variables. Figure 1 illustrates the adult developmental and family stress model that guided the present study. This study did not test the model per se, but used the model to guide the selection of variables that were examined in this work. Antecedents refer to influencing factors, namely developmental life stage, concurrent concerns, self-efficacy, and illness-related factors of symptom experience and phase of illness. Appraisal refers to the patients’ perception of the illness experience and caregivers’ perception of the caregiving experience. Adaptation refers to quality of life outcomes including physical and mental health for both patients and their spouses.
Figure 1.
Adult Developmental and Family Stress Framework
Both theoretical frameworks make important contributions to understanding the cancer experience of adult patients at different life stages and their spouses. Developmental theory suggests that there are different stages people experience as they age and each stage has its own biopsychosocial character that provides an underlying order to the flow of life[2]. Recognition of where a person is in life, physically, socially, and psychologically is particularly useful in interpreting the impact of cancer on the person [3]. Family stress theory [4] contends that a stressor such as an illness affects both patients and family members. According to the theory, certain antecedent factors such as developmental life stage as well as appraisal factors influence the family’s ability to adapt to a stressor. The ability of the family to appraise the situation as manageable helps the family in their adaptation process.
Review of Literature
Developmental Perspective
A life span perspective suggests that adults experience a series of gains and losses across all stages of the life span [5]. Typically, gains are more pronounced in young adulthood, tend to be balanced in middle adulthood and tip in later adulthood with losses exceeding the gains. Efforts to balance gains and losses in these different life stages are essential to personal development over the life span [6].
Adults in their fifties and early sixties (middle age) often view themselves in the prime of their lives. Fewer financial responsibilities and a higher earning potential result in more economic stability for some, while for others perceived lack of financial preparation for retirement is a constant concern [7]. Additionally, a significant challenge of this stage is effectively responding to the additional responsibilities related to caring for aging parents.
Most adults in their mid-sixties and early seventies (young-old) enter retirement and a transitional status. There is a life review of the successes and failures and a demand to adjust to new roles [8]. While many people may welcome retirement, for others retirement is a stressor that challenges their self-esteem related to the absence of work-related responsibilities and lack of a defined role. While many adults in this stage of life continue to experience good health, concurrently, they are beginning to experience physical changes related to aging and the development of co-morbid conditions [7].
As couples move into the mid-seventies and beyond (old-old), they begin to experience a greater decline in physical abilities resulting in increased susceptibility to illness, greater difficulty in managing a complex medical regimen, less adaptability to unfamiliar environments and treatment, and they require a longer period to recover from illness [3]. A large proportion of these couples have one or more chronic illnesses and symptoms related to them. Couples in a long-standing relationship rely on each other to meet daily challenges and maintain independence.
Age, often used as a proxy for life stage, can affect one’s response to a cancer illness [3]. According to the research literature, age is a factor that can affect couples’ belief in their ability to manage the treatment regimen as well as their ability to manage symptoms created by an illness or its treatment [3]. Research has shown that younger individuals (under 65) compared to older individuals diagnosed with cancer are at higher risk for psychological problems [9]. In addition, younger individuals with cancer have lower quality of life [10] and more life disruption [11]. Older individuals, however, are at increased risk of functional disability [12, 13] and greater risk of developing cognitive changes [14].
In general, studies examining age and caregiving in a variety of populations have provided inconsistent findings. Some researchers have reported that older female caregivers (>65 yrs) experienced less disruption to their schedules and thus, view caregiving as less negative over time [15]. Further in some research, older caregivers were more satisfied with their role than younger caregivers [16]. However, other researchers have found that the physical vulnerabilities of older caregivers put them at risk for decreased physiological functioning [17], increased health problems and increased mortality [18]. Social isolation, decreased family resources and co-morbid conditions also can cause caregiver distress to be more pronounced in older spousal caregivers [19, 20]. In contrast, other researchers have found that age does not play a role in the caregiver’s reaction to caregiving [21]. Overall, studies on age and caregiving have reported mixed findings, underscoring the need for further research in this area.
Quality of Life
Health-related quality of life has been defined as an overall experience of physical, functional, psychological and social well-being [22]. Research has shown that in prostate cancer patients (mean age 73.4) physical dysfunction left the person vulnerable to problems with emotional health [23], suggesting the inter-relatedness of physical and emotional aspects of quality of life. While recognizing the interconnectedness of physical and psychological influences on quality of life, in this study of dyads, quality of life was operationalized as the physical and mental responses to a diagnosis of prostate cancer for both patients and their spouses.
Physical quality of life is often related to patients’ symptom experiences. In particular, symptom bother in prostate cancer has been related to specific treatment effects associated with changes in function and exhibited in problems such as urinary frequency, leakage, diarrhea and hot flashes. Many investigators have reported that bowel problems, bladder difficulties and sexual dysfunction occurring after treatment for prostate cancer are common side effects of treatment [24–26]. Sexual dysfunction, very common following a radical prostatectomy, can negatively affect men’s quality of life [27–30] and have a profound effect on the men’s emotional well-being [31]. Incontinence, impotence, loss of libido and fatigue resulting from treatment affect the lives of both patients and their spouses and can diminish the quality of life of both [32–36].
Mental quality of life can also be affected by the stress and uncertainty experienced during the cancer experience. Fatigue and stress associated with caregiving can diminish the quality of life of the caregiver [20]. Equally important, research suggests that the quality of life of the partner also can affect the quality of life of the patient [21, 27, 37–39].
Psychosocial Factors
Concurrent concerns are factors such as work and family obligations that dyads must manage in addition to those presented by a prostate cancer diagnosis. Higher levels of concurrent concerns have been associated with poorer adjustment to cancer [40]. Because prostate cancer is most common in older men, stressors associated with work and family obligations may be fewer than in younger dyads. On the other hand, stressors related to other chronic illnesses may be greater in older dyads than in younger dyads [41]. Still, studies have found that older adults reported fewer concurrent concerns and rated them as less stressful than younger adults [37, 42–44].
Self-efficacy is a person’s belief in one’s capabilities to manage new tasks. Self-efficacy can affect a person’s thought processes and emotions and ultimately affect his/her motivation to master the challenge presented [47]. Research in elderly post-operative patients has shown that those who had confidence in their ability to perform a given activity recovered more quickly [48]. Self-efficacy predicted perseverance, performance and the selection of appropriate strategies to optimize functional ability. Further, prostate cancer survivors and their partners who have higher self-efficacy for managing symptoms related to prostate cancer treatment reported better quality of life [49].
Appraisal of illness is the person’s evaluation or judgment of the illness situation that determines the stressfulness of this event in terms of personal significance, goals and resources [48]. Appraisal of illness can have a profound effect on an individual’s overall adaptation to illness and therefore quality of life. For example, breast cancer patients who appraised their illness as more positive had better quality of life outcomes than those who had more negative appraisals [40]. Bowman and associates found that being younger was associated with more negative appraisal while being older was related to less stressful appraisal [38]. Negative appraisal has been associated with poorer quality of life outcomes in cancer patients and their caregivers [27, 28, 37, 49]. Appraisal of caregiving is associated with caregiver responsibilities that can take a toll on spouses in the form of stress and fatigue. Spouses often find themselves physically, mentally and emotionally drained [50]. Negative feelings, such as guilt, anger, fear, bitterness, isolation, depression, helplessness and anxiety may be far more disruptive to life and daily function than physical demands [51]. Because spouses are a major source of support for patients [39], spouses’ negative appraisal of caregiving can potentially affect the quality of life of patients [52, 50, 53].
Summary
In summary, the literature reviewed suggests that age and other factors may affect a patient’s and a spouse’s ability to positively adapt to an illness. The compounding effect of age has been studied in some cancer populations; however, it has not been systematically examined in the prostate cancer population nor has it been studied according to developmental life stage. Because of the prevalence of prostate cancer in the aging population, understanding how age affects the response of men and their partners to prostate cancer is important to healthcare personnel providing care and developing interventions designed to improve quality of life for these dyads.
Based on the review of literature, this study was designed to address the following research objectives:
Determine if there is a difference in the physical and mental domains of quality of life reported by men and their partners living with prostate cancer according to age cohort (middle age, young-old, old-old).
Determine if men and their partners in various age cohorts differ in their number of concurrent concerns, levels of self-efficacy and appraisal of illness/caregiving.
Method
Design
A descriptive, comparative design was used to obtain data from men with prostate cancer and their partners in the Midwest. Participants were recruited from two large comprehensive cancer centers. Data for this study were obtained from baseline assessments of couples recruited to take part in an intervention study. The study was approved by the Human Subjects Committees of both medical institutions.
Sample
The sample consisted of 69 patients with prostate cancer and their spouses/partners who were in one of three age groups, middle age (50–64 yrs), young-old (65–74yrs), and old-old (75–84 yrs). Because stratification was based on the patient’s age, spouses/partners were not necessarily in the same discrete age group. Power analysis indicated that 23 couples in each group were required to detect a moderate effect (d=.33) with a power of .80 and alpha of .05 in statistical tests for age group comparisons [54].
Men were eligible to participate if they had a confirmed diagnosis of prostate cancer, were mentally and physically able to participate, spoke sufficient English to participate and had a spouse willing to participate. The majority of patients were diagnosed with localized prostate cancer and had completed primary treatment. Spouses were eligible for the study if they were: identified by the patient as his spouse (included female or male partners without marital ties, but who cohabited in the same household and were involved with the patient for more than a year); mentally and physically able to participate in the study and spoke and understood sufficient English to complete the surveys. Each member of the couple provided informed consent. Table 1 describes demographic characteristics of the sample. The majority of patients were Caucasian, well educated and had a moderate or high family income. The partners were all female spouses of the men with diagnosed prostate cancer. Most of the spouses were Caucasian and reported diverse educational backgrounds.
Table 1.
Sample Characteristics
| Characteristics | Middle Age | Young-Old | Old-Old | |||
|---|---|---|---|---|---|---|
| Age in years | Patient | Spouse | Patient | Spouse | Patient | Spouse |
| n = 23 | n = 23 | n = 23 | n = 23 | n = 23 | n = 23 | |
| Mean | 57.4 | 53.6 | 69.3 | 65.9 | 76.3 | 71.6 |
| SD | 4.3 | 5.0 | 2.5 | 4.9 | 1.8 | 5.5 |
| Range | 50–64 | 45–62 | 65–73 | 53–73 | 75–80 | 58–77 |
| Education in years | ||||||
| Mean | 15.5 | 14.4 | 15.0 | 13.9 | 14.9 | 14.2 |
| SD | 3.5 | 2.5 | 3.4 | 2.9 | 3.3 | 2.8 |
| Range | 8–25 | 12–19 | 9–24 | 8–20 | 8–21 | 8–20 |
| Race (%) | ||||||
| Caucasian | 22 (96%) | 22 (96%) | 19 (82%) | 19 (82%) | 18 (78%) | 16 (69%) |
| African American | 0 | 0 | 2 (9%) | 4 (18%) | 5 (22%) | 4 (18%) |
| Hispanic | 0 | 1 (4%) | 2 (9%) | 0 | 0 | 1 (4%) |
| Native American | 1 (4%) | 0 | 0 | 0 | 0 | 2 (9%) |
| Occupation (%) | ||||||
| Professional | 17 (74%) | 14 (61%) | 15 (65%) | 13 (57%) | 16 (70%) | 13 (57%) |
| Non-professional | 6 (26%) | 8 (35%) | 8 (35%) | 10 (43%) | 7 (30%) | 10 (43%) |
| Missing | 1 (4%) | |||||
| Employment Status | ||||||
| In the labor force | 15 (65%) | 12 (52%) | 5 (22%) | 3 (14%) | 4 (18%) | 4 (18%) |
| Not in labor force | 8 (35%) | 4 (18%) | 18 (78%) | 10 (43) | 18 (78%) | 13 (56%) |
| Homemaker | 0 | 7 (30%) | 0 | 10 (43%) | 1 (4%) | 6 (26 %) |
| Length of Marriage | Middle Age | Young-Old | Old-Old | |||
| Mean | 28.7 | 40.9 | 41.9 | |||
| SD | 8.2 | 11.0 | 13.8 | |||
| Range | 7–39 | 14–57 | 5–56 | |||
| Income: % couples | ||||||
| $5,000–15,000 | 0 | 4% | 4% | |||
| $15,001–30,000 | 4% | 13% | 26% | |||
| $30,001–50,000 | 17% | 22% | 13% | |||
| $50,001–75,000 | 13% | 44% | 22% | |||
| $>75,000 | 66% | 13% | 27% | |||
| Missing | 0 | 4% | 8% | |||
| Diagnosis: (%) | ||||||
| Localized | 15 (65%) | 13 (57%) | 10 (44%) | |||
| Rising PSA | 2 (9%) | 4 (17%) | 5 (22%) | |||
| Advanced | 6 (26%) | 6 (26%) | 8 (34%) | |||
Instruments
Quality of Life
The Medical Outcomes Study Short Form Health Survey (MOS SF-12), a general health survey scale of physical and mental health [55], was used as a measure of quality of life. The general nature of the instrument makes it appropriate for both patients and spouses. Higher scores indicate better quality of life. Extensive psychometric testing has been completed on this instrument, including studies with cancer patients, and the instrument has shown strong evidence of validity and reliability [55].
In addition, the Expanded Prostate Cancer Index Composite (EPIC) scale was used as a specific measure of physical symptoms associated with prostate cancer and its treatment. The EPIC is a 50-item, self-report instrument assessing function and bother related to urinary, sexual, bowel and hormonal symptoms in men with prostate cancer occurring during the previous four weeks [56]. Scores are rated on a Likert-type scale from no trouble (0) to a lot of trouble (3). Summative scores for each of the four symptom subscales were used, with higher scores indicating less bother and better function. Internal consistency for the EPIC was established at .82 and test-retest reliability was .80. Content and construct validity has been reported by Wei and colleagues [56]. Internal consistency alpha coefficients for each subscale were satisfactory in this sample (urinary = .86; bowel = .77; sexual = .93; and hormone = .79).
The spouse version of the EPIC is a measure of the spouse’s perception of the bother that her husband’s symptoms caused her. The instrument consists of four questions developed to measure spouses’ perception of bother caused by their husbands’ prostate cancer treatment (urinary, sexual, bowel and hormonal symptoms) during the previous four weeks. Scores are rated on a five-point Likert-type scale from no problem (1) to big problem (5) (e.g. How much has your husband’s or partner’s sexual function, such as his degree of sexual desire, the frequency and quality of his erections, or the level of sexual activity, been a problem for you during the last four weeks?). Each question is scored independently with lower scores indicating less perceived bother. Concurrent validity of the spouse version of the EPIC was obtained from the significant correlation between patient’s EPIC scores and spouses’ scores on the spouse version of the EPIC [53].
Appraisal of Illness/Caregiving
Appraisal of illness/caregiving was assessed using the Appraisal of Illness Scale (AIS) for the patient and the Appraisal of Caregiving Scale (ACS) for spouses [57]. Both scales have 27 items. Summative scores were used, with higher scores indicating more negative appraisal. The intensity of each item is measured on a five-point Likert-type scale from very false to very true (e.g. I haven’t been doing very well since this situation started). Construct validity of the scales was established by Oberst [57], and the internal consistency alpha coefficient of the AIS in the present study was .96 and of the ACS was .83.
Self-Efficacy
Self-efficacy was measured using a modified version of the Lewis Cancer Self-Efficacy Scale (CASE) [58]. This original Likert-type scale was modified to a 17-item scale used previously in a study of dyads with breast cancer [59]. An item example is: I have the ability to take the necessary steps to work through the demands from cancer and its treatment. A summative score was obtained, with higher scores indicating greater confidence in the ability to manage the illness. The internal consistency alpha coefficient of the original scale was .97 and evidence of content and criterion validity has been reported by Lewis [58]. The internal consistency alpha coefficient for the current study was .96 for the patient group and .97 for the spouse group.
Concurrent concerns
Concurrent stressors were measured using the Omega Screening Questionnaire (OSQ) as developed by Weisman and Worden and adapted by Mood and associates (1989). The OSQ is composed of 4 parts, (a) demographic and background information, (b) health history, (c) inventory of current concerns, and (d) symptom scale. The Inventory of Current Concerns (ICC) is a 40-item scale that asks subjects to rate the extent to which they had concerns about finances, children, work, etc. during the last month. Higher scores indicated more concurrent concerns. Internal consistency of the ICC was reported as .93 in research by Northouse et al. (1999). In the present study the internal consistency alpha coefficient was .95 for the patients and .93 for the caregivers.
Phase of Illness
Phase of illness was identified by the referral source and validated in the patient’s medical record. Phases of illness included newly diagnosed, rising PSA, and advanced. Participants were categorized as advanced disease vs. newly diagnosed or rising PSA for the analysis.
Patient-Spouse Age Discordance
Dyads were stratified into age groups using the patient’s age. Consequently, spouses were not always in the same discreet age group as the patient (67% were in the same age group and 23% were younger than the patients; no spouses were in an older age category than the patients). We categorized this discordance as younger vs. same age category for the analysis.
Data Analysis
Descriptive statistics were calculated for all study variables. ANCOVAs (controlling for phase of illness and patient-spouse age discordance) were used to determine differences in quality of life among the three age groups. Individual multivariate ANCOVAs (MANCOVA) were used to determine differences in prostate cancer symptoms, concurrent concerns, self-efficacy and appraisal of illness by age groups for patients and for spouses. MANCOVA permits the analysis of several dependent variables simultaneously by combining the information from different variables into an overall significance test and is appropriate for use with a small sample size [61]. When significant differences between groups were found, univariate analyses and Tukey post-hoc comparison tests were performed to further explain the differences.
Results
Quality of Life by Age Cohort
Patient results
One-way ANCOVAs conducted on the quality of life measure, the SF-12, for the patient group showed that the age groups differed on both the physical and the mental components scores of the SF-12 (Table 2). Tukey post-hoc comparisons indicated that the young-old group had significantly better physical quality of life than the middle age group and the old-old group. In addition, the young-old group had better mental quality of life than the middle age group.
Table 2.
Age Differences in Quality of Life Among Patients
| Variable | Late Middle Age (1) M ( SD) | Young-old (2) M (SD) | Old-old (3) M (SD) | F | p | Post-hoc |
|---|---|---|---|---|---|---|
| QOL (SF-12) a | ||||||
| Physical component | 43.05 (13.78) | 51.66 (5.2) | 39.06 (9.53) | 9.16 | .001 | 2>1,3 |
| Mental component | 49.15 (9.18) | 54.95 (5.56) | 50.23 (6.74) | 4.03 | .02 | 2>1 |
| Physical Symptoms b,* | 2.89 | .006 | ||||
| EPIC Urinary c | 73.77 (20.05) | 85.33 (12.95) | 76.36 (16.72) | 2.40 | .09 | N.S. |
| Epic Bowel c | 86.68 (10.25) | 87.11 (14.23) | 83.40 (9.68) | .36 | .70 | N.S. |
| EPIC Sexual c | 23.57 (26.17) | 36.41 (28.61) | 20.66 (17.95) | 2.40 | .09 | N.S |
| EPIC Hormonal c | 70.26 (21.05) | 86.77 (12.67) | 82.02 (13.10) | 7.40 | .001 | 1>2,3 |
| Psychosocial Variables b | 3.97 | .005 | ||||
| Concurrent concernsb | 17.96 (12.75) | 12.51 (12.89) | 21.95 (18.17) | 2.35 | .10 | N.S. |
| Self-efficacy c,** | 136.57 (25.05) | 154.92 (17.76) | 142.48(25.10) | 3.06 | .05 | 2>1 |
| Appraisal of Illness c,*** | 2.69 (1.02) | 1.87 (.67) | 2.84 (.89) | 8.38 | .001 | 1,3>2 |
ANOVA;
MANOVA
Post-hoc test
All analyses controlled for phase of illness and patient-spouse age discordance (younger vs. same age category)
higher scores = less bother and better function
higher scores= more positive results; more self-efficacy to manage the illness or treatment associated with it
higher scores = more negative results of the illness
Spouse results
One-way ANCOVAs used to examine possible differences in quality of life by age groups for spouses revealed a significant difference between the three age groups on the physical component score of the SF-12, but there was no significant difference among groups on the mental component score (Table 3). Tukey post-hoc testing of the significant univariate effects indicated that the spouses in the middle age group had better physical quality of life than spouses in the old-old group but not better than spouses in the young-old age group.
Table 3.
Age Differences in Quality of Life, Self-Efficacy, and Appraisal of Caregiving Among Spouses
| Variable | Middle Age (1) M (SD) | Young-old (2) M (SD) | Old-old (3) M (SD) | F | p | Post-hoc |
|---|---|---|---|---|---|---|
| Quality of Life a | ||||||
| Physical component | 53.52 (10.18) | 48.51 (8.9) | 43.70 (11.68) | 5.85 | .005 | 1>3 |
| Mental component | 49.38 (11.70) | 53.70 (6.3) | 50.77 (11.58) | 1.94 | .15 | N.S. |
| Physical Symptoms b, * | 3.24 | .002 | ||||
| EPIC Urinary c | 2.04 (1.46) | 1.35 (.77) | 1.90 (1.18) | 2.10 | .13 | N.S. |
| EPIC Bowel c | 1.17 (.49) | 1.39 (1.19) | 1.81 (1.03) | 1.75 | .18 | N.S. |
| EPIC Sexual c | 3.17 (1.37) | 1.65 (1.19) | 1.95 (1.32) | 8.63 | .001 | 1>2,3 |
| EPIC Hormonal c | 2.30 (1.22) | 1.39 (.89) | 2.38 (1.20) | 5.70 | .005 | 1,3 >2 |
| Psychosocial Variables b | 0.19 | .94 | ||||
| Concurrent Concerns | 16.07 (10.66) | 16.23 (12.63) | 17.17 (16.23) | -- | -- | N.S. |
| Self-efficacy ** | 138.96 27.57) | 143.00(24.45) | 141.48(23.96) | -- | -- | N.S. |
| Appraisal of Caregiving*** | 2.52 (.51) | 2.43 (.53) | 2.51 (.60) | -- | -- | N.S. |
ANOVA;
MANOVA
Post-hoc test
All analyses controlled for phase of illness and patient-spouse age discordance (younger vs. same age category)
lower scores = less perceived bother
higher scores= more positive results; more self-efficacy to manage the illness or treatment associated with it
higher scores = more negative results of the illness
Patient symptom results
Using symptom subscales scores, MANCOVA showed a significant multivariate age group effect, F = 2.53(10), p = .01 (Table 2). Inspection of the univariate results showed that age groups for patients were significantly different for hormonal symptom distress, but not for distress related to urinary, sexual and bowel symptoms. Post-hoc testing showed that the middle age patient group had significantly more bother related to hormonal problems than the young-old or the old-old groups. Group differences for urinary (p =.06) and sexual (p = .08) problems approached statistical significance. The mean scores for both urinary and sexual symptom distress were higher for the young-old group than either of the other two groups indicating a trend toward less bother and better function for the young-old group. Variance in scores for each group in the sexual component was high indicating that men experienced varying degrees of difficulty.
Spouse symptom results
Using the spouses’ symptom subscale scores (EPIC), MANCOVA showed significant multivariate age group effects. Inspection of the univariate effects showed a significant difference among the three age groups in their perception of symptom problems related to sexual and hormonal problems. Post-hoc testing showed middle age spouses reported significantly more bother related to their husbands’ sexual problems than spouses in the young-old group and old-old group. The young-old group reported significantly less bother related to their husbands’ hormonal problems than either the middle age or old-old groups.
Psychosocial Variables
Patient results
Using the self-efficacy and appraisal of illness scores, MANCOVA indicated that there was a significant multivariate age group effect for these psychosocial variables, F = 3.12(6), p =.01. Inspection of the univariate results showed that age groups for patients were significantly different in levels of self-efficacy and appraisal of illness, but there was no difference on concurrent concerns (Table 2). Post-hoc testing, used to determine the nature of the differences, indicated that the young-old group had higher self-efficacy scores than both the middle age group and the old-old group. In addition, the young-old group had lower negative appraisal of illness than the middle age group and the old-old group.
Spouse results
MANCOVA showed no significant multivariate effect for age group differences related to self-efficacy, appraisal of caregiving or concurrent concerns.
Relationship between Patient and Spouse
Correlation coefficients were used to determine if there was a relationship between patients’ and spouses’ physical and mental quality of life. Results indicated an inverse relationship between patients’ physical quality of life and spouses’ physical quality of life for the young-old group only (r = −.44). There were no other significant relationships between the patients’ physical and/or mental quality of life and the spouses’ physical and/or mental quality of life.
Discussion
This study examined physical and mental quality of life, appraisal, self-efficacy and concurrent concerns among couples with prostate cancer according to age cohort: middle age (50–64); young-old (65–74); and old-old (75–84).
Quality of Life
Findings from this study indicate that patients in the young-old group (65–74) experienced better outcomes in several areas than did patients in the middle age and old-old groups. First, young-old patients reported better physical quality of life than patients in the other two groups. As expected, patients in the old-old group had the lowest mean score for physical quality of life of the three groups. Results of this study suggest that older men who are experiencing physical decline as a result of aging may find it difficult to manage the additional burden of a prostate cancer treatment regimen, which may affect not only their physical quality of life but also their mental quality of life. The lower physical quality of life reported by patients in the old-old group in this study is consistent with findings from other studies of older prostate cancer patients [11, 34, 62, 63].
Second, young-old patients also had better mental health QOL than the middle age group. Developmentally, members in the young-old group (65–74 years) have completed many of their life goals, reached retirement and still experienced fairly good overall health prior to the diagnosis of prostate cancer. The lower quality of life of patients in the middle age group (50–64 years) may be due to disruption from cancer in their daily lives, including work and social activities. Reports of lower mental health in younger men is consistent with recent research which has shown that cancer and its treatment may affect involvement in valued activities and interests more in younger people [11]. The old-old group scores for mental quality of life were significantly lower than the young-old group. This finding of lower mental quality of life was in contrast to other research findings which indicate that older people experience less psychological distress when diagnosed with cancer than younger people.
In regard to spouses, there was a significant difference in the physical quality of life of spouses in the middle age and old-old groups: spouses in the middle age group (50–64 years) reported better physical QOL than spouses in the old-old group (75–84 years). Because co-morbid conditions develop as a person ages, this is not a surprising finding. Spouses in the middle age group (mean age 53.6 years) were much younger than spouses in the old-old group (mean age 71.6 years). There were no significant differences for mental QOL for the spouses in the three age groups.
Symptom distress related to hormonal therapy was more problematic for men in the middle age group than men in the young-old group. Side effects of hormone therapy include hot flashes and loss of libido. Because of the increased desire for companionship and intimacy common during middle age [3], these side effects may have created more bother for middle age men than men in the young-old group. While there was not a significant difference among the three age groups for distress related to urinary or sexual symptoms, it should be noted that the variance in scores for each group in the EPIC sexual component was high, indicating that within each group some of the men did experience a higher level of bother and decreased function than the mean scores would indicate. Treatment for prostate cancer can affect urinary and sexual function, and research also has shown that the impact of both urinary and sexual function is affected by older age [25, 33, 34, 64].
The spouse’s version of the EPIC, which was discussed earlier, is a measure of the spouse’s perception of bother her husband’s symptoms caused her. Results indicated that spouses of middle age men (50–64 years) experienced more bother related to their husbands’ sexual problems than spouses in the other two groups. This is in contrast to patients’ findings related to sexual problems, which did not differ significantly by age group. In this study, wives may have been more willing to report problems related to the change in their sexual relationship with their husband than their husbands reported, a result also found in a study by Gray, Phillips and associates [39]. In addition, other research also supports the partner’s concern with their changing sexual relationship [27, 65]. Litwin and colleagues found that erectile dysfunction had a significant negative correlation with marital interaction [66]. Findings would suggest that sexual counseling offered following a diagnosis of prostate cancer should be extended to partners to help facilitate the dyads’ successful adaptation to treatment outcomes.
It is interesting to note that spouses in the middle age and old-old groups reported significantly more problems with the symptoms experienced by their husbands related to hormone therapy than the young-old group. Changes from hormone therapy include loss of libido, sexual dysfunction and fatigue [67]. In other studies, women have reported that the husbands’ lack of interest in them caused by loss of libido affected their own self-esteem [32]. It is possible that the middle aged spouse had more problems with the hormone effect on sexual function and libido while spouses in the old-old group had more problems with the hormone effect causing fatigue. Wives’ level of perceived disturbance related to hormone therapy is an interesting finding and needs further exploration.
Psychosocial Factors
Patients in the young-old group (65–74 years) reported higher self-efficacy when compared to patients in the middle age group but not so compared with the old-old group. Reaching life goals and flexibility of schedules, more common in the young-old group, could contribute to feelings of confidence and be associated with higher levels of self-efficacy. Middle age men who are still active in the workforce may have had less confidence that they could manage the additional challenges presented by the side effects of prostate cancer, including urinary incontinence.
Patients in the young-old group had a less negative appraisal of illness than either of the other age groups. In other words, patients in the young-old group (65–74) found the diagnosis of prostate cancer less threatening than either the middle age or old-old groups of men. These findings were consistent with those of other researches who have found that younger age was associated with more stressful appraisal of the cancer experience [38]. Since more middle age men are working, they may experience more disruption to their work schedule and may experience more negative effects on their financial situation as a result of treatment for prostate cancer than young-old men. Old-old men may already be experiencing stress related to aging and other co-existing health conditions so that the diagnosis of prostate cancer and its treatment is seen as one more stress to manage which in turn negatively affects their appraisal of illness. Ficarra and colleagues found that older men had more emotional limitations following prostate cancer treatment, difficulty with role performance and lower energy/fatigue status [68].
For the most part there were no significant relationships between patients’ and caregivers’ quality of life, with one exception. Among the young-old age group, there was an inverse relationship between patient and spouse physical QOL. As patients reported more physical problems their spouses reported fewer. This finding was unexpected given that the literature indicates that if the patient has more problems, the spouse may be at risk for immediate and long term physical and psychological negative effects [69, 70]. It is possible that the sample (N=69) may not have been large enough to demonstrate other significant correlations between patient and partner quality of life. It is also possible that, in this young-old group, spouses may have minimized their own physical concerns because of concern for their husband.
Limitations of this study include use of a convenience sample. Because this was a cross sectional design, causal directions cannot be inferred. Second, the quality of life variable was classified as physical and mental QOL to be consistent with the literature and the way the construct was operationalized and measured using the SF-12. However, it should be noted that there could be a degree of conceptual overlap between physical and mental functioning. Finally, the sample was composed primarily of middle and upper middle class, well-educated, Caucasian couples and, therefore, is not reflective of the ethnic/racial diversity of the population as a whole.
Conclusions
Findings in this study indicate that differences in quality of life exist among age groups of prostate cancer patients and spouses above and beyond the extent of disease. More research, however, is needed to further understanding of how each life stage affects the ability of couples to adjust to illness. The findings provide preliminary information about how life stage might affect patients’ and spouses’ experiences with prostate cancer. Middle age, young-old and old-old prostate cancer survivors and their partners may benefit from tailored interventions designed to improve quality of life and confidence in managing treatment outcomes during the survivorship period. Information from this study provides a starting point in understanding the influence of age on the individuals’ response to prostate cancer.
Acknowledgments
This study was funded in part by a NCI grant (R01CA90739-01) to L. Northouse (P.I.)
References
- 1.Jemal A, Siegal R, Ward E, Murray T, Xu J, Thun M. Cancer Statistics, 2007. CA: A Cancer Journal for Clinicians. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Levinson D. A conception of adult development. American Psychologist. 1986;41(1):1–13. [Google Scholar]
- 3.Rowland J. Developmental states and adaptation: Adult model. In: Holland J, Rowland J, editors. Handbook of Psycho-oncology: Psychological Care of the Patient with Cancer. New York: Oxford University Press, Inc; 1990. pp. 25–43. [Google Scholar]
- 4.McCubbin M. Family Stress theory and the development of nursing knowledge about family adaptation. In: Fathom SL, et al., editors. The Nursing of Families: Theory, Research, Education, and Practice. Newborn Park: Sage Publiscations, Inc; 1993. pp. 46–58. [Google Scholar]
- 5.Baltes PB. Theorectical propositions of life-span developmental psychology: on dynamics between growth and decline. Developmental Psychology. 1987;23(5):611–626. [Google Scholar]
- 6.Brandtstadter J, Renner G. Tenacious goal pursuit and flexible goal adjustment: explication and age-related analysis of assimilative and accommodative strategies of coping. Psychology and Aging. 1990;5(1):58–67. doi: 10.1037//0882-7974.5.1.58. [DOI] [PubMed] [Google Scholar]
- 7.Newman B, Newman P. Development through Life: A Psychosocial Approach. Belmont, CA: Wadsworth Publishing Company; 1999. [Google Scholar]
- 8.Baltrusch HF, et al. Psychosocial stress, aging, and cancer. Annals New York Academy of Sciences. 1988;521:1–15. doi: 10.1111/j.1749-6632.1988.tb35261.x. [DOI] [PubMed] [Google Scholar]
- 9.Deimling GT, et al. Cancer-related health worries and psychological distress amond older adult, long-term cancer survivors. Psycho-Oncology. 2006;15:306–320. doi: 10.1002/pon.955. [DOI] [PubMed] [Google Scholar]
- 10.Rusteon T, Moum T, Wiklund I, Hanestad BR. Quality of life in newly diagnosed cancer patients. Journal of Advanced Nursing. 1999;29(2):490–498. doi: 10.1046/j.1365-2648.1999.00912.x. [DOI] [PubMed] [Google Scholar]
- 11.Devins G, et al. Context moderates illness-induced lifestyle disruptions across life domains: A test of the illness instrusiveness theoretical framework in six common cancers. Psycho-Oncology. 2006;15:221–233. doi: 10.1002/pon.940. [DOI] [PubMed] [Google Scholar]
- 12.Deimling GT, et al. The health of older-adult, long-term cancer survivors. Cancer Nursing. 2005;28(6):415–424. doi: 10.1097/00002820-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Hewitt M, Rowland J, Yancik R. Cancer survivors in the United States: Age, health, and disability. Journal of Gerontology: Medical Sciences. 2003;58(1):82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 14.Cimprich B, Ronis DL. Age at diagnosis and quality of life in breast cancer survivors. Cancer Practice. 2002;10(2):85–93. doi: 10.1046/j.1523-5394.2002.102006.x. [DOI] [PubMed] [Google Scholar]
- 15.Nijboer C, Triemstra M, Tempelaar R, Mulder M, Sanderman R, van de Bos G. Patterns of caregiving experiences among partners of cancer patients. The Gerontologist. 2000;40(6):738–746. doi: 10.1093/geront/40.6.738. [DOI] [PubMed] [Google Scholar]
- 16.Bull M. Factors influencing family caregiver burden and health. Western Journal of Nursing Research. 1990;12(6):758–776. doi: 10.1177/019394599001200605. [DOI] [PubMed] [Google Scholar]
- 17.Carter PA, Acton GJ. Personality and Coping: Predictors of depression and sleep problems among caregivers of individuals who have cancer. Journal of Gerontological Nursing. 2006;32(2):45–53. doi: 10.3928/0098-9134-20060201-11. [DOI] [PubMed] [Google Scholar]
- 18.Schulz R, Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. JAMA. 1999;282(23):2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 19.McCorkle R, Pasacreta JV. Enhancing Caregiver Outcomes in Palliative Care. Cancer Control. 2001;8(1):36–45. doi: 10.1177/107327480100800106. [DOI] [PubMed] [Google Scholar]
- 20.Grov EK, Dahl AA, Moun T, Fossa SD. Anxiety, depression, and quality of life in caregivers of patients with cancer in late palliative phase. Anals of Oncology. 2005;16:1185–1191. doi: 10.1093/annonc/mdi210. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz M, et al. Depression and physical health among family caregivers of geriatric patients with cancer - a longitudinal view. Medical Science Monitor. 2004;10(8):CR447–456. [PubMed] [Google Scholar]
- 22.Zahn L. Quality of life: Conceptual and measurement issues. Journal of Advanced Nursing. 1992;17:795–800. doi: 10.1111/j.1365-2648.1992.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 23.Krongrad A, Litwin S, Lai H, Lai S. Dimensions of quality of life in prostate cancer. The Journal of Urology. 1998;160:807–810. doi: 10.1016/S0022-5347(01)62792-7. [DOI] [PubMed] [Google Scholar]
- 24.Eton DT, Lepore S. Prostate cancer and health-related quality of life: A review of the literature. Psycho-Oncology. 2002;11:307–326. doi: 10.1002/pon.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu JC, Elkin EP, Pasta DJ, Lubeck DP, Katttan MW, Carroll PR, Litwin MS. Predicting quality of life after radical prostatectomy: results from CaPSURE. The Journal of Urology. 2004;171:703–708. doi: 10.1097/01.ju.0000107964.61300.f6. [DOI] [PubMed] [Google Scholar]
- 26.Litwin MS, Sadetsky N, Pasta DJ, Lubeck DP. Bowel function and bother after treatment for early stage prostate cancer: a longitudinal quality of life analysis from CaPSURE. The Journal of Urology. 2004;172:515–519. doi: 10.1097/01.ju.0000129236.56712.e7. [DOI] [PubMed] [Google Scholar]
- 27.Boehmer UaRB. Facing erectile dysfunction due to prostate cancer treatment: Perspectives of men and their partners. Cancer Investigation. 2004;22(6):840–848. doi: 10.1081/cnv-200039641. [DOI] [PubMed] [Google Scholar]
- 28.Brar R, Maliski SL, Swan L, Krupski T, Litwin M. Changes in quality of life among low-income men treated for prostate cancer. Urology. 2005;66(2):344–349. doi: 10.1016/j.urology.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Deliveliotis C, Liakouras C, Delis A, Skolarikos A, Varkarakis J, Protogerou V. Prostate operations: long-term effects on sexual and urinary function and quality of life. Comparison with an age-matched control population. Urologic Research. 2004;32(4):283–289. doi: 10.1007/s00240-004-0411-0. [DOI] [PubMed] [Google Scholar]
- 30.Weber BaPS-N. Psychosocial consequences of prostate cancer: 30 years of research. Geriatric Nursing. 2005;26(3):166–75. doi: 10.1016/j.gerinurse.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Fitch M, Gray R, Franssen R, Johnson E. Men’s perspective on the impact of prostate cancer: implications for oncology nurses. Oncology Nursing Forum. 2000;27(8):1255–63. [PubMed] [Google Scholar]
- 32.Harden J, Schafenacker A, Northouse LL, Mood D, Smith D, Pienta K, Hussain M, Baranowski K. Couples’ experiences with prostate cancer: focus group research. Oncology Nursing Forum. 2002;29(4):701–709. doi: 10.1188/02.ONF.701-709. [DOI] [PubMed] [Google Scholar]
- 33.Hedestig O, Sandman P, Tomic R, Widmark A. Living after external beam radiotherapy of localized prostate cancer. Cancer Nursing. 2005;28(4):310–317. doi: 10.1097/00002820-200507000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Ward-Smith P, Kapitan D. Quality of life among men treated with radiation therapy for prostate cancer. Urologic Nursing. 2005;25(4):263–268. [PubMed] [Google Scholar]
- 35.Yang bK, Crisci A, Young MD, Silverstein AD, Peterson B, Dahm P. Cross-sectional survey of long-term quality of life after radical perineal prostatectomy. Urology. 2005;65(1):120–125. doi: 10.1016/j.urology.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 36.Novan LMA. Advanced prostate cancer patients’ relationships with their spouses following hormonal therapy. European Journal of Oncology Nurses. 2003;7:73–80. doi: 10.1016/s1462-3889(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 37.Northouse LLMD, Templin T, Mellon S, George T. Couples patterns of adjustment to colon cancer. Social Science Medicine. 2000;50:271–284. doi: 10.1016/s0277-9536(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 38.Bowman KF, Deimling GT, Smerglia V, Sage P, Kahana B. Appraisal of the cancer experience by older long-term survivors. Psycho-Oncology. 2003;12:226–238. doi: 10.1002/pon.630. [DOI] [PubMed] [Google Scholar]
- 39.Gray RE, Fitch M, Phillips C, Labrecque M, Fergus K. Managing the Impact of Illness: the experiences of men with prostate cancer and their spouses. Journal of Health Psychology. 2000;5(4):531–548. doi: 10.1177/135910530000500410. [DOI] [PubMed] [Google Scholar]
- 40.Northouse LL, Dorris G, Charron-Moore CT, Templin Mood D. Couples’ adjustment to breast cancer during the first year following diagnosis. Journal of Behavioral Medicine. 2001;24(2):115–136. doi: 10.1023/a:1010772913717. [DOI] [PubMed] [Google Scholar]
- 41.McPherson CP, Swenson KK, Kjellberg J. Quality of life inpatients with prostate cancer. Seminars in Oncology Nursing. 2001;17(2):138–146. doi: 10.1016/s0749-2081(01)80021-8. [DOI] [PubMed] [Google Scholar]
- 42.Given BA, Given CW, Stommel M, Azzouz F. The impact of new demands for assistance on caregiving depression: Tests using the inception cohort. The Gerontologist. 1999;39(1):76–85. doi: 10.1093/geront/39.1.76. [DOI] [PubMed] [Google Scholar]
- 43.Aldwin CM, et al. Age differences in stress, coping, and appraisal: Findings from the Normative Aging Study. The Journals of Gerontology. 1996;51B(4):P179–P188. doi: 10.1093/geronb/51b.4.p179. [DOI] [PubMed] [Google Scholar]
- 44.Kim J, Keshian J. Old, Old caregivers: a growing challenge for community health nurses. Journal of Community Health Nursing. 1994;11(2):63–70. doi: 10.1207/s15327655jchn1102_1. [DOI] [PubMed] [Google Scholar]
- 45.Bandura A. Self-efficacy. In: Ramachaudran VS, editor. Encyclopedia of Human Behavior. Vol. 4. 1994. pp. 71–81. [Google Scholar]
- 46.Kurlowicz LH. Perceived self-efficacy, functional ability, and depressive symptoms in older elective surgery patients. Nursing Research. 1998;47(4):219–226. doi: 10.1097/00006199-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Campbell LC, et al. Prostate cancer in African Americans: relationship of patient and partner self-efficacy to quality of life. Journal of Pain and Symptom Management. 2004;28(5):433–444. doi: 10.1016/j.jpainsymman.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 48.McCubbin M. Family stress theory and the development of nursing knowledge about family adaptation. Newborn Park: Sage Publications; 1993. [Google Scholar]
- 49.Kornblith A, Herr HW, Ofman US, Scher HI, Holland JC. Quality of life of patient’s with prostate cancer and their spouses: the value of data base in clinical care. Cancer. 1994;73(11):2791–2802. doi: 10.1002/1097-0142(19940601)73:11<2791::aid-cncr2820731123>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 50.Eton DT, Lepore SJ, Helgeson VS. Psychological distress in spouses of men trated for early-stage prostate carcinoma. Cancer. 2005;103(11):2412–2418. doi: 10.1002/cncr.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holicky R. Caring for the caregivers: The hidden victim of illness and disability. Rehabilitation Nursing. 1996;21:247–252. doi: 10.1002/j.2048-7940.1996.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 52.Given CW, Stommel M, Collins C, Kings S, Given BA. Responses of elderly spouse caregivers. Research in Nursing and Health. 1990;13:77–85. doi: 10.1002/nur.4770130204. [DOI] [PubMed] [Google Scholar]
- 53.Northouse LL, Mood DW, Montie JE, Sandler HM, Forman JD, Hussain M, Pienta KJ, Smith DC, Sanda MG, Kershaw T. Living with prostate cancer: Patients’ and spouses’ psychosocial status and quality of life. Journal of Clinical Oncology. 2007 doi: 10.1200/JCO.2006.09.6503. (accepted for publication) [DOI] [PubMed] [Google Scholar]
- 54.Cohen J. A Power Primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 55.Ware JJ, Kosinski M, Keeler SD. A 12-item Short-Form Health Survey: Construction of scale and preliminary tests of reliability and validity. Medical Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 57.Oberst MT. Appraisal of Caregiving Scale: Manual for Use. Detroit, MI: Wayne State University; 1991. [Google Scholar]
- 58.Lewis F. Family Visitation Study Final Report: National Cancer Institute. 1996. [Google Scholar]
- 59.Northouse LLMD, Kershaw T, Schafenacker A, Mellon S, Walker J, Galvin E, Decker V. Quality of life of women with recurrent breast cancer and their family members. Journal of Clinical Oncology. 2002;20(19):4050–4064. doi: 10.1200/JCO.2002.02.054. [DOI] [PubMed] [Google Scholar]
- 60.Northouse LL, Caffey M, Deichelbohrer L, Schmidt L, Guziatek-Trojniak L, West S, et al. The quality of life of African American women with breast cancer. Research in Nursing & Health. 1999;22:444–460. doi: 10.1002/1098-240x(199912)22:6<449::aid-nur3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 61.Nunnally JCBIH. Psychometric Theory. 3. New York: McGraw-Hill, Inc; 1994. [Google Scholar]
- 62.Crowe H, Costello A. Prostate cancer: Perspectives on quality of life and impact of treatment on patients and their partners. Urologic Nursing. 2003;23(4):279–285. [PubMed] [Google Scholar]
- 63.Clark JA, et al. Changes in quality of life following treatment for early prostate cancer. Urology. 1999;53(1):161–168. doi: 10.1016/s0090-4295(98)00457-9. [DOI] [PubMed] [Google Scholar]
- 64.Litwin MS, Melmed GY, Nakazon T. Life after radical prostatectomy: a longitudinal study. The Journal of Urology. 2001;166:587–592. [PubMed] [Google Scholar]
- 65.Soloway CT, et al. Sexual, psychological and dyadic qualities of the prostate cancer ‘couple’. BJU International. 2005;95:780–785. doi: 10.1111/j.1464-410X.2005.05400.x. [DOI] [PubMed] [Google Scholar]
- 66.Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. Journal of General Internal Medicine. 1998;13:159–165. doi: 10.1046/j.1525-1497.1998.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Litwin MS, Shpall A, Dorey F, Nguyen T. Quality of life outcomes in long term survivors of advanced prostate cancer. Americal Journal of Oncology. 1998;21(4):327–332. doi: 10.1097/00000421-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Ficarra V, Novara G, Galfano A, Stringari C, Baldassarre R, Cavelleri S, Artibani W. Twelve-month self-reported quality of life after retropubic radical prostatectomy: a prospective study with Rand 36-Item Health Survey (Short Form-36) BJU International. 2005;97:274–278. doi: 10.1111/j.1464-410X.2005.05893.x. [DOI] [PubMed] [Google Scholar]
- 69.Neundorfer MM. Coping and health outcomes in spouse caregivers of persons with dementia. Nursing Research. 1991;40(5):260–265. [PubMed] [Google Scholar]
- 70.Wallhagen MI. Caregiving demands: their difficulty and effects on the well being of elderly caregivers. Scholarly Inquiry for Nursing Practice: An International Journal. 1992;6(2):111–127. [PubMed] [Google Scholar]