Abstract
Although the role of systemic activation of the nuclear factor κB (NF-κB) pathway in septic coagulation has been well documented, little is known about the contribution of endothelial-specific NF-κB signaling in this pathologic process. Here, we used transgenic mice that conditionally overexpress a mutant I-κBα, an inhibitor of NF-κB, selectively on endothelium, and their wild-type littermates to define the role of endothelial-specific NF-κB in septic coagulation. In wild-type mice, lipopolysaccharide (LPS) challenge (5 mg/kg intraperitoneally) caused markedly increased plasma markers of coagulation, decreased plasma fibrinogen level, and widespread tissue fibrin deposition, which were abrogated by endothelial NF-κB blockade in transgenic mice. Endothelial NF-κB blockade inhibited tissue factor expression in endothelial cells, but not in leukocytes. Endothelial NF-κB blockade did not inhibit LPS-induced tissue factor expression in heart, kidney, and liver. Endothelial NF-κB blockade prevented LPS down-regulation of endothelial protein C receptor (EPCR) and thrombomodulin protein expressions, inhibited tissue tumor necrosis factor-α converting enzyme activity, reduced EPCR shedding, and restored plasma protein C level. Our data demonstrate that endothelial intrinsic NF-κB signaling plays a pivotal role in septic coagulation and suggests a link between endothelial-specific NF-κB activation and the impairment of the thrombomodulin-protein C-EPCR anticoagulation pathway.
Introduction
Sepsis is a systemic inflammatory response syndrome that can lead to multiple sequelae,1,2 including shock, disseminated intravascular coagulation (DIC), and multiple organ failure/injury (MOF/I). Although interrelated and generally coexistent, each of the septic complications can be an independent clinical entity and cause of mortality. Sepsis as it progresses leads almost invariably to hemostatic abnormalities and coagulopathies,2,3 which are thought to be a major cause of septic MOF/I.
Sepsis causes coagulation by multiple mechanisms. One mechanism that has been extensively studied is the activation of nuclear factor κB (NF-κB)–mediated procoagulant pathways. NF-κB mediates the expression of tissue factor (TF) and factor VIII,3–6 which activate the extrinsic and intrinsic coagulation pathway, leading to thrombin generation and coagulation. Thrombin produced during coagulation activates NF-κB–dependent genes,7 forming a positive feedback loop that further amplifies coagulation. NF-κB mediates type 1 plasminogen-activator inhibitor (PAI-1) expression.8 An increased level of PAI-1 impairs fibrinolysis, promoting coagulation.
Sepsis or inflammation suppresses anticoagulant mechanisms, particularly the thrombomodulin, protein C, and endothelial protein C receptor (TM-PC-EPCR) anticoagulation pathway.3,9–17 The TM-PC-EPCR system is the most important natural anticoagulation mechanism.9–11 During thrombosis and inflammation, thrombin released binds to its receptor TM, and PC binds to its receptor, EPCR, forming a thrombin-TM-PC-EPCR complex on the endothelial surface. This leads to the formation of activated protein C (APC). APC has multiple anticoagulant and anti-inflammatory properties.9–13,18–22 Suppression of the TM-PC-EPCR system during sepsis disrupts the balance between procoagulant and anticoagulant mechanisms, resulting in enhanced coagulation and DIC.
Although the significance of systemic activation of the NF-κB pathway in the pathogenesis of septic coagulation has been well documented, the role of activation of the endothelial cell–specific NF-κB pathway in this pathologic process remains unknown because of lack of an investigative tool. We have recently generated transgenic mice (TG) that conditionally overexpress a mutant I-κBα (I-κBαmt), an inhibitor of NF-κB, selectively on the endothelium using the tetracycline-regulated gene expression system.23 Using this mouse strain, we have previously shown that selective blockade of the endothelial NF-κB pathway inhibited adhesion molecule expression, reduced neutrophil infiltration, alleviated MOF/I, and improved survival in septic mice,23 demonstrating a crucial role for endothelial NF-κB in septic MOF/I. We have also observed that TG mice had a reduced plasma level of thrombin-antithrombin, suggesting that endothelial NF-κB may play a role in septic coagulation.23 However, it is difficult to draw a conclusion based on one plasma marker. Thus, the role that endothelial intrinsic NF-κB plays in septic coagulation has not been definitively elucidated. More importantly, the mechanisms by which activation of the endothelial intrinsic NF-κB pathway leads to septic coagulation and DIC have not been identified. In addition, although sepsis is well known to impair the TM-PC-EPCR anticoagulation pathway,3,9–17 the mechanisms underlying this impairment remain elusive.
The aims of this study were to define the role of endothelial intrinsic NF-κB signaling in septic coagulation and DIC and to elucidate the mechanisms involved. We also examined the interrelation between endothelial NF-κB activation and impairment of the TM-PC-EPCR anticoagulation pathway. Our data demonstrate that endothelial intrinsic NF-κB signaling plays a pivotal role in septic coagulation and suggests a link between endothelial NF-κB signaling and the impairment of TM-PC-EPCR anticoagulation pathway.
Methods
Animal groups
The generation and characterization of the TG mice that conditionally overexpress I-κBαmt selectively on endothelium have been previously described in detail.23 Here, we used this mouse strain to define the role of endothelial intrinsic NF-κB signaling in septic coagulation. We studied 4 groups of mice: TG-negative control (wild-type [WT]-Con), TG-negative lipopolysaccharide (WT-LPS), TG-positive control (TG-Con), and TG-positive LPS (TG-LPS) mice. To avoid any potential effect of doxycycline (Dox), which was used to induce I-κBαmt expression in TG mice, we fed all mice with Dox (1.5 mg/mL) in drinking water for 4 days before experimentation. Mice in WT-Con and TG-Con groups were injected with saline (1 mL/kg, intraperitoneally), and in WT-LPS and TG-LPS groups injected with Escherichia coli LPS (5 mg/kg, intraperitoneally). At 6 or 24 hours after LPS injection, blood and organs were collected for further analysis. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research and complied with the National Institutes of Health Guide for the Care and Use of Experimental Animals.
Assays for plasma levels of coagulation parameters
Blood was withdrawn into a syringe containing 3.2% trisodium citrate(9 volumes of blood to 1 volume of trisodium citrate) via cardiac puncture, centrifuged at 1500g for 15 minutes, and plasma collected and kept at −70°C for use. Plasma levels of fibrinogen, D-dimer, and PAI-1 were measured using the fibrinogen determination kit (Dade Behring), the D-dimer enzyme immunoassay kit (Diagnostica Stago), and the mouse PAI-1 ELISA kit (Molecular Innovations).
Immunohistochemical staining
For detection of fibrin deposition, mice were anticoagulated by intravenous injection of heparin (200 U) at 6 hours after LPS, killed, and perfused with 10% formalin. Heart, liver, and kidney were removed, fixed in 10% zinc formalin solution, and embedded in paraffin. Tissue sections (6 μm) were prepared, dewaxed, rehydrated, and washed with acidic formalin to wash out soluble fibrinogen/fibrin. Sections were blocked with blocking solution and incubated overnight with a rabbit anti–human fibrin antibody (Dako North America). Specific binding was detected with biotinylated secondary antibody–horseradish peroxidase complexes using VECTASTAIN Elite ABC kits (Vector Laboratories). Antigen-antibody complexes were visualized using 3′, 3′-diaminobenzidine (Vector Laboratories). Sections were counterstained with hematoxylin, mounted, and viewed under light microscope. Similar protocol was used to stain for cellular TF expression. In a separate set of experiments, heart, liver, and kidney sections were prepared at 24 hours after LPS injection and stained for fibrin deposition.
Immunofluorescence staining
Cryosections (6 μm) were prepared from kidney of each group of mice at 6 hours after saline or LPS injection, fixed with paraformaldehyde, permeabilized, blocked, and incubated with rabbit anti–mouse TF antibody (Santa Cruz Biotechnology) overnight at 4°C followed by washing and incubation with fluorescein isothiocyanate–conjugated secondary antibody. After washing 3 times, sections were incubated with rat anti–mouse CD31 antibody (BD Biosciences) overnight at 4°C and than Alexa 594–conjugated secondary antibody (BD Biosciences). Sections were mounted and viewed under confocal microscope.
Assay for protein C and activated protein C
Blood was collected into citrate buffer containing 20 mM benzamidine. Plasma levels of PC and APC were determined by enzyme capture assay. Briefly, anti–mouse PC and APC antibody (100 μg/mL; GenWay Biotech) was immobilized in microtiter plates overnight. After washing and blocking of the plates, plasma samples supplemented with benzamidine were incubated in the wells for 4 hours to capture PC and APC. The plates were then washed to remove plasma constituents and benzamidine. The SPECTROZYME Pca chromogenic substrate (American Diagnostica) was added to each well. Absorbance at 405 nm was read immediately (time 0) and again after a 30-minute incubation at 37°C (time 30). The amidolytic activity of the captured APC was determined by subtracting the absorbance at time 0 from that at time 30.
Total PC was measured by activating the bound PC in each well by Protac (American Diagnostica), a rapid PC activator, and then measuring the amidolytic activity with the SPECTROZYME APC chromogenic substrate (American Diagnostica) as described earlier in “Assay for protein C and activated protein C.”
Cell isolation and culture
Microvascular endothelial cells (ECs) were isolated from lungs of WT and TG mice, cultured, and plated out as we have previously described.23 Subconfluent cells were incubated with Dox (0.5 μg/mL) for 48 hours to induce I-κBαmt expression. ECs were then left untreated or stimulated with 100 ng/mL mouse recombinant tumor necrosis factor-α (TNF-α; R&D Systems) for 1 (for p65 detection), 6 (for TF detection), or 14 hours (for EPCR and TM determination) before cell harvest and protein extraction.
Whole-blood white blood cells were obtained by a 20-minute centrifugation of blood samples collected from WT and TG mice at 6 hours after intraperitoneal saline or LPS injection (5 mg/kg). Erythrocytes were depleted by washing the cell pellets with red blood cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM ethylenediaminetetraacetic acid). Protein was extracted from remaining cells for TF determination.
Western blot
Membrane, nuclear, or total protein was extracted from heart, kidney, and liver or from endothelial or white blood cells. Equal amounts of protein were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electroblotted onto nitrocellulose membrane. Western blot was performed as we have previously described23 using TM or EPCR-specific antibodies (R&D Systems) or TF (Santa Cruz Biotechnology, no. sc-30201; and American Diagnostics, no. 4515), p65 (Santa Cruz Biotechnology), or actin (Santa Cruz Biotechnology) specific antibodies.
Quantitative RT-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) and treated with RNase-free DNase I to remove traces of genomic DNA. Each RNA sample was subjected to quantitative reverse transcription and polymerase chain reaction (RT-PCR) using TaqMan One-Step RT-PCR Master Mix Reagents (Applied Biosystems), and gene-specific primers and probes (nos. 26 and 81 of the Universal Probe Library; Roche). Primers used were: TM, forward, 5′-ATGCGTGGAGCATGAGTG-3, and reverse, 5′-CTGGCATCGAGGAAGGTC-3′; and EPCR, forward, 5′-AGCGCAAGGAGAACGTGT-3′ and reverse, 5′-GGGTTCAGAGCCCTCCTC-3′. Quantitative RT-PCR was performed in 7900 HT thermocycler (Applied Biosystems). Results were analyzed using the δ-δ Ct method. TM and EPCR gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase mRNA (TaqMan RNA Reagents; Applied Biosystems).
Measurement of tissue TNF-α converting enzyme activity
TNF-α converting enzyme (TACE) activity was measured using fluorogenic peptide substrate as previously described.24 In brief, cytoplasmic protein (40 μg for kidney and 20 μg for liver) was mixed with 10 μM peptide substrate, Abz-LAQAVRSSSR-Dpa, in TACE reaction buffer and incubated at room temperature. Increase in fluorescence reading was monitored using spectrofluorophotometer (Shimadzu) at excitation of 320 nm and emission of 420 nm for 15 minutes. TACE activity was expressed as fluorescence unit per minute.
Survival studies
WT and TG mice were injected with E coli LPS (5 mg/kg intraperitoneally), and survival was monitored for up to 14 days.
Statistical analysis
Data are mean plus or minus SEM and analyzed using analysis of variance or Kruskal-Wallis rank test, followed by multiple pairwise comparisons using the Holm-Sidak method or Tukey test. We considered P values less than .05 significant.
Results
Blockade of endothelial-specific NF-κB signaling reduced plasma markers of coagulation
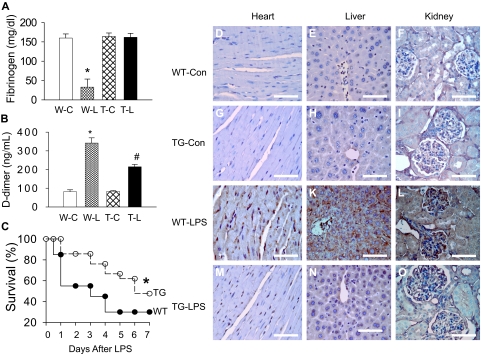
We have previously demonstrated that our TG mice overexpress I-κBαmt selectively on endothelial cells and displayed endothelial restricted blockade of NF-κB activation.23 Here, we used this mouse strain to define the role of endothelial-specific NF-κB signaling in septic coagulation. We measured plasma markers of coagulation at 6 hours after LPS challenge. Plasma level of fibrinogen was comparable between WT-Con and TG-Con mice but was reduced by approximately 80% in WT-LPS mice (Figure 1A), indicating consumption of fibrinogen as a consequence of clot formation. In contrast, the plasma fibrinogen level in TG-LPS mice was comparable with that of control mice (Figure 1A). Consistent with the reduced plasma level of fibrinogen, WT-LPS mice showed a significantly elevated plasma level of D-dimer, the degradation product of fibrin (Figure 1B). The LPS-induced plasma level of D-dimer was reduced by approximately 37% in TG-LPS (Figure 1B).
Figure 1.
Blockade of endothelial NF-κB signaling attenuated coagulation and improved survival. (A-B) Endothelial NF-κB blockade restored plasma fibrinogen level and reduced plasma D-dimer level. Plasma was collected from WT-Con (W-C), WT-LPS (W-L), TG-Con (T-C), and TG-LPS (T-L) groups of mice at 6 hours after saline or LPS injection. Plasma levels of D-dimer and fibrinogen were determined using commercial kits. Data are mean ± SEM of 6 mice. *P < .001 compared with any other group. #P < .001 compared with the WT-LPS group. (C) Endothelial NF-κB blockade improved survival. WT and TG mice were injected with LPS (5 mg/kg intraperitoneally) and followed for 14 days (no further mortality after 7 days). *P < .001, compared with WT mice (log-rank test, 20 mice per group). (D-O) Endothelial NF-κB blockade reduced tissue fibrin deposition. Paraffin-embedded sections were prepared at 6 hours after saline or LPS injection, dewaxed, rehydrated, blocked, reacted with fibrin/fibrinogen-specific antibody, and counterstained with hematoxylin. The dark brown horseradish peroxidase reaction product shows fibrin deposition in microvasculature and arterioles of heart (J), liver (K), and kidney (L) sections from WT-LPS mice. No fibrin deposition was observed in the 3 organs of WT-Con (D-F) and TG-Con (G-I) mice. The dark brown staining was significantly reduced in tissue sections from TG-LPS (M-O) mice. Bar represents 85 μm. Slides were viewed with an Olympus BH2 microscope (Olympus America) using an Splan 40PL lens at 40×/0.70 and Permount mounting medium (Fisher Scientific). Images were acquired using a Nikon DS camera (model DS-U2 cooled), and were processed with Nikon NIS-Elements basic research software and Adobe Photoshop Version 7.0 software.
Blockade of endothelial-specific NF-κB signaling reduced tissue fibrin deposition
The effect of blocking the endothelial intrinsic NF-κB pathway on coagulation was further evaluated by immunohistochemical staining of fibrin deposition. Heart, kidney, and liver were collected from the 4 groups of mice at 6 hours after saline or LPS injection, and stained for fibrin. No fibrin deposition was detected in any tissue section from WT-Con and TG-Con mice (Figure 1D-I). Fibrin deposition was evident in tissue sections of heart, liver, and kidney of WT-LPS mice (Figure 1J-L), but not evident in tissue sections from TG-LPS mice (Figure 1M-O). These results define a pivotal role of endothelial intrinsic NF-κB signaling in LPS-induced coagulation and clot formation.
To ascertain that inhibition of NF-κB activation within endothelium does not simply delay coagulation activation, we stained for fibrin deposition on sections of heart, liver, and kidney at 24 hours after LPS injection. Likewise, tissue sections from WT-LPS mice showed a significant fibrin deposition, which was not evident in tissue sections from TG-LPS mice (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These results confirm that blockage of endothelial intrinsic NF-κB signaling mitigated, not simply delayed, LPS-induced coagulation.
Blockade of endothelial-specific NF-κB signaling improved survival
Compared with WT-LPS mice, TG-LPS mice displayed a significantly improved survival. The 2-day survival rate was 55% and 86%, and 7-day survival rate was 30% and 48% for WT-LPS and TG-LPS groups of mice (Figure 1C), respectively. Taken together, these results demonstrate a pathogenic role of endothelial intrinsic NF-κB signaling in LPS-induced coagulation and mortality.
Blockade of endothelial-specific NF-κB signaling had no effect on tissue TF level
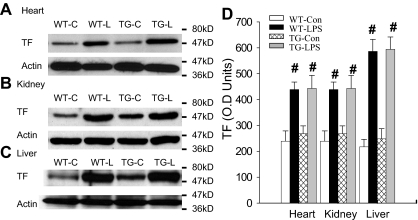
LPS initiates the coagulation cascade primarily by activating TF-mediated extrinsic coagulation pathway.3–5 LPS activates NF-κB, which up-regulates TF expression,3–5 resulting in the activation of coagulation. We have therefore examined whether inhibition of endothelial NF-κB signaling prevents or reduces LPS-induced TF expression. Figure 2A-C depicts Western blot photographs showing the tissue level of TF protein in heart, kidney, and liver. Figure 2D summarizes the densitometry quantification of TF bands. Depending on organ, WT-Con and TG-Con mice showed a variable level of basal TF protein expression, which was significantly up-regulated in WT-LPS and TG-LPS mice. Tissue TF protein levels were comparable between WT-LPS and TG-LPS mice in all 3 organs examined (Figure 2), indicating that blockade of endothelial NF-κB signaling had no significant effect on overall tissue level of TF induced by LPS.
Figure 2.
Blockade of endothelial NF-κB signaling had no effects on tissue levels of TF. (A-C) Western blot photographs showing levels of TF in heart (A), kidney (B), and liver (C) of WT-Con (WT-C), WT-LPS (WT-L), TG-Con (TG-C), and TG-LPS (TG-L) mice at 6 hours after saline or LPS injection. Actin indicates membrane for TF blotting was reblotted with actin antibody. (D) Densitometry quantification of TF bands. Compared with WT-Con and TG-Con, WT-LPS and TG-LPS mice showed significantly increased tissue levels of TF in all 3 organs. Data are mean ± SEM of 5 mice. #P < .01 compared with the WT-Con and TG-Con groups.
To confirm the specificity of TF antibody, we analyzed the same group of 4 liver protein samples on parallel Western blots using 2 anti-TF antibodies from different suppliers. The 2 antibodies detected the same size TF band and revealed an identical pattern of changes in TF expression in the 4 groups of mice (supplemental Figure 2). We repeated the same experiment using another group of 4 liver proteins and obtained similar result (supplemental Figure 2). These results confirm the specificity of anti-TF antibody.
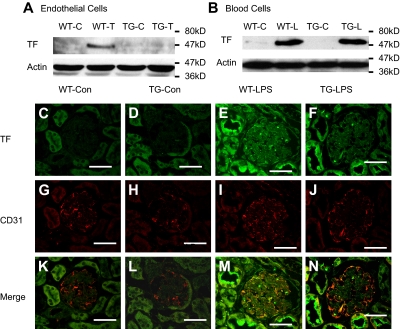
Failure to inhibit LPS-induced TF expression in TG mice raised several questions. Do ECs of those mice express TF in response to LPS? If so, is the inducible TF expression regulated by NF-κB? To answer these questions, we examined TF expression in ECs and white blood cells isolated from WT and TG mice. As depicted in Figure 3A, the TF band was detected in proteins from TNF-α–stimulated WT ECs, but not TG ECs. By contrast, identical TF bands were detected in proteins from LPS-stimulated WT and TG white blood cells (Figure 3B). These data demonstrate that ECs of those mice express TF and that the expression of TF is NF-κB dependent. This conclusion is further supported by our immunofluorescence double-staining results, which showed that TF and CD31 double-positive cells increased significantly in kidney section from WT-LPS, but not TG-LPS mice (Figure 3M-N). Immunohistochemical stains of kidney sections from WT-LPS mice showed TF expression in vascular wall cells, which was greatly reduced in kidney sections from TG-LPS mice (supplemental Figure 3).
Figure 3.
Endothelial NF-κB blockade inhibited endothelial TF expression. (A-B) Endothelial NF-κB blockade diminished TNF-α–induced TF protein in ECs (A), but not LPS-induced TF protein in white blood cells (B). WT and TG ECs were treated with Dox (0.5 μg/mL) for 48 hours and then left untreated (WT-C and TG-C) or stimulated with TNF-α (100 ng/mL) for 6 hours (WT-T and TG-T). White blood cells were isolated from WT-Con (WT-C), WT-LPS (WT-L), TG-Con (TG-C), and TG-LPS (TG-L) mice at 6 hours after saline or LPS injection. TF band was not detected in proteins from control cells but induced in TNF-α–stimulated ECs (WT-T) or LPS-stimulated blood cells (WT-L). The TNF-α–induced TF band was prevented in TG ECs (TG-T). The LPS-induced TF band was not affected in TG white blood cells (TG-L). Blots are representative of 3 independent experiments. Actin indicates membrane for TF blotting was reblotted with actin antibody. (C-N) Representative immunofluorescence staining showing endothelial TF expression. Cryosections of kidney were prepared from mice at 6 hours after LPS injection and stained with anti-TF antibody (green) and anti-CD31 (an endothelial-specific marker) antibody (red). Positive staining for TF (E-F) was detected on sections from WT-LPS and TG-LPS mice. TF and CD31 double-positive staining (yellow) localizes TF-expressing ECs (M). TF-expressing ECs (yellow) were not detected in sections from WT-Con and TG-Con mice (K-L), increased in section from WT-LPS mice (M), and significantly reduced on section from TG-LPS mice (N). Bar represents 50 μm. Data are representative of 3 independent experiments. Slides were viewed with a confocal laser-scanning microscope system (FluoView 300-IX; Olympus) using a PLAN APO 60×/1.4 oil objective lens and VECTASHIELD Mounting Medium (Vector Laboratories). Images were processed and analyzed using Image J with colocalization plug-ins that statistically evaluate colocalization using Pearson correlation coefficient (r), which varies from −1 to 1, where 1 equals complete colocalization.
Blockade of endothelial-specific NF-κB signaling restored plasma level of APC
Coagulation during sepsis is a combined result of activating procoagulant pathways and impairing anticoagulant pathways. Because endothelial NF-κB blockade had no significant effect on tissue level of TF, we next examined whether selective blockade of endothelial NF-κB signaling protects anticoagulant pathways by determining plasma levels of APC in WT-LPS and TG-LPS mice, which is a major natural anticoagulation mechanism. Plasma levels of total PC and APC were identical between WT-Con and TG-Con groups of mice but diminished in WT-LPS mice (Table 1). Compared with WT-LPS mice, TG-LPS mice showed significantly higher plasma levels of total PC and APC (Table 1). This result indicates that blockade of endothelial NF-κB activation reverses LPS-induced suppression of APC production, implying that activation of endothelial intrinsic NF-κB pathway impairs the APC anticoagulation mechanism.
Table 1.
Blockade of endothelial NF-κB signaling restored plasma levels of TPC and APC
| WT-Con | TG-Con | WT-LPS | TG-LPS | |
|---|---|---|---|---|
| TPC, mOD units | 73.4 ± 2.4 | 72.0 ± 3.2 | 26.0 ± 6.2* | 58.5 ± 4.7† |
| APC, mOD units | 45.6 ± 1.6 | 43.0 ± 1.3 | 2.8 ± 1.9* | 29.9 ± 4.1† |
Plasma was collected at 6 hours after LPS. Plasma levels of total (TPC) and activated protein C (APC) were determined by enzyme capture assay. Data are mean ± SEM of 7 mice.
mOD indicates milli-optical density.
P < .02 compared with any other group.
P < .02 compared with WT-LPS group.
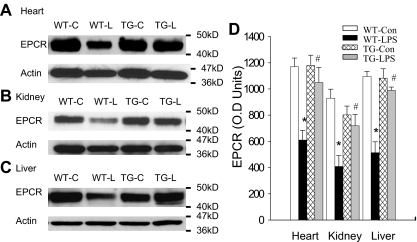
Blockade of endothelial NF-κB signaling prevented EPCR down-regulation
EPCR and TM are the 2 key components of the TM-PC-EPCR anticoagulation system9–11 that controls APC production. To elucidate how endothelial NF-κB activation impairs APC production during endotoxemia, we compared the tissue level of EPCR protein in the absence or presence of endothelial NF-κB blockade. A high tissue level of EPCR protein was detected in heart, kidney, and liver of WT-Con and TG-Con mice (Figure 4). Compared with WT-Con and TG-Con mice, WT-LPS mice displayed a dramatically reduced tissue level of EPCR protein in all 3 organs (Figure 4). By contrast, tissue levels of EPRC protein in the 3 organs of TG-LPS mice were significantly higher than that of WT-LPS mice and were similar to that of control mice (Figure 4). These results illustrate that blockade of endothelial specific NF-κB signaling prevents LPS-induced EPCR down-regulation.
Figure 4.
Blockade of endothelial NF-κB signaling reversed LPS-induced EPCR down-regulation. (A-C) Western blot photographs showing levels of EPCR protein in heart (A), kidney (B), and liver (C) of WT-Con (WT-C), WT-LPS (WT-L), TG-Con (TG-C), and TG-LPS (TG-L) mice at 6 hours after saline or LPS injection. Actin indicates membrane for EPCR blotting was reblotted with actin antibody. (D) Densitometry quantification of EPCR bands. Tissue levels of EPCR protein were comparable in all 3 organs between WT-Con and TG-Con mice and were greatly reduced in these organs of WT-LPS mice. Reduction in tissue levels of EPCR protein was not observed in these organs of TG-LPS mice. Data are mean ± SEM of 6 to 8 mice. *P < .001, compared with any other group. #P < .01, compared with the WT-LPS group.
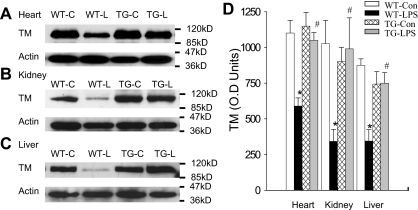
Blockade of endothelial NF-κB signaling prevented TM down-regulation
Likewise, endothelial NF-κB blockade abrogated LPS down-regulation of TM protein. As expected, WT-Con and TG-Con mice had a high tissue level of TM protein, and WT-LPS mice had a very low tissue level of TM protein in heart, kidney, and liver (Figure 5). TG-LPS mice showed a tissue level of TM protein that was comparable with that of WT-Con and TG-Con mice, and was significantly higher than that of WT-LPS mice in all 3 organs (Figure 5), indicating that activation of the endothelial intrinsic NF-κB pathway results in a reduced TM protein level.
Figure 5.
Blockade of endothelial NF-κB signaling prevented LPS-induced TM down-regulation. (A-C) Western blot photographs showing levels of TM protein in heart (A), kidney (B), and liver (C) of WT-Con (WT-C), WT-LPS (WT-L), TG-Con (TG-C), and TG-LPS (TG-L) mice. Actin indicates membrane for TM blotting was reblotted with actin antibody. (D) Densitometry quantification of TM bands. Tissue level of TM protein was high in all 3 organs of WT-Con and TG-Con mice, greatly reduced in organs of WT-LPS mice, and restored in the 3 organs of TG-LPS mice. Data are mean ± SEM of 6 to 8 mice. *P < .001 compared with any other group. #P < .02 compared with the WT-LPS group.
LPS up-regulated EPCR and TM mRNA expression
We next examined whether LPS reduces EPCR and TM proteins by inhibiting their gene expression. We quantified tissue levels of EPCR and TM mRNA in kidney using quantitative RT-PCR. Compared with WT-Con and TG-Con, WT-LPS mice showed a 7.2- and 5.4-fold increase in tissue levels of EPCR and TM mRNAs (Table 2). In TG-LPS mice, the LPS-induced EPCR mRNA expression was significantly inhibited, and the LPS-induced TM mRNA expression was not significantly affected, although there was a trend toward inhibition (Table 2). These results exclude the possibility that LPS reduces tissue levels of EPCR and TM proteins by inhibiting their mRNA expression.
Table 2.
LPS up-regulated EPCR and TM mRMA expression
| WT-Con | TG-Con | WT-LPS | TG-LPS | |
|---|---|---|---|---|
| EPCR, % GAPDH | 119 ± 28 | 88 ± 22 | 863 ± 170* | 189 ± 84*† |
| TM, % GAPDH | 75 ± 12 | 65 ± 7 | 411 ± 170* | 252 ± 74* |
Kidney was collected at 6 hours after LPS. EPCR and TM mRMA levels were quantified using quantitative RT-PCR and normalized to the level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. Data are mean ± SEM of 8 mice.
P < .01 compared with WT-Con and TG-Con groups.
P < .01 compared with WT-LPS group.
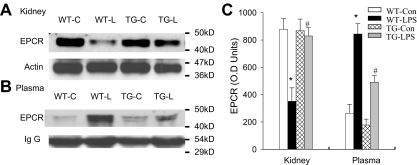
Blockade of endothelial NF-κB signaling reduced EPCR shedding
Because LPS did not inhibit EPCR and TM mRNA expression in our mouse model, we next examined whether LPS promotes EPCR protein degradation. We compared tissue and plasma levels of EPCR protein in the same groups of WT and TG mice. In WT-LPS mice, LPS caused a marked reduction in tissue level of EPCR in kidney (Figure 6A,C), and in parallel with this reduction, a significant elevation in plasma level of EPCR protein (Figure 6B-C). In TG-LPS mice whose endothelial NF-κB was inhibited, both the reduction in tissue EPCR level and the elevation in plasma EPCR level were prevented (Figure 6). The concomitant increase in plasma EPCR level and decrease in tissue EPCR level in WT-LPS mice indicate that LPS reduces tissue EPCR level by causing EPCR shedding from ECs. The parallel prevention of decrease in tissue EPCR level and increase in plasma EPCR level in TG-LPS mice illustrates that activation of endothelial intrinsic NF-κB signaling promotes endothelial EPCR shedding.
Figure 6.
Blockade of endothelial NF-κB signaling reduced endothelial EPCR shedding. (A-B) Western blot photographs comparing tissue (A, kidney) and plasma (B) levels of EPCR protein in same groups of WT-Con (WT-C), WT-LPS (WT-L), TG-Con (TG-C), and TG-LPS (TG-L) mice. Actin and IgG indicate membrane for EPCR blotting was reblotted with actin or IgG antibody. (C) Densitometry quantification of EPCR bands. Compared with WT-Con and TG-Con mice, WT-LPS mice showed a markedly reduced tissue level of EPCR, in parallel with a significantly elevated plasma level of EPCR, indicating EPCR shedding. TG-LPS mice abrogated the LPS-induced reduction in tissue EPCR level and elevation in plasma EPCR level. Data are mean ± SEM of 5 mice. *P < .001 compared with any other group. #P < .001 compared with the WT-LPS group.
Blockade of endothelial NF-κB signaling inhibited LPS-induced TACE activity
It is reported that TACE is responsible for EPCR shedding in cultured cells.14 To investigate how activation of endothelial specific NF-κB pathway promotes endothelial EPCR shedding, we examined the effects of endothelial NF-κB blockade on LPS-induced TACE activity in liver and kidney, 2 organs that showed a significantly reduced tissue level of EPCR. Compared with that in WT-Con and TG-Con mice, there was a 2- to 4-fold increase in TACE activity in liver and kidney of WT-LPS mice (Table 3). Tissue level of TACE activity was markedly reduced in the 2 organs of TG-LPS mice (Table 3). Taken together with the previous report demonstrating a critical role of TACE activity in EPCR shedding,14 these data suggest that increased tissue TACE activity is a likely mechanism linking endothelial NF-κB activation and endothelial EPCR shedding.
Table 3.
Blockade of endothelial NF-κB signaling reduced tissue TNF-α converting enzyme activity and plasma level of PAI-1
| WT-Con | TG-Con | WT-LPS | TG-LPS | |
|---|---|---|---|---|
| Kidney, FU/min | 0.111 ± 0.019 | 0.127 ± 0.015 | 0.454 ± 0.074* | 0.271 ± 0.041† |
| Liver, FU/min | 0.107 ± 0.006 | 0.111 ± 0.013 | 0.269 ± 0.032* | 0.150 ± 0.017† |
| PAI-1, ng/mL | 0.07 ± 0.05 | 0.00 ± 0.00 | 31.35 ± 4.79‡ | 18.14 ± 3.29§ |
Organs were collected at 6 hours after LPS. TNF-α converting enzyme activity was measured using fluorogenic peptide substrate and expressed as fluorescence units (FU) per minute. Data are mean ± SEM of 6 mice. Plasma was collected at 6 hours after LPS. Plasma level of PAI-1 was determined using mouse PAI-1 ELISA kit. Data are mean ± SEM of 6 mice.
P < .008 compared with any other group.
P < .008 compared with WT-LPS group.
P < .001, compared with any other group.
P < .001, compared with WT-LPS group.
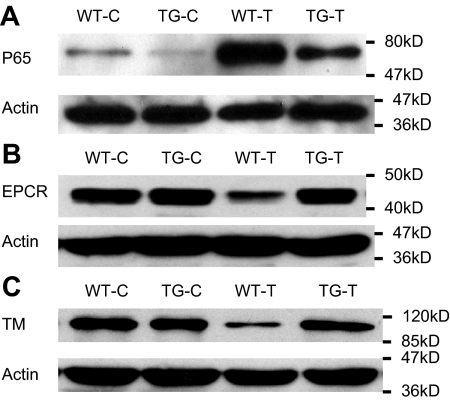
NF-κB activation resulted in reduced cellular levels of EPCR and TM proteins in cultured ECs
To further establish a link between endothelial NF-κB activation and EPCR/TM down-regulation, we examined whether alteration in cellular NF-κB activity directly affects cellular levels of EPCR and TM proteins in cultured ECs. Stimulation of WT ECs with TNF-α caused a marked NF-κB activation as indicated by increased p65 nuclear translocation (Figure 7A, WT-T), and significantly reduced cellular levels of EPCR and TM proteins (Figure 7B-C, WT-T). By contrast, TNF-α stimulation of TG ECs resulted in a significantly attenuated NF-κB activation (Figure 7A, TG-T) and no reduction in cellular levels of EPCR and TM proteins (Figure 7B-C, TG-T). These results support a link between endothelial NF-κB activation and EPCR/TM down-regulation.
Figure 7.
NF-κB activation resulted in reduced cellular levels of EPCR and TM in cultured ECs. WT and TG ECs were incubated with Dox (0.5 μg/mL) for 48 hours to induce I-κBαmt expression in TG ECs, and left untreated (WT-C and TG-C) or stimulated with 100 ng/mL TNF-α (WT-T and TG-T) for 1 (for measuring NF-κB activity) or 14 hours (for detecting EPCR and TM proteins). Bolts are representative of 3 experiments. (A) Representative Western blot photograph showing NF-κB activation measured by p65 nuclear translocation. TNF-α markedly activated NF-κB in WT ECs (WT-T), which was significantly inhibited in TG ECs (TG-T). (B) Representative Western blot photograph showing that TNF-α stimulation decreased cellular level of EPCR protein in WT ECs (WT-T), but not in TG ECs (TG-T). (C) Representative Western blot photograph showing that TNF-α stimulation decreased cellular level of TM protein in WT ECs (WT-T), but not in TG ECs (TG-T). Actin indicates membrane for p65, EPCR, or TM blotting was reblotted with actin antibody.
Blockade of endothelial NF-κB signaling reduced LPS-induced plasma PAI-1 level
NF-κB mediates PAI-1 gene expression.8 We have therefore examined the effect of endothelial NF-κB blockade on plasma levels of PAI-1 in the 4 groups of mice. Compared with the WT-Con and TG-Con groups, WT-LPS mice showed markedly elevated plasma level of PAI-1, which was significantly reduced in the TG-LPS group of mice (Table 3). This result suggests that increased PAI-1 production may also contribute to the coagulant activity of endothelial NF-κB activation.
Discussion
Despite the well-documented roles of systemic activation of NF-κB pathway and vascular endothelium in the regulation of coagulation during sepsis, the contribution of endothelial specific NF-κB signaling in this pathologic process has not been defined. We demonstrated here that endothelial intrinsic NF-κB signaling plays a pivotal role in the pathogenesis of coagulation and DIC during endotoxemia. WT-LPS mice displayed markedly elevated plasma markers of coagulation, decreased plasma level of fibrinogen, and widespread tissue fibrin deposition in multiple organs. By contrast, TG-LPS showed a normal plasma level of fibrinogen, significantly reduced plasma levels of coagulation markers, and diminished tissue fibrin deposition. Because NF-κB inhibition was restricted to ECs and the NF-κB pathway in other cell types was not affected in TG mice as we have previously demonstrated,23 the markedly attenuated coagulation in TG-LPS mice reveals a pivotal role of endothelial intrinsic NF-κB signaling in activation of coagulation during endotoxemia.
Several factors may contribute to the procoagulant activity of endothelial NF-κB activation. In the current study, we demonstrate that endothelial NF-κB activation impaired the TM-PC-EPCR anticoagulant mechanism. LPS challenge of WT mice resulted in dramatic reductions in plasma levels of total and activated protein C and markedly decreased tissue levels of TM and EPCR proteins in multiple organs, which were prevented by blockade of endothelial NF-κB pathway in TG mice. The anticoagulant, anti-inflammatory, and cytoprotective actions of APC and TM-PC-EPCR system are well documented.3,9–13,18–22 APC attenuates thrombin formation,18 enhances fibrinolysis,18 inhibits NF-κB activation,19 reduces the inflammatory cytokine release,19 maintains endothelial integrity,20 and protects cell from apoptosis.20,21 Heterozygous PC-deficient mice exhibited more thrombin generation, more severe coagulation, exacerbated systemic inflammation and organ damage, and reduced survival, compared with WT mice after LPS challenge.25–27 Reconstitution of the PC-deficient mice with exogenous APC improved survival.25 In addition to controlling APC generation, TM and EPCR also have direct anticoagulant and anti-inflammatory actions.28–31 Ablation of the EPCR gene exacerbated LPS-induced inflammation and tissue damage and increased mortality,30 whereas EPCR overexpression protected mice from LPS lethality.31 Collectively, these results suggest that impairment of TM-PC-EPCR anticoagulant pathway could contribute to development of coagulation and thrombosis after activation of endothelial intrinsic NF-κB pathway. We also demonstrate that selective blockade of endothelial NF-κB pathway reduced the plasma level of PAI-1, suggesting that increased PAI-1 production may also contribute to the development of coagulation caused by endothelial NF-κB activation.
APC was previously reported to inhibit NF-κB activation.19 Here, we show that NF-κB activation suppresses the TM-PC-EPCR pathway and inhibits APC production, illustrating a reciprocal inhibitory interaction between the NF-κB pathway and the TM-PC-EPCR pathway. This adds new information to our current knowledge on crosstalk between the 2 pathways.
Selective blockade of the endothelial NF-κB pathway failed to inhibit LPS-induced TF expression in multiple organs. This lack of effect cannot be interpreted as that ECs do not express TF or that EC TF expression is NF-κB independent. We showed that ECs expressed TF in vivo in response to LPS and in vitro in response to TNF-α and that the LPS- and TNF-α–induced TF expressions were inhibited by endothelial NF-κB blockade. A probable explanation is that the contribution of endothelial TF to the overall tissue level of TF is not significant compared with that of other cell types. Because the LPS-induced NF-κB activation and TF up-regulation in other cells were not affected by selective NF-κB blockade in ECs, the overall tissue level of TF was consequently not affected. This explanation is consistent with several previous reports showing that hematopoietic cells are the major source of the inducible TF during endotoxemia or sepsis.3,32 However, our results cannot exclude the possibility that endothelial TF contributes to the activation of coagulation cascades during endotoxemia. Such a conclusion would have to come from studies using mutant mice whose endothelial TF gene is selectively inactivated or deleted.
We observed an up-regulation of both TM and EPCR mRNAs in WT-LPS mice, which is in good agreement with a previous report showing that LPS up-regulates EPCR mRNA expression in endotoxemic mice.33 However, our results contrast sharply with the results from several prior studies demonstrating that TNF-α down-regulates EPCR16,17 and TM15 mRNA expression in cultured ECs. These conflicting results suggest differences in the regulation of in vitro and in vivo EPCR and TM gene expressions, and highlight the importance of in vivo investigation.
In parallel with gene up-regulation, EPCR and TM proteins were down-regulated by LPS in our mouse model. This excludes the possibility that LPS reduces EPCR or TM protein by suppressing their gene expression. We found that, in parallel with the diminished tissue level of EPCR, the plasma level of EPCR was significantly elevated in WT-LPS mice. Blockade of endothelial intrinsic NF-κB pathway in TG-LPS mice restored the tissue level of EPCR and concurrently reduced the plasma level of EPCR. These results suggest that LPS may down-regulate EPCR protein by promoting endothelial EPCR shedding, in which activation of endothelial intrinsic NF-κB pathway plays a role. This contention is consistent with our observation that NF-κB activation in WT ECs resulted in significantly reduced cellular levels of EPCR and TM proteins, and blockade of NF-κB activation in TG ECs resulted in increased cellular levels of EPCR and TM proteins. Up-regulation of EPCR mRNA in WT-LPS mice probably represents a compensatory mechanism to maintain cellular EPCR level. This explanation is consistent with our observation that decreased tissue EPCR protein level was associated with an increased tissue EPCR mRNA level in WT-LPS mice and that an increased tissue EPCR protein level was associated with a decreased tissue EPCR mRNA level in TG-LPS mice.
One likely mechanism underlying the increased endothelial EPCR shedding caused by endothelial NF-κB activation is up-regulation or activation of TACE, which results in endothelial EPCR shedding. In supporting this notion, Qu et al have previously shown that EPCR shedding is mediated by TACE activity in cultured cells.14 We demonstrated in current study that LPS increased TACE activity in organs where tissue level of EPCR protein was diminished. Blockade of endothelial NF-κB activation in TG-LPS mice reduced tissue TACE activity, which was associated with increased tissue levels of EPCR protein in the same organs.
Supplementary Material
Acknowledgments
The authors thank Dr J. W. Pollard and staff at Albert Einstein College of Medicine Transgenic and Gene Targeting Facility for help in generating transgenic mice.
This study was supported by the National Institutes of Health (grant GM063907) and the Faculty Award Program of the Feinstein Institute for Medical Research.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.S. and X.Y. performed research and analyzed data; H.X. performed research; and S.F.L. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shu Fang Liu, Long Island Jewish Medical Center, 270-05 76th Ave, Research Bldg, RM B371, New Hyde Park, NY 11040; e-mail: Sliu@lij.edu.
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Liu SF, Malik AB. NF-κB activation as a pathologic mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 3.Schouten M, Wiersinga WJ, Levi M, Van Der Poll T. Inflammation, endothelium and coagulation in sepsis. J Leukoc Biol. 2008;83(3):536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 4.Oeth PA, Parry GC, Kunsch C, Nantermet P, Rosen CA, Mackman N. Lipopolysaccharide induction of tissue factor gene expression in monocytic cells is mediated by binding of c-Rel/p65 heterodimers to a kappa B-like site. Mol Cell Biol. 2004;14(6):3772–3781. doi: 10.1128/mcb.14.6.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackman N, Brand K, Edgington TS. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med. 1991;174(6):1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueiredo MS, Brownlee GG. cis-acting elements and transcription factors involved in the promoter activity of the human factor VIII gene. J Biol Chem. 1995;270(20):11828–11838. doi: 10.1074/jbc.270.20.11828. [DOI] [PubMed] [Google Scholar]
- 7.Anrather D, Millan MT, Palmetshofer A, et al. Thrombin activates nuclear factor-kappaB and potentiates endothelial cell activation by TNF. J Immunol. 1997;159(11):5620–5628. [PubMed] [Google Scholar]
- 8.Hou B, Eren M, Painter CA, et al. Tumor necrosis factor alpha activates the human plasminogen activator inhibitor-1 gene through a distal nuclear factor kappaB site. J Biol Chem. 2004;279(18):18127–18136. doi: 10.1074/jbc.M310438200. [DOI] [PubMed] [Google Scholar]
- 9.Esmon CT. New mechanisms for vascular control of inflammation mediated by natural anticoagulatant proteins. J Exp Med. 2002;16(5):561–564. doi: 10.1084/jem.20021088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24(8):1374–1383. doi: 10.1161/01.ATV.0000134298.25489.92. [DOI] [PubMed] [Google Scholar]
- 11.Riewald M, Schuepbach RA. Protective signaling pathways of activated protein C in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(1):1–3. doi: 10.1161/ATVBAHA.107.157321. [DOI] [PubMed] [Google Scholar]
- 12.Taylor BF, Chank A, Esmon CT, et al. Protein C prevents the coagulopathic and lethal effects of E. coli infusion in the baboon. J Clin Invest. 1987;79(3):918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 14.Qu D, Wang Y, Esmon NL, Esmon CT. Regulated endothelial protein C receptor shedding is mediated by tumor necrosis factor-alpha converting enzyme/ADAM17. J Thromb Haemost. 2007;5(2):395–402. doi: 10.1111/j.1538-7836.2007.02347.x. [DOI] [PubMed] [Google Scholar]
- 15.Sohn RH, Deming CB, Johns DC, et al. Regulation of endothelial thrombomodulin expression by inflammatory cytokines is mediated by activation of nuclear factor-kappa B. Blood. 2005;105(10):3910–3917. doi: 10.1182/blood-2004-03-0928. [DOI] [PubMed] [Google Scholar]
- 16.Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269(42):26486–26491. [PubMed] [Google Scholar]
- 17.Nan B, Lin P, Lumsden AB, Yao Q, Chen C. Effects of TNF-alpha and curcumin on the expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Thromb Res. 2005;115(5):417–426. doi: 10.1016/j.thromres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Sakata Y, Loskutoff DJ, Gladson CL, Hekman CM, Griffin JH. Mechanism of protein C-dependent clot lysis: role of plasminogen activator inhibitor. Blood. 1986;68(6):1218–1223. [PubMed] [Google Scholar]
- 19.White B, Schmidt M, Murphy C, et al. Activated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kappaB (NF-kappaB) and tumour necrosis factor alpha (TNF-alpha) production in the THP-1 monocytic cell line. Br J Haematol. 2000;110(1):130–134. doi: 10.1046/j.1365-2141.2000.02128.x. [DOI] [PubMed] [Google Scholar]
- 20.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105(8):3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 21.Isermann B, Vinnikov IA, Madhusudhan T, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13(11):1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 22.Kerschen EJ, Fernandez JA, Cooley BC, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204(10):2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye X, Ding D, Zhou X, Chen G, Liu SF. Divergent roles of endothelial NF-κB in multiple organ injury and bacterial clearance in murine models of sepsis. J Exp Med. 2008;205(6):1303–1315. doi: 10.1084/jem.20071393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin G, Huang X, Black R, et al. Continuous fluorimetric assay for tumor necrosis factor-alpha converting enzyme. Anal Biochem. 2002;302(2):269–275. doi: 10.1006/abio.2001.5549. [DOI] [PubMed] [Google Scholar]
- 25.Lay AJ, Donahue D, Tsai MJ, Castellino FJ. Acute inflammation is exacerbated in mice genetically predisposed to a severe protein C deficiency. Blood. 2007;109(5):1984–1991. doi: 10.1182/blood-2006-07-037945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganopolsky JG, Castellino FJ. A protein C deficiency exacerbates inflammatory and hypotensive responses in mice during polymicrobial sepsis in a cecal ligation and puncture model. Am J Pathol. 2004;165(4):1433–1446. doi: 10.1016/S0002-9440(10)63401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levi M, Dörffler-Melly J, Reitsma P, et al. Aggravation of endotoxin-induced disseminated intravascular coagulation and cytokine activation in heterozygous protein-C-deficient mice. Blood. 2003;101(12):4823–4827. doi: 10.1182/blood-2002-10-3254. [DOI] [PubMed] [Google Scholar]
- 28.Uchiba M, Okajima K, Murakami K, Johno M, Okabe H, Takatsuki K. Recombinant thrombomodulin prevents endotoxin-induced lung injury in rats by inhibiting leukocyte activation. Am J Physiol. 1996;271(3):L470–L475. doi: 10.1152/ajplung.1996.271.3.L470. [DOI] [PubMed] [Google Scholar]
- 29.Conway EM, Van de Wouwer M, Pollefeyt S, et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappa B and mitogen-activated protein kinase pathways. J Exp Med. 2002;196(5):565–577. doi: 10.1084/jem.20020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwaki T, Cruz DT, Martin JA, Castellino FJ. A cardioprotective role for the endothelial protein C receptor in lipopolysaccharide-induced endotoxemia in the mouse. Blood. 2005;105(6):2364–2371. doi: 10.1182/blood-2004-06-2456. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Zhang X, Gu J, et al. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J Thromb Haemost. 2005;3(7):1351–1359. doi: 10.1111/j.1538-7836.2005.01385.x. [DOI] [PubMed] [Google Scholar]
- 32.Pawlinski R, Pedersen B, Schabbauer G, et al. Role of tissue factor and protease-activated receptors in a mouse model of endotoxemia. Blood. 2004;103(4):1342–1347. doi: 10.1182/blood-2003-09-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu JM, Katsuura Y, Ferrell GL, Grammas P, Esmon CT. Endotoxin and thrombin elevate rodent endothelial cell protein C receptor mRNA levels and increase receptor shedding in vivo. Blood. 2000;95(5):1687–1693. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.