Abstract
Purpose
To compare the sensitivity of the TonoLab rebound tonometer with the Tono-Pen in awake Brown Norway rats and to compare their ability to predict optic nerve damage induced by experimental IOP elevation.
Methods
TonoLab and Tono-Pen tonometers were calibrated in cannulated rat eyes connected to a pressure transducer. The TonoLab was used in awake animals housed in standard lighting to measure IOP during light and dark phases. Both instruments were used to monitor chronically elevated IOP produced by episcleral vein injection of hypertonic saline. Measured IOPs were correlated with quantified optic nerve damage in injected eyes.
Results
Although they were lower than transducer and Tono-Pen measurements at all levels, TonoLab readings showed an excellent linear fit with transducer readings from 20 to 80 mm Hg (R2 = 0.99) in cannulated eyes. In awake animals housed in standard lighting, the TonoLab documented significantly higher pressures during the dark phase (27.9 ± 1.7 mm Hg) than during the light phase (16.7 ± 2.3 mm Hg). With elevated IOP, correlation between TonoLab and Tono-Pen readings (R2 = 0.86, P < 0.0001) was similar to that in cannulated eyes. Although both instruments provided measurements that correlated well with optic nerve injury grade, only the Tono-Pen documented significant IOP elevation in eyes with the least amount of injury (P < 0.05).
Conclusions
The TonoLab is sensitive enough to be used in awake Brown Norway rats, though instrument fluctuation may limit its ability to identify significant pressure elevations in eyes with minimal optic nerve damage.
Glaucoma remains the second leading cause of blindness in the world, yet the mechanism of damage is unknown.1,2 Of the major risk factors for glaucoma, elevated intraocular pressure (IOP) remains the best known, and major trials suggest that lowering IOP has an important role in reducing the development of glaucomatous optic nerve damage in patients with ocular hypertension and in minimizing progressive vision loss in patients with early and advanced glaucoma.3–6 However, even in these studies, glaucoma continued to progress in some patients even after the desired level of pressure control was achieved. Thus, there remains a need for methods to preserve vision that can be used to augment traditional pressure control therapies.
To this end, significant interest has developed in understanding the mechanisms of glaucomatous optic neuropathy. Although several models have been used, those that rely on IOP elevation are immediately applicable to most patients with glaucoma. Such models, originally developed in primates,7,8 are anatomically relevant for the disease in humans.9–11 However, these models are unfeasible for research requiring large numbers of animals, such as cell biology studies and preclinical in vivo evaluations of prospective neuroprotective agents.
In the past 15 years, investigators have developed more cost-effective models of chronically elevated IOP in rodents, incorporating a wide array of experimental methods.12–16 Recently developed genetic models and experimental techniques to elevate IOP in mice have further improved the potential for understanding the mechanisms of damage from elevated IOP.17–19
All these models require accurate, reproducible, and non-invasive measurement of IOP. For more than a decade, the Tono-Pen (Mentor, Norwell, MA) has been used for this purpose in rats. Although initially used with general anesthesia,20,21 we later showed that the Tono-Pen could provide meaningful data in awake animals, with enough sensitivity to identify even subtle, circadian fluctuations in IOP.22 Measuring IOP in awake animals avoids the pressure-lowering effects of general anesthesia23 and the possibility of overlooking abnormally large changes in IOP.24 It has been possible to correlate a range of elevated pressures with optic nerve damage and alterations in a variety of cellular retinal and optic nerve head responses.13,24–31 However, the Tono-Pen requires extensive operator experience.20 In addition, though it has been adapted to the mouse eye, use in mice is difficult and remains controversial.32,33
Several years ago, rebound tonometry was introduced. This method relies on the propulsion of a lightweight magnetized probe against the cornea by a solenoid. Several motion parameters, including the deceleration of the probe as it strikes the cornea, are then analyzed and used to calculate IOP.34,35 Prototype instruments have been used to measure IOP in rats,36,37 mice,38–40 and humans.41
The TonoLab (Colonial Medical Supply, Franconia, NH), a rebound tonometer designed specifically for rodents, is now commercially available. Wang et al.42 have shown the accuracy of this instrument in cannulated eyes connected to a pressure transducer for Wistar rats and four strains of mice and have demonstrated methods for using it in awake animals. Pease et al.43 have corroborated these findings in anesthetized rats and C57/BL6 mice and directly compared TonoLab with Tono-Pen readings in rat eyes with laser-induced IOP elevation.
Extensive analysis of the sensitivity and reliability of the TonoLab in awake rats is lacking, and no reports are available on the performance of this instrument in the Brown Norway rat, an animal widely used in modeling glaucomatous optic nerve injury.12,25,44–50 In this study, we compared the sensitivity and reliability of the TonoLab with the Tono-Pen in awake Brown Norway rats. For sensitivity, we compared the ability of these two instruments to detect subtle diurnal IOP fluctuations in normal eyes and their ability to document IOP elevation in eyes with experimental obstruction of aqueous humor outflow. In addition, we compared the ability of Tono-Lab readings to predict optic nerve damage in eyes with various levels of IOP elevation with that of the Tono-Pen, providing an independent corroboration of the reliability of readings generated by this instrument when used in glaucoma research.
Methods
All experiments complied with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Tonometer Calibrations
Adult Brown Norway rats were anesthetized by intraperitoneal injection of 1 mL/kg of 5 mL ketamine (100 mg/mL), 2.5 mL xylazine (20 mg/mL), 1 mL acepromazine (10 mg/mL), and 1.5 mL sterile water. Anterior chambers were cannulated with a blunted 23-gauge needle and were simultaneously attached to a Hamilton syringe (Hamilton Company, Reno, NV) with screw plunger for adjusting IOP and a pressure transducer connected to a chart recorder, as described previously.20 The transducer was calibrated to a manometer before each calibration. IOP was then manually adjusted by one experimenter using the screw plunger on the Hamilton syringe in 5-mm Hg increments, and tonometer readings were taken and recorded at each increment by a second experimenter masked to the transducer IOP. Previous work with this arrangement has demonstrated that intraocular pressure responds immediately to changes in transducer pressure.20 Fourteen calibrations in separate eyes were performed with the Tono-Lab. In eight of these eyes, calibrations were also performed with the Tono-Pen XL.
The TonoLab takes six measurements deemed reliable by internal software and, after elimination of high and low readings, generates and displays an average.43 For the purpose of our study, we considered this machine-generated average as one reading and have designated it the machine-generated reading throughout this article. For each time point, we recorded multiple machine-generated readings in each eye. An average of these readings was then calculated and is reported as calculated mean IOP. The Tono-Pen, in contrast, produces individual readings along with instrument-generated averages. Because these averages are unreliable,20 valid individual readings were collected each time and were used to calculate the mean IOP.
For TonoLab IOP measurements, the instrument was clamped to a ring stand with the probe oriented horizontally, and the animal was placed at the edge of an adjustable table, as described by Wang.42 Animals were positioned, and the height of the table was adjusted to locate the probe tip at the center of the cornea, poised 2 mm from its surface. Actual IOP, as measured by the transducer, was increased from 10 to 100 mm Hg in 5-mm Hg increments, and at least five machine-generated readings were recorded for every IOP setting.
For the Tono-Pen, IOP was varied from 10 to 70 mm Hg in 5-mm Hg increments, and every valid reading was recorded for a total of 10 readings for each pressure level. We have previously determined that, when used on the rat eye, valid TonoPen readings are those that are displayed immediately after instrument contact with the eye with force sufficient to move the eye slightly posteriorly. Readings that occur after only slight contact with the eye (without causing eye movement) are unreliable. “Off” readings (those that occur when the TonoPen tip breaks contact with the eye) are also invalid, as are instrument-generated averages, because the instrument itself is unable to eliminate “off” and other invalid readings from this average.20 For each instrument, calculated mean IOP at each pressure level was compared with the transducer IOP.
TonoLab IOP Measurements in Awake Animals under Standard Light- and Dark-Phase Lighting Conditions
Six rats were housed in a room illuminated by fluorescent lights (330 lux) that were turned on and off automatically every 12 hours; lights were on from 1:00 AM to 1:00 PM (light phase) and off from 1:00 PM to 1:00 AM (dark phase). Light-phase IOPs were determined 2 hours before the onset of the dark phase, and dark phase readings were taken 2 hours after the lights were turned off. Dark-phase measurements were performed using a Bright Lab Junior bulb (CPM Inc., Dallas, Texas) to avoid influencing the circadian rhythm. These conditions duplicated those used in a previous evaluation of the Tono-Pen to detect circadian fluctuation in IOP.22 IOP was measured under light and dark conditions periodically for both eyes over a 45-day period.
All TonoLab measurements were performed without general or topical anesthesia. The animal was gently restrained by hand, and five machine-generated readings were recorded at each session and used to determine the calculated mean IOP for that time point.
Awake IOP Measurements after Experimental Aqueous Humor Outflow Obstruction
Forty-three animals were kept in low-level constant light, with lights on 24 hours a day at 40 to 90 lux, to minimize circadian fluctuations in IOP.13,51 One eye of each animal received an episcleral vein injection of 50 μL of 1.75 M hypertonic saline solution to scar the aqueous humor outflow pathway, as described previously.12 Awake IOPs were measured in both eyes using the TonoLab, as described, before injection and two to three times per week for 4 weeks after injection. Tono-Pen measurements were taken at the same frequency with topical 0.5% proparacaine hydrochloride (Akorn, Buffalo Grove, IL) anesthesia. Calculated mean IOP was determined for each time point by averaging five machine-generated readings with the TonoLab and 10 valid individual readings with the Tono-Pen. Because the intervals between IOP readings for this group of animals were irregular (2–3 days), mean IOP over the experimental period was calculated from cumulative IOP (as determined by the area under the curve of IOP readings vs. days after injection with GraphPad Prism [GraphPad Software, La Jolla, CA]) divided by the total number of days after injection (28 days for 4 weeks).
After 4 weeks, animals were anesthetized and killed. Optic nerve segments 2 mm from the back of the globe were dissected, washed, postfixed, dehydrated, and embedded. Cross-sections were cut on a microtome and stained with 1% toluidine blue. Optic nerve sections from injected eyes were masked and assessed for damage by five independent observers using light microscopy. A grading scale of nerve injury ranging from 1 (normal) to 5 (degeneration involving the entire nerve cross-section) was used.24 Each eye was assigned a grade of injury determined by calculating the mean of the five independent grade scores. Eyes with different degrees of damage were then grouped by severity, and the mean Tono-Pen and TonoLab IOPs of each group for the duration of observation were compared with the mean IOPs of their fellow, unoperated eyes.
Effect of Number of Pressure Readings on Pressure Determination with the TonoLab in Awake Animals
Given the relatively high fluctuation in TonoLab IOP readings, we also wanted to study the effect of using more than one machine-generated reading to calculate pressure determination. IOP data collected from 15 additional adult Brown Norway rats were used to determine the standard deviation of the calculated mean when using different numbers of machine-generated readings. These animals were housed in constant low-level light and received injections of hypertonic saline in one eye, as described. Awake IOP was then measured in both eyes (N = 30) with the TonoLab two to three times per week for 5 weeks. Baseline IOP was determined in both eyes before the injection. At each time of IOP measurement, one set of 10 consecutive machine-generated readings was recorded, making a total of 488 sets of IOP readings over the whole experiment. For each set of 10 machine-generated readings, standard deviation was calculated for the first three readings and whenever another reading was added.
Statistical Analysis
Statistical analyses were performed with statistical software packages (Excel [Redmond, WA] and GraphPad Prism [GraphPad Software]). Polynomial regression was used to correlate transducer IOP levels to tonometer readings for Tono-Pen and TonoLab calibrations over the entire range of transducer IOP, with emphasis on the ranges with the most linear relationship. An F test was used to compare variability by determining the difference of the mean IOP for each eye with the group mean as determined by each instrument, and an F value was then calculated to determine the significance of the difference. In eyes with experimental IOP elevation, linear regression was used to correlate IOPs measured by one tonometer with those measured by the other. For determination of the effect of the number of readings on calculated mean with the TonoLab, standard deviation distribution was visualized in a box plot as a function of the number of machine-generated readings.
Results
TonoLab and Tono-Pen Calibrations
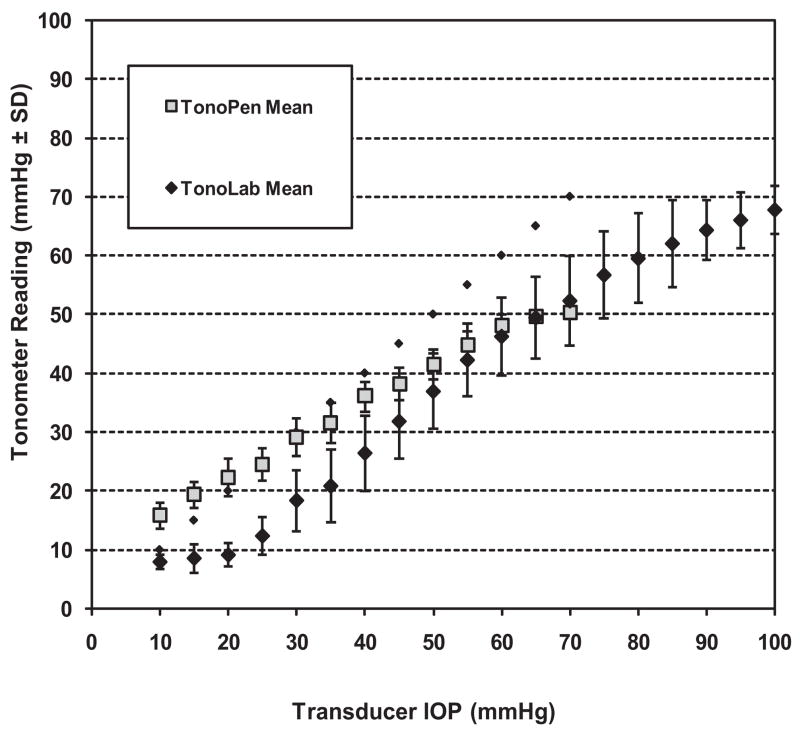
Figure 1 summarizes the calibration curves and displays the average readings at each pressure level for the Tono-Pen and TonoLab. When plotted individually, the spread of all these curves for the TonoLab is greater, and this is reflected in the greater standard deviations for this instrument. For the Tono-Pen, the best linear correlation with transducer IOP was found for pressures between 10 and 60 mm Hg (y = 0.6442x + 9.363; R2 = 0.9981). As noted previously, the Tono-Pen overestimated IOP at actual pressures in the lower ranges and progressively underestimated IOP above 30. For the TonoLab, an excellent linear fit was found between actual IOP of 20 and 80 mm Hg (y = 0.88x − 8.23; R2 = 0.99). At actual IOPs below 20 mm Hg, the TonoLab curve flattened, making it difficult to interpret actual IOP from readings of 10 and below with this instrument. A linear regression calculation for both instruments over the range of 10 to 50 mm Hg, used in previous studies,42,43 yielded a regression of y = 0.65x + 9.29 (R2 = 0.99) for the TonoPen and y = 0.76x − 3.80 (R2 = 0.95) for the TonoLab.
Figure 1.
Comparison of cumulative TonoLab and Tono-Pen calibrations against a pressure transducer. Note the greater variability between calibrations for the TonoLab as opposed to those for the Tono-Pen, illustrated by larger SD bars at every pressure level. Note also a consistent underestimation of IOP with the TonoLab and a “flattening” of the curve at transducer IOP <20 mm Hg.
Light- and Dark-Phase IOP Measurements with the TonoLab
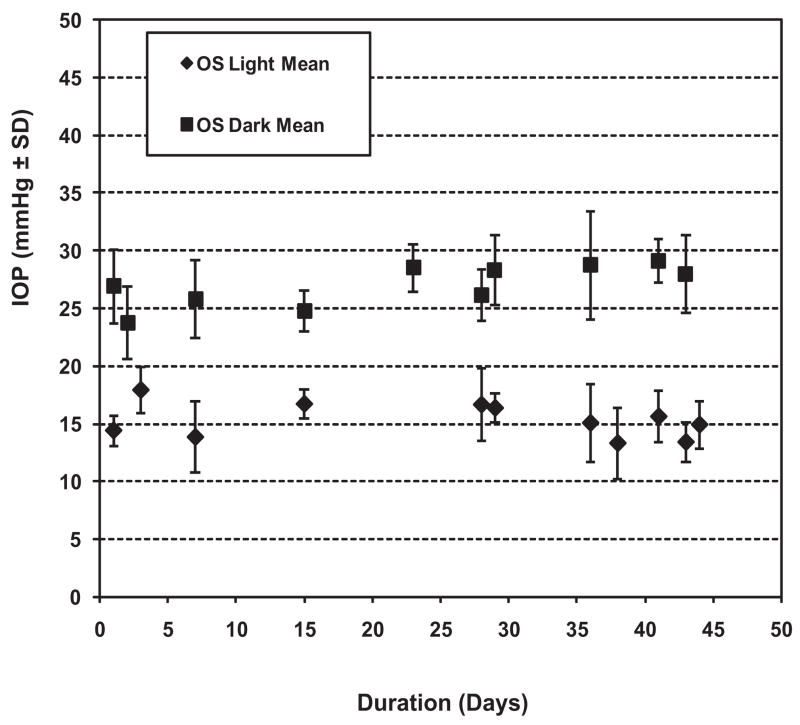
For animals in light/dark conditions (standard lighting), the TonoLab documented distinctly higher pressure (±SD) during the dark phase (27.9 ± 1.7 mm Hg) than during the light phase (16.7 ± 2.3 mm Hg). The difference was highly statistically significant (P < 0.0001) and was in agreement with previous observations with the Tono-Pen.22,24,51 Figure 2 illustrates IOP readings for one eye of all six animals over the 7-week observation period, demonstrating the distinct IOP difference between the two phases, with moderate fluctuation.
Figure 2.
IOP as determined by TonoLab in normal left eyes of six animals housed in a 12-hour light/12-hour dark environment. Note the consistent, significantly higher IOP readings in eyes during the dark phase of the light cycle as opposed to the light phase.
Constant Light IOP Measurements with TonoLab and Tono-Pen in Normal Eyes and in Eyes after Hypertonic Saline Injection
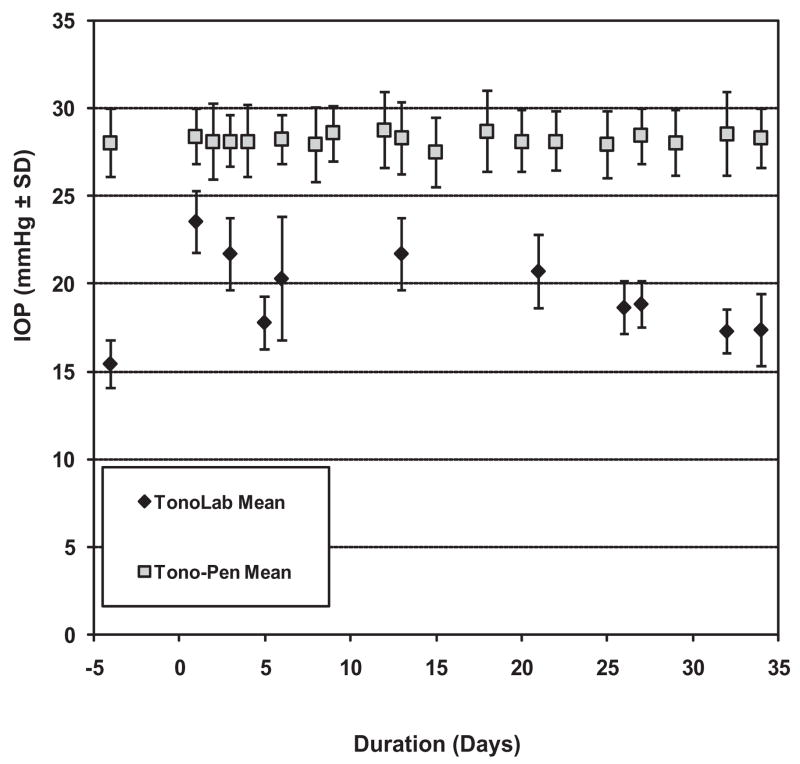
In low-level constant lighting, IOPs measured with the Tono-Pen over the period of observation in fellow (uninjected) eyes averaged 28.5 ± 0.2 mm Hg, an excellent agreement with prior Tono-Pen experience.13,51 With the TonoLab, measured IOPs over the same time period in these eyes averaged 20.8 ± 1.8 mm Hg. Figure 3 illustrates typical comparative readings for the Tono-Pen versus the TonoLab in the fellow eye of a single animal. For both instruments, measurements were roughly between the light- and dark-phase readings. However, the TonoLab readings under these conditions appeared more variable day to day compared with TonoPen readings. Further comparison of the variability between the two instruments for the entire group of fellow eye IOP determinations confirmed that variance with the TonoLab was greater than with the Tono-Pen to a highly statistically significant degree (F = 4.4 × 10−30).
Figure 3.
Comparison of TonoLab and Tono-Pen readings in the uninjected fellow eye of an animal housed in constant low-level light. Note that TonoLab IOP readings were lower than those of the Tono-Pen and showed greater variability.
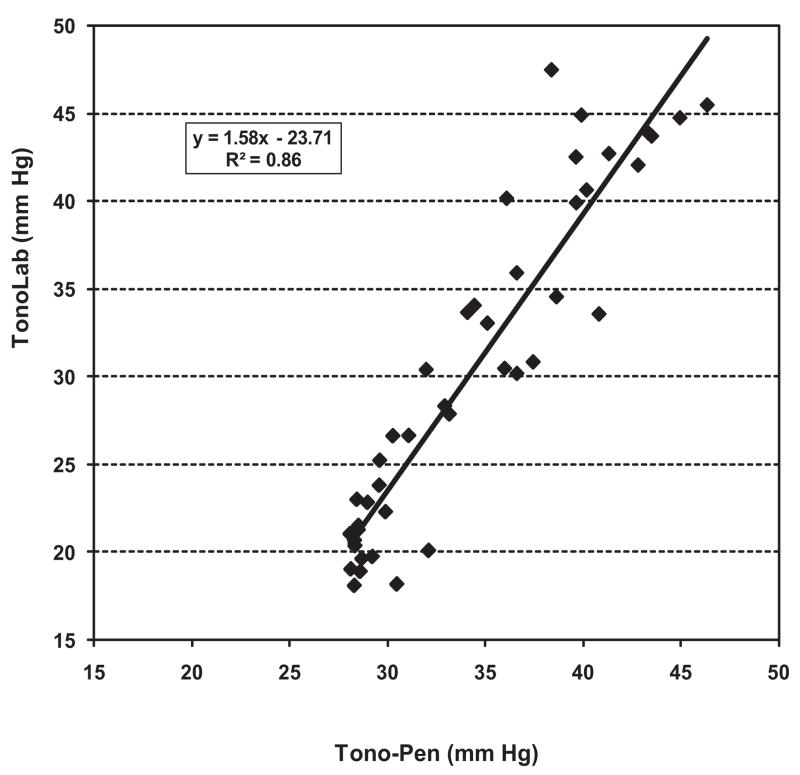
Eyes with elevated IOP after hypertonic saline injection exhibited a range of pressure responses. As shown in Figure 4, the correlation between mean IOP with the two instruments over the experimental period of 4 weeks was excellent (R2 = 0.86; P < 0.0001). The regression equation for this relationship was y = 1.58x − 23.71. This was similar to the regression equation we obtained when we used the data shown in Figure 1 from our calibration experiments to compare the performance of the two tonometers with each other over the same IOP range. The line for this regression is y = 1.41x − 22.84 [R2 = 0.99].
Figure 4.
Linear relationship between TonoLab and Tono-Pen mean IOPs in eyes with experimentally elevated IOP after hypertonic saline injection. This relationship is nearly identical with that seen in calibration experiments using cannulated eyes.
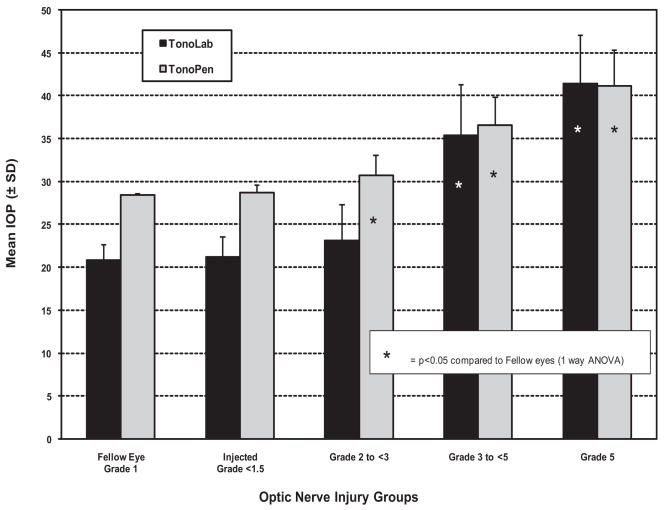
This group of eyes also demonstrated an array of optic nerve damage ranging from grades 1 through 5. When eyes were grouped by grade of optic nerve injury and then compared with mean IOP, both instruments provided IOP measurements that correlated well with optic nerve injury grade (Fig. 5). Eyes with greater degrees of optic nerve injury were associated with significantly elevated IOP compared with fellow eyes for both instruments. However, in eyes with the least amount of injury (grades 2 to <3), only the Tono-Pen was capable of detecting significant elevations in IOP (P < 0.05) compared with IOP in fellow eyes. Although a slight increase in IOP was found with the TonoLab in this group, it was not statistically significant primarily because of the greater variability of pressure measurement with this instrument in fellow and experimental eyes.
Figure 5.
Comparison of mean IOPs as determined by the TonoLab and the Tono-Pen for fellow eyes and experimental eye groups with different grades of optic nerve injury. Note that, in the group with the least amount of injury (grades 2 to <3), the group mean IOP of experimental eyes determined with the Tono-Pen was significantly elevated compared with the mean of their fellow eyes, whereas that obtained with the Tono-Lab for this same group was not.
Effect of Number of Pressure Measurements on Reliability with the TonoLab
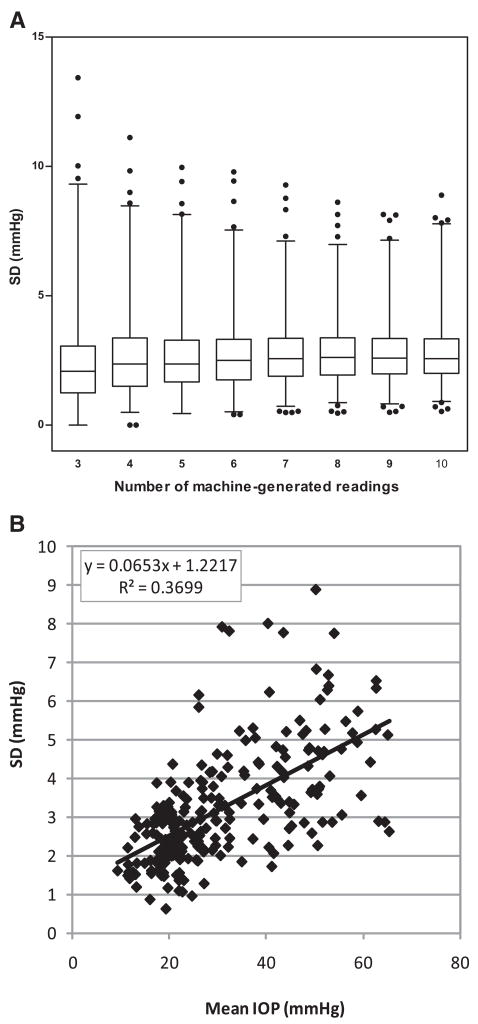
A wide range of pressure levels was covered by this IOP data set. In fellow eyes, IOP readings averaged 21.1 ± 4.2 mm Hg; in injected eyes, IOP readings varied from 8 to 73 mm Hg. The boxes-and-whiskers plot in Figure 6A illustrates the distribution of standard deviations and their changing trend with increased numbers of machine-generated IOP readings. For mean IOPs calculated from fewer machine-generated readings, standard deviations were more scattered and had higher maximal values than those based on higher numbers of readings. The 99th percentile and maximal values dropped steadily as the number of readings increased from three (99%, 9.3 mm Hg; maximum, 13.4 mm Hg) to seven (99%, 7.1 mm Hg; maximum, 9.3 mm Hg) and changed relatively little with higher numbers of readings.
Figure 6.
(A) Distribution of the SD values for different numbers of instrument-generated readings with the TonoLab (3–10) for all eyes. Boxes and whiskers represent the 25th to 75th percentiles and the 1st to 99th percentiles, respectively. Outliers above the 99th and below the 1st percentiles are shown as black dots. Note the steady decrease of the 99th percentile and maximal values from three readings to seven readings, with relatively little change in this effect above seven readings. (B) SD plot representing the SD of each 10 readings against the mean IOP from all injected eyes. Representing chance variation, the largest SD variations are associated with IOPs above 30 mm Hg.
To analyze the effect of the extent of IOP elevation on this relationship, we plotted the standard deviation of each of 10 readings against the mean IOP for all the injected eyes (Fig. 6B). Representing chance variations, the standard deviation should be an indication of the precision of repeated measures. This clearly shows the relation of the standard deviation to the IOP level (y = 0.07x + 1.22; R2 = 0.37), with the largest variations associated with IOPs above 30 mm Hg.
Discussion
In experienced hands, the Tono-Pen can provide meaningful IOP measurements in awake rats.22,25,27,29 This instrument requires operators with extensive experience because measurements can be influenced by the speed at which the probe contacts the eye. The operator must learn to distinguish valid from invalid readings.20,43 The TonoLab should eliminate a large source of this error because the probe is not manually propelled toward the eye,43 thereby enhancing the learning curve for this instrument and providing more uniform measurements.
Although several reports assessing a rebound tonometer prototype have been published,34–37 only two are available that use the commercially available TonoLab in rats.42,43 Both found that the instrument calibrated favorably with pressures in cannulated eyes connected to a reservoir in rats and mice over a range of 10 to 50 mm Hg.
Our findings in Brown Norway rats provide additional insights into the advantages of the TonoPen, along with some limitations that have not been previously reported. Our calibrations in cannulated eyes showed that TonoLab readings provide an excellent linear fit to actual IOP. However, in contrast to previous work,42,43 we found that average IOP readings with the TonoLab were 10 mm Hg less than actual IOP over the range of highest linear fit (Fig. 1).
Another unexpected finding of our calibration studies was that, when actual IOP was brought below 20 mm Hg, TonoLab readings tended to flatten. This means that TonoLab pressure readings below 10 mm Hg do not correctly distinguish between actual pressures below 20 mm Hg. Fortunately, we and others23,51 have found that awake readings in this range do not occur in normal eyes in either a light/dark environment or in constant light. Thus, we would not expect this to be a limitation of this instrument in our hands.
Our Tono-Pen calibrations, performed in tandem with the TonoLab, were similar to our original calibrations20 and to those reported by Pease43 and, more recently, by Pang.51 In all these studies, average Tono-Pen IOP yields only a mild underestimate of the actual IOP when pressure is set at 30 mm Hg (Fig. 1). Given these similarities in Tono-Pen calibrations across studies, it seems unlikely that methodological differences would produce the TonoLab calibration differences noted in this article. In addition, the relationship between Tono-Pen and TonoLab readings in the current calibration experiments was close to that found over a range of elevated IOP in uncannulated eyes with experimental aqueous humor outflow obstruction (Fig. 4), again suggesting that the cannulation itself did not induce a systematic error. All these considerations reinforce our contention that users should perform their own calibrations in the appropriate animals to understand the behavior of this instrument before they use it in experimental studies.
Previous TonoLab calibrations have been limited to pressures up to 50 mm Hg.42,43 However, electrophysiology experiments in anesthetized rats with acute IOP elevations show that retinal functions diminish from 30 to 50 mm Hg, with further nonspecific changes and slower functional recovery above 50 mm Hg.52,53 This suggests that detecting fluctuations of IOP above this level are important to avoid overlooking IOP high enough to produce injury through mechanisms unlikely to occur in chronic glaucoma. We have calibrated the TonoLab up to transducer pressures as high as 100 mm Hg and found that it has a range of best linear fit from 20 to 85 mm Hg actual pressure. This indicates that the TonoLab may be better suited for detecting pressures above this critical level than the Tono-Pen, which appears to have a practical limit of 60 mm Hg.
Our evaluation of awake animals in a normal light/dark cycle and under conditions of constant light further demonstrates the versatility of the TonoLab. We found that awake animals in light/dark conditions had a mean pressure of 16.7 ± 2.3 mm Hg during the light portion of the cycle, which agrees well with Wang et al.,42 who noted a mean pressure of approximately 18 mm Hg during light in Wistar rats. However, we noted that pressures taken during the dark phase were significantly elevated to 27.9 ± 1.7 mm Hg (P < 0.0001), confirming that, in awake rats, the TonoLab is just as capable as the Tono-Pen in detecting physiological, circadian variations in IOP22 and provides a sensitive demonstration that its measurements are meaningful and valid. When animals were housed in constant light, we found a mean TonoLab reading of 20.8 ± 1.8 mm Hg that was intermediate between the light- and dark-phase readings, an observation previously demonstrated with the Tono-Pen by our laboratory and others.13,23,51
Our comparison between the TonoLab and the Tono-Pen in normal, fellow eyes in constant light confirms that TonoLab readings are consistently lower than those of the Tono-Pen and illustrates that IOP readings with the TonoLab are more variable than with the Tono-Pen. This difference was found to be highly statistically significant. It is possible that this variability results from the steeper relationship between measured and actual IOP with the TonoLab (illustrated in Fig. 1) and its greater sensitivity to modest pressure fluctuations. Our assessment of the impact of number of consecutive readings on mean IOP (Fig. 6) suggests that averaging multiple instrument-generated averages could help minimize, but not eliminate, this problem, which appears to increase in eyes with elevated IOP.
Overall, we found a high degree of correlation between TonoLab and Tono-Pen readings in eyes after hypertonic saline injection, as shown in Figure 4. In addition, TonoLab IOP measurements correlated well with optic nerve injury grade, suggesting that this instrument should be useful for predicting optic nerve injury, even though measured IOP is consistently lower than actual IOP.
In the group of eyes with the least amount of optic nerve injury, however, the mild elevation in IOP determined by the TonoLab was not significantly greater than that of the uninjected fellow eyes, whereas that observed with the Tono-Pen was significant. This is likely the result of the greater variability with the TonoLab pressure measurements in fellow eyes compared with those of the Tono-Pen and may have significant bearing on the future usefulness of this instrument for neuro-protection studies or other work that requires close assessment of the relationship between mild increases in IOP and optic nerve damage. Although the use of increased numbers of machine-generated pressure readings should reduce this problem, our analysis of the impact of pressure measurement numbers (Fig. 6) suggests that this strategy has already been nearly fully exploited because these numbers represent the mean of a minimum of five readings.
Acknowledgments
Supported in part by National Eye Institute Grants EY10145 and EY016866 and by Research to Prevent Blindness.
Footnotes
Disclosure: J.C. Morrison, None; L. Jia, None; W. Cepurna, None; Y. Guo, None; E. Johnson, None
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman DS, Wolfs RC, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drance SM. The Collaborative Normal-Tension Glaucoma Study and some of its lessons. Can J Ophthalmol. 1999;34:1–6. [PubMed] [Google Scholar]
- 4.AGIS-Investigators. The Advanced Glaucoma Intervention Study (AGIS), 7: the relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 5.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- 6.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 7.Gaasterland D, Kupfer C. Experimental glaucoma in the rhesus monkey. Invest Ophthalmol. 1974;13:455–457. [PubMed] [Google Scholar]
- 8.Quigley HA, Hohman RM. Laser energy levels for trabecular mesh-work damage in the primate eye. Invest Ophthalmol Vis Sci. 1983;24:1305–1307. [PubMed] [Google Scholar]
- 9.Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Harwerth RS, Crawford ML, Frishman LJ, Viswanathan S, Smith EL, 3rd, Carter-Dawson L. Visual field defects and neural losses from experimental glaucoma. Prog Retin Eye Res. 2002;21:91–125. doi: 10.1016/s1350-9462(01)00022-2. [DOI] [PubMed] [Google Scholar]
- 11.Harwerth RS, Smith EL, 3rd, DeSantis L. Experimental glaucoma: perimetric field defects and intraocular pressure. J Glaucoma. 1997;6:390–401. [PubMed] [Google Scholar]
- 12.Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997;64:85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- 13.Morrison JC, Johnson EC, Cepurna W, Jia L. Understanding mechanisms of pressure-induced optic nerve damage. Prog Retin Eye Res. 2005;24:217–240. doi: 10.1016/j.preteyeres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Sawada A, Neufeld AH. Confirmation of the rat model of chronic, moderately elevated intraocular pressure. Exp Eye Res. 1999;69:525–531. doi: 10.1006/exer.1999.0732. [DOI] [PubMed] [Google Scholar]
- 15.Shareef SR, Garcia-Valenzuela E, Salierno A, Walsh J, Sharma SC. Chronic ocular hypertension following episcleral venous occlusion in rats [letter] Exp Eye Res. 1995;61:379–382. doi: 10.1016/s0014-4835(05)80131-9. [DOI] [PubMed] [Google Scholar]
- 16.Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci. 2002;43:402–410. [PubMed] [Google Scholar]
- 17.John SW, Anderson MG, Smith RS. Mouse genetics: a tool to help unlock the mechanisms of glaucoma. J Glaucoma. 1999;8:400–412. [PubMed] [Google Scholar]
- 18.John SW, Smith RS, Savinova OV, et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- 19.Aihara M, Lindsey JD, Weinreb RN. Experimental mouse ocular hypertension: establishment of the model. Invest Ophthalmol Vis Sci. 2003;44:4314–4320. doi: 10.1167/iovs.03-0137. [DOI] [PubMed] [Google Scholar]
- 20.Moore CG, Milne ST, Morrison JC. Noninvasive measurement of rat intraocular pressure with the Tono-Pen. Invest Ophthalmol Vis Sci. 1993;34:363–369. [PubMed] [Google Scholar]
- 21.Moore CG, Epley D, Milne ST, Morrison JC. Long-term non-invasive measurement of intraocular pressure in the rat eye. Curr Eye Res. 1995;14:711–717. doi: 10.3109/02713689508998499. [DOI] [PubMed] [Google Scholar]
- 22.Moore CG, Johnson EC, Morrison JC. Circadian rhythm of intraocular pressure in the rat. Curr Eye Res. 1996;15:185–191. doi: 10.3109/02713689608997412. [DOI] [PubMed] [Google Scholar]
- 23.Jia L, Cepurna WO, Johnson EC, Morrison JC. Effect of general anesthetics on IOP in rats with experimental aqueous outflow obstruction. Invest Ophthalmol Vis Sci. 2000;41:3415–3419. [PubMed] [Google Scholar]
- 24.Jia L, Cepurna WO, Johnson EC, Morrison JC. Patterns of intraocular pressure elevation after aqueous humor outflow obstruction in rats. Invest Ophthalmol Vis Sci. 2000;41:1380–1385. [PubMed] [Google Scholar]
- 25.Ahmed F, Brown KM, Stephan DA, Morrison JC, Johnson EC, Tomarev SI. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2004;45:1247–1258. doi: 10.1167/iovs.03-1123. [DOI] [PubMed] [Google Scholar]
- 26.Fortune B, Bui BV, Morrison JC, et al. Selective ganglion cell functional loss in rats with experimental glaucoma. Invest Ophthalmol Vis Sci. 2004;45:1854–1862. doi: 10.1167/iovs.03-1411. [DOI] [PubMed] [Google Scholar]
- 27.Johnson EC, Cepurna WO, Jia L, Morrison JC. The use of cyclodialysis to limit exposure to elevated intraocular pressure in rat glaucoma models. Exp Eye Res. 2006;83:51–60. doi: 10.1016/j.exer.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 28.Pang IH, Johnson EC, Jia L, et al. Evaluation of inducible nitric oxide synthase in glaucomatous optic neuropathy and pressure-induced optic nerve damage. Invest Ophthalmol Vis Sci. 2005;46:1313–1321. doi: 10.1167/iovs.04-0829. [DOI] [PubMed] [Google Scholar]
- 29.Schlamp CL, Johnson EC, Li Y, Morrison JC, Nickells RW. Changes in Thy1 gene expression associated with damaged retinal ganglion cells. Mol Vis. 2001;7:192–201. [PubMed] [Google Scholar]
- 30.Johnson EC, Deppmeier LM, Wentzien SK, Hsu I, Morrison JC. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000;41:431–442. [PubMed] [Google Scholar]
- 31.Johnson EC, Jia L, Cepurna WO, Doser TA, Morrison JC. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2007;48:3161–3177. doi: 10.1167/iovs.06-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reitsamer HA, Kiel JW, Harrison JM, Ransom NL, McKinnon SJ. TonoPen measurement of intraocular pressure in mice. Exp Eye Res. 2004;78:799–804. doi: 10.1016/j.exer.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Dalke C, Pleyer U, Graw J. On the use of Tono-Pen XL for the measurement of intraocular pressure in mice. Exp Eye Res. 2005;80:295–296. doi: 10.1016/j.exer.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Kontiola A. A new electromechanical method for measuring intraocular pressure. Doc Ophthalmol. 1996;93:265–276. doi: 10.1007/BF02569066. [DOI] [PubMed] [Google Scholar]
- 35.Kontiola AI. A new induction-based impact method for measuring intraocular pressure. Acta Ophthalmol Scand. 2000;78:142–145. doi: 10.1034/j.1600-0420.2000.078002142.x. [DOI] [PubMed] [Google Scholar]
- 36.Goldblum D, Kontiola AI, Mittag T, Chen B, Danias J. Non-invasive determination of intraocular pressure in the rat eye: comparison of an electronic tonometer (TonoPen), and a rebound (impact probe) tonometer. Graefes Arch Clin Exp Ophthalmol. 2002;240:942–946. doi: 10.1007/s00417-002-0571-y. [DOI] [PubMed] [Google Scholar]
- 37.Kontiola AI, Goldblum D, Mittag T, Danias J. The induction/impact tonometer: a new instrument to measure intraocular pressure in the rat. Exp Eye Res. 2001;73:781–785. doi: 10.1006/exer.2001.1088. [DOI] [PubMed] [Google Scholar]
- 38.Danias J, Kontiola AI, Filippopoulos T, Mittag T. Method for the noninvasive measurement of intraocular pressure in mice. Invest Ophthalmol Vis Sci. 2003;44:1138–1141. doi: 10.1167/iovs.02-0553. [DOI] [PubMed] [Google Scholar]
- 39.Morris CA, Crowston JG, Lindsey JD, Danias J, Weinreb RN. Comparison of invasive and non-invasive tonometry in the mouse. Exp Eye Res. 2006;82:1094–1099. doi: 10.1016/j.exer.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Filippopoulos T, Matsubara A, Danias J, et al. Predictability and limitations of non-invasive murine tonometry: comparison of two devices. Exp Eye Res. 2006;83:194–201. doi: 10.1016/j.exer.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Kontiola A, Puska P. Measuring intraocular pressure with the Pulsair 3000 and Rebound tonometers in elderly patients without an anesthetic. Graefes Arch Clin Exp Ophthalmol. 2004;242:3–7. doi: 10.1007/s00417-003-0671-3. [DOI] [PubMed] [Google Scholar]
- 42.Wang WH, Millar JC, Pang IH, Wax MB, Clark AF. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Invest Ophthalmol Vis Sci. 2005;46:4617–4621. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]
- 43.Pease ME, Hammond JC, Quigley HA. Manometric calibration and comparison of TonoLab and TonoPen tonometers in rats with experimental glaucoma and in normal mice. J Glaucoma. 2006;15:512–519. doi: 10.1097/01.ijg.0000212276.57853.19. [DOI] [PubMed] [Google Scholar]
- 44.Neufeld AH, Das S, Vora S, et al. A prodrug of a selective inhibitor of inducible nitric oxide synthase is neuroprotective in the rat model of glaucoma. J Glaucoma. 2002;11:221–225. doi: 10.1097/00061198-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Grozdanic SD, Betts DM, Sakaguchi DS, Kwon YH, Kardon RH, Sonea IM. Temporary elevation of the intraocular pressure by cauterization of vortex and episcleral veins in rats causes functional deficits in the retina and optic nerve. Exp Eye Res. 2003;77:27–33. doi: 10.1016/s0014-4835(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 46.Bui BV, Fortune B. Ganglion cell contributions to the rat full-field electroretinogram. J Physiol. 2004;555:153–173. doi: 10.1113/jphysiol.2003.052738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danias J, Shen F, Kavalarakis M, et al. Characterization of retinal damage in the episcleral vein cauterization rat glaucoma model. Exp Eye Res. 2006;82:219–228. doi: 10.1016/j.exer.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartwick AT, Zhang X, Chauhan BC, Baldridge WH. Functional assessment of glutamate clearance mechanisms in a chronic rat glaucoma model using retinal ganglion cell calcium imaging. J Neurochem. 2005;94:794–807. doi: 10.1111/j.1471-4159.2005.03214.x. [DOI] [PubMed] [Google Scholar]
- 49.Huang W, Fileta JB, Dobberfuhl A, et al. Calcineurin cleavage is triggered by elevated intraocular pressure, and calcineurin inhibition blocks retinal ganglion cell death in experimental glaucoma. Proc Natl Acad Sci U S A. 2005;102:12242–12247. doi: 10.1073/pnas.0505138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Pernet V, Hauswirth WW, Di Polo A. Activation of the extracellular signal-regulated kinase 1/2 pathway by AAV gene transfer protects retinal ganglion cells in glaucoma. Mol Ther. 2005;12:402–412. doi: 10.1016/j.ymthe.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Pang IH, Wang WH, Clark AF. Acute effects of glaucoma medications on rat intraocular pressure. Exp Eye Res. 2005;80:207–214. doi: 10.1016/j.exer.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Bui BV, Edmunds B, Cioffi GA, Fortune B. The gradient of retinal functional changes during acute intraocular pressure elevation. Invest Ophthalmol Vis Sci. 2005;46:202–213. doi: 10.1167/iovs.04-0421. [DOI] [PubMed] [Google Scholar]
- 53.He Z, Bui BV, Vingrys AJ. The rate of functional recovery from acute IOP elevation. Invest Ophthalmol Vis Sci. 2006;47:4872–4880. doi: 10.1167/iovs.06-0590. [DOI] [PubMed] [Google Scholar]