Abstract
Background
There are few patient-reported data regarding quality of life after taxane-based adjuvant chemotherapy and none regarding mental health outcomes.
Methods
This was a naturalistic, longitudinal study that used a case–control design. Data were derived from a randomized clinical trial in patients who had stage II/III breast cancer (N = 227). Paclitaxel (Taxol) was approved for use midway during the accrual period (1994–1999). Patients who received taxanes as part of their adjuvant chemotherapy (the taxane group; n = 55) were matched with patients receiving regimens without taxanes (the no-taxane group; n = 83) on trial arm, lymph node status, surgery type, menopausal status, and partner status. Mixed-effects models tested for group differences in nurse evaluations of patients' symptoms and Karnofsky performance status and in patient-reported quality of life (the 36-item Medical Outcomes Study Short Form) and emotional distress (Profile of Mood States; Center for Epidemiological Studies Depression scale).
Results
As expected, patients in the taxane group experienced significantly higher rates of selected toxicities, including arthralgia/myalgia (45% vs 26%) and ataxia (20% vs 5%). Patients in the taxane group also had significantly worse emotional distress and mental quality of life throughout adjuvant treatment. Rates of probable clinical depression also were high. In contrast, these outcomes were improving for patients in the no-taxane group (all P <.023). Emotional recovery for patients in the taxane group required 2 years on average versus 6 to 12 months for patients in the no-taxane group. During Years 3 through 5, the groups had similar outcomes.
Conclusions
These data suggested that taxane-based chemotherapies confer risk for significant psychological symptoms. Depression, in particular, should be monitored.
Keywords: breast neoplasms, depression, adverse effects, paclitaxel, quality of life
Taxanes are used increasingly for the adjuvant treatment of early and locally advanced breast cancer.1,2 Little is known about long-term quality-of-life outcomes after patients receive these drugs. Data available from other patients (ie, patients with ovarian or lung cancer) or from patients with metastatic disease may not generalize to the adjuvant breast cancer setting because of differing symptom profiles, dosing, or agents used in combination with taxanes.
Paclitaxel (PTX) (Taxol; Bristol-Myers Squibb, NY), which was approved for adjuvant use in breast cancer in October 1999,3 and docetaxel (T) (Taxotere; Sanofi-Aventis, Paris, France), which was approved in August 2004,3 were shown in Phase 2 clinical trials to cause significant neurotoxicity, arthralgia, myalgia, skin reactions, neurosensory disturbance, and peripheral neuropathy, in addition to side effects that are common to many chemotherapy drugs (eg, neutropenia, alopecia4–7). Although clinically manageable, the toxicity profile of taxanes in phase 3 trials continues to be noteworthy for neurologic toxicities.8–12
Three phase 3 clinical trials have suggested that quality of life declines during the period of taxane delivery, as can occur with other agents, but it also declines differentially. Fountzilas and colleagues10 reported that patients who received PTX with a regimen that included epirubicin (E), cyclophosphamide (C), methotrexate (M), and 5-fluoracil (F) (E-CMF) reported poorer social functioning, emotional functioning, and pain during chemotherapy relative to patients who received E-CMF without PTX. Similar results were reported in 2 trials by Martin and colleagues,11,12 who compared patients who received T, doxorubicin (A), and C (TAC) with patients who received FAC. Only Martin and colleagues11,12 have reported quality-of-life data regarding outcomes after chemotherapy. They reported that global quality of life was similar for the TAC and the FAC group at a 6-month follow-up. Additional data are needed to determine whether this positive evaluation is reliable.
The objective of the current study was, with a case-control design, to determine short-term (during treatment), moderate-term (2 year), and long-term (up to 5 years) toxicity and quality of life for patients who received taxanes compared with patients who did not. A phase 3 randomized controlled trial (RCT) of a psychological intervention that accrued patients with breast cancer (N = 227) prior the start of adjuvant therapy and followed them for 5 years provided data. Midway through the accrual period (1994–2000), taxane-containing trials and clinical use began. Thus, a naturalistic observational study resulted, with 2 patient groups that differed primarily in their exposure to taxane-based chemotherapy. We tested the hypothesis that taxane treatment is associated with poorer outcomes—signs, symptoms, toxicities, psychological outcomes, and quality of life—during treatment delivery and recovery.
Materials and Methods
Clinical Trial Sample
Patients (N = 227) with newly diagnosed, surgically treated stage II or III breast cancer (TNM staging system13; International Classification of Diseases, 9th Revision codes 174.0–174.9) were eligible and were accrued between 1994 and 2000 through a university-affiliated Comprehensive Cancer Center. Details of the informed-consent procedures and accrual have been published.14,15 All participants were provided, in person, oral and written informed consent in keeping with institutional guidelines and in accordance with an assurance approved by and filed with the U.S. Department of Health and Human Services. After surgery and before randomization and adjuvant therapy, patients completed face-to-face interviews and questionnaires, and a nurse completed a health status evaluation. Patients were stratified and randomized to an assessment-only arm or to a psychological intervention with assessment arm. Follow-up assessments occurred every 4 months during Year 1 and every 6 months during Years 2 through 5. Patients remained in the trial only as long as they remained recurrence-free. Patients in the intervention arm experienced reduced emotional distress at 4 months and 12 months, as reported previously.14,15
Research Design and Patients
A case–control, repeated-measures design was used. With data from a subset of patients from the RCT (N = 227), 2 patient groups were defined: the taxane group and the no-taxane group. Patients who had received taxanes were matched to patients who had not. Matching variables were RCT study arm (the psychological intervention and assessment arm vs the assessment-only arm), lymph node status, tumor size, surgery type, menopausal status, and partner status. For each patient in the taxane group, 1 or 2 matches were identified. At each chemotherapy cycle, patients in both groups received from 8 mg to 20 mg dexamethasone as an antiemetic.
Taxane group
Of 227 patients, 55 received taxanes as part of the following regimens: AC-PTX (n = 45), A-T (n = 5), or AC-T (n = 5). Of those 55 patients, 37 (67%) were participating in chemotherapy clinical trials (Southwest Oncology Group [SWOG] 94–10; National Surgical Adjuvant Breast and Bowel Project [NSABP] B-28 and B-30; and Cancer and Leukemia Group B [CALGB] 49,802, 49,906, and C9741), and a subset of those 37 patients (n = 22) had been randomized to a taxane study arm. The remaining 18 of 55 patients (33%) received taxanes as part of standardized, off-protocol regimens (eg, 4 cycles of 600 mg/m2 C with 60 mg/m2 A followed by 4 cycles of 175 mg/m2 PTX). Of the 55 patients who received taxanes, 1 patient reacted adversely to her first cycle of T and thereafter received A-PTX (n = 1). Across all drugs, the median relative dose intensity16 was 91.9%; for taxanes, the median relative dose intensity was 93.4%.
No-taxane group
Matched patients for the no-taxane group (n = 83) were identified as described above. Among those patients, approximately half (n = 41) were in chemotherapy clinical trials, including trials in which patients were randomized to a no-taxane arm (n = 24 patients; 29%; SWOG 94-10, NSABP B-28, and CALGB 49,802) and other clinical trials that did not include taxanes (n = 17 patients; 20%; NSABP B-23, SWOG 88-14, SWOG 9061, and SWOG 93-13). The remaining patients (n = 42) received standardized, off-protocol regimens (n = 42 patients; 51%; eg, 6 cycles of 500 mg/m2 C, 50 mg/m2 A, and 500 mg/m2 F). The regimens were as follows: AC (n = 62), CMF (n = 14), FAC (n = 5), MF (n = 1), and CAMF (n = 1). The median relative dose intensity across all drugs was 91.3%.
Measures
Nurse-rated health
Signs, symptoms, and toxicities
A research nurse documented chemotherapy delays due to toxicities and completed a rating scale (1994 version) used by the SWOG14,17 documenting the type and severity of toxicities from chemotherapy. The current analyses focused on 9 items that were chosen a priori based on the most common effects of taxane treatments reported in phase 3 trials.8–12 We examined 3 items relevant to peripheral neuropathy (parasthesia/numbness, motor weakness, and incontinence), 3 items relevant to neurosensory dysfunction (incoordination/ataxia, loss of reflexes, and change in hearing, vision, or taste), and 1 item each for neurotoxicity (ie, disorientation, somnolence, or agitation), arthralgia/myalgia, and dermatologic toxicity. All items were coded as 0 (sign/symptom absent), 1 (mild/moderate; grade 1 or 2 toxicity), or 2 severe/life-threatening; grade 3 or 4 toxicity) as specified by the SWOG rating scale.
Performance status
The Karnofsky performance status (KPS) measure ranges from 0 to 100 with higher scores indicating better functional status.18
Patient-reported outcomes
Emotional distress
The 65-item Profile of Mood States (POMS) assessed emotional distress, including anxiety, depression, and fatigue.19 Scores range from −32 to 200, and higher scores indicate greater distress. In the current study, the Cronbach α reliability of the POMS was 0.92.
Depressive symptoms
The Iowa short form of the Center for Epidemiological Studies Depression scale (CES-D) was used.20,21 Scores range from 0 to 22, and scores ≥10 indicate clinically significant depressive symptoms. In the current study, the Cronbach α reliability was 0.77.
Health-related quality of life
The 36-item Medical Outcomes Study Short Form (SF-36) yields a Physical Component score (PCS) and a Mental Component score (MCS) that summarize quality of life.22 The component scores are standardized to have a mean score of 50 and a standard deviation of 10. Higher scores reflect better quality of life. The Cronbach α reliability for the PCS and the MCS was 0.94 and 0.89, respectively.
Analytic Strategy
The incidence and severity of side effects and toxicities also were noted. For these data, chi-square analyses tested for group differences. Prechemotherapy symptoms, signs, and toxicities were examined first, and only signs/symptoms that appeared or worsened with chemotherapy were analyzed. There were 11 follow-up assessments. To enhance reliability and examine critical phases of follow-up, assessments were collapsed for analysis into early symptoms (Year 1: during and soon after adjuvant chemotherapy; 4-, 8-, and 12-month assessments; and Year 2: 18- and 24-month assessments) and late symptoms (Year 3: 30- and 36-month assessments; Year 4: 42- and 48-month assessments; and Year 5: (54- and 60-month assessments).
Multilevel mixed-effects models were used to test for group differences in emotional distress and quality-of-life trajectories over the 5-year follow-up. Preliminary analyses revealed that the raw self-report data (emotional distress, depressive symptoms, and quality of life) showed a rapid improvements from the initial assessment to 12 months with further improvement although less rapid, from 12 to 24 months, and little change thereafter. Because a polynomial function (eg, linear or quadratic) cannot adequately represent such a complex pattern of change, 2 models were estimated for each outcome. An ‘early’ model described the period of change (prechemotherapy baseline assessment and 4-, 8-, 12-, 18-, and 24-month assessments), and a ‘late’ model described stable, long-term outcomes (30-, 36-, 42-, 48-, 54-, and 60-month assessments).
Six fixed effects were tested: intercept, linear slope, quadratic change, group differences in intercept, group differences in slope, and group differences in quadratic change. Factors that did not improve the fit of the model (based on the Bayseian Information Criterion and the Akaike Information Criterion) were removed for parsimony. Time was coded as months. With these analyses, we tested the hypotheses that the groups would show differential changes during chemotherapy and the year thereafter in the early models and would show different long-term outcomes in the late models.
Results
Preliminary Analyses
Table 1 provides descriptive data. The taxane and no-taxane groups were equivalent on baseline demographic, prognostic, and treatment variables with 1 exception. As expected, the number of weeks between the first and last chemotherapy administration was significantly higher in the taxane group (P <.001). Therefore, the number of weeks of chemotherapy was included as a control in the multilevel models.
TABLE 1. Equivalence of the No-Taxane and Taxane Groups on Sociodemographic, Prognostic, Treatment, and Outcome Variables at Baseline.
| No. of patients (%) or mean [SD] | |||
|---|---|---|---|
| Variable | No taxane, n = 83 |
Taxane, n = 55 |
P |
| Sociodemographic and prognostic | |||
| Age, y | 48 [8.9] | 49 [9.6] | .747 |
| Tumor size, cm | 2.8 [1.8] | 3.1 [1.6] | .364 |
| Stage, stage II | 76 (92) | 48 (87) | .413 |
| Lymph nodes, positive | 67 (81) | 49 (89) | .189 |
| ER/PR status, positive | 55 (66) | 37 (67) | .902 |
| Menopausal status, postmenopausal | 26 (31) | 24 (44) | .141 |
| Race, minority | 10 (12) | 5 (9) | .585 |
| Partner status, partnered | 67 (81) | 43 (78) | .716 |
| Education, y | 14.9 [2.9] | 14.6 [2.9] | .463 |
| Family income, $K/y | 68 [56] | 85.6 [113] | .238 |
| Treatment | |||
| Surgery, modified radical mastectomy | 43 (52) | 35 (64) | .170 |
| Radiation therapy, yes | 42 (51) | 31 (56) | .507 |
| Hormone therapy, yes | 62 (75) | 41 (75) | .984 |
| Psychological intervention, yes | 41 (49) | 28 (51) | .862 |
| Duration of chemotherapy, wk | 14.4 [7.8] | 20.2 [5.6] | <.001 |
| Emotional distress and depressive symptoms | |||
| POMS, total mood disturbance | 22.5 [22.9] | 25.6 [23.9] | .445 |
| CES-D, depressive symptoms | 5.9 [3.6] | 6.4 [4.2] | .525 |
| Quality of life | |||
| SF-36 Physical Component Summary | 41.0 [7.0] | 39.0 [6.6] | .095 |
| SF-36 Mental Component Summary | 42.2 [10.9] | 43.6 [11.6] | .466 |
SD indicates standard deviation; ER/PR, estrogen receptor/progesterone receptor; POMS, Profile of Mood States; CES-D, Center for Epidemiological Studies-Depression; SF-36: Medical Outcomes Study 36-item Short Form.
The baseline assessment was conducted before the receipt of adjuvant therapy, as noted above. All patients began treatment after the initial assessment. By the 4-month assessment, some patients (n = 38; 18%) had completed adjuvant therapy. By 8 months, the majority of patients had completed treatment (n = 129; 93%), and by 12 months, all patients (100%) had completed treatment.
At 60 months, follow-up data were available for 87 of 138 patients (63%). Of the 51 patients without 60-month data, 30 patients had developed recurrent disease or had died, and 21 patients had withdrawn from the study. The groups did not differ in the rates of recurrence, death, study withdrawal, or participation at 60 months (all P ≥.205). Regarding the number of data points, there were 1216 completed assessments; 216 assessments were missing because of patient recurrence or death, 140 assessments were missing because of patient withdrawal from the study, and 94 assessments were missed by patients who continued participating in the study. All available data were used.
Signs, Symptoms, and Toxicities: Incidence and Group Differences
Patients who received taxanes experienced more symptoms during chemotherapy, as expected. Moreover, they were more likely to have cycles prolonged in days because of toxicities (50% vs 32% in the no-taxane group; chi-square [df = 1] = 4.12; P =.042). Across all patients, the most common reasons for chemotherapy delay were leukopenia (n = 28), infections (n = 15), pain (n = 11), fever (n = 8), and nausea (n = 8). Group comparisons of the reasons for chemotherapy delays indicated that patients in the taxane group were more likely to require delays because of pain (18% vs 3%; Fisher exact P =.003). Other reasons for delay were distributed equally across groups (P >.06).
In addition, patients in the taxane group experienced a greater number and severity of 6 of the 9 signs/symptoms during the months of chemotherapy (4-, 8-, and 12-month assessments) (Fig. 1a). No life-threatening (grade 4) reactions were recorded. Compared with patients in the no-taxane group, patients in the taxane group experienced greater peripheral neuropathy (motor weakness: chi-square [df = 2] = 9.64; P =.008; incoordination/ataxia: chi-square [de = 1] = 6.12; P =.013; incontinence: chi-square [df = 1] = 3.99; P =.046), greater neurosensory dysfunction (loss of reflexes: chi-square [df = 1] = 9.14; P =.002; neurologic reactions: chi-square [df = 1] = 9.72; P =.008), and more arthral-gia/myalgia (chi-square [df = 2] = 7.15; P =.028), as hypothesized. There were no group differences for sensory changes (hearing, vision, or taste: chi-square [df = 2] = 0.21; P =.899) or dermatologic reactions (chi-square [df = 2] = 0.48; P =.788). Unfortunately, the final item, parasthesia/numbness, referred to sensation at the surgical site rather than the periphery, which precluded our examination of the symptom in hands and feet. Greater than 90% of patients reported this symptom before receiving any chemotherapy. Therefore, it is not included in Figure 1.
FIGURE 1.
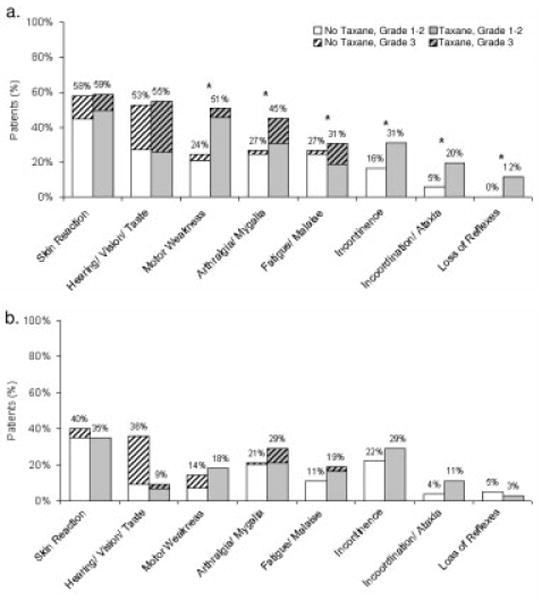
Percentage of patients experiencing signs, symptoms, and toxicities that began or worsened after the initial (prechemotherapy) assessment. Data indicate the percentage of patients reporting symptoms that differed from the initial symptoms at Months 4 through 12 (a), and at Months 42 and 48 (b).
* P < .05
Data from Years 2 through 5 indicated no differences between groups on any of the 9 symptoms (all P >.11). For illustration, Figure 1b provides the percentage of patients reporting symptoms in Year 4.
Psychological and Quality-of-life Trajectories
Emotional distress (POMS) and depressive symptoms (CES-D)
Results of the multilevel models are provided in Table 2. The predicted trajectories produced by the analyses closely approximated the raw data, as illustrated in Figure 2. Figure 2a illustrates the observed mean scores by group for the POMS across time. Figure 2b illustrates the predicted trajectories by group for the POMS across 2 time periods. The groups did not differ significantly at baseline, as indicated by the intercept parameters. The early model (from the initial assessment to 24 months) suggested that the no-taxane group enjoyed early, rapid relief from distress from the initial assessment through 12 months, whereas distress relief for the taxane group began significantly later, as indicated by a significant group difference in quadratic change (P < .001) (see Table 2). Descriptively, no distress reduction was reported by patients in the taxane group until 12 months. The late model (30–60 months) demonstrated no change over time in POMS and no significant group differences, as indicated by a nonsignificant group effect for intercept (P = .429). Thus, from Year 3 though Year 5, the groups reported reduced and comparable levels of emotional distress.
TABLE 2. Multilevel Models Contrasting the No-Taxane and Taxane Group Trajectories During Early (Baseline to 24 Months) and Late (30 Months to 60 Months) Periods.
| Estimates for no-taxane group | Effects of the taxane group | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control: Weeks of chemotherapy |
Intercept* | Slope† | Quadratic‡ | Difference in intercept§ |
Difference in slope‖ | Difference in quadratic¶ |
||||||||
| Outcome | Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P | Estimate (SE) | P |
| Early trajectories: Baseline to 24 mo | ||||||||||||||
| Emotional distress | −0.53 (0.24) | .029 | 28.7 (4.26) | <.001 | −1.40 (0.33) | <.001 | .044 (.013) | .001 | +7.54 (4.23) | .076 | +1.49 (0.52) | .004 | −.063 (.021) | .003 |
| Depressive symptoms | −0.08 (0.04) | .029 | 7.03 (0.68) | <.001 | −0.27 (0.05) | <.001 | .008 (.002) | <.001 | +0.92 (0.68) | .174 | +0.23 (0.09) | .009 | −.008 (.004) | .023 |
| Physical quality of life | — | — | 42.1 (0.94) | <.001 | 0.86 (0.13) | <.001 | −.023 (.005) | <.001 | −2.88 (1.46) | .050 | +0.01 (0.21) | .974 | −.002 (.008) | .826 |
| Mental quality of life | 0.17 (0.10) | .090 | 40.2 (1.75) | <.001 | 1.26 (0.16) | <.001 | −.043 (.006) | <.001 | +0.46 (1.79) | .797 | −0.75 (0.25) | .003 | +.028 (.010) | .006 |
| Late trajectories: 30–60 mo | ||||||||||||||
| Emotional distress | — | — | 13.6 (2.89) | <.001 | — | — | — | — | +3.69 (4.65) | .429 | — | — | — | — |
| Depressive symptoms | — | — | 3.91 (0.48) | <.001 | — | — | — | — | +1.25 (0.81) | .126 | — | — | — | — |
| Physical quality of life | — | — | 50.8 (1.04) | <.001 | −0.09 (0.03) | .004 | — | — | −3.31 (1.72) | .056 | +0.07 (0.05) | .174 | — | — |
| Mental quality of life | — | — | 49.4 (1.14) | <.001 | 0.05 (0.04) | .162 | — | — | +0.74 (1.99) | .698 | −0.13 (0.06) | .034 | — | — |
SE indicates standard error; —, the parameter did not improve the model (based on the Akaike Information Criterion and the Bayseian Information Criterion) and, thus, was not included in the final model.
Intercept gives the estimate for the no-taxane group mean at Time 0. For early models, Time 0 indicates the pretreatment baseline. For late models, Time 0 was defined as the beginning of the late period, 30 months after baseline. P value indicates whether the estimate differed significantly from zero.
Slope provides an estimate of the average rate of change for the no-taxane group in units per month.
Quadratic provides an indication of acceleration or deceleration in rate of change. If the sign of the quadratic change parameter (positive or negative) matches the sign of the slope, then the rate of change is accelerating over time, with the majority of change occurring late in the period. If the sign is the opposite of that of slope, then the majority of change occurred early in the period.
Estimate of the difference between the groups' intercepts. A significant test indicates a group difference at Time 0 (ie, baseline for the early models, 30 months for the late models.)
Estimate of the group difference in slope. A significant test indicates different rates of change between groups.
Estimate of the group difference in quadratic change. A significant test indicates different curvilinear trajectories for the groups.
FIGURE 2.
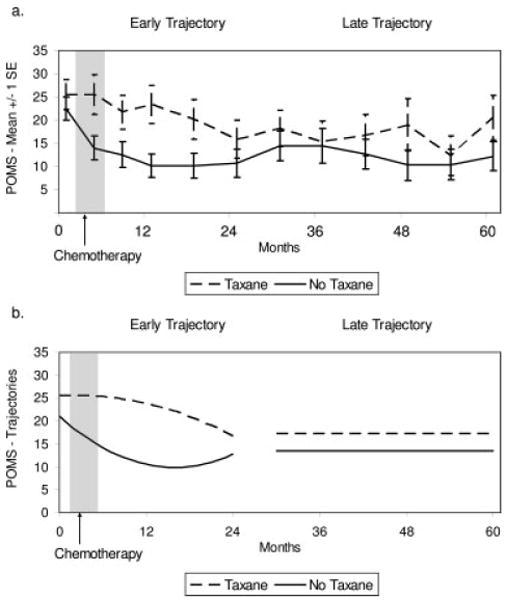
Scores on the Profile of Mood States (POMS) by group across time. (a) Observed group mean scores and standard errors (SE). (b) Predicted group scores during the early (initial to 24 months) and late (30–60 months) periods.
Figure 3 provides data on patient reports of depressive symptoms (CES-D). Figure 3a illustrates the predicted trajectories for depressive symptoms by group during the 2 periods. The early model demonstrated that the groups were equivalent at diagnosis (P =.174), and this was followed again by rapid improvement for the no-taxane group and significantly slower improvement for the taxane group (P <.023). Like the POMS, the late model for the CES-D demonstrated no change over time and no difference between groups (P =.126). To determine the clinical significance of the symptom reports, we calculated the percentage of patients that reported a clinically significant level of depressive symptoms (ie, a CES-D Short Form score ≥10). The proportion of patients in each group with clinical elevations reported across time is illustrated in Figure 3b. Chi-square analysis indicated statistically significant group differences at the 12-month (P = .013) and 18-month (P = .016) assessments and a trend at the 24-month assessment (P = .053).
FIGURE 3.
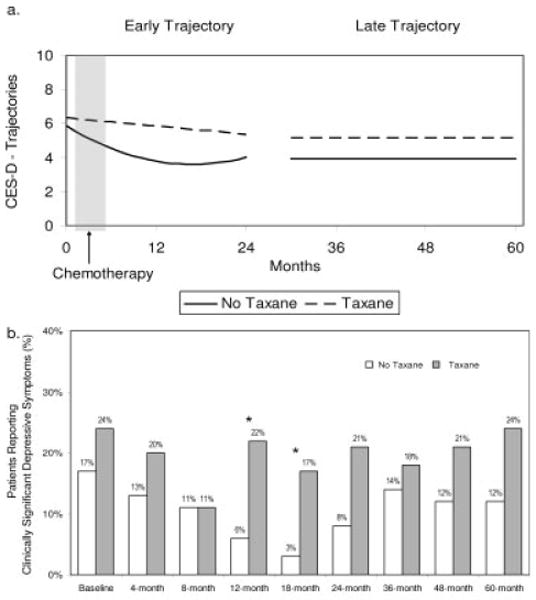
Scores on the Center for Epidemiological Studies-Depression scale (CES-D) (Iowa Short Form) by group across time. (a) Predicted group scores during the early (initial to 24 months) and late (30–60 months) periods. (b) Percent of patients reporting clinically significant depressive symptoms (scores ≥10).
* P < .05
Health-related quality of life (SF-36)
Figure 4 illustrates the trajectories for quality of life. For the PCS (see Fig. 4a), the early model demonstrated that the taxane group had more disruption of physical quality of life at baseline (P =.050). Thereafter, both groups improved in a similar pattern, with the rate of change slowing over time. The late model demonstrated that both groups declined slowly over Years 3 through 5 (P =.004). The groups did not differ in the late model (P =.174). At all time points in the late period, both groups were at or within 1/2 standard deviation of the PCS norm (ie, PCS = 5022).
FIGURE 4.
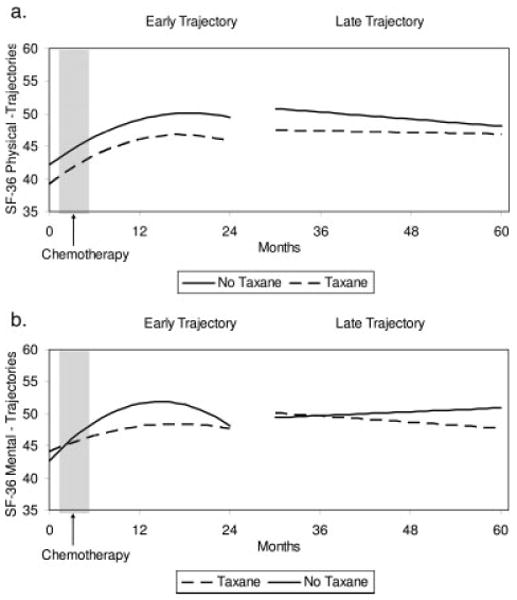
Scores on the 36-item Medical Outcomes Study Short Form (SF-36) by group across time. Predicted group scores during the early (initial to 24 months) and late (30–60 months) periods for the Physical Component score (a) and the Mental Component score (b).
Figure 4b illustrates the trajectories for the MCS. Like the POMS and CES-D, the early model demonstrated that the groups were similar at baseline (P =.797), and there were differential changes thereafter. Patients in the taxane group recovered later and more slowly than patients in the no-taxane group (P <.006). There was, however, a significant group difference observed in the late model. Although similar at 30 months (P =.698), the groups changed differentially over the follow-up period: The no-taxane group had an increase and the taxane group had a decline in mental quality of life (P =.034). Again, the means for both groups in the late model were estimated in the range of the normative mean (ie, MCS = 5022).
Post-hoc Analyses
Post-hoc analyses were conducted to rule out alternative explanations for the findings. First, because the taxane group initially reported significantly lower physical quality of life than the no-taxane group, we tested the hypothesis that physical functioning may account for the differential psychological recovery. To do so, multilevel model analyses were repeated using concurrent nurse-rated functional status (KPS) as a time-varying covariate. The results were identical to those reported above for the POMS, CES-D, and MCS. That is, controlling for concurrent functional performance status, the taxane and no-taxane groups were equivalent at baseline (all P >.061), but, once again, they diverged, with patients in the taxane group exhibiting delayed psychological recovery (all P <.024). Among the late models, a slight divergence of the groups in mental quality of life was observed (P =.031), with decreasing quality of life for the taxane group. All other effects were nonsignificant, as in the primary analyses. Thus, the greater impairments in physical functioning of the taxane group at baseline did not appear to account for the psychological differences between the groups at follow-up.
For current study, we used a convenience sample of patients (N = 138) who did or did not receive taxanes. However, there was a subset of patients (n = 46) enrolled in randomized trials that were testing the therapeutic efficacy of taxanes. Post hoc, we repeated our analyses and included only those patients who were randomized either to receive a taxane (n = 22 patients) or to a no-taxane arm (n = 24 patients). We then compared the effect sizes of the differences between groups (Cohen d) observed in all patients (N = 138) with those observed in analyses with the randomized patients (see Table 3). The effect sizes for the subsample were equivalent to those observed with all patients, and differences between the estimates were minimal (range, .01-.07). Thus, effects of the same magnitude were observed in both randomized and nonrandomized samples. These data support the interpretation that the observed differences between groups resulted from the receipt of taxane therapy by some patients rather than from other, unidentified differences.
TABLE 3. Cohen d Effect Size Estimates for Early Group Effects: A Comparison of Results From All Patients (N = 138) With Results From the Subsample of Patients Randomized to Taxane or No-Taxane Conditions (n = 46).
| Effects with all patients | Effects with patients in taxane vs no-taxane randomized trials | |||
|---|---|---|---|---|
| Difference in intercept | Difference in quadratic | Difference in intercept | Difference in quadratic | |
| Emotional distress | .24 | .25 | .28 | .31 |
| Depressive symptoms | .22 | .19 | .21 | .18 |
| Physical quality of life* | — | — | — | — |
| Mental quality of life | .26 | .24 | .30 | .31 |
Dash (—) indicates that the group difference was not significant, and the effect size was not calculated.
Discussion
Patients' emotional and quality-of-life trajectories in the years after taxane treatment have not been studied previously. These new data indicated that patients who receive taxanes have significantly slower psychological recovery after a diagnosis of cancer. In fact, for patients in the taxane group, emotional ‘recovery’ required an average of 2 years compared with 6 to 12 months for patients in the notaxane comparison group. From a clinical perspective, there also were high rates of probable depression. Depressive symptoms, even when they are severe, are not equivalent to a diagnosis of major depression; however, it is noteworthy that the rates of probable depression in the no-taxane group declined to <10% by the 12-month follow-up assessment, whereas the rates in the taxane group remained high at approximately 20%. National prevalence estimates for depression in women, as determined by diagnostic interview, range from 5% to 9%.23
The current data demonstrate, as have others,10–12 that patients who receive taxanes experience neurologic side effects to a greater extent than patients on other regimens, including peripheral neuropathy, ataxia, and neurotoxicity. However, the data also highlight the limitations of toxicity documentation. It has been suggested that standard toxicity assessments are inadequate to understand the impact of adjuvant treatment on a patient's quality of life.24,25 Indeed, the current psychological and quality-of-life data provide an important context for the toxicity findings. The group differences in quality of life and emotional distress persisted for approximately 1 year longer than the differences in nurse-rated toxicity. Data such as these can provide an important contribution as the efficacy of the taxanes continues to be evaluated.
A limitation of this study is the use of a convenience sample and the case–control design. The post-hoc analyses with patients in randomized taxane treatment trials, however, provide strong suggestive evidence that the observed group differences were not caused by an unmeasured variable. However, we were not able to test for differential effects among the taxanes (PTX and T). Additional research is needed to determine whether taxanes produce different mood and quality-of-life disruption. Treatment intent also may be important. For example, it is known that T and PTX have a different spectrum of toxicities when they are used to control metastatic disease.26
Finally, we provide clinical observations. The current data are useful because, to our knowledge, no study has provided data on any psychosocial outcomes with taxanes beyond 6 months after adjuvant treatment. Moreover, 5-year follow-up is unusual in survivorship research. If these novel findings are confirmed in randomized trials, then monitoring of psychological symptoms would seem to be in order, with referral of patients who become significantly symptomatic. Currently, interventions are in development for preventing or treating the physical sequelae of the taxanes. Results from a recent phase 2 clinical trial, for example, suggested that vitamin E supplementation may reduce the risk of peripheral neuropathy from PTX.27 For patients with lesser, but still significant, distress, psychological interventions tailored to the context of cancer are efficacious.28,29 Should the taxanes pose a risk for depression, it is important for oncology professionals to be aware that psychotherapy or pharmacotherapy are efficacious, but psychotherapy appears to be more effective in preventing a relapse of depression.30 In closing, oncology professionals' awareness of the possibility of mood alteration with taxanes is important, because depression in cancer patients frequently is not recognized; and, even when it is recognized, it may be under treated.31 The referral of patients for the management of depressive sequelae may need to become a more salient component of comprehensive medical oncology care for patients who receive taxanes.
Acknowledgments
Preparation of this article was supported in part by grants from the American Cancer Society (PF-07-169-01-CPPB, PBR-89, and RSGPB-03-248-01-PBP), by a Longaberger Company-American Cancer Society Grant for Breast Cancer Research (PBR-89A), by U.S. Army Medical Research Acquisition Activity grants (DAMD17-94-J-4165; DAMD17-96-1-6294; DAMD17-97-1-7062), by the National Institutes of Mental Health (1 RO1 MH51487), by the National Cancer Institute (KO5 CA098133 and RO1 CA92704), by the General Clinical Research Center (MO1-RR0034), and by the Ohio State University Comprehensive Cancer Center (P30 CA16058).
We thank the participants and the professional and research staff of the Stress and Immunity Cancer Projects. Special thanks to Carolyn Hagopian, Shruti Patel, and Caroline Helmick.
We have complied with all institutional and international ethical standards in the treatment (assessment) of participants in this study.
Footnotes
L. M. Thornton, W. E. Carson III, C. L. Shapiro, W. B. Farrar, and B. L. Andersen have no relationships, financial or otherwise, that might lead to a conflict of interest with regard to this manuscript.
References
- 1.Hudis C. The use of taxanes in early breast cancer. EJC Supplements. 2003;1:1–10. [Google Scholar]
- 2.Ferguson T, Wickedn N, Vagg R, Ghersi D, Nowak AK. Taxanes for adjuvant treatment of early breast cancer [serial online] Cochrane Database Syst Rev. 2007;(4):CD004421. doi: 10.1002/14651858.CD004421.pub2. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration: Center for Drug Evaluation and Research. Listing of Approved Oncology Drugs With Approved Indications. Washington, DC: Department of Health and Human Services; 2007. [Google Scholar]
- 4.Fornier MN, Seidman AD, Theodoulou M, et al. Doxorubicin followed by sequential paclitaxel and cyclophosphamide versus concurrent paclitaxel and cyclophosphamide: 5-year results of a phase II randomized trial of adjuvant dose-dense chemotherapy for women with node-positive breast carcinoma. Clin Cancer Res. 2001;7:3934–3941. [PubMed] [Google Scholar]
- 5.Cella DF, Peterman A, Hudgens S, Webster K, Socinski MA. Measuring the side effects of taxane therapy in oncology: the Functional Assessment of Cancer Therapy-Taxane (FACT-taxane) Cancer. 2003;98:822–831. doi: 10.1002/cncr.11578. [DOI] [PubMed] [Google Scholar]
- 6.Chevallier B, Fumoleau P, Kerbrat P, et al. Docetaxel is a major cytotoxic drug for the treatment of advanced breast cancer: a phase II trial of the Clinical Screening Cooperative Group of the European Organization for Research and Treatment of Cancer. J Clin Oncol. 1995;13:314–322. doi: 10.1200/JCO.1995.13.2.314. [DOI] [PubMed] [Google Scholar]
- 7.Holmes FA, Walters RS, Theriault RL, et al. Phase II trial of taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst. 1991;83:1979–1805. doi: 10.1093/jnci/83.24.1797-a. [DOI] [PubMed] [Google Scholar]
- 8.Mamounas EP, Bryant J, Leinbersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 9.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–537. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 10.Fountzilas G, Skarlos D, Dafni U, et al. Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high-risk operable breast cancer: a randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol. 2005;16:1762–1771. doi: 10.1093/annonc/mdi366. [DOI] [PubMed] [Google Scholar]
- 11.Martin M, Lluch A, Segui MA, et al. Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. Ann Oncol. 2006;17:1205–1212. doi: 10.1093/annonc/mdl135. [DOI] [PubMed] [Google Scholar]
- 12.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 13.Fleming ID, Cooper JS, Hensen DE, et al., editors. AJCC Cancer Staging Manual. 5th. Philadelphia, Pa: Lippincott-Raven; 1998. [Google Scholar]
- 14.Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007;7:953–961. doi: 10.1016/j.bbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Psychological, behavioral, and immune changes following a psychosocial intervention: a clinical trial. J Clin Oncol. 2004;17:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hryniuk WM. The importance of dose intensity in the outcome of chemotherapy. Important Adv Oncol. 1988:121–141. [PubMed] [Google Scholar]
- 17.Moinpour CM, Feigl P, Metch B, Hayden KA, Meyskens FL, Crowley J. Quality of life end points in cancer clinical trials: review and recommendations. J Natl Cancer Inst. 1989;81:485–495. doi: 10.1093/jnci/81.7.485. [DOI] [PubMed] [Google Scholar]
- 18.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- 19.McNair DM, Lorr M, Droppleman LF. EITS Manual for the Profile of Mood States. San Diego, Calif: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 20.Kohout FJ, Berkman LF, Evans DA. Two shorter forms of the CES-D Depression Symptoms Index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychological Meas. 1977;1:385–401. [Google Scholar]
- 22.Ware JE, Snow KK, Kosinski M. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 24.Kayl AE, Meyers CA. Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr Opin Obstet Gynecol. 2006;18:24–28. doi: 10.1097/01.gco.0000192996.20040.24. [DOI] [PubMed] [Google Scholar]
- 25.Groenvold M, Fayers PM, Petersen MA, Sprangers MAG, Aaronson NK, Mouridsen HT. Breast cancer patients on adjuvant chemotherapy report a wide range of problems not identified by health-care staff. Breast Cancer Res Treat. 2007;103:185–195. doi: 10.1007/s10549-006-9365-y. [DOI] [PubMed] [Google Scholar]
- 26.Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542–5551. doi: 10.1200/JCO.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 27.Argyriou AA, Chroni E, Koutras A, et al. Preventing paclitaxel-induced peripheral neuropathy: a phase II trial of vitamin E supplementation. J Pain Symptom Manage. 2006;32:237–244. doi: 10.1016/j.jpainsymman.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Andersen BL. Biobehavioral outcomes following psychological interventions for cancer patients. J Consult Clin Psychol. 2002;70:590–610. doi: 10.1037//0022-006X.70.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer TJ, Mark MM. Effects of psychosocial interventions with adult cancer patients: a meta-analysis of randomized experiments. In: Suinn RM, Gary RV, editors. Cancer Patients and Their Families: Readings on Disease Course, Coping, and Psychological Interventions. Washington, DC: American Psychological Association; 1999. pp. 163–177. [DOI] [PubMed] [Google Scholar]
- 30.Pedro L, Delgado PZ. Treatment of Mood Disorders. In: Panksepp J, editor. Textbook of Biological Psychiatry. Hoboken, NJ: John Wiley & Sons, Inc.; 2004. pp. 231–266. [Google Scholar]
- 31.Ell K, Sanchez K, Vourlekis B, et al. Depression, correlates of depression, and receipt of depression care among low-income women with breast or gynecologic cancer. J Clin Oncol. 2005;23:3052–3060. doi: 10.1200/JCO.2005.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]


