Abstract
Variables influencing the risk of dissemination and outcome of C. neoformans infection were assessed in 111 organ transplant recipients with cryptococcosis in a prospective, multicenter, international study. Sixty one percent (68/111) of the patients had disseminated infection. The risk of disseminated cryptococcosis was significantly higher for liver transplant recipients (adjusted HR 6.65, p = 0.048). Overall mortality rate at 90 days was 14% (16/111). Mortality rate was higher in patients with abnormal mental status (p = 0.023), renal failure at baseline (p= 0.028), fungemia (p=0.006) and disseminated infection (p = 0.035), and lower in those receiving a calcineurin-inhibitor agent (p= 0.003). In multivariate analysis, the receipt of a calcineurin-inhibitor agent was independently associated with a lower mortality (adjusted HR 0.21, p = 0.008), and renal failure at baseline with a higher mortality rate (adjusted HR 3.14, p = 0.037). Thus, outcome in transplant recipients with cryptococcosis appears to be influenced by the type of immunosuppressive agent employed. Additionally, discerning the basis for transplant type-specific differences in disease severity has implications relevant for yielding further insights into the pathogenesis of C. neoformans infection in transplant recipients.
INTRODUCTION
Invasive fungal infections occur in 15–42% of the organ transplant recipients (1, 2). Refinements in surgical techniques and antifungal prophylaxis have led to a decline in the overall incidence of fungal infections in the early post-transplant period, particularly that of invasive candidiasis (3, 4). The risk factors for cryptococcal infections, however, are poorly understood. Cryptococcosis generally occurs in the late post-transplant period, well beyond the usual period of employment of antifungal prophylaxis (5, 6). Furthermore, most cases represent reactivation of latent infection (5, 7–9) such that limiting the exposure is unlikely to curtail the risk of cryptococcosis.
Mortality rate in transplant recipients with cryptococcosis typically ranges from 15–20%, and approaches 40% in those with central nervous system infection (5, 6, 10), suggesting a need to better understand the variables that affect outcome in these patients. Factors that impact outcome in other hosts have yielded insights that are relevant to prognosis in transplant recipients as well (11–14). However, organ transplant patients are unique in that the calcineurin-inhibitor based immunosuppressive regimens employed in these patients have antifungal activity in vitro (15–17), and could potentially modify the extent of infection or its prognosis. Thus, assessment of characteristics and outcome of C. neoformans infection specifically in organ transplant recipients is important. In a multicenter study, we determined the extent to which the risk of dissemination and mortality in organ transplant recipients with cryptococcosis can be predicted by readily assessable clinical and laboratory variables.
METHODS
Patients
Study population included 111 organ transplant recipients with C. neoformans infection at the participating centers in the United States, Canada, Spain, France, and India. These patients represented 98.2%(111/113) of the cases of cryptococcosis in transplant recipients at our institutions during the study period; two patients diagnosed and followed at a site remote from the transplant center could not be enrolled. Patients included from France were transplant recipients who developed cryptococcosis during the study period and were enrolled in a nationwide, multicenter, prospective study of the French Cryptococcosis Study Group. The study was conducted between December 1999 and March 2006; the timing of initiation at different sites varied. Institutional Review Board approval was obtained as per local requirements.
Definitions
C. neoformans infection was defined as per criteria proposed by the European Organization for Research and Treatment in Cancer and the Mycoses Study Group, i.e., positive cultures for C. neoformans in a clinical specimen, including blood cultures; histopathologic or cytopathologic examination of specimens of needle aspiration or biopsy showing encapsulated yeast cells; or positive cryptococcal antigen in the blood or cerebrospinal fluid in a patient with compatible clinical presentation (18). Variables assessed included demographic characteristics, immunosuppressive regimen at the time of diagnosis, rejection episodes or antifungal agent use within 6 months prior to the onset of infection, cytomegalovirus infection, renal failure (defined as creatinine ≥ 2 mg/dl) at the time of diagnosis, sites of infection, cerebrospinal fluid characteristics, antifungal therapy employed, and patient outcome. In all cases, the primary immunosuppressive agent at diagnosis was the patients’ stable immunosuppressive regimen that had remained unchanged within the previous 6 months.
Organ sites involved were classified as central nervous system (CNS); pulmonary; skin, soft-tissue, osteoarticular; or other (5, 19). Disseminated infection was defined as CNS infection or fungemia or involvement of ≥ 2 noncontiguous organ sites (5, 19). As in previous studies on opportunistic mycoses, including cryptococcosis, the mortality rate was assessed at 90 days (11, 20).
Statistical analysis
Stata (Intercooled Stata 9.2, College Station, TX) was used for all analyses. Logistic regression models were used to calculate odds ratios and confidence intervals for factors associated with disseminated infection; no adjustments were made for multiple comparisons. A multivariable model was developed to assess for the effect of several factors as risks for disseminated infection. For this model, backward selection was used with factors removed at p > 0.20. Interaction terms were generated and evaluated for the main effects factors in this model. Interaction terms were entered one at a time and dropped from the model if not statistically significant at p < 1.0. The Pearson goodness-of-fit was used to evaluate the final model. The Cox proportional hazards model was used to evaluate factors associated with mortality. Entry time was the date of diagnosis, and follow-up ended with death or 90 day post-diagnosis. A multivariable model was generated using backward selection with factors removed at p > 0.20. Interaction terms were generated and evaluated for the main effects factors in this model. Interaction terms were entered one at a time and dropped from the model if not statistically significant at p < 1.0. Schoenfeld residuals were used to test the proportional-hazard assumption. Treatment with amphotericin B was forced into the final model to adjust for potential effect of therapy.
RESULTS
The clinical and demographic characteristics of the study patients are outlined in Table 1. Cryptococcosis occurred a median of 21 months after transplantation; 68.5% of the infections developed >1 year post-transplant.
Table 1.
Demographic and clinical characteristics of the study patients (n = 111)
| Age, years, median (range) | 52 (19–77) |
| Gender, male | 67% (74) |
| Type of transplant | |
| Kidney | 51% (57) |
| Liver | 25% (28) |
| Heart | 8% (9) |
| Lung | 7% (8) |
| Other/multiorgan | 8% (9) |
| Kidney-pancreas | (5) |
| Kidney-heart | (2) |
| Kidney-liver | (1) |
| Small bowel-pancreas | (1) |
| Immunosuppressive regimens | |
| Tacrolimus based | 69% (76) |
| Tacrolimus + mycophenolate mofetil + prednisone | 36 |
| Tacrolimus + prednisone | 21 |
| Tacrolimus + azathioprine + prednisone | 10 |
| Tacrolimus + mycophenolate mofetil | 4 |
| Tacrolimus only | 5 |
| Cyclosporine A based | 18% (20) |
| CsA + mycophenolate mofetil + prednisone | 11 |
| CsA + azathioprine + prednisone | 4 |
| CsA + prednisone | 4 |
| CsA + mycophenolate mofetil | 1 |
| Other | 14% (15) |
| Azathioprine + prednisone | 10 |
| Mycophenolate mofetil + prednisone | 5 |
| T-cell agent use | 3% (4/111) |
| As induction therapy | |
| Antithymocyte globulin | 1 |
| As rejection therapy | |
| Antithymocyte globulin | 2 |
| Campath-1H | 1 |
| Prednisone dose mg/qd, median* | 10 |
| Retransplant** | 1% (2) |
| Rejection† | 30% (33) |
| Cytomegalovirus infection | 27% (30) |
| Renal failure at baseline†† | 26% (29) |
| Prior antifungal agent use§ | 7% (6/11) |
| Antifungal therapy | |
| Amphotericin B | 67% (74) |
| Fluconazole | 28% (31) |
| Other§§ | 5% (6) |
Numbers represent actual values unless identified as percentages.
In those receiving prednisone.
Retransplant implies prior receipt of an organ transplant.
Episodes occurring within 6 months prior to the onset of cryptococcosis.
Renal failure refers to creatinine ≥ 2 mg/dl at the time of diagnosis of infection.
Only one of these patients had received fluconazole.
Includes 3 patients who received no therapy and 3 who received a triazole agent.
Disseminated infection
Of 111 patients, 54% (60/111) had pulmonary infection, 52.2% (58/111) had CNS, and 8.1% (15/111) had skin, soft-tissue, or osteoarticular infections (Table 2). Sixty-one percent (68/111) of the patients had disseminated cryptococcosis and in 32.4% (36/111) of the patients, the infection was limited to the lungs. Patients receiving a calcineurin-inhibitor based regimen (tacrolimus or cyclosporine A) were significantly less likely to have CNS infection (48%, 46/96 vs. 80%, 12/15, p = 0.02), and were more likely to have cryptococcosis limited to the lungs (36.6%, 35/96 vs. 6.6%, 1/15, p = 0.02). CNS infection was present in 47.3% (36/76) of the tacrolimus recipients, 50% (10/20) of the cyclosporine A recipients, and 80% (12/15) of the patients who received azathioprine or mycophenolate mofetil, without a calcineurin-inhibitor agent (p = 0.004).
Table 2.
Characteristics of C. neoformans infection in the study patients (n = 111)
| Sites of infection | |
| Central nervous system | 52.2% (58) |
| Pulmonary | 54% (60) |
| Skin, soft-tissue, osteoarticular | 18% (20) |
| Other | 3.6% (4) |
| Renal abscess | 2 |
| Abdominal abscess | 1 |
| Spinal and iliac mass | 1 |
| Disseminated infection | 61% (68) |
| Central nervous system | 52.2% (58) |
| Fungemia* | 20.7% (23) |
| ≥ 2 noncontiguous sites** | 9% (10) |
| Serum cryptococcal antigen titer | |
| Median | 1:64 |
| Interquartile range | 1:4–1:512 |
| Cerebrospinal fluid values (in patients with CNS infection) | |
| White blood cell, median (interquartile range) | 81 (2–131) |
| No. with positive culture of the cerebrospinal fluid | 88% (49/56) |
| Cryptococcal antigen titer, median (interquartile range) | 1:64 (1:2 – 1:1024) |
| Time to onset of infection post-transplant | |
| Median (interquartile), months | 21 (9.4 – 53) |
| Infection occurring within | |
| 0–30 days | 2.7% (3) |
| 31–90 days | 5.4% (6) |
| 91days – 1 year | 23.4% (26) |
| > 1 year post-transplant | 68.5% (76) |
These included 20 patients with central nervous system infection.
2 of 10 patients also had fungemia.
Univariable logistic regression analysis of factors associated with disseminated as compared with non-disseminated or localized infection is shown in Table 3. No association was found between rejection, cytomegalovirus infection, or time to onset post-transplant and dissemination (Table 3). However, the type of organ transplanted and the immunosuppressive agent employed appeared to be associated with the risk of dissemination, although statistical significance was not achieved (Table 3). A multivariable model was constructed to determine if the immunosuppressive regimen and the specific organ transplant type were independently associated with the risk of disseminated infection (Table 3). The effect of type of transplant was assessed in comparison to lung transplant recipients who had the lowest risk of dissemination in univariable analysis (Table 3). The risk of disseminated infection was significantly higher for liver transplant recipients (adjusted hazard ratio 6.65, 95% CI, 1.01 – 43.64, p = 0.048), even when controlled for the type of immunosuppression (Table 3). Of 28 liver transplant recipients, 61% (17/28) had hepatitis C virus or alcohol as underlying liver disease. The incidence of disseminated infection was 80% (8/10) in patients with hepatitis C with virus, 71% (5/7) in those with alcohol, and 64% (7/11) for patients with other underlying liver diseases (p = 0.71)
Table 3.
Variables associated with disseminated versus localized cryptococcosis
| Variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Univariable analysis | |||
| Age | 0.99 | 0.97 – 1.03 | 0.98 |
| Type of transplant | |||
| Liver | 1.19 | 0.76 – 4.84 | 0.17 |
| Heart | 0.81 | 0.20 – 3.18 | 0.75 |
| Kidney | 1.58 | 0.65 – 3.81 | 0.31 |
| Lung | 0.19 | 0.04 – 1.01 | 0.05 |
| Multiorgan | 0.47 | 0.12 – 1.88 | 0.29 |
| Renal failure at baseline | 1.77 | 0.72 – 4.35 | 0.21 |
| Cytomegalovirus infection | 0.68 | 0.29 – 1.58 | 0.36 |
| Rejection | 1.04 | 0.45 – 2.39 | 0.93 |
| Time from onset to diagnosis | 0.99 | 0.99 – 1.00 | 0.35 |
| Months post-transplant to diagnosis | 1.10 | 0.48 – 2.52 | 0.81 |
| Onset < 1 year post-transplant | 1.17 | 0.51 – 2.66 | 0.71 |
| Receipt of prednisone | 4.32 | 0.79 – 1.13 | 0.09 |
| Prednisone, dose | 1.03 | 0.97 – 1.10 | 0.34 |
| Receipt of a calcineurin inhibitor agent* | 0.35 | 0.09 – 1.32 | 0.12 |
| Receipt of tacrolimus** | 0.34 | 0.08 – 1.31 | 0.12 |
| Receipt of cyclosporine A† | 0.37 | 0.079 – 1.76 | 0.21 |
| Multivariable analysis | |||
| Receipt of a calcineurin-inhibitor agent | 0.37 | 0.09 – 1.52 | 0.17 |
| Receipt of prednisone | 1.03 | 0.96 – 1.10 | 0.34 |
| Type of Transplant†† | |||
| Liver | 6.65 | 1.01 – 43.64 | 0.048 |
| Heart | 3.21 | 0.38 – 26.75 | 0.28 |
| Kidney | 4.07 | 0.69 – 23.88 | 0.11 |
| Multiorgan | 1.46 | 0.15 – 13.46 | 0.73 |
Includes tacrolimus or cyclosporine. Comparison is made with the receipt of a non-calcineurin-inhibitor based regimen (azathioprine or mycophenolate mofetil).
Compared to non-tacrolimus regimen.
Compared to non-cyclosporine A regimen.
Comparison is made with lung transplant recipients as reference.
Mortality
Mortality rate in the patients at 90 days was 14% (16/111). Mortality was 7.9% (6/76) in patients receiving tacrolimus, 20% (4/20) in those receiving cyclosporine A, and 40% (6/15) in patients who received azathioprine or mycophenolate mofetil without the aforementioned agents (p = 0.004, Figure 1). When stratified by the site of involvement, the mortality rate was 19% (11/58) in patients with CNS infection, 20.6% (14/68) in those with disseminated infection, and 33.3% (8/24) in patients with fungemia. Mortality in patients with infection limited to the lungs was 2.8% (1/36).
Figure 1.
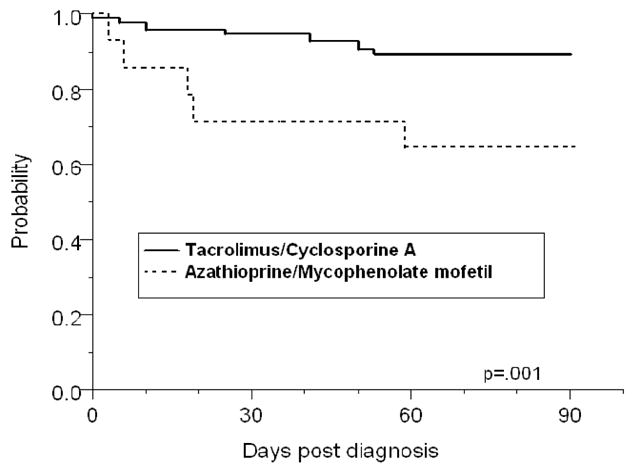
Kaplan-Meier survival analysis showing that the probability of survival after the diagnosis of cryptococcosis was significantly higher in patients who received a calcineurin-inhibitor agent (tacrolimus or cyclosporine A) as compared to those who received azathioprine or mycophenolate mofetil without a calcineurin-inhibitor agent (p=.001, log rank test).
Univariate Cox regression analysis showed that the mortality rate was significantly higher in patients with abnormal mental status (HR 3.11, 95% CI, 1.17–8.31, p = 0.023), renal failure at baseline (HR 2.99, 95% CI, 1.12 – 7.98, p= 0.028), fungemia (HR 3.94, 95% CI 1.48–10.51, p=0.006) and disseminated infection (HR 4.93, 95% CI, 1.11 – 21.69, p = 0.035), and lower in patients receiving a calcineurin-inhibitor agent (HR 0.21, 95 % CI, 0.07 – 0.59, p= 0.001). Patients receiving a calcineurin-inhibitor compared to a non-calcineurin-inhibitor based regimen were older (mean 52 versus 41 years, p = 0.01), more likely to have cryptococcosis >1 year post-transplant (100% versus 63%, p = 0.003), and less likely to be kidney transplant recipients (46% versus 87%, p = 0.004). However, age, time to onset of infection, and type of transplant were not significantly associated with mortality (Table 4). Details of antifungal therapy have been discussed elsewhere (19) and are not the focus of this report. Briefly, 66.6% (74/111) of all patients and 90% (60/66) of those with disseminated infections were treated with amphotericin B preparations (amphotericin B deoxycholate or lipid formulations of amphotericin B). Fluconazole, on the other hand was employed primarily for localized infections. Of 27.9% (31/111) of the patients who received fluconazole, 80.6% (25/31) had pulmonary, skin/soft tissue or other single site involvement, and only 19.3% (6/31) had disseminated infections. When adjusted for the site of infection (disseminated versus localized) there was no significant difference in outcome with the use of amphotericin B formulations as compared to fluconazole (Table 4).
Table 4.
Variables associated with mortality at 90 days in the study patients based on Cox proportional hazard analysis
| Variable | Hazard ratio (95% C.I.) | P-value |
|---|---|---|
| Univariable analysis | ||
| Age | 0.98 (0.94 – 1.02) | 0.35 |
| Gender, female | 1.16 (0.39 – 3.40) | 0.78 |
| Type of transplant (compared to the lung) | ||
| Liver | 1.10 (0.12 – 9.65) | 0.95 |
| Heart | 0.89 (0.06 – 14.24) | 0.94 |
| Kidney | 1.36 (0.17 – 10.65) | 0.77 |
| Retransplant | 0,56 (0.07 – 4.29) | 0.58 |
| Rejection | 0.75 (0.24 – 2.33) | 0.62 |
| Cytomegalovirus infection | 0.35 (0.08 – 1.52) | 0.16 |
| Renal failure at baseline | 2.99 (1.12 – 7.98) | 0.028 |
| Infection within 1 year post-transplant | 1.28 (0.46 – 3.53) | 0.63 |
| Duration of symptoms prior to therapy | 0.99 (0.98 – 1.01) | 0.69 |
| Site of infection | ||
| Central nervous system | 2.15 (0.75 – 6.20) | 0.15 |
| Pulmonary | 0.89 (0.33 – 2.36) | 0.81 |
| Skin, soft-tissue, osteoarticular only | 1.08 (0.14 – 8.20) | 0.94 |
| Fungemia | 3.94 (1.48 – 10.51) | 0.006 |
| Disseminated infection | 4.93 (1.11 – 21.69) | 0.035 |
| Abnormal mental status at presentation | 3.11 (1.17 – 8.31) | 0.023 |
| Primary immunosuppressive agent | ||
| Calcineurin-inhibitor agent (compared to non-calcineurin-inhibitor agent use) | 0.21 (0.07 – 0.59) | 0.003 |
| Tacrolimus | 0.15 (0.05 – 0.49) | .001 |
| CsA | 0.45 (0.13 – 1.59) | .21 |
| Antifungal therapy with amphotericin B (compared to fluconazole) | ||
| Localized infection | 1.85 (0.11 – 29.64) | 0.66 |
| Disseminated infection | 1.06 (0.13 – 8.21) | 0.95 |
| 5 flucytosine use as initial therapy (compared to no 5 flucytosine use) | ||
| Localized infection* | -- | -- |
| Disseminated infection | 0.66 (0.22 – 1.97) | 0.46 |
| Multivariable analysis** | ||
| Disseminated infection | 4.13 (0.92 – 18.42) | 0.063 |
| Receipt of a calcineurin-inhibitor agent | 0.21 (0.06 – 0.66) | 0.008 |
| Renal failure | 3.14 (1.06 – 9.26) | 0.037 |
Unable to calculate as there were no deaths in this group. Variables included in the model were abnormal mental status, renal failure, disseminated infection, and receipt of calcineurin-inhibitor agent. Using backward selection with factors removed at 0.20, abnormal mental status fell from the model.
There were no significant interactions and no significant violation of the proportional hazard assumption.
A multivariate Cox regression model was constructed with abnormal mental status, disseminated infection, receipt of a calcineurin-inhibitor agent, and renal failure in the model. Since fungemia was considered a manifestation of disseminated infection (Methods), only the latter was included in the model. Renal failure and receipt of a calcineurin-inhibitor agent correlated independently and significantly with outcome even when controlled for disseminated infection and abnormal mental status at baseline (Table 4). Mortality was significantly higher in patients with renal failure (adjusted HR 3.14, 95% CI 1.06 – 9.26, p= 0.037), and lower in those receiving a calcineurin-inhibitor agent (adjusted HR 0.21, 95% CI, 0.06 – 0.66, p = 0.008) (Table 4). When amphotericin B as antifungal therapy was added to this model, the findings remained unchanged. Renal failure (adjusted HR 3.40, 95% CI, 1.14 – 10.06, p = 0.027) remained significantly associated with higher mortality and calcineurin-inhibitor agent use with a lower mortality rate (adjusted HR 0.16, 95% CI, 0.05 – 0.48, p = 0.001).
DISCUSSION
Several observations from our study are relevant with regards to cryptococcosis in transplant recipients. In all, 61% of the infections were disseminated and the risk of dissemination was significantly higher for liver transplant recipients even when controlled for the immunosuppressive regimen. A number of possible reasons could account for this. Liver disease per se appears to be associated with more severe presentation and poorer outcome in cryptococcosis. Cirrhotic patients were more likely to develop septic shock and cirrhosis of the liver was an independent predictor of mortality in cryptococcocemia (21). Specific deficits in chemotaxis, complement deficiency, and monocyte suppressor cell activity in liver dysfunction were proposed to be the basis for these findings (21).
While intact cell-mediated immunity is critical, antibody responses also contribute to the pathogenesis of cryptococcal disease (22–24). Transplant recipients with cryptococcosis had higher IgM and IgG titers to glucoronoxylomannan than those who did not develop this infection after transplantation (25). That antibody promotes disease expression may seem intuitively paradoxical, but is plausible since a prozone-like effect enhances the severity and increases mortality in experimental cryptococcosis (26, 27). Liver transplant recipients have a lower frequency of posttransplant hypogammaglobulinemia due immunonusuppressive therapy than other transplant recipients (28–30). Given that hepatic sinusoidal and Kupffer cells play a role in the clearance of immunoglobulins (31), a decline in cryptococcal-specific or nonspecific immunoglobulins may be substantially less or protracted in liver than in other transplant recipients, thus enhancing their susceptibility (loss of resistance) to cryptococcosis. Finally, hepatic iron overload in liver transplant recipients may also enhance fungal virulence (32).
We note that a greater propensity of liver transplant recipients to develop disseminated infection has also been observed for aspergillosis. Historically, disseminated invasive aspergillosis has been documented in 50–60% of the liver as compared to 6–35% of the other organ transplant recipients (33–36). Notably, despite the requirement of a higher degree of immunosuppression, most Aspergillus infections in lung transplant recipients are limited to the lungs with disseminated infections occurring in ~6–16% of the patients (36, 37). This suggests that immune defects that facilitate the evasion of host defenses by these opportunistic mycoses are greater in magnitude or that certain deficits occur uniquely in liver transplant recipients.
Tacrolimus (FK506) and cyclosporine exert their immunosuppressive effect by inhibiting calcineurin, a T-cell signaling molecule (16, 38). Although highly conserved from man to yeast, calcineurin is also identified in fungi and plays a vital role in cell biology in pathogenic fungi, including cellular morphogenesis and virulence in C. neoformans (39, 40). Calcineurin-inhibitor agents have potent in vitro antifungal activity against C. neoformans that is mediated through inhibition of fungal homologs of calcineurin (16, 41). The minimum inhibitory concentration of FK506 at 37°C for C. neoformans was < 0.09 μg/mL and that for cyclosporine A was 0.39 – 5μg/mL (15). Despite in vitro activity against C. neoformans, cyclosporine A was associated with progressive infection in an animal model of cryptococcal meningitis (42). Cyclosporine A, however, penetrates the CNS less effectively than tacrolimus (5, 42). Given that transplant recipients receiving calcineurin-inhibitor agents develop cryptococcosis, the immunosuppressive effect as compared to the antifungal effect appears to predominate in the clinical setting. However, the use of these agents appeared to confer a protective effect on mortality that was particularly notable for tacrolimus. Whether this association is due to antifungal attributes of tacrolimus or other unmeasured variables pertaining to the host or infection in our patients remains to be determined. The association of renal failure with poor outcome in opportunistic mycoses, including cryptococcosis has previously been reported (5).
Cryptococcosis has been reported following zoonotic exposure and in outbreak settings (43, 44). However, a vast majority of the cases are considered to be due to reactivation of strains acquired long before clinical disease – likely during early childhood, and sequestered in alveolar macrophages (7, 9). Patients receiving a calcineurin-inhibitor agent in our study were less likely to have CNS involvement and more likely to have infection limited to the lungs. Thus, these agents might inhibit fungal calcineurin in strains emerging from the dormant phase and decrease dissemination from lungs and hilar lymph nodes to the CNS. However, the association of calcineurin-inhibitor agents with mortality was much stronger than the association of these agents with the risk of dissemination, suggesting that their protective effect on mortality may be mediated by other mechanisms as synergy with the azoles.
The combination of FK506 and fluconazole is synergistic in vitro for C. neoformans and resulted in ~30-fold decrease in the minimum inhibitory concentration of FK506 and 4-fold decrease in that of fluconazole for this yeast (45). Whether outcomes in patients receiving immunophilin-binding immunosuppressive agents can be further improved by employing therapeutic interventions that synergistically target calcineurin or signaling pathways distinct from it is an important question.
There are limitations of our study that deserve to be acknowledged. Since this was not a clinical trial, neither the immunosuppressive regimen nor antifungal therapy was randomized. There was also a significant difference in the time of onset of infection posttransplant with more patients who received a non-calcineurin inhibitor based regimen having later onset of cryptococcosis. However, this finding, if at all, would tend to bias the outcome in favor of the non-calcineurin inhibitor agent group as cummulative immunosuppression is generally lower and outcomes in opportunistic infections are better in the late posttransplant period. Although we found no statistically significant association between the time to onset and the risk of disseminated infection or mortality, it is possible that timing or yet unknown factors influenced the course of infection. Amongst the strengths of our study is that it included a large cohort of patients in a prospective, multicenter design which renders our findings generalizable to other transplant recipients with cryptococcosis.
In summary, our data show that cryptococcosis remains a significant complication in organ transplant recipients. The outcome, and to some extent the spectrum of infection appears to be influenced by the receipt of calcineurin-inhibitor based immunosuppression. Calcineurin-inhibitor agents remain the mainstay of immunosuppression; however, long-term outcomes in transplant recipients receiving these drugs are suboptimal. Renal dysfunction, metabolic toxicity, and cardiovascular complications due to cumulative exposure to calcineurin-inhibitor agents (46, 47) have spawned a growing interest in the use of induction therapy with the aim of achieving calcineurin-free/sparing maintenance immunosuppression after transplantation (48, 49). The impact of these evolving strategies on the spectrum of infectious complications, including cryptococcosis remains to be determined. Finally, future studies to discern the precise basis for organ-specific differences in disease expression and severity of opportunistic mycoses have the potential to yield further insights into the pathogenesis of these infections in transplant recipients.
Acknowledgments
The following is a complete list of the investigators in alphabetical order by the country and the participants: Canada: Andrew A. House, University of Western Ontario, London, ON; Atul Humar, University Health Network, Toronto General Hospital, Toronto, ON.
France: Olivier Lortholary (Institut Pasteur and U. Paris V, Necker-Enfants Malades Hospital, Paris), and Françoise Dromer (Centre National de Référence Mycologie et Antifongiques, Unité de Mycologie Moléculaire, Institut Pasteur, Paris) for the French Cryptococcosis Study Group, the members of which are listed below in alphabetical order: Corinne Antoine (Saint-Louis Hosp, Paris); Benoît, Barrou (Pitié-Salpétriére Hosp, Paris); Anne-Elisabeth Heng (Gabriel Montpied Hosp, Clermont-Ferrand); Christophe Legendre (Necker-Enfants aladies Hosp, Paris); Christian Michelet (Pontchaillou Hosp, Rennes); Bénédicte Ponceau (Croix-Rousse Hosp, Lyon); Nacéra Ouali (Tenon Hosp, Paris); Marc Stern (Foch Hosp, Suresnes).
India: Krishan L. Gupta, Postgraduate Institute of Medical Education and Research, Chandigarh; George T. John, Christian Medical College, Vellore.
Spain: Patricia Munoz, Gregorio Mãranón, Madrid.
United States: Barbara D. Alexander and Joseph Heitman (Duke University Medical Center, Durham, NC); Ramon del Busto and Theresa Sheppard (Henry Ford Hospital, Detroit, MI); Shahid Husain, Nina Singh, and Marilyn M. Wagener (University of Pittsburgh Medical Center, Pittsburgh, PA); Lorraine Dowdy (University of Miami, Miami, FL); Robert A. Fisher (Virginia Commonwealth University, Richmond, VA); Julia Garcia-Diaz (Ochsner Clinic, New Orleans, LA); Sally Houston (University of South Florida, Tampa, FL), Goran B. Klintmalm (Baylor University Medical Center, Dallas, TX); Andre C. Kalil (University of Nebraska, Omaha, NE); Ajit P. Limaye (University of Washington, Seattle, WA); Marshall Lyon, and Jyoti Somani (Emory University, Atlanta, GA); Susan Orloff (Oregon Health Sciences University, Portland, OR); Timothy L. Pruett (University of Virginia, Charlottesville, VA); Kenneth Pursell (University of Chicago, Chicago, IL); Valentina Stosor (Northwestern University, Chicago, IL), Dannah Wray (Medical University of South Carolina, Charleston, SC).
Sources of support: Supported by NIH/NIAID award R01 AI054719-01 to NS. There was no other source of support for this study.
Footnotes
Conflict of Interest Disclosure: Lorraine Dowdy has received research support from Enzon and Astellas. Shahid Husain has received research support from Enzon. G. Marshall Lyon is on the Speaker’s Bureau of Pfizer and Astellas and has received research support from Merck and Astellas. Olivier Lortholary is on the Speaker’s Bureau of Pfizer and Astellas. Kenneth Pursell is on the Speaker’s Bureau for Merck, Pfizer, and Schering-Plough. Nina Singh has received research grant support from Schering-Plough and Enzon. There are no conflict of interest disclosures for the other authors.
References
- 1.Kanj SS, Welty-Wolf K, Madden J, Tapson V, Baz MA, Davis D, Perfect JR. Fungal infections in lung and heart-lung transplant recipients: report of 9 cases and review of the literature. Medicine. 1996;75:142–156. doi: 10.1097/00005792-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Paya CV. Fungal infections in solid-organ transplantation. Clin Infect Dis. 1993;16:677–688. doi: 10.1093/clind/16.5.677. [DOI] [PubMed] [Google Scholar]
- 3.Fortun J, Martin-Davila P, Moreno S, Barcena R, de Vicente E, Honrubia A, Garcia M, Nuno J, Candela A, Uriarte M, Pintado V. Prevention of invasive fungal infections in liver transplant recipients: the role of prophylaxis with lipid formulations of amphotericin B in high-risk patients. J Antimicrob Chemother. 2003;52:813–819. doi: 10.1093/jac/dkg450. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Wagener MM, Marino IR, Gayowski T. Trends in invasive fungal infections in liver transplant recipients: correlation with evolution in transplantation practices. Transplantation. 2002;73:63–67. doi: 10.1097/00007890-200201150-00011. [DOI] [PubMed] [Google Scholar]
- 5.Husain C, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375–381. doi: 10.3201/eid0703.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John GT, Mathew M, Snehaltha E, Anandi V, Date A, Jacob CK, Shastry JCM. Cryptococcosis in renal allograft recipients. Transplantation. 1994;58:855–856. [PubMed] [Google Scholar]
- 7.Goldman DL, Khine H, Abadi J, Lindenberg DJ, Pirofski L, Niang R, Casadevall A. Serologic evidence of Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107:E66. doi: 10.1542/peds.107.5.e66. [DOI] [PubMed] [Google Scholar]
- 8.Dromer F, Robin O, Dupont B. Isolation of Cryptococcus neoformans from Asian patient in France: evidence for dormant infection in healthy subjects. J Med Vet Mycol. 1992;30:395–397. [PubMed] [Google Scholar]
- 9.Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol. 1999;37:3204–3209. doi: 10.1128/jcm.37.10.3204-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander BD. Cryptococcosis after solid organ transplantation. Transplant Infect Dis. 2005;7:1–3. doi: 10.1111/j.1399-3062.2005.00079.x. [DOI] [PubMed] [Google Scholar]
- 11.Saag MS, Powderly WG, Cloud GA, Robinson P, Grieco MH, Sharkey PK, Thompson SE, Sugar AM, Tuazon CU, Fisher JF, Hyslop N, Jacobson JM, Hafner R, Dismukes WE. The NIAID Mycoses Study Group and The AIDS Clinical Trials Group. Comparison of amphotericin with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. N Engl J Med. 1992;326:83–89. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- 12.Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis: A study in 111 cases. Ann Intern Med. 1974;80:176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- 13.Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, Lancaster DJ, Henderson H, Kauffman CA, Haas DW, Saccente M, Hamill RJ, Holloway S, Warren RM, Dismukes WE. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33:690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 14.Dromer F, Mathoulin S, Dupont B, Brugiére O, Letenneur L the French Cryptococcosis Study Group. Comparison of the efficacy of amphotericin B and fluconazole in the treatment of cryptococcosis in human immunodeficiency virus-negative patients: retrospective analysis of 83 cases. Clin Infect Dis. 1996;22:154–160. doi: 10.1093/clinids/22.supplement_2.s154. [DOI] [PubMed] [Google Scholar]
- 15.Cruz MC, Del Poeta M, Wang P, Wenger R, Zenke G, Quesniaux VFJ, Movva NR, Perfect JR, Cardenas ME, Heitman J. Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob Agents Chemother. 2000;44:143–149. doi: 10.1128/aac.44.1.143-149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumont FJ, Staruch MJ, Koprak SL, Siekierka JJ, Lin CS, Harrison R, Sawell T, Kindt VM, Beattie TR, Wyvratt M, Sigal NH. The immunosuppressive and toxic affects of FK-506 are mechanically related: pharmacology of a novel antagonist of FK-506 and rapamycin. J Exp Med. 1992;178:751–760. doi: 10.1084/jem.176.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ascioglu S, Rex JH, Bennett JE, Billie J, Croksert F, Denning DW, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 19.Singh N, Lortholary O, Alexander BD, Gupta KL, John GT, Pursell KJ, Munoz P, Klintmalm GB, Stosor V, Del Busto R, Limaye AP, Somani J, Lyon M, Houston S, House AA, Pruett TL, Orloff S, Humar A, Dowdy LA, Garcia-Diaz J, Kalil AC, Fisher RA, Heitman J, Husain S. Antifungal management practices and evolution of infection in organ transplant recipients with C. neoformans infection. Transplantation. 2005;80:1033–1039. doi: 10.1097/01.tp.0000173774.74388.49. [DOI] [PubMed] [Google Scholar]
- 20.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 21.Jean SS, Fang CT, Shau WY, Chen YC, Chang SC, Hsueh PR, Hung CC, Luh KT. Cryptococcaemia: clinical features and prognostic factors. Q J Med. 2002;95:511–518. doi: 10.1093/qjmed/95.8.511. [DOI] [PubMed] [Google Scholar]
- 22.Fleuridor R, Lyles RH, Pirolski L. Quantitative and qualitative differences in the serum antibody profiles of human immunodeficiency virus-infected persons with and without Cryptococcus neoformans meningitis. J Infect Dis. 1999;180:1526–1535. doi: 10.1086/315102. [DOI] [PubMed] [Google Scholar]
- 23.Subramaniam KS, French N, Pirofski L. Cryptococcus neoformans - reactive and total immunoglobulin profiles of HIV-infected and HIV-uninfected Ugandans. Clin Diagn Lab Immunol. 2006;12:1168–1176. doi: 10.1128/CDLI.12.10.1168-1176.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeShaw M, Pirofski L. Antibodies to Cryptococcus neoformans capsular polysaccharide glucoronoxylomannan are ubiquitious in the serum of HIV+ and HIV− individuals. Clin Exp Immunol. 1995;99:425–432. doi: 10.1111/j.1365-2249.1995.tb05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalali Z, Ng N, Singh N, Pirofski L. Antibody response to Cryptococcus neoformans capsular pollysaccharide glucoronoxylomannan in patients with solid organ transplants. Clin Vaccine Immunology. 2006;13:740–746. doi: 10.1128/CVI.00139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maitta RW, Datta K, Chang Q, et al. Protective and non-protective human IgM monoclonal antibodies to Cryptococcus neoformans glucoronoxylomannan manifest different specificity and gene use. Infect Immun. 2004;72:4810–4818. doi: 10.1128/IAI.72.8.4810-4818.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabora C, Rivera J, Zaragoza O, Casadevall A. More is not necessarily better: prozone-like effects in passive immunization with IgG1. J Immunol. 2003;170:3621–3630. doi: 10.4049/jimmunol.170.7.3621. [DOI] [PubMed] [Google Scholar]
- 28.Doron S, Ruthazer R, Werner BG, Rabson A, Snydman DR. Hypogammaglobulinemia in liver transplant recpients: incidence, timing, risk factors, and outcomes. Transplantation. 2006;81:697–703. doi: 10.1097/01.tp.0000180531.66518.9e. [DOI] [PubMed] [Google Scholar]
- 29.Corales R, Chua J, Mawhorter S, Young JB, Starling R, Tomford JW, McCarthy P, Braun WE, Smedira N, Hobbs R, Haas G, Pelegrin D, Majercik M, Hoercher K, Cook D, Avery RK. Significant post-transplant hypogammaglobulinemia in six heart transplant recipients; an emerging clinical phenomenon? Transpl Infect Dis. 2000;2:133–139. doi: 10.1034/j.1399-3062.2000.020306.x. [DOI] [PubMed] [Google Scholar]
- 30.Goldfarb NS, Avery RK, Goormastic M, et al. Hypogammaglobulinemia in lung transplant recipients. Transplantation. 2001;71:242–246. doi: 10.1097/00007890-200101270-00013. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Quintela A, Lopez-Ben S, Perez LF, Grana B, Varela M. Time-course changes of serum immunogobulins (IgA, IgG, IgM) after liver transplantation for alcoholic cirrhosis. Transpl Immunol. 2003;11:73–77. doi: 10.1016/S0966-3274(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 32.Alexander J, Limaye AP, Ko CW, Bronner MP, Kowdley KV. Association of hepatic iron overload with invasive fungal infection in liver transplanat recipients. Liver Transpl. 2006 doi: 10.1002/lt.20827. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Boon AP, Adams DH, Buckels J, McMaster P. Cerebral aspergillosis in liver transplantation. J Clin Pathol. 1990;43:114–118. doi: 10.1136/jcp.43.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torre-Cisneros J, Lopez OL, Kusne S, Martinez AJ, Starzl TE. CNS aspergillosis in organ transplantation: a clinicopathologic study. J Neurol Neurosurg Psychiatry. 1993;56:188–193. doi: 10.1136/jnnp.56.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18:44–49. doi: 10.1128/CMR.18.1.44-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sole A, Morant P, Salavert M, Pemán J, Morales P the Valencia Lung Transplant Group. Aspergillus in lung transplant recipients: risk factors and outcome. Clin Microbiol Infect. 2005;11:359–365. doi: 10.1111/j.1469-0691.2005.01128.x. [DOI] [PubMed] [Google Scholar]
- 37.Mehrad B, Paciocco G, Martinez FJ, et al. Spectrum of Aspergillus infection in lung transplant recipients: case series and review of the literature. Chest. 2001;119:169–175. doi: 10.1378/chest.119.1.169. [DOI] [PubMed] [Google Scholar]
- 38.Cardenas ME, Lim E, Heitman J. Mutations that perturb cyclophilin A ligand binding pocket confer cyclosporin A resistance in Saccharomyces cerevisiae. J Biol Chem. 1995;270:20997–21002. doi: 10.1074/jbc.270.36.20997. [DOI] [PubMed] [Google Scholar]
- 39.Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, Cardenas ME, et al. Calcineurin is essential for survival during membrane stress in Candida albicans. The EMBO Journal. 2002;21:546–559. doi: 10.1093/emboj/21.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruz MC, Sia RAL, Olson M, Cox CM, Heitman J. Comparisons of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans. Infect Immun. 2000;68:982–985. doi: 10.1128/iai.68.2.982-985.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorlach J, Fox DS, Cutler NS, Cox GM, Perfect JR, Heitman J. Identification and characterization of a highly conserved calcineurin binding protein, CBP1/calcipressin, in Cryptococcus neoformans. The EMBO Journal. 2000;19:1–12. doi: 10.1093/emboj/19.14.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perfect JR, Durack DT. Effects of cyclosporine in experimental cryptococcal meningitis. Infect and Immun. 1985;50:22–26. doi: 10.1128/iai.50.1.22-26.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nosanchuk JD, Shoham S, Fries BC, Shapiro DS, Levitz SM, Casadevall A. Evidence of zoonotic transmission of Cryptococcus neoformans from a pet cockatoo to an immunocompromised patient. Ann Intern Med. 2000;132:205–208. doi: 10.7326/0003-4819-132-3-200002010-00006. [DOI] [PubMed] [Google Scholar]
- 44.Hoang LM, Maguire JA, Doyle P, Fyfe M, Roscoe DL. Cryptococcus neoformans infections in Vancouver Hospital and Health Sciences Centre (1997–2002): epidemiology, microbiology and histopathology. J Med Microbiol. 2004;53:935–940. doi: 10.1099/jmm.0.05427-0. [DOI] [PubMed] [Google Scholar]
- 45.Del Poeta M, Cruz MC, Cardenas ME, Perfect J, Heitman J. Synergistic antifungal activities of bafilomycin A1, fluconazole and the pneumocandin MD-0991 caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans. Antimicrob Agents Chemother. 2000;44:739–746. doi: 10.1128/aac.44.3.739-746.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 47.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 48.Morris PJ, Russell NK. Alemtuzumab (Campath-1H): A systematic review in organ transplantation. Transplantation. 2006;81:1361–1367. doi: 10.1097/01.tp.0000219235.97036.9c. [DOI] [PubMed] [Google Scholar]
- 49.Fung J, Kelly D, Kadry Z, Patel-Tom K, Eghtsad B. Immunosuppression in liver transplantation: beyond calcineurin inhibitors. Liver Transpl. 2005;11:267–280. doi: 10.1002/lt.20373. [DOI] [PubMed] [Google Scholar]


