Abstract
PURPOSE One-quarter of adolescent mothers bear another child within 2 years, compounding their risk of poorer medical, educational, economic, and parenting outcomes. Most efforts to prevent rapid subsequent birth to teenagers have been unsuccessful but have seldom addressed motivational processes.
METHODS We conducted a randomized trial to determine the effectiveness of a computer-assisted motivational intervention (CAMI) in preventing rapid subsequent birth to adolescent mothers. Pregnant teenagers (N = 235), aged 18 years and older who were at more than 24 weeks’ gestation, were recruited from urban prenatal clinics serving low-income, predominantly African American communities. After completing baseline assessments, they were randomly assigned to 3 groups: (1) those in CAMI plus enhanced home visit (n = 80) received a multi-component home-based intervention (CAMI+); (2) those in CAMI−only (n = 87) received a single component home-based intervention; (3) and those in usual-care control (n = 68) received standard usual care. Teens in both intervention groups received CAMI sessions at quarterly intervals until 2 years’ postpartum. Those in the CAMI+ group also received monthly home visits with parenting education and support. CAMI algorithms, based on the transtheoretical model, assessed sexual relationships and contraception-use intentions and behaviors, and readiness to engage in pregnancy prevention. Trained interventionists used CAMI risk summaries to guide motivational interviewing. Repeat birth by 24 months’ postpartum was measured with birth certificates.
RESULTS Intent-to-treat analysis indicated that the CAMI+ group compared with the usual-care control group exhibited a trend toward lower birth rates (13.8% vs 25.0%; P = .08), whereas the CAMI-only group did not (17.2% vs 25.0%; P = .32). Controlling for baseline group differences, the hazard ratio (HR) for repeat birth was significantly lower for the CAMI+ group than it was with the usual-care group (HR = 0.45; 95% CI, 0.21–0.98). We developed complier average causal effects models to produce unbiased estimates of intervention effects accounting for variable participation. Completing 2 or more CAMI sessions significantly reduced the risk of repeat birth in both groups: CAMI+ (HR = 0.40; 95% CI, 0.16–0.98) and CAMI−only (HR = 0.19; 95% CI, 0.05–0.69).
CONCLUSIONS Receipt of 2 or more CAMI sessions, either alone or within a multicomponent home-based intervention, reduced the risk of rapid subsequent birth to adolescent mothers.
Keywords: Adolescent, outcome assessment (health care), health behavior, pregnancy in adolescence, reproductive behavior, community health services
INTRODUCTION
Almost one-quarter of adolescent mothers give birth to another child within 24 months of having a baby,1,2 despite national objectives to increase birth spacing3 and evidence that additional child-bearing during adolescence may compound the risk of poorer medical, educational, economic, and developmental outcomes.2,4,5 Compared with white adolescent mothers, African Americans and Latinas are more likely to experience another birth during adolescence (17%, 23%, 22%, respectively).1
Multiple interventions conducted in clinic and community settings by a range of service providers have attempted to reduce repeat adolescent pregnancy by providing birth control, social support, service coordination, health education, life skills, and employment training.3,6–15 Impacts have been modest, perhaps because of insufficient attention to motivation and support for behavior change.16–18 Ensuring access to contraception is ineffective if teens are not interested in preventing pregnancy.12,19–21 Without alternative life choices, such as career options, the benefits of becoming pregnant may outweigh the costs.20,22
Motivational interviewing is a counseling style that emphasizes an individual’s personal goals and self-efficacy in relation to complex health behaviors.16,23–25 Motivational interviewing aims to highlight discrepancies between current behaviors and personal goals, thereby promoting an intention and optimism for change.16,21,26 Brief motivational interviewing interventions with adolescents have been successful in motivating substance use and dieting behavior change.27–30 School-based programs incorporating motivational components have increased safer sexual practices.31
Few studies have examined the effect of motivational interviewing on adolescent contraceptive behaviors.32 A recent randomized trial compared the effect of receiving 2 motivational interviewing sessions with that of 2 general counseling sessions on contraceptive use and unintended pregnancy. Their findings did not support a significant effect on pregnancy prevention.32 We could find no published studies evaluating the effect of motivational interviewing on adolescent repeat birth.
Our main objective was to evaluate the effectiveness of a computer-assisted motivational intervention (CAMI) in preventing rapid repeat birth to adolescent mothers. We tested CAMI in 2 contexts: (1) as part of a multicomponent home-visiting intervention (CAMI+); and (2) as a single-component home-based intervention (CAMI−only). We hypothesized that repeat birth rates in both CAMI groups would be lower than for usual-care control group, and would be lowest for the CAMI+ group because of its greater intensity. As have others, we encountered substantial variation in participant adherence in prevention interventions.33–36 Because adherence may be related to a complex mix of behavioral risks, motivational processes, and social factors,37 as well as our outcome of interest, we also examined how intervention adherence moderated the CAMI effects.
METHODS
Study Setting and Participants
This randomized controlled trial was carried out in Baltimore, Maryland, which has a teenage birth rate of almost twice the national average.1 Participants were observed from enrollment to 24 months after the index birth. Study methods were approved by the institutional review boards of the University of Maryland School of Medicine and the Maryland Department of Health and Mental Hygiene.
Recruitment occurred between February 2003 and April 2005 from 5 prenatal care clinics serving low-income, predominantly African American communities. Adolescents were eligible if they were aged 12 to 18 years and if their pregnancy was 24 or more weeks’ gestation. They were excluded if the pregnancy did not result in a live birth and withdrawn if the infant died in the neonatal period, since parenting was an intervention focus. After the teen and her parent provided informed consent, the teen completed a baseline interview and was randomly assigned to a CAMI+, CAMI−only, or usual-care control group. Randomization was applied to consecutively consenting teens using computer-generated permuted blocks of 6. Because service delivery was an important goal, by design more teens were assigned to the intervention groups than the usual-care control group.
Interventions
Study Interventions
CAMI uses software developed for this study and programmed with algorithms based on the transtheoretical model.38–41 Using a laptop computer, the teen answered questions about her current sexual relationships and contraceptive and condom use intentions and behaviors. CAMI algorithms computed the adolescent’s stage of change (ie, for contraceptive and condom use) and produced a summary printout depicting whether she was at no, low, medium, or high risk for pregnancy and sexually transmitted infections. Interventionists, called CAMI counselors, then conducted a 20-minute stage-matched motivational interviewing session to enhance the teen’s motivation to use contraception and remain nonpregnant.24 Counselors were African American paraprofessional women, members of the communities from which participants were recruited, and hired for their empathetic qualities, rapport with adolescents, and knowledge of the community.
CAMI Frequency
CAMI sessions were initiated by 6 weeks’ postpartum and continued quarterly through 24 months’ postpartum. Although a total of 9 sessions was possible, we defined full CAMI adherence as receipt of 7 or more CAMI sessions, since a conception occurring after the seventh session (ie, after 18 months’ postpartum) was unlikely to result in a live birth before 24 months’ postpartum. If the adolescent became pregnant, we stopped administering the CAMI, because many of its questions were no longer relevant (eg, use of contraception), and the CAMI algorithms did not permit items to be skipped.
CAMI Training
CAMI counselors completed an initial 2.5 days of training on the transtheoretical model, motivational interviewing, and the CAMI protocol. Each counselor’s proficiency was ascertained by scoring a videotaped standardized-patient CAMI session using the Motivational Interviewing Process Code.42 After the intervention began, counselors recorded selected CAMI sessions (with the teen’s consent), often choosing to record sessions they believed were most challenging (eg, the mother engaged in high-risk behaviors yet was resistant to change). In biweekly group meetings during the project’s first 4 months, CAMI counselors reviewed and discussed their audiotapes with a motivational interviewing supervisor (E.P.), who provided assessment and feedback.
Description of Intervention Groups
In the CAMI+ and CAMI−only groups, the intervention was conducted in home- and community-based settings. Two-thirds of intervention encounters occurred in teens’ homes, and the remainder occurred elsewhere in the community because of safety concerns related to drug trafficking in the home. The CAMI+ group received CAMI sessions as part of a multicomponent home-visiting program with biweekly to monthly visits. The CAMI−only group received CAMI sessions as a single-component intervention. Each CAMI counselor was assigned to 1 group and carried an equivalent case-load: CAMI+ counselors visited a maximum of 25 adolescents monthly, and the CAMI−only counselors visited a maximum of 60 adolescents quarterly. If a participant in either group experienced a repeat pregnancy, she received no further CAMI sessions; however, if she was in the CAMI+ group, she continued to receive non-CAMI home-visiting components.
Description of the Home-Visiting Program
Upon enrollment, the CAMI+ participants received biweekly to monthly home visitation, parent training, and case management, as well as quarterly CAMI sessions after delivery. Parent training was accomplished by means of a 16-module curriculum grounded in social cognitive theory and created specifically for urban African-American adolescent mothers.14,43,44 Modules addressed age- and developmentally-appropriate feeding, growth, play, and discipline; 3 modules focused on safer sex, negotiation, and goal setting.14
Data Collection
Study participants completed baseline structured interviews administered by research staff before randomization. The interview measured characteristics associated with adolescent repeat pregnancy, as well as factors that might influence intervention participation.3,10,45–48 We assessed demographic characteristics, insurance status (whether insured, by whom, continuous or interrupted), living arrangements, relationship with the baby’s father, school, parity, future contraceptive and pregnancy intentions, sexual decision-making competence (Decision-Making-Competency Inventory, or DMCI scale),49 depressive symptoms (Center for Epidemiologic Studies Depression Scale, or CES-D),50 substance use,51 and social support.52 To measure intervention adherence, CAMI counselors in both groups completed standardized forms at each encounter.
Repeat births occurring within 24 months of the index birth were identified from Maryland birth certificates. We provided the Maryland Department of Health and Mental Hygiene Vital Statistics Administration (VSA) with a file containing 6 data elements with identifying information for the index child (study identification, birth date of index child, mother’s first name, last name, date of birth, social security number). VSA looked for records of these index births and then searched for records of subsequent births to mothers identified from our file, sending us both full and partial matches. VSA found 100% of our index birth cohort, suggesting that if a mother in our sample did have a subsequent birth in Maryland, the files contained it.
Structured follow-up interviews were carried out at 24 months’ postpartum. For this study we used data from these interviews to provide details about repeat pregnancy outcomes (ie, abortion, miscarriage), because vital statistics do not provide this information.
Analysis
We defined statistical significance as a 2-sided P value of ≤.05 and evidence of a trend as P ≤.10. Baseline comparisons among and between groups were carried out using χ2, analysis of variance, Fisher exact test, and Student t test. All outcome analyses controlled for significant baseline group differences. We tested the bivariate association of each baseline characteristic with repeat birth; variables with a significant association were included in multivariable models. Primary outcome analyses measuring effects of group assignment on repeat birth were conducted using an intention-to-treat approach.53 We calculated hazard ratios (risk) for repeat birth using Cox proportional hazards models and compared mean time to repeat birth using Mantel-Cox log rank.54 Survival was censored at 24 months’ postpartum for those without a subsequent birth.
When intervention trials encounter variable adherence, complier average causal effect (CACE) models can be used to obtain unbiased estimates of intervention causal effects.55,56 Intention-to-treat analysis may underestimate causal effects because adherence is measured only in the intervention group; control group participants who would have adhered if assigned to the experimental group are not identified.55,57,58 CACE modeling produces a summary measure of individual-level treatment effects enabling comparison between actual intervention compliers and the subpopulation of control participants who meet criterion for adherence.55,59,60
Construction of the CACE models is described in the Supplemental Appendix, available at http://www.annfammed.org/cgi/content/full/7/5/436/DC1. Statistical analyses were conducted using the Statistical Package for the Social Sciences, SPSS version 15.0 (SPSS Inc, Chicago, Illinois) and Stata 8 (StataCorp LP, College Station, Texas).
RESULTS
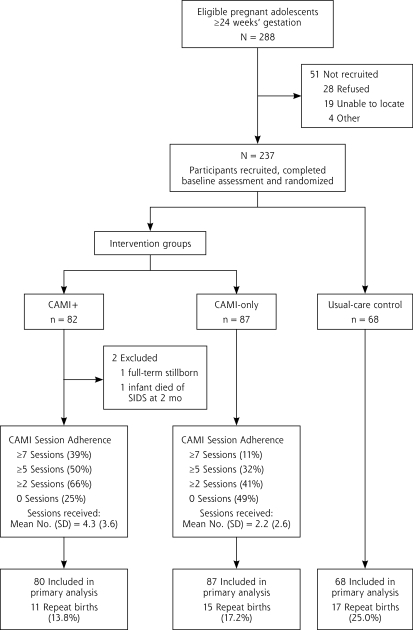
More than 80% (237 of 288) of eligible participants agreed to participate and were randomly assigned to CAMI+ (n = 82), CAMI-only (n = 87), or usual-care control (n = 68) (Figure 1 ▶). Those refusing were similar in age to participants (17.3 years [SD 1.0 years] vs 17.0 years [SD 1.2 years]; P=.18). Our final sample comprised 235 participants, with 80 in the CAMI+ group after excluding 1 participant with a stillborn infant and withdrawing 1 participant when her 2-month-old died.
Figure 1.
Participant flow through intervention and study.
CAMI = computer-assisted motivational intervention; CAMI+ = CAMI plus a multicomponent home-based intervention; SIDS = sudden infant death syndrome.
Baseline characteristics overall and by group are displayed in Table 1 ▶. Significant baseline differences among groups were observed for prior birth, substance use, history of sexually transmitted infections, and contraceptive intentions, although none of these variables were associated with repeat birth (Table 2 ▶).
Table 1.
Baseline Characteristics Overall and by Group
| Characteristic | Overall n=235 | CAMI+ n=80 | CAMI-Only n=87 | UCC n=68 | PValue |
| Demographic and education variables | |||||
| Maternal age,a mean, (SD), y | 17.0±1.2 | 17.2±1.1 | 17.0±1.2 | 16.9±1.4 | .24 |
| African American, % | 97 | 99 | 95 | 99 | .39 |
| Medicaid insurance, % | 86 | 80 | 89 | 90 | .18 |
| Continuous health insurance, past 12 months, % | 61 | 53 | 66 | 63 | .25 |
| Dropped out of school, % | 42 | 39 | 43 | 46 | .69 |
| Relationships and support | |||||
| Lives with her mother, % | 61 | 63 | 63 | 57 | .73 |
| Age of baby’s father,b mean (SD), y | 19.8±3.2 | 20.4±3.4 | 19.3±2.6 | 19.7±3.6 | .11 |
| Age difference between teen mother and baby’s father, mean (SD), y | 2.7±3.0 | 3.2±3.2 | 2.3±2.4 | 2.8±3.4 | .21 |
| Married (n = 2), living together, going with baby’s father, % | 74 | 78 | 72 | 72 | .66 |
| Social support satisfaction score, mean (SD) | 15.6 (2.9) | 16.0 (2.4) | 15.0 (3.1) | 15.8 (3.0) | .07 |
| Pregnancy history | |||||
| Age at first pregnancy, mean, (SD), y | 14.3±1.4 | 14.4±1.4 | 14.2±1.5 | 14.3±1.5 | .60 |
| Prior pregnancy, % | 31 | 38 | 30 | 24 | .19 |
| Prior birth, % | 11 | 16 | 5 | 13 | .04 |
| Prior abortion, % | 14 | 14 | 18 | 7 | .14 |
| Prior miscarriage/stillbirth, % | 12 | 14 | 12 | 10 | .85 |
| Mental health and violence exposure | |||||
| Depressive symptoms (CES-D ≥24), % | 32 | 25 | 38 | 32 | .20 |
| Maternal substance use | |||||
| Tobacco use in past 30 days, % | 8 | 5 | 13 | 4 | .11 |
| Alcohol use in past 30 days, % | 3 | 0 | 7 | 3 | .04 |
| Marijuana use in past 30 days, % | 3 | 3 | 5 | 0 | .25 |
| Sexually transmitted infection history, contraceptive practices and plans, decision making | |||||
| Ever diagnosed with a sexually transmitted infection, % | 37 | 23 | 41 | 49 | .003 |
| Any condom use in the past 12 months, % | 81 | 83 | 78 | 82 | .76 |
| Always uses a condom for STD protection, % | 22 | 21 | 24 | 19 | .76 |
| Plans to use condom at next intercourse, % | 76 | 78 | 69 | 82 | .14 |
| Plans to use hormonal contraceptionc after delivery, % | 6 | 65 | 53 | 75 | .02 |
| DMCI score,d mean (SD) | 86.2±11.0 | 86.4±11.3 | 85.9±10.0 | 86.3±11.9 | .96 |
| Wants another child within 2 years, % | 3 | 5 | 4 | 2 | .54 |
CAMI = computer-assisted motivational intervention; CAMI+ = CAMI plus multicomponent home-based intervention; DMCI = Decision-Making-Competency Inventory; STD=sexually transmitted disease; UCC=usual-care control.
a Range from 12 to 19 years.
b Range from 14 to 39 years.
c Oral contraceptive pills, patch, ring, medroxyprogesterone depot.
d Range from 55.0 to 113.0; higher scores are more favorable (greater competency, greater self-efficacy).
Table 2.
Baseline Characteristics of Adolescent Mothers With and Without a Repeat Birth
| Characteristic | Overall n=235 | Repeat Birth n=43 | No Repeat Birth n=192 | PValue |
| Demographic and education variables | ||||
| Maternal age,a mean (SD), y | 17.0±1.2 | 17.1±1.0 | 17.0±1.3 | 0.59 |
| African American, % | 97 | 98 | 97 | 1.00 |
| Medicaid insurance, % | 86 | 88 | 85 | 0.81 |
| Continuous health insurance in past 12 mo, % | 61 | 47 | 64 | 0.04 |
| Dropped out of school, % | 42 | 47 | 41 | 0.61 |
| Relationships and support | ||||
| Lives with her mother, % | 61 | 65 | 60 | 0.61 |
| Age of baby’s father,b mean (SD), y | 19.8±3.2 | 19.6±2.9 | 19.9±3.3 | 0.59 |
| Age difference between teen mother and baby’s father, mean (SD), y | 2.7 ± 3.0 | 2.4 ± 2.7 | 2.8 ± 3.1 | 0.44 |
| Married (n = 2), living together, going with baby’s father, % | 74 | 81 | 72 | 0.25 |
| Social support satisfaction score, mean (SD) | 15.6 (2.9) | 15.7 ± 2.9 | 15.6 ± 2.9 | 0.98 |
| Pregnancy history | ||||
| Age at first pregnancy, mean (SD), y | 14.3±1.4 | 16.0±1.3 | 15.9±1.4 | 0.85 |
| Prior pregnancy, % | 31 | 23 | 32 | 0.28 |
| Prior birth, % | 11 | 12 | 11 | 1.00 |
| Prior abortion, % | 14 | 2 | 16 | 0.01 |
| Prior miscarriage/stillbirth, % | 12 | 4 | 12 | 0.61 |
| Mental health and violence exposure | ||||
| Depressive symptoms (CES-D ≥24), % | 32 | 33 | 32 | 1.00 |
| Maternal substance use | ||||
| Tobacco use in past 30 days, % | 8 | 2 | 9 | 0.21 |
| Alcohol use in past 30 days, % | 3 | 0 | 4 | 0.36 |
| Marijuana use in past 30 days, % | 3 | 0 | 3 | 0.60 |
| Sexually transmitted infection history, contraceptive practices and plans, and decision making | ||||
| Ever diagnosed with a sexually transmitted infection, % | 37 | 332 | 38 | 0.60 |
| Any condom use in the past 12 months, % | 81 | 79 | 81 | 0.83 |
| Always uses a condom for STD protection, % | 22 | 28 | 20 | 0.31 |
| Plans to use condom at next intercourse, % | 76 | 65 | 78 | 0.08 |
| Plans to use hormonal contraceptionc after delivery, % | 6 | 56 | 65 | 0.29 |
| DMCI score,d mean (SD) | 86.2±11.0 | 87.6±11.5 | 85.8±10.9 | 0.34 |
| Wants another child within 2 years, % | 3 | 2 | 4 | 1.00 |
CES-D = Center for Epidemiologic Studies Depression Scale; DMCI = Decision-Making-Competency Inventory; STD = sexually transmitted disease.
a Range = 12 to 19 years.
b Range = 14 to 39 years.
c Oral contraceptive pills, patch, ring, medroxyprogesterone depot.
d Range from 55.0 to 113.0; higher scores are more favorable (greater competency, greater self-efficacy)
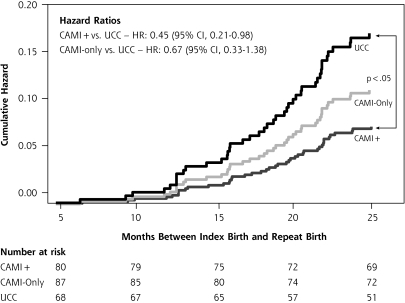
There were 43 (18%) participants who experienced a repeat birth by 24 months’ postpartum (Figure 1 ▶). Compared with controls, participants in the CAMI+ group showed a trend toward lower repeat birth rates (25.0% vs 13.8%; P = .08) but those in the CAMI−only group did not (25.0% vs 17.2%; P = .32). Repeat birth was less likely for adolescent mothers who at baseline reported having continuous health insurance, a history of abortion, and the intention to use condoms (Table 2 ▶). Compared with mothers in the control group, mothers in the CAMI+ group were significantly more likely to defer a repeat birth (HR = 0.45; P <.05), but mothers in the CAMI−only group were not (Figure 2 ▶). Time to repeat birth did not differ among the groups overall (mean time to subsequent birth in months: CAMI+, 23.0; CAMI−only, 22.8; usual care control, 22.6; P = .10).
Figure 2.
Effects of CAMI on the risk of subsequent birth intent-to-treat model.
CAMI = computer-assisted motivational intervention; CAMI+ = CAMI plus a multicomponent home-based intervention; UCC=usual-care control.
Continuous insurance coverage was independently associated with lower risk of repeat birth (HR = 0.53; 95% CI, 0.29–0.98; P <.05) and showed a moderating effect on repeat birth risk for mothers in the CAMI+ group: insured continuously (HR = 0.20; 95% CI, 0.04–0.83; P <.05) vs not-insured-continuously (HR = 0.78; 95% CI, 0.29–2.14; P = .63).
Supplemental Table 1 (available at: http://www.annfammed.org/cgi/content/full/7/5/436/DC1) shows that adherence to the CAMI intervention was significantly greater among participants in the CAMI+ than in the CAMI−only group. The most common reason for nonadherence in the CAMI+ group was failure to keep confirmed home visits, and the most common reason in the CAMI−only group was inability to locate the adolescent. Several trends distinguished CAMI adherers from nonadherers. We examined whether adherence was affected by our CAMI termination protocol for pregnancy. Eight intervention teens with a repeat birth stopped receiving CAMI sessions when counselors became aware of their repeat pregnancy; however, 7 of 8 received 2 or more CAMI sessions.
To test the impact of CAMI on the subpopulation of adherers, we computed hazard ratios for repeat birth with CACE models. Mothers in both intervention groups were at significantly lower risk of repeat birth compared with the usual-care control group (Supplemental Table 1). We computed additional CACE models examining other thresholds for adherence (receipt of 3 or fewer CAMI sessions, and 4 or more CAMI sessions) and adjusting for factors associated with repeat birth, and we found similar results.
Finally, we looked at whether differences in repeat birth among groups occurred because of differences in rates of elective abortions. The 2-year postpartum follow-up interviews were successfully completed for 191 participants (81%) (CAMI+ 83%; CAMI−only 84%; usual care 76%). There were no significant baseline differences (age, insurance, schooling, relationship with baby’s father, prior birth, contraception intentions, substance use, or group assignment) between participants with and without a follow-up interview. Overall, 40 (21%) reported having an abortion since the index child’s birth, but there were no significant differences by group (CAMI+ 22%; CAMI−only 20%; usual care 21%; P=.96).
DISCUSSION
Our study provides evidence that this computer-assisted motivational intervention, conducted by paraprofessionals in community-based settings, is effective in reducing a subsequent birth within 24 months to low-income, African-American teenage mothers. Similar to other samples of adolescent mothers receiving usual care,2,14,48 one-quarter of adolescent mothers in the control group experienced a rapid repeat birth. In contrast, adolescent mothers who received CAMI within a multicomponent, home-based intervention showed a 44% reduction in repeat birth. Repeat birth risk reduction was greatest for mothers reporting continuous insurance coverage in the CAMI+ group. Findings suggest that a motivational intervention aimed at reducing repeat birth risk is effective, but its impact may be attenuated when insurance coverage is inadequate.
Some adolescents are highly motivated to postpone additional childbearing, whereas others may be conflicted, actively seeking pregnancy or skeptical about their risks.61–63 If an adolescent is ambivalent or unmotivated, access to medical care, contraception services, and traditional counseling will not prevent pregnancy.12,19,20,22,23 Our findings support prior research that personalized and tailored interventions, geared to an adolescent’s readiness to change, are more effective at reducing high-risk sexual behavior than approaches that offer standardized messages and advice.64,65
During sessions with adolescent mothers, CAMI counselors observed notable inconsistencies and ambivalence regarding contraception use. For example, teens commonly maintained they did not want another pregnancy but reported having sexual intercourse without the use of contraceptives. Even so, they often disagreed when their CAMI assessment ranked them as high risk. CAMI counselors provided factual reproductive health information, explored reasons for nonuse of contraception, gave feedback, and tried not to engage in direct argumentation.25 Although some adolescents remained resistant to change, their disputed CAMI risk assessment was still a powerful tool for engaging in a motivational interviewing counseling discussion and collaborative goal setting.
Similar to other community-based interventions, we encountered challenges to implementation fidelity, particularly with respect to participant engagement and adherence.58 Participant adherence was about twice as great in the CAMI+ group as it was in the CAMI−only group. This variation may have resulted from differences in how teens in each group became engaged in the study.58,66 Random assignment to group occurred on average at 32-weeks’ gestation. Although CAMI sessions for participants in both groups began after delivery, participants in the CAMI+ group began monthly in-person contact with their CAMI counselor shortly after randomization. In contrast, CAMI−only participants did not begin in-person contact with their CAMI counselor until after delivery. The CAMI−only group counselor tried to maintain engagement through telephone contact with her case load of pregnant adolescents, but such contact was challenging because telephones were frequently disconnected, and the adolescents became difficult to locate. These findings support the importance of engaging the participant soon after recruitment, minimizing appointment delays, and building trust early between interventionists and adolescent mothers.67
Our data add to The National Institutes of Health Behavior Change Consortium recommendations for improving the quality of behavior change interventions.66 Implementation fidelity frameworks posit that participant responsiveness to an intervention moderates intervention adherence, which influences delivery of the intervention’s content and frequency.58 Implementation procedures should therefore minimize the gap between recruitment and onset of intervention activities. We speculate that CAMI−only participant responsiveness was adversely affected by inadequate contact with their counselor in the early phases of program engagement. Ongoing intervention differences, such as monthly vs quarterly contact, may have enhanced the quality of the teen-counselor relationship within the CAMI+ group and resulted in more-favorable outcomes.3,14
Our study has several strengths. First, our use of vital statistics enabled collection of complete repeat birth data for the sample, eliminating bias effects of differential group follow-up. Second, unlike several similar interventions,14,68 we did not limit our sample to first-time adolescent mothers. Because one-quarter of all births to teens are second or more,1,2 we believe inclusion of multiparous adolescents increases the generalizeability of our findings. Third, our CACE analysis strengthens the conclusion that CAMI is an effective intervention if an adolescent can be induced to adhere to 2 or more sessions, as compliers in the CAMI−only group also showed robust reductions in repeat birth.
This study has several limitations. CAMI counselors demonstrated use of motivational interviewing skills under ideal training conditions, but translating these skills into unpredictable community settings amidst crowded households, lack of electricity, homelessness, and abusive partners presented challenges. Although we assessed the interviewing delivery quality of the CAMI counselors, both initially and during the course of the intervention, with a standardized instrument, we did not systematically record quality ratings. Thus we are unable to determine any moderating effects of the quality of the motivational interviewing delivery.
Second, likely by chance, groups were not balanced on a few key variables, although we controlled for these in multivariable analyses. Third, we lost some participants for the 2-year postpartum interview during which we measured contraceptive behaviors across all 3 groups. Consequently, we are unable to examine potential mediating factors in the causal pathway to repeat birth for the full sample. Fourth, effectiveness findings in the CAMI+ group may have resulted from other intervention elements, such as the parenting curriculum, or from more frequent contact with their CAMI counselor. The CACE analysis, however, makes these latter explanations less likely, because CAMI adherers in the CAMI−only and CAMI+ groups experienced similar birth reductions.
Fifth, our protocol that required stopping further CAMI sessions if the adolescent mother disclosed she was pregnant again may have resulted in a confounding effect of CAMI adherence and repeat birth. We do not believe this actually occurred, because only 1 adolescent with a repeat birth and fewer than 2 CAMI sessions stopped receiving CAMI sessions as a result of becoming pregnant. We are currently testing a new CAMI program that can be administered to adolescents regardless of pregnancy status. Finally, we do not know whether CAMI effects on repeat birth reductions continued throughout the mothers’ adolescence.
Health risk behaviors are the most common cause of disease burden in the United States,69 but large gaps remain in how best to promote positive behavior change. Interventions that attend to adolescent contextual factors, such as partner influences on motivation, may have a greater impact on behavior change.70 Our findings suggest, however, that in tandem to a focus on motivation for behavior change, to reduce risk of adolescent repeat birth, having health insurance matters.
It is possible that CAMI can be adapted and used in primary care to address general pregnancy prevention and other high-risk adolescent behaviors. New models of service delivery stress patient-centered, integrated care that spans clinical practice and the community. For adolescents seen in practice settings, interactive behavior change technologies with tailored feedback could be coordinated with efforts of community-based interventions. Such strategies are current best practice for addressing multiple risks in primary care.17,71 Results from this study support the use of interactive behavior change technology with adolescents and show that receipt of at least 2 CAMI sessions reduces the risk of rapid subsequent birth to low-income, African American adolescent mothers.
Table 3.
CACE Model of the Hazard Ratio of Subsequent Birth, by Group
| Group | Hazard Ratioa | 95% CI |
| Control | ref | – |
| CAMI+ | 0.40a | 0.16–0.98 |
| CAMI−only | 0.19a | 0.05–0.69 |
CAMI=computer-assisted motivational intervention; CAMI+=CAMI plus a multicomponent home-based intervention; CACE = complier average causal effect; CI=confidence interval; ref = reference group.
Note: Model adjusted for significant baseline differences between intervention and control groups.
aP <.05.
Acknowledgments
We thank Bob Hayman, Department of Health and Mental Hygiene, Vital Statistics Administration, for his work linking our data to Maryland birth certificates. We gratefully acknowledge the dedicated contributions of the CAMI counselors.
Conflicts of interest: none reported
Funding support: This research was supported by Grant APRPA006010 from the Department of Health and Human Services, Office of Population Affairs, Office of Adolescent Pregnancy Programs and Cooperative Agreement MM-0452-03/03 from the Centers for Disease Control/Association of American Medical Colleges.
REFERENCES
- 1.Schelar E, Franzetta K, Manlove J. Repeat teen childbearing: differences across states and by race and ethnicity. Washington DC: Child Trends Research Brief. http://www.childtrends.org/Files//Child_Trends-2007_11_27_RB_RepeatCB.pdf Accessed May 12, 2008.
- 2.Klerman LV. Another Chance: Preventing Additional Births to Teen Mothers. Washington DC: National Campaign to Prevent Teen Pregnancy; 2004.
- 3.US Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. 2nd ed. Washington, DC: US Government Printing Office; 2000.
- 4.Furstenberg FF Jr, Levine JA, Brooks-Gunn J. The children of teenage mothers: patterns of early childbearing in two generations. Fam Plann Perspect. 1990;22(2):54–61. [PubMed] [Google Scholar]
- 5.Pogarsky G, Thornberry TP, Lizotte AJ. Developmental outcomes for children of young mothers. J Marriage Fam. 2006;68(2):332–344. [Google Scholar]
- 6.Polit DF, Kahn JR. Project Redirection: evaluation of a comprehensive program for disadvantaged teenage mothers. Fam Plann Perspect. 1985;17(4):150–155. [PubMed] [Google Scholar]
- 7.Ruch-Ross HS, Jones ED, Musick JS. Comparing outcomes in a statewide program for adolescent mothers with outcomes in a national sample. Fam Plann Perspect. 1992;24(2):66–71, 96. [PubMed] [Google Scholar]
- 8.Maynard R, Rangarajan A. Contraceptive use and repeat pregnancies among welfare dependent teenage mothers. Fam Plann Perspect. 1994;26(5):198–205. [Google Scholar]
- 9.Barnet B, Liu J, DeVoe M, Alperovitz-Bichell K, Duggan AK. Home visiting for adolescent mothers: effects on parenting, maternal life course, and primary care linkage. Ann Fam Med. 2007;5(3):224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polit DF, Kahn JR. Early subsequent pregnancy among economically disadvantaged teenage mothers. Am J Public Health. 1986;76(2):167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweet MA, Appelbaum MI. Is home visiting an effective strategy? A meta-analytic review of home visiting programs for families with young children. Child Dev. 2004;75(5):1435–1456. [DOI] [PubMed] [Google Scholar]
- 12.Stevens-Simon C, Kelly L, Singer D. Preventing repeat adolescent pregnancies with early adoption of the contraceptive implant. Fam Plann Perspect. 1999;31(2):88–93. [PubMed] [Google Scholar]
- 13.Olds DL, Henderson CR Jr, Kitzman HJ, Eckenrode JJ, Cole RE, Tatel-baum RC. Prenatal and infancy home visitation by nurses: recent findings. Future Child. 1999;9(1):44–65, 190–191. [PubMed] [Google Scholar]
- 14.Black MM, Bentley ME, Papas MA, et al. Delaying second births among adolescent mothers: a randomized, controlled trial of a home-based mentoring program. Pediatrics. 2006;118(4): e1087–e1099. [DOI] [PubMed] [Google Scholar]
- 15.Barnet B, Duggan AK, Devoe M, Burrell L. The effect of volunteer home visitation for adolescent mothers on parenting and mental health outcomes: a randomized trial. Arch Pediatr Adolesc Med. 2002;156(12):1216–1222. [DOI] [PubMed] [Google Scholar]
- 16.Prochaska JO, Velicer WF, Rossi JS, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychol. 1994;13(1):39–46. [DOI] [PubMed] [Google Scholar]
- 17.Glasgow RE, Goldstein MG, Ockene JK, Pronk NP. Translating what we have learned into practice. Principles and hypotheses for interventions addressing multiple behaviors in primary care. Am J Prev Med. 2004;27(2)(Suppl):88–101. [DOI] [PubMed] [Google Scholar]
- 18.Woolf SH, Chan EC, Harris R, et al. Promoting informed choice: transforming health care to dispense knowledge for decision making. Ann Intern Med. 2005;143(4):293–300. [DOI] [PubMed] [Google Scholar]
- 19.Miller WB. Why some women fail to use their contraceptive method: a psychological investigation. Fam Plann Perspect. 1986;18(1):27–32. [PubMed] [Google Scholar]
- 20.Stevens-Simon C, Kelly L, Singer D, Nelligan D. Reasons for first teen pregnancies predict the rate of subsequent teen conceptions. Pediatrics. 1998;101(1):e8–e14. [DOI] [PubMed] [Google Scholar]
- 21.DiClemente RJ. The psychological basis of health promotion for adolescents. Adolesc Med. 1999;10(1):13–22, v. [PubMed] [Google Scholar]
- 22.Hotz VJ, McElroy SW, Sanders SG. Teenage childbearing and its life cycle consequences: exploiting a natural experiment. J Hum Resour. 2005;40(3):683–715. [Google Scholar]
- 23.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. [DOI] [PubMed] [Google Scholar]
- 24.Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: The Guilford Press; 1991.
- 25.Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21(6):835–842. [DOI] [PubMed] [Google Scholar]
- 26.Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):305–314. [DOI] [PubMed] [Google Scholar]
- 27.Dijkstra A, De Vries H, Roijackers J, van Breukelen G. Tailored interventions to communicate stage-matched information to smokers in different motivational stages. J Consult Clin Psychol. 1998;66(3):549–557. [DOI] [PubMed] [Google Scholar]
- 28.Tevyaw TO, Monti PM. Motivational enhancement and other brief interventions for adolescent substance abuse: foundations, applications and evaluations. Addiction. 2004;99(Suppl 2):63–75. [DOI] [PubMed] [Google Scholar]
- 29.Colby SM, Monti PM, O’Leary Tevyaw T, et al. Brief motivational intervention for adolescent smokers in medical settings. Addict Behav. 2005;30(5):865–874. [DOI] [PubMed] [Google Scholar]
- 30.Colby SM, Monti PM, Barnett NP, et al. Brief motivational interviewing in a hospital setting for adolescent smoking: a preliminary study. J Consult Clin Psychol. 1998;66(3):574–578. [DOI] [PubMed] [Google Scholar]
- 31.Fisher JD, Fisher WA, Bryan AD, Misovich SJ. Information-motivation-behavioral skills model-based HIV risk behavior change intervention for inner-city high school youth. Health Psychol. 2002;21(2):177–186. [PubMed] [Google Scholar]
- 32.Petersen R, Albright J, Garrett JM, Curtis KM. Pregnancy and STD prevention counseling using an adaptation of motivational interviewing: a randomized controlled trial. Perspect Sex Reprod Health. 2007;39(1):21–28. [DOI] [PubMed] [Google Scholar]
- 33.Hollis JF, Polen MR, Whitlock EP, et al. Teen reach: outcomes from a randomized, controlled trial of a tobacco reduction program for teens seen in primary medical care. Pediatrics. 2005;115(4):981–989. [DOI] [PubMed] [Google Scholar]
- 34.Korfmacher J, Kitzman H, Olds DL. Intervention processes as predictors of outcomes in a preventative home visitation program. J Clin Child Adolesc Psychol. 1998;26(1):49–64. [Google Scholar]
- 35.Wald ER, Ewing L, Cluss P, Goldstrohm S, Cipriani L, Colborn K. Establishing a family-based intervention for overweight children in pediatric practice. Ann Fam Med. 2005;3(Suppl 2):S45–S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duggan AK, McFarlane EC, Windham AM, et al. Evaluation of Hawaii’s Healthy Start Program. Future Child. 1999;9(1):66–90, discussion 177–178. [PubMed] [Google Scholar]
- 37.Rust G, Cooper LA. How can practice-based research contribute to the elimination of health disparities? J Am Board Fam Med. 2007;20(2):105–114. [DOI] [PubMed] [Google Scholar]
- 38.DiClemente CC, Prochaska JO. Self-change and therapy change of smoking behavior: a comparison of processes of change in cessation and maintenance. Addict Behav. 1982;7(2):133–142. [DOI] [PubMed] [Google Scholar]
- 39.Galavotti C, Cabral RJ, Lansky A, Grimley DM, Riley GE, Prochaska JO. Validation of measures of condom and other contraceptive use among women at high risk for HIV infection and unintended pregnancy. Health Psychol. 1995;14(6):570–578. [DOI] [PubMed] [Google Scholar]
- 40.Gold MA, Parker AM, Young AJ, DiClemente CC. Using the stages of change model to explore female adolescent contraceptive behaviors [abstract]. J Adolesc Health. 1999;24(2):97. [Google Scholar]
- 41.Gold MA, Parker AM, Young AJ. Exploring the stages of change model as a framework for creating new contraceptive counseling strategies [abstract]. J Pediatr Adolesc Gynecol. 1999;12(2):112. [Google Scholar]
- 42.Barsky A, Coleman H. Evaluating skill acquisition in motivational interviewing: the development of an instrument to measure practice skills. J Drug Educ. 2001;31(1):69–82. [DOI] [PubMed] [Google Scholar]
- 43.Schweingruber HA, Kalil A. Decision making and depressive symptoms in black and white multigenerational teen-parent families. J Fam Psychol. 2000;14(4):556–569. [PubMed] [Google Scholar]
- 44.Black MM, Papas MA, Hussey JM, Dubowitz H, Kotch JB, Starr RH Jr. Behavior problems among preschool children born to adolescent mothers: effects of maternal depression and perceptions of partner relationships. J Clin Child Adolesc Psychol. 2002;31(1):16–26. [DOI] [PubMed] [Google Scholar]
- 45.Deal LW, Holt VL. Young maternal age and depressive symptoms: results from the 1988 National Maternal and Infant Health Survey. Am J Public Health. 1998;88(2):266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eamon MK. Antecedents and socioemotional consequences of physical punishment on children in two-parent families. Child Abuse Negl. 2001;25(6):787–802. [DOI] [PubMed] [Google Scholar]
- 47.Jacoby M, Gorenflo D, Black E, Wunderlich C, Eyler AE. Rapid repeat pregnancy and experiences of interpersonal violence among low-income adolescents. Am J Prev Med. 1999;16(4):318–321. [DOI] [PubMed] [Google Scholar]
- 48.Raneri LG, Wiemann CM. Social ecological predictors of repeat adolescent pregnancy. Perspect Sex Reprod Health. 2007;39(1):39–47. [DOI] [PubMed] [Google Scholar]
- 49.Miller DC, Byrnes JP. Adolescents’ decision making in social situations: A self- regulation perspective. J Appl Dev Psychol. 2001;22(3): 237–256. [Google Scholar]
- 50.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3): 385–401. [Google Scholar]
- 51.Sieving RE, Beuhring T, Resnick MD, et al. Development of adolescent self-report measures from the National Longitudinal Study of Adolescent Health. J Adolesc Health. 2001;28(1):73–81. [DOI] [PubMed] [Google Scholar]
- 52.Barrera M. Social support in the adjustment of pregnant adolescents: assessment Issues. In: Social Networks and Social Supports. Gottlieb BH, ed. Beverly Hills, CA: Sage Publications;1981:69–96.
- 53.McKinlay SM, Stone EJ, Zucker DM. Research design and analysis issues. Health Educ Q. 1989;16(2):307–313. [DOI] [PubMed] [Google Scholar]
- 54.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York, NY: John Wiley & Sons; 1999.
- 55.Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annu Rev Public Health. 2000;21:121–145. [DOI] [PubMed] [Google Scholar]
- 56.Nagelkerke N, Fidler V, Bernsen R, Borgdorff M. Estimating treatment effects in randomized clinical trials in the presence of non-compliance. Stat Med. 2000;19(14):1849–1864. [DOI] [PubMed] [Google Scholar]
- 57.Forgatch MS, Patterson GR, Degarmo DS. Evaluating fidelity: predictive validity for a measure of competent adherence to the Oregon model of parent management training. Behav Ther. 2005;36(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implementation Science. 2007;2:40. http://implementationscience.com/content/2/1/40. Accessed May 11, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Little RJ and Yau LH. Statistical techniques for analyzing data from prevention trials: treatment of no-shows using Rubin’s Causal Model. Psychol Methods. 1998;3(2):147–159. [Google Scholar]
- 60.Bellamy SL, Lin JY, Ten Have TR. An introduction to causal modeling in clinical trials. Clin Trials. 2007;4(1):58–73. [DOI] [PubMed] [Google Scholar]
- 61.Bloom KC, Hall DS. Pregnancy wantedness in adolescents presenting for pregnancy testing. Am J Maternal/Child Nurs. 1999;24(6): 296–300. [DOI] [PubMed] [Google Scholar]
- 62.Cowley C, Farley T. Adolescent girls’ attitudes toward pregnancy: the importance of asking what the boyfriend wants. J Fam Pract. 2001;50(7):603–607. [PubMed] [Google Scholar]
- 63.Rubin V, East PL. Adolescents’ pregnancy intentions: relations to life situations and caretaking behaviors prenatally and 2 years post-partum. J Adolesc Health. 1999;24(5):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamb ML, Fishbein M, Douglas JM Jr, et al. Project RESPECT Study Group. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. JAMA. 1998;280(13):1161–1167. [DOI] [PubMed] [Google Scholar]
- 65.Shrier LA, Ancheta R, Goodman E, Chiou VM, Lyden MR, Emans SJ. Randomized controlled trial of a safer sex intervention for high-risk adolescent girls. Arch Pediatr Adolesc Med. 2001;155(1):73–79. [DOI] [PubMed] [Google Scholar]
- 66.Coday M, Boutin-Foster C, Goldman Sher T, et al. Strategies for retaining study participants in behavioral intervention trials: retention experiences of the NIH Behavior Change Consortium. Ann Behav Med. 2005;29(Suppl):55–65. [DOI] [PubMed] [Google Scholar]
- 67.Chariatte V, Berchtold A, Akré C, Michaud PA, Suris JC. Missed appointments in an outpatient clinic for adolescents, an approach to predict the risk of missing. J Adolesc Health. 2008;43(1):38–45. [DOI] [PubMed] [Google Scholar]
- 68.Olds DL, Kitzman H, Cole R, et al. Effects of nurse home-visiting on maternal life course and child development: age 6 follow-up results of a randomized trial. Pediatrics. 2004;114(6):1550–1559. [DOI] [PubMed] [Google Scholar]
- 69.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270(18):2207–2212. [PubMed] [Google Scholar]
- 70.Johnston-Briggs BD, Liu J, Carter-Pokras O, Barnet B. Effect of partner relationship on motivation to use condoms among adolescent mothers. J Natl Med Assoc. 2008;100(8):929–935. [DOI] [PubMed] [Google Scholar]
- 71.Prochaska JO. Multiple health behavior research represents the future of preventive medicine. Prev Med. 2008;46(3):281–285. [DOI] [PubMed] [Google Scholar]