Abstract
Hepatocellular carcinoma is a well-known malignancy in the world. However, the molecular mechanism of carcinogenesis and tumour progression remains unclear. Recently, reduced E-cadherin expression due to transcriptional suppressor Snail was proven in a panel of epithelial and dedifferentiated cells derived from carcinomas of various etiologies. In the present study, we examined Snail and E-cadherin mRNA/protein expression in five hepatocellular carcinoma cell lines with variable phenotypes (HuL-1, Hep-G2, Changliver, HLE, and HLF). The results demonstrated that the presence of Snail mRNA in HuL-1, Changliver, HLE and HLF cells detected by RT–PCR, which was further proven by in situ hybridization in tumours induced by HuL-1, Changliver, and HLF cells where Snail mRNA signals expressed in each of the sections. By contrast, E-cadherin mRNA and protein expression were only detected in Hep-G2 cells by RT–PCR and Western blot, respectively. These results were also consistent with the data obtained from in vivo immunohistochemical staining where membranous expression of endogenous E-cadherin protein was revealed only in tumour sections induced by Hep-G2 cells. Here we are the first to report that there is an inverse correlation between Snail and E-cadherin expression in HCC cells as well.
British Journal of Cancer (2002) 86, 98–101. DOI: 10.1038/sj/bjc/6600017 www.bjcancer.com
© 2002 The Cancer Research Campaign
Keywords: hepatocellular carcinoma, cell line, Snail, E-cadherin
Hepatocellular carcinoma (HCC), the major type of primary liver cancer, is one of the most common malignancies worldwide (Hussain and Harris, 2000; Yu et al, 2000). In Japan, HCC is one of the leading causes of cancer death (Okuda, 2000). Chronic hepatitis B virus and hepatitis C virus infections are the major contributing factors of hepatocarcinogenesis (Oda, 1999). However, the basic molecular mechanism of hepatocarcinogenesis remains unclear (Buendia, 2000; Durr and Caselmann, 2000).
E-cadherin (E-cad), one of the key cadherins, plays a major role in the establishment and maintenance of intercellular adhesion, cell polarity and tissue architecture (Takeichi, 1991). Abnormalities in expression and cellular distribution of E-cad are frequently associated with dedifferentiation, invasiveness, and lymph node or distance metastasis in a variety of human malignancies including primary HCC (Bracke et al, 1996; Endo et al, 2000; Takeichi, 1993). E-cad is also a critical factor in the process of intrahepatic metastasis of HCC (Osada et al, 1996). Thus, elucidating the mechanisms leading to disturb E-cad function in carcinomas has become a crucial issue because it is expected to provide new insights into the process of tumour invasion and consequently open avenues for therapy. It has been reported that E-cad expression is under strict spatiotemporal control and may be regulated at genetic (e.g. E-cad CDH1 gene mutation, LOH, (Kondoh et al, 2001) and/or epigenetic (e.g. CDH1 promoter methylation, SNP) (Kanai et al, 1997; Li et al, 2000) level. Most recently, there is an evidence that functional perturbation of E-cad expression during the process of epithelial dedifferentiation occurs at the transcriptional level (Hayashi et al, 1999; Hennig et al, 1996).
The transcriptional regulator Snail is the prototype of a family of zinc finger proteins that participate in various developmental and physiological processes (Cook, 1999). Snail expression in epithelial cells induces both epithelial-mesenchymal transitions and the acquisition of tumorigenic as well as invasive properties (Hemavathy et al, 2000). Most recently, Snail was described to contribute to repress transcription of E-cad gene by binding to the E-boxes of the CDH1 promoter (Giroldi et al, 1997). There is a strong inverse correlation between the Snail and E-cad expression in a panel of epithelial and dedifferentiated cells derived from carcinomas of various etiologies, including oral squamous carcinoma, breast, pancreas, colon, bladder cancer, melanomas, and fibroblast (Batlle et al, 2000; Cano et al, 2000; Yokoyama et al, 2001). However, the correlation of Snail and E-cad is not yet known in human HCC.
In the present study, we examined relationship between Snail and E-cad expression in five cell lines with variable phenotypes derived from human HCC. The results were unambiguously revealed that Snail inverses correlation with E-cad expression at mRNA and protein levels in HCC cells in vitro as well as in vivo.
MATERIALS AND METHODS
Cell culture and generation of tumours
Five established human HCC cell lines (HuL-1, Hep-G2, Changliver, HLE and HLF) (Chang, 1978; Dor et al, 1975; Huh and Utakoji, 1981; Knowles et al, 1980) were subjected to this study. All of these cells were obtained from RIKEN Cell Bank (Ibaraki, Japan). Cells were routinely cultured in Williams' medium E (W/E, ICN Biomedicals Inc, Costa Mesa, CA, USA) supplemented with 10% heat-inactivated FBS (JRH Biosciences, Lenexa, KS, USA), 100 μg ml−1 streptomycin, and 100 IU ml−1 penicillin, and incubated at 37°C under 5% CO2 in a humidified atmosphere. Tumours were induced in congenitally athymic male BALB/cA jcl nu/nu mice between 5 to 6-weeks-old by subcutaneous injection (5×106 cells per injection site). Mice were purchased from CLEA (Tokyo, Japan), and housed in a laminar flow cabinet under specific-pathogen free conditions according to the institutional guidelines. Injected animals were observed every 3 days and sacrificed when the tumours reached an external diameter of 1.5–2.0 cm. The tumours were fixed in 10% formalin neutral buffered solution (WAKO, Osaka, Japan), paraffin-embedded, and 5 μm sections then cut and sequentially stained with H&E, and used for in situ hybridization (ISH) and immunohistochemical (IHC) staining.
RT–PCR
Total RNA was isolated from each cell line using ISOGEN (Nippongene, Toyama, Japan). RT–PCR was carried out as described previously (Jiao et al, 2001) with specific primers. The primer pairs of human Snail (hSnail), E-cadherin (hE-cad) and GAPDH was designed as follows: hSnail (GenBank AF125377) forward 5′-TTC TTC TGC GCT ACT GCT GCG-3′ and reverse 5′-GGG CAG GTA TGG AGA GGA AGA-3′; hE-cad (GenBank Z13009) forward 5′-TCC CAT CAG CTG CCC AGA AA-3′ and reverse 5′-TGA CTC CTG TGT TCC TGT TA-3′; GAPDH (GenBank NM002046) forward 5′-TGG TAT CGT GGA AGG ACT CAT GAC-3′ and reverse 5′-ATG CCA GTG AGC TTC CCG TTC AGC-3′, respectively. The PCR product was an 883 bp fragment of hSnail gene, and a 502 bp fragment of the extracellular domain of the hE-cad gene. GAPDH was simultaneously amplified in each sample as the internal marker. All reactions were repeated at least twice.
Western blot
Whole cell lysate without trypsin treatment was prepared from 5×106 cultured cells using lysis buffer (0.1% SDS, 150 mM NaCl, 1 mM PMSF, 5 mM EDTA pH 8.0, 10 μg ml−1 trypsin inhibitor, 50 mM iodoacetamide, 50 mM Tris pH 7.4). After sonication and centrifugation, aliquots of each cell extracts containing equal amount of protein (50 μg) was resolved by 10% SDS–PAGE, and electrophoretically transferred onto Hybond™ ECL™ nitrocellulose membrane. Blots were probed with E-cad monoclonal antibody HECD-1 (1 μg ml−1, Takara Biochemicals, Tokyo, Japan), and developed with chemiluminescence as described previously (Jiao et al, 2001).
Immunohistochemical staining
IHC staining on sections was performed essentially as described elsewhere (Jiao et al, 2001). HECD-1 was used at 1:250 dilutions. No staining was obtained when PBS was used instead of the primary antibody.
Pretreatment of tumour sections for in situ hybridization
As a result of no satisfactory commercial specific antibodies against hSnail-peptide available yet, we developed an ISH method to detect endogenous Snail mRNA expression in tumour cells with an appropriate biotinylated hSnail cDNA probe. The 5 μm sections were mounted onto poly-L-lysine coated slides and dried at 37°C overnight. Sections were deparaffinized in xylene and dehydrated in ethanol. To obtain an optimal recovery of cells and proper removal of cellular protein for improvement of DNA probe penetration, a proteolytic digestion step was applied. The digestion was performed with proteinase K at a concentration of 10 μg ml−1 in 10 mM PBS (pH 7.4) for 20 min at 37°C. Sections were immersed in PBS containing 3% hydrogen peroxide to inhibit endogenous peroxidase activity.
cDNA probe
To identify endogenous Snail gene expression in the sections, we designed a hybridizing specific cDNA probe, complementary to the sequence of the hSnail mRNA. The PCR primer for the hydbridization probe was: forward, 5′-AGG CTT GGG CCA AGT GCC CA-3′; reverse, 5′-GGT GGG CCC GCA GGT TGG AG-3′. The thermal cycle profile consisted of an initial denaturation at 94°C for 2 min, followed by 35 cycles with a 30 s denaturation at 94°C, a 30 s annealing of primers at 60°C, and a 1.5 min extension at 72°C, and a final 10 min extension at 72°C. The PCR product, corresponding to the complete hSnail cDNA sequence extending from 451–743 positions, was electrophoresed in 1.5% agarose gels. The 292 bp fragment in length of interest was then sliced cleanly under UV illumination, and recovered by using MagExtractor® DNA Fragment Purification Kit (TOYOBO Co., Ltd. Osaka, Japan). The cDNA probe was labelled with Biotin-16-dUTP using Biotin-High Prime Kit (Roche Molecular Biochemical, Mannheim, Germany) according to the manufacturer's instructions.
In situ hybridization
ISH for Snail mRNA with appropriate positive and negative controls was performed. The probes were hybridized to the sections according to the standard protocol of the In Situ Hybridization Detection Kit (ISH-B1, Sigma). Twenty μl of Biotin-conjugated probe (1 ng 1 μl−1) were applied to the each slide under a coverslip. Denaturation was carried out by heating the slides in an oven to 95°C for 10 min. Hybridization was then performed for 16 h at 37°C in a sealed moist chamber. Coverslips were removed by submersing the slides in PBS. After blocking, the slides were applied of ExtrAvidin®-Peroxidase solution, the ISH signal was immunologically amplified using monoclonal anti-avidin-biotin conjugated solution followed by re-applying ExtrAvidin®-Peroxidase solution. Colour development was achieved by adding a freshly prepared substrate solution DAB. The sections were counterstained with haematoxylin, then dehydrated and mounted. We used poly d(T) Probe-Biotin labelled as a positive control and ISH solution as a negative control.
RESULTS
In this study, four cell lines, i.e. HuL-1, Hep-G2, Changliver, and HLF, developed tumours in mice. However, HLF cells needed longer latency periods, grew slower, and reached an average size of less than 20% of the formers after 1 month. HLE cells had no tumorigenicity. Histological analysis of tumours derived from respective HuL-1, Changliver and HLF cells revealed dedifferentiated characteristics, whereas the tumour induced by Hep-G2 cells showed differentiated features (data not shown). All these histopathologic properties resemble those of the original tumour as reported elsewhere (Chang, 1978; Dor et al, 1975; Huh and Utakoji, 1981; Knowles et al, 1980).
To examine the expression of Snail and E-cad mRNA in these cell lines, RT–PCR analysis was performed. A clear inversed correlation between Snail and E-cad expression was observed (Figure 1). Snail mRNA expression was found in HuL-1, Changliver, HLE and HLF cells but not in Hep-G2 cells, whereas E-cad mRNA expression was detected in Hep-G2 cells merely. In addition, we analyzed E-cad protein expression in these cell lines by Western blot method, E-cad protein was detected only in Hep-G2 cells as well with monoclonal antibody HECD-1 (Figure 2).
Figure 1.
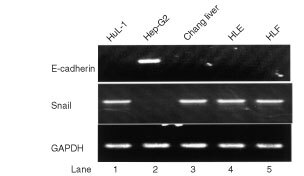
RT–PCR analyses of E-cad expression in Hep-G2 cells and Snail expression in HuL-1, Changliver, HLE, and HLF cells showed a strong inverse correlation between the expression of both mRNAs (upper and middle panels). A housekeeping gene, GAPDH, mRNA was simultaneously expressed in each sample (lower panels).
Figure 2.
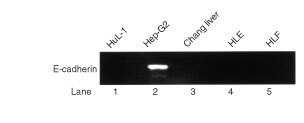
Detection of E-cad protein only in Hep-G2 cells was obtained on Western blot method with monoclonal antibody HECD-1.
By IHC staining of tumour sections, we found membranous expression of endogenous E-cad protein only in Hep-G2 cells (Figure 3B). ISH with biotinylated hSnail probe detected positive brown staining in the cytoplasm of HuL-1, Changliver and HLF cells, but not in Hep-G2 cells at all. These in vivo IHC of E-cadherin expression and ISH of Snail mRNA expression staining patterns were consistent with the same expression profiles as found in their respective in vitro RT–PCR and Western blot analyses.
Figure 3.
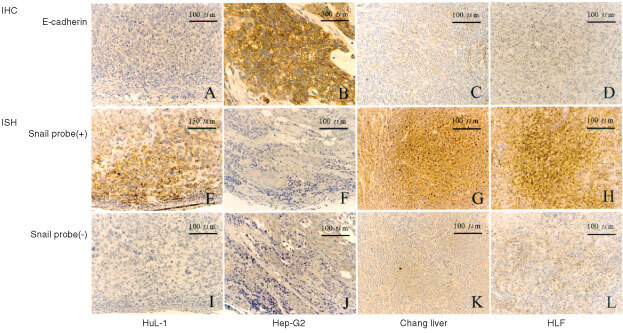
IHC staining of endogenous E-cad protein expression in the tumour sections induced by HuL-1 (A), Hep-G2 (B), Changliver (C) and HLF (D) cells (upper panels). Only Hep-G2 tumours clearly showed membranous expression of E-cad. ISH with biotinylated Snail probe detected positive brown staining in the cytoplasm of HuL-1 (E), Changliver (G) and HLF (H) tumour sections, representative of endogenous Snail mRNA expression, but not in Hep-G2 tumours (F) at all (middle panels). Lower panels (I, J, K and L) represented negative controls of each respective section for ISH.
DISCUSSION
HCC is known to have a poor prognosis because of multicentric development and intrahepatic metastasis (Ariizumi et al, 2001; Feitelson, 1998; Kojiro, 2000). However, the molecular mechanisms of hepatocarcinogenesis and intrahepatic metastasis are not yet well understood (Bergsland and Venook, 2000). Loss of both cell-cell adhesion and cellular differentiation is one of the characteristics of malignant cells, and this has been reported extensively to correlate with E-cad down-regulation (Bracke et al, 1996; Endo et al, 2000; Huang et al, 1999; Takeichi, 1993). Reduced E-cad expression due to transcriptional repressor Snail around CDH1 promoter region may participate in certain steps of carcinogenesis by reduction of intercellular adhesiveness, which may result in initiation of invasion and destruction of normal tissue morphology (Batlle et al, 2000; Cano et al, 2000). The present study demonstrated the presence of Snail mRNA in HuL-1, Changliver, HLE and HLF cells, but not in Hep-G2 cells detected by RT–PCR, these patterns were further proved by ISH in tumours, where Snail mRNA signals expressed in each tumour sections induced by HuL-1, Changliver, and HLF, respectively. By contrast, E-cad mRNA and protein expression were only detected in Hep-G2 cells by RT–PCR and Western blot analyses, these results were confirmed by the finding in vivo IHC staining where endogenous E-cad protein membranous expression was revealed only in tumour sections induced by Hep-G2 cells. To our knowledge, no reports have yet been published on the use of in vivo as well as in vitro molecular techniques to show that the zinc finger protein Snail has reversed correlation with E-cad at mRNA and protein levels in HCC cell lines. Our subsequent study will include expanding the number of HCC cell lines to further investigate the relationship between E-cad and Snail; Ectopic expression of Snail in hepatocytes to assess whether or not the transformed phenotype in HCC can be reversed by abrogating Snail function. Meanwhile, extending correlation analysis of Snail and E-cad expression patterns in HCC surgical specimens so as to evaluate whether Snail may be used as a screen for the invasion and progression of HCC in human is also in consideration.
Transcription factors critically regulate cell physiologic processes because they control the protein repertoire expression (Cook, 1999). Alterations in transcription factors have significant impact on disease and their treatment (Hedin et al, 2000). From the standpoint of hepatocellular carcinogenesis, unveil the mechanism of functions and regulation of Snail are not only important for understanding of hepatocarcinogenesis and intrahepatic metastasis processes, but will facilitate the design of novel therapies for HCC and the control of biologic responses after surgery (Guyton and Kensler, 1997; Hayashi et al, 1999).
Acknowledgments
We acknowledge Dr Yongxin Chen for histopathological judgement and photography, and Miss Yuko Ichimaru for helping histologic sections.
References
- AriizumiSITakasakiKYamamotoMOtsuboTKatagiriSSaitoA2001Eight multicentric hepatocellular carcinomas occurring in the same segment of the liver J Hepatobiliary Pancreat Surg 8383386 [DOI] [PubMed] [Google Scholar]
- BatlleESanchoEFranciCDominguezDMonfarMBaulidaJGarcie De HerrerosA2000The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells Nat Cell Biol 28489 [DOI] [PubMed] [Google Scholar]
- BergslandEKVenookAP2000Hepatocellular carcinoma Curr Opin Oncol 12357361 [DOI] [PubMed] [Google Scholar]
- BrackeMEVan RoyFMMareelMM1996The E-cadherin/catenin complex in invasion and metastasis Curr Top Microbiol Immunol 213123161 [DOI] [PubMed] [Google Scholar]
- BuendiaM2000Genetics of hepatocellular carcinoma Semin Cancer Biol 10185200 [DOI] [PubMed] [Google Scholar]
- CanoAPerez-MorenoMARodrigoILocascioABlancoMJdel BarrioMGPortilloFNietoMA2000The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression Nat Cell Biol 27683 [DOI] [PubMed] [Google Scholar]
- ChangRS1978HeLa marker chromosomes, Chang liver cells, and liver-specific functions Science 199567568 [DOI] [PubMed] [Google Scholar]
- CookPR1999The organization of replication and transcription Science 28417901795 [DOI] [PubMed] [Google Scholar]
- DorINambaMSatoJ1975Establishment and some biological characteristics of human hepatoma cell lines Gann 66385392 [PubMed] [Google Scholar]
- DurrRCaselmannWH2000Carcinogenesis of primary liver malignancies Langenbecks Arch Surg 385154161 [DOI] [PubMed] [Google Scholar]
- EndoKUedaTUeyamaJOhtaTTeradaT2000Immunoreactive E-cadherin, alpha-catenin, beta-catenin, and gamma-catenin proteins in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, and patients' survival Hum Pathol 31558565 [DOI] [PubMed] [Google Scholar]
- FeitelsonMA1998Hepatitis B x antigen and p53 in the development of hepatocellular carcinoma J Hepatobiliary Pancreat Surg 5367374 [DOI] [PubMed] [Google Scholar]
- GiroldiLABringuierPPde WeijertMJansenCvan BokhovenASchalkenJA1997Role of E boxes in the repression of E-cadherin expression Biochem Biophys Res Commun 241453458 [DOI] [PubMed] [Google Scholar]
- GuytonKZKenslerTW1997Prevention of liver cancer Curr Opin Oncol 9492496 [DOI] [PubMed] [Google Scholar]
- HayashiYWangWNinomiyaTNaganoHOhtaKItohH1999Liver enriched transcription factors and differentiation of hepatocellular carcinoma Mol Pathol 521924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HedinKEKaczynskiJAGibsonMRUrrutiaR2000Transcription factors in cell biology, surgery, and transplantation Surgery 12815 [DOI] [PubMed] [Google Scholar]
- HemavathyKAshrafSIIpYT2000Snail/slug family of repressors: slowly going into the fast lane of development and cancer Gene 257112 [DOI] [PubMed] [Google Scholar]
- HennigGLowrickOBirchmeierWBehrensJ1996Mechanisms identified in the transcriptional control of epithelial gene expression J Biol Chem 271595602 [DOI] [PubMed] [Google Scholar]
- HuangGTLeeHSChenCHSheuJCChiouLLChenDS1999Correlation of E-cadherin expression and recurrence of hepatocellular carcinoma Hepatogastroenterology 4619231927 [PubMed] [Google Scholar]
- HuhNUtakojiT1981Production of HBs-antigen by two new human hepatoma cell lines and its enhancement by dexamethasone Gann 72178179 [PubMed] [Google Scholar]
- HussainSPHarrisCC2000Molecular epidemiology and carcinogenesis: endogenous and exogenous carcinogens Mutat Res 462311322 [DOI] [PubMed] [Google Scholar]
- JiaoWMiyazakiKKitajimaY2001Exogenous expression of E-cadherin in gallbladder carcinoma cell line G-415 restores its cellular polarity and differentiation Int J Oncol 1910991107 [DOI] [PubMed] [Google Scholar]
- KanaiYUshijimaSHuiAMOchiaiATsudaHSakamotoMHirohashiS1997The E-cadherin gene is silenced by CpG methylation in human hepatocellular carcinomas Int J Cancer 71355359 [DOI] [PubMed] [Google Scholar]
- KnowlesBBHoweCCAdenDP1980Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen Science 209497499 [DOI] [PubMed] [Google Scholar]
- KojiroM2000Premalignant lesions of hepatocellular carcinoma: pathologic viewpoint J Hepatobiliary Pancreat Surg 5367374 [DOI] [PubMed] [Google Scholar]
- KondohNWakatsukiTHadaAShudaMTanakaKAraiMYamamotoM2001Genetic and epigenetic events in human hepatocarcinogenesis Int J Oncol 1812711278 [DOI] [PubMed] [Google Scholar]
- LiLCChuiRMSasakiMNakajimaKPerincheryGAuHCNojimaDCarrollPDahiyaP2000A single nucleotide polymorphism in the E-cadherin gene promoter alters transcriptional activities Cancer Res 60873876 [PubMed] [Google Scholar]
- OdaT1999Viral hepatitis and hepatocellular carcinoma prevention strategy in Japan Jpn J Cancer Res 9010511060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OkudaK2000Hepatocellular carcinoma J Hepatol 32225237 [DOI] [PubMed] [Google Scholar]
- OsadaTSakamotoMInoYIwamatsuAMatsunoYMutoTHirohashiS1996E-cadherin is involved in the intrahepatic metastasis of hepatocellular carcinoma Hepatology 2414601467 [DOI] [PubMed] [Google Scholar]
- TakeichiM1991Cadherin cell adhesion receptors as a morphogenetic regulator Science 25114511455 [DOI] [PubMed] [Google Scholar]
- TakeichiM1993Cadherins in cancer: implications for invasion and metastasis Curr Opin Cell Biol 5806811 [DOI] [PubMed] [Google Scholar]
- YokoyamaKKamataNHayashiEHoteiyaTUedaNFujimotoRNagayamaM2001Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro Oral Oncol 376571 [DOI] [PubMed] [Google Scholar]
- YuMCYuanJMGovindarajanSRossRK2000Epidemiology of hepatocellular carcinoma Can J Gastroenterol 14703709 [DOI] [PubMed] [Google Scholar]


