Summary
There has been an increase in our understanding of the complexity of the T cell response to mycobacterial infection recently. Improved tools have allowed determination of the location and kinetics of naive T cell activation in the mouse as well the variety of function of mycobacteria-specific cells in humans. There is also an increased appreciation of the balance required during mycobacterial infection between anti-bacterial activity and control of the immunopathologic response. The integration of the T cell functional data with the consequences of infection should improve rational vaccine design.
Introduction
Development of a vaccine to substantially impact tuberculosis (TB) has been a goal of the research community since Koch first identified Mycobacterium tuberculosis (Mtb) as the causative agent of TB. While the current vaccine, BCG, has proven invaluable in limiting childhood disease, it is not effective at limiting pulmonary disease. BCG generates a circulating population of mycobacteria-responsive cells, however these cells are not efficient at rapidly limiting bacterial growth within the lung. To improve upon BCG we need to determine what constitutes protective immunity and what regulates this immunity [1].
Activation of the phagocytic host cell is required to limit growth of Mtb; in the absence of activation, disease outcome is extremely poor. Effective phagocyte activation requires an acquired specific cellular response, as infected hosts lacking specific components of the acquired response have a poor outcome [1]. Based on this knowledge new vaccines have been developed, however they have not significantly improved upon BCG in animal models. New data gathered over the last 2 years has improved our understanding of the immune response to Mtb both in animal models and humans. However, we have not yet identified the crucial protective mechanism capable of mediating effective vaccine-induced immunity in the lung.
Acquired immune responses are slow to be expressed in the lung
While acquired cellular protection is expressed rapidly following systemic challenge with Mtb, it is less rapid in the lung [1]. Slow expression of protection in the lung allows mycobacteria to grow and modulate the infection site. With the lung being the primary route of infection and also the primary organ exhibiting disease, understanding induction and expression of immunity in this organ is key to the rational development of an effective vaccine.
Until recently it has not been clear whether the slow response to aerosol delivery of bacteria resulted from limited availability of antigen or inhibition of antigen-presentation by Mtb. To define when effective antigen presentation occurs in vivo, several groups have used naive CD4 T cells isolated from T cell receptor (TCR) transgenic (Tg) mice that express a TCR specific for Mtb antigens. These studies show that the first T cell activation occurs in the draining lymph node (DLN) of the lung 8–10 days following initial challenge. Two different antigens and three separate TCRTg models give the same location and time frame for this activation [2–4]. The activation of T cells correlated temporally with the arrival of bacteria and availability of antigen in the DLN, however conditions for T cell activation were unique to the DLN as the presence of antigen-producing bacteria in the lung and spleen did not result in initial activation of T cells [2,3].
While delivery of LPS to the Mtb-infected lung failed to accelerate T cell priming [2], increasing the bacterial dose did accelerate the response modestly suggesting that both antigen burden and refractory cells serve to slow the response [3]. The slowness of the host to recognize infection is problematic for vaccine design as protective memory cells will not become activated until they see antigen, i.e. more than 8 days post infection.
Once cells become activated they differentiate into effector cells that migrate to the lung [2,3]. By day 14 of infection, when activated T cells first arrive in the lung, bacteria are within alveolar macrophages, myeloid DC's and neutrophils [5]. The activated myeloid DC's from the lung are not efficient inducers of cytokine production by antigen-specific T cells suggesting that antigen presentation is not optimal in the lung [5]. It has been shown however that mycobacterially-infected lung tissue is better at inducing T cell responses than uninfected tissue when exogenous antigen is used to stimulate T cells in the lung [6,7]. These data suggest that T cells can recognize antigen within the lung but that it is likely the response is not optimal.
T cell location
In most models of vaccine-induced immunity to Mtb, it takes 14 days for the protective T cells to reach sufficient numbers to stop bacterial growth [1]. Having T cells within the lung ready to detect bacteria when they arrive should be advantageous. Using two different models to induce CD8 T cells specific for the mycobacterial antigen Ag85, it has been shown that these cells are protective only when they are already in the airway lumen at the time of challenge [8,9] (Fig 1). Antibody blocking of specific chemokines suggests that accumulation of these antigen-specific cells into the lumen requires IP-10 and MIP-1a [10]. In a different model, in vitro differentiated Th1 TCRTg T cells transferred into mice can populate the lung, however upon challenge these cells do not limit bacterial growth until day 7 [4]. This delay may be due to failure of the T cells to recognize infection either as a function of bacterial burden or active inhibition by Mtb. Alternatively, phagocytic cells in the lung for the first 7 days maybe poor stimulators of T cells and newly migrating phagocytes maybe required for efficient T cell activation (Fig 1).
Figure 1. The importance of location and functionality of both antigen-specific lymphocytes and infected phagocytes in rapid vaccine-induced control of Mtb.
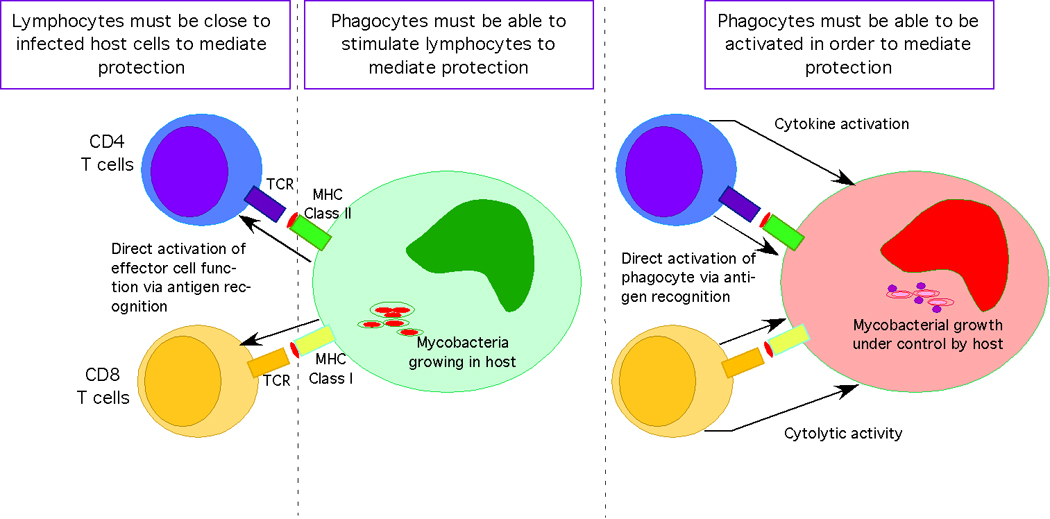
In order for vaccine-induced memory T cells to be effective against pulmonary challenge with Mtb they must be in the correct location i.e. in the lung close to where phagocytes first become infected. In addition, the infected phagocyte must be expressing signals (i.e. mycobacterial antigen in class I or class II) in order to activate the memory effector cells to locally express their effector function. The infected phagocyte within the lung must also be able to be responsive to the signals (both IFNγ dependent and independent) in order for mycobacterial growth to come under rapid control. While CD4 T cells mediate primary control of Mtb in infection, CD8 T cells can be activated by vaccination and mediate protection.
Expression of immunity in the lung
Expression of immunity occurs through the activated phagocyte, thus if the phagocyte is unable to be activated then antigen-specific T cells will fail to protect the host. Mice with a susceptible sst1 (sst1(S)) locus generate a potentially protective T cell response to vaccination but macrophages from these mice are poorly activated by T cells compared to sst1(R) macrophages [11]. Furthermore, macrophages from the sst1(S) mice are efficiently activated by IFNγ and also bacterial burden outside the lung is normal suggesting that T cells in the lung induce a non-IFNγ-dependent protective function in lung phagocytes (Fig 1). In a further study, linkage analysis by whole genome scanning of resistant crossed to susceptible F2 mice, detected a TB-resistance locus that regulates pulmonary replication of Mtb. This locus was associated with increased macrophage capacity to control bacterial growth [12]. In a second model, mice lacking the common adaptor molecule Myd88 are highly susceptible to disease while expressing a normal acquired immune response; this is due to an inability of the phagocytes to respond to activating signals [13] likely via IL-1R [14] (Fig 1). Together these data demonstrate the importance of understanding phagocyte control of mycobacterial growth, particularly in the lung.
Cytokine regulation of T cell response
The chronic nature of mycobacterial infection in the lung makes it an excellent model for the study of the balance between expression of immunity and pathological damage to the host (Fig 2).
Figure 2. Regulation of the protective response to Mtb occurs on several levels.

Infection with Mtb results in accumulation of mononuclear phagocytes and lymphocytes in the lung; granulocytes are associated with detrimental inflammation. These cellular accumulations occur within the normal interstitium of the lung and interfere with lung function. Regulating inflammation and tissue damage is essential to maintaining the critical function of the lung. Cytokines (IL-10, TGFβ) provide control of phagocyte activation, while IFNγ and TNFα provide feedback loops that induce regulatory T cells, limit effector T cell survival and limit production of proinflammatory molecules. Regulatory T cells expand with effector cells and infiltrate the inflammation and likely limit effector cell function. Regulators of intracellular signaling (TIR8, DAP12) as well as decoy and inhibitory receptors (DR6, FcγRIIB) curtail immunopathologic consequences during Mtb infection.
IFNγ has long been implicated as a regulator of T cell responses in mycobacterial disease [15]. In the mouse, IFNγ acts on T cells to promote apoptosis by modulating both T cell susceptibility to apoptosis and altering the level of apoptotic signals during mycobacterial disease [16]. In humans lacking a functional IFNγ receptor 1 binding chain, CD4 (but not CD8) T cells express low levels of FasL, are less susceptible to activation induced cells death and have reduced ability to kill mycobacteria-infected compared to controls [17]. IFNγ is also responsible for the loss of established CD8 T cell memory following mycobacterial infection [18]. In an M. avium model, the presence of the IFNγ-inducible immunity related GTPase lrgm1 is required to protect mature effector CD4 T cells from IFNγ-induced cell death [19]. It seems therefore that the Th1 pathway induces responses within effector cells that protect them from IFNγ-mediated negative feedback control (Fig 2).
Deletion of TNF during the chronic phase of Mtb leads to breakdown of the granuloma and an enhanced proinflammatory response [20]. In an M. abscessus model, absence of TNF leads to rapid death with liver necrosis [21] while in M. avium infection of TNF deficient mice, the granuloma disintegrates [22]. In this latter model, disintegration correlates with expansion of T cells and an increase in T cell IFNγ production (Fig 2).
IL-10 is a regulatory cytokine implicated in controlling Mtb immunity. Production of IL-10 during infection with the immunomodulatory strain of Mtb (HN878) limits Th1 immunity and exacerbates disease [23]. In the CBA mice, IL-10 is produced at high levels and when IL-10 is inhibited there is a reduction in bacterial burden and enhancement of type 1 immunity [24].
The cytokine balance during mycobacterial infection is key to control of bacterial growth and maintenance of tissue function.
T cell control of cellular response
Regulatory T cells (Tregs) expand in the lung during experimental Mtb infection and upon deletion bacterial control is improved [25]. Recent data links IFNγ in Treg development as, during Mtb infection the expansion of Tregs is dependent upon IFNγ-induction of T-bet and in the absence of this transcription factor, Tregs fail to persist [26].
In humans, tuberculin responders but not naïve individuals, respond to mannose-capped lipoarabinomannan (ManLAM) by expansion of CD4+CD25+FoxP3+ cells [27]. The ManLAM binds to phagocytes inducing PGE2 which promotes expansion of Tregs further, depletion of Tregs from the culture results in more IFNγ producing T cells [27]. There are more Treg cells in patients than in healthy tuberculin responders [27]. Treg activity may be limited in humans with TB via NK cells lysing expanded Tregs, in this way ongoing activation of NK cells by infection would limit the Tregs [28]. Interestingly, in AIDS patients with mycobacterial disease, both effector T cells and Tregs emerge during anti-viral therapy however the regulatory activity of the Tregs is compromised [29]. Finally, there are more programmed death (PD)-1 positive cells in the PBMC and pleural fluid of TB patients and delivery of anti-PD-1 to these cells in vitro increases responses suggesting that PD-1 may regulate the function of T cells during TB [30].
Regulation of immunopathology
Recent data support the hypothesis that regulation of immunopathology is crucial to survival during chronic mycobacterial disease as mice lacking a negative regulator of both TLR and IL-1R signaling, TIR8, die rapidly from Mtb despite their ability to control bacterial growth. These mice exhibit a high inflammatory response, an excess of IL-1β and liver necrosis importantly, blocking IL-1 and TNF prolong survival [31] (Fig 2).
DAP12 is also a negative regulator of Th1 immunity and in its absence Mtb infection results in an immunopathologic proinflammatory type 1 response with excess CD4 and CD8 T cells [32]. In contrast, mice lacking NOD2 are able to initially control bacterial growth but exhibit reduced inflammation and reduced Th1 responses. This reduced Th1 response results in exacerbated bacterial growth later in infection [33]. Finally, D6 is a decoy scavenger for inflammatory CC chemokines and in its absence Mtb-infected mice rapidly die; this death is associated with excess mononuclear inflammation despite normal bacterial control (Fig 2). Blocking specific chemokines results in reduced tissue damage [34].
The immunoglobulin-binding inhibitory receptor FcγRIIB is also regulatory, as Mtb-infected mice lacking this receptor exhibit better control of bacterial growth [35] (Fig 2). In contrast, mice lacking the γ-chain shared by the activating FcγR exhibit enhanced bacterial growth, more pathologic consequences and higher IL-10 levels [35].
Human effector response induced by Mycobacteria
In South Africa where there is a high incidence of disease, mycobacterial stimulation of PBMCs from healthy humans induces cells to produce IFNγ, IL-17 or IL-22. The cells have a memory T cell phenotype and are reduced in TB patients relative to the healthy exposed individuals; likely due to the cells being sequestered in the lungs as IFN and IL-22 are present in the BAL [36]. Also in South Africa, BCG is given to all infants and induces both CD4 and CD8 T cells. In the responding CD4 T cell population IFNγ, IL-2 and TNFα are produced in a variety of combinations whereas responding CD8 T cells are predominantly IFNγ producers. A minor population of cells making IL-4 or IL-10 are detectable [37]. A recent study in The Gambia compared the PBMC response of patients with healthy household contacts to show that multi-functional cells (making IFNγ, TNFα and IL-2) and cells making TNF alone were higher in patients than contacts, whereas IL-2 single producers were lower. As for the South African study, the TB patients had a reduced peripheral IL-17 response [38].
Specific antigens have been used to investigate the differences between TB patients and controls. Differential IFNγ responses to two mycobacterial antigens, Ag85B and the HspX, demonstrate that the response to HspX is dependent upon Mtb exposure while the response to Ag85B is not. It appears that BCG does not induce responses to HspX and that this may help distinguish between patients and vaccinees [39]. Novel antigens capable of driving human T cell responses such as MPT51 [40] and very novel lipopeptides which induce CD4+, class II-restricted cytokine-producing and cytolytic T cells [41] are being investigated. Further, a stimulatory M. leprae antigen that must be glycosylated to maintain immunogenicity LprG has also been identified [42].
Mycobacteria can interact with human innate cells and modulate their function. Human DC’s infected with BCG activate γδ T cells and these cells can kill mycobacteria-infected primary macrophages but not infected DC’s by a perforin-mediated mechanism [43]. Further, neutrophils take up BCG and cluster with human DC's to promote DC activation with less IL-10 and better presentation compared to BCG and DC's alone [44]. Finally, the human DC response to mycobacteria is very finely tuned as differential induction of immunomodulatory cytokines such as IL-12 and IL-23 depends upon sequential ligation of specific receptors [45]. Further, the presentation of specific antigens is defined by the rapidity with which presenting molecules relocate to the plasma membrane [46].
Vaccination
Any vaccine program against TB will involve BCG at some level therefore determining why BCG works (and doesn't work) is important. There are many strains of BCG in use and these differ in immunogenicity [47]. Further BCG differs from Mtb in that it lacks RD-1 and absence of RD-1 alters induction of cells specific for antigens dependent upon RD-1 [48,49].
BCG delivered to the lung, induces CD4+ T cells that make IFNγ and this response is greater than the CD8 response however the CD8 response can be protective in the absence of CD4 help [50]. Comparison of oral and systemic delivery of BCG demonstrates that although bacteria go to distinct locations and induce antigen-specific cells within different locations, the ability of either route to induce protection is similar. As protection correlates with the arrival in the lung of IFNγ-producing T cells at day 14, it is the kinetics of cell accumulation in the lung that is key to protection [1,51]
To improve BCG vaccination it is possible to boost the initial BCG response by delivery of specific antigen(s). The route of this boost is important as BCG vaccinated mice boosted intranasally (but not intradermally) using a recombinant adenovirus expressing Ag85, are better protected than BCG alone. This protection correlates with the presence of multifunctional high cytokine producers within the lung at the time of challenge [52]. Importantly, using a modified vaccinia virus expressing Ag85A to boost BCG-induced responses in humans also results in expansion of long-lived multifunctional T cells expressing IFNγ TNFα, and IL2 [53].
Several investigators have altered BCG in order to induce better immune responses. Manipulating BCG to express the DC stimulant Flt3-ligand or GM-CSF increases the cellular response to vaccination but only GM-CSF improves protection [54,55]. Manipulation of BCG to express human Cathepsin S overcomes the ability of BCG to inhibit Cathepsin S and thereby allows for better Class II expression and antigen presentation by human macrophages [56]. Further, deletion of the SecA2 gene in BCG reduces its ability to inhibit apoptosis in host cells and results in improved induction of CD8 T cells [57]. While deletion of Treg cells during BCG vaccination does improve vaccine-induced responses it does not improve protection [58].
Despite the focus on CD4 T cells as mediators of vaccine-induced protection, CD8 T cells are capable of performing this function. Delivery of a defined antigen (CFP-10) by DNA vaccination induces CD8 but not CD4 T cells in C3H mice and these cells accumulate in the lung upon challenge and mediate protection [59]. It is likely that these cells use cytolytic activity mediated by perforin to mediate protection [60].
Conclusions
Current knowledge of the immune response to Mtb has not allowed for dramatic improvement in the design of vaccines. We know that early recognition of bacterial infection is important but it appears that the specific immune response is ignorant of lung infection for up to 8 days post infection. Cells resident in the lung are most effective at limiting bacterial growth early but they are dependent upon the infected phagocytes to both present antigen and to respond to activating signals (Fig 1). Once disease has developed a delicate balance between protective and pathologic responses is key to survival (Fig 2). This balance must be considered when developing vaccines.
Acknowledgements
This work was supported by the Trudeau Institute, Inc. and by NIH grants AI046530, AI067723, AI069121 and an ALA DeSouza grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooper A. Cell mediated immune responses in Tuberculosis. Ann. Rev. Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf A, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst J. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reiley W, Calayag M, Wittmer S, Huntington J, Pearl J, Fountain J, Martino C, Roberts A, Cooper A, Winslow G, et al. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in mediastinal lymph nodes. Proc. Natl. Acad. Sci. USA. 2008;105:10961–10966. doi: 10.1073/pnas.0801496105. Using TCRTg T cells, these two references definitively demonstrate the location and kinetics of the first naive T cell activation in response to two different Mtb antigens following low dose aerosol infection. The delay in activation of naive T cells is an important aspect of disease development in the lung.
- 4. Gallegos A, Pamer E, Glickman M. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J. Exp. Med. 2008;205:2359–2368. doi: 10.1084/jem.20080353. Demonstrates that even though effector T cells are in the lung at the time of infection they are not immediately effective at activating phagocytes and limiting bacterial growth. This is important as it suggests that other factors can limit expression of immunity early in lung infection (bacterial burden, phagocyte function or bacterial regulation of responses).
- 5.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 6.Anis M, Fulton S, Reba S, Harding C, Boom W. Modulation of naive CD4+ T-cell responses to an airway antigen during pulmonary mycobacterial infection. Infect. Immun. 2007;75:2260–2268. doi: 10.1128/IAI.01709-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anis M, Fulton S, Reba S, Liu Y, Harding C, Boom W. Modulation of pulmonary dendritic cell function during mycobacterial infection. Infect. Immun. 2008;76:671–677. doi: 10.1128/IAI.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santosuosso M, McCormick S, Roediger E, Zhang X, Zganiacz A, Lichty BD, Xing Z. Mucosal luminal manipulation of T cell geography switches on protective efficacy by otherwise ineffective parenteral genetic immunization. J. Immunol. 2007;178:2387–2395. doi: 10.4049/jimmunol.178.4.2387. [DOI] [PubMed] [Google Scholar]
- 9.McCormick S, Santosuosso M, Small C-L, Shaler C, Zhang X, Jeyanathan M, Mu J, Takenaka S, Ngai P, Gauldie J, et al. Mucosally delivered dendritic cells activate T cells independently of IL-12 and endogenous APCs. J. Immunol. 2008;181:2356–2367. doi: 10.4049/jimmunol.181.4.2356. [DOI] [PubMed] [Google Scholar]
- 10.Jeyanathan M, Mu J, Kugathasan K, Zhang X, Damjanovic D, Small C, Divangahi M, Petrof B, Hogaboam C, Xing Z. Airway delivery of soluble mycobacterial antigens restores protective mucosal immunity by single intramuscular plasmid DNA tuberculosis vaccination: role of proinflammatory signals in the lung. J. Immunol. 2008;181:5618–5626. doi: 10.4049/jimmunol.181.8.5618. [DOI] [PubMed] [Google Scholar]
- 11. Yan BS, Pichugin AV, Jobe O, Helming L, Eruslanov EB, Gutierrez-Pabello JA, Rojas M, Shebzukhov YV, Kobzik L, Kramnik I. Progression of pulmonary tuberculosis and efficiency of bacillus Calmette-Guerin vaccination are genetically controlled via a common sst1-mediated mechanism of innate immunity. J. Immunol. 2007;179:6919–6932. doi: 10.4049/jimmunol.179.10.6919. The essential role of the phagocyte in mediating control of bacterial growth following vaccination is demonstrated here. The data also suggest a unique T cell activation mechanism for the lung phagocytes.
- 12.Marquis J, Lacourse R, Ryan L, North R, Gros P. Genetic and functional characterization of the mouse Trl3 locus in defense against tuberculosis. J. Immunol. 2009;182:3757–3767. doi: 10.4049/jimmunol.0802094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hölscher C, Reiling N, Schaible U, Hölscher A, Bathmann C, Korbel D, Lenz I, Sonntag T, Kröger S, Akira S, et al. Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, -4 and -9. Eur. J. Immunol. 2008;38:680–694. doi: 10.1002/eji.200736458. [DOI] [PubMed] [Google Scholar]
- 14.Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J. Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 15.Cooper AM, Adams LB, Dalton DK, Appelberg R, Ehlers S. IFN-γ and NO in mycobacterial disease: new jobs for old hands. Trends Microbiol. 2002;10:221–226. doi: 10.1016/s0966-842x(02)02344-2. [DOI] [PubMed] [Google Scholar]
- 16.Li X, McKinstry K, Swain S, Dalton D. IFN-gamma acts directly on activated CD4+ T cells during mycobacterial infection to promote apoptosis by inducing components of the intracellular apoptosis machinery and by inducing extracellular proapoptotic signals. J. Immunol. 2007;179:939–949. doi: 10.4049/jimmunol.179.2.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boselli D, Losana G, Bernabei P, Bosisio D, Drysdale P, Kiessling R, Gaston J, Lammas D, Casanova J, Kumararatne D, et al. IFN-gamma regulates Fas ligand expression in human CD4+ T lymphocytes and controls their anti-mycobacterial cytotoxic functions. Eur. J. Immunol. 2007;37:2196–2204. doi: 10.1002/eji.200636541. [DOI] [PubMed] [Google Scholar]
- 18.Dudani R, Murali-Krishna K, Krishnan L, Sad S. IFN-gamma induces the erosion of preexisting CD8 T cell memory during infection with a heterologous intracellular bacterium. J. Immunol. 2008;181:1700–1709. doi: 10.4049/jimmunol.181.3.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feng C, Zheng L, Jankovic D, Báfica A, Cannons J, Watford W, Chaussabel D, Hieny S, Caspar P, Schwartzberg P, et al. The immunity-related GTPase Irgm1 promotes the expansion of activated CD4+ T cell populations by preventing interferon-gamma-induced cell death. Nat. Immunol. 2008;9:1279–1287. doi: 10.1038/ni.1653. The long observed ability of IFNγ to limit cellular responses during mycobacterial infection has been addressed here. The data demonstrate, for the first time, the pathway whereby IFNγ modulates the survival of effector T cells during chronic mycobacterial infection.
- 20.Chakravarty S, Zhu G, Tsai M, Mohan V, Marino S, Kirschner D, Huang L, Flynn J, Chan J. Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect. Immun. 2008;76:916–926. doi: 10.1128/IAI.01011-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rottman M, Catherinot E, Hochedez P, Emile J, Casanova J, Gaillard J, Soudais C. Importance of T cells, gamma interferon, and tumor necrosis factor in immune control of the rapid grower Mycobacterium abscessus in C57BL/6 mice. Infect. Immun. 2007;75:5898–5907. doi: 10.1128/IAI.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flórido M, Appelberg R. Characterization of the deregulated immune activation occurring at late stages of mycobacterial infection in TNF-deficient mice. J. Immunol. 2007;179:7702–7708. doi: 10.4049/jimmunol.179.11.7702. [DOI] [PubMed] [Google Scholar]
- 23.Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, Basaraba RJ, Orme IM. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 2007;179:522–531. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 24.Beamer G, Flaherty D, Assogba B, Stromberg P, Gonzalez-Juarrero M, de Waal Malefyt R, Vesosky B, Turner J. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice. J. Immunol. 2008 doi: 10.4049/jimmunol.181.8.5545. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scott-Browne J, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot J, Rudensky A, Bevan M, Urdahl K. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. Here the ability of regulatory T cells to limit control of Mtb growth in the lung was demonstrated. Although the impact of Treg depletion was modest, this paper established a function for these cells in Mtb.
- 26. Koch M, Tucker-Heard G, Perdue N, Killebrew J, Urdahl K, Campbell D. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009 doi: 10.1038/ni.1731. Here a role for the Th1 associated transcription factor T-bet is demonstrated, for the first time, in the maintenance of regulatory T cells during chronic Th1 inflammation. This is important as it shows the adaptability of the immune response and the multiple levels at which it undergoes self-regulation.
- 27.Garg A, Barnes P, Roy S, Quiroga M, Wu S, García V, Krutzik S, Weis S, Vankayalapati R. Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur. J. Immunol. 2008;38:459–469. doi: 10.1002/eji.200737268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy S, Barnes P, Garg A, Wu S, Cosman D, Vankayalapati R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J. Immunol. 2008;180:1729–1736. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 29.Seddiki N, Sasson S, Santner-Nanan B, Munier M, van Bockel D, Ip S, Marriott D, Pett S, Nanan R, Cooper D, et al. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur. J. Immunol. 2009;39:391–403. doi: 10.1002/eji.200838630. [DOI] [PubMed] [Google Scholar]
- 30.Jurado J, Alvarez I, Pasquinelli V, Martínez G, Quiroga M, Abbate E, Musella R, Chuluyan H, García V. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J. Immunol. 2008;181:116–125. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- 31. Garlanda C, Di Liberto D, Vecchi A, La Manna M, Buracchi C, Caccamo N, Salerno A, Dieli F, Mantovani A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J. Immunol. 2007;179:3119–3125. doi: 10.4049/jimmunol.179.5.3119. Here the control of inflammation during a chronic mycobacterial infection is shown to be as important to survival as is the control of bacterial growth.
- 32.Divangahi M, Yang T, Kugathasan K, McCormick S, Takenaka S, Gaschler G, Ashkar A, Stampfli M, Gauldie J, Bramson J, et al. Critical negative regulation of type 1 T cell immunity and immunopathology by signaling adaptor DAP12 during intracellular infection. J. Immunol. 2007;179:4015–4026. doi: 10.4049/jimmunol.179.6.4015. [DOI] [PubMed] [Google Scholar]
- 33.Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, Kobayashi K, Flavell R, Gros P, Behr M. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J. Immunol. 2008;181:7157–7165. doi: 10.4049/jimmunol.181.10.7157. [DOI] [PubMed] [Google Scholar]
- 34. Di Liberto D, Locati M, Caccamo N, Vecchi A, Meraviglia S, Salerno A, Sireci G, Nebuloni M, Caceres N, Cardona P, et al. Role of the chemokine decoy receptor D6 in balancing inflammation, immune activation, and antimicrobial resistance in Mycobacterium tuberculosis infection. J. Exp. Med. 2008;205:2075–2084. doi: 10.1084/jem.20070608. As for ref #31, this paper demonstrates that control of immunopathology is essential in order to survive pulmonary Mtb infection.
- 35.Maglione P, Xu J, Casadevall A, Chan J. Fc{gamma} Receptors Regulate Immune Activation and Susceptibility during Mycobacterium tuberculosis Infection. J. Immunol. 2008;180:3329–3338. doi: 10.4049/jimmunol.180.5.3329. [DOI] [PubMed] [Google Scholar]
- 36.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, Hawkridge A, Hussey GD, Maecker H, Kaplan G, et al. Bacillus calmette-guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J. Immunol. 2008;180:3569–3577. doi: 10.4049/jimmunol.180.5.3569. In these studies comprehensive analysis of the immune response of vaccinees, exposed controls and TB patients is performed in detail. The complexity of the human response to mycobacteria is reported more fully than previously.
- 38.Sutherland J, Adetifa I, Hill P, Adegbola R, Ota M. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur. J. Immunol. 2009;39:723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 39.Geluk A, Lin M, van Meijgaarden K, Leyten E, Franken K, Ottenhoff T, Klein M. T-cell recognition of the HspX protein of Mycobacterium tuberculosis correlates with latent M. tuberculosis infection but not with M. bovis BCG vaccination. Infect. Immun. 2007;75:2914–2921. doi: 10.1128/IAI.01990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aoshi T, Nagata T, Suzuki M, Uchijima M, Hashimoto D, Rafiei A, Suda T, Chida K, Koide Y. Identification of an HLA-A*0201-restricted T-cell epitope on the MPT51 protein, a major secreted protein derived from Mycobacterium tuberculosis, by MPT51 overlapping peptide screening. Infect. Immun. 2008;76:1565–1571. doi: 10.1128/IAI.01381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bastian M, Braun T, Bruns H, Rollinghoff M, Stenger S. Mycobacterial lipopeptides elicit CD4+ CTLs in Mycobacterium tuberculosis-infected humans. J. Immunol. 2008;180:3436–3446. doi: 10.4049/jimmunol.180.5.3436. [DOI] [PubMed] [Google Scholar]
- 42.Sieling P, Hill P, Dobos K, Brookman K, Kuhlman A, Fabri M, Krutzik S, Rea T, Heaslip D, Belisle J, et al. Conserved mycobacterial lipoglycoproteins activate TLR2 but also require glycosylation for MHC class II-restricted T cell activation. J. Immunol. 2008;180:5833–5842. doi: 10.4049/jimmunol.180.9.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martino A, Casetti R, Sacchi A, Poccia F. Central memory Vgamma9Vdelta2 T lymphocytes primed and expanded by bacillus Calmette-Guerin-infected dendritic cells kill mycobacterial-infected monocytes. J. Immunol. 2007;179:3057–3064. doi: 10.4049/jimmunol.179.5.3057. [DOI] [PubMed] [Google Scholar]
- 44.Morel C, Badell E, Abadie V, Robledo M, Setterblad N, Gluckman J, Gicquel B, Boudaly S, Winter N. Mycobacterium bovis BCG-infected neutrophils and dendritic cells cooperate to induce specific T cell responses in humans and mice. Eur. J. Immunol. 2008;38:437–447. doi: 10.1002/eji.200737905. [DOI] [PubMed] [Google Scholar]
- 45.Gerosa F, Baldani-Guerra B, Lyakh L, Batoni G, Esin S, Winkler-Pickett R, Consolaro M, De Marchi M, Giachino D, Robbiano A, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hava D, van der Wel N, Cohen N, Dascher C, Houben D, León L, Agarwal S, Sugita M, van Zon M, Kent S, et al. Evasion of peptide, but not lipid antigen presentation, through pathogen-induced dendritic cell maturation. Proc. Natl. Acad. Sci. USA. 2008;105:11281–11286. doi: 10.1073/pnas.0804681105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aguirre-Blanco A, Lukey P, Cliff J, Dockrell H. Strain-dependent variation in Mycobacterium bovis BCG-induced human T-cell activation and gamma interferon production in vitro. Infect. Immun. 2007;75:3197–3201. doi: 10.1128/IAI.01611-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Billeskov R, Vingsbo-Lundberg C, Andersen P, Dietrich J. Induction of CD8 T cells against a novel epitope in TB10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J. Immunol. 2007;179:3973–3981. doi: 10.4049/jimmunol.179.6.3973. [DOI] [PubMed] [Google Scholar]
- 49.Woodworth J, Fortune S, Behar S. Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect. Immun. 2008;76:4199–4205. doi: 10.1128/IAI.00307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ngai P, McCormick S, Small C, Zhang X, Zganiacz A, Aoki N, Xing Z. Gamma interferon responses of CD4 and CD8 T-cell subsets are quantitatively different and independent of each other during pulmonary Mycobacterium bovis BCG infection. Infect. Immun. 2007;75:2244–2252. doi: 10.1128/IAI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mittrücker H, Steinhoff U, Köhler A, Krause M, Lazar D, Mex P, Miekley D, Kaufmann S. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl. Acad. Sci. USA. 2007;104:12434–12439. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Forbes E, Sander C, Ronan E, McShane H, Hill A, Beverley P, Tchilian E. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J. Immunol. 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. A clear study demonstrating that it is the location and functionality of T cell that correlates with their protective capacity.
- 53.Beveridge N, Price D, Casazza J, Pathan A, Sander C, Asher T, Ambrozak D, Precopio M, Scheinberg P, Alder N, et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur. J. Immunol. 2007;37:3089–3100. doi: 10.1002/eji.200737504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan AA, Wozniak TM, Shklovskaya E, O'Donnell MA, Fazekas de St Groth B, Britton WJ, Triccas JA. Improved protection against disseminated tuberculosis by Mycobacterium bovis bacillus Calmette-Guerin secreting murine GM-CSF is associated with expansion and activation of APCs. J. Immunol. 2007;179:8418–8424. doi: 10.4049/jimmunol.179.12.8418. [DOI] [PubMed] [Google Scholar]
- 55.Triccas J, Shklovskaya E, Spratt J, Ryan A, Palendira U, Fazekas de St Groth B, Britton W. Effects of DNA- and Mycobacterium bovis BCG-based delivery of the Flt3 ligand on protective immunity to Mycobacterium tuberculosis. Infect. Immun. 2007;75:5368–5375. doi: 10.1128/IAI.00322-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soualhine H, Deghmane AE, Sun J, Mak K, Talal A, Av-Gay Y, Hmama Z. Mycobacterium bovis bacillus Calmette-Guerin secreting active cathepsin S stimulates expression of mature MHC class II molecules and antigen presentation in human macrophages. J. Immunol. 2007;179:5137–5145. doi: 10.4049/jimmunol.179.8.5137. [DOI] [PubMed] [Google Scholar]
- 57.Hinchey J, Lee S, Jeon B, Basaraba R, Venkataswamy M, Chen B, Chan J, Braunstein M, Orme I, Derrick S, et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Invest. 2007;117:2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quinn K, Rich F, Goldsack L, de Lisle G, Buddle B, Delahunt B, Kirman J. Accelerating the secondary immune response by inactivating CD4(+)CD25(+) T regulatory cells prior to BCG vaccination does not enhance protection against tuberculosis. Eur. J. Immunol. 2008;38:695–705. doi: 10.1002/eji.200737888. [DOI] [PubMed] [Google Scholar]
- 59.Wu Y, Woodworth J, Shin D, Morris S, Behar S. Vaccine-elicited 10-kilodalton culture filtrate protein-specific CD8+ T cells are sufficient to mediate protection against Mycobacterium tuberculosis infection. Infect. Immun. 2008;76:2249–2255. doi: 10.1128/IAI.00024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woodworth J, Wu Y, Behar S. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J. Immunol. 2008;181:8595–8603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]


