Abstract
The basic helix-loop-helix transcription factor Hand2 is essential for the proliferation and noradrenergic differentiation of sympathetic neuron precursors during development. Here we address the function of Hand2 in postmitotic, differentiated sympathetic neurons. Knockdown of endogenous Hand2 in cultured E12 chick sympathetic neurons by siRNA results in a significant (about 60%) decrease in the expression of the noradrenergic marker genes dopamine-β-hydroxylase (DBH) and tyrosine hydroxylase (TH). In contrast, expression of the pan-neuronal genes TuJ1, HuC and SCG10 was not affected. To analyze the in vivo role of Hand2 in differentiated sympathetic neurons we used mice harboring a conditional Hand2-null allele and excised the gene by expression of Cre recombinase under control of the DBH promotor. Mouse embryos homozygous for Hand2 gene deletion showed decreased sympathetic neuron number and TH expression was strongly reduced in the residual neuron population. The in vitro Hand2 knockdown also enhances the CNTF-induced expression of the cholinergic marker genes vesicular acetylcholine transporter (VAChT) and choline acetyltransferase (ChAT). Taken together, these findings demonstrate that the Hand2 transcription factor plays a key role in maintaining noradrenergic properties in differentiated neurons.
Keywords: Hand2, siRNA knockdown, TH, DBH, ChAT, VAChT, sympathetic neuron survival, proliferation, sympathetic ganglia
Introduction
The development of sympathetic neurons is initiated by bone morphogenetic proteins (BMPs) acting on neural crest cells that have migrated to the dorsal aorta. BMPs control the expression of a group of transcription factors, including Ascl1, Phox2b, Phox2a, Hand2 and Gata2/3 that play important roles in sympathetic neuron specification and differentiation (reviewed in (Goridis and Rohrer, 2002; Howard, 2005; Rohrer, 2003). Sympathetic neuron development is completely prevented in the absence of Phox2b, which is essential for development of the autonomic nervous system (Pattyn et al., 1999). Ascl1 mainly affects the timing of sympathetic neuron development, as shown by delayed sympathetic neuron differentiation in Ascl1-deficient mouse embryos (Pattyn et al., 2006). A similar role has been demonstrated for the transcription factor Insm1, which in addition also affects sympathetic neuron proliferation (Wildner et al., 2008). Whereas the knockouts of Phox2b, Ascl1 and Insm1 displayed pleiotropic functions on noradrenergic and generic neuronal differentiation, more selective effects on the expression of noradrenergic marker genes were observed in the absence of Gata2/3 and Hand2, in particular during early development. In the absence of Gata3 a selective decrease of TH expression was found at E10.5, followed by impaired neuronal differentiation and cell death (Lim et al., 2000; Tsarovina et al., 2004). TH and DBH are strongly reduced in developing sympathetic ganglia of the Hand2 zebrafish mutant hands off (Hendershot et al., 2008; Lucas et al., 2006) and in conditional Hand2 mouse knockouts where the Hand2 gene was excised in neural crest cells, using Wnt1-Cre (Hand2Wnt1Cre) (Hendershot et al., 2008; Morikawa et al., 2007). HuC, TuJ1 and NF160 are less affected and reduced to a variable extent in different model sytems analyzed (Hendershot et al., 2008; Morikawa et al., 2007; Lucas et al., 2006). Interestingly, Hand2 is also required for the proliferation of sympathetic neuron precursors and immature sympathetic neurons, resulting in a massive decrease in neuron numbers in the conditional Hand2Wnt1Cre knockout (Hendershot et al., 2008).
The knockouts demonstrate that Hand2 is important for the proliferation and noradrenergic differentiation in sympathetic ganglia. The marked decrease in TH and DBH expression implies an essential function for Hand2 in the initial onset of noradrenergic differentiation (Hendershot et al., 2008; Morikawa et al., 2007; Lucas et al., 2006). However, Hand2 is not required for the initial expression of TH and DBH in young parasympathetic ciliary ganglion neurons that are devoid of Hand2 (Müller and Rohrer, 2002). TH and DBH are expressed during normal ciliary neuron development only transiently, but the loss of TH and DBH could be prevented by ectopic Hand2 expression. We thus proposed that the function of Hand2 in sympathetic neurons includes the maintenance of noradrenergic differentiation (Müller and Rohrer, 2002).
To address the role of Hand2 in sustaining noradrenergic gene expression, a siRNA knockdown approach was used in cultures of postmitotic, differentiated neurons from E12 chick sympathetic ganglia. The Hand2 knockdown resulted in a strongly decreased expression of TH and DBH, without affecting neuron number and the expression of pan-neuronal genes like TuJ1, HuC and SCG10. In parallel, we analyzed sympathetic ganglia of a mouse line where Hand2 has been conditionally eliminated in differentiated, DBH-expressing neurons (Hand2DBHCre). Sympathetic ganglia of E11.5 and E14.5 Hand2DBHCre mouse embryos show decreased neuron numbers and a significantly diminished TH expression in the residual neuron population, confirming the results of the in vitro Hand2 knockdown. Thus, we conclude that Hand2 is essential for the maintenance of the noradrenergic phenotype of sympathetic neurons.
Materials and methods
Construction of plasmids
pCAGGS-mHand2 and pCAGGS-zHand2 PCR technology was used to insert a Kozak sequence linked to a NheI site and a ClaI site flanking the coding sequence of pcDNA3-mus musculus Hand2 (kindly provided by E. Doxakis) and pCS2+-zebrafish Hand2 (kindly provided by P. D. Henion). mHand2-Primer: sense: 5′-AAG CTA GCA CCA CCA TGA GTC TGG TGG GGG GC-3′; antisense: 5′-TTA TCG ATT CAC TGC TTG AGC TCC AGG G-3′; zHand2-Primer: sense: 5′-AAG CTA GCA CCA CCA TGA GTT TGA TTG GAG GGT TTC-3′; antisense: 5′-TTA TCG ATT CAT TGC TTC AGT TCC AAT GCC-3′ (sense primer: bold, NheI site; underlined, Kozak sequence; bold + underlined, Start) (antisense primer: bold, ClaI site; bold+underlined, Stop). The PCR product was then cloned directly into the pCAGGS vector.
Primary culture preparation and electroporation
Chick sympathetic neurons were prepared by dissociation of paravertebral lumbosacral sympathetic chain ganglia, dissected at E7/E12 (Ernsberger et al., 1989a; Ernsberger et al., 1989b). Cells were cultivated in the presence of either nerve growth factor (NGF, 15ng/ml, (Peprotech)) or of both NGF and ciliary neurotrophic factor (CNTF, 30ng/ml, (Peprotech)). For Hand2 knockdown experiments, 200.000 cells were mixed with either 3μg siRNA against Hand2 or control siRNA and electroporated with the Amaxa Nucleofector device according to the Amaxa nucleofection protocol for primary chicken neurons (Program G13). For rescue experiments, cells were transfected by electroporation as described above with 3μg siRNA plus expression plasmids (1μg control siRNA plus 1μg pCAGGS-eGFP (GFP) for controls, 1μg control siRNA plus 1μg pCAGGS-eGFP and 1μg pCAGGS-z/mHand2 for overexpression, 1μg siRNA against Hand2 plus 1μg pCAGGS-eGFP for knockdown and 1μg siRNA against Hand2 plus 1μg pCAGGS-eGFP and 1μg pCAGGS-z/mHand2 for rescue). 200.000 cells per electroporation were plated on a 35mm 4-well dish or 35mm dish precoated with poly-DL-ornithine (Sigma) and Laminin (Invitrogen). The cultures were incubated for 2 days in MEM media containing 5% FCS, 10% HS, 1% glutamine and 1% Penicillin/Streptomycin at 37°C and 5% CO2. Cells were harvested after 4 days and total RNA was isolated using the QIAGEN RNeasy Mini Kit, following the manufacturer’s protocol. The siRNAs against ggHand2 target the following sequence: 5′-CACAGTTAGCAGCAGCGATAA-3′ (Hand2 siRNA1; Qiagen) and 5′-GAAGAGGAAGAAGGAGCTGAA-3′ (Hand2 siRNA2; Qiagen). Hand2 siRNA1 does not target mouse and zebrafish Hand2 due to 3 and 5 nucleotide mismatches, respectively (Dahlgren et al., 2008). The control siRNA against GFP (siGFP; Qiagen) targets the sequence 5′-GCAAGCTGACCCTGAGTTC-3′. The control siRNA against NP25 is non-functional and targets the sequence 5′GCACCTCTGTCTGTAGAGA.
In situ hybridization in cell culture
Non-radioactive in situ hybridization in cell culture and preparation of digoxigenin-labelled riboprobes for SCG10, DBH, TH and Hand2 were carried out as described previously (Ernsberger et al., 1997; Stanke et al., 1999).
Immunocytochemistry in cell culture
Cell culture dishes were washed once with PBS and cells were fixed using 4% paraformaldehyde in 0.1M sodium phosphate buffer for 15min. Cells were washed with PBS and incubated for 15min in staining buffer (PBS, 5% FCS, 0.2% Triton-X-100). Primary antibody for Hand2 (Santa Cruz) was diluted 1/100, for TH (Rohrer et al., 1986), for βIII-Tubulin (Tuj1 antigen, HISS Diagnostics, MMS-435P), for HuC/HuD (Molecular Probes) were diluted 1/1000 in staining buffer and incubated for 1h at RT. Cell culture dishes were washed twice with PBST (PBS, 0.2% Triton-X-100). Fluorophore coupled secondary antibody (in PBST + DAPI (1μg/ml)) was incubated for 30 min at RT and washed off twice with PBS. Sections were covered with AquaPolyMount covering media (Polysciences, Inc.) and glass cover slips. Neurons counts were obtained by examining phase-bright or fluorescent neurons in randomly selected fields of the culture dish. At least 10 visual fields were counted for each well. Two to four wells of a dish were examined for each parameter in each experiment. The number of independent experiments analyzed is indicated by n. The results are given as the mean number per dish ± s.e.m. of at least five experiments analyzed. Student’s t-test was used for statistical analysis.
Semiquantitative RT- PCR
cDNA synthesis on total RNA from sympathetic ganglia cells was performed using the M-MLV Reverse Transcriptase Kit (Invitrogen). For detection of ChAT, VAChT, SCG10 and GAPDH mRNA levels, the following primer combinations and the Taq Polymerase Kit (Invitrogen) for PCR reaction were used: ggChAT-forward (for) 5′-CAACATTAGGTCTGCTACGGCG-3′, ggChAT-reverse (rev) 5′-GCAACTGTGTGGCTTCTTCTTG-3′, ggVAChT-for 5′-TTTCTGGCAGGTCATCATCCC-3′, ggVAChT-rev 5′-GGTGTCGTAGAGTCCCTTAGGTCC-3′; ggSCG10-for 5′-TTTAATGCCCGGAGATTCTG-3′, ggSCG10-rev 5′-TCAGCTTTTCCTCTGCCATT-3′ ggGAPDH-for 5′-CAGAGGTGCTGCCCAGAA-3′, ggGAPDH-rev 5′-GCAGGGGCTCCAACAAAG-3′.
All PCR reactions were performed within the linear range of amplification; this was determined empirically for each primer pair and cell preparation. GAPDH was analyzed after 20 and 22 cycles, SCG10 after 27 and 29 cycles, VAChT after 29 and 31 cycles and ChAT after 31 und 33 cycles. The PCR products were separated on a 1.5% agarose gel and visualized with ethidium bromide. Quantification was done with the ScionImage Software version 1.63 (Scion Corporation). ChAT, VAChT, SCG10 mRNA level were normalized to GAPDH mRNA level. In some cases ChAT and VAChT mRNA levels were referred to SCG10 mRNA levels. Quantification for each experiment and gene was done in triplicate. Data shown represent mean ± s.e.m. of at least 4 independent experiments. Statistical analysis was done using paired Student’s t-test.
Transfection of stable cell lines expressing Hand2-myc (Hand2-myc-DF1)
Chicken fibroblasts of the DF1 cell line were transfected with 1μg RCAS(B)-Hand2-myc in 10 cm dishes according to the Qiagen Effectene protocol and incubated in DMEM with 10% FCS and 1% Penicillin/Streptomycin at 37°C and 10% CO2. Expression levels of Hand2 were assessed by western blotting of 2-day-cultures.
Protein Blotting
Hand2-myc-DF1 cells were harvested and pelleted by centrifugation (100 × g, 10min). Pelleted cells were lysed with cold lysis buffer (50mM Tris/HCl pH7.4, 150mM NaCl, 40mM NaF, 5mM EDTA, 5mM EGTA, 1mM Na3VO4, 1% Triton-X100, 0.1% Na-deoxycholat, 0.1% SDS, 1mM PMSF, 10μg/ml Aprotinin) for 10min on ice. After centrifugation (10.000 × g, 10min, 4°C) the supernatant was collected and protein content determined with the DC protein assay (BioRad). After conventional SDS-PAGE and semi-dry blotting to PVDF membrane (BioRad), the membrane was blocked with 5% instant milk powder in TBST (10mM Tris/HCl pH8, 150mM NaCl, 0.05% Tween20) for 1h before antibody incubation. The anti-myc- (Abcam) and anti-actin-antibodies (Dianova, MS-1295) were diluted 1/2500 in blocking solution and incubated overnight at 4°C. After washing 3 times with TBST the appropriate HRP-coupled secondary antibody (1/5000 in TBST) was incubated for 1h at RT and washed twice with TBS. The blots were developed using standard ECL according to manufacturer’s instructions (Pierce, SuperSignal). For quantification of protein bands the software ScionImage version 4.03.2 from (Scion Corporation) was used with the macro “gel blot 2”.
Mouse Strains
All animal care, breeding procedures, and experimental protocols were approved by the Medical University of Ohio (renamed University of Toledo Health Sciences Campus) animal care and use committee. A description of our strategy for targeting Hand2 is published elsewhere (Hendershot et al., 2007). To generate Hand2DBHCre mice, DBHCre+/0;Hand2wt/del males were mated to Hand2fl/fl females. The DBHCre mouse line has been described previously (Matsushita et al., 2004). Plug date was counted as E0.5. Because targeted deletion of Hand2 is embryonic lethal at around E11 we used a pharmacological approach to rescue embryos making it possible to assess effects of Hand2 deletion in embryos older than E10-12. For rescue, pregnant dams received water containing a cocktail of catecholamines (200 μg/ml L-phenylephrine, 100 μg/ml isoproterenol, and 2 mg/ml ascorbic acid) beginning at E8.
Immunocytochemistry and cell counts of tissue sections
Preparation of tissue sections for cell counts and immunostaining were carried out using our established methods (Hendershot et al., 2008; Hendershot et al., 2007). Following fixation in 4% paraformaldehyde, pH. 7.4, embryos are washed in PBS and stored in 30% sucrose. Embryos are fixed from 4 hours (E10) to overnight (E12 and E14) at 4°C. For cell counts, embryos were embedded in Cryo-gel™ (Instrumedics, St. Louis, MO) and 20μm serial sections were cut on a Leica CM 3050 S freezing microtome. Cell counts were compiled from trunk sympathetic ganglia on serial sections spanning the region from just above the forelimb and inclusive to the hindlimb. For immunostaining, tissue sections were blocked (0.1M Tris, pH 7.4, 1.5% NaCl, 0.3% Triton-X 100 and 10% horse serum) for 30 minutes and then incubated in primary antibodies (0.1M Tris, pH 7.4, 1.5% NaCl, 0.3% Triton-X 100 and 4% horse serum) overnight at 4°C. After washing with 0.1M Tris pH 7.4, 15% NaCl, sections were incubated at room temperature for 3 hours in secondary antibodies (0.1M Tris, pH 7.4, 1.5% NaCl, 0.3% Triton-X 100 and 4% horse serum). Sections were washed 3 × 5 minutes (0.1M Tris, pH 7.4, 15% NaCl) and mounted in Vectashield (Vector Laboratories, Burlingame, CA) or Fluoromount-G (Southern Biotech, Birmingham, Al). The anti-TH antibody (Pel Freez, Rogers, AK) was used at a dilution of 1:200. The anti-Hu antibody (1:50.000) was the generous gift of Vanda A. Lennon (Mayo Clinic, Rochester, MN). Species specific secondary antibodies used were goat anti-rabbit FITC (TH) (Abcam, Cambridge, MA) and Donkey anti-human TRITC (Hu) (Jackson ImmunoResearch, Inc. West Grove, PA). Data of cell counts are presented as the mean ± s.e.m. Statistical significance was determined using ANOVA and Bonferroni post hoc test.
Results
siRNA knockdown of Hand2
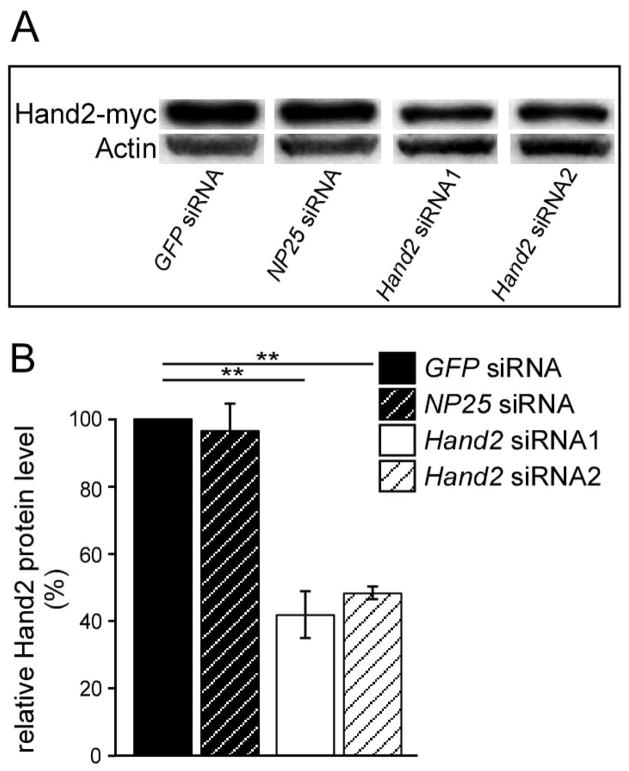
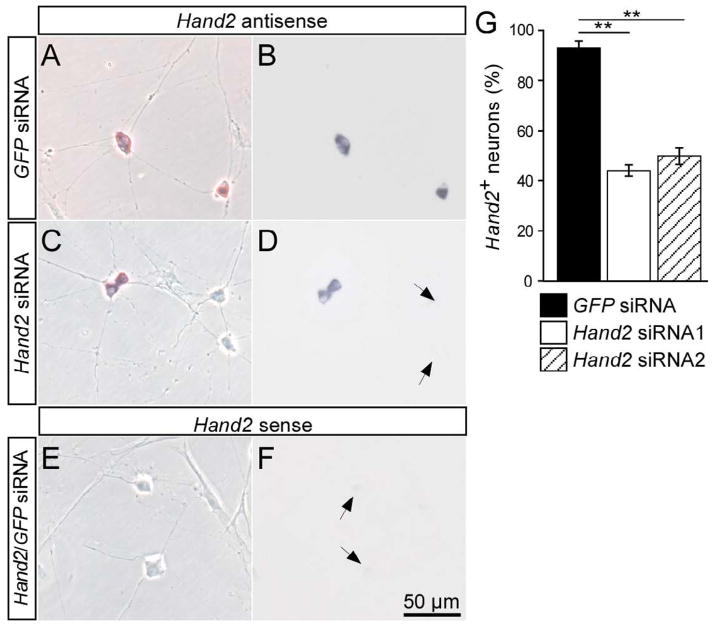
Two Hand2 siRNA oligonucleotides that reduce Hand2 protein expression by 50–60% were selected using the chick DF-1 cell line previously transfected with Hand2-myc expressing RCAS virus (Fig. 1). Control siRNA were used that are directed either against the pan-neuronal gene NP25 (Pape et al., 2008) or against GFP, which is not expressed in vertebrate cells. To assess the extent of Hand2 knockdown in sympathetic neurons, E12 (Fig. 2A,B) and E7 (not shown) chick sympathetic ganglion cells were transfected with control and Hand2 siRNA before plating and analyzed by in situ hybridization for Hand2 expression in 2-day-cultures. Whereas 93±2% of sympathetic neurons displayed an in situ hybridization signal for Hand2 in GFP siRNA transfected control cultures, (Fig. 2) only 44±2% and 50±3% Hand2-positive cells were observed upon transfection with Hand2 siRNA1 and Hand2 siRNA2, respectively. In control hybridizations with sense Hand2 RNA no labeled cells were observed. Antibody staining for Hand2 was less sensitive, but revealed a similar extent of Hand2 knockdown, from about 60% neurons with nuclear Hand2-staining in siGFP-controls to 20% in Hand2 siRNA transfections (not shown).
Fig. 1.
Knockdown of Hand2 by siRNA. (A) Western blot analysis of Hand2 expression in a chick DF-1 cell line with permanent expression of Hand2-myc. DF-1 cells were transfected with control siRNA directed against GFP and NP25 and with two different siRNAs directed against ggHand2. Protein extracts were subjected to PAGE electrophoresis and analyzed by western blotting for Hand2 and β-actin. (B) The intensity of Hand2 protein bands were quantified and normalized to β-actin. Hand2 expression levels were compared to siGFP control transfections. Hand2 siRNA1 and Hand2 siRNA2 reduced Hand2 protein levels to about 40% and 50% of control, respectively, whereas NP25 siRNA had no effect. **P<0,05.
Fig. 2.
Knockdown of Hand2 in cultured sympathetic neurons by siRNA. E12 sympathetic neurons were transfected with control GFP siRNA (A,B) or Hand2 siRNA (C,D) and analyzed after 2 days in culture for the expression of Hand2 mRNA by in situ hybridization. To control for the specificity of the signal obtained in the in situ hybridization also a sense RNA probe was used (E,F). Morphology of neurons and non-neuronal cells is shown in phase contrast (A,C,E), the in situ hybridization signal is shown in bright field optics (B,D,F). Hand2-positive neurons display a strong in situ hybridization signal (B,D), which is completely absent in the sense controls (F). Non-neuronal cells were also devoid of Hand2. In cultures treated with Hand2 siRNA only 44–50% of neurons were Hand2+, whereas >90% Hand2+ neurons were observed in control transfections. Data shown in (G) represent mean ± s.e.m. (n=3), **P<0,05.
Hand2 knockdown results in a strong decrease in TH and DBH expression
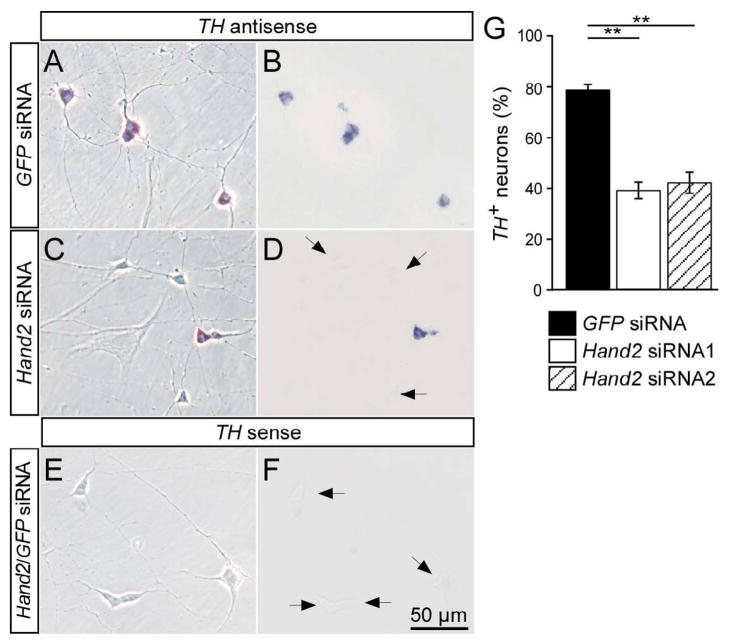
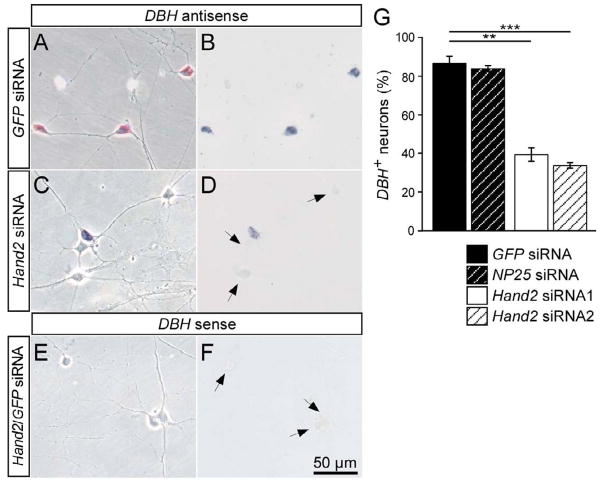
Hand2 knockdown by siRNA affected TH mRNA expression in both E12 (Fig. 3) and E7 (not shown) sympathetic neuron cultures. Whereas 79±2% of neurons express TH in E12 cultures transfected with control siRNA, only 39±3% and 40±4% TH-positive cells were present in cultures with Hand2 siRNA1 and Hand2 siRNA2, respectively (Fig. 3). A sense probe for TH did not produce a signal in the in situ hybridization. The Hand2 knockdown also reduced the proportion of TH-immunoreactive neurons from 62±2% in E12 cultures transfected with control siRNA, to 31±3 % TH-immunoreactive in cultures transfected with Hand2 siRNA1 or Hand2 siRNA2 (Supplementary Fig. 1; not shown). To exclude the possibility that the response to Hand2 siRNA treatments are due to off-target effects, rescue experiments were done using parallel transfections with Hand2 siRNA1 and mouse and zebrafish Hand2 that are not targeted by the chick Hand2 siRNA1. The transfection of both mouse and zebrafish Hand2 completely rescued the effect of chick Hand2 siRNA on the expression of TH (Supplementary Fig. 1), demonstrating that the effects of chick Hand2 siRNA are due to reduced Hand2 levels. The best studied target gene of Hand2 in the sympathoadrenal lineage is DBH, where DNA binding and transactivation have been characterized in detail (Rychlik et al., 2003; Xu et al., 2003). Therefore, we analysed the proportion of DBH-expressing cells to assess the role of Hand2 in the maintenance of noradrenergic differentiation. DBH-expressing neurons with strong in situ hybridization signal could be clearly distinguished from DBH-negative satellite cells and fibroblasts in E12 (Fig. 4) and E7 cultures (not shown). The specificity of the DBH in situ signal is demonstrated by the complete lack of hybridization with a DBH sense probe. In control GFP and NP25 siRNA transfections of E12 ganglion cells, about 85% of cells with neuronal morphology were distinctly DBH-positive (Fig. 4A,B,G). Hand2 siRNA transfections resulted in a strong decrease in the proportion of DBH-positive neurons to 40±2 % and 35±1 % for Hand2 siRNA1 and Hand2 siRNA2, respectively (Fig. 4C,D,G).
Fig. 3.
Effect of Hand2 knockdown on TH expression in cultured sympathetic neurons. E12 sympathetic neurons were transfected with control GFP siRNA (A,B) or Hand2 siRNA (C,D) and analyzed after 2 days in culture for the expression of TH mRNA expression by in situ hybridization. A sense RNA probe was used to control for the specificity of the TH in situ hybridization signal (E,F). TH-positive neurons display a strong in situ hybridization signal (B,D), which is completely absent in non-neuronal cells and in neurons treated with the sense control RNA (F). In cultures treated with Hand2 siRNA the proportion of TH-expressing sympathetic neurons decreased from 80% in control transfections to about 40% in cultures transfected with Hand2 siRNA. (A,C,E) Phase contrast; (B,D,F) bright field optics. Data shown in (G) represent mean ± s.e.m. (n=3), **P<0,01; ***P<0,001.
Fig. 4.
Effect of Hand2 knockdown on DBH expression in cultured sympathetic neurons. E12 sympathetic neurons were transfected with control GFP siRNA (A,B) or Hand2 siRNA (C,D) and analyzed after 2 days in culture for the expression of DBH mRNA expression by in situ hybridization as described for TH in Fig. 3. In cultures treated with Hand2 siRNA the proportion of DBH-expressing sympathetic neurons decreased from 83–87% in control transfections to 35–40% in cultures transfected with Hand2 siRNA. (A,C,E) Phase contrast; (B,D,F) bright field optics. Data shown represent mean ± s.e.m. (n=3), **P<0,01.
Taken together, these results demonstrate that Hand2 knockdown produces a strong decrease in the proportion of noradrenergic, TH- and DBH-expressing neurons.
Hand2 knockdown does not affect sympathetic neuron survival
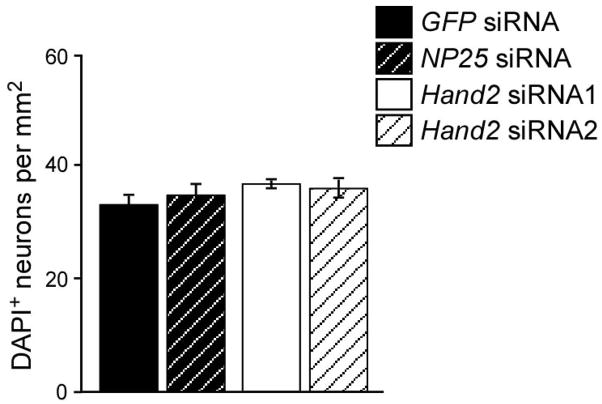
The decreased proportion of noradrenergic neurons observed upon Hand2 knockdown could either be due to the selective survival of non-noradrenergic neurons or due to a general decrease in TH and DBH expression which leads in some cells to levels below the detection limit. An effect of Hand2 knockdown on chick sympathetic neuron survival was expected, since Hand2 has been shown to control trkA expression and NGF-dependent survival in cultures of postnatal mouse sympathetic neurons (Doxakis et al., 2008). To investigate effects of Hand2 knockdown on neuron survival, the number of sympathetic neurons per culture, identified as cells with neuronal morphology and DAPI-positive nucleus, was determined in 2-day-cultures. As shown in Fig. 5, the same neuron density was observed in cultures transfected either with control or Hand2 siRNA in E12 neuron cultures. This result excludes survival effects of the Hand2 knockdown under the culture conditions used, thus supporting the notion that the levels of TH and DBH expression in differentiated sympathetic neurons are Hand2 dependent. The lack of survival effects, in contrast to findings in mouse neuron cultures (Doxakis et al., 2008), is most likely due to the shorter culture period, different culture conditions and the knockdown of Hand2 rather than of both Hand2 and Hand1. As sympathetic neuron proliferation has ceased in the chick embryo at E12, neuron numbers in these cultures are also not affected by the strong antiproliferative effects of Hand2 elimination observed at earlier stages (Hendershot et al., 2008).
Fig. 5.
Survival of E12 sympathetic neurons is not affected by Hand2 knockdown. E12 sympathetic neurons were transfected with control GFP siRNA, NP25 siRNA or Hand2 siRNAs and analyzed after 2 days in culture for the number of sympathetic neurons. Neuron number was determined after fixation and nuclear staining for DAPI to distinguish individual neurons in small neuron groups. Data represent mean ± s.e.m. (n=6).
The expression of generic neuronal genes in differentiated E12 chick sympathetic neurons does not depend on Hand2
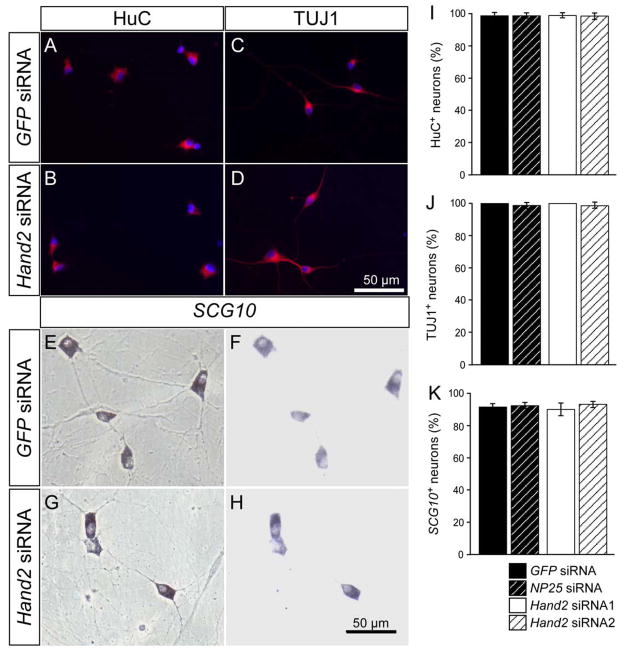
In the conditional mouse Hand2 knockouts and the zebrafish Hand2 mutant, discrepant results were observed with respect to the expression of pan-neuronal genes (Hendershot et al., 2008; Lucas et al., 2006; Morikawa et al., 2007). Thus, it was of considerable interest to investigate to which extent Hand2 controls generic neuronal gene expression in differentiated sympathetic neurons. The expression of three pan-neuronal gene products was analyzed in chick sympathetic ganglion neurons in vitro: HuC and TuJ1 by immunostaining and SCG10 by in situ hybridization (Fig. 6). Interestingly, all neuronal markers were unaffected by the Hand2 knockdown, which demonstrated that the siRNA-mediated reduction of Hand2 expression levels is essential for TH and DBH expression but has no functional relevance for the expression of HuC, TuJ1 and SCG10. To directly demonstrate the correlation between Hand2, noradrenergic and pan-neuronal gene expression, Hand2 siRNA-transfected cells were analyzed for Hand2, TH and TUJ1 (Supplementary Fig. 2A–D). Only a minority (8±1%) of Hand2-negative sympathetic neurons was TH-positive, whereas virtually all expressed TuJ1 (Supplementary Fig. 2E). In contrast, Hand2-positive sympathetic neurons, reflecting incomplete knockdown, displayed both TH and TuJ1 (Supplementary Fig. 2F). Together, these results implicate a selective role of Hand2 in the sustained expression of noradrenergic but not pan-neuronal genes in postmitotic neurons.
Fig. 6.
Effect of Hand2 knockdown on the expression of HuC, TuJ1 and SCG10 in cultured sympathetic neurons. E12 sympathetic neurons were transfected with control GFP siRNA (A,C,E,F) or Hand2 siRNA (B,D,G,H) and analyzed after 2 days in culture for the expression of HuC and TuJ1 by antibody staining (A–D) and for SCG10 expression by in situ hybridization (E–H). In cultures treated with Hand2 siRNA the proportion of HuC-, TuJ1- and SCG10-expressing sympathetic neurons was not significantly different from control transfections using GFP siRNA or NP25 siRNA (I–K). (A–D) Immunofluorescence for HuC and TuJ1 (red) combined with nuclear DAPI-staining (blue). (E, G) phase contrast; (F,H) bright field optics. Data in (I–K) represent mean ± s.e.m. (n=3).
Epistatic relationship of Hand2 and Gata2
Hand2 acts in a network of cross-regulatory transcription factors (Goridis and Rohrer, 2002; Howard, 2005). Previous studies of sympathetic neuron development in the zebrafish Hand2 mutant hands off revealed a progressive loss of Gata2 expression (Lucas et al., 2006), suggesting that Hand2 might maintain Gata2 expression and that the reduced Gata2 levels participate in mediating effects of Hand2 on noradrenergic gene expression. An action of Gata2 downstream of Hand2 was implied from the finding that Gata2 is expressed after Hand2 during chick sympathetic ganglion development (Tsarovina et al., 2004). Whereas in chick sympathetic neurons Gata2 but not Gata3 is expressed, Gata3 is essential for sympathetic neuron differentiation in the mouse (Tsarovina et al., 2004). In the conditional Hand2 knockouts in the mouse, Gata3 was found to be reduced in one mouse line (Hendershot et al., 2008) but not affected in the other (Morikawa et al., 2007). Is Gata2 expression dependent on Hand2 in differentiated chick sympathetic neurons? Hand2 knockdown produced only a very minor reduction of Gata2 expression (Supplementary Fig. 3). In view of the delayed decrease of Gata2 expression in zebrafish, Gata2 levels were analyzed also at a later time point. However, in 4-day-old cultures no significant effects on Gata2 expression were found (Supplementary Fig. 3). As Gata3 responds much less to a reduction in Hand2 expression levels than TH and DBH (Hendershot et al., 2008; Lucas et al., 2006) it seems very unlikely that Hand2 effects on noradrenergic differentiation are mediated through effects on Gata2/3 expression. However, Gata2/3 may be required, at least in part, to mediate other downstream effects of Hand2.
Hand2 and cholinergic differentiation in sympathetic neurons
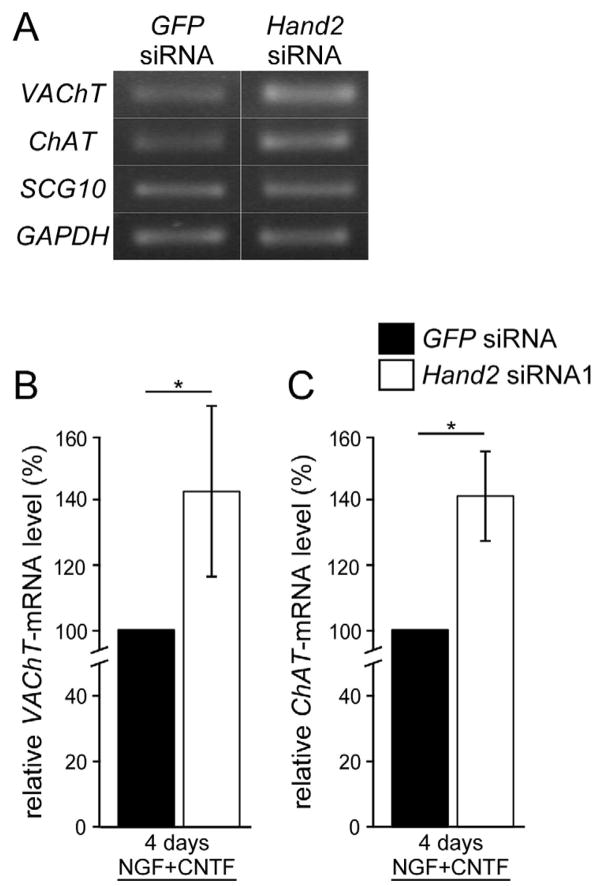
During early development, sympathetic and parasympathetic neurons transiently co-express noradrenergic and cholinergic markers (Burau et al., 2004; Ernsberger et al., 1997; Ernsberger and Rohrer, 1999; Huber and Ernsberger, 2006; Lee et al., 2003; Müller and Rohrer, 2002). Subsequently, ChAT and VAChT expression are lost in most, if not all sympathetic neurons and, vice versa, TH and DBH expression disappears from most parasympathetic ganglia. Interestingly, Hand2 is not expressed in the parasympathetic ciliary ganglion, but ectopic Hand2 expression maintains noradrenergic properties (Müller and Rohrer, 2002). The correlation between Hand2 expression and reduced cholinergic differentiation raised the question whether Hand2 not only induces noradrenergic properties but may also reduce the expression of cholinergic markers in sympathetic (autonomic) neurons. To address this issue, we have investigated the expression of ChAT and VAChT in sympathetic neurons treated with Hand2 siRNA. Semiquantitative RT-PCR analysis of ChAT and VAChT expression in E12 sympathetic neurons, maintained under standard culture conditions, showed unaltered ChAT and VAChT expression upon Hand2 knockdown (not shown). We then investigated whether Hand2 knockdown would enhance the expression of cholinergic marker genes stimulated by CNTF (Ernsberger et al., 2000; Ernsberger et al., 1989b). ChAT and VAChT expression levels increased in response to CNTF to 160±27% and 230±28% of controls without CNTF (not shown). Hand2 knockdown in the presence of CNTF further increased VAChT and ChAT expression (Fig. 7). These results suggest that Hand2 in parallel maintains noradrenergic and antagonizes cholinergic gene expression in differentiated sympathetic neurons. This notion is supported by the finding that Hand2 expression levels in mature, E20 sympathetic ganglia are very low in the subpopulation of VAChT-expressing cholinergic sympathetic neurons as shown by double-in situ hybridisation for Hand2 and VAChT (Supplementary Fig. 4A, B). Double-in situ hybridization for Hand2 and SCG10 was carried out as control, demonstrating similar SCG10 expression levels in Hand2-positive and Hand2-negative sympathetic neurons (Supplementary Fig. 4C, D).
Fig. 7.
Effect of Hand2 knockdown on CNTF-mediated expression of VAChT and ChAT. E12 sympathetic neurons were transfected with control GFP siRNA or Hand2 siRNA1, cultured with NGF plus CNTF and analyzed after 4 days for the expression of VAChT, ChAT, SCG10 and GAPDH by semiquantitative RT-PCR. (A) RT-PCR bands for VAChT, ChAT, SCG10 and GAPDH from cultures transfected with control GFP siRNA or Hand2 siRNA (B,C) Quantitation of the levels of VAChT mRNA (B) and ChAT mRNA (C), normalized to SCG10 mRNA. The knockdown of Hand2 resulted in significantly increased VAChT and ChAT expression, as compared to control siGFP-treated cultures. * P<0,05; (n=3).
Hand2 is required in vivo for neuron proliferation and the maintenance of noradrenergic properties in sympathetic neurons
To investigate the in vivo role of Hand2 in differentiated sympathetic neurons, Hand2 was selectively eliminated in DBH-expressing neurons of Hand2DBHCre mouse embryos. Hand2DBHCre mice were generated by cross-breeding Hand2fl/fl (Hendershot et al., 2008) and DBHCre mice (Matsushita et al., 2004). Although DBH (and DBHCre) is expressed very early, from E10.5 onwards in sympathetic ganglia, Hand2 is eliminated after noradrenergic and generic neuronal gene expression has proceeded (Goridis and Rohrer, 2002; Howard, 2005). This allowed us to investigate the function of Hand2 in the maintenance rather than the initial induction of noradrenergic and generic neuronal gene expression.
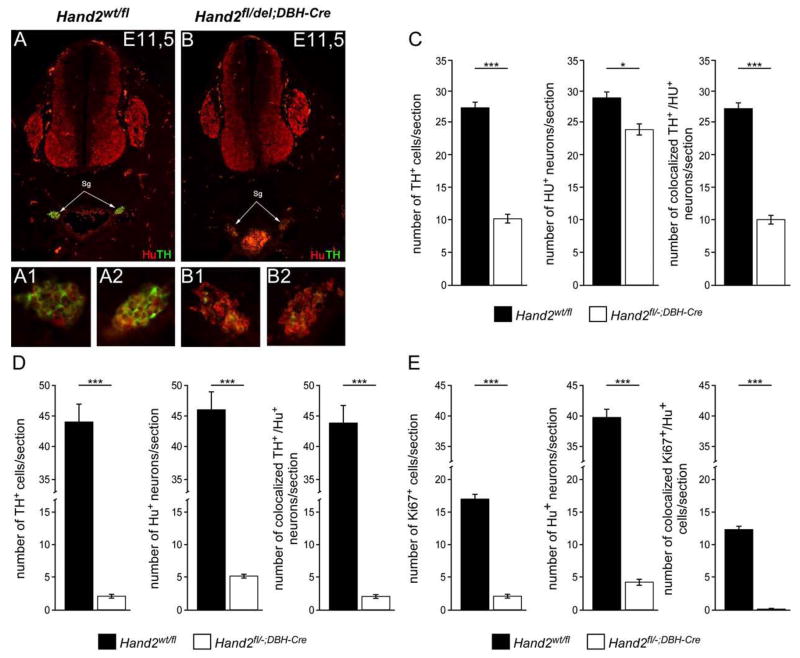
The analysis of sympathetic ganglia in Hand2DBHCre embryos at E10.5 revealed no significant difference in the number of HuC- and TH-positive cells as compared to controls (data not shown). This is in contrast to the reduced number of TH- and HuC-positive cells in Hand2Wnt1Cre embryos (Hendershot et al., 2008) but is expected due to the delayed onset of Cre expression in DBH-expressing cells in primary sympathetic ganglia (Hand2DBHCre), as compared to migrating neural crest cells before the onset of differentiation (Hand2Wnt1Cre). However, at E11.5, TH is strongly reduced, with a smaller effect on Hu expression (Fig. 8A,B). Quantification shows that the number of Hu-positive neurons is lowered in Hand2DBHCre to about 84% of controls and only about 43% of these remaining cells express TH-immunoreactivity (Fig. 8C). At E14.5 the number of Hu-positive neurons is reduced to 12% of controls and only 41% of this residual neuron population express TH (Fig. 8D). Thus, less than half of the sympathetic neurons display TH-immunoreactivity in ganglia where Hand2 has been eliminated in DBH-expressing cells, whereas virtually all Hu-positive sympathetic neurons co-express TH in control ganglia (Fig. 8D). This result confirms the conclusions from our in vitro analysis and demonstrates that TH expression depends on Hand2 not only during initial differentiation but also in DBH-expressing sympathetic neurons.
Fig. 8.
Effect of conditional Hand2 knockout on the number of TH-, Hu- and Ki67-positive cells in sympathetic ganglia of E11.5 and E14 mouse embryos. (A,B) Sections from E11.5 control Hand2wt/fl (A) and Hand2DBHCre (B) mouse embryos double-stained for Hu (red) and TH (green). Sympathetic ganglia (sg) are indicated by arrows and show strongly reduced TH expression in the conditional knockout (B). Enlargements of left (A1) and right (A2) sympathetic ganglia from control and conditional knockout (B1, B2). (C) Number of TH- and Hu-positive neurons/section of E11.5 Hand2wt/fl (black bars) and Hand2DBHCre (white bars) embryos. Double-labeling reveals that all TH-positive cells co-express TuJ1 in both control and Hand2-knockout animals (right graph). The number of TH-positive cells is significantly lower in Hand2DBHCre ganglia as compared to Hand2wt/fl controls (P<0.001) (D) Number of TH- and Hu-positive neurons/section of E14.5 Hand2wt/fl (black bars) and Hand2DBHCre (white bars) embryos. The number of TH-positive cells is significantly lower in Hand2DBHCre ganglia as compared to Hand2wt/fl controls (P<0,001). Double-labeling reveals that all TH-positive cells co-express TuJ1 in both control and Hand2-knockout animals (right graph). (E) Number of Ki67- and Hu-positive cells/section of E14.5 Hand2wt/fl (black bars) and Hand2DBHCre (white bars) embryos. Double-labeling reveals that none of residual Ki67-positive cells co-express Hu in Hand2-knockout animals whereas many Hu-positive neurons are Ki67-positive in control animals (right graph). *** significantly different from control P<0.001. Data shown are the mean ± s.e.m. (n=3).
The strongly reduced sympathetic ganglion size in the Hand2Wnt1Cre conditional knockout is mainly due to reduced proliferation, reflected by a massive decrease in the proportion of Ki67-expressing cells (Hendershot et al., 2008). Also in the Hand2DBHCre conditional knockout the proliferation of immature sympathetic neurons is affected. At E14.5, about 31% of Hu-positive cells express Ki67 in controls, but almost no Hu-positive cells are dividing in sympathetic ganglia of Hand2DBHCreembryos (Fig. 8E).
Discussion
The bHLH transcription factor Hand2 is expressed in developing and mature sympathetic neurons. During specification and early differentiation of sympathetic neurons, Hand2 has been shown to play important roles in the control of the noradrenergic marker genes DBH and TH and in the proliferation of progenitors and immature sympathetic neurons. Here, we provide evidence that Hand2 is also required for the continued expression of DBH and TH in cultured avian postmitotic, differentiated sympathetic neurons, whereas the expression of the generic neuronal marker genes HuC, TuJ1 and SCG10 in these cells does not depend on Hand2. In parallel, Hand2 seems to interfere with cholinergic differentiation of sympathetic neurons, as Hand2 knockdown facilitates the expression of cholinergic marker genes. By eliminating Hand2 in DBH-expressing noradrenergic neurons of Hand2DBHCre mice the important role of Hand2 for the maintenance of TH-expression was confirmed in vivo. This analysis also revealed a continued Hand2 requirement for sympathetic neuron proliferation.
Hand2 in the induction and maintenance of noradrenergic gene expression
In contrast to the transient expression of the proneural gene Ascl1, all other members of the network of transcription factors controlling sympathetic neuron specification and differentiation are also expressed in differentiated sympathetic neurons. Their function in differentiated neurons, in particular whether these functions are similar to their role in early development, is not known. We focus here on Hand2 since we previously had suggested a function for Hand2 in the maintenance of the noradrenergic phenotype of sympathetic neurons (Müller and Rohrer, 2002). This notion was deduced from our finding that Hand2 overexpression in parasympathetic ciliary neurons was able to sustain the normally only transiently expressed TH and DBH (Müller and Rohrer, 2002). The analysis of the Hand2 knockout in zebrafish (Lucas et al., 2006) and the conditional Hand2 knockouts in mouse neural crest cells (Hendershot, 2008; Morikawa, 2007) revealed impaired TH and DBH expression, suggesting a major role for Hand2 in the initiation of noradrenergic differentiation. In addition, proliferation in the sympathetic neuron lineage was virtually completely blocked, which results in ganglia with only few residual neurons (Hendershot et al., 2008). In Hand2Wnt1Cre sympathetic ganglia Hu-positive neurons, most without TH-expression, are still present at E12 (Hendershot et al., 2008; Morikawa et al., 2007) but virtually no neurons are detectable at E18 (Hendershot et al., 2008). The interpretation of effects observed with a considerable delay after the elimination of Hand2 is difficult, due to eventual secondary or compensatory effects. Thus, to address the function of Hand2 in differentiated neurons we analyzed i.) the effect of Hand2 knockdown in short-term cultures of postmitotic, differentiated neurons from E12 chick sympathetic ganglia and ii.) the effect of the conditional Hand2 knockout in noradrenergic neurons of Hand2DBHCremice.
Using a Hand2 siRNA knockdown protocol which strongly reduces Hand2 protein and Hand2 mRNA expression in sympathetic neurons, we observed a selective inhibition of TH and DBH expression, however no effect on SCG10, TuJ1 and HuC. A similar reduction in DBH expression has recently been obtained upon Hand2 knockdown in cultured sympathetic neurons from newborn mouse superior cervical ganglia (Doxakis et al., 2008). Also in vivo, TH expression was detectable only in about 40% of E11.5 and E14.5 mouse sympathetic neurons after eliminating Hand2 in DBH-expressing cells, which supports the concept that noradrenergic marker gene expression in differentiated sympathetic neurons continues to depend on Hand2. The major effect observed in vivo is, however, the strongly reduced neuron number due to lack of proliferation. The effects of excising the Hand2 gene in DBH-expressing sympathetic neurons are very similar to the effects observed previously when the Hand2 gene was eliminated in Wnt1-expressing neural crest cells, using Wnt1Cre (Hendershot, 2008; Morikawa, 2007). This demonstrates that the function of Hand2 is not restricted to the onset of sympathetic neuron differentiation, e.g. the initiation of TH and DBH expression in sympathetic precursors, but continues in DBH-expressing cells. In addition, it is concluded that Hand2 is important for the maintenance of TH expression and continued proliferation of noradrenergic, DBH- and Hu/TuJ1-expressing sympathetic neurons.
Hand2 in the control of sympathetic neuron numbers and pan-neuronal gene expression
Why is the number of Hu-expressing sympathetic neurons affected in the conditional Hand2 knockout in vivo but not in the Hand2 knockdown in vitro? The DBHCre-mediated Hand2 elimination takes place in proliferating immature sympathetic neurons and neural precursor cells, at E10.5 in the mouse, corresponding to E3/4 in the chick, whereas the Hand2 knockdown has been performed in postmitotic, mature E12 chick sympathetic neurons. The reduced proliferation of Hu-positive immature sympathetic neurons in Hand2DBHCre embryos is the major cause for the low number of Hu-positive cells. In contrast, E12 chick sympathetic neurons do not proliferate in vitro and cell numbers are not affected by the in vitro Hand2 knockdown.
The continued expression of TuJ1, SCG10 and HuC in E12 chick sympathetic neurons with reduced Hand2 levels due to siRNA treatment cannot be explained by an insufficient knockdown of Hand2 as strong TuJ1 expression was observed in sympathetic neurons without detectable Hand2 and TH expression. The expression of TuJ1, HuC and peripherin in E11/12 mouse embryos homozygous for neural crest Hand2 deletion (Hendershot et al., 2008; Morikawa et al., 2007), demonstrates that initial generic neuronal differentiation also does not fully depend on Hand2.
The results of the present combination of in vitro and in vivo loss-of-function approaches support a role for Hand2 in the continuation of noradrenergic but not generic neuronal gene expression. It is very likely that other aspects of sympathetic neuron function are also dependent on Hand2 in mature sympathetic neurons. As recently shown, this includes the control of sympathetic neuron survival as Hand2 affects the NGF-dependent expression of the NGF receptor trkA and NGF-dependent survival in cultured mouse sympathetic neurons (Doxakis et al., 2008).
Cholinergic versus noradrenergic differentiation in the autonomic nervous system
During development, noradrenergic and cholinergic marker genes are co-expressed in sympathetic neurons (Burau et al., 2004; Ernsberger et al., 1997; Ernsberger and Rohrer, 1999). The extrinsic signals that initiate ChAT and VAChT expression in embryonic sympathetic neurons are not known but the strongly decreased cholinergic differentiation in the c-ret knockout implicates members of the GFL family in the control of cholinergic differentiation (Burau et al., 2004). In mature mammalian ganglia only a small subpopulation of sympathetic neurons, innervating sweat glands and periosteum, display cholinergic properties that are dependent on cytokines released from the innervated targets (Ernsberger and Rohrer, 1999; Francis and Landis, 1999; Stanke et al., 2006). In the chick, the proportion of cholinergic sympathetic neurons in mature ganglia is considerably larger than in rodents, with vasculature as the major target (Ernsberger and Rohrer, 1999). The generation of distinct subpopulations of cholinergic and noradrenergic neurons, including the downregulation of ChAT and VAChT in noradrenergic neurons, is thought to be regulated by signals from the innervated targets (Ernsberger and Rohrer, 1999; Francis and Landis, 1999).
Transcriptional control of the cholinergic locus is not understood in any detail and may involve different sets of transcription factors in different neuronal lineages. The present finding that Hand2 knockdown facilitates cytokine-induced VAChT expression suggests that Hand2 may participate, directly or indirectly, in the downregulation and subsequent repression of cholinergic marker genes in noradrenergic sympathetic neurons. This notion is also supported by the finding that ChAT-expressing neurons in mature sympathetic ganglia are characterized by low-level expression of Hand2, in contrast to noradrenergic sympathetic neurons. However, in the Hand2Wnt1Cre embryos no significant effects on early cholinergic marker gene expression were observed (Hendershot et al., 2008). This is in agreement with the lack of effect observed upon Hand2 knockdown without cholinergic stimulation by CNTF. A reduction of Hand2 expression seems not to be sufficient to affect cholinergic gene expression but requires cytokine-mediated signaling, whereas Hand2 knockdown is sufficient to reduce TH and DBH expression. In conclusion, the present results indicate a potential dual-function for Hand2, stimulating noradrenergic expression and antagonizing cytokine-mediated cholinergic gene expression.
Conclusion
The function of noradrenergic sympathetic neurons and adrenal chromaffin cells in the adult organism is subject to both short-term and long-term regulation (Kumer and Vrana, 1996; Mallet, 1996). TH, the rate-limiting enzyme in catecholamine biosynthesis represents the major target. Short-term regulation of TH-activity involves phosphorylation of the enzyme by a variety of kinases. Long-term increases in TH activity elicited by electrical stimulation (trans-synaptic induction) or environmental stress are, in contrast, due to increased TH mRNA and protein levels (Kumer and Vrana, 1996; Mallet, 1996). As important regulatory regions in the TH promotor were found to be essential for TH expression in adult but not in developing tissues, it was suggested that distinct mechanisms underlie the establishment and maintenance of the catecholaminergic phenotype (Trocmé et al., 1998). The present results, together with data from the Hand2 knockouts (Hendershot et al., 2008; Morikawa et al., 2007; Lucas et al., 2006), argue against this notion and demonstrate that Hand2 is important both for initial and sustained expression of TH and DBH. It will be of great interest to delineate in more detail the role of Hand2 in the regulation of the mature noradrenergic transmitter phenotype.
Supplementary Material
Acknowledgments
We thank Tyler Hendershot and Ellen Binder for helpful discussions and Konstantina Tsarovina, Leslie Huber and Tobias Reiff for critical comments on the manuscript. The authors have been supported by grants from the DFG (H.R., U.E.), Schram-Stiftung and Wilhelm Sander Stiftung (H.R.) and National Institutes of Health (NIDDK067064, NS040644 (M.H.)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burau K, Stenull I, Huber K, Misawa H, Berse B, Unsicker K, Ernsberger U. C-ret regulates cholinergic properties in mouse sympathetic neurons: evidence from mutant mice. Eur J Neurosci. 2004;20:353–362. doi: 10.1111/j.1460-9568.2004.03500.x. [DOI] [PubMed] [Google Scholar]
- Dahlgren C, Zhang HY, Du Q, Grahn M, Norstedt G, Wahlestedt C, Liang Z. Analysis of siRNA specificity on targets with double-nucleotide mismatches. Nucleic Acids Res. 2008;36:e53. doi: 10.1093/nar/gkn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxakis E, Howard L, Rohrer H, Davies AM. HAND transcription factors are required for neonatal sympathetic neuron survival. EMBO Rep. 2008 doi: 10.1038/embor.2008.161. online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernsberger U, Edgar D, Rohrer H. The survival of early chick sympathetic neurons in vitro is dependent on a suitable substrate but independent of NGF. Dev Biol. 1989a;135:250–262. doi: 10.1016/0012-1606(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Patzke H, Rohrer H. The developmental expression of choline acetyltransferase (ChAT) and the neuropeptide VIP in chick sympathetic neurons: evidence for different regulatory events in cholinergic differentiation. Mech Dev. 1997;68:115–126. doi: 10.1016/s0925-4773(97)00135-4. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Reissmann E, Mason I, Rohrer H. The expression of dopamine β-hydroxylase, tyrosine hydroxylase, and Phox2 transcription factors in sympathetic neurons: evidence for common regulation during noradrenergic induction and diverging regulation later in development. Mech Dev. 2000;92:169–177. doi: 10.1016/s0925-4773(99)00336-6. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Rohrer H. Development of the cholinergic neurotransmitter phenotype in postganglionic sympathetic neurons. Cell Tissue Res. 1999;297:339–361. doi: 10.1007/s004410051363. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Sendtner M, Rohrer H. Proliferation and differentiation of embryonic chick sympathetic neurons:effects of ciliary neurotrophic factor. Neuron. 1989b;2:1275–1284. doi: 10.1016/0896-6273(89)90312-7. [DOI] [PubMed] [Google Scholar]
- Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Ann Rev Neurosci. 1999;22:541–566. doi: 10.1146/annurev.neuro.22.1.541. [DOI] [PubMed] [Google Scholar]
- Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci. 2002;3:531–541. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- Hendershot TJ, Liu H, Clouthier DE, Shepherd IT, Coppola E, Studer M, Firulli AB, Pittman DL, Howard MJ. Conditional deletion of Hand2 reveals critical functions in neurogenesis and cell type-specific gene expression for development of neural crest-derived noradrenergic sympathetic ganglion neurons. Dev Biol. 2008;319:179–91. doi: 10.1016/j.ydbio.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot TJ, Liu H, Clouthier DE, Shepherd IT, Coppola E, Studer M, Firulli AB, Pittman DL, Howard MJ. Expression of Hand2 is sufficient for neurogenesis and cell type-specific gene expression in the enteric nervous system. Dev Dyn. 2007;236:93–105. doi: 10.1002/dvdy.20989. [DOI] [PubMed] [Google Scholar]
- Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol. 2005;277:271–286. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Huber K, Ernsberger U. Cholinergic differentiation occurs early in mouse sympathetic neurons and requires Phox2b. Gene Expressions. 2006;13:133–139. doi: 10.3727/000000006783991854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–62. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Lee VM, Sechrist JW, Luetolf S, Bronner-Fraser M. Both neural crest and placode contribute to the ciliary ganglion and oculomotor nerve. Dev Biol. 2003;263:176–190. doi: 10.1016/j.ydbio.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Lim K-C, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nature Genetics. 2000;25:209–212. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- Lucas ME, Müller F, Rüdiger R, Henion PD, Rohrer H. The bHLH transcription factor hand2 is essential for noradrenergic differetiation of sympathetic neurons. Development. 2006;133:4015–4024. doi: 10.1242/dev.02574. [DOI] [PubMed] [Google Scholar]
- Mallet J. The TiPS/TINS lecture catecholamines: From gene regulation to neuropsychiatric disorders. Trends Neurosci. 1996;19:191–196. doi: 10.1016/s0166-2236(96)10029-1. [DOI] [PubMed] [Google Scholar]
- Matsushita N, Kobayashi K, Miyazaki J. Fate of transient catecholaminergic cell types revealed by site-specific recombination in transgenic mice. J Neurosci Res. 2004;78:7–15. doi: 10.1002/jnr.20229. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, D’Autreaux F, Gershon MD, Cserjesi P. Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol. 2007;307:114–26. doi: 10.1016/j.ydbio.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Rohrer H. Molecular control of ciliary neuron development: BMPs and downstream transcriptional control in the parasympathetic lineage. Development. 2002;129:5707–5717. doi: 10.1242/dev.00165. [DOI] [PubMed] [Google Scholar]
- Pape M, Doxakis E, Reiff T, Duong CV, Davies A, Geissen M, Rohrer H. A function for the calponin family member NP25 in neurite outgrowth. Dev Biol. 2008;321:434–443. doi: 10.1016/j.ydbio.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Guillemot F, Brunet J-F. Delays in neuronal differentiation in Mash1/Ascl1 mutants. Dev Biol. 2006;295:67–75. doi: 10.1016/j.ydbio.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet J-F. The homeobox gene Phox2b is essential for the development of all autonomic derivatives of the neural crest. Nature. 1999;399:366–370. doi: 10.1038/20700. [DOI] [PubMed] [Google Scholar]
- Rohrer H. The role of bone morphogenetic proteins in sympathetic neuron development. Drug News Perspect. 2003;16:589–596. doi: 10.1358/dnp.2003.16.9.829341. [DOI] [PubMed] [Google Scholar]
- Rohrer H, Acheson AL, Thibault J, Thoenen H. Developmental potential of quail dorsal root ganglion cells analyzed in vitro and in vivo. J Neurosci. 1986;6:2616–2624. doi: 10.1523/JNEUROSCI.06-09-02616.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik JL, Gerbasi V, Lewis EJ. The interaction between dHand and Arix at the dopamine b-hydroxylase promotor region is independent of direct dHand binding to DNA. J Biol Chem. 2003;278:49652–49660. doi: 10.1074/jbc.M308577200. [DOI] [PubMed] [Google Scholar]
- Stanke M, Duong CV, Pape M, Geissen M, Burbach G, Deller T, Gascan H, Otto C, Parlato R, Schütz G, Rohrer H. Target-dependent specification of the neurotransmitter phenotype: cholinergic differentiation of sympathetic neurons is mediated in vivo by gp130 signaling. Development. 2006;133:141–150. doi: 10.1242/dev.02189. [DOI] [PubMed] [Google Scholar]
- Stanke M, Junghans D, Geissen M, Goridis C, Ernsberger U, Rohrer H. The Phox2 homeodomain proteins are sufficient to promote the development of sympathetic neurons. Development. 1999;126:4087–4094. doi: 10.1242/dev.126.18.4087. [DOI] [PubMed] [Google Scholar]
- Trocmé C, Sarkis C, Hermel JM, Duchateau R, Harrison S, Simonneau M, Al-Shawi R, Mallet J. CRE and TRE sequences of the rat tyrosine hydroxylase promoter are required for TH basal expression in adult mice but not in the embryo. Eur J Neurosci. 1998;10:508–521. doi: 10.1046/j.1460-9568.1998.00059.x. [DOI] [PubMed] [Google Scholar]
- Tsarovina K, Pattyn A, Stubbusch J, Müller F, Van der Wees J, Schneider C, Brunet J-F, Rohrer H. Essential role of Gata transcription factors in sympathetic neuron development. Development. 2004;131:4775–4786. doi: 10.1242/dev.01370. [DOI] [PubMed] [Google Scholar]
- Wildner H, Gierl MS, Strehle M, Pla P, Birchmeier C. Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development. 2008;135:473–81. doi: 10.1242/dev.011783. [DOI] [PubMed] [Google Scholar]
- Xu HM, Firulli AB, Zhang XTHoward MJ. HAND2 synergistically enhances transcription of dopamine-β-hydroxylase in the presence of Phox2a. Dev Biol. 2003;262:183–193. doi: 10.1016/s0012-1606(03)00361-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.