Abstract
Purpose
To perform an immunohistochemical evaluation of corneas with INTACS for post–laser in situ keratomileusis (LASIK) keratectasia and keratoconus, obtained after corneal transplantation.
Methods
Corneas from 1 patient with INTACS for post-LASIK keratectasia and 2 patients with INTACS for keratoconus were obtained within 3 hours after penetrating keratoplasty, and cryostat sections were analyzed by immunostaining for 35 extracellular matrix (ECM) components and proteinases.
Results
In the stroma of all corneas next to an INTACS implant, ECM components typically associated with fibrosis were observed. These included tenascin-C, fibrillin-1, and types III, IV (α1/α2 chains), and XIV collagen. Also, significant deposition of perlecan, nidogen-2, and cellular fibronectin was revealed in the same locations. The keratoconus cases displayed typical Bowman layer breaks and subepithelial fibrosis with deposition of various ECM components. In all cases, some keratocytes around INTACS were positive for specific proteinases associated with stromal remodeling, including cathepsins F and H, matrix metalloproteinase (MMP)-1, MMP-3, and MMP-10. Staining for MMP-7 was variable; MMP-2 and MMP-9 were mostly negative. Patterns of type IV collagen α3, α4, and α6 chains; types VI and VIII collagen; laminin-332,α4, α5,β1, β2, and γ1 laminin chains; vitronectin; thrombospondin-1; urokinase; EMMPRIN; and cathepsins B and L were unchanged around INTACS in all 3 cases compared with normal.
Conclusions
Abnormal accumulation of fibrotic ECM components and proteinases near INTACS suggests ongoing lysis and remodeling of corneal stroma. Specific changes observed in each case may be related to underlying pathology.
Keywords: INTACS, keratoconus, laser in situ keratomileusis, cornea, extracellular matrix, fibrosis, matrix metalloproteinase, tenascin-C, cathepsin, nidogen
INTACS involves the insertion of 2 semicircular plastic segments into the corneal stroma, thereby creating central flattening and correcting small amounts of myopia.1,2 The parameters influencing the final surgery outcome are the diameter and thickness of these intrastromal corneal segments. Intrastromal corneal rings were approved by the US Food and Drug Administration in 1999 for the correction of a low degree of myopia.3,4 Partly because of the popularity of laser in situ keratomileusis (LASIK), which was approved at about the same time, INTACS technology did not gain widespread use.
Recently, INTACS has acquired new interest as an alternative treatment of keratoconus.5–10 The rationale is that INTACS not only flattens the central cornea but also corrects some of the astigmatic component. The corneal profile becomes more symmetrical, usually allowing better correction with glasses and contact lenses. Given that, for these patients, the alternative would be penetrating keratoplasty, INTACS may delay the need for this option.10,11
Midstromal semicircular channels for INTACS ring insertion are created by a mechanical system with a blunt spiral device.1,12,13 Despite the streamlined mechanistic procedure, attempts were made to improve control over the depth and shape of the channels. A femtosecond laser mounted on a delivery system capable of creating an intrastromal pattern of virtually any shape was recently used to create channels for INTACS insertion.10 This method may be technically easier and more precise, reflecting better final outcomes.
Keratectasia is a rare but serious complication of laser vision correction of any refractive errors. It occurs mostly after LASIK but was also described after surface excimer laser ablation (photorefractive keratectomy).14–16 It involves severe refractive changes mostly occurring several years after the surgery, which are caused by thinning and sagging of the corneal profile, similar to keratoconus.
The etiology of this complication remains uncertain. In some cases, preexisting forme fruste keratoconus was documented.17,18 Other cases could be caused by a mechanical collapse of the corneal structure because of thinning induced by surgery.18 Extracellular matrix (ECM) changes and increased matrix metalloproteinases (MMPs)19 may also be contributing factors. The affected patients can for the most part be fitted with gas-permeable contact lenses, but some do need corneal transplantation to resume functional vision.
Because of similarities between keratoconus and keratectasia, INTACS was tried in post-LASIK patients with some degree of success.13,15,16 In rare cases, INTACS implantation could not resolve ectatic changes in keratoconus and after LASIK surgery. It is unclear what factors contribute to progressing ectasia after INTACS and what reaction INTACS implantation elicits in the corneal stroma at the molecular level. We describe here 3 cases of unsuccessful INTACS surgery for keratoconus and post-LASIK keratectasia and provide data on ECM and proteinase changes around INTACS in these corneas.
MATERIALS AND METHODS
Case 1 With Keratoconus
A 36-year-old white woman diagnosed with bilateral keratoconus in her early 20s was followed up by her referring physician and fitted with gas-permeable lenses. She sustained several corneal abrasions and developed grade 3 giant papillary conjunctivitis (GPC). In an attempt to facilitate contact lens wear, she underwent INTACS implantation OD in December 2004. The patient remained contact lens intolerant. On May 20, 2005, she was seen in initial consultation. Best-corrected visual acuity (BCVA) was OD = 20/100 with soft contact lens and OS = 20/30 with gas-permeable lens. Central pachymetry was OD = 0.510 mm and OS = 0.499 mm. Topography of both corneas is shown in Figure 1. Slit-lamp examination of the right eye showed grade 3 giant papillae on tarsal conjunctiva. The cornea showed apical thinning and scarring with significant epithelial irregularity. Two INTACS were noted OD with a temporal entry incision and superior/inferior placement. Nasal INTACS ends were touching. The outer diameter of the channel was slightly <8 mm. The patient underwent penetrating keratoplasty on June 21, 2005. The GPC was controlled with a supratarsal injection of 10 mg/mL Kenalog (triamcinolone acetonide, Bristol-Myers Squibb, New York, NY). The postoperative course was uneventful except for conservatively managed steroid-induced glaucoma. Her last BCVA with a spherical soft contact lens was 20/20.
FIGURE 1.
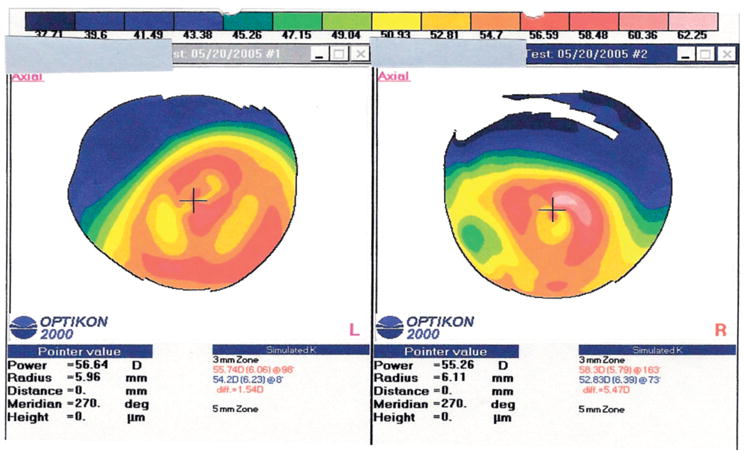
Case 1 with keratoconus (topographies after INTACS). Corneal topography of both eyes before corneal transplantation. There is bilateral severe inferior corneal steepening in both eyes with surface irregularity in the steep areas. The INTACS (in the right eye, R) did not seem to affect topography in any significant way, because both eyes’ topographic patterns are similar.
Case 2 With Keratoconus
A 30-year-old black woman was diagnosed with bilateral keratoconus 15 years ago. There was no known family history of keratoconus. The patient wore gas-permeable lenses for 14 years, at which point she became contact lens intolerant in the left eye. She underwent INTACS implantation surgery in both eyes 6 months before being seen in initial consultation. On initial examination, BCVA was OD = 20/40 and OS = 20/200. INTACS were present in both eyes with their edges at the 6 and 12 o’clock hours. The right nasal ring segment migrated, and its inferior edge was overlapping the inferior edge of the temporal ring segment. The left corneal apex was thinned and showed apical scarring. Central pachymetry was OD = 0.577 mm and OS = 0.340 mm. Corneal topography (Fig. 2) showed an asymmetric and skewed bowtie with inferior steepening in the right eye and significant inferonasal steepening in the left, which involved the visual axis. The patient underwent penetrating keratoplasty in the left eye with INTACS removal. The surgery was uneventful. Uncorrected visual acuity (UCVA) 1 month postoperatively was 20/60.
FIGURE 2.
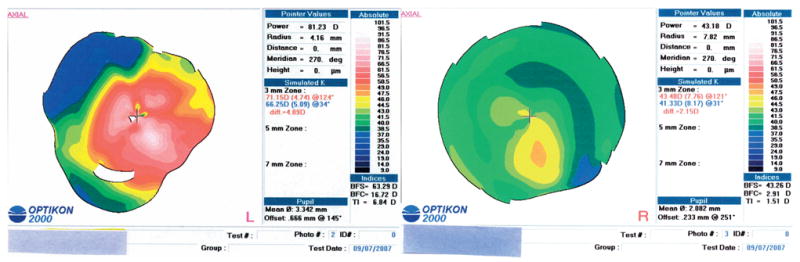
Case 2 with keratoconus (topographies after INTACS). Corneal topography of both eyes before corneal transplantation. Left eye (L), severe inferonasal steepening involving the visual axis. There is an irregular asymmetric bowtie centered on the visual axis; right eye (R), inferior steepening with asymmetric and skewed bowtie. There is a curved area of flattening in the nasal midcornea corresponding to the nasal INTACS.
Case 3 Post-LASIK
A 53-year-old white man, healthy with no known family history of keratoconus, underwent bilateral LASIK on February 26, 1999. Preoperative refraction was OD = −3.00 + 3.25 × 172 = 20/50 and OS = −2.50 + 1.25 × 173 = 20/25. Central pachymetry was OD = 0.501 mm and OS = 0.518 mm. Topography OD is shown in Figure 3A. Over the next several months, the left eye remained stable with UCVA of 20/30. The right eye UCVA became worse with progressing inferior steepening (Fig. 3B) and was 20/400 in 2002, at which point an attempt was made to fit the eye with a gas-permeable lens. Even though BCVA was 20/30, the patient could not tolerate lens wear. In July 2002, he underwent INTACS implantation. On initial consultation, BCVA OD was 20/200. Central pachymetry was OD = 0.410 mm and OS = 0.510 mm. Topography OD is shown in Figure 3C. Slit-lamp examination showed thinning and inferior steepening OD > OS. Two INTACS were noted OD with a temporal entry incision and superior/inferior placement. The nasal ends of the INTACS were touching. The outer diameter of the channel was slightly <8 mm. The patient underwent penetrating keratoplasty on April 26, 2005. INTACS segments could not be removed and were included in the trephination of the recipient. The postoperative course was uneventful. The most recent BCVA was 20/30 with a well-tolerated gas-permeable contact lens.
FIGURE 3.
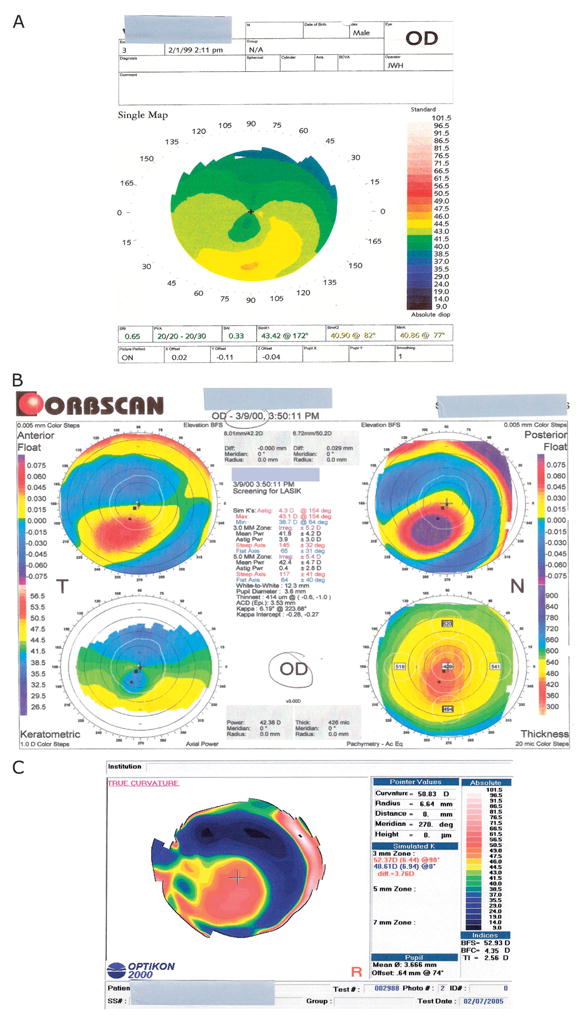
Case after LASIK keratectasia topographies. A, Corneal topography of the right eye before LASIK. A typical crab claw pattern is visible, suggesting the presence of pellucid marginal degeneration preoperatively. B, Corneal topography of the right eye after LASIK. Orbscan shows abnormal anterior and posterior floats, and the keratometric tracing shows again a pattern consistent with pellucid marginal degeneration. C, Corneal topography of the right eye after INTACS and before corneal transplantation. It shows an area of inferior steepening involving the optical center and the lack of the crab claw pattern previously observed.
All diseased corneas were obtained under Institutional Review Board protocol 3201 as of August 1, 2001. None of the patients had a history of diabetes.
Controls
Two postmortem corneas were from a 56-year-old white man who underwent uneventful LASIK on both eyes in 2002. He died in February 2005 from a ruptured aortic dissection. There was a history of heart valve replacement and schizophrenia, and no diabetes was noted. One postmortem cornea was from a 57-year-old white man who underwent uneventful LASIK before 1999. He died in March 1999 from end-stage heart disease. He had a history of type I diabetes. Postmortem corneas were obtained 24 hours after death.
Control postmortem corneas were purchased from the National Disease Research Interchange (Philadelphia, PA).
Immunohistochemistry
Freshly excised patient corneas and control ones were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek USA, Torrance, CA), snap-frozen in liquid nitrogen, and cryosectioned. Indirect immunofluorescent staining of cryostat sections was performed as described.20 Routine specificity controls without primary or secondary antibodies were negative. Well-characterized antibodies20–24 were used for α1/α2, α3, α4, and α6 chains of type IV collagen; α2, α4, α5, β1, β2, and γ1 chains of laminin; laminin-332; fibronectin 8th type III repeat and cellular (ED-A repeat containing) fibronectin; nidogen-1; nidogen-2; types III, VI, VIII, and XIV collagen; perlecan; tenascin-C; vitronectin; thrombospondin-1; fibrillin-1; α–smooth-muscle actin; matrix metalloproteinases (MMP)-1, -2, -3, -7, -9, and -10; urokinase; and cathepsins B, F, H, and L. Monoclonal antibody III-53 to type III collagen was from EMD Biosciences/Calbiochem (La Jolla, CA), monoclonal antibody HIM6 to EMMPRIN/CD147/neurothelin was from BD Biosciences Pharmingen (San Jose, CA), and polyclonal antibody to MMP-10 hinge region was from AnaSpec (San Jose, CA).
RESULTS
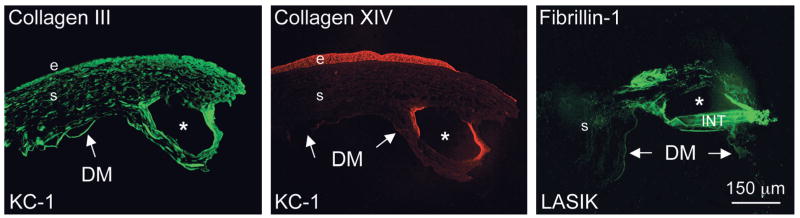
Three corneas were analyzed that developed ectasia after LASIK surgery or keratoconus and subsequent INTACS placement. Immunohistochemical analyses were performed on these corneas in comparison with corneas from patients after uneventful LASIK. The focus was on 2 classes of corneal molecules that are important participants in wound healing including cases of refractive surgery, that is, ECM components and proteinases.19,25–27 Particular attention was paid to the area around the INTACS channel (Table 1). Figure 4 shows low magnification of diseased corneas stained for some ECM markers. One can see stromal INTACS channels of ~200 μm in diameter surrounded by deposits of ECM molecules including types III and XIV collagen and fibrillin-1. In 1 case (post-LASIK), remnants of INTACS are seen in the channel.
TABLE 1.
Occurrence of Studied Components Around INTACS Channels
| Marker | Post-LASIK Case | Keratoconus Case 1 | Keratoconus Case 2 |
|---|---|---|---|
| Accumulated | |||
| Tenascin-C | ++ | ++ | ++ |
| Fibrillin-1 | ++ | ++ | ++ |
| Type III collagen | + | + | + |
| Type IV collagen α1–α2 | + | + | ++ |
| Type XIV collagen | ++ | ++ | ++ |
| Fibronectin total | ++ | ++ | ++ |
| Fibronectin cellular | ++ | ++ | ++ |
| Perlecan | + | ++ | + |
| Nidogen-2 | ++ | ++ | ++ |
| Nidogen-1 | + | + | +/− |
| Laminin α2 | − | + | +/− |
| MMP-1 | + | + | + |
| MMP-3 | ± | ± | ± |
| MMP-7 | ± | ± | ± |
| MMP-10 | + | + | + |
| Cathepsin F | + | + | + |
| Cathepsin H | + | ± | ± |
| Not accumulated | |||
| Type IV collagen α3 | − | − | − |
| Type IV collagen α4 | − | − | − |
| Type IV collagen α6 | − | − | − |
| Type VI collagen α3 | − | − | − |
| Type VIII collagen α3 | − | − | − |
| Laminin-332 (α3β3γ2) | − | − | − |
| Laminin α4 | − | − | − |
| Laminin α5 | − | − | − |
| Laminin β1 | − | − | − |
| Laminin β2 | − | − | − |
| Laminin γ1 | − | − | − |
| Thrombospondin-1 | − | − | − |
| Vitronectin | − | − | − |
| MMP-2 | − | − | − |
| MMP-9 | − | − | − |
| EMMPRIN/CD147 | − | − | − |
| Urokinase | − | − | − |
| Cathepsin B | − | − | − |
| Cathepsin L | − | − | − |
| α–Smooth-muscle actin | ± | ± | − |
Staining was arbitrarily assessed as strong (++), distinct (+), weak (+/−), and negative (−). Positive staining of occasional cells is marked as ±.
FIGURE 4.
Low magnification of diseased corneas stained for some ECM components. INTACS channels of ~200 μm in diameter have irregular edges and display deposits of studied ECM proteins around them (collagens III and XIV and fibrillin-1); distant corneal stroma is not compromised. In case of post-LASIK, INTACS remnant (INT) is seen in the channel. E, epithelium; s, stroma; arrows point to the Descemet membrane (DM); KC-1, case 1 with keratoconus. INTACS channels are marked by asterisks. Bar = 150 μm.
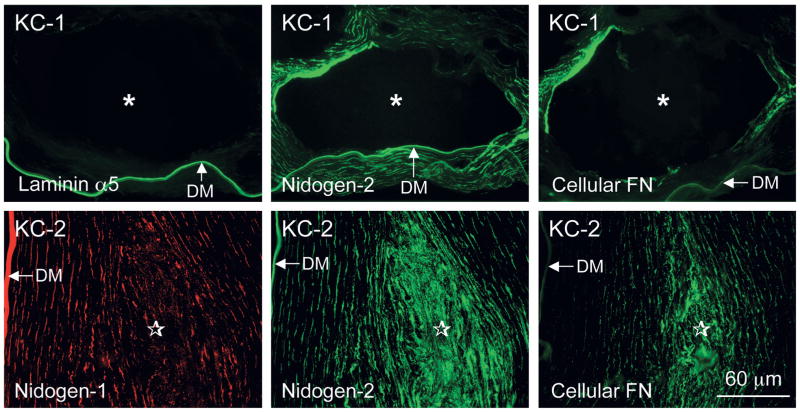
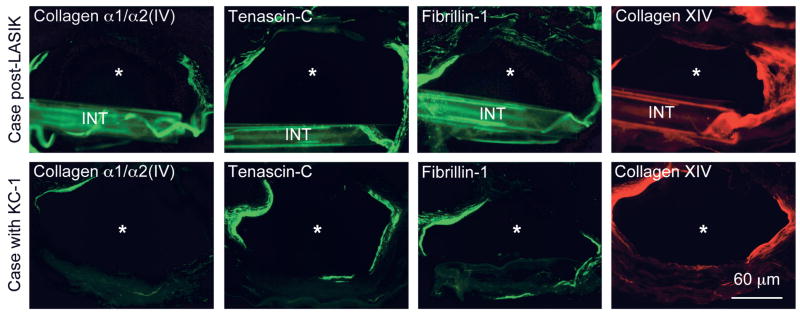
Unlike control corneas after uneventful LASIK surgery, all INTACS corneas displayed significant stromal deposition of known fibrotic ECM components (Table 1) found in keratoconus, bullous keratopathy, or after radial keratotomy.21,28,29 Deposits were observed both along and at the ends of the INTACS channels (Figs. 4–6). These components included tenascin-C; fibrillin-1; and types III, IV (α1/α2 chains), and XIV collagen and were concentrated in the corneal stroma around the INTACS channels (Figs. 4 and 5). In the same locations, stromal deposits of basement membrane (BM) components, such as nidogen-1, nidogen-2 (much stronger than −1), perlecan, and cellular fibronectin, were also seen (Fig. 6). Control corneas had only moderate staining of the whole LASIK flap margin for cellular fibronectin.30,31 Some other BM components, such as α4 and α5 laminin chains (Fig. 6), thrombospondin-1, and types IV (α3-α4 and α6 chains) and VIII collagen, were seen only in normal locations in the INTACS corneas, that is, in the epithelial BM and/or in the Descemet membrane. In the keratoconus corneas, there were also weak to moderate amounts of laminin α2 chain (most probably part of laminin-211) around INTACS channels (data not shown). Also, keratoconus corneas had small areas of Bowman layer breaks and subepithelial fibrosis that were positive for most markers mentioned above. α–Smooth-muscle actin–positive myofibroblasts typical for actively remodeling tissues and healing wounds were only occasionally seen in all cases and were associated mostly with subepithelial fibrosis (data not shown).
FIGURE 6.
Basement membrane components around and at the edge of an INTACS channel. Top row, Case 1 with keratoconus (KC-1). Laminin α5 chain (component of laminin-511) only highlights the Descemet membrane (bottom). Cellular fibronectin (FN; ED-A repeat-containing) and nidogen-2 (but not as much nidogen-1; data not shown) are also prominent in the corneal stroma around the INTACS channel marked by an asterisk. DM, Descemet membrane. Bottom row, Case 2 with keratoconus (KC-2). No channel is visible on these sections because they show the area next to the INTACS insert tip (stars). Double-labeled section is shown at the left and middle, showing much stronger stromal accumulation of nidogen-2 than of nidogen-1. The Descemet membrane (DM) and keratocytes are positive for both nidogens, as in normal corneas. Cellular fibronectin (FN, right) is also accumulated next to the INTACS tip, similar to KC-1. Bar = 60 μm.
FIGURE 5.
Fibrotic ECM components around the INTACS channels. Note significant accumulation of α1/α2 type IV collagen, tenascin-C, fibrillin-1, and type XIV collagen around INTACS channels in both cases. None of these components can be detected in normal corneal stroma.21 Samples in the top row had a remnant of INTACS (INT). INTACS channels are marked by asterisks; KC-1, case 1 with keratoconus. Bar = 60 μm.
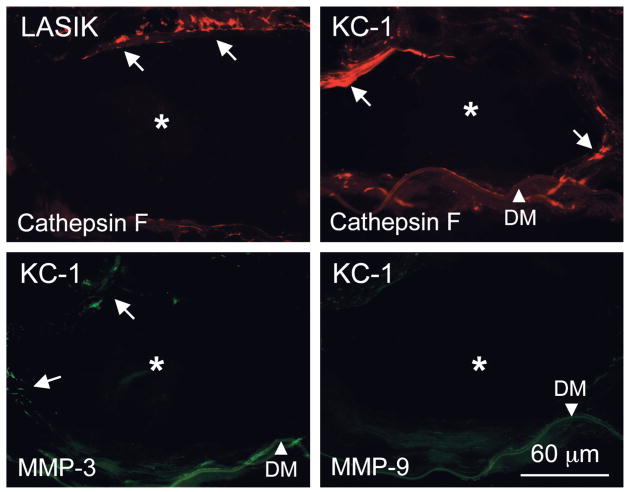
All experimental corneas displayed positive immunoreactivity for specific proteinases near INTACS channels (Table 1). Keratocytes around these channels distinctly stained in all cases for cathepsin F (Fig. 7) and, somewhat less regularly, for cathepsin H (data not shown). Certain keratocytes close to INTACS channels were positive in all cases for MMP-1 and stromelysins [MMP-3 (Fig. 7) and MMP-10]. Staining for MMP-7 was seen in rare cells and that for MMP-2 and MMP-9 (Fig. 7) was weak to negligible. Immunostaining for urokinase and cathepsins B and L was virtually negative (data not shown). A known MMP inducer, EMMPRIN/CD147/-neurothelin, was negative around INTACS areas (data not shown). Control corneas after uneventful LASIK showed only weak and irregular staining for cathepsin F at the flap edge30; other proteinases were largely negative (data not shown).
FIGURE 7.
Proteinase expression in stromal cells around the INTACS channel. Cathepsin F appears in both shown cases (arrows, top row). Staining for cathepsins B and L was largely negative (data not shown). Occasional cells around INTACS area are positive for MMP-3 (arrows, bottom left) in case 1 with keratoconus (KC-1). At the same time, no MMP-9 expression is observed (bottom right). INTACS channel is marked by an asterisk; arrowhead, Descemet membrane (DM). Bar = 60 μm.
DISCUSSION
INTACS have been used for many years with reasonable success to correct myopia and keratectasia in keratoconus and after LASIK surgery.1–10,12,15,32,33 The procedure is generally safe, with only rare cases of corneal infections.34 Morphologic studies have shown local stromal reaction with keratocyte activation, lipid accumulation, moderate fibrosis, mild epithelial changes, and formation of new collagen and hypocellular scars.16,35–37
In this study, we analyzed the molecular composition of the corneal stromal reactive tissue around the INTACS used in 3 cases of keratectasia caused by keratoconus or LASIK surgery. Similar data were obtained in all cases for most markers. The main differences in the expression of ECM proteins and proteinases were seen outside the INTACS area and were more pronounced in keratoconus, in agreement with our previous results.29
Despite mild histopathologic changes around INTACS channels,16,37 significant accumulation of several fibrotic ECM markers was observed in these areas. Similar deposits were detected in radial keratotomy scars,21 in cases of failed LASIK,19,30 and in scarred areas of keratoconus corneas.29 They may be related to a wound healing process that may be incomplete even years after surgically induced stromal injury,21 despite the attempts of corneal cells to remove abnormal ECM deposits by secreted proteinases.
Fibrotic stromal deposits can contain certain BM components as well.19,21,29 In the studied corneas, some BM components but not others were indeed detected around INTACS channels. An interesting example concerns nidogens. They are laminin-binding proteins, important for joining together laminin and type IV collagen networks and for the proper assembly of various BMs.38 Two nidogens are similar in structure and have a largely overlapping repertoire of protein interactions and, possibly, functions.24,38 In these cases, mostly nidogen-2 was found around INTACS channels; nidogen-1 showed significantly weaker staining (Fig. 6, case KC-2). The mechanisms of differential expression of BM proteins in fibrotic areas in the cornea remain to be established. They may relate to specific phenotypic properties of activated keratocytes and/or myofibroblasts that are thought to be the cellular sources of stromal fibrosis.25,27,39
Fibrosis often occurs as a result of abnormal ECM remodeling during wound healing and is thought to be dependent on specific cytokines, such as transforming growth factor-β.27 ECM deposits accumulated during wound healing are often removed over time by specific proteinases expressed in the affected areas. Indeed, we found cells overexpressing MMP-3, MMP-10, and cathepsins F and H around INTACS channels; however, little expression of gelatinases, MMP-2 and MMP-9, was observed. MMP-3 and MMP-10 (stromelysin-1 and -2, respectively), as well as MMP-7, which can degrade ECM and BM components, have been implicated in corneal wound healing and stromal remodeling.23,40,41 Although cathepsins are largely considered lysosomal proteinases, some of them may be secreted (cathepsins B, H, K) or have extracellular localization, such as cathepsin V/L2.42–45 Cathepsins B, G, and F have been implicated in fibrosis at Bowman layer breaks of keratoconus corneas and in corneal wound healing.23,46,47 These data suggest the occurrence of ongoing proteolysis around the INTACS channels, possibly as an attempt of corneal stromal cells to remodel the stroma and remove iatrogenic accumulations of fibrotic ECM. Future studies of this process may focus on cytokines that control ECM accumulation and proteinase activation.27,48
Fibrosis resorption by extracellular proteolysis may take several months to several years, as in the presented cases. At the same time, this process does not seem to require myofibroblasts around INTACS, which is also typical for old radial keratotomy and LASIK scars. Apparently, myofibroblasts are necessary at the early stages of fibrotic scar formation; at later stages and in chronic scars, active stromal remodeling may be continued by activated keratocytes in the fibrosed scar area. In line with virtual absence of myofibroblasts, the MMP inducer, EMMPRIN, which associates with these cells,49 was not found around INTACS channels in any of the studied cases. Our results also suggest that stromal fibrosis and accompanying proteolytic remodeling around INTACS is a local process that may not contribute to cornea-wide disease. However, this stromal wound healing response seems to be slow, and the corneal stroma remains abnormal for some time. In this respect, INTACS implants elicit a stronger stromal response than LASIK surgery.30,31
In summary, fibrotic deposits of abnormal ECM components were found around the INTACS implants in 3 corneas with keratoconus and keratectasia. These components included tenascin-C; fibrillin-1; and types III, IV (α1/α2), and XIV collagen that are absent from normal corneas but appear during the wound healing process. Some BM proteins were also accumulated around the implants. Increased expression of specific proteinases around INTACS implants, notably of stromelysins and some cathepsins, may result from the attempt of keratocytes to locally remodel the stroma and remove fibrotic deposits.
Acknowledgments
Supported by the Skirball Program in Molecular Ophthalmology at Cedars-Sinai Medical Center, NIH RR00425 and Winnick Family Foundation, and the Discovery Eye Foundation.
The authors thank Drs. Eva Engvall (The Burnham Institute, La Jolla, CA), Ralph J. Butkowski (Augsburg College, Minneapolis, MN), Yoshikazu Sado (Shigei Medical Research Institute, Okayama, Japan), and Robert E. Burgeson (CBRC, Massachusetts General Hospital East, Charlestown, MA) for the generous gift of antibodies, and Dr. Eric E. Gabison (Department of Ophthalmology, Fondation Ophtalmologique A. de Rothschild and Bichat Hospital, APHP, Paris, France) for helpful discussions about EMMPRIN expression in wound healing. Antibodies to laminin β2 chain produced by Dr. Joshua R. Sanes and to type IV collagen α1/α2 chains produced by Dr. Heinz Furthmayr were obtained from the Developmental Studies Hybridoma Bank (DSHB), Department of Biology, University of Iowa (Iowa City, IA), under contract N01-HD-2-3144 from the NICHD.
Footnotes
Presented at the Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO), April 29 to May 4, 2006, Fort Lauderdale, FL.
References
- 1.Linebarger EJ, Song D, Ruckhofer J, et al. Intacs: the intrastromal corneal ring. Int Ophthalmol Clin. 2000;40:199–208. doi: 10.1097/00004397-200007000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Ruckhofer J. Klinische und histologische studien zu den intrastromalen cornealen Ringsegmenten (ICRS®, Intacs®) Klin Monatsbl Augenheilkd. 2002;219:557–574. doi: 10.1055/s-2002-34421. [DOI] [PubMed] [Google Scholar]
- 3.Rapuano CJ, Sugar A, Koch DD, et al. Intrastromal corneal ring segments for low myopia. A report by the American Academy of Ophthalmology. Ophthalmology. 2001;108:1922–1928. doi: 10.1016/s0161-6420(01)00804-1. [DOI] [PubMed] [Google Scholar]
- 4.Tham VM, Hwang DG. The intrastromal corneal ring segments. Intacs. Ophthalmol Clin North Am. 2001;14:295–299. [PubMed] [Google Scholar]
- 5.Colin J, Cochener B, Savary G, et al. Correcting keratoconus with intracorneal rings. J Cataract Refract Surg. 2000;26:1117–1122. doi: 10.1016/s0886-3350(00)00451-x. [DOI] [PubMed] [Google Scholar]
- 6.Siganos CS, Kymionis GD, Kartakis N, et al. Management of keratoconus with Intacs. Am J Ophthalmol. 2003;135:64–70. doi: 10.1016/s0002-9394(02)01824-x. [DOI] [PubMed] [Google Scholar]
- 7.Levinger S, Pokroy R. Keratoconus managed with Intacs: one-year results. Arch Ophthalmol. 2005;123:1308–1314. doi: 10.1001/archopht.123.10.1308. [DOI] [PubMed] [Google Scholar]
- 8.Kanellopoulos AJ, Pe LH, Perry HD, et al. Modified intracorneal ring segment implantations (INTACS) for the management of moderate to advanced keratoconus: efficacy and complications. Cornea. 2006;25:29–33. doi: 10.1097/01.ico.0000167883.63266.60. [DOI] [PubMed] [Google Scholar]
- 9.Sharma M, Boxer Wachler BS. Comparison of single-segment and double-segment Intacs for keratoconus and post-LASIK ectasia. Am J Ophthalmol. 2006;141:891–895. doi: 10.1016/j.ajo.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Rabinowitz YS. Intacs for keratoconus. Curr Opin Ophthalmol. 2007;18:279–283. doi: 10.1097/ICU.0b013e3281fc94a5. [DOI] [PubMed] [Google Scholar]
- 11.Cezón J. El por qué de los anillos intracorneales. Arch Soc Esp Oftalmol. 2005;80:321–322. doi: 10.4321/s0365-66912005000600001. [DOI] [PubMed] [Google Scholar]
- 12.Mian SI, Jarade EF, Scally A, et al. Combined ICRS insertion and LASIK to maximize postoperative residual bed thickness in high myopia. J Cataract Refract Surg. 2004;30:2383–2390. doi: 10.1016/j.jcrs.2004.02.069. [DOI] [PubMed] [Google Scholar]
- 13.Pokroy R, Levinger S, Hirsh A. Single Intacs segment for post-laser in situ keratomileusis keratectasia. J Cataract Refract Surg. 2004;30:1685–1695. doi: 10.1016/j.jcrs.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 14.Seiler T, Koufala K, Richter G. Iatrogenic keratectasia after laser in situ keratomileusis. J Refract Surg. 1998;14:312–317. doi: 10.3928/1081-597X-19980501-15. [DOI] [PubMed] [Google Scholar]
- 15.Alió JL, Salem TF, Artola A, et al. Intracorneal rings to correct corneal ectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2002;28:1568–1574. doi: 10.1016/s0886-3350(01)01275-5. [DOI] [PubMed] [Google Scholar]
- 16.Spirn MJ, Dawson DG, Rubinfeld RS, et al. Histopathological analysis of post-laser-assisted in situ keratomileusis corneal ectasia with intrastromal corneal ring segments. Arch Ophthalmol. 2005;123:1604–1607. doi: 10.1001/archopht.123.11.1604. [DOI] [PubMed] [Google Scholar]
- 17.Binder PS. Ectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2003;29:2419–2429. doi: 10.1016/j.jcrs.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Randleman JB, Russell B, Ward MA, et al. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110:267–275. doi: 10.1016/S0161-6420(02)01727-X. [DOI] [PubMed] [Google Scholar]
- 19.Maguen E, Zorapapel NC, Zieske JD, et al. Extracellular matrix and matrix metalloproteinase changes in human corneas after complicated laser-assisted in situ keratomileusis (LASIK) Cornea. 2002;21:95–100. doi: 10.1097/00003226-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Ljubimov AV, Burgeson RE, Butkowski RJ, et al. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72:461–473. [PubMed] [Google Scholar]
- 21.Ljubimov AV, Alba SA, Burgeson RE, et al. Extracellular matrix changes in human corneas after radial keratotomy. Exp Eye Res. 1998;67:265–272. doi: 10.1006/exer.1998.0511. [DOI] [PubMed] [Google Scholar]
- 22.Saghizadeh M, Brown DJ, Castellon R, et al. Overexpression of matrix metalloproteinase-10 and matrix metalloproteinase-3 in human diabetic corneas: a possible mechanism of basement membrane and integrin alterations. Am J Pathol. 2001;158:723–734. doi: 10.1016/S0002-9440(10)64015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saghizadeh M, Kramerov AA, Tajbakhsh J, et al. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest Ophthalmol Vis Sci. 2005;46:3604–3615. doi: 10.1167/iovs.04-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miosge N, Sasaki T, Timpl R. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol. 2002;21:611–621. doi: 10.1016/s0945-053x(02)00070-7. [DOI] [PubMed] [Google Scholar]
- 25.Zieske JD. Extracellular matrix and wound healing. Curr Opin Ophthalmol. 2001;12:237–241. doi: 10.1097/00055735-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Kuo IC. Corneal wound healing. Curr Opin Ophthalmol. 2004;15:311–315. doi: 10.1097/00055735-200408000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24(8 Suppl):S2–S11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- 28.Ljubimov AV, Burgeson RE, Butkowski RJ, et al. Extracellular matrix alterations in human corneas with bullous keratopathy. Invest Ophthalmol Vis Sci. 1996;37:997–1007. [PubMed] [Google Scholar]
- 29.Kenney MC, Nesburn AB, Burgeson RE, et al. Abnormalities of the extracellular matrix in keratoconus corneas. Cornea. 1997;16:345–351. [PubMed] [Google Scholar]
- 30.Maguen E, Maguen B, Regev L, et al. Immunohistochemical evaluation of two corneal buttons with post-LASIK keratectasia. Cornea. 2007;26:983–991. doi: 10.1097/ICO.0b013e3180de1d91. [DOI] [PubMed] [Google Scholar]
- 31.Priglinger SG, May CA, Alge CS, et al. Immunohistochemical findings after LASIK confirm in vitro LASIK model. Cornea. 2006;25:331–335. doi: 10.1097/01.ico.0000183535.99651.7b. [DOI] [PubMed] [Google Scholar]
- 32.Kymionis GD, Siganos CS, Kounis G, et al. Management of post-LASIK corneal ectasia with Intacs inserts: 1-year results. Arch Ophthalmol. 2003;121:322–326. doi: 10.1001/archopht.121.3.322. [DOI] [PubMed] [Google Scholar]
- 33.Barbara A, Shehadeh-Masha’our R, Garzozi HJ. Intacs after laser in situ keratomileusis and photorefractive keratectomy. J Cataract Refract Surg. 2004;30:1892–1895. doi: 10.1016/j.jcrs.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 34.Hofling-Lima AL, Branco BC, Romano AC, et al. Corneal infections after implantation of intracorneal ring segments. Cornea. 2004;23:547–549. doi: 10.1097/01.ico.0000126434.95325.24. [DOI] [PubMed] [Google Scholar]
- 35.Ruckhofer J, Bohnke M, Alzner E, et al. Confocal microscopy after implantation of intrastromal corneal ring segments. Ophthalmology. 2000;107:2144–2151. doi: 10.1016/s0161-6420(00)00387-0. [DOI] [PubMed] [Google Scholar]
- 36.Twa MD, Ruckhofer J, Kash RL, et al. Histologic evaluation of corneal stroma in rabbits after intrastromal corneal ring implantation. Cornea. 2003;22:146–152. doi: 10.1097/00003226-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Dawson DG, Edelhauser HF, Grossniklaus HE. Long-term histopathologic findings in human corneal wounds after refractive surgical procedures. Am J Ophthalmol. 2005;139:168–178. doi: 10.1016/j.ajo.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 38.Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Määttä M, Väisänen T, Väisänen MR, et al. Altered expression of type XIII collagen in keratoconus and scarred human cornea: increased expression in scarred cornea is associated with myofibroblast transformation. Cornea. 2006;25:448–453. doi: 10.1097/01.ico.0000183537.45393.1f. [DOI] [PubMed] [Google Scholar]
- 40.Lu PC, Ye H, Maeda M, et al. Immunolocalization and gene expression of matrilysin during corneal wound healing. Invest Ophthalmol Vis Sci. 1999;40:20–27. [PubMed] [Google Scholar]
- 41.Daniels JT, Geerling G, Alexander RA, et al. Temporal and spatial expression of matrix metalloproteinases during wound healing of human corneal tissue. Exp Eye Res. 2003;77:653–664. doi: 10.1016/j.exer.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Adachi W, Kawamoto S, Ohno I, et al. Isolation and characterization of human cathepsin V: a major proteinase in corneal epithelium. Invest Ophthalmol Vis Sci. 1998;39:1789–1796. [PubMed] [Google Scholar]
- 43.Yan S, Sameni M, Sloane BF. Cathepsin B and human tumor progression. Biol Chem. 1998;379:113–123. [PubMed] [Google Scholar]
- 44.Waghray A, Keppler D, Sloane BF, et al. Analysis of a truncated form of cathepsin H in human prostate tumor cells. J Biol Chem. 2002;277:11533–11538. doi: 10.1074/jbc.M109557200. [DOI] [PubMed] [Google Scholar]
- 45.Buth H, Wolters B, Hartwig B, et al. HaCaT keratinocytes secrete lysosomal cysteine proteinases during migration. Eur J Cell Biol. 2004;83:781–795. doi: 10.1078/0171-9335-00428. [DOI] [PubMed] [Google Scholar]
- 46.Abbott RE, Corral CJ, MacIvor DM, et al. Augmented inflammatory responses and altered wound healing in cathepsin G-deficient mice. Arch Surg. 1998;133:1002–1006. doi: 10.1001/archsurg.133.9.1002. [DOI] [PubMed] [Google Scholar]
- 47.Brookes NH, Loh IP, Clover GM, et al. Involvement of corneal nerves in the progression of keratoconus. Exp Eye Res. 2003;77:515–524. doi: 10.1016/s0014-4835(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 48.Philipp WE, Speicher L, Gottinger W. Histological and immunohistochemical findings after laser in situ keratomileusis in human corneas. J Cataract Refract Surg. 2003;29:808–820. doi: 10.1016/s0886-3350(02)01611-5. [DOI] [PubMed] [Google Scholar]
- 49.Huet E, Vallée B, Szul D, et al. Extracellular matrix metalloproteinase inducer/CD147 promotes myofibroblast differentiation by inducing α-smooth muscle actin expression and collagen gel contraction: implications in tissue remodeling. FASEB J. doi: 10.1096/fj.07-8748com. in press. [DOI] [PubMed] [Google Scholar]