Abstract
Integrins have been reported to mediate cell survival, proliferation, differentiation, and migration programs. For this reason, the past few years have seen an increased interest in the implications of integrin receptors in cancer biology and tumor cell aggression. This review considers the potential role of integrins in cancer and also addresses why integrins are present attractive targets for drug design. It discusses of the several properties of the integrin-based chemotherapeutic agents currently under consideration clinically and provides an insight into cancer drug development using integrin as a target.
Keywords: integrin, cancer, disintegrin, ligand
Introduction
Integrins are a large family of eukayotic cell-surface receptors that mediate dynamic interaction between cells and extracellular adhesion molecules (Humphries, 2000). The integrins recognize extracellular matrix (ECM) proteins or counter-receptors on adjacent cells. ECM molecules that affect cell adhesion include glycoproteins such as fibronectin (Fn) (Gardner and Hynes, 1985), von Willebrand factor (vWF) (Chow et al. 1992), vitronectin (Vn) (Pytela et al. 1985), thrombospondin (Tsp) (Karczewski et al. 1989), tenascin (Tn) (Joshi et al. 1993), collagen (Coll) (Heino, 2000), laminin (Ln) (Burgeson and Christiano, 1997), osteopontin (Opn) (Green, 2001), and other unidentified molecules. A key finding in the discovery of the integrins was that the well known amino acid sequence Arg-Gly-Asp (RGD) which was initially found in fibronectin, serves as a primary cell recognition motif. Subsequently, the RGD sequence was found in many ECM molecules and, in many cases, was responsible for cell attachment (Karczewski et al. 1989; Joshi et al. 1993; Ruggeri et al. 1983; Davis, 1992; Schnapp et al. 1995; Kimura et al. 1998). The recent crystal structures of the extracellular domains of αVβ3 (Xiong et al. 2001; 2002) have provided new insights into integrin activation and ligand recognition. The interaction of integrins with their ligands is dependent upon signals transduced from the cytoplasmic tails to the extracellular domains (Travis et al. 2003). The binding of integrins to their ligands is critically important to many diverse physiological phenomena, such as attachment, cell proliferation (Miyata et al. 2000; Hollenbeck et al. 2004; Hedin et al. 2004; Zhou et al. 2004), migration (Hirsch et al. 1996; Sakai et al. 1998; Fujiwara et al. 2001; Paulhe et al. 2001). Integrins also contribute to the initiation and/or progression of many diseases including tumor invasion, angiogenesis and metastasis (Tsuji et al. 2004; Takanami et al. 2005; Guo et al. 2005; Enserink et al. 2004; Chung et al. 2004; Felding-Habermann et al. 2002; Gladson et al. 1996; Zheng et al. 1999; Zheng et al. 2000).
Integrin Family
Integrin family have atleast 18 α- and 8 β-subunits that are known to comprise least 24 members (Berman et al. 2000; Parise et al. 2000; Humphries, 2000; Hynes, 2002). Additionally, in a recent survey of human genome, 24 α- and 9 β-subunits have been identified (Venter et al. 2001), which implies 6 novel α- and 1 novel β-subunits. However, their existence is not yet firmly established. Integrins are found in many species, ranging from sponges to mammals (Brower et al. 1997; Gettner et al. 1995).
Cell adhesion requires integrin occupancy. The binding of integrins to their ligands has been intensively studied employing proteolytic fragments and synthetic peptides corresponding to selected regions in Fg, Fn and several other matrix components (Table 1).
Table 1.
The integrin family of proteins and their ligands.
| β1 | ||
| α1 | Colls, laminins | |
| α2 | Colls, laminins, chondroadherin | |
| α3 | Laminins (such as laminin-1, -5, -8, -10, | |
| and -11), Fn, thrombospondin, TIMP-2, uPAR, collagen, epiligrin, entactin | ||
| α4 | Fn, VCAM | |
| α5 | Fn, Fg, uPAR | |
| α6 | Laminins, merosin (laminin α2 chain), kalinin | |
| α7 | Laminins, merosin (laminin α2 chain), | |
| α8 | Fn, vitronectin, Tn-C, osteopontin, and nephronectin | |
| α9 | angiostatin, Tn-C, osteopontin, and ADAMs, VCAM-1, tTG, | |
| α10 | Colls | |
| α11 | Colls | |
| αV | Fn, vitronectin | |
| β2 | ||
| αL | ICAM-1, -2 and -3 | |
| αM | Fg, ICAMs, iC3b, factor-Xa, denatured ovalbumin | |
| αX | Fg, iC3b | |
| αD | VCAM, ICAMs | |
| β3 | ||
| αIIb | Coll, Fn, vitronectin, Fg, vWF, thrombospondin | |
| αV | Fn, vitronectin, Fg, vWf, thrombospondin, FGF-2, MMP-2 and some ADAM proteins | |
| β4 | ||
| α6 | Laminins | |
| β5 | ||
| αV | Vitronectin, uPAR | |
| β6 | ||
| αV | Fn, Tn | |
| β7 | ||
| α4 | Fn, VCAM, MAdCAM | |
| αE | E-cadherin | |
| αV | Colls, laminins, Fn | |
| βN | ||
| αV | Fn, Colls | |
| β8 | ||
| αV | Vitronectin, Fn | |
The various heterodimeric combinations of α and β subunits. Abbreviations used are: Colls: collagens; Fn: fibronectin; TIMP-2: tissue inhibitor of metalloproteinase; uPAR: urokinase-type plasminogen activator (uPA) receptor; VCAM: vascular cell adhesion molecule; Fg: fibrinogen; Tn-c: tenacin-C; ADAMs: a disintegrin and metalloproteinase proteins; tTG: tissue-type transglutaminase; iC3b: inactivated complement component 3b; ICAM: intercellular cell adhesion molecule; vWf: von Willebrand factor; FGF-2: fibroblast growth factor 2; MMP: matrix metallo-proteinases and MAdCAM: mucosal addressin cell adhesion molecule.
As shown in Table 1, the two distinct subunits form noncovalent heterodimers where each subunit has a large extracellular domain (700–100 residues), a single transmembrane domain and a short cytoplasmic domain (20–70 residues). The exception to this is the β4 subunit which has an extended cytoplasmic domain containing four Fn type III-like domains (Colombatti et al. 1993). All integrin dimers are dissociated by ionic detergents, indicating that the subunits are noncovalently held together.
The α subunits are subdivided into two groups based on some structural differences. The first group is comprised of the α1, α2, αD, αE, αL, αM or αX subunits, respectively. The second group is composed of α3, α4, α5, α6, α7, α8, α9, α10, α11, αIIb or αV subunits, respectively, and is bridged by a disulphide bond with an exceptional α4 subunit subjected to a post translational cleavage at a site close to the transmembrane domain of the precursor. Thus, there are two chains linked by a disulphide bridge, a light chain and a heavy chain. The light chain is composed of the cytoplasmic domain, the transmembrane region and a part of the extra-cellular domain (about 25 kD), while the heavy chain contains the rest of the extracellular domain (about 120 kD). Integrin α4 is unique among all known integrin α subunit sequences in that it (i) has neither an inserted I-domain, nor a disulfide-linked C-terminal fragment, and (ii) a potential protease cleavage site, near the middle of the extra-cellular portion of the polypeptide rather than close to the transmembrane domain of other integrin α subunits (Xiong et al. 2001, 2002).
The β1 integrins generally mediate interaction between cells and ECM (Perlino et al. 2000). The β2 integrins subfamily including αLβ2, αMβ2, αXβ2, αDβ2, are immunologically restricted to leukocytes and typically have other cell surface molecules as their ligands, for example, αLβ2 interacts with counter receptors ICAM-1, ICAM-2, and ICAM-3 (Marlin and Springer, 1987; de Fouerolles and Springer, 1992; Binnerts et al. 1996; Woska et al. 1996) while αMβ2 recognizes iC3b (Wright and Silverstein, 1983), fibrinogen (Zhang and Plow, 1996) and neutrophil inhibitory factor (NIF) (Muchowski et al. 1994). There are two integrins αIIbβ3 and αVβ3 in integrin family, that share the common β3 subunit, have been reported to function as promiscuous receptors for the RGD-containing adhesive proteins such as fibrinogen, vitronectin, fibronectin, von Willebrand factor, and thrombospondin (Scarborough et al. 1999). The β4 integrin facilitates key functions of carcinoma cells, including their ability to migrate, invade, and evade apoptosis (Folgiero et al. 2007). The β5, β6, β7, β8 and βN subunits can form a dimmer with an αV subunit binding to different ligands (Table 1) and showing different functions.
Integrin Structure
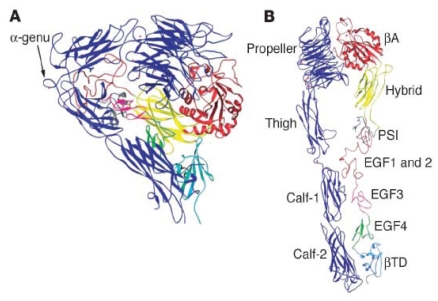
The first three-dimensional structure of the extracellular domain of an integrin was published in October 2001, a decade and a half after the family was first defined (Xiong et al. 2001, 2002, 2004) (Fig. 1).
Figure 1.
Structure of the extracellular segment of αVβ3 derived with permission from Xiong et al. 2002. (A) Bent conformation of αVβ3 as it was present in the crystal. (B) Extension of the structure to reveal its domains.
Crystal structure of integrin αVβ3 showing the dimer and individual subunits (Xiong et al. 2002). An unliganded ectodomain from the αA-lacking integrin αVβ3 contains the two subunts assembled into a globular head built by two predicted domains: the N-terminal seven-bladed propeller domain of αV and an αA-like domain (βA) from the β3. βA loops out from the “Hybrid” domain (β3 residues 55–108 and 353–432), which itself is inserted in the N-terminal plexin/semaphorin/integrin (PSI) domain (residues 1–54 and residues 433–435) of β3. The PSI domain and the beta-tail domain (βTD), together forming the β3 leg. Ig-like thigh domain and calf-1 and calf-2 domains formed the αV leg. Two legs are bent at the “knees” and folded back against the head of the same molecule. This sharp bending takes place between the thigh and calf-1 of αV (α-genu) and approximately corresponding to between EGF domains 1 and 2 of β3 (β-genu). A metal ion (Ca2+ or Mn2+) occupies the α-genu on both the ligand and unliganded structures. At the base of propeller, blades 4–7 each contain a metal ion coordinated in a β-hairpin loop.
Integrin and Cancer
Cancer occurs when cells become abnormal and keep dividing and forming more cells without control or order. If cells keep dividing when new cells are not needed, a mass undifferentiated tissue forms. This mass of extra tissue, called a growth or tumour, can constitute either a benign or a malignant tumour respectively. Benign tumors can usually be removed and, in most cases, they do not come back. Most importantly, cells from benign tumors do not spread to other parts of the body. Benign tumors are rarely a threat to life. In contrast, malignant tumours are truly cancerous. Cancer cells can invade and damage nearby tissues and organs. Cancer cells can break away from a malignant tumor and enter the bloodstream or the lymphatic system. The spread of cancer is called metastasis which appears to be a complex multistep process that involves the invasion of cancer cells from primary neoplasm followed by their dissemination through the lymphatic vessels and systemic circulation. New blood vessels form either by vasculogenesis, which refers to initial events of vascular growth in which endothelial cell precursors (angioblasts) differentiate and assemble into primitive vessels or by angiogenesis, which refers to a combination of sprouting of new vessels from pre-existing ones, and longitudinal separation of pre-existing vessels in a process named intussusception (Conway et al. 1993). The angiogenesis can be triggered in pathological conditions such as tumor growth and chronic wounding. Angiogenetic process involves functional cooperativity between cytokines and endothelial cell (EC) surface integrins. Cell bound integrins by their physical interaction with ligands necessary are essential for cell adhesion, migration and positioning, and induce signaling events essential for cell survival, proliferation and differentiation. They also trigger a variety of signal transduction pathways which are involved in mediating invasion, metastasis and squamous-cell carcinoma which can be reviewed as follows. The review focuses mainly on specific α and β subtypes which have been most extensively investigated in cancer.
β1 class of integrins
Although little clear correlation between tumor formation, invasion and β1 integrin expression has yet been demonstrated in human patients, it has been possible to show a crucial role of β1 integrin in tumor formation and metastasis in mice. Tumor cells expressing β1 integrin formed significantly larger primary tumors and had a dramatically increased metastasis into liver and lung (Brakebusch et al. 1999). In another study, which used a T cell lymphoma line in which both β1 integrin alleles was deleted by homologous recombination, metastasis formation in mice was significantly reduced (Stroeken et al. 2000). Recently, it was shown that ablation of the β1 integrin gene in mammary epithelium dramatically impaired mammary tumorigenesis in mice (White et al. 2004). Sudhakar et al. have reported that human collagen α1(IV)NC1 binds to α1β1 integrin, competes with type IV collagen binding to α1β1 integrin, and inhibits migration, proliferation, and tube formation by ECs, indicating that α1 (IV)NC1 is a potential therapeutic candidate for targeting tumor angiogenesis (Sudhakar et al. 2005). A study using β1 integrin double knockout lymphocytes and retransfection of β1 integrin deletion mutants have shown that different parts of the cytoplasmic domain of β1 integrin are required either for adhesion or for invasion and metastasis (Stroeken et al. 2000).
Integrin α1β1 and α2β1 were shown to regulate hepatocarcinoma cell invasion across the fibrotic matrix microenvironment (Yang et al. 2003). A potent selective inhibitor of α1β1 integrin, obtustatin purified from the venom of the Vipera lebetina obtusa viper was reported to have a marked ability to inhibit angiogenesis in vivo in the chicken chorioallantoic membrane assay, and in the Lewis lung syngeneic mouse model (Marcinkiewicz et al. 2003). Grzesiak and Bouvet have demonstrated that the certain cancer cell lines including CFPAC (a ductal epithelioid cell line established from a cystic fibrosis patient with pancreatic adenocarcinoma), BxPC-3 (human pancreas adenocarcinoma), Colo-357 (human lymph node metastasis), and Panc-1 (Pancreatic Cancer Cell Line) attach to 3D type I collagen scaffolds in an α2β1-specific manner and that this integrin-specific adhesion is required for subsequent cell proliferation. Such evidences support the notion that targeting α2β1 integrin-specific type I collagen adhesion may have therapeutic value in the treatment of pancreatic cancer (Grzesiak and Bouvet, 2007). Integrin α2β1 was also reported to mediate the anti-angiogenic and anti-tumor activities of angiocidin, a novel tumour-associated protein which is capable of binding to both α2β1 and type I collagen. This protein promoted α2β1-dependent cell adhesion and inhibited tumor growth and angiogenesis (Sabherwal et al. 2006). Combined antagonism of α1β1 and α2β1 was shown to reduce tumor growth substantially as well as angiogenesis of human squamous cell carcinoma xenografts (Senger et al. 2002).
The interaction of α3β1 with ligand laminin-5 has been demonstrated to promote the migration and invasion of malignant glioma and melanoma cells (Tsuji, 2004; Tsuji et al. 2002; Giannelli et al. 2007) and to promote binding to virus glycoprotein. A significant increase in proliferation and adhesion in response to collagen 1 and laminin for integrin receptor α3β1 was also observed in ovarian cancer cell lines (Ahmed et al. 2005). More recently, uPAR (urokinase-type plasminogen activator receptor), and TIMP (tissue inhibitors of metalloproteinases)-2 were also proposed as ligands of α3β1 integrin in mediating uPA/uPAR interaction and intracellular signaling (Wei et al. 2007). In an animal model it was shown that soluble uPAR antagonizes cancer progression (Jo et al. 2003).
The Src family kinases are classified as oncogenic proteins due to their ability to activate cell migration (Rodier et al. 1995; Rahimi et al. 1998) in many cell types including epithelial tumor cells. Studies with chimeric α4 integrin subunits have shown that α4 cytoplasmic domain can enhances cell migration via c-Src activation (Chan et al. 1992; Hsia et al. 2005).
α5β1 integrin interacts with Fn which is implicated in several cellular activities including cell proliferation, differentiation, and migration. A high-affinity interaction that occurs with the central cell binding domain, a region involved in many fundamental aspects of cell growth and morphogenesis, is dependent on the RGD sequence and other recognition sequences (Li et al. 2003; Murillo et al. 2004). The interaction with Fn has been demonstrated with both lung epithelial cells and fibroblasts. In addition, the inhibition of cell surface α5 integrin expression was found to decrease phosphoinositide-3 kinase (PI3K) activity and inhibit colon cancer cell attachment, suggesting that agents which selectively target α5 integrin subunit expression may enhance the effects of standard chemotherapeutic agents and provide a novel adjuvant treatment for selected colon cancers (Lopez-Conejo et al. 2002). Furthermore, cells expressing the α5β1 integrin displayed a dramatic enhancement in the ability of growth factors to activate PI3K and protein kinase B (PKB), indicating this stimulation may also involve the interaction between α5β1 and the PI3 K and PKB signalling pathways (Lee et al. 2000). Wei et al. recently reported that urokinase receptor binding to α5β1 is required for maximal responses to Fn and tumor cell invasion (Wei et al. 2007). Kuwada et al. demonstrated that expression of integrin α5β1 in colon cancer cells decreases HER (human epidermal growth factor receptor)-2-mediated proliferation, crystal violet assays were showing inhibition of the cell proliferation of Caco-2 control cells with the antagonistic HER-2 antibody mAb 4D5 (Kuwada et al. 2005). MAb 4D5 is also indicated clinically active in cancer patients to target HER2-overexpression (Baselga et al. 1996; Rhodes, 2005). Furthermore, mAb 4D5 has been shown great promise as targeted agents in the treatment of patients with cancer (Bartsch et al. 2007).
It has been reported that α6 integrin-mediated neutrophil migration through the perivascular basement membrane (PBM) is platelet-endothelial cell adhesion molecule1 (PECAM-1) dependent, a response associated with PECAM-1-mediated increased expression of α6β1 on transmigrating neutrophils (Dangerfield et al. 2002). Significantly increased ovarian cancer cell line proliferation and adhesion to collagen 1 and laminin (ligands of integrin receptor α6β1) were also reported (Ahmed et al. 2005). In addition, an α6 integrin is found to be overexpressed in human oesophageal carcinomas, suggesting an important role in oesophageal tumor invasion (Tanaka et al. 2000). This notion has since been confirmed by other studies (Mercurio et al. 2001; Demetriou et al. 2004).
The α7β1 integrin is a laminin-binding receptor that was originally identified in melanoma (Kramer et al. 1991). Ziober et al. reported that during melanoma progression, acquisition of a highly tumorigenic and metastatic melanoma phenotype is associated with loss of the α7β1 (Ziober et al. 1999). Integrin α7β1 serves an important mechanical function in the diaphragm by contributing to passive compliance, viscoelasticity, and modulation of muscle contractile properties (Lopez et al. 2005).
Integrin α10β1 is a major collagen-binding integrin during cartilage development and in mature hyaline cartilage while α11β1 was originally found in fetal muscle (Gullberg et al. 1995). Integrin α11β1 recognizes the triple-helical GFOGER sequence (where single letter amino acid nomenclature is used, O = hydroxyproline) found in interstitial collagens (Tulla et al. 2001). Little is known about the biology of these recently identified integrins. Integrin α10β1 is expressed on chondrocytes and some fibrous tissues. Integrin α11β1 is involved in cell migration and collagen reorganization in mesenchymal non-muscle cells (Tiger et al. 2001). Recently, α11β1 integrin is required on periodontal ligament fibroblasts for cell migration and collagen reorganization by assisting axial tooth movement (Popova et al. 2007).
αV class of integrin
The first integrin found associated with tumor angiogenesis was αVβ3 (Eliceiri et al. 1998; Eliceiri, 2001; Ruegg et al. 2003). Integrin αVβ3 has a broad distribution and is found on endothelial cells, smooth muscle cells (SMCs) and hematopoietic cell types such as platelets and osteoclasts. The interaction of αVβ3 with its ligands plays a crucial role in angiogenesis and neointimal formation after vascular injury. In addition, during osteoclast-mediated bone resorption, αVβ3 regulates the cytoskeletal organization required for cell migration and formation of the sealing zone (McHugh et al. 2000). Prostate cancer specific integrin αVβ3 was demonstrated to modulate bone metastatic growth and tissue remodeling (McCabe et al. 2007). The study of co-expression of bone sialoprotein, integrin αVβ3, and MMP-2 in papillary thyroid carcinoma cells demonstrated that cancer cells appear to become more invasive when bone sialoprotein forms a cell-surface trimolecular complex that links MMP-2 to integrin αVβ3 (Karadag et al. 2004). Bayless et al. present very convincing data showing that integrin αVβ3 as well as integrin α5β1 regulate human endothelial cell vacuolation and lumen formation, implicating a major role contributed by these two integrins for endothelial cell morphogenesis (Bayless et al. 2000). It is also clear that the integrin αVβ3 plays an important role in virtually every stage of cancer progression. Indeed, neuroblastoma aggressiveness has been identified to be correlated with the expression of integrin αVβ3 and αVβ5 by microvascular endothelium (Erdreich-Epstein et al. 2000). Other studies also demonstrate that increased αVβ3 expression level is closely associated with increased cell invasion and metastasis (Feldin-Habermann et al. 2002). Li et al. reported that antisense αV suppressed tumour growth more strongly than antisense β3, antisense therapy but simultaneous targeting at both integrin subunits was more effective than the respective monotherapies (Li et al. 2007). Integrin αVβ3 has been demonstrated to interact with the activated forms of the platelet-derived growth factor, insulin, and vascular endothelial growth factor (VEGF) cell receptors faciliting optimal activation of cell proliferative signalling pathways (Giancotti and Ruoslahti, 1999; Kumar, 2003). The functional activity of αvβ3 on endothelial and tumor cells may well be regulated by VEGF (Byzova et al. 2000). VEGF has been also implicated in prostate carcinogenesis and metastasis as well as in angio-genesis. Both VEGF and its receptor are expressed by prostate carcinoma cells at a high level (Ferrer et al. 1998; 1999).
A role for αVβ6-mediated production in the regulation of MMP-9 and MMP-3 have been reported in several tumor types and in untransformed keratinocytes (Ramos et al. 2002; Ahmed et al. 2002). MMP-9 plays a critical role in the recruitment of bone marrow derived CD45 positive cells into the primary tumor and the establishment of a mature vasculature (Jodele et al. 2005). Integrin αVβ6 also plays a role in wound healing and cancer of the oral cavity (Thomas et al. 2006). In addition, αVβ6 has been implicated in the regulatory control of the uPA proteolytic cascade (Ahmed et al. 2002). A gradual increase in the expression of αVβ6 integrin from borderline to malignant tumors has been reported in oral squamous carcinomas (Jones et al. 1997) and breast carcinomas (Arihiro et al. 2000). In malignant keratinocytes and colon cancer cells, increased expression of this integrin enhances MMP-9 secretion and MMP-9-mediated invasion (Thomas et al. 2000; Agrez et al. 1999). Inhibition of αVβ6 function using inhibitory antibodies results in total abrogation of MMP-9 activation (Thomas et al. 2001) suggesting that the expressions of αVβ6 integrin and MMP-9 are linked, and their coordinate expression appears to promote invasion by squamous and colon carcinoma cells. The integrin αVβ6 interacts with Fn, Vn (Huang et al. 1998), tenascin (Weinacker et al. 1995), and latency-associated peptide (Munger et al. 1999), a protein derived from the N-terminal region of the transforming growth factor(TGF)-β gene product that mediates cell adhesion, spreading, migration, proliferation, and activation of latent TGF-β (Weinreb et al. 2004; Thomas et al. 2001).
Until recently, there has been little information about integrin αVβ8 which has been reported to function as an additional receptor for foot-and-mouth disease virus (FMDV) (Jackson et al. 2004) in addition to the three RGD-dependent integrins αVβ1, αVβ3, and αVβ6, which have been shown to be receptors for FMDV previously (Jackson et al. 1997, 2000, 2002; Duque et al. 2004). Notably, αVβ8 as well as αVβ6 may promote epithelial-mesenchymal transition (EMT) by contributing to the activation of TGF-β (Munger et al. 1999). Additionally, αVβ8—mediated activation of TGF-β was shown to block the proliferation of certain cancer cells (Mu et al. 2002). Several recent studies have demonstrated that both up-regulation and down-regulation of expression of αV integrins and other integrins can be effective markers of malignant diseases and patient prognosis.
Although there are few reports of enhanced expression of αIIbβ3 (than of αVβ3) integrin in tumour cells, one observation indicated an important role in tumour progression. A study on human melanoma biopsies showed that αIIbβ3 expression increased with tumour thickness (Trikha et al. 2002a). In addition, a single pretreatment of human melanoma cells with c7E3 Fab, an αIIbβ3 antibody inhibited lung colonization of the tumor cells in severe combined immunodeficient mice (Trikha et al. 2002b).
Other sub-classes of integrins
Parathyroid hormone-related protein (PTHrP) was reported to not only increase transcriptional activity of the integrin subunit α5 (Anderson et al. 2007) but also upregulate integrin α6β4 expression and activate Akt in breast cancer cells (Dittmer et al. 2006; Shen and Falzon, 2006; Shen et al. 2007). Falcioni et al. first identified a tumor antigen (TSP-180) associated with metastasis that was shown to be identical to the β4 integrin subunit (Falcioni et al. 1997; Kennel et al. 1989). Subsequently other studies showed that expression of α6β4 persists in some aggressive carcinomas and that its expression may be linked to the behavior of these tumors (Guo et al. 2004). At earlier of the year in 2001, Davis and his colleagues demonstrated that α6β4 integrin has an influence on tumour biology as this integrin and its ligand, laminin-5, are essential gene products for the maintenance and remodeling of a stratified epithelium (Davis et al. 2001). The β4 integrin, for example, was lost in the lesions of prostatic intraepithelial neoplasia together with basal cell-lining and in prostate carcinoma the expression of β4 integrins was totally lost (Davis et al. 2001). In normal skin keratinocytes, expression of the α6β4 integrin is restricted to the proliferative basal layer and mediates stable adhesion to the underlying basement membrane. Observations in carcinoma cells show a functional and spatial dissociation of the α6β4 integrin from the hemidesmosomal complex, which stimulates cell migration and, therefore, may contribute to carcinoma invasion (Kippenberger et al. 2004). Indeed, many carcinomas express elevated levels of α6β4 (Herold-Mende et al. 2001), particularly breast carcinomas (Chung and Mercurio, 2004).
Pawar et al. recently have shown that the uPA-mediated cell surface cleavage of the alpha6 integrin extracellular domain is involved in tumor cell invasion and migration on laminin (Pawar et al. 2007). In addition, observations in carcinoma cells show a functional and spatial dissociation of the α6β4 integrin from the hemidesmosomal complex, which stimulates cell migration (Kippenberger et al. 2004). Furthermore, blocking antibodies to either α6 or β4 integrin subunits suppress the formation of apoptosis-resistant acinar structures in Matrigel by mammary epithelial cells (Weaver et al. 2002), suggesting a role for β4-mediated cellular polarity in mediating antiapoptotic signaling. Integrin α6β4 was recently noted only at the cell’s basal interface with the basement membrane in normal pancreatic ducts. But in pancreatic adenocarcinomas, 92% demonstrated overexpression of integrin α6β4 and altered localization to the cytoplasm and membranous regions, this upregulation and redistribution of integrin α6β4 expression implicated a role of integrin in pancreatic adenocarcinoma progression (Cruz-Monserrate et al. 2007). Interestingly, the expression of β4 was inversely correlated with dissemination of ten human gastric cancer cell lines in SCID (severe combined immunodeficiency) mice (Ishii et al. 2000). In addition, strong evidence suggests that reduced expression of α6 and β4 subunits may contribute to the higher tumorigenicity of androgen-independent prostate tumor cells (Bonaccorsi et al. 2000).
Several key signalling molecules in carcinoma cells are also involved in the mechanisms of α6β4 integrin-mediated tumour behaviour (Bon et al. 2007; Folgiero et al. 2007) since β4 has been demonstrated to interact with ERBB2 (erythroblastic leukemia viral oncogene homolog 2, encoding an 185-kDa, 1255 amino acids, orphan receptor tyrosine kinase) that displays potent oncogenic activity when overexpressed) in some cultured breast tumour cells, and the two proteins synergize in promoting cellular proliferation and invasion (Falcioni et al. 1997). In addition, Guo et al. established that integrin α6β4 may be required for mammary tumourigenesis driven Lu et al by the expression of ErBB2 (Guo et al. 2006). Folgiero et al. revealed that α6β4 can regulate the expression of ErBB-3 at the level of protein translation, resulting in a significant induction of ErBB-2/ErBB-3 heterodimerization and consequent activation of PI3K (Folgiero et al. 2007; Liu et al. 2007). Introduction of β4 in β4-negative breast carcinoma cells activates signalling from PI3K to Rac (a member of the Rho family of small guanosine triphosphatases) and increases the invasion of these cells in vitro (Shaw, 2001).
The integrin αEβ7 (also known as cell marker CD103) is expressed by most intra-epithelial lymphocytes (IEL). An important ligand for this molecule is the epithelial cell adhesion molecule E-cadherin. Cresswell et al. have demonstrated that the up-regulation of integrin αEβ7 by lymphocytes increases adhesion to E-cadherin expressing bladder cancer targets, indicating a role of integrin αEβ7 in cancer invasion (Cresswell et al. 2002).
Integrins as Targets for the Treatment of Cancer
From what has been discussed above, integrins play a key role in tumor angiogenesis and cancer. Because they are cell surface receptors interacting with extracellular ligands, they represent ideal pharmacological targets. A variety of integrin antagonists such as low molecular weight inhibitors, peptidomimetics, or monoclonal antibodies are in various stages of development as anti-cancer therapeutics (Kerr et al. 2002; Mousa 2002; Tucker et al. 2003).
In-vivo study has demonstrated that the addition of inhibitory anti-β1-integrin antibodies or the re-expression of α2β1 integrins leads to the reversal of the malignant phenotype in a 3-dimensional cell culture model and to a reduction in tumour formation in animal models (Zutter et al. 1995). Yao et al. recently show that β1 integrin expression has potential prognostic value in invasive breast cancer and that coexpression of fibronectin may help identify patients with more aggressive tumors who may benefit from targeted therapy (Yao et al. 2007). More studies have been focused on αVβ3, since αVβ3 has been identified as a prognostic indicator of survival and a specific potential target for control of angiogenesis, therapies directed against integrin against αVβ3, have been developed (Brooks et al. 1994; Gladson et al. 1996; Zhang et al. 2007; Gramoun et al. 2007).
Antibodies Against Integrins as Inhibitors
MEDI-552
Brooks and his coworkers first showed that a monoclonal antibody specific for αVβ3, MEDI-552 (LM609), could block angiogenesis in a murine model (Brooks et al. 1994). In addition, there is an ongoing phase I dose escalation study evaluating the safety of MEDI-522 in patients with advanced malignancies. This antibody was chosen for its unique ability to selectively target multiple and different cell types. In a phase I trial on various solid tumours, MEDI-522 appeared to be without significant toxicity (McNeel et al. 2005). MEDI-522 was detectable both in quiescent and in angiogenically active skin blood vessels as well as in the dermal interstitial space. The levels of phosphorylated focal adhesion kinase (pFAK) were reduced during MEDI-522 treatment, suggesting a modulating effect on this signaling molecule (Zhang et al. 2007; Gramoun et al. 2007).
CNTO 95
A fully humanized monoclonal antibody to anti-αV integrins, CNTO 95, has been shown to inhibit angiogenesis and tumor growth in preclinical studies (Mullamitha et al. 2007). CNTO 95 is likely to be less immunogenic in humans compared to chimeric or humanized antibodies (Trikha et al. 2004). CNTO 95 bound to purified αVβ3 and αVβ5 with higher affinity (a Kd of approximately 200 pM and to αV integrin-expressing human cells with a Kd of 1–24 nM). In vitro, CNTO 95 potentially inhibited human melanoma cell adhesion, migration and invasion (doses ranging 7–20 nM) and appeared to be safe without inhibition of normal physiologic angiogenesis (Martin et al. 2005; Trikha et al. 2004).
17E6
The 17E6 antibody strongly perturbs cell attachment mediated by αV associated integrins, by reacting with αVβ3, αVβ5, and αVβ1, and has the ability to disrupt stable interaction between vitronectin and αVβ3, and blocks the growth of M21 tumours in nude mice. In two nude mouse tumor models, injection of 17E6 strongly inhibited tumor development (Mitjans et al. 2000; Mitjans et al. 1995).
Integrin antibodies that block specific integrins for treatment of cancer are still in clinical trial stages as lessons should be learnt from integrin antibodies for the treatment of other diseases. For example, Tysabri (also called natalizumab), an antibody which blocks α4 integrins and inhbits the α4-mediated adhesion of leukocytes to their counterreceptor (s) (Minagar et al. 2000; O’Connor et al. 2004, 2005). Although the specific mechanism(s) by which tysabri exerts its effects in multiple sclerosis (MS) have not been fully characterized, Tysabri was initially approved by the Food and Drug Administration (FDA) in U.S.A. in November, 2004 for the treatment of patients with relapsing forms of MS, but was withdrawn by the manufacturer three months later after three patients developed progressive multifocal leukoencephalopathy (PML), a serious viral infection of the brain, in the drug’s clinical trials, FDA then put clinical trials of the drug on hold, allowing them to resume a year later after confirming that there were no additional cases of PML. In June 2006, the FDA resumed marketing of Tysabri with a restricted distribution program. Tysabri is indicated for use as monotherapy, because we do not know enough about how its use with other immune modifying drugs could impact risk. (www.fda.gov/cder/drug/infopage/natalizumab).
Other antibodies
α1β1 and α2β1 integrins play a significant role in the VEGF-driven angiogenesis. Ha 31/8 and Ha 1/29 are antibodies against α1 and α2 integrin subunits which were reported to inhibit endothelial cells in a gradient of immobilized collagen I assay (haptotaxis) by <40%, whereas the combination of both antibodies synergized to reach <90% inhibition (Alghisi and Ruegg, 2006). Consistent with these results, administration of both the anti-α1 and the anti-α2 antibodies to nude mice bearing a human A431 squamous cell carcinoma xenograft suppressed angiogenesis by <60% and tumor growth by >40% (Senger et al. 2002). Interestingly, preclinical studies with monoclonal antibodies (MAbs) against lactadherin, a glycoprotein of the milk fat globule membrane was found that there was a clear increase in VEGF-like proangiogenic activity (Taylor et al. 1997; Silvestre et al. 2005) when lactadherin is added back exogenously to the ischemic muscles. An investigation has further identified lactadherin as a physiological ligand of αVβ3 and αVβ5, thus confirming a proangiogenic activity of these integrins in the VEGF-dependent neovascularization in adult mice, but not in embryos. Animal test showed that the expression of Flk-1 (VEGFR-2) is elevated in β3-deficient mice, indicating that αVβ3 can control the amplitude of the VEGF response by controlling the Flk-1 level or activity (Reynolds et al. 2004). In vitro, anti-α5β1 function-blocking mAbs (NKI-SAM-1, JBS5, or IIA1) inhibited adhesion in a 72% to 100% range depending on the cell line used. This result was further confirmed in vivo in an angiogenesis assay treated with fibroblast growth factor 2 (Kim et al. 2000). The anti-α5β1 M200 antibody (Volociximab) is another chimeric monoclonal antibody of α5β1 integrin that blocks tumor growth and metastasis. M200 binds to α5β1 integrin on activated endothelial cells with high binding affinity and inhibits in vitro tube formation induced by VEGF and/or bFGF, suggesting a mechanism of action independent of growth factor stimulus. In fact, inhibition of α5β1 function by M200 induced apoptosis of actively proliferating, but not resting endothelial cells (Ramakrishnan et al. 2006).
Disintegrins, RGD-Based Peptides and Small Molecule Integrin Antagonists
The “disintegrin” terminology was initially applied in 1990 to describe a family of cysteine-rich, RGD-containing proteins from viper venom toxins that inhibit platelet aggregation and integrin-mediated cell adhesion (Gould et al. 1990; Niewiarowski et al. 1994; McLane et al. 2004). Studies of RGD-containing proteins in venom toxins have been found that a number of them, such as contortrostatin, salmosin and bitistatin (Markland et al. 2001; Zhou et al. 1999; Swenson et al. 2004; Golubkov et al. 2003; Kang et al. 1999; Chung et al. 2003; McQuade et al. 2004), are able to inhibit tumor growth and angiogenesis. Echistatin has been found to induce a decrease of both auto-phosphorylation and kinase activity of pp125FAK, suggesting inhibitory activity in processes integral to angiogenesis, such as cell growth, survival, and migration (Della et al. 2000). Triflavin was found to interact with either αIIbβ3 on platelet membranes, resulting in inhibition of platelet adhesion, secretion, and aggregation in injured arteries, or αVβ3 on SMCs subsequently inhibiting cell migration and proliferation (Sheu et al. 2001). Triflavin also blocks neuronal sprouting and the induction of hyperalgesia induced by peripheral nerve injury (Fu et al. 2004). Recently, soluble RGD peptides have been demonstrated to induce apoptosis by inducing conformational changes in procaspases, leading to increased oligomerization and subsequent autoprocessing of these enzymes (Buckley et al. 1999). In addition, RGD-containing proteins from venom toxins (e.g. salmosin, contortrostatin, rhodostomin and accutin) were also found to induce apoptosis (Chung et al. 2003; Zhou et al. 1999; Wu et al. 2003; Yeh et al. 1998). It is still not clear whether these proteins’ apoptotic induction is through interaction with integrins or through a different apoptotic pathway since Jan et al. in a recent issue of Cell have shown that integrins may regulate apoptosis, through caspase-independent mechanisms (Jan et al. 2004). These data have shown the potential for these RGD-containing snake venom proteins to function as integrin antagonists as well as anti-angiogenic and antimetastatic compounds, leading to drug development for therapeutic usage (Markland et al. 2001; Kerr et al. 2002; Hallahan et al. 2001; Coller, 2001).
The integrins that bind to RGD peptides are generally over-expressed in angiogenic vessels. In certain cancer, the tumor cells also express RGD-binding integrins. A vast body of preclinical and clinical literature exists on the use of RGD-based integrin antagonists in cardiovascular disease and cancer (Tucker, 2003; McQuade and Knight, 2003; Kumar et al. 2003; Shimaoka and Springer, 2004). A cyclic pentapeptide called EMD66203 [cyclic L-Arg-L-Gly-L-Asp-D-Phe-L-Val (RGDfV) peptide or cyclo(-Arg-GlyAsp-D-Phe-Val)](Aumailley et al. 1991) was shown preferential inhibition of vitronectin binding to the αVβ3 rather than to the αIIbβ3 (Frieser et al. 1996). Further modification of the EMD66203 led to the synthesis of EMD121974, an RGD-containing pseudopeptide (c(RGDfV)) or cyclo(Arg-Gly-Asp-D-Phe-[NMe]Val) also known as cilengitide (Dechantsreiter et al. 1999). Structural study revealed that the D-amino acid in this peptide is found preferentially in position i + 1 of a β II’ turn, a characteristic for its biological activity. EMD121974 is also a dual αVβ3/αVβ5 integrin antagonist with interesting biochemical and biological features to be tested in cancer therapy (Belvisi et al. 2005, 2006). The crystal structure of the extracellular segment of integrin αVβ3 in complex with EMD121974 revealed that the pentagonal peptide inserted into a crevice between the propeller and βA domains on the integrin head (Xiong et al. 2002). EMD 121974 was demonstrated to be an αV-integrin antagonist and a potent inhibitor of angiogenesis, by inducing apoptosis of growing endothelial cells through inhibition of their αV-integrin interaction with the matrix proteins vitronectin and tenascin (Taga et al. 2002).
ST1646, an RGD-containing pseudopeptide, is a potent, highly selective αVβ3/αVβ5 integrin antagonist, equipotent to or more potent than the well-characterized integrin antagonists c(RGDfV) (Belvisi et al. 2006; Haier et al. 2002). The structure docking model for the ST1646-αVβ3 complex has confirmed that, similarly to the crystal structure of the EMD121974-αVβ3 complex, the ligand seems to interact mainly through electrostatic forces in a rather shallow cleft and that essentially no hydrophobic interactions can be observed (Belvisi et al. 2005). In an in vitro anti-angiogenic activity assay, ST1646 inhibited HUVEC proliferation with potency similar to EMD121974 (IC50, 2.9 and 4.4 μmol/L for the two compounds, respectively). The inhibitory effect was reversible. In an in vivo antiangiogenic activity assay as determined by daily administration of ST1646 (30 μg/embryo) with CAM (chick chorioallantoic membrane) assay at day 9 via a gelatin sponge implant and at day 12 for histologic analysis, showed significant inhibition of the angiogenic response triggered by both FGF2 and VEGF (p < 0.001) (Belvisi et al. 2005).
SCH 221153, an RGD-based peptidomimetic, inhibits the binding of the disintegrin, echistatin to αVβ3 and αVβ5 with similar potency, according to IC50 values of 3.2 nM and 1.7 nM, respectively (Kumar et al. 2001). SCH 221153 inhibits FGF2 and VEGF-induced endothelial cell proliferation in vitro according to IC50 equal to 3–10 μM (Kumar et al. 2001). Monsanto-Searle (St. Louis, MO) has reported an orally compound SC-68448 which inhibited αVβ3-mediated endothelial cell proliferation in a dose-dependent manner but did not inhibit tumor cell proliferation, suggesting that effects on endothelial cell proliferation were not due to SC-68448-induced cytotoxicity. SC68448 was 100-fold more potent as a functional inhibitor of αVβ3 versus αIIbβ3 (Carron et al. 2000). Integrin αIIBβ3 expressed mainly on platelet membrane plays a crucial role in platelet aggregation and thrombus formation, and recently was reported to have a role in increasing the risk of metastases in renal cell carcinoma in men (Kallio et al. 2006). Haubner and his co-workers reported that 18F-Galacto-RGD is a highly αVβ3-selective tracer for positron emission tomography (PET) (Haubner et al. 2004, 2001). Molecular imaging with 18F-Galacto-RGD and PET provides important information for planning and monitoring anti-angiogenic therapies targeting the αVβ3 integrin (Beer et al. 2006). Meerovitch et al. demonstrated BCH-14661 and BCH-15046, RGD peptidomimetic compounds are as apoptotic inducers for endothelial cells by causing cell detachment-dependent when cells are grown on RGD-containing integrin ligand vitro-nectin and fibronectin. BCH-14661 was specific for integrin αVβ3, whereas BCH-15046 nonselectively antagonized αVβ3, αVβ5, and α5β1 (Meerovitch et al. 2003). A 20 amino acid N-terminal peptide of angiocidin was reported to promote α2β1- dependent adhesion of K562 cells, disrupt human umbilical vein endothelial cell tube formation and inhibit tumour growth as well as angiogenesis in a mouse model (Sabherwal et al. 2006). Angiocidin has also been reported to inhibit angiogenesis through binding collagen and integrin α2β1 present on many tumour cells (Sabherwal et al. 2006).
The most selective nonpeptidic α5β1 antagonist SJ749 showed a reduced proliferation of astrocytoma cell lines dependent on α5β1 expression levels and cell culture conditions, underlining the importance of α5β1 as a target for anticancer therapies (Marinelli et al. 2005; Maglott et al. 2006). A non—peptide RGD mimetic, S36578-2, was also developed and demonstrated as highly selective antagonist of both αVβ3 and αVβ5 integrins that was able to induce detachment, caspase-8 activation, and apoptosis in human umbilical endothelial cells (HUVECs) plated on vitronectin (Maubant et al. 2006). Reinmuth and his co-works demonstrated that S-247, another αVβ3/αVβ5 integrin antagonist, showed significant antimetastatic and antiangiogenic activity and impaired both endothelial and hVSMC/pericyte function in vitro and in vivo (Reinmuth et al. 2003; Harms et al. 2004).
The integrin-induced signaling cascades have also been demonstrated to impact tumor cell survival, cell migration, and angiogenesis. It is known that transforming growth factor (TGF)-beta suppresses breast cancer formation by preventing cell cycle progression in mammary epithelial cells (MECs). During the course of mammary tumorigenesis, genetic and epigenetic changes negate the cytostatic actions of TGF-beta, thus enabling TGF-beta to promote the acquisition and development of metastatic phenotypes. TGF-β stimulation can induce αVβ3 integrin expression in a manner that coincides with epithelial-mesenchymal transition (EMT) in MECs. Introduction of siRNA against β3 integrin can block TGF-β induction and also prevent TGF-β stimulation of EMT in MECs (Galliher and Schiemann, 2006). Therefore, antagonists of growth factor receptors (Cardones et al. 2006; Wick et al. 2006) can be used for anti-cancer therapy. Indeed, the recognition of potent, sequence-selective gene inhibition by siRNA oligonucleotides and rapid adoption as the tool of choice in cell culture has generated the expectation for their use to improve targeted therapeutics (Elbashir et al. 2002; Paddison et al. 2003; Carpenter and Sabatini, 2004; Ganju and Hall, 2004). The prospects of siRNA to be a therapeutic tool were enhanced by their double-stranded RNA (dsRNA) oligonucleotide nature, resembling antisense, ribozymes and gene therapy (Song et al. 2003; Davidson et al. 2004). Silencing integrin αV expression by siRNA can inhibit proliferation and induce apoptosis in integrin αV over-expressing MDA-MB-435 human breast cancer cells (Cao et al. 2006). Lipscomb and his co-workers demonstrated that siRNA oligonucleotides targeted to either subunit of the α6β4 integrin reduced cell surface expression of this integrin and resulted in decreased invasion of MDA-MB-231 breast carcinoma cells (Lipscomb et al. 2003). Recently gene transfer of antisense αV and β3 expression vectors was demonstrated to downregulate αV and β3 in HepG2 tumours established in nude mice, inhibit tumour vascularization and growth, and enhance tumour cell apoptosis, suggesting that anti-sense gene therapy targeting αV integrins could be as an approach to treat hepatocellular carcinomas (Li et al. 2007).
A study using SUM-159 breast carcinoma cell line showed that decreased expression of the α6β4 integrin led to enhanced apoptosis. Recombinant VEGF is able to significantly inhibit the cell death observed in the β4-deficient cell line. The specificity of α6β4 in both in vitro and in vivo assays showed that reexpression of the β4 subunit into the β4-deficient cell line could rescue the functional phenotype (Lipscomb et al. 2005).
Conclusions
In this review the potential roles of integrin in tumor progression and cancer were discussed. Evidence presented here indicates that integrins represent highly appropriate pharmacological targets as based upon the beneficial effect of integrin antibodies and antagonists in cancer treatment. A number of the integrin antibodies and antagonists are now in clinical trials, determining their effect on angiogenesis, metastasis and tumour growth (Table 2).
Table 2.
Integrin inhibitors in clinical development as anticancer agents.
| Antibodies | Other names | Target integrin | Comments on highest phase reached | Company | References |
|---|---|---|---|---|---|
| LM609 | Vitaxin, MEDI-552 | αVβ3 | Currently in Phase II | Scripps Research Institute | Brooks et al. 1994; Gutheil et al. 2000,McNeel et al. 2005 |
| CNTO95 | αVβ3, αVβ5 | Currently in phase I | Centocor. Medarex | Trikha et al. 2004,Mullamitha et al. 2007 | |
| Ha31/8 | α1β1 | Senger et al. 2002 | |||
| 17E6 | αVβ3 | Merck | Mitjans et al. 1995, 2000 | ||
| Ha1/29 | α2β1 | Senger et al. 2002 | |||
| NKI-SAM-1, JBS5 | α5β1 | Francis et al. 2002 | |||
| M200 | Volociximab Eos-200-4 | α5β1 | Currently in phase II | Protein design Labs | Protein Design Labs, www.pdl.com |
| Peptides | |||||
| SCH 221153 | Kumar et al. 2001 | ||||
| EMD 121974 | Cilengitide | αVβ5 | Currently in phase II | Merck KGaA, EMD Pharmaceuticals, National cancer Institute | Taga et al. 2002 |
| ST1646 | αVβ3 | Belvisi et al. 2005 | |||
| angiocidin | α2β1 | Sabherwal et al. 2006 | |||
| 18F-Galacto-RGD | Haubner et al. 2004, 2001 | ||||
| Nonpeptidic | |||||
| SJ749 | α5β1 | Marinelli et al. 2005; Maglott et al. 2006 | |||
| E7820 | α2 subunit | Phase I | Eisai Medical Research | ||
| S36578-2 | αVβ3, αVβ5 | Maubant et al. 2006 | |||
| S-247 | αVβ3, αVβ5 | Reinmuth et al. 2003; Harms et al. 2004 | |||
Biography
Biography: Xinjie Lu is a principal investigator of on-going project “Development a DNA-based vaccine against atherosclerosis”. Dr. Xinjie Lu received a D. Phil in Biochemistry in Imperial College in the U.K. in 1995. Since then he worked as a postdoctoral research fellow, a lecturer, a senior lecturer and the head of the Protein Biochemistry section, then the head of Molecular Immunology section at Thrombosis Research Institute in U.K. His principal research interests have been in the protein-protein interaction area on BHF founded projects (BHF Project Grant No. PG/97047, PG/99159 and PG/02/126) since 1977.

References
- Ahmed N, Riley C, Rice GE, et al. Alpha(v)beta(6) integrin-A marker for the malignant potential of epithelial ovarian cancer. J. Histochem. Cytochem. 2002;50:1371–80. doi: 10.1177/002215540205001010. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Riley C, Rice G, et al. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin. Exp. Metastasis. 2005;22:391–402. doi: 10.1007/s10585-005-1262-y. [DOI] [PubMed] [Google Scholar]
- Agrez M, Gu X, Turton J, et al. The alpha v beta 6 integrin induces gelatinase B. secretion in colon cancer cells. Int. J. Cancer. 1999;81:90–7. doi: 10.1002/(sici)1097-0215(19990331)81:1<90::aid-ijc16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Alghisi GC, Ruegg C. Vascular integrins in tumor angiogenesis: mediators and therapeutic targets. Endothelium. 2006;13:113–35. doi: 10.1080/10623320600698037. [DOI] [PubMed] [Google Scholar]
- Anderson JA, Grabowska AM, Watson SA. PTHrP increases transcriptional activity of the integrin subunit alpha(5) Br J Cancer. 2007 doi: 10.1038/sj.bjc.6603720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arihiro K, Kaneko M, Fujii S, et al. Significance of alpha 9 beta 1 and alpha v beta 6 integrin expression in breast carcinoma. Breast Cancer. 2000;7:19–26. doi: 10.1007/BF02967183. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Gurrath M, Muller G, et al. Arg-Gly-Asp constrained within cyclic pentapeptides. Strong and selective inhibitors of cell adhesion to vitronectin and laminin fragment P1. FEBS Lett. 1991;291:50–4. doi: 10.1016/0014-5793(91)81101-d. [DOI] [PubMed] [Google Scholar]
- Bartsch R, Wenzel C, Zielinski CC, et al. HER-2-Positive Breast Cancer: Hope Beyond Trastuzumab. Bio. Drugs. 2007;21:69–77. doi: 10.2165/00063030-200721020-00001. [DOI] [PubMed] [Google Scholar]
- Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER.2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 1996;14:737–44. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- Bayless KJ, Salazar R, Davis GE. RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. Am. J. Pathol. 2000;156:1673–83. doi: 10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer AJ, Haubner R, Sarbia M, et al. Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin alpha(v)beta3 expression in man. Clin. Cancer. Res. 2006;12:3942–9. doi: 10.1158/1078-0432.CCR-06-0266. [DOI] [PubMed] [Google Scholar]
- Belvisi L, Riccioni T, Marcellini M, et al. Biological and molecular properties of a new alpha(v)beta3/alpha(v)beta5 integrin antagonist. Mol. Cancer. Ther. 2005;4:1670–80. doi: 10.1158/1535-7163.MCT-05-0120. [DOI] [PubMed] [Google Scholar]
- Belvisi L, Bernardi A, Colombo M, et al. Targeting integrins: insights into structure and activity of cyclic RGD pentapeptide mimics containing azabicycloalkane amino acids. Bioorg. Med. Chem. 2006;14:169–80. doi: 10.1016/j.bmc.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Berman AE, Kozlova NI. Integrins: structure and functions. Membr. Cell. Biol. 2000;13:207–44. [PubMed] [Google Scholar]
- Binnerts ME, van Kooyk Y, Edwards CP, et al. Antibodies that selectively inhibit leukocyte function-associated antigen 1 binding to intercellular adhesion molecule-3 recognize a unique epitope within the CD11a I domain. J. Biol. Chem. 1996;271:9962–8. doi: 10.1074/jbc.271.17.9962. [DOI] [PubMed] [Google Scholar]
- Bon G, Folgiero V, Di Carlo S, et al. Involvement of alpha6beta4 integrin in the mechanisms that regulate breast cancer progression. Breast Cancer Res. 2007;9:203. doi: 10.1186/bcr1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorsi L, Carloni V, Muratori M, et al. Androgen receptor expression in prostate carcinoma cells suppresses alpha6beta4 integrin-mediated invasive phenotype. Endocrinology. 2000;141:3172–82. doi: 10.1210/endo.141.9.7640. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Wennerberg K, Krell HW, et al. β1 integrin promotes but is not essential for metastasis of ras-myc transformed fibroblasts. Oncogene. 1999;18:3852–61. doi: 10.1038/sj.onc.1202770. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brower DL, Brower SM, Hayward DC. Molecular evolution of integrins: genes encoding integrin beta subunits from a coral and a sponge. Proc. Natl. Acad. Sci., U.S.A. 1997;94:9182–87. doi: 10.1073/pnas.94.17.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Pilling D, Henriquez NV, et al. RGD peptides induce apoptosis by direct caspase-3 activation. Nature. 1999;397:534–9. doi: 10.1038/17409. [DOI] [PubMed] [Google Scholar]
- Burgeson RE, Christiano AM. The dermal-epidermal junction. Curr. Opin. Cell. Biol. 1997;9:651–8. doi: 10.1016/s0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- Byzova TV, Goldman CK, Pampori N. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell. 2000;6:851–60. [PubMed] [Google Scholar]
- Cardones AR, Banez LL. VEGF inhibitors in cancer therapy. Curr. Pharm. Des. 2006;12:387–94. doi: 10.2174/138161206775201910. [DOI] [PubMed] [Google Scholar]
- Cao Q, Cai W, Li T, et al. Combination of integrin siRNA and irradiation for breast cancer therapy. Biochem. Biophys. Res. Commun. 2006;351:726–32. doi: 10.1016/j.bbrc.2006.10.100. [DOI] [PubMed] [Google Scholar]
- Carron CP, Meyer DM, Engleman VW, et al. Peptidomimetic antagonists of alphavbeta3 inhibit bone resorption by inhibiting osteoclast bone resorptive activity, not osteoclast adhesion to bone. J. Endocrinol. 2000;165:587–98. doi: 10.1677/joe.0.1650587. [DOI] [PubMed] [Google Scholar]
- Carpenter AE, Sabatini DM. Systematic genome-wide screens of gene function. Nature Rev. Genet. 2004;5:11–22. doi: 10.1038/nrg1248. [DOI] [PubMed] [Google Scholar]
- Chan BM, Kassner PD, Schiro JA, et al. Distinct cellular functions mediated by different VLA integrin alpha subunit cytoplasmic domains. Cell. 1992;68:1051–60. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Chow TW, Hellums JD, Moake JL, et al. Shear stress-induced von Willebrand factor binding to platelet glycoprotein Ib initiates calcium influx associated with aggregation. Blood. 1992;80:113–20. [PubMed] [Google Scholar]
- Cresswell J, Wong WK, Henry MJ, et al. Adhesion of lymphocytes to bladder cancer cells: the role of the alpha(E)beta(7) integrin. Cancer Immunol. Immunother. 2002;51:483–91. doi: 10.1007/s00262-002-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KH, Kim SH, Han KY, et al. Inhibitory effect of salmosin, a Korean snake venom-derived disintegrin, on the integrin alphaV-mediated proliferation of SK-Mel-2 human melanoma cells. J. Pharm. Pharmacol. 2003;55:1577–82. doi: 10.1211/0022357022160. [DOI] [PubMed] [Google Scholar]
- Chung J, Mercurio AM. Contributions of the alpha6 integrins to breast carcinoma survival and progression. Mol. Cells. 2004;17:203–9. [PubMed] [Google Scholar]
- Coller BS. Anti-GPIIb/IIIa drugs: current strategies and future directions. Thromb. Haemost. 2001;86:427–43. [PubMed] [Google Scholar]
- Colombatti A, Bonaldo P, Doliana R. Type A modules: interacting domains found in several non-fibrillar collagens and in other extracellular matrix proteins. Matrix. 1993;13:297–306. doi: 10.1016/s0934-8832(11)80025-9. [DOI] [PubMed] [Google Scholar]
- Conway EE., Jr Central nervous system findings and intussusception: how are they related? Pediatr. Emerg. Care. 1993;9:15–8. doi: 10.1097/00006565-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Cruz-Monserrate Z, Qiu S, Evers BM, et al. Upregulation and redistribution of integrin alpha6beta4 expression occurs at an early stage in pancreatic adenocarcinoma progression. Mod. Pathol. 2007;20:656–67. doi: 10.1038/modpathol.3800782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangerfield J, Larbi KY, Huang MT, et al. PECAM-1 (CD31) homophilic interaction up-regulates alpha6beta1 on transmigrated neutrophils in vivo and plays a functional role in the ability of alpha6 integrins to mediate leukocyte migration through the perivascular basement membrane. J. Exp. Med. 2002;196:1201–11. doi: 10.1084/jem.20020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BL, Paulson HL. Molecular medicine for the brain: silencing of disease genes with RNA interference. Lancet Neurol. 2004;3:145–9. doi: 10.1016/S1474-4422(04)00678-7. [DOI] [PubMed] [Google Scholar]
- Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem. Biophys. Res. Commun. 1992;182:1025–31. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- Davis TL, Cress AE, Dalkin BL, et al. Unique expression pattern of the alpha6beta4 integrin and laminin-5 in human prostate carcinoma. Prostate. 2001;46:240–8. doi: 10.1002/1097-0045(20010215)46:3<240::aid-pros1029>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fougerolles AR, Springer TA. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J. Exp. Med. 1992;175:185–90. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechantsreiter MA, Planker E, Matha B, et al. N-methylated cyclic RGD peptides as highly active and selective a(V)h(3) integrin antagonists. J. Med. Chem. 1999;42:3033–40. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- Della Morte R, Squillacioti C, Garbi C, et al. Echistatin inhibits pp125FAK autophosphorylation, paxillin phosphorylation and pp125FAK-paxillin interaction in fibronectin-adherent melanoma cells. Eur. J. Biochem. 2000;267:5047–54. doi: 10.1046/j.1432-1327.2000.01561.x. [DOI] [PubMed] [Google Scholar]
- Demetriou MC, Pennington ME, Nagle RB, et al. Extracellular alpha 6 integrin cleavage by urokinase-type plasminogen activator in human prostate cancer. Exp. Cell. Res. 2004;294:550–8. doi: 10.1016/j.yexcr.2003.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer A, Vetter M, Schunke D, et al. Parathyroid hormone-related protein regulates tumor-relevant genes in breast cancer cells. J. Biol. Chem. 2006;281:14563–72. doi: 10.1074/jbc.M510527200. [DOI] [PubMed] [Google Scholar]
- Duque H, LaRocco M, Golde WT, et al. Interactions of foot-and-mouth disease virus with soluble bovine alpha v beta 3 and alphav beta 6 integrins. J. Virol. 2004;78:9773–81. doi: 10.1128/JVI.78.18.9773-9781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Weber K. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Klemke R, Stromblad S, et al. Integrin alphaV-beta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. Journal of Cellular Biology. 1998;140:1255–63. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri BP. Integrin and growth factor receptor crosstalk. Circ. Res. 2001;89:1104–10. doi: 10.1161/hh2401.101084. [DOI] [PubMed] [Google Scholar]
- Enserink JM, Methi T, Mahic M, et al. The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. J. Biol. Chem. 2004;279:44889–96. doi: 10.1074/jbc.M404599200. [DOI] [PubMed] [Google Scholar]
- Erdreich-Epstein A, Shimada H, Groshen S, et al. Integrins alpha(v)beta3 and alpha(v)beta5 are expressed by endothelium of high-risk neuroblastoma and their inhibition is associated with increased endogenous ceramide. Cancer Res. 2000;60:712–21. [PubMed] [Google Scholar]
- Falcioni R, Antonini A, Nistico P, et al. Alpha 6 beta 4 and alpha 6 beta 1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp. Cell. Res. 1997;236:76–85. doi: 10.1006/excr.1997.3695. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B, Fransvea E, O’Toole TE, et al. Involvement of tumor cell integrin alpha v beta 3 in hematogenous metastasis of human melanoma cells. Clin. Exp. Metastasis. 2002;19:427–36. doi: 10.1023/a:1016377114119. [DOI] [PubMed] [Google Scholar]
- Ferrer FA, Miller LJ, Andrawis RI, et al. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology. 1998;51:161–7. doi: 10.1016/s0090-4295(97)00491-3. [DOI] [PubMed] [Google Scholar]
- Ferrer FA, Miller LJ, Lindquist R, et al. Expression of vascular endothelial growth factor receptors in human prostate cancer. Urology. 1999;54:567–72. doi: 10.1016/s0090-4295(99)00156-9. [DOI] [PubMed] [Google Scholar]
- Folgiero V, Bachelder RE, Bon G, et al. The alpha6beta4 integrin can regulate ErbB-3 expression: implications for alpha6beta4 signaling and function. Cancer Res. 2007;67:1645–52. doi: 10.1158/0008-5472.CAN-06-2980. [DOI] [PubMed] [Google Scholar]
- Francis SE, Goh KL, Hodivala-Dilke K, et al. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arteriosclerosis Thrombosis and Vascular Biology. 2002;22:927–33. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- Frieser M, Hallmann R, Johansson S, et al. Mouse polymorpho-nuclear granulocyte binding to extracellular matrix molecules involves beta 1 integrins. Eur. J. Immunol. 1996;26:3127–36. doi: 10.1002/eji.1830261245. [DOI] [PubMed] [Google Scholar]
- Fu WM, Chang TK, Sun WZ, et al. Inhibition of neuropathic pain by a potent disintegrin—triflavin. Neurosci Lett. 2004;368:263–8. doi: 10.1016/j.neulet.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Kikkawa Y, Sanzen N, et al. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins. J. Biol. Chem. 2001;276:17550–8. doi: 10.1074/jbc.M010155200. [DOI] [PubMed] [Google Scholar]
- Galliher AJ, Schiemann WP. Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R.42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganju P, Hall J. Potential applications of siRNA for pain therapy. Expert Opin. Biol. Ther. 2004;4:531–42. doi: 10.1517/14712598.4.4.531. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Hynes RO. Interaction of fibronectin with its receptor on platelets. Cell. 1985;42:439–48. doi: 10.1016/0092-8674(85)90101-1. [DOI] [PubMed] [Google Scholar]
- Gettner SN, Kenyon C, Reichardt LF. Characterization of beta pat-3 heterodimers, a family of essential integrin receptors in C. elegans. J. Cell. Biol. 1995;129:1127–41. doi: 10.1083/jcb.129.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Sgarra C, Di Naro E, et al. Endometriosis is characterized by an impaired localization of laminin-5 and alpha3beta1 integrin receptor. Int. J. Gynecol. Cancer. 2007;17:242–7. doi: 10.1111/j.1525-1438.2006.00750.x. [DOI] [PubMed] [Google Scholar]
- Gladson CL, Hancock S, Arnold MM, et al. Stage-specific expression of integrin alphaVbeta3 in neuroblastic tumors. Am. J. Pathol. 1996;148:1423–34. [PMC free article] [PubMed] [Google Scholar]
- Golubkov V, Hawes D, Markland FS. Anti-angiogenic activity of contortrostatin, a disintegrin from Agkistrodon contortrix contortrix snake venom. Angiogenesis. 2003;6:213–24. doi: 10.1023/B:AGEN.0000021396.47009.b0. [DOI] [PubMed] [Google Scholar]
- Gould RJ, Polokoff MA, Friedman PA, et al. Disintegrins: a family of integrin inhibitory proteins from viper venoms. Proc. Soc. Exp. Biol. Med. 1990;195:168–71. doi: 10.3181/00379727-195-43129b. [DOI] [PubMed] [Google Scholar]
- Gramoun A, Shorey S, Bashutski JD, et al. Effects of Vitaxin(R), a novel therapeutic in trial for metastatic bone tumors, on osteoclast functions in vitro. J. Cell. Biochem. 2007;102:341–52. doi: 10.1002/jcb.21296. [DOI] [PubMed] [Google Scholar]
- Green PM, Ludbrook SB, Miller DD, et al. Structural elements of the osteopontin SVVYGLR. motif important for the interaction with alpha(4) integrins. FEBS Lett. 2001;503:75–9. doi: 10.1016/s0014-5793(01)02690-4. [DOI] [PubMed] [Google Scholar]
- Grzesiak JJ, Bouvet M. Determination of the ligand-binding specificities of the alpha2beta1 and alpha1beta1 integrins in a novel 3-dimensional in vitro model of pancreatic cancer. Pancreas. 2007;34:220–8. doi: 10.1097/01.mpa.0000250129.64650.f6. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Velling T, Sjoberg G, et al. Up-regulation of a novel integrin alpha-chain (alpha mt) on human fetal myotubes. Dev. Dyn. 1995;204:57–65. doi: 10.1002/aja.1002040108. [DOI] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumor progression. Nat. Rev. Mol. Cell. Biol. 2004;5:816–26. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Guo HB, Lee I, Bryan BT, et al. Deletion of mouse embryo fibroblast N. acetylglucosaminyltransferase V stimulates alpha5beta1 integrin expression mediated by the protein kinase C signaling pathway. J. Biol. Chem. 2005;280:8332–42. doi: 10.1074/jbc.M413532200. [DOI] [PubMed] [Google Scholar]
- Guo W, Pylayeva Y, Pepe A, et al. β4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 2006;126:489–502. doi: 10.1016/j.cell.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Gutheil JC, Campbell TN, Pierce PR, et al. Targeted antiangiogenic therapy for cancer using vitaxin: A humanized monoclonal antibody to the integrin αVβ3. Clinical Cancer Research. 2000;6:3056–61. [PubMed] [Google Scholar]
- Haier J, Goldmann U, Hotz B, et al. Inhibition of tumor progression and neoangiogenesis using cyclic RGD-peptides in a chemically induced colon carcinoma in rats. Clin. Exp. Metastasis. 2002;19:665–72. doi: 10.1023/a:1021316531912. [DOI] [PubMed] [Google Scholar]
- Hallahan DE, Qu S, Geng L, et al. Radiation-mediated control of drug delivery. Am. J. Clin. Oncol. 2001;24:473–80. doi: 10.1097/00000421-200110000-00012. [DOI] [PubMed] [Google Scholar]
- Harms JF, Welch DR, Samant RS, et al. A small molecule antagonist of the alpha(v)beta3 integrin suppresses MDA-MB-435 skeletal metastasis. Clin. Exp. Metastasis. 2004;21:119–28. doi: 10.1023/b:clin.0000024763.69809.64. [DOI] [PubMed] [Google Scholar]
- Haubner R, Kuhnast B, Mang C, et al. [18F]Galacto-RGD: synthesis, radiolabelling, metabolic stability and radiation dose estimates. Bioconjug. Chem. 2004;15:61–9. doi: 10.1021/bc034170n. [DOI] [PubMed] [Google Scholar]
- Haubner R, Wester HJ, Weber WA, et al. Noninvasive imaging of vß3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001;61:1781–5. [PubMed] [Google Scholar]
- Hedin U, Roy J, Tran PK. Control of smooth muscle cell proliferation in vascular disease. Curr. Opin. Lipidol. 2004;15:559–65. doi: 10.1097/00041433-200410000-00010. [DOI] [PubMed] [Google Scholar]
- Heino J. The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biol. 2000;19:319–23. doi: 10.1016/s0945-053x(00)00076-7. [DOI] [PubMed] [Google Scholar]
- Herold-Mende C, Kartenbeck J, Tomakidi P, et al. Metastatic growth of squamous cell carcinomas is correlated with upregulation and redistribution of hemidesmosomal components. Cell. Tissue. Res. 2001;306:399–408. doi: 10.1007/s004410100462. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Iglesias A, Potocnik AJ, et al. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996;380:171–5. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- Hollenbeck S, Itoh H, Louie O, et al. Type I collagen synergistically enhances PDGF-induced smooth muscle cell proliferation through pp60src-dependent crosstalk between the alpha2beta1 integrin and PDGF beta receptor. Biochem. Biophys. Res. Commun. 2004;325:328–37. doi: 10.1016/j.bbrc.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Huang X, Wu J, Zhu W, et al. Expression of the human integrin beta6 subunit in alveolar type II cells and bronchiolar epithelial cells reverses lung inflammation in beta6 knockout mice. Am. J. Respir. Cell. Mol. Biol. 1998;19:636–42. doi: 10.1165/ajrcmb.19.4.3293. [DOI] [PubMed] [Google Scholar]
- Humphries MJ. Integrin structure. Biochem. Soc. Trans. 2000;28:311–39. [PubMed] [Google Scholar]
- Hsia DA, Lim ST, Bernard-Trifilo JA, et al. Integrin alpha4beta1 promotes focal adhesion kinase-independent cell motility via alpha4 cytoplasmic domain-specific activation of c-Src. Mol. Cell. Biol. 2005;25:9700–12. doi: 10.1128/MCB.25.21.9700-9712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Ochiai A, Yamada T, et al. Integrin alpha6beta4 as a suppressor and a predictive marker for peritoneal dissemination in human gastric cancer. Gastroenterology. 2000;118:497–506. doi: 10.1016/s0016-5085(00)70255-1. [DOI] [PubMed] [Google Scholar]
- Jackson T, Clark S, Berryman S, et al. Integrin {alpha}v{beta}8 Functions as a Receptor for Foot-and-Mouth Disease Virus: Role of the {beta}-Chain Cytodomain in Integrin-Mediated Infection. J. Virol. 2004;78:4533–40. doi: 10.1128/JVI.78.9.4533-4540.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T, Mould AP, Sheppard D, et al. Integrin alpha v beta1 is a receptor for foot-and-mouth disease virus. J. Virol. 2002;76:935–41. doi: 10.1128/JVI.76.3.935-941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T, Sharma A, Ghazaleh RA, et al. Arginineglycine-aspartic acid-specific binding by foot-and-mouth disease viruses to the purified integrin alpha v beta 3 in vitro. J. Virol. 1997;71:8357–61. doi: 10.1128/jvi.71.11.8357-8361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T, Sheppard D, Denyer M, Blakemore W, King AM. The epithelial integrin alpha v beta 6 is a receptor for foot-and-mouth disease virus. J. Virol. 2000;74:4949–56. doi: 10.1128/jvi.74.11.4949-4956.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y, Matter M, Pai JT, et al. A mitochondrial protein, Bit1, mediates apoptosis regulated by integrins and Groucho/TLE corepressors. Cell. 2004;116:751–62. doi: 10.1016/s0092-8674(04)00204-1. [DOI] [PubMed] [Google Scholar]
- Jo M, Thomas KS, Wu L, et al. Soluble urokinase-type plasminogen activator receptor inhibits cancer cell growth and invasion by direct urokinase-independent effects on cell signaling. J. Biol. Chem. 2003;278:46692–8. doi: 10.1074/jbc.M308808200. [DOI] [PubMed] [Google Scholar]
- Jodele S, Chantrain CF, Blavier L, et al. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005;65:3200–8. doi: 10.1158/0008-5472.CAN-04-3770. [DOI] [PubMed] [Google Scholar]
- Jones J, Watt FM, Speight PM. Changes in the expression of alpha v integrins in oral squamous cell carcinomas. J. Oral Pathol. Med. 1997;26:63–8. doi: 10.1111/j.1600-0714.1997.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Joshi P, Chung CY, Aukhil I, et al. Endothelial cells adhere to the RGD domain and the fibrinogen-like terminal knob of tenascin. J. Cell. Sci. 1993;106:389–400. doi: 10.1242/jcs.106.1.389. [DOI] [PubMed] [Google Scholar]
- Kallio JP, Mikkelsson J, Tammela TL, et al. Genetic variation in platelet integrin alpha IIb beta 3 (GPIIb/IIIa) and the metastatic potential of renal cell carcinoma. BJU Int. 2006;9:201–4. doi: 10.1111/j.1464-410X.2006.06196.x. [DOI] [PubMed] [Google Scholar]
- Kang IC, Lee YD, Kim DS. A novel disintegrin salmosin inhibits tumor angiogenesis. Cancer Res. 1999;59:3754–60. [PubMed] [Google Scholar]
- Karczewski J, Knudsen KA, Smith L. The interaction of thrombospondin with platelet glycoprotein GPIIb-IIIa. J. Biol. Chem. 1989;264:21322–6. [PubMed] [Google Scholar]
- Karadag A, Ogbureke KU, Fedarko NS, et al. Bone sialoprotein, matrix metalloproteinase 2, and alpha(v)beta3 integrin in osteotropic cancer cell invasion. J. Natl. Cancer Inst. 2004;96:956–65. doi: 10.1093/jnci/djh169. [DOI] [PubMed] [Google Scholar]
- Kennel SJ, Foote LJ, Falcioni R, et al. Analysis of the tumor-associated antigen TSP-180. Identity with α6β4 in the integrin superfamily. J. Biol. Chem. 1989;264:15515–21. [PubMed] [Google Scholar]
- Kerr JS, Slee AM, Mousa SA. The alpha V integrin antagonists as novel anticancer agents: an update. Expert Opin. Investig. Drugs. 2002;11:1765–74. doi: 10.1517/13543784.11.12.1765. [DOI] [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa SA, et al. Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. American Journal of Pathology. 2000;156:1345–62. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Toyoshima T, Kojima T, et al. Entactin-2: a new member of basement membrane protein with high homology to entactin/nidogen. Exp. Cell. Res. 1998;241:36–45. doi: 10.1006/excr.1998.4016. [DOI] [PubMed] [Google Scholar]
- Kippenberger S, Loitsch S, Muller J, et al. Ligation of the beta4 integrin triggers adhesion behavior of human keratinocytes by an “inside-out” mechanism. J. Invest. Dermatol. 2004;123:444–51. doi: 10.1111/j.0022-202X.2004.23323.x. [DOI] [PubMed] [Google Scholar]
- Kloczewiak M, Timmons S, Bednarek MA. Platelet receptor recognition domain on the gamma chain of human fibrinogen and its synthetic peptide analogues. Biochemistry. 1989;28:2915–9. doi: 10.1021/bi00433a025. [DOI] [PubMed] [Google Scholar]
- Kramer RH, Vu MP, Cheng YF, et al. Laminin-binding integrin alpha 7 beta 1: functional characterization and expression in normal and malignant melanocytes. Cell. Regul. 1991;2:805–17. doi: 10.1091/mbc.2.10.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar CC. Integrin αvβ3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr. Drug. Targets. 2003;4:123–31. doi: 10.2174/1389450033346830. [DOI] [PubMed] [Google Scholar]
- Kumar CC, Malkowski M, Yin Z, et al. Inhibition of angiogenesis and tumor growth by SCH221153, a dual alpha(v)beta3 and alpha(v)beta5 integrin receptor antagonist. Cancer Res. 2001;61:2232–8. [PubMed] [Google Scholar]
- Kuwada SK, Kuang J, Li X. Integrin alpha5/beta1 expression mediates HER-2 down-regulation in colon cancer cells. J. Biol. Chem. 2005;280:19027–35. doi: 10.1074/jbc.M410540200. [DOI] [PubMed] [Google Scholar]
- Lee JW, Juliano RL. Alpha5beta1 integrin protects intestinal epithelial cells from apoptosis through a phosphatidylinositol 3-kinase and protein kinase B-dependent pathway. Mol. Biol. Cell. 2000;11:1973–87. doi: 10.1091/mbc.11.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Redick SD, Erickson HP, et al. Force measurements of the alpha5beta1 integrin-fibronectin interaction. Biophys. J. 2003;84:1252–62. doi: 10.1016/S0006-3495(03)74940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tan H, Dong X, et al. Antisense integrin alphaV and beta3 gene therapy suppresses subcutaneously implanted hepatocellular carcinomas. Dig. Liver Dis. 2007;39:557–65. doi: 10.1016/j.dld.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Lipscomb EA, Dugan AS, Rabinovitz I, et al. Use of RNA interference to inhibit integrin (alpha6beta4)-mediated invasion and migration of breast carcinoma cells. Clin. Exp. Metastasis. 2003;20:569–76. doi: 10.1023/a:1025819521707. [DOI] [PubMed] [Google Scholar]
- Lipscomb EA, Simpson KJ, Lyle SR, et al. The alpha6beta4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res. 2005;65:10970–6. doi: 10.1158/0008-5472.CAN-05-2327. [DOI] [PubMed] [Google Scholar]
- Liu B, Ordonez-Ercan D, Fan Z, et al. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int. J. Cancer. 2007;120:874–82. doi: 10.1002/ijc.22423. [DOI] [PubMed] [Google Scholar]
- Lopez-Conejo MT, Olmo N, Turnay J, et al. Interaction of fibro-nectin with human colon adenocarcinoma cells: effect on the in vivo tumorigenic capacity. Oncology. 2002;62:371–80. doi: 10.1159/000065070. [DOI] [PubMed] [Google Scholar]
- Lopez MA, Mayer U, Hwang W, et al. Force transmission, compliance, and viscoelasticity are altered in the alpha7-integrin-null mouse diaphragm. Am. J. Physiol. Cell. Physiol. 2005;288:C282–9. doi: 10.1152/ajpcell.00362.2003. [DOI] [PubMed] [Google Scholar]
- Maglott A, Bartik P, Cosgun S, et al. The small alpha5beta1 integrin antagonist, SJ749, reduces proliferation and clonogenicity of human astrocytoma cells. Cancer Res. 2006;66:6002–7. doi: 10.1158/0008-5472.CAN-05-4105. [DOI] [PubMed] [Google Scholar]
- Marinelli L, Meyer A, Heckmann D, et al. Ligand binding analysis for human alpha5beta1 integrin: strategies for designing new alpha5beta1 integrin antagonists. J. Med. Chem. 2005;48:4204–7. doi: 10.1021/jm040224i. [DOI] [PubMed] [Google Scholar]
- Markland FS, Shieh K, Zhou Q, et al. A novel snake venom dis-integrin that inhibits human varian cancer dissemination and angiogenesis in an orthotopic nude mouse model. Haemostasis. 2001;31:183–91. doi: 10.1159/000048062. [DOI] [PubMed] [Google Scholar]
- Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–9. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz C, Weinreb PH, Calvete JJ, et al. Obtustatin: a potent selective inhibitor of alpha1beta1 integrin in vitro and angiogenesis in vivo. Cancer Res. 2003;63:2020–3. [PubMed] [Google Scholar]
- Martin PL, Jiao Q, Cornacoff J, et al. Absence of adverse effects in cynomolgus macaques treated with CNTO 95, a fully human anti-alpha V integrin monoclonal antibody, despite widespread tissue binding. Clin. Cancer. Res. 2005;11:6959–65. doi: 10.1158/1078-0432.CCR-04-2623. [DOI] [PubMed] [Google Scholar]
- Maubant S, Saint-Dizier D, Boutillon M, et al. Blockade of alpha V beta3 and alpha v beta5 integrins by RGD mimetics induces anoikis and not integrin-mediated death in human endothelial cells. Blood. 2006;108:3035–44. doi: 10.1182/blood-2006-05-023580. [DOI] [PubMed] [Google Scholar]
- McCabe NP, De S, Vasanji A, et al. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–43. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh KP, Hodivala-Dilke K, Zheng MH, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 2000;105:433–40. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLane MA, Sanchez EE, Wong A, et al. Disintegrins. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004;4:327–55. doi: 10.2174/1568006043335880. [DOI] [PubMed] [Google Scholar]
- McNeel DG, Eickhoff J, Lee FT, et al. Phase I trial of a monoclonal antibody specific for alphavbeta3 integrin (MEDI-522) in patients with advanced malignancies, including an assessment of effect on tumor perfusion. Clin. Cancer Res. 2005;11:7851–60. doi: 10.1158/1078-0432.CCR-05-0262. [DOI] [PubMed] [Google Scholar]
- McQuade P, Knight LC. Radiopharmaceuticals for targeting the angiogenesis marker αvβ3. Q. J. Nucl. Med. 2003;47:209–20. [PubMed] [Google Scholar]
- McQuade P, Knight LC, Welch MJ. Evaluation of 64Cu- and 125I-radiolabeled bitistatin as potential agents for targeting alphavbeta3 integrins in tumor angiogenesis. Bioconjug Chem. 2004;15:988–96. doi: 10.1021/bc049961j. [DOI] [PubMed] [Google Scholar]
- Meerovitch K, Bergeron F, Leblond L, et al. A novel RGD antagonist that targets both alphavbeta3 and alpha5beta1 induces apoptosis of angiogenic endothelial cells on type I collagen. Vascul Pharmacol. 2003;40:77–89. doi: 10.1016/s1537-1891(02)00339-7. [DOI] [PubMed] [Google Scholar]
- Mercurio AM, Rabinovitz I, Shaw LM. The alpha 6 beta 4 integrin and epithelial cell migration. Curr. Opin. Cell. Biol. 2001;13:541–5. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- Minagar A, Sheremata WA, Vollmer TL, et al. Reduction of relapses in multiple sclerosis after anti-alpha4 integrin antibody (natalizumab) International Journal of MS Care. 2000;3:1–6. Available at http://www.mscare.com. [Google Scholar]
- Mitjans F, Meyer T, Fittschen C, et al. In vivo therapy of malignant melanoma by means of antagonists of alphav integrins. Int. J. Cancer. 2000;87:716–23. [PubMed] [Google Scholar]
- Mitjans F, Sander D, Adan J, et al. An anti-alpha v-integrin antibody that blocks integrin function inhibits the development of a human melanoma in nude mice. J. Cell. Sci. 1995;108:2825–38. doi: 10.1242/jcs.108.8.2825. [DOI] [PubMed] [Google Scholar]
- Miyata S, Koshikawa N, Yasumitsu H, et al. Trypsin stimulates integrin alpha(5)beta(1)-dependent adhesion to fibronectin and proliferation of human gastric carcinoma cells through activation of proteinase-activated receptor-2. J. Biol. Chem. 2000;275:4592–8. doi: 10.1074/jbc.275.7.4592. [DOI] [PubMed] [Google Scholar]
- Mousa SA. Anti-integrin as novel drug-discovery targets: potential therapeutic and diagnostic implications. Curr. Opin. Chem. Biol. 2002;6:534–41. doi: 10.1016/s1367-5931(02)00350-2. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, et al. The integrin alpha v beta 8 mediates epithelial homeostasis through MT1-MMPdependent activation of TGF-beta1. J. Cell. Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ, Zhang L, Chang ER, et al. Functional interaction between the integrin antagonist neutrophil inhibitory factor and the I domain of CD11b/CD18. J. Biol. Chem. 1994;269:26419–23. [PubMed] [Google Scholar]
- Mullamitha SA, Ton NC, Parker GJ, et al. Phase I evaluation of a fully human anti-alphav integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin. Cancer Res. 2007;13:2128–35. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Murillo CA, Rychahou PG, Evers BM. Inhibition of alpha5 integrin decreases PI3K activation and cell adhesion of human colon cancers. Surgery. 2004;136:143–9. doi: 10.1016/j.surg.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Niewiarowski S, McLane MA, Kloczewiak M, et al. Disintegrins and other naturally occurring antagonists of platelet fibrinogen receptors. Semin. Hematol. 1994;31:289–300. [PubMed] [Google Scholar]
- O’Connor P, Goodman A, Willmer-Hulme AJ, et al. Randomized multicentre trial of natalizumab in acute MS relapses: clinical and MRI effects. Neurology. 2004;62:2038–43. doi: 10.1212/01.wnl.0000128136.79044.d6. [DOI] [PubMed] [Google Scholar]
- O’Connor P, Miller D, Riester K, et al. Relapse rates and enhancing lesions in a phase II trial of natalizumab in multiple sclerosis. Multiple Sclerosis. 2005;11:568–72. doi: 10.1191/1352458505ms1205oa. [DOI] [PubMed] [Google Scholar]
- Paddison PJ, Hannon GJ. siRNAs and shRNAs: skeleton keys to the human genome. Curr. Opin. Mol. Ther. 2003;5:217–22. [PubMed] [Google Scholar]
- Parise LV, Lee J, Juliano RL. New aspects of integrin signaling in cancer. Semin. Cancer Biol. 2000;10:407–14. doi: 10.1006/scbi.2000.0337. [DOI] [PubMed] [Google Scholar]
- Paulhe F, Racaud-Sultan C, Ragab A, et al. Differential regulation of phosphoinositide metabolism by alphaVbeta3 and alphaVbeta5 integrins upon smooth muscle cell migration. J. Biol. Chem. 2001;276:41832–40. doi: 10.1074/jbc.M105459200. [DOI] [PubMed] [Google Scholar]
- Pawar SC, Demetriou MC, Nagle RB, et al. Integrin alpha6 cleavage: a novel modification to modulate cell migration. Exp. Cell. Res. 2007;313:1080–9. doi: 10.1016/j.yexcr.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlino E, Lovecchio M, Vacca RA, et al. Regulation of mRNA and protein levels of beta1 integrin variants in human prostate carcinoma. Am. J. Pathol. 2000;157:1727–34. doi: 10.1016/s0002-9440(10)64809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova SN, Barczyk M, Tiger CF, et al. {alpha}11{beta}1 Integrin-Dependent Regulation of Periodontal Ligament Function in the Erupting Mouse Incisor. Mol. Cell. Biol. 2007;27:4306–16. doi: 10.1128/MCB.00041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc. Natl. Acad. Sci., U.S.A. 1985;82:5766–70. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi N, Hung W, Tremblay E, et al. c-Src kinase activity is required for hepatocyte growth factor-induced motility and anchorage-independent growth of mammary carcinoma cells. J. Biol. Chem. 1998;273:33714–21. doi: 10.1074/jbc.273.50.33714. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan V, Bhaskar V, Law DA, et al. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J. Exp. Ther. Oncol. 2006;5:273–86. [PubMed] [Google Scholar]
- Ramos DM, But M, Regezi J, et al. Expression of integrin beta 6 enhances invasive behavior in oral squamous cell carcinoma. Matrix Biol. 2002;21:297–307. doi: 10.1016/s0945-053x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- Reinmuth N, Liu W, Ahmad SA, et al. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–87. [PubMed] [Google Scholar]
- Reynolds AR, Reynolds LE, Nagel TE, et al. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in β3-integrin-deficient mice. Cancer Research. 2004;64:8643–50. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- Rhodes A. Developing a cell line standard for HER2/neu. Cancer Biomark. 2005;1:229–32. doi: 10.3233/cbm-2005-14-505. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Rodier JM, Valles A, Denoyelle M. pp60c-src is a positive regulator of growth factor-induced cell scattering in a rat bladder carcinoma cell line. J. Cell. Biol. 1995;131:761–73. doi: 10.1083/jcb.131.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg C, Mariotti A. Vascular integrins: Pleiotropic adhesion and signaling molecules in vascular homeostasis and angiogenesis. Cellular and Molecular Life Sciences. 2003;60:1135–57. doi: 10.1007/s00018-003-2297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri ZM, De Marco L, Gatti L. Platelets have more than one binding site for von Willebrand factor. J. Clin. Invest. 1983;72:1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabherwal Y, Rothman VL, Dimitrov S, et al. Integrin alpha2beta1 mediates the anti-angiogenic and anti-tumor activities of angiocidin, a novel tumor-associated protein. Exp. Cell. Res. 2006;312:2443–53. doi: 10.1016/j.yexcr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Sakai T, Zhang Q, Fassler R, et al. Modulation of beta1A integrin functions by tyrosine residues in the beta1 cytoplasmic domain. J. Cell. Biol. 1998;141:527–38. doi: 10.1083/jcb.141.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough RM, Kleiman NS, Phillips DR. Platelet Glyco-protein IIb/IIIa Antagonists : What Are the Relevant Issues Concerning Their Pharmacology and Clinical Use? Circulation. 1999;100:437–44. doi: 10.1161/01.cir.100.4.437. [DOI] [PubMed] [Google Scholar]
- Schnapp LM, Breuss JM, Ramos DM, et al. Sequence and tissue distribution of the human integrin alpha 8 subunit: a beta 1-associated alpha subunit expressed in smooth muscle cells. J. Cell. Sci. 1995;108:537–44. doi: 10.1242/jcs.108.2.537. [DOI] [PubMed] [Google Scholar]
- Shen X, Falzon M. PTH-related protein upregulates integrin alpha6beta4 expression and activates Akt in breast cancer cells. Exp. Cell. Res. 2006;312:3822–34. doi: 10.1016/j.yexcr.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Shen X, Mula RV, Evers BM, et al. Increased cell survival, migration, invasion, and Akt expression in PTHrP-overexpressing LoVo colon cancer cell lines. Regul. Pept. 2007;141:61–72. doi: 10.1016/j.regpep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, Streit M, et al. The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am. J. Pathol. 2002;160:195–204. doi: 10.1016/s0002-9440(10)64363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the alpha6beta4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol. Cell. Biol. 2001;21:5082–93. doi: 10.1128/MCB.21.15.5082-5093.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu JR, Wu CH, Chen YC, et al. Mechanisms in the inhibition of neointimal hyperplasia with triflavin in a rat model of balloon angioplasty. J. Lab. Clin. Med. 2001;137:270–8. doi: 10.1067/mlc.2001.114065. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Springer TA. Therapeutic antagonists and the conformational regulation of the beta2 integrins. Curr. Top. Med. Chem. 2004;4:1485–95. doi: 10.2174/1568026043387575. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Thery C, Hamard G, et al. Lactadherin promotes VEGF-dependent neovascularization. Nature Medicine. 2005;11:499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- Song E, Lee SK, Wang J, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nature Med. 2003;9:347–51. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- Stroeken PJ, van Rijthoven EA, Boer E, et al. Cytoplasmic domain mutants of beta1 integrin, expressed in beta 1-knockout lymphoma cells, have distinct effects on adhesion, invasion and metastasis. Oncogene. 2000;19:1232–8. doi: 10.1038/sj.onc.1203423. [DOI] [PubMed] [Google Scholar]
- Sabherwal Y, Rothman VL, Dimitrov S, et al. Integrin alpha2beta1 mediates the anti-angiogenic and anti-tumor activities of angiocidin, a novel tumor-associated protein. Exp. Cell. Res. 2006;312:2443–53. doi: 10.1016/j.yexcr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Sudhakar A, Nyberg P, Keshamouni VG, et al. Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin. J. Clin. Invest. 2005;115:2801–10. doi: 10.1172/JCI24813. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Swenson S, Costa F, Minea R, et al. Intravenous liposomal delivery of the snake venom disintegrin contortrostatin limits breast cancer progression. Mol. Cancer. Ther. 2004;3:499–511. [PubMed] [Google Scholar]
- Taga T, Suzuki A, Gonzalez-Gomez I, et al. Alpha v-Integrin antagonist EMD 121974 induces apoptosis in brain tumor cells growing on vitronectin and tenascin. Int. J. Cancer. 2002;98:690–7. doi: 10.1002/ijc.10265. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Mimori K, Shiraishi T, et al. Alpha6 integrin expression in esophageal carcinoma. Int. J. Oncol. 2000;16:725–9. doi: 10.3892/ijo.16.4.725. [DOI] [PubMed] [Google Scholar]
- Takanami I. Increased expression of integrin-linked kinase is associated with shorter survival in non-small cell lung cancer. BMC Cancer. 2005;5:1–7. doi: 10.1186/1471-2407-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MR, Couto JR, Scallan CD, et al. Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA Cell. Biol. 1997;16:861–9. doi: 10.1089/dna.1997.16.861. [DOI] [PubMed] [Google Scholar]
- Thomas GJ, Lewis MP, Hart IR, et al. AlphaVbeta6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int. J. Cancer. 2000;92:641–50. doi: 10.1002/1097-0215(20010601)92:5<641::aid-ijc1243>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Thomas GJ, Nystrom ML, Marshall JF. Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J. Oral. Pathol. Med. 2006;35:1–10. doi: 10.1111/j.1600-0714.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- Thomas GJ, Poomsawat S, Lewis MP, et al. Alphavbeta6 integrin upregulates matrix metalloproteinase 9 and promotes migration of normal oral keratinocytes. J. Invest Dermatol. 2001;116:898–904. doi: 10.1046/j.1523-1747.2001.01352.x. [DOI] [PubMed] [Google Scholar]
- Tiger CF, Fougerousse F, Grundstrom G, et al. Alpha11beta1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev. Biol. 2001;237:116–29. doi: 10.1006/dbio.2001.0363. [DOI] [PubMed] [Google Scholar]
- Travis MA, Humphries JD, Humphries MJ. An unraveling tale of how integrins are activated from within. Trends. Pharmacol. Sci. 2003;24:192–7. doi: 10.1016/S0165-6147(03)00069-5. [DOI] [PubMed] [Google Scholar]
- Trikha M, Timar J, Zacharek A, et al. Role for beta3 integrins in human melanoma growth and survival. Int. J. Cancer. 2002a;101:156–67. doi: 10.1002/ijc.10521. [DOI] [PubMed] [Google Scholar]
- Trikha M, Zhou Z, Timar J, et al. Multiple roles for platelet GPIIb/IIIa and alphavbeta3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Res. 2002b;62:2824–33. [PubMed] [Google Scholar]
- Trikha M, Zhou Z, Nemeth JA, et al. CNTO 95, a fully human monoclonal antibody that inhibits alphav integrins, has antitumor and antiangiogenic activity in vivo. Int. J. Cancer. 2004;110:326–35. doi: 10.1002/ijc.20116. [DOI] [PubMed] [Google Scholar]
- Tsuji T. Physiological and pathological roles of alpha3beta1 integrin. J. Memb. Biol. 2004;200:115–32. doi: 10.1007/s00232-004-0696-5. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Kawada Y, Kai-Murozono M, et al. Regulation of melanoma cell migration and invasion by laminin-5 and alpha3beta1 integrin (VLA-3) Clin. Exp. Metastasis. 2002;19:127–34. doi: 10.1023/a:1014573204062. [DOI] [PubMed] [Google Scholar]
- Tucker GC. Alpha v integrin inhibitors and cancer therapy. Curr. Opin. Investig Drugs. 2003;4:722–31. [PubMed] [Google Scholar]
- Tulla M, Pentikainen OT, Viitasalo T, et al. Selective binding of collagen subtypes by integrin alpha 1I, alpha 2I, and alpha 10I domains. J. Biol. Chem. 2001;276:48206–12. doi: 10.1074/jbc.M104058200. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Lelievre S, Lakins JN, et al. Beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–16. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Tang CH, Kim Y, et al. Urokinase receptors are required for alpha 5 beta 1 integrin-mediated signaling in tumor cells. J. Biol. Chem. 2007;282:3929–39. doi: 10.1074/jbc.M607989200. [DOI] [PubMed] [Google Scholar]
- Weinacker A, Ferrando R, Elliott M, et al. Distribution of integrins alpha v beta 6 and alpha 9 beta 1 and their known ligands, fibronectin and tenascin, in human airways. Am. J. Respir. Cell. Mol. Biol. 1995;12:547–56. doi: 10.1165/ajrcmb.12.5.7537970. [DOI] [PubMed] [Google Scholar]
- Weinreb PH, Simon KJ, Rayhorn P, et al. Function-blocking integrin alphavbeta6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J. Biol. Chem. 2004;279:17875–87. doi: 10.1074/jbc.M312103200. [DOI] [PubMed] [Google Scholar]
- White DE, Kurpios NA, Zuo D, et al. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–70. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Wick W, Naumann U, Weller M. Transforming growth factor-beta: a molecular target for the future therapy of glioblastoma. Curr. Pharm. Des. 2006;12:341–9. doi: 10.2174/138161206775201901. [DOI] [PubMed] [Google Scholar]
- Woska JR, Jr, Morelock MM, Jeanfavre DD, et al. Characterization of molecular interactions between intercellular adhesion molecule-1 and leukocyte function- associated antigen-1. J. Immunol. 1996;156:4680–5. [PubMed] [Google Scholar]
- Wright SD, Silverstein SC. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J. Exp. Med. 1983;158:2016–23. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WB, Peng HC, Huang TF. Disintegrin causes proteolysis of beta-catenin and apoptosis of endothelial cells. Involvement of cell-cell and cell-ECM interactions in regulating cell viability. Exp. Cell. Res. 2003;286:115–27. doi: 10.1016/s0014-4827(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Diefenbach B, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science. 2001;294:339–45. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Goodman SL, et al. A novel adaptation of the integrin PSI domain revealed from its crystal structure. J. Biol. Chem. 2004;279:40252–4. doi: 10.1074/jbc.C400362200. [DOI] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Zhang R, et al. Crystal structure of the extra-cellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–5. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- Yang C, Zeisberg M, Lively JC, et al. Integrin alpha1beta1 and alpha2beta1 are the key regulators of hepatocarcinoma cell invasion across the fibrotic matrix microenvironment. Cancer Res. 2003;63:8312–7. [PubMed] [Google Scholar]
- Yao ES, Zhang H, Chen YY, et al. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007;67:659–64. doi: 10.1158/0008-5472.CAN-06-2768. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Peng HC, Huang TF. Accutin, a new disintegrin, inhibits angiogenesis in vitro and in vivo by acting as integrin alpha V beta 3 antagonist and inducing apoptosis. Blood. 1998;92:3268–76. [PubMed] [Google Scholar]
- Zhang D, Pier T, McNeel DG, Wilding G, et al. Effects of a monoclonal anti-alphavbeta3 integrin antibody on blood vessels—a pharmacodynamic study. Invest. New Drugs. 2007;25:49–55. doi: 10.1007/s10637-006-9013-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, Plow EF. A discrete site modulates activation of I domains. Application to integrin alphaMbeta2. J. Biol. Chem. 1996;271:29953–7. doi: 10.1074/jbc.271.47.29953. [DOI] [PubMed] [Google Scholar]
- Zheng DQ, Woodard AS, Fornaro M, et al. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–64. [PubMed] [Google Scholar]
- Zheng DQ, Woodard AS, Tallini G, et al. Substrate specificity of alpha(v)beta(3) integrin-mediated cell migration and phosphati-dylinositol 3-kinase/AKT pathway activation. J. Biol. Chem. 2000;275:24565–74. doi: 10.1074/jbc.M002646200. [DOI] [PubMed] [Google Scholar]
- Zhou X, Murphy FR, Gehdu N, et al. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J. Biol. Chem. 2004;279:23996–4006. doi: 10.1074/jbc.M311668200. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nakada MT, Arnold C, et al. Contortrostatin, a dimeric disintegrin from Agkistrodon contortrix contortrix, inhibits angiogenesis. Angiogenesis. 1999;3:259–69. doi: 10.1023/a:1009059210733. [DOI] [PubMed] [Google Scholar]
- Ziober BL, Chen YQ, Ramos DM, et al. Expression of the alpha7beta1 laminin receptor suppresses melanoma growth and metastatic potential. Cell. Growth Differ. 1999;10:479–90. [PubMed] [Google Scholar]
- Zutter MM, Santoro SA, Staatz WD, et al. Re-expression of the alpha 2 beta 1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc. Natl. Acad. Sci., U.S.A. 1995;92:7411–5. doi: 10.1073/pnas.92.16.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]