Abstract
Claudin-16 (paracellin-1) is a tight junction protein localized mainly in the thick ascending limb of Henle's loop and also in the distal nephron. Its defect causes familial hypomagnesaemia with hypercalciuria and nephrocalcinosis. This had been taken as an indication that claudin-16 conveys paracellular Mg2+ and Ca2+ transport; however, evidence is still conflicting. We studied paracellular ion permeabilties as well as effects of claudin-16 on the driving forces for passive ion movement. MDCK-C7 cells were stably transfected with wild-type (wt) and mutant (R146T, T233R) claudin-16. Results indicated that paracellular permeability to Mg2+ but not to Ca2+ is increased in cells transfected with wt compared to mutant claudin-16 and control cells. Increased basolateral Mg2+ concentration activated a transcellular Cl− current which was greatly enhanced in cells transfected with wt and T233R claudin-16, as compared to R146T claudin-16-transfected or control cells. This current was triggered by the basolateral calcium-sensing receptor causing Ca2+ release from internal stores, thus activating apical Ca2+-sensitive Cl− channels and basolateral Ca2+-sensitive K+ channels. Immunohistochemical data suggest that the Cl− channel involved is bestrophin. We conclude that claudin-16 itself possesses only moderate paracellular Mg2+ permeability but governs transcellular Cl− currents by interaction with apical Ca2+-activated Cl− channels, presumably bestrophin. As the transepithelial voltage generated by such a current alters the driving force for all ions, this may be the major mechanism to regulate Mg2+ and Ca2+ absorption in the kidney.
The kidneys play a pivotal role in maintaining Mg2+ homeostasis of the human body. The main location of Mg2+ absorption within the kidney is the thick ascending limb (TAL) of Henle's loop, which accounts for some 70% of Mg2+ re-uptake and thus the bulk of the filtrated Mg2+. While the proximal tubule appears to play only a marginal role in Mg2+ absorption, the fine-tuning of Mg2+ homeostasis takes place in the distal convoluted tubule (DCT; for review see Praga et al. 1995; Cole & Quamme, 2000; Meij et al. 2002).
Mechanisms of Mg2+ absorption differ greatly in the different nephron segments. Mg2+ transport in the DCT has been shown to be active transcellular transport, driven by a basolateral Na+/Mg2+ antiport combined with an apical Mg2+ influx through Mg2+-permeable channels (such as TRPM6 and 7; for review see Schlingmann & Gudermann, 2005). In contrast, Mg2+ transport in the TAL has been demonstrated to be predominantly paracellular, driven by the lumen-positive potential (e.g. Di Stefano et al. 1993).
In 1999, Simon et al. identified the tight junction protein paracellin-1 which is defective in patients with the rare pathological condition familial hypomagnesaemia with hypercalciuria and nephrocalcinosis (FHHNC, OMIM 248250 and 603959). This protein was found to belong to the claudin family. The paracellin-1 orthologue in cattle was therefore dubbed claudin-16 by Hirano et al. (2000) and both terms have since been used synonymously in the literature. Claudins are membrane proteins with four membrane-spanning domains and two extracellular loops that are localized in the tight junctions of epithelial and endothelial cells. They interact with intracellular scaffolding proteins of the tight junction complex, such as ZO-1, through a C-terminal PDZ domain binding motif (Itoh et al. 1999) and primarily determine paracellular permeability. While many claudins contribute to paracellular barrier formation, at least one, claudin-2, has been shown to form a paracellular channel, rendering specific permeability for small cations to the tight junctions (Amasheh et al. 2002). It has therefore been hypothesized that claudin-16 increases paracellular permeability for divalent cations, in particular Mg2+ and Ca2+, by forming a paracellular channel, the unusual number of negatively charged amino acids of the first extracellular loop acting as a selectivity filter.
Different forms of claudin-16 exist in rodents and man, designated here as the short and long version. The short version of claudin-16, consisting of 235 amino acids with only 3 intracellular amino acids preceding the first transmembrane region, corresponds to the protein found in mouse and rat and has been suggested to be the physiologically relevant form of claudin-16. The long version is found in man: here, the open reading frame of the claudin-16 mRNA contains two start codons resulting in two proteins that differ in length by 70 amino acids at the cytoplasmic N-terminus (Weber et al. 2001a). The uncertainty about the two versions led us to study both the short and the long version.
To date, more than 40 mutants of claudin-16 from patients exhibiting FHHNC have been described (Simon et al. 1999; Weber et al. 2000, 2001b; Müller et al. 2003, 2006a,b; for review see Günzel et al. 2009a). Interestingly, none of the mutations involve the negative charges of the first extracellular loop.
In mouse kidney, claudin-16 is exclusively expressed in the epithelial cells of the TAL (Kiuchi-Saishin et al. 2002), which appears to support the hypothesis that claudin-16 forms Mg2+- and Ca2+-permeable channels in this segment of the nephron. However, in rat and human kidney there is evidence for additional expression in the DCT and the collecting duct (rat, Weber et al. 2001a; human, Simon et al. 1999), but in these nephron segments the lumen-negative voltage would not favour but rather counteract paracellular cation absorption. Physiological data are conflicting as well. Ikari et al. (2004) report that Ca2+ flux in low resistance MDCK cells transfected with claudin-16 cDNA is increased only in the apical-to-basolateral direction. In spite of this increased permeability, claudin-16 expression caused an increase in transepithelial resistance in these cells. Hou et al. (2005), on the other hand, found an only slightly enhanced Mg2+ permeability without directional preference in low resistance MDCK and LLC-PK1 cells, accompanied by a large increase in Na+ permeability in the latter cell line. They concluded that the Mg2+-pore hypothesis for claudin-16 is not valid.
In contrast, Kausalya et al. (2006) found a moderately increased Mg2+ permeability in claudin-16-transfected, high-resistance MDCK-C7 cells compared to mock-transfected controls and cells transfected with various claudin-16 mutants. No changes in the Na+vs Cl− permeability ratios were observed and no correlation between Mg2+ permeability and transepithelial resistance was detected.
This indicates that – although the effects of claudin-16 mutations on Mg2+ loss and Ca2+ homeostasis are clinically without doubt – the mechanism of how claudin-16 enhances Mg2+ absorption is far from being clear and deserves basic investigation.
In the present study we stably transfected high resistance MDCK cells (MDCK-C7; Gekle et al. 1994) (i) with the long or the short version of the functionally intact wild-type (wt) claudin-16, (ii) with the claudin-16 mutant R146T which causes FHHNC phenotype in patients carrying this mutant and which affects the net charge of the second extracellular loop (Weber et al. 2001b), or (iii) with the claudin-16 mutant T233R which is located in the PDZ-binding motif of claudin-16 and causes a relatively mild form of FHHNC in affected patients (Müller et al. 2003). This enabled us to compare the effects of divalent cations on paracellular and transcellular fluxes in claudin-16-transfected cells and mock-transfected control cells.
First, we confirmed and thus corroborated the puzzling fact that claudin-16 only moderately increases paracellular Mg2+ permeability. At the same time, basic Ca2+ permeability was not significantly altered by claudin-16. However, as a dominating effect, Mg2+ and other divalent cations triggered a transcellular Cl− current by activating the basolateral Ca2+-sensing receptor (CaSR). This current was greatly enhanced in cells transfected with wt claudin-16 compared to control cells and cells transfected with the claudin-16 mutant R146T. In native epithelium, such a current would alter the transepithelial voltage and thus modulate the driving force for the paracellular flux of divalent cations.
Methods
Cell culture and solutions
Monolayers of the high resistance MDCK-C7 cell strain (Gekle et al. 1994) were grown in 25 cm2 culture flasks containing MEM-EARLE (Biochrom, Berlin, Germany), supplemented with 10% (v/v) fetal bovine serum, 100 U ml−1 penicillin and 100 mg ml−1 streptomycin (Biochrom, Berlin, Germany). Cells were cultured at 37°C in a humidified 5% CO2 atmosphere.
For electrophysiological measurements and molecular analyses, epithelial cell monolayers were grown on culture plate inserts (pore size 0.45 μm, effective area 0.6 cm2, Millicell-HA, Millipore, Bedford, MA, USA). Cells were grown to confluence and used on days 6–8 after seeding. Inserts were mounted in Ussing chambers, and water-jacketed gas lifts were filled with 10 ml circulating fluid on each side. The standard bath solution contained: 119 mm NaCl, 21 mm NaHCO3, 5.4 mm KCl, 1.2 mm CaCl2, 3 mm Hepes, and 10 mm d(+)-glucose. The solution was constantly bubbled with 95% O2 and 5% CO2, to ensure a pH value of 7.4 at 37°C. To this solution, Mg2+ was added as MgCl2 (1 m stock solution, FLUKA, Buchs, Switzerland) or MgSO4 at the desired concentrations (stated for individual experiments). Alternatively, to increase the Mg2+ concentration without major changes in osmolarity, appropriate amounts of the standard bath solution were replaced with a bath solution containing 100 mm MgSO4, 19 mm NaCl, 21 mm NaHCO3, 5.4 mm KCl, 1.2 mm CaCl2, 3 mm Hepes, and 10 mm d(+)-glucose.
To obtain Na+-free bath solutions, NaCl was replaced with equimolar amounts of NMDG-Cl, while NaHCO3 was replaced with equimolar amounts of cholin-HCO3. In low K+ bath solutions, KCl was omitted without substitution. In high K+ bath solutions the desired amount of NaCl was replaced with KCl to maintain osmolarity. Several different Cl−-free solutions were applied: all Cl− was either replaced with gluconate, with nitrate or with pyruvate. As gluconate binds divalent cations, Ca2+ concentrations in gluconate-containing solutions were increased to 10 mm.
Cloning and transfection procedures
Molecular cloning of claudin-16 cDNA was performed by PCR with respective sense (5′-CTTCGGATAATGACCTCCAGG-3′) and antisense (5′-ACGTGCATTTTACACCCTTGT-3′) oligonucleotides (Simon et al. 1999) using human universal quick clone cDNA as template (Clontech Laboratories Inc., Mountain View, CA, USA). The resulting 936 bp PCR product encompassing the complete claudin-16 cDNA was cloned into pGEMT-Easy (Promega, Madison, WI, USA). Similarly, claudin-11 cDNA was cloned from kidney cDNA (generous gift from Dr Dominik Müller, Charité, Berlin) using sense (5′-ATGGTGGCCACGTGCCTGCAGG-3′) and antisense (5′-CTATACGTGGGCACTCTTCGCATG-3′) oligonucleotides. Claudin-16 mutants R146T and T233R were cloned as recently reported (Kausalya et al. 2006). The correctness of the cDNA was verified by sequencing and the mutants, the short (‘rodent’, 708 bp), and the long version of claudin-16 (‘human’, 918 bp) and claudin-11 were subcloned into the eukaryotic expression vectors pcDNA3 (Invitrogen, Carlsbad, CA, USA) and pFLAG-CMV4 (Sigma Aldrich, St Louis, MO, USA), respectively. The long version of claudin-16 was also subcloned into a modified pcDNA3.1 vector (generous gift from O. Huber, Charité, Berlin), tagging the N-terminus of claudin-16 with yellow fluorescence protein (YFP). MDCK-C7 cells were stably transfected employing the Lipofectamine plus method (Gibco BRL, Invitrogen Corp., Gaithersburg, MD, USA). G418-resistant cell clones were screened for claudin-16 expression by Western blot analysis and immunohistochemistry. MDCK-C7 cells mock-transfected with respective empty vectors and with claudin-11 served as controls.
PCR
RNA was extracted from claudin-16- and vector-transfected MDCK-C7 cells using the NucleoSpin RNA/protein kit (Macherey-Nagel, Düren, Germany) according to the instructions of the manufacturer. cDNA was synthesized from 4 μg total RNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) in a 40 μl reaction volume.
PCR for bestrophin-1 to -4 was performed on MDCK-C7 cDNA and on commercial dog kidney cDNA (BioChain, Hayward, CA, USA) using HOTSTAR DNA polymerase (Qiagen, Hilden, Germany) and the following primers: bestrophin-1, forward 5′-GGAGGAAGAGGAAGGCACTC-3′, reverse 5′-AGGCTTGAGGGTGCTGACT-3′, alternative reverse 5′-TGTGGACGGCTGTATGGCTGTGAC-3′; bestrophin-2, forward 5′-GCCAAAGAGGACATGCAGTT-3′, reverse 5′-GCATAGGCACAGTGGTGAAG-3′; bestrophin-3, forward 5′-GGAGGAAGAGGAAGGCACTC-3′, reverse 5′-ACTCGGCTCGACTCCTGTAA-3′; bestrophin-4 forward 5′-AAACCTCGGGAGCCTTTAGA-3′, reverse 5′-CGAAGGTTGAAGGTGGAG-3′. Cycling conditions were: 45 s 95°C, 60 s 60°C and 120 s 72°C for 35 cycles with an initial denaturation of 15 min and final extension of 8 min.
TaqMan real-time PCR was performed using the following probes (Applied Biosystems, Foster City, CA, USA): bestrophin-1, Cf02697409_gH; bestrophin-4, Cf02718712_m1, calcium sensing receptor, Cf02741809_s1, and β-Actin (part number 4310881E) as an endogenous control.
Western blot analysis
Western blot analysis was carried out as described in detail by Amasheh et al. (2002). Claudin-16 and claudin-16 mutants were detected by immunoblotting, employing antibodies raised against human claudin-16 (Zymed Laboratories, Invitrogen Immunodetection, South San Francisco, CA, USA), against the FLAG-tag or against the HA-tag (Sigma Aldrich), as appropriate. Control blots showed no differences for the major MDCK claudins (1–5, 7, 8) between mock-transfected control cells and claudin-16-transfected cells (see online Supplementary Fig. S1).
Immunohistochemistry/laser scanning microscopy
For immunohistochemical studies, cells were grown on transparent culture plate inserts (pore size 0.4 μm, effective area 0.6 cm2, Millicell-PCF, Millipore). Cells were rinsed with PBS, fixed with methanol, and permeabilized with PBS containing 0.5% Triton X-100. Primary antibodies employed were mouse anti-occludin and rabbit anti-claudin-16 (Zymed Laboratories), and rabbit anti-bestrophin-1, Abcam ab14927 (Abcam, Cambridge, MA, USA). Concentrations of primary antibody were 10 μg ml−1. Secondary antibodies Alexa Fluor 488 goat anti-mouse and Alexa Fluor 594 goat anti-rabbit (both used at concentrations of 2 μg ml−1) were purchased from Molecular Probes (Eugene, OR, USA). DAPI (4′,6-diamidino-2-phenylindole dihydrochloride, 1 μm) was used to stain cell nuclei. Fluorescence images were obtained with a confocal microscope (Zeiss LSM510, Carl Zeiss, Jena, Germany) using excitation wavelengths of 543 nm, 488 nm and 405 nm.
Electrophysiology and flux measurements
Short circuit current (ISC, μA cm−2) was measured in Ussing chambers specially designed for insertion of Millicell filters (Kreusel et al. 1991). Resistance of bath solutions and filter support was measured prior to each single experiment and taken into account for ISC data. In all figures, positive ISC values indicate anion secretion (i.e. movement from the basolateral to the apical side) and/or cation absorption (i.e. movement from the apical to the basolateral side). In some experiments, ion gradients were applied across the cell layer, so that, strictly, the term short circuit current does not apply. In these experiments, the current necessary to clamp the transepithelial potential to 0 mV is dubbed ‘holding current’, Ih. All traces shown in Figs 3–6 are typical examples of at least three independent experiments.
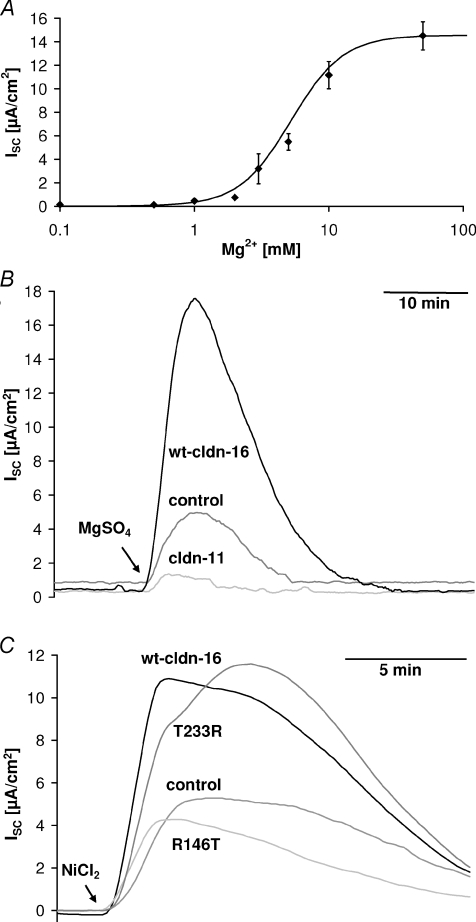
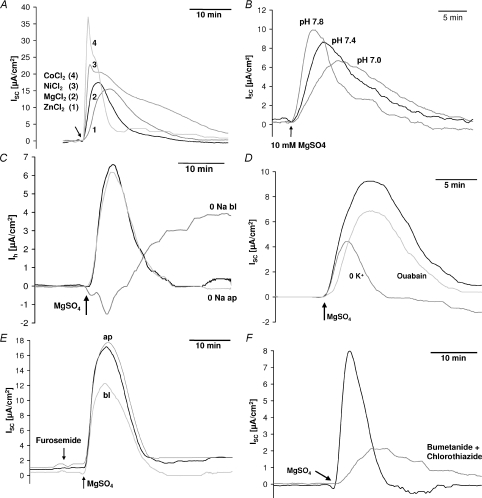
Figure 3. Divalent cation-induced transcellular current.
A, dose–response curve for the transcellular current induced by basolateral Mg2+ application in wt claudin-16-transfected MDCK cells. EC50, 5.2 mm; Hill coefficient, 2.3. B and C, application of divalent cations (B, 10 mm MgSO4; C, 100 μm NiCl2) to the basolateral side induced a transcellular current (short circuit current, ISC) that was considerably larger in wt claudin-16-transfected (B, long version; C, short version) and T233R mutant-transfected cell layers than in R146T mutant claudin-16-transfected, in mock-transfected control cell layers, and, for comparison in claudin-11-transfected cells.
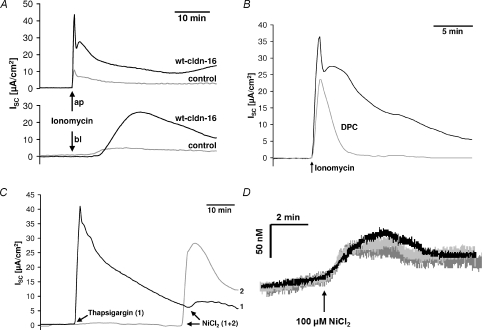
Figure 6. Involvement of intracellular Ca2+.
A–C, black traces, wt claudin-16-transfected cells; grey traces, mock-transfected cells. A, application of 10−8m ionomycin induced a current which was considerably larger in wt claudin-16-transfected cells than in mock-transfected controls. Application of ionomycin to the apical side caused an instantaneous, rapid increase in current while application to the basolateral side slowed the current increase and delayed it by several minutes. B, the current induced by ionomycin (10−8m) was sensitive to low concentrations (50 μm) of DPC (diphenylamine-2-carboxylate). C, thapsigargin (10−6m)-induced release of Ca2+ from intracellular stores caused an instantaneous rise in current. Subsequent application of 100 μm NiCl2 induced only a minor increase in current compared to the current elicited in cell layers not pretreated with thapsigargin. D, divalent cation-induced increases in [Ca2+]i measured with fura-2 did not differ between wt claudin-16-transfected (black trace), R146T mutant-transfected (light grey trace) or mock-transfected control cells (dark grey trace). ISC, short circuit current.
Measurement of unidirectional tracer flux was performed under short circuit conditions with 50 kBq ml−145Ca (Amersham Biosciences, Piscataway, NJ, USA). The medium also contained non-labelled tracer molecules (1.2 mm CaCl2). Ten minutes after addition of the tracer, two 100 μl samples were taken from the donor side, and 900 μl standard bathing solution and 4 ml of Ultima Gold high flash-point liquid scintillation cocktail (Packard Bioscience, Groningen, The Netherlands) were added to each sample. Samples (1 ml) of the receiving side, replaced with fresh standard bath solution, were mixed with 4 ml of the liquid scintillation cocktail. All 5 ml samples were subsequently analysed with a Tri-Carb 2100TR Liquid Scintillation counter (Packard, Meriden, CT, USA).
As radioactive Mg isotopes are not readily available, Mg2+ flux was measured employing atomic absorption sprectrometry (AAS). Prior to these experiments Ussing chambers were scrupulously cleaned, using a 10 mm EDTA solution, and rinsed several times with bidistilled water.
Cells grown on filter supports were washed twice in nominally Mg2+-free solution, mounted in the Ussing chambers and equilibrated in nominally Mg2+-free bath solutions for 15 min. A 1 ml sample was taken from the appropriate side (basolateral or apical) and the solution was replaced with 1 ml nominally Mg2+-free bath solution before 10 mm Mg2+ was added to the other side, by replacing 1 ml Mg2+-free bath solution with 1 ml 100 mm Mg2+ bath solution. After the addition of Mg2+, 1 ml samples were taken from the low Mg2+ side every 30 min (replaced with 1 ml nominally Mg2+-free solution). Five microlitres of a solution containing 20% La2O3 in 32% HCl were added to the samples to minimize anion interference and the Mg2+ content was measured with a Perkin-Elmer 2380 atomic absorption spectrometer (Perkin-Elmer, Waltham, MA, USA).
As this method requires the use of a nominally Mg2+-free solution on the acceptor side, dilution potentials were measured as an alternative method to evaluate permeabilities to mono- and divalent cations, as described in detail by Günzel et al. 2009b.
Microfluorimetric measurements
All microfluorimetric measurements were carried out in nominally HCO3−-free, Hepes-buffered solutions (composition see above). Filter membranes from culture plate inserts (pore size 0.4 μm, effective area 0.6 cm2, Millicell-PCF) covered with confluent cell monolayers were carefully cut out and incubated with 10 μm fura-2 AM (Molecular Probes) in Hepes-buffered saline for 30 min at room temperature. Subsequently, filters were mounted in the perfusion chamber (apical side down) attached to an inverted microscope (Zeiss Axiovert 100) and washed for 30 min to remove excess extracellular dye and to allow complete conversion of intracellular fura-2 AM to the free fura-2 acid. Fluorescence was excited with light from a xenon lamp (XPO 75 W/2; Osram, Munich, Germany) filtered by two rotating filters (6 s−1) at 340 and 380 nm. Emitted light was detected at 510 nm by a photomultiplier (928 SF; Hamamatsu, Hamamatsu, Japan) connected to an EPC-9 patch-clamp amplifier. Data were stored and processed on a PC using TIDA for Windows. Relative changes in [Ca2+]i are represented by changes in the 340/380 nm fluorescence ratio while absolute [Ca2+]i was calculated using the equation and dissociation constant of Grynkiewicz et al. (1985) as previously described by Hochstrate et al. (1995).
Results
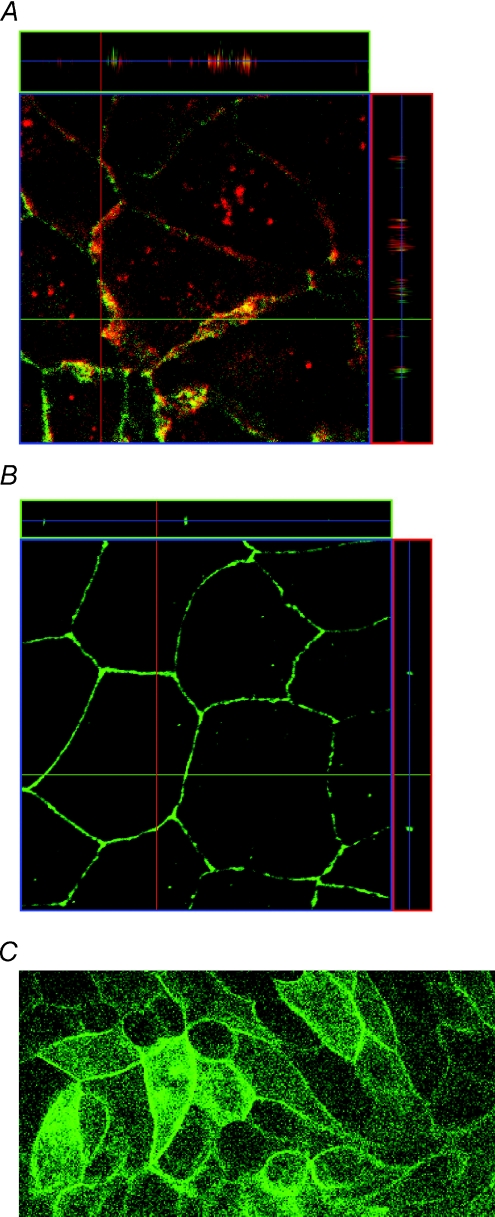
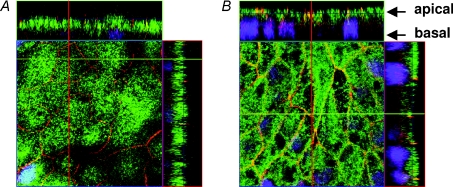
After transfection, the proper localization of the long (‘human’) version of claudin-16 within the tight junction was derived from staining against the FLAG-tag or from the fluorescence signal of the YFP fused to the N-terminus of the claudin-16 (Fig. 1). Localization of the short (‘rodent’) version has been described in a previous study (Kausalya et al. 2006).
Figure 1. Localization of wt claudin-16 (long version) expressed in MDCK cells.
A, immunohistochemical staining indicates co-localization of the long ‘human’ wt claudin-16 version in cells expressing claudin-16 (red: anti-Flag antibody) with the tight junction marker occludin (green). B, mock-transfected control. C, also the YFP–claudin-16 fusion protein is localized within the tight junction (green, YFP fluorescence). Localization of the short wt claudin-16 variant and the mutants R146T and T233R has been studied previously (Kausalya et al. 2006).
Paracellular Mg2+ and Ca2+ permeability of claudin-16-expressing cell layers
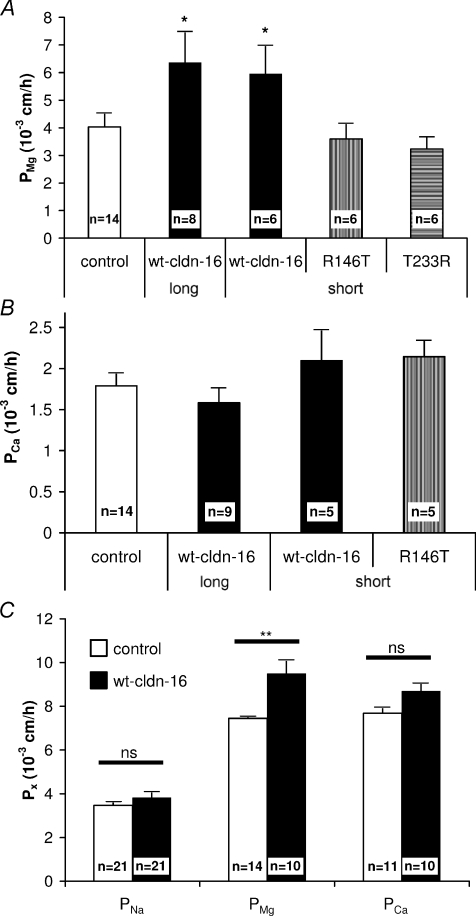
Mg2+ permeabilities (PMg) of MDCK cell layers kept in Ussing chambers (transepithelial potential, Vepi, clamped to 0 mV) were investigated using atomic absorption spectrometry, as previously described (Kausalya et al. 2006). This technique requires the application of a Mg2+ gradient (cis 10 mm, trans nominally 0 mm). Under these conditions, PMg was found to be increased in wt claudin-16-expressing cells (long and short version) compared to R146T or T233R claudin-16-expressing cells and control cells (Fig. 2A).
Figure 2. Paracellular Mg2+ and Ca2+ permeability.
A, paracellular Mg2+ permeability, calculated from Mg2+ flux measurements, was increased (P < 0.05, unpaired t test) in MDCK-C7 cell layers transfected with either the long or the short version of wt claudin-16 compared to mock-transfected control cells while Mg2+ permeability of mutant (R146T, T233R) claudin-16-transfected cell layers was unchanged. B, paracellular Ca2+ permeability, calculated from 45Ca2+ flux measurements, did not differ in wt or mutant claudin-16-transfected MDCK-C7 cell-layers compared to mock-transfected control cells. n, number of independent experiments. C, Na+, Mg2+ and Ca2+ permeabilities, calculated from dilution potential/biionic potential measurements on wt claudin-16 (long version) and mock-transfected MDCK-C7 cell layers. Mg2+ permeability was increased in claudin-16-transfected compared to mock-transfected cells (P < 0.01, unpaired t test), while no significant differences were found for Ca2+ or Na+ permeabilities. All values mean ±s.e.m.
Ca2+ permeabilities (PCa) were determined from 45Ca2+ flux measurements in Ussing chambers (Vepi clamped to 0 mV). In contrast to PMg, PCa was determined under symmetrical Ca2+ conditions (1.2 mm Ca2+ on the apical and basolateral side) to avoid disruption of tight junctions at low Ca2+ concentrations. Under these conditions, PCa was approximately 50% of PMg determined in mock-transfected cells and did not differ significantly between mock-transfected, claudin-16-transfected and R146T mutant-transfected cells (Fig. 2B).
As transport kinetics under symmetrical and zero-trans conditions may differ, Ca2+, Mg2+ and Na+ permeabilities were additionally determined by measuring dilution/biionic potentials in control and wt claudin-16-transfected cell layers. As shown in Fig. 2C, under these conditions PMg and PCa were similar. While control and wt claudin-16-transfected cell layers did not differ in PNa and PCa, PMg was significantly increased in wt claudin-16-transfected cell layers.
Increased Mg2+-induced transcellular current in wt claudin-16-transfected MDCK cells
Wt claudin-16-expressing cells differed from controls not only with respect to their paracellular transport properties, but unexpectedly also with respect to a transient, transcellular current elicited by an increase in extracellular Mg2+. This current was concentration dependent (Fig. 3A) with a half-maximal response concentration (EC50) of 5.2 mm and a Hill coefficient of 2.3. The current could be elicited by Mg2+ simultaneously applied to both sides of the epithelium or by application to the basolateral side alone, but not by application to the apical side alone, and was thus solely due to Mg2+ applied to the basolateral side (Supplementary Fig. S2). The direction of the current indicated either net anion secretion or net cation absorption. The current was accompanied by a decrease in transepithelial resistance (Rt) and an increase in the transepithelial voltage difference (Supplementary Fig. S3).
The Mg2+-induced current was larger in cells expressing the long or the short version of wt claudin-16 than in mock-transfected control cells or, as a further control, in claudin-11-transfected cells. In cells transfected with the claudin-16 mutant R146T, the current was comparable to the current observed in control cells, while in cells transfected with the claudin-16 mutant T233R the current was similar to that observed in wt claudin-16-transfected cells (Fig. 3B and C). As cells expressing the long or the short version of wt claudin-16 showed the same properties, no further distinction is made between these two versions and the pooled results are referred to as wt claudin-16-transfected cells. The current was independent of the anion of the Mg2+ salts used (e.g. MgCl2vs MgSO4) and could not be elicited by osmotically equivalent amounts of NaCl or mannitol (not shown), ruling out that the current might be induced by cell volume changes.
A transient current with the same properties as the Mg2+-induced current, but with different magnitude, could also be activated by a basolateral application of millimolar concentrations of CaCl2 or the polyamine spermidine, or (as depicted in Fig. 4A) by micromolar concentrations of CdCl2, CoCl2, NiCl2, MnCl2, and ZnCl2, but not e.g. by cobalt hexaammine (an analogue of the hydrated Mg2+ ion), CuSO4 or SnCl2. Independent of the ion used for activation, the divalent cation-induced current was strongly dependent on the extracellular pH value. It was greatly enhanced at alkaline pH values and reduced at acidic pH values (Fig. 4B). Again, the current was larger in cells expressing the long or the short version of wt claudin-16 than in mock-transfected control cells. The current could be activated both in the presence and in the nominal absence of extracellular Mg2+.
Figure 4. Divalent cation-induced transcellular current: cation contribution.
A, comparison of current responses to a basolateral application of 100 μm ZnCl2, 10 mm MgCl2, 100 μm NiCl2 and 100 μm CoCl2, respectively, to wt claudin-16-transfected cell layers. B, the current response (elicited by the application of 10 mm MgSO4, short wt claudin-16-transfected cells) was strongly dependent on the extracellular pH. C, reduction of Na+ to nominally 0 mm on the basolateral side inhibited the divalent cation-induced current (10 mm MgSO4). D, reduction of K+ to nominally 0 mm on the basolateral side partially inhibited the divalent cation-induced current (10 mm MgSO4). This was not due to an inhibition of the Na+/K+ pump, as preincubation with 400 μm ouabain had only small effects. E, the divalent cation-induced current (10 mm MgSO4) was partially blocked by an application of 200 μm furosemide to the basolateral but not to the apical side. F, a combined application of 100 μm bumetanide and 100 μm chlorothiazide almost completely blocked the divalent cation-induced current (10 mm MgSO4). ISC, short circuit current. Ih, holding current necessary to clamp the transepithelial potential to 0 mV in the presence of an ion gradient across the cell layer.
The fact that the current could only be stimulated from the basolateral side, the spectrum of activating substances, the EC50 and Hill coefficient derived from the dose–response curve and the pH dependence, suggested that the current was due to an activation of the basolateral calcium (and magnesium)-sensing receptor (CaSR, for review see e.g. Chang & Shoback, 2004).
This assumption implies that wt claudin-16 interacts either with CaSR or with one of the processes triggered by CaSR activation. To investigate this hypothesis, this divalent cation-induced current was further characterized. As the relatively high MgSO4 concentrations needed to elicit the current would impair strict short circuit conditions, many of the following experiments were carried out using 100 μm NiCl2.
Na+ and K+ dependence of the claudin-16-dependent cation-induced current
The divalent cation-induced current was completely inhibited in nominally Na+-free solutions (Na+ replaced with NMDG+). Further experiments demonstrated that it was sufficient to replace Na+ on the basolateral side, while replacement on the apical side had no effect (Fig. 4C).
All changes in the extracellular K+ concentration (reduction to nominally 0 K+ and 0.1 mm K+, increase to 10, 20, 30, 60 or 120 mm K+) decreased the divalent cation-induced current, irrespective of the direction of the change. As seen with changes in the extracellular Na+ concentration, variations in the extracellular K+ concentration were only effective on the basolateral side.
Thus, effects of variations in the extracellular K+ concentration were difficult to interpret. This was attributed to the fact that changes in the extracellular K+ concentration cause changes in the membrane potential and thus in the driving force for ion transport. At the highest K+ concentrations of 60 or 120 mm, the observed inhibition of the divalent cation-induced current might well be due to the concommitant decrease in Na+. Conversely, using nominally K+-free solutions on the basolateral side would, in addition, block the Na+/K+ pump. Control experiments showed, however, that using up to 0.4 mm ouabain to block the divalent cation-induced current was considerably less potent than the use of nominally K+-free solutions, so that there appeared to be an absolute requirement for basolateral K+ (Fig. 4D). This conclusion was supported by the finding that furosemide (up to 0.2 mm) also partially blocked the divalent cation-induced current (Fig. 4E) and the combined application of bumetanide (0.1 mm) and chlorothiazide (0.1 mm, Fig. 4F) caused an almost complete inhibition.
The divalent cation-induced current was also partially blocked by a basolateral application of the Ca2+-activated K+ channel blocker charybdotoxin (10−7m), and the K+ channel blockers glibenclamide (0.2 mm) and Ba2+ (5 mm). In contrast, neither an apical application of Ba2+ nor the application of the maxi-K+ channel blocker iberiotoxin (10−7m) had any effect (not shown).
HCO3−- and Cl−-dependence of the claudin-16-dependent cation-induced current
Using HCO3−/CO2-free solutions (Hepes-buffered and equilibrated with 100% O2) had no effect on the divalent cation-induced current (not shown).
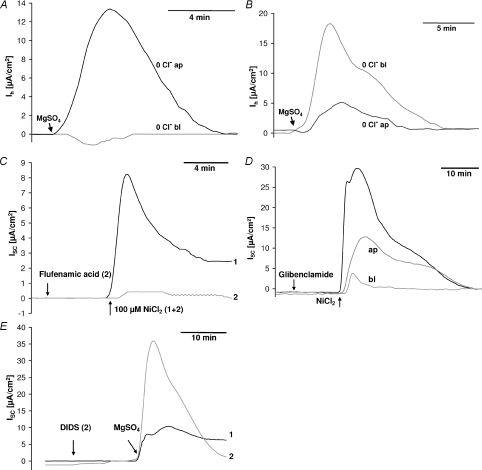
The dependence of the divalent cation-induced current on Cl− was investigated by replacing Cl− with gluconate, pyruvate or nitrate. Gluconate has the disadvantage of binding divalent cations, so that the free Mg2+ and Ca2+ concentrations in gluconate-based solutions with unchanged total Mg2+ and Ca2+ concentrations are lower than in Cl−-based solutions. Nevertheless, the effect of gluconate- and pyruvate-based solutions on the divalent cation-induced current was identical: the current was completely blocked if Cl− was replaced on the basolateral side, but it was enhanced if Cl− was replaced on the apical side of the cell layer (Fig. 5A). Replacement of Cl− by nitrate had the opposite effect; it enhanced the current, if nitrate was present on the basolateral side, but inhibited the current if it was present on the apical side. This indicates that the divalent cation-induced current is due to the movement of Cl− from the basolateral side to the apical side, but that in the presence of nitrate, nitrate is preferred over Cl− (Fig. 5B).
Figure 5. Divalent cation-induced transcellular current: anion contribution.
A and B, Cl− was replaced with pyruvate or nitrate, either in the basolateral or the apical side throughout the entire experiment. A, the divalent cation-induced current (10 mm MgSO4) was completely blocked if basolateral Cl− was replaced with pyruvate (or gluconate, not shown), while an apical replacement had no effect or even caused a slight increase in current. B, in contrast to A, replacing basolateral Cl− with nitrate increased the divalent cation-induced current (10 mm MgSO4) while an apical replacement greatly reduced the current. C, the divalent cation-induced current (100 μm NiCl2) was inhibited by very low concentrations (0.2 μm) of the Cl− channel blocker flufenamic acid. D, the K+ and CFTR Cl− channel blocker glibenclamide (0.2 mm) was more effective in blocking the divalent cation-induced current (100 μm NiCl2) when applied to the basolateral than to the apical side of the cell layer. E, basolateral application of 100 μm DIDS (4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid) greatly enhanced the divalent cation-induced current (10 mm MgSO4). ISC, short circuit current. Ih, holding current necessary to clamp the transepithelial potential to 0 mV in the presence of an ion gradient across the cell layer.
The divalent cation-induced current was completely blocked by low concentrations of the Cl− channel blockers flufenamic acid (0.2 μm) and DPC (diphenylamine-2-carboxylatem, 50 μm) (Fig. 5C).
As reported above, the CFTR and K+ channel blocker glibenclamide (0.2 mm) also inhibited the divalent cation-induced current, but it was more effective when applied to the basolateral side, so that this effect is likely to be due to an inhibition of basolateral K+ channels, rather than CFTR channels (Fig. 5D).
The blocker of Cl− channels and HCO3−/Cl− exchangers, 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS), had no effect on the divalent cation-induced current when applied to the apical side, but greatly enhanced the current when applied to the basolateral side (Fig. 5E). This effect was observed both in the presence and the nominal absence of HCO3−.
Effects of increased intracellular Ca2+ concentrations
[Ca2+]i was manipulated by the application of the Ca2+ ionophores ionomycin and A23187, respectively or by the Ca2+ releasing drug thapsigargin. In all cases a current was elicited which greatly exceeded the divalent cation-induced current. Similar to the divalent cation-induced current, the current was enhanced in claudin-16-expressing cells, compared to control cells (Fig. 6A) or cells transfected with the claudin-16 mutant R146T. In contrast, responses of cells transfected with the claudin-16 mutant T233R showed currents of almost the same amplitude as those observed in wt claudin-16-transfected cells. The ionomycin-induced current was again clearly sensitive to 50 μm DPC (Fig. 6B).
Depletion of the intracellular Ca2+ stores with thapsigargin almost completely suppressed any subsequent response of the cell layers to divalent cations, indicating that the release of Ca2+ from intracellular stores is responsible for triggering the divalent cation-induced current (Fig. 6C).
Effect of divalent cations on the intracellular Ca2+ concentration
Differences in response of claudin-16-transfected and control cells to ionomycin and thapsigargin rule out direct interactions between CaSR and claudin-16. The effects, however, might still be due to claudin-16 interacting with the loading of Ca2+ stores or with the Cl− channel.
We therefore measured the effect of an application of 0.1 mm NiCl2 on [Ca2+]i, using the fluorescent indicator fura-2. Under these conditions an increase in [Ca2+]i was observed as would be expected for CaSR activation. There was no significant difference between [Ca2+]i, increases between wt claudin-16-transfected, R146T mutant claudin-16-transfected, and control cells (Fig. 6D).
Furthermore, Ni2+ is known to quench fura-2 fluorescence, but no quenching was observed during micro-fluorimetric experiments, when exposing the cells to 100 μm Ni2+. As fura-2 fluorescence quenching allows detection of nanomolar Ni2+ concentrations (McCall & Fierke, 2000), an uptake of Ni2+ can also be ruled out.
Crosstalk mechanism between claudin-16 and the Ca2+-activated Cl− channel bestrophin
Recent findings suggest various bestrophin isoforms as candidates for Ca2+-activated Cl− channels (Qu et al. 2004; Tsunenari et al. 2006). The presence of bestrophin mRNA in MDCK cells and, as control, in commercial dog kidney cDNA, was therefore further investigated. Two bestrophin isoforms, Best1 and Best4 could be detected and were verified by sequencing. Real-time PCR using commercial probes for dog Best1, Best4 and CaSR revealed the presence of all three RNAs (see Supplementary Fig. S4). There was no difference in mRNA abundance between mock-transfected control cells and wt claudin-16-transfected cells.
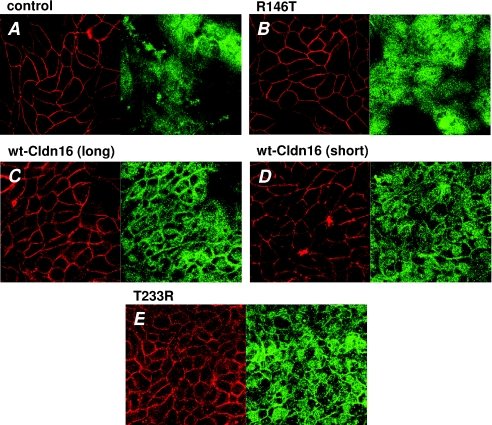
We therefore carried out immunohistochemical staining for bestrophin using the antibodies ab14927 (Abcam). As shown in Fig. 7, mock-transfected control cells and claudin-16-transfected cells differed in bestrophin distribution. In claudin-16-transfected cells, the staining pattern indicated bestrophin accumulation in the tight junction region while bestrophin was not localised at cell–cell contacts in control cells. Staining of R146T mutant-transfected cells showed a bestrophin distribution similar to control cells (Fig. 8A and B). In contrast, T233R mutant-transfected cells showed the same staining pattern as wt claudin-16 (Fig. 8C–E). Thus, accumulation of bestrophin was only found in cells exhibiting enhanced Cl− secretion when stimulated with divalent cations.
Figure 7. Localization of bestrophin in mock-transfected and claudin-16-transfected cells.
A, confocal laser-scanning micrographs of mock-transfected, and B, wt claudin-16 (short version)-transfected MDCK-C7 cells. Red, occludin; green, bestrophin; blue, nuclei (DAPI). The yellow colour along the cell–cell contacts in B indicates co-localization of occludin and bestrophin. This was not found in control cells. Z scans indicate that bestrophin accumulated not only at tight junctions but generally in the apical membrane.
Figure 8. Localization of bestrophin in mutant claudin-16-transfected cells.
Confocal laser-scanning micrographs of wt claudin-16-, mutant claudin-16-, and mock-transfected MDCK-C7 cells. Red, occludin; green, bestrophin; blue, nuclei (DAPI). All micrographs were obtained in the plane of the tight junction, as indicated by the clearly visible occludin signal. A and B, distribution of bestrophin in mock-transfected control cells and R146T mutant-transfected cells was diffuse. C–E, cells exhibiting strong divalent cation-induced current (wt claudin-16- and T233R mutant-transfected cells) showed a prominent accumulation of bestrophin in the tight junction region.
Based on these data, we propose that increased Cl− secretion in claudin-16-expressing cells may be due to direct or indirect interaction between claudin-16 and the apical Ca2+-activated Cl− channel bestrophin.
Discussion
Paracellular ion transport depends on tight junction permeability as well as on transcellular driving forces. As a general result, we found that both were altered by claudin-16 and that this may explain the full effect of defective forms of claudin-16 on Mg2+ and Ca2+ transport.
Claudin-16 and paracellular ion permeability
Changes in paracellular permeabilities have been studied in different claudin-16-transfected cell models with conflicting results. Hou et al. (2005) found that claudin-16 increased the Na+ permeability (PNa) in layers of transfected LLC-PK1 cells, without affecting Cl− permeability (PCl). Effects on Mg2+ permeability (PMg) in these cells were significant but small. Both effects were augmented in the presence of claudin-19 which additionally decreased PCl (Hou et al. 2008). In contrast to these findings in LLC-PK1 cells, Hou et al. (2005) found no significant effects of claudin-16 on PNa, PCl or PMg in low resistance MDCK-II cells.
Ikari et al. (2004, 2006) also used low resistance MDCK cells and found a decrease in PNa when expressing claudin-16, but an increase in Ca2+ and Mg2+ flux (apical to basolateral), while Ca2+ flux in the opposite direction remained unchanged.
In our previous (Kausalya et al. 2006) as well as in the present study, we transfected high resistance MDCK-C7 cells with claudin-16 and found an increased PMg, but no effects on PNa, PCl or PCa. Mg2+ and Ca2+ fluxes occurred similarly in both directions.
Thus, the only consistent effect in the different cell types compared to control cells is the increase, albeit small, in PMg in wt claudin-16-transfected cells, which is absent in cells transfected with mutant claudin-16. Considering the dramatic effect of defects in claudin-16 on Mg2+ homeostasis in FHNNC patients, the effect of claudin-16 on PMg appears surprisingly small.
The reason for the variability of the effects of claudin-16 expression on all other paracellular parameters observed in the different studies is unclear. A possible reason may be the use of different cell lines (i.e. MDCK-C7 vs MDCK-II or LLC-PK1), which differ in their endogenous claudin repertoire that may alter the effects of exogenous claudins (Van Itallie et al. 2003). Furthermore, it cannot be ruled out that claudin-16 specifically needs claudin-19 as an interaction partner to fully unfold its function (Hou et al. 2008).
In addition, differences in findings relating to PCa may also be related to methodological differences. Low extracellular Ca2+ concentrations, as used by Ikari et al. (2004, 2006) for example, are known to open tight junctions. To avoid this problem in the present study, extracellular Ca2+ concentrations during Ca2+ flux experiments were kept at 1.2 mm on both the apical and the basolateral side of the cell layers.
Transcellular current
The possibility that the Mg2+-induced transcellular current observed in the present study might be caused by reversal of an electrogenic, Na+-dependent, transcellular Mg2+ transport could be ruled out by the finding that a current with identical properties could be induced in the nominal absence of extracellular Mg2+ by the application of various other divalent cations. Some of these cations, such as Ni2+, are known to quench fura-2 fluorescence. As no quenching was observed during application of 100 μm Ni2+, Ni2+ uptake could also be ruled out.
The features of the divalent cation-induced current are consistent with those published for CaSR and cation-sensing receptor (CSR) activation (CaSR, Arthur et al. 1997; Faurskov & Bjerregaard, 2002; CSR, Chang & Shoback, 2004; Ward, 2004): CaSR can be activated through extracellular divalent and trivalent cations. For Mg2+ stimulation, an EC50 of 4.5 mm (Ruat et al. 1996) and 4.7 mm (Bräuner-Osborne et al. 1999) and a Hill coefficient of 2.3 (Bräuner-Osborne et al. 1999) have been reported, values that are strikingly similar to those found in the present study (see Fig. 3A). EC50 values are surprisingly high; however, there are estimates that interstitial concentrations in this order of magnitude may be reached at least in the medulla of the kidney (Brunette et al. 1978).
CaSR is coupled to a G-protein and is known to act through a cAMP decrease and through IP3-induced Ca2+ release from intracellular stores (Arthur et al. 1997; Ward, 2004). Furthermore, in MDCK cells an increase in [Ca2+]i triggers Cl− secretion (Lahr et al. 2000).
In a recent study, Ikari et al. (2008) postulate an interaction between CaSR and claudin-16-mediated Mg2+ transport via PKA inhibition, decreased claudin-16 phosphorylation and a resulting claudin-16 translocation to lysosomes.
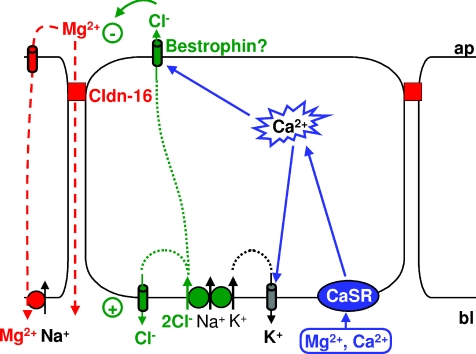
Our present model for the divalent cation-induced transcellular current in claudin-16-transfected MDCK-C7 cells is depicted in Fig. 9 and differs fundamentally from the model suggested by Ikari et al. (2008). In part the present model is comparable with a model published by Lahr et al. (2000) for antidiuretic hormone (ADH) stimulation of MDCK cells, where activation of V1 receptors causes Ca2+ release from intracellular stores and the activation of apical Ca2+-sensitive Cl− channels.
Figure 9. Model of components involved in the generation of the divalent cation-induced Cl− current in claudin-16-transfected MDCK-C7 cells.
Application of divalent cations to the basolateral side of claudin-16-expressing MDCK-C7 cell layers activates the Ca2+-sensing receptor, CaSR. This causes release of Ca2+ from intracellular stores and thus an increase in [Ca2+]i. Intracellular Ca2+ activates the apical Ca2+-sensitive Cl− channel bestrophin, which, in claudin-16-expressing cells, is recruited to the apical membrane and to the tight junction, i.e. close to the release sites of Ca2+ from intracellular stores. Cl− enters the cells through basolateral Na+-dependent, K+-dependent or independent symport mechanisms and, under resting conditions, probably leaves the cells through basolateral, DIDS-sensitive Cl− channels. When, upon CaSR stimulation, apical Cl− channels open, Cl− is able to leave the cells on the apical side, thus carrying the observed current. K+ entering the cells together with Cl− on the basolateral side can leave the cells on the basolateral side through Ca2+-sensitive K+ channels. This helps to further enhance the observed divalent cation-induced current. This current changes the transepithelial potential and thus the driving force for both para- and transcellular transport of Mg2+ (and Ca2+).
In the present model, application of divalent cations to the basolateral side of the cell layer causes CaSR activation, which triggers an increase in [Ca2+]i, thereby activating a transcellular current (Cl− secretion, see below). The [Ca2+]i increase appears to be predominantly due to Ca2+ release from intracellular stores, as store depletion by thapsigargin prevents consecutive activation of the current by application of divalent cations. Conversely, the transcellular current can be activated by direct manipulations of [Ca2+]i, e.g. by the application of Ca2+ ionophores (ionomycin, A23187) or during the depletion of Ca2+ stores through the application of thapsigargin and/or caffein.
The increase in [Ca2+]i causes activation of apical Ca2+-sensitive Cl− channels and basolateral Ca2+-sensitive K+ channels and thus induces Cl− secretion, which is energized by a basolateral furosemide/bumetanide-sensitive Na+-K+-2Cl− symport and a basolateral chlorothiazide-sensitive Na+-Cl− symport. Therefore, a respective removal of basolateral Na+ and Cl− (replaced with non-permeant anions) completely inhibits the transcellular current, while removal of basolateral K+ only causes partial inhibition. K+ has to leave the cells through the basolateral Ca2+-sensitive K+ channels, as an inhibition of these channels reduces the observed current. Cl− partially leaks out at the basolateral side through DIDS-sensitive channels or transporters. Hence, basolateral application of DIDS increases the current.
So, how does claudin-16 modulate the pathway leading to Cl− secretion? One possibility is that claudin-16 interacts with CaSR. Alternatively, claudin-16 could affect the loading of intracellular Ca2+ stores, or interact with the apical, Ca2+-sensitive Cl− channels.
An interaction between claudin-16 and CaSR is unlikely based on the observation that intracellular Ca2+ increases induced by thapsigargin and ionomycin are able to mimick the divalent cation-induced currents. Furthermore, a claudin-16 interaction with the loading of intracellular Ca2+ stores is also unlikely. First, [Ca2+]i transients observed after CaSR activation did not differ between claudin-16- and mock-transfected control cells (Fig. 6D). Second, ionomycin effects differ between wt claudin-16-transfected, mutant claudin-16-transfected and control cells. As ionomycin-induced [Ca2+]i increases should be store independent, this indicates that components down-stream of the [Ca2+]i increases are responsible for the observed differences.
Bestrophin
An interaction between claudin-16 and apical Ca2+-activated Cl− channels therefore seems most likely. Unfortunately, the molecular identity of Ca2+-activated Cl− channels is still controversial (Qu et al. 2003). While members of the CLCA (chloride channel, calcium-activated) family are either not blocked by niflumic acid or only at near-millimolar concentrations, the Cl− current observed in the present study was completely blocked by micromolar concentrations of the structurally similar flufenamic acid. Another Ca2+-activated Cl− channel candidate is bestrophin, which is predominantly expressed in the retinal pigment epithelium and has been linked to Best disease (Petrukhin et al. 1998). However, bestrophin expression has been reported for other tissues such as liver, spleen, colon and, interestingly, kidney (Qu et al. 2003; Barro Soria et al. 2006). In this context, it is noteworthy that about one third to one half of the FHNNC patients present ocular abnormalities (Meij et al. 2002). In contrast to the CLCA Cl− channels, bestrophin is inhibited by niflumic acid at concentrations as low as 10 μm (Pifferi et al. 2006).
Interestingly, we observed that the subcellular distribution of bestrophin differed in wt claudin-16 and T233R mutant claudin-16-transfected cells compared to R146T mutant claudin-16 or control cells. Bestrophin showed a diffuse distribution in control cells, similar to the recently reported localization of bestrophin-1 in the endoplasmic reticulum of HEK293 cells (Aldehni et al. 2009). Howewer, bestrophin was concentrated near the apical membrane and at the site of cell–cell contacts in claudin-16-overexpressing cells, where it partially colocalized with occludin. This relocalization correlated with the presence of strong divalent cation-induced currents as induced by wt claudin-16 or the T233R mutant. Incidentally, release of Ca2+ from the endoplasmic reticulum has been reported to occur from this region in polarized MDCK cells (Colosetti et al. 2003). Claudin-16 may thus optimise the localization of Ca2+-activated Cl− channels to areas of high [Ca2+]i during the release of Ca2+ from intracellular stores. Bestrophin is thus an attractive candidate for mediating the claudin-16-dependent transcellular current observed in MDCK cells and further research will test this hypothesis.
Wt-claudin-16 and claudin-16 mutants
We did not detect a functional diversity between ‘human’ and ‘rodent’ claudin-16: MDCK cells expressing either the long or the short version of claudin-16 showed identical properties with regard to paracellular Mg2+ and Ca2+ flux and to the increase in divalent cation-induced Cl− current.
The two mutants investigated showed contrasting behaviour. The R146T mutant, which affects net charge of the second extracellular loop, still resides within the tight junction (Kausalya et al. 2006), but transfected cells show neither increased paracellular fluxes nor increased transcellular currents and thus lack both components of wt claudin-16. In affected patients, this mutation causes FHHNC (Weber et al. 2001b). The T233R mutant affects the PDZ binding motif of claudin-16 and thus correct localization within the tight junction (Kausalya et al. 2006). Cells expressing this mutant do not show increased paracellular Mg2+ flux but still exhibit the increased transcellular current. Patients carrying this mutation only show a mild form of FHHNC (Müller et al. 2003).
It is thus concluded that the increased paracellular flux requires an intact claudin-16 and a correct localization within the tight junction. Both the long and the short claudin-16 version serve equally well.
Together with the observation that cells overexpressing another claudin, namely claudin-11, do not exhibit the increased divalent cation-induced Cl− current, it can be concluded that this current is not a mere artefact of the overexpression of a tight junction protein. The observation that the T233R mutant does not reside within the tight junction but retains the increased divalent cation-induced Cl− current argues against a direct interaction between bestrophin and claudin-16 or at least against an interaction via the PDZ-binding motif. The molecular details of this interaction will be investigated in a future study.
Physiological implications
TAL cells and MDCK cells are both equipped with Na+-K+-2Cl− symporters and Cl− channels, but these transporters are arranged in a mirror-like orientation in both cell types. Therefore, net Cl− transport is directed from the basolateral to the apical side in MDCK cell layers but in the reverse direction in TAL cells.
Nevertheless, due to the lack of TAL cell lines that can be grown to form confluent monolayers, claudin-16-transfected MDCK cells represent the best model system currently available to explore the functional roles of claudin-16.
It might be tempting to extrapolate the findings obtained in MDCK cells to TAL cells since, similar to MDCK cells, these cells also possess a basolateral CaSR (Chattopadhyay et al. 1996; Desfleurs et al. 1998). Although stimulation of CaSR in the TAL is known to dissipate the transepithelial potential and thus the driving force for Mg2+ absorption, the underlying mechanisms are different from those observed in MDCK cells.
Thus, the situation in MDCK cells may better reflect that of more distal parts of the nephron, where claudin-16 is also expressed (Simon et al. 1999). In this region of the nephron, fine-tuning of renal Mg2+ homeostasis takes place and claudin-16 may crosstalk with the CaSR to control Mg2+ transport.
Acknowledgments
We gratefully acknowledge the skilful technical assistance of Sieglinde Lüderitz, Brigitte Papanis and Detlef Sorgenfrei. Supported by the Deutsche Forschungsgemeinschaft (DFG GU447/11-1 and DFG Research Unit FOR 721).
Glossary
Abbreviations
- CaSR
calcium sensing receptor
- CFTR
cystic fibrosis transmembrane conductance regulator
- CLCA
chloride channel, calcium-activated
- DCT
distal convoluted tubule
- DIDS
4,4′-diisothiocyanatostilbene-2, 2′-disulphonic acid
- DPC
diphenylamine-2-carboxylatem
- FHHNC
familial hypomagnesaemia, hypercalciuria and nephrocalcinosis
- LLC-PK1 cells
porcine kidney cells
- MDCK cells
Madin–Darby canine kidney cells
- NMDG
N-methyl-d-glucamine
- TAL
thick ascending limb of Henle's loop
- ZO-1
zonula occludens-1
Author contributions
D.G., M.F.: conception and design of data. D.G., S.A., S.P., P.J.K.: performing experiments. D.G.: drafting the article. All authors: analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published.
Supplemental material
References
- Aldehni F, Barro Soria R, Almaca J, Raquel Martins J, Ousingsawat J, Schreiber R, Kunzelmann K. ER localized and Pak2 phosphorylated bestrophin 1 confers receptor activation of epithelial Ca2+ dependent ion channels. Acta Physiol. 2009;195(Suppl 669):P472. [Google Scholar]
- Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- Arthur JM, Collinsworth GP, Gettys TW, Quarles LD, Raymond JR. Specific coupling of a cation-sensing receptor to G proteina-subunits in MDCK cells. Am J Physiol Renal Physiol. 1997;273:F129–F135. doi: 10.1152/ajprenal.1997.273.1.F129. [DOI] [PubMed] [Google Scholar]
- Barro Soria R, Spitzner M, Schreiber R, Kunzelmann K. Bestrophin 1 enables Ca2+ activated Cl− conductance in epithelia. J Biol Chem. 2006 doi: 10.1074/jbc.M605716200. DOI 10.1074/jbc.M605716200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuner-Osborne H, Jensen AA, Sheppard PO, O’Hara P, Krogsgaard-Larsen P. The agonist-binding domain of the calcium-sensing receptor is located at the amino-terminal domain. J Biol Chem. 1999;274:18382–18386. doi: 10.1074/jbc.274.26.18382. [DOI] [PubMed] [Google Scholar]
- Brunette MG, Vigneault N, Carriere S. Magnesium handling by the papilla of the young rat. Pflügers Arch. 1978;373:229–235. doi: 10.1007/BF00580829. [DOI] [PubMed] [Google Scholar]
- Chang W, Shoback D. Extracellular Ca2+-sensing receptors – an overview. Cell Calcium. 2004;35:183–196. doi: 10.1016/j.ceca.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N, Baumm M, Bai M, Riccardi D, Hebert SC, Harris HW, Brown EM. Ontogeny of the extracellular calcium-sensing receptor in rat kidney. Am J Physiol Renal Physiol. 1996;271:F736–F743. doi: 10.1152/ajprenal.1996.271.3.F736. [DOI] [PubMed] [Google Scholar]
- Cole DEC, Quamme GA. Inherited disorders of renal magnesium handling. J Am Soc Nephrol. 2000;11:1937–1947. doi: 10.1681/ASN.V11101937. [DOI] [PubMed] [Google Scholar]
- Colosetti P, Tunwell REA, Cruttwell C, Jean-Pierre Arsanto J-P, Mauger JP, Cassio D. The type 3 inositol 1,4,5-trisphosphate receptor is concentrated at the tight junction level in polarized MDCK cells. J Cell Sci. 2003;116:2791–2803. doi: 10.1242/jcs.00482. [DOI] [PubMed] [Google Scholar]
- Desfleurs E, Wittner M, Simeone S, Pajaud S, Moine G, Rajerison R, Di Stefano A. Calcium-sensing receptor: regulation of electrolyte transport in the thick ascending limb of Henle's loop. Kidney Blood Press Res. 1998;21:401–412. doi: 10.1159/000025892. [DOI] [PubMed] [Google Scholar]
- Di Stefano A, Roinel N, de Rouffignac C, Wittner M. Transepithelial Ca2+ and Mg2+ transport in the cortical thick ascending limb of Henle's loop of the mouse is a voltage-dependent process. Ren Physiol Biochem. 1993;16:157–166. doi: 10.1159/000173762. [DOI] [PubMed] [Google Scholar]
- Faurskov B, Bjerregaard HF. Evidence for cadmium mobilization of intracellular calcium through a divalent cation receptor in renal distal epithelial A6 cells. Pflügers Arch. 2002;445:40–50. doi: 10.1007/s00424-002-0912-z. [DOI] [PubMed] [Google Scholar]
- Gekle M, Wünsch S, Oberleithner H, Silbernagl S. Characterization of two MDCK-cell subtypes as a model system to study principal cell and intercalated cell properties. Pflügers Arch. 1994;428:157–162. doi: 10.1007/BF00374853. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Günzel D, Haisch L, Pfaffenbach S, Krug SM, Milatz S, Amasheh S, Hunziker W, Müller D. Claudin function in the thick ascending limb of Henle's loop. Ann NY Acad Sci. 2009a;1165:1507–1517. doi: 10.1111/j.1749-6632.2009.04051.x. [DOI] [PubMed] [Google Scholar]
- Günzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Müller D. Claudin-10 exists in six alternatively spliced isoforms which exhibit distinct localization and function. J Cell Sci. 2009b;122:1507–1517. doi: 10.1242/jcs.040113. [DOI] [PubMed] [Google Scholar]
- Hirano T, Kobayashi N, Itoh T, Takasuga A, Nakamaru T, Hirotsune S, Sugimoto Y. Null mutation of PCLN-1/claudin-16 results in bovine chronic interstitial nephritis. Genome Res. 2000;10:659–663. doi: 10.1101/gr.10.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrate P, Piel C, Schlue WR. Effect of extracellular K+ on the intracellular free Ca2+ concentration in leech glial cells and Retzius neurones. Brain Res. 1995;696:231–241. doi: 10.1016/0006-8993(95)00883-r. [DOI] [PubMed] [Google Scholar]
- Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118:5109–5118. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikari A, Hirai N, Shiroma M, Harada H, Sakai H, Hayashi H, Suzuki Y, Degawa M, Takagi K. Association of paracellin-1 with ZO-1 augments the reabsorption of divalent cations in renal epithelial cells. J Biol Chem. 2004;279:54826–54832. doi: 10.1074/jbc.M406331200. [DOI] [PubMed] [Google Scholar]
- Ikari A, Matsumoto S, Harada H, Takagi K, Hayashi H, Yuichi Suzuki Y, Degawa M, Miwa M. Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions. J Cell Sci. 2006;119:1781–1789. doi: 10.1242/jcs.02901. [DOI] [PubMed] [Google Scholar]
- Ikari A, Okude C, Sawada H, Sasaki Y, Yamazaki Y, Sugatani J, Degawa M, Miwa M. Activation of a polyvalent cation-sensing receptor decreases magnesium transport via claudin-16. Biochim Biophys Acta. 2008;1778:283–290. doi: 10.1016/j.bbamem.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausalya PJ, Amasheh S, Günzel D, Wurps H, Müller D, Fromm M, Hunziker W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of claudin-16. J Clin Invest. 2006;116:878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- Kreusel KM, Fromm M, Schulzke JD, Hegel U. Cl− secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6) Am J Physiol Cell Physiol. 1991;261:C574–C582. doi: 10.1152/ajpcell.1991.261.4.C574. [DOI] [PubMed] [Google Scholar]
- Lahr TF, Record RD, Hoover DK, Hughes CL, Blazer-Yost BL. Characterization of the ion transport responses to ADH in the MDCK-C7 cell line. Pflügers Arch. 2000;439:610–617. doi: 10.1007/s004249900222. [DOI] [PubMed] [Google Scholar]
- McCall KA, Fierke CA. Colorimetric and fluorimetric assays to quantitate micromolar concentrations of transition metals. Anal Biochem. 2000;284:307–315. doi: 10.1006/abio.2000.4706. [DOI] [PubMed] [Google Scholar]
- Meij IC, Van Den Heuvel LP, Knoers NV. Genetic disorders of magnesium homeostasis. BioMetals. 2002;15:297–307. doi: 10.1023/a:1016039101747. [DOI] [PubMed] [Google Scholar]
- Müller D, Kausalya PJ, Bockenhauer D, Thumfart J, Meij IC, Dillon MJ, van't Hoff W, Hunziker W. Unusual clinical presentation and possible rescue of a novel claudin-16 mutation. J Clin Endocrinol Metab. 2006a;91:3076–30769. doi: 10.1210/jc.2006-0200. [DOI] [PubMed] [Google Scholar]
- Müller D, Kausalya PJ, Claverie-Martin F, Meij IC, Eggert P, Garcia-Nieto V, Hunziker W. A novel claudin-16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am J Hum Genet. 2003;73:1293–1301. doi: 10.1086/380418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D, Kausalya PJ, Meij IC, Hunziker W. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: blocking endocytosis restores surface expression of a novel claudin-16 mutant that lacks the entire C-terminal cytosolic tail. Hum Mol Genet. 2006b;15:1049–1058. doi: 10.1093/hmg/ddl020. [DOI] [PubMed] [Google Scholar]
- Petrukhin K, Koisti MJ, Bakall B, Li W, Xie G, Marknell T, et al. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19:241–247. doi: 10.1038/915. [DOI] [PubMed] [Google Scholar]
- Pifferi S, Pascarella G, Boccaccio A, Mazzatenta A, Gustincich S, Menini A, Zucchelli S. Bestrophin-2 is a candidate calcium-activated chloride channel involved in olfactory transduction. Proc Natl Acad Sci U S A. 2006;103:12929–12934. doi: 10.1073/pnas.0604505103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praga M, Vara J, Gonzalez-Parra E, Andres A, Alamo C, Araque A, Ortiz A, Rodicio JL. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Kidney Int. 1995;47:1419–1425. doi: 10.1038/ki.1995.199. [DOI] [PubMed] [Google Scholar]
- Qu Z, Fischmeister R, Hartzell C. Mouse bestrophin-2 is a bona fide Cl− channel: identification of a residue important in anion binding and conduction. J Gen Physiol. 2004;123:327–340. doi: 10.1085/jgp.200409031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Wei RW, Hartzell HC. Characterization of Ca2+-activated Cl− currents in mouse kidney inner medullary collecting duct cells. Am J Physiol Renal Physiol. 2003;285:F326–F335. doi: 10.1152/ajprenal.00034.2003. [DOI] [PubMed] [Google Scholar]
- Ruat M, Snowman AM, Hester LD, Snyder SH. Cloned and expressed rat Ca2+-sensing receptor. Differential cooperative responses to calcium and magnesium. J Biol Chem. 1996;271:5972–5975. doi: 10.1074/jbc.271.11.5972. [DOI] [PubMed] [Google Scholar]
- Schlingmann KP, Gudermann T. A critical role of TRPM channel-kinase for human magnesium transport. J Physiol. 2005;566:301–308. doi: 10.1113/jphysiol.2004.080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- Tsunenari T, Nathans J, Yau KW. Ca2+-activated Cl− current from human bestrophin-4 in excised membrane patches. J Gen Physiol. 2006;127:749–754. doi: 10.1085/jgp.200609527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–F1084. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- Ward DT. Calcium receptor-mediated intracellular signalling. Cell Calcium. 2004;35:217–228. doi: 10.1016/j.ceca.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Weber S, Hoffmann K, Jeck N, Saar K, Boeswald M, Kuwertz-Broeking E, et al. Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur J Hum Genet. 2000;8:414–422. doi: 10.1038/sj.ejhg.5200475. [DOI] [PubMed] [Google Scholar]
- Weber S, Schlingmann KP, Peters M, Nejsum LN, Nielsen S, Engel H, et al. Primary gene structure and expression studies of rodent paracellin-1. J Am Soc Nephrol. 2001a;12:2664–2672. doi: 10.1681/ASN.V12122664. [DOI] [PubMed] [Google Scholar]
- Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2001b;12:1872–1881. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.