Abstract
Reduced availability of tetrahydrobiopterin (BH4) contributes to the age-related decline of nitric oxide (NO)-mediated vasodilatation of soleus muscle arterioles. Depending on availability of substrate and/or necessary co-factors, endothelial nitric oxide synthase (eNOS) can generate NO and/or superoxide (O2−). We evaluated the effects of age and chronic exercise on flow-induced vasodilatation and levels of NO and O2− in soleus muscle arterioles. Young (3 months) and old (22 months) male rats were exercise trained or remained sedentary (SED) for 10 weeks. Flow-stimulated NO and O2−, as well as BH4 and l-arginine content, were determined in soleus muscle arterioles. Flow-induced vasodilatation was assessed under control conditions and during the blockade of O2− and/or hydrogen peroxide. Exercise training enhanced flow-induced vasodilatation in arterioles from young and old rats. Old age reduced, and exercise training restored, BH4 content and flow-stimulated NO availability. Flow-stimulated, eNOS-derived O2− levels were higher in arterioles from old SED compared to those from young SED rats. Exercise training increased flow-stimulated eNOS-derived O2− levels in arterioles from young but not old rats. O2− scavenging with Tempol reduced flow-induced vasodilatation from all groups except young SED rats. Addition of catalase to Tempol-treated arterioles eliminated flow-induced vasodilatation in arterioles from all groups. Catalase reduced flow-induced vasodilatation from all groups. In Tempol-treated arterioles, flow-induced vasodilatation was restored by deferoxamine, an iron chelator. These data indicate that uncoupling of eNOS contributes to the age-related decline in flow-induced vasodilatation; however, reactive oxygen species are required for flow-induced vasodilatation in soleus muscle arterioles from young and old rats.
Maximal exercise capacity declines with advancing age (Ogawa et al. 1992; Fitzgerald et al. 1997) and although part of this decline is associated with a reduction of maximal cardiac output (Lakatta, 1995), alterations in the local control of skeletal muscle blood flow and declines in vascular conductance also contribute to this abatement (Dinenno et al. 1999). Numerous studies have demonstrated that age impairs endothelial function in conduit and skeletal muscle resistance arteries in human and animal models (Gerhard et al. 1996; DeSouza et al. 2000; Muller-Delp et al. 2002; Woodman et al. 2002). Old age-associated reductions in endothelium-dependent dilatation of skeletal muscle resistance arterioles occur in part through reduced nitric oxide (NO) signalling (Spier et al. 2004). Mechanisms that may underlie this age-associated impairment in NO signalling include reduced availability of substrate (l-arginine) (Morris, 2000) or cofactors (e.g. tetrahydrobiopterin (BH4)) (Cosentino & Katusic, 1995; Tiefenbacher, 2001), reduced endothelial NO synthase (eNOS) protein levels and/or activity, and increased superoxide production (Darley-Usmar et al. 1995). Depending on the availability of l-arginine and/or BH4, eNOS can become uncoupled, resulting in the generation of NO and superoxide (O2−) (Vasquez-Vivar et al. 1998; Cosentino & Luscher, 1999). Ageing-induced diminution of BH4 contributes to the decline of NO-mediated dilatation to flow, and sepiapterin, a precursor to BH4 synthesis (Bagi et al. 2004), improves flow-induced vasodilatation in soleus muscle arterioles of aged rats (Delp et al. 2008). BH4 supplementation ameliorates endothelium-dependent dilatation in humans with cardiovascular diseases or elevated risk factors (Stroes et al. 1997; Maier et al. 2000); however, the impact of reduced BH4 availability on eNOS function has not been directly evaluated in the skeletal muscle resistance vasculature.
Reactive oxygen species (ROS) potentially decrease NO bioavailability by two mechanisms. First, O2− rapidly interacts with NO to form peroxynitrite (ONOO−), a potent ROS, which elicits cellular damage (Darley-Usmar et al. 1995; Cai & Harrison, 2000). Secondly, the presence of ROS, and primarily ONOO−, reduces BH4 to the inactive trihydrobiopterin radical (BH3), thereby reducing this necessary co-factor and subsequent NO production. Recent reports indicate that insufficient BH4 is the major determinant of whether eNOS produces O2− or NO (Bevers et al. 2006; Crabtree et al. 2009).
Aerobic exercise training restores age-associated reductions in NO-mediated dilatation in human and animal models (Taddei et al. 1995; DeSouza et al. 2000; Spier et al. 2004). Exercise training may increase NO bioavailability, in part, through enhanced regulation of ROS (Rush et al. 2000) or through increased expression of eNOS protein (Spier et al. 2004); however, the effects of age and exercise training on the regulation of eNOS activity have not been evaluated. Eskurza et al. (2005) demonstrated that an acute bolus of BH4 augments endothelial function in the brachial artery of old sedentary men, but had no effect on endothelium-dependent dilatation in habitually active, age-matched counterparts. These data suggest that aerobic exercise training prevents the loss of NO bioavailability by preserving BH4 in aged individuals; however, the effects of exercise training on BH4 levels in the resistance vasculature of skeletal muscle have not been determined. Here, we investigate the effects of age and exercise training on BH4 availability and putative eNOS uncoupling in soleus muscle arterioles of male rats.
Therefore, the purpose of this study was threefold. First, we sought to determine whether age-induced reductions of arteriolar BH4 are associated with eNOS uncoupling. Second, we investigated the possibility that exercise training restores arteriolar BH4 availability and reverses eNOS uncoupling with old age. And third, we determined whether eNOS uncoupling in skeletal muscle arterioles is accompanied by significant increases in ROS and a concomitant decrease in NO bioavailability.
Methods
Animals
All procedures in this study were approved by the Institutional Animal Care and Use Committees at West Virginia University. All methods complied fully with guidelines set in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, revised 1996). Young (3 months) and old (22 months) male Fischer 344 rats were obtained from Harlan (Indianapolis, IN, USA), housed under a 12: 12 h light–dark cycle, and given food and water ad libitum. This particular strain was chosen because cardiovascular function decreases with age in these rats, without the development of atherosclerosis or hypertension (Lakatta, 1995).
Exercise training
All rats were habituated to treadmill exercise, during which each rat walked on a motor-driven treadmill at 5 m min−1 (0 deg incline), 5 min per day for 3 days. After habituation, young and old rats were randomly assigned to either a control sedentary (SED) group (young SED, n= 30, and old SED, n= 32) or an exercise-trained (ET) group (young ET, n= 37, and old ET, n= 26). ET rats performed treadmill running at 15 m min−1 (15 deg incline), 5 days per week, for 10–12 weeks. The duration of running was gradually increased in the first 3 weeks until a 60 min duration was reached. The rats continued to run 5 days per week for 60 min per day for the remainder of the 10–12 week training period. Vascular responses were determined at least 48 h after the last exercise bout in ET rats.
Muscle oxidative enzyme activity
To determine the efficacy of the training protocol, sections of soleus muscle were stored at −80°C for determination of citrate synthase activity, a measure of muscle oxidative capacity (Srere, 1969; Delp & Duan, 1996).
Microvessel preparation
Rats were anaesthetized with isoflurane (5%, O2 balance) and killed by decapitation. The gastrocnemius–plantaris–soleus muscle group was dissected free from both hindlimbs and placed in a cold (4°C), filtered physiological saline solution (PSS) containing 145.0 mm NaCl, 4.7 mm KCl, 2.0 mm CaCl2, 1.17 mm MgSO4, 1.2 mm NaH2PO4, 5.0 mm glucose, 2.0 mm pyruvate, 0.02 mm EDTA, 3.0 mm Mops buffer, and 1 g/100 ml BSA, pH 7.4. With the aid of a dissecting microscope (Olympus SVH10), first-order (1A) arterioles were isolated and dissected from the soleus muscle, which is composed primarily of high-oxidative fibres (Delp & Duan, 1996), as previously described (Muller-Delp et al. 2002; Spier et al. 2004). The arterioles were then transferred to a Lucite chamber containing PSS with 1% albumin (pH 7.4) equilibrated with room air. Each end of the arteriole was cannulated with micropipettes filled with PSS-albumin solution and secured with a nylon suture. The sizes and resistances of the pipettes were matched to within 1%. The chamber was placed on the stage of an inverted microscope (Zeiss Axio40), equipped with a video camera (Panasonic BP310), video caliper (Colorado Video), and data-acquisition system (PowerLab; ADInstruments). Arterioles were pressurized via two independent reservoirs and checked for leaks. If leaks were present, the arterioles were discarded. Vessels that were free from leaks were pressurized to 70 cmH2O, gradually warmed to 37°C, and allowed to develop spontaneous tone during an initial equilibration period. The bathing solution was changed every 20 min during the course of the experiment.
Vasodilator responses to intraluminal flow
Upon displaying a steady level of spontaneous tone, arterioles were exposed to graded increases in intraluminal flow in the absence of changes in intraluminal pressure (Muller-Delp et al. 2002; Delp et al. 2008). This was accomplished by altering the heights of independent pressure reservoirs in equal and opposite directions so that a pressure difference was created across the vessel without altering mean intraluminal pressure. Diameter measurements were determined in response to incremental pressure differences of 2, 4, 10, 20, 40 and 60 cmH2O. Volumetric flow (Q) was then calculated from inner diameter (d) and mean red cell velocity (Vrbc), according the following equation (Davis, 1987; Kuo et al. 1990; Muller-Delp et al. 2002):
At the end of the experiment, arterioles were placed in Ca2+-free PSS with 100 μm of the NO donor, sodium nitroprusside for 1 h to obtain the maximal passive diameter (Muller-Delp et al. 2002; Spier et al. 2004).
Effects of ROS scavengers on flow-induced vasodilatation
To determine the role of O2−, hydrogen peroxide (H2O2), and hydroxyl ion (OH−) in old age-related reductions of flow-induced vasodilatation, responses to flow were evaluated in the presence of the following: (1) a superoxide dismutase mimetic, Tempol (100 μm; Sigma) (Chen et al. 2007), (2) a H2O2 scavenger, catalase (100 U; Sigma) (Emsley et al. 1999), (3) Tempol (100 μm) plus catalase (100 U), (4) a NADPH oxidase inhibitor, apocynin (100 μm; Sigma) (Zhu et al. 2007), and (5) Tempol plus deferoxamine (100 μm; Sigma) (Thengchaisri et al. 2006), an iron chelator, and inhibitor of OH− formation (Thengchaisri et al. 2006).
Detection of flow-stimulated NO availability
To evaluate flow-stimulated bioavailability of NO, isolated soleus muscle arterioles were cannulated as described above. 4,5-Diaminofluorescein diacetate (DAF-2DA) (Calbiochem) is a non-fluorescent dye until it reacts with NO in the presence of oxygen to form a fluorescent compound, triazolofluorescein (2DAF-2T) (Kojima et al. 1998). The intensity of the fluorescence signal is proportional to NO levels. Following intraluminal loading of DAF-2DA (2.5 μm) for 20 min, soleus muscle arterioles were exposed to a flow rate of 34 nl s−1 and fluorescence images were acquired every 15 s over 2 min. To confirm that availability of NO occurred through eNOS, flow-induced DAF-2DA fluorescence was evaluated in the presence of NG-nitro-l-arginine methyl ester (l-NAME). DAF-2DA fluorescence during exposure to 10 μm Dea-NONOate, an NO donor, was used as a positive control to ensure that the dye was not saturated and to determine maximal fluorescence intensity as a control for loading of dye. A user-defined region of interest (ROI), which encompassed the entire vessel diameter and the central portion of the length of each vessel, was analysed for fluorescence intensity using ImageJ software (National Institutes of Health). Basal fluorescence levels varied among ROIs and in different experiments. Therefore, after background subtraction, changes in fluorescence intensity were expressed as the ratio of DAF stimulated/DAF at baseline. Both baseline and flow-induced fluorescence intensity values were measured within the same ROI in the same focal plane of each vessel.
Detection of flow-stimulated O2− generation
Dihydroethidium (DHE) (Calbiochem) fluorescence was used to evaluate flow-stimulated O2− generation in real time (Suzuki et al. 1995). To evaluate O2− generation in response to flow stimulation, soleus muscle arterioles were cannulated as described above. DHE fluorescence was evaluated in arterioles similar to those described by Suzuki et al. (1995). Following intraluminal loading of DHE (40 μm) for 20 min, soleus muscle arterioles were exposed to a flow rate of 34 nl s−1 and fluorescence images were acquired every 15 s over 2 min. The specificity of the DHE dye for superoxide was confirmed by application of the superoxide dismutase (SOD) mimetic, Tempol, which significantly reduced DHE fluorescence. To determine whether generation of O2− occurred through eNOS, flow-stimulated DHE fluorescence was evaluated in the presence of L-NAME. DHE fluorescence during exposure to 100% ethanol was used as a positive control to ensure that the dye was not saturated and to control for equal loading of dye. A region of interest was selected for each vessel and changes in fluorescence intensity for each respective ROI were analysed using ImageJ software as described above.
Arteriolar l-arginine and BH4 measurements
Arteriolar l-arginine and BH4 levels were determined using the HPLC method as previously described (Wu & Meininger, 1995; Meininger et al. 2000; Delp et al. 2008). Briefly, soleus muscle arterioles were dissected from each animal and pooled. For l-arginine analysis, vessels (10 to 15 mg) were homogenized with 0.2 ml 1.5 m HClO4, and then 0.1 ml 2 m K2CO3 was added. The homogenates were centrifuged at 10 000 g for 1 min, and an aliquot (0.2 ml) of the supernatant was used for sample determination of l-arginine content (Wu & Meininger, 1995). For BH4 analysis, arterioles (10 to 15 mg) were homogenized in 0.1 ml 0.1 m phosphoric acid containing 5 mm dithioerythritol (an antioxidant), to which 17.5 μl 2 m trichloroacetic acid was added. Extracts were oxidized with acidic or basic iodine. Acidic oxidation quantitatively converts BH4 and dihydrobiopterin to biopterin; basic oxidation converts dihydrobiopterin and BH4 to biopterin and pterin, respectively. Samples were incubated in the dark for 1 h. Excess iodine was removed by adding ascorbic acid (final concentration, 0.1 m). The final solution was analysed on a C18 reversed-phase column using fluorescence detection and authentic biopterin as a standard. The amount of BH4 in the arteriolar extracts was determined from the difference between acidic and basic iodine-generated biopterin (Meininger et al. 2000). The sensitivity of l-arginine and BH4 analyses by HPLC, which was assessed using detection limits defined as a signal-to-noise ratio of 3, was 5 and 2 μm, respectively. The reliability of the assays was indicated by the precision (agreement between replicate measurements), evaluated by the relative deviation (mean of absolute deviation/mean of replicate measurements × 100%), and by the accuracy (the nearness of an experimental value to the true value), determined with known amounts of standards and expressed as the relative errors ((measurement value − true value)/true value × 100%). The precision and accuracy for the l-arginine analysis were 1.4% and 1.6%, respectively, and for the BH4 analysis were 2.0% and 2.3%, respectively. The values in fmol (mg tissue)−1 and pmol (mg tissue)−1 for BH4 and arginine concentrations, respectively, were calculated on the basis of tissue weight.
Data analysis
Data are expressed as means ± standard error of the mean. Spontaneous tone was calculated as a percent constriction in relation to maximal diameter as determined by the following equation:
where DM is the maximal diameter recorded at 70 cmH2O and DT is the steady-state baseline diameter recorded at the same pressure. The vasodilator responses to flow are expressed as percent relaxation as calculated by the formula:
where DS is the arteriolar diameter at each respective stage, DB is the diameter recorded immediately prior to initiation of the flow–diameter curves, and DM is the maximal diameter for the arteriole. For statistical analyses, changes in diameter in response to flow were expressed as a percentage of maximal vasodilatation as previously described (Muller-Delp et al. 2002). Flow–diameter curves were evaluated by a three-way ANOVA with repeated measures on one factor in order to detect differences within (flow rate) and between (animal groups) factors. Two-way ANOVA was used to determine group differences in animal and vessel characteristics, and group differences in l-arginine and BH4 content in soleus muscle arterioles. Three-way ANOVA was used to determine the effect of age, training and l-NAME treatment on DAF fluorescence. Three-way ANOVA was used to determine the effect of age, training, and l-NAME treatment on DHE fluorescence, and planned contrasts were used as post hoc analysis to compare treatment combinations of interest. All data are presented as means ±s.e.m. In all statistical analyses, n indicates the number of animals in each group. Significance was defined as P≤ 0.05.
Results
Animals
Body mass increased with age and exercise training reduced body mass in both young and old rats (Table 1). Soleus muscle mass increased with age, but was unaltered by exercise training (Table 1). In contrast, soleus muscle mass to body mass ratio decreased with age and increased with exercise training (Table 1). Exercise training increased citrate synthase activity by 18.3% in soleus muscles of young rats, and by 20.1% in soleus muscles of old rats, confirming the efficacy of the exercise training as previously reported (Spier et al. 2004).
Table 1.
Soleus muscle and vessel characteristics of young and old, sedentary and exercise trained rats
| Young SED (n= 30) | Young ET (n= 37) | Old SED (n= 32) | Old ET (n= 26) | |
|---|---|---|---|---|
| Animal characteristics body wt (g) | 371 ± 5 | 348 ± 4 | 437 ± 6* | 379 ± 5† |
| Soleus muscle wt (mg) | 156 ± 4 | 165 ± 3 | 166 ± 3* | 169 ± 4 |
| Soleus wt/body wt (mg g–1) | 0.41 ± 0.01 | 0.47 ± 0.01* | 0.37 ± 0.01* | 0.45 ± 0.01† |
| Vessel characteristics maximal diameter (μm) | 130 ± 4 | 127 ± 4 | 123 ± 4 | 124 ± 5 |
| Spontaneous tone (%) | 51 ± 2 | 54 ± 2 | 51 ± 2 | 57 ± 2 |
| (n= 30) | (n= 37) | (n= 33) | (n= 26) | |
| With Tempol | 48 ± 5 | 47 ± 5 | 61 ± 6 | 59 ± 3 |
| (n= 7) | (n= 8) | (n= 7) | (n= 10) | |
| With Tempol + catalase | 50 ± 5 | 53 ± 4 | 54 ± 3 | 57 ± 3 |
| (n= 5) | (n= 10) | (n= 10) | (n= 7) | |
| With catalase | 45 ± 2 | 53 ± 5 | 55 ± 3 | 56 ± 6 |
| (n= 6) | (n= 9) | (n= 7) | (n= 9) | |
| With apocynin | 46 ± 3 | 52 ± 5 | 57 ± 4 | 56 ± 4 |
| (n= 6) | (n= 6) | (n= 7) | (n= 6) | |
| With Tempol + deferoxamine | 54 ± 4 | 48 ± 5 | 64 ± 3 | 60 ± 5 |
| (n= 6) | (n= 9) | (n= 7) | (n= 3) |
Maximal diameter was recorded in Ca2+-free physiological saline solution with 100 μm sodium nitroprusside. Tone (%) =[(maximal diameter − diameter with tone)/maximal diameter]× 100. *P < 0.05 vs. young SED and †P < 0.05 vs. old SED.
Maximal intraluminal diameter and levels of spontaneous tone of soleus muscle arterioles were not different among groups (Table 1). Treatment with Tempol, Tempol plus catalase, catalase alone, apocynin and Tempol plus deferoxamine had no effect on spontaneous tone among groups (Table 1).
Vasodilator responses to flow
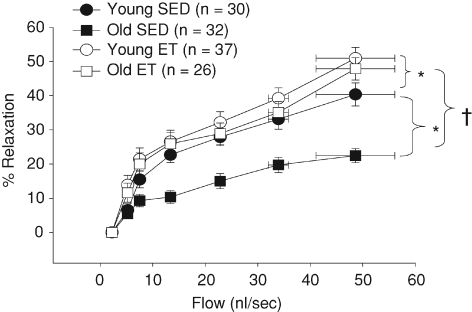
Vasodilatation to intraluminal flow was diminished in soleus muscle arterioles from old SED rats (Fig. 1). Exercise training restored flow-induced vasodilatation in soleus muscle arterioles from old rats to that of young SED rats and exercise training improved flow-induced dilatation in soleus muscle arterioles from young rats (Fig. 1).
Figure 1. Diameter changes in response to increasing intraluminal flow in soleus muscle arterioles.
Exercise training restores flow-induced vasodilatation in arterioles from old rats and improves flow-induced vasodilatation in arterioles from young rats. *P < 0.05 vs. young SED and †P < 0.05 vs. old SED.
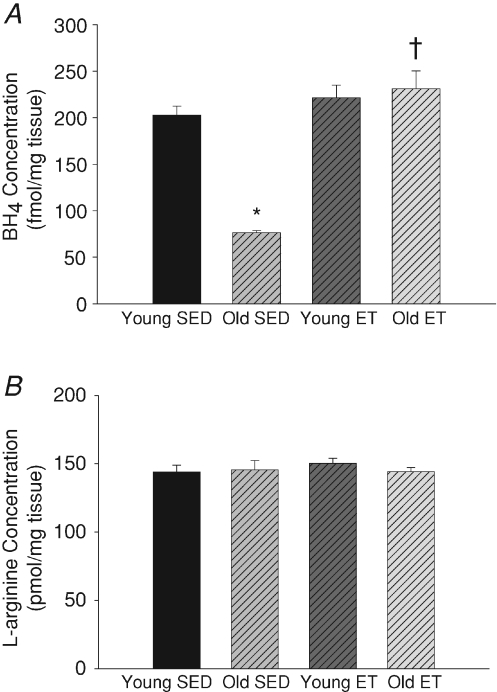
Arteriolar BH4 and l-arginine content
Similar to previous findings (Delp et al. 2008), age decreased arteriolar BH4 levels (Fig. 2A). Exercise training restored arteriolar BH4 levels of old rats to that of young rats (Fig. 2A). Exercise training did not alter BH4 levels in soleus muscle arterioles from young rats. As previously reported (Delp et al. 2008), ageing had no effect on l-arginine levels and exercise training did not effect arteriolar l-arginine levels in either young or old rats (Fig. 2B).
Figure 2. BH4 and l-arginine levels in soleus muscle arterioles.
A, exercise training reverses age-related declines in arteriolar BH4 levels in soleus muscle arterioles. B, ageing and exercise training had no effect on arteriolar l-arginine levels in soleus muscle arterioles. *P < 0.05 vs. young SED and †P < 0.05 vs. old SED. n= 6–8 per group.
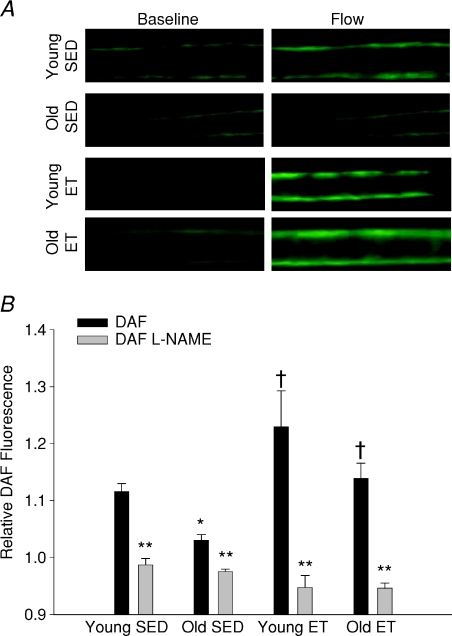
Flow-stimulated NO availability in soleus muscle arterioles
Exposure to intraluminal flow increased DAF fluorescence by 10% above baseline values in arterioles from young SED rats, and by approximately 5% in arterioles from old SED rats (Fig. 3B). In arterioles from young ET and old ET rats, DAF fluorescence increased by approximately 23% and 14% above baseline, respectively, during exposure to flow. l-NAME eliminated flow-stimulated increases in DAF fluorescence in arterioles from all groups of rats, confirming DAF specificity for NO (Fig. 3B). Exposure to Dea-NONOate (10 μm) elicited maximal DAF fluorescence that was similar in arterioles from all groups (young SED, 2.27 ± 0.30; old SED 2.17 ± 0.23; young ET, 2.17 ± 0.27; old ET, 2.23 ± 0.20 fluorescence units), indicating that the dye was not saturated, and that loading of dye did not differ between groups.
Figure 3. Flow-induced stimulation of arteriolar NO.
A, DAF fluorescence indicates NO bioavailability in soleus muscle arterioles from young and old, sedentary and exercise trained rats at baseline and during flow stimulation (34 nl s−1). B, flow-stimulated changes in NO bioavailability, shown here as flow-induced to baseline fluorescence ratio (relative fluorescence), is reduced in soleus muscle arterioles from old SED rats. Exercise training restored flow-stimulated NO availability in arterioles from old rats and increased flow-stimulated NO availability in young rats. l-NAME inhibited flow-induced increases in DAF fluorescence in soleus muscle arterioles from all rats. *P < 0.05 vs. young SED, †P < 0.05 vs. respective SED group, and **P < 0.05 control vs.l-NAME. n= 8–10 per group.
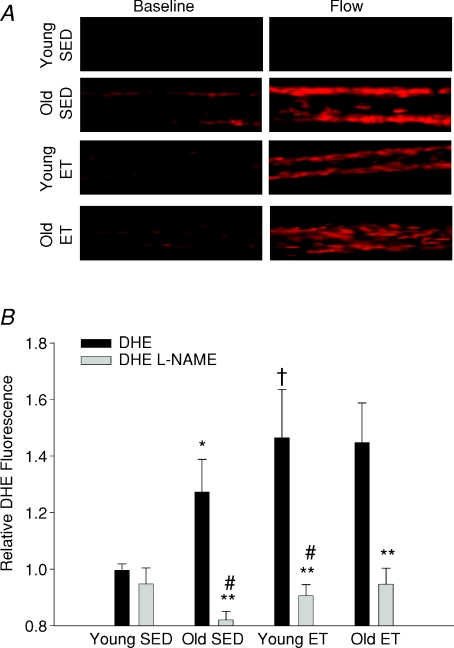
Flow-stimulated O2− generation in soleus muscle arterioles
Flow-stimulated increases in DHE fluorescence were eliminated by treatment with Tempol, confirming O2−-specific oxidation of DHE (data not shown). The relative increase in DHE fluorescence that occurred in response to exposure to intraluminal flow was greater in soleus muscle arterioles from old SED rats as compared to those from young SED rats (Fig. 4B). Exercise training augmented the relative increase in O2− produced by intraluminal flow exposure in arterioles of young rats, but did not alter the relative amount of O2− generated by flow stimulation in arterioles from old rats, indicating that augmented O2− signalling contributes to exercise training-induced improvement of flow-induced vasodilatation in young rats. Inhibition of eNOS with l-NAME reduced the flow-induced DHE fluorescence signal significantly in arterioles from all but young SED rats (Fig. 4B), implicating eNOS as a significant source of flow-induced O2− generation. l-NAME inhibition of flow-induced DHE fluorescence was significant in arterioles from old SED rats but not in arterioles from young SED rats, suggesting that flow stimulation of eNOS-derived O2− increases with age. l-NAME inhibited flow-induced increases in DHE fluorescence in arterioles from both young and old ET rats, suggesting that exercise training increases signalling through eNOS-derived O2− in arterioles from rats of both ages. Exposure to ethanol induced maximal DHE fluorescence that was not different between groups (young SED, 315 ± 0.50; old SED 290 ± 0.29; young ET, 296 ± 0.41; old ET, 315 ± 0.35 fluorescence units), confirming that loading of dye was similar in arterioles from all groups of rats.
Figure 4. Flow-induced stimulation of arteriolar O2−.
A, DHE fluorescence indicates O2− levels in soleus muscle arterioles from young and old, sedentary and exercise trained rats at baseline and during flow stimulation (34 nl s−1). B, flow-stimulated changes in O2− levels, indicated here as flow-induced to baseline DHE fluorescence ratio (relative fluorescence), is increased in soleus muscle arterioles from old SED rats. Exercise training increased relative DHE fluorescence in young rats, but had no effect in old rats. l-NAME reduced DHE fluorescence significantly in soleus muscle arterioles from all but young SED rats. *P < 0.05 vs. young SED, †P < 0.05 vs. old SED, **P < 0.05 control vs.l-NAME, and #P < 0.05 change in DHE fluorescence with l-NAME vs. young SED. n= 9–11 per group.
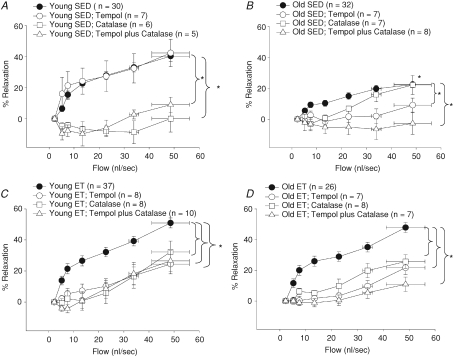
Role of O2− and H2O2 and in flow-induced vasodilatation
In young SED rats, scavenging of O2− with Tempol did not alter flow-induced vasodilatation (Fig. 5A). Catalase and Tempol plus catalase inhibited flow-induced vasodilatation indicating dependence on H2O2 in young SED rats (Fig. 5A).
Figure 5. Effects of Tempol and catalase on flow-induced vasodilatation of soleus muscle arterioles from young and old, SED and ET rats.
A, Tempol had no effect, but catalase and Tempol + catalase completely abolished flow induced vasodilatation in young SED. B, in old SED rats, catalase, Tempol, and Tempol + catalase reduced flow-induced vasodilatation. C, all treatments reduced flow-induced vasodilatation in young ET and (D) old ET rats. *P < 0.05 vs. control response.
Scavenging of O2− with Tempol inhibited flow-induced vasodilatation in arterioles from old SED rats (Fig. 5B). Scavenging of H2O2 with catalase reduced flow-induced vasodilatation between flow rates of 5.2 ± 0.75 and 22.8 ± 0.42 nl s−1 but not at higher flow rates, and Tempol plus catalase eliminated flow-induced vasodilatation in the old SED rats, suggesting that despite an overall reduction of flow-induced dilatation, H2O2 functions as a significant vasodilator in arterioles from old SED rats (Fig. 5B).
In young and old ET rats, scavenging of O2− with Tempol reduced flow-induced vasodilatation (Fig. 5C and D). Likewise, the scavenging of H2O2 with catalase alone reduced flow-induced dilatation in arterioles from young and old ET rats (Fig. 5C and D). Simultaneous scavenging of both O2− and H2O2 with Tempol plus catalase nearly abolished flow-induced vasodilatation in arterioles from old ET rats (Fig. 5D). The lack of a significant residual flow-induced vasodilatation following simultaneous scavenging of O2− and H2O2 (Fig. 5D) indicates that H2O2 is critical for flow-induced vasodilatation in arterioles from old ET rats. In contrast, although simultaneous scavenging of O2− and H2O2 reduced flow-induced vasodilatation in arterioles from young ET rats (Fig. 5C), significant dilatation remained in the presence of these scavengers, suggesting that NO contributes substantially to flow-induced dilatation in arterioles from these rats.
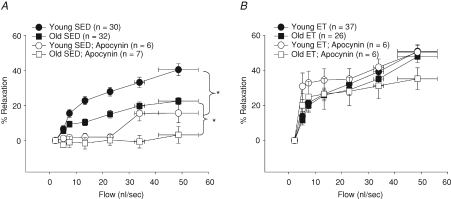
The inhibition of NADPH oxidase with apocynin reduced flow-induced vasodilatation in young and old SED rats (Fig. 6A). However, apocynin had no effect on flow-induced vasodilatation in young and old ET rats (Fig. 6B), suggesting that O2−-derived H2O2 required for flow-induced vasodilatation in the ET rats is not generated from NADPH oxidase-derived O2−.
Figure 6. Role of NADPH oxidase-derived O2− production in flow-induced vasodilatation in soleus muscle arterioles of young and old, SED and ET rats.
A, NADPH oxidase inhibition with Apocynin reduced flow-induced vasodilatation in soleus muscle arterioles of young and old SED rats. B, inhibition of NADPH oxidase had no effect on flow-induced vasodilatation in soleus muscle arterioles of young and old ET rats. *P < 0.05 vs. control response.
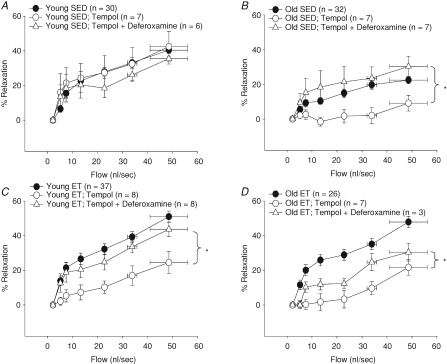
Role of OH− in flow-induced vasodilatation
SOD has the potential to generate OH− in the presence of iron, and OH− may cause vasoconstriction (Bulvik et al. 2008). The addition of deferoxamine, an iron chelator, to Tempol-treated arterioles had no effect in young SED rats (Fig. 7A); however, iron chelation restored the Tempol-induced loss of flow-induced vasodilatation in arterioles from old SED (Fig. 7B) suggesting that OH− generation in Tempol-treated arterioles elicits vasoconstriction. Deferoxamine added to Tempol-treated arterioles improved the Tempol-induced loss of flow-induced vasodilatation observed in young and old ET rats (Fig. 7C and D), confirming that O2− production increases with exercise training and may contribute to increased OH−-induced vasoconstriction in the presence of exogenous SOD and sufficient free iron.
Figure 7. Effects of Tempol and deferoxamine on flow-induced vasodilatation in soleus muscle arterioles of young and old, SED and ET rats.
A, neither Tempol or deferoxamine, an iron chelator, reduced flow-induced vasodilatation in young SED. B, Tempol reduced and Tempol + deferoxamine improved flow-induced vasodilatation in old SED. C, Tempol reduced and Tempol + deferoxamine restored flow-induced vasodilatation in young ET. D, Tempol reduced flow-induced vasodilatation; however, Tempol + deferoxamine only partially restored flow-induced vasodilatation in the old ET. *P < 0.05 vs. control response.
Discussion
Several important new findings emerge from this study. First, the balance between NO and O2− signalling is altered by age and exercise training in skeletal muscle arterioles. Second, BH4 content is reduced with age and restored with exercise training in skeletal muscle arterioles from old rats, whereas neither ageing nor training altered arteriolar arginine levels. The age-related decline in arteriolar BH4 content is concomitant to a decrease in NO signalling and an increase in ROS signalling. Further, the exercise-induced restoration of BH4 content coincides with an increased NO signalling in skeletal muscle arterioles of old ET rats. Third, under conditions of limited BH4, such as occurs with old age, NO-mediated signalling declines and eNOS-generated ROS (O2−) increases in skeletal muscle arterioles. And finally, exercise training increases both NO- and ROS-mediated signalling in skeletal muscle arterioles of young rats, but a balance in these signalling systems is maintained, resulting in robust flow-induced vasodilatation.
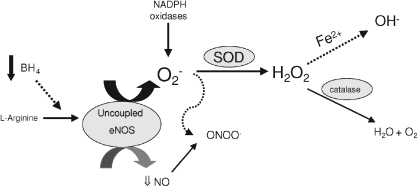
Ageing is associated with declines in flow-induced NO-dependent vasodilatation in conduit and resistance arterioles of humans and rats (Gerhard et al. 1996; DeSouza et al. 2000; Muller-Delp et al. 2002; Woodman et al. 2002). Limited NO bioavailability may be mediated by several possible mechanisms, including reduced eNOS protein abundance or activity, limited availability of substrate (l-arginine) or co-factor (BH4), or increased degradation of NO by ROS. We recently reported that a deficit in availability of BH4 is associated with an age-dependent decline of endothelium-dependent vasodilatation in skeletal muscle arterioles, and that supplementation with the BH4 precursor sepiapterin partially restores flow-induced, endothelium-dependent vasodilatation in skeletal muscle arterioles from old rats (Delp et al. 2008). Eskurza et al. (2005) reported that an acute bolus of BH4 restored flow-mediated dilatation in older sedentary humans. These findings support the hypothesis that the old age-associated decrements in BH4 contribute to reduced NO bioavailability (Fig. 8). When BH4 is limited, eNOS has been shown to become uncoupled, producing both O2− and NO (Bevers et al. 2006; Crabtree et al. 2009); thus, the current study was designed to test the hypothesis that eNOS uncoupling increases with age in skeletal muscle arterioles. To our knowledge, this is the first study to demonstrate that an old age-related reduction of BH4 (Fig. 2A) is accompanied by impaired flow-induced NO signalling (Fig. 3) and increased O2− signalling in skeletal muscle arterioles (Fig. 8). Although DHE can be oxidized by various ROS including NO, our fluorescence data also suggest that the age-associated decrement in BH4 availability contributes to uncoupling of eNOS and an increase in eNOS-derived O2− generaton in response to flow stimulation. Further study is required to determine whether augmentation of endothelial BH4 can reverse eNOS uncoupling and restore NO signalling in arterioles from old rats.
Figure 8. The proposed effects of ageing on eNOS uncoupling.
BH4 content is reduced in soleus muscle arterioles of aged rats. With limited availability of BH4, eNOS becomes uncoupled and leads to generation of O2− rather than NO. Peroxynitrite (ONOO−), a product of NO and O2− generated through eNOS uncoupling, NADPH oxidases and other sources, could further reduce BH4 availability. Vascular SOD scavenges O2−, which can produce the vasodilator H2O2. In the presence of catalytic transition metals (i.e. Fe2+), H2O2 can produce the potent vasoconstrictor OH−. H2O2 is converted to H2O and O2 in the presence of catalase.
Increases in O2− generation, observed with age, scavenge NO and may alter endothelial function (Donato et al. 2007). In soleus muscle feed arteries, SOD-1 protein content, but not mRNA expression, is reduced in aged rats (Woodman et al. 2002); however, SOD-1 activity appears to increase in the aorta of aged rats (Demaree et al. 1999). Additionally, catalase activity declines in aorta from aged rats (Demaree et al. 1999); however, catalase protein content does not change with age in human endothelial cells (Donato et al. 2007). Exercise training potentially improves NO bioavailability by enhanced regulation of O2− and ROS by inducing antioxidant defence systems and reducing O2− generating systems. Rush et al. (2000) demonstrated in aortic endothelial cells (AEC) from young pigs that exercise training increased SOD-1 protein abundance and activity but had no effect on catalase content. In contrast, exercise training decreased the NADPH pro-oxidant subunit, p67phox protein. Graham & Rush (2004) further demonstrated that the cytosolic subunit of NADPH oxidase, gp91phox, is reduced with training in aorta homogenates from young rats. Based on these observations, it appears that exercise training improves the management of superoxide (and subsequent ROS) generation by enhancing antioxidant mechanisms and reducing pro-oxidant pathways. However, a paradox emerges as O2− generation and oxidant stress increases with exercise training (Davies et al. 1982; Alessio, 1993; Alessio et al. 2000). Our data suggest that exercise training-induced enhancement of flow-induced vasodilatation occurs because both NO- and ROS-mediated signalling increase in a balanced fashion.
Even though high levels of ROS may potentiate the pathology of cardiovascular diseases (Harrison, 1997), it is becoming increasingly clear that ROS can serve as signalling molecules, which mediate normal cellular functions (Droge, 2002). H2O2 generated by the dismutation of O2− is emerging as an important vasodilator and signalling molecule in the vasculature. Endothelium-derived H2O2 contributes to flow-induced vasodilatation in isolated coronary arterioles (Koller & Bagi, 2004). Marvar et al. (2007) demonstrated that H2O2 produced by contracting skeletal muscle elicits a vasodilatory response on nearby skeletal muscle arterioles. Furthermore, exogenous H2O2 is vasodilatory in a variety of vascular beds including pulmonary (Burke & Wolin, 1987), skeletal muscle (Cseko et al. 2004), cerebral (Wei & Kontos, 1990) and mesenteric beds (Matoba & Shimokawa, 2003). H2O2 also indirectly alters vascular reactivity by activating eNOS via phosphoinositide 3-kinase (PI3K) (Thomas et al. 2002). Our data indicate that H2O2 is an important mediator of flow-induced vasodilatation of skeletal muscle arterioles from young SED rats. In addition, our data suggest that flow-induced H2O2 signalling declines with age, but is restored by exercise training.
SOD is an important anti-oxidant enzyme that reduces oxidant stress by dismutating O2− into H2O2; however, in the presence of catalytic transition metals, SOD can rapidly form OH− (Pattwell et al. 2004). H2O2 generates OH− through metal-catalysed reactions, such as the Fenton reaction as follows: H2O2+ Fe2+→ Fe3++•OH + OH−. The formation of OH− is further promoted by the presence of O2−, which reacts with Fe3+ to produce Fe2+ through the Haber–Weiss reaction (Halliwell, 1989; Powers & Jackson, 2008). The net effect of SOD is the dismutation of O2− to produce either the vasodilatory H2O2 or in the presence of Fe2+, OH−, which is a potent vasoconstrictor (Fig. 8). Iron chelators, such as deferoxamine, inhibit the generation of OH− by preventing iron ions from catalysing redox reactions, thereby improving vasodilatation in coronary arterioles (Nitenberg et al. 1998; Thengchaisri et al. 2006). Iron accumulation increases with age in skeletal muscle, liver and cardiac muscle, and is associated with increased oxidative stress and diminished functional capacity (Bulvik et al. 2008; Xu et al. 2008). Old age-associated increases in iron accompanied by diminished catalase activity (Demaree et al. 1999) could produce excess OH− contributing to decreased flow-induced vasodilatation in skeletal muscle arterioles of old rats (Fig. 2B). Exercise training increases iron in skeletal muscle of rats (Navas & Cordova, 2000) and catalase activity in the vasculature remains unchanged with exercise training (Rush et al. 2000). Our data indicate that exercise training increases flow-induced O2− generation in arterioles from young and old rats. Therefore, it seems plausible that this increase in flow-induced O2− generation could also result in elevated OH− production in arterioles treated with an exogenous SOD mimetic. In addition, our data indicate that iron chelation by deferoxamine improves flow-induced vasodilatation in skeletal muscle arterioles treated with the SOD mimetic Tempol, suggesting that inhibition of OH− production reverses the Tempol-induced reduction of flow-induced vasodilatation.
In conclusion, the results of the present study confirm previous findings that ageing impairs endothelium-dependent, NO-mediated vasodilatation and BH4 levels in rat skeletal muscle arterioles (Muller-Delp et al. 2002; Delp et al. 2008). The present findings further demonstrate that limited availability of BH4, as observed in old SED rats, may contribute to eNOS uncoupling, and a subsequent decrease in NO signalling and an increase in eNOS-derived O2− generation as illustrated in Fig. 8. Exercise training restores BH4 levels and improves flow-stimulated NO availability in arterioles from aged rats. Furthermore, exercise training increases both NO and ROS-mediated signalling in skeletal muscle arterioles, suggesting that exercise training-induced enhancement of flow-induced vasodilatation in skeletal muscle arterioles involves a balance between NO and O2−-derived ROS. Our results suggest that the beneficial effects of exercise training in the skeletal muscle resistance vasculature involve much more than improvement of NO bioavailability.
Acknowledgments
This work was supported by NIH ROI HL077224 and AHA-TX 0755024Y.
Glossary
Abbreviations
- DHE
dihydroethidium
- eNOS
endothelial nitric oxide synthase
- ET
exercise trained
- NO
nitric oxide
- ROS
reactive oxygen species
- SED
sedentary
- SOD
superoxide dismutase
Author contributions
A.L.S. contributed to experimental design, analysis and interpretation, and writing of this article. M.D.D. contributed to experimental design, data interpretation, and critical revision of this article. R.R. contributed to data collection, data analysis and interpretation, and critical revision of this article. G.W. contributed to experimental design, data analysis and interpretation, and critical revision of this article. J.M.D. contributed to conception and design of experiments, data interpretation, and writing of this article.
References
- Alessio HM. Exercise-induced oxidative stress. Med Sci Sports Exerc. 1993;25:218–224. [PubMed] [Google Scholar]
- Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med Sci Sports Exerc. 2000;32:1576–1581. doi: 10.1097/00005768-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Toth E, Koller A, Kaley G. Microvascular dysfunction after transient high glucose is caused by superoxide-dependent reduction in the bioavailability of NO and BH4. Am J Physiol Heart Circ Physiol. 2004;287:H626–633. doi: 10.1152/ajpheart.00074.2004. [DOI] [PubMed] [Google Scholar]
- Bevers LM, Braam B, Post JA, van Zonneveld AJ, Rabelink TJ, Koomans HA, Verhaar MC, Joles JA. Tetrahydrobiopterin, but not L-arginine, decreases NO synthase uncoupling in cells expressing high levels of endothelial NO synthase. Hypertension. 2006;47:87–94. doi: 10.1161/01.HYP.0000196735.85398.0e. [DOI] [PubMed] [Google Scholar]
- Bulvik B, Grinberg L, Eliashar R, Berenshtein E, Chevion MM. Iron, ferritin and proteins of the methionine-centered redox cycle in young and old rat hearts. Mech Ageing Dev. 2008;130:139–144. doi: 10.1016/j.mad.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol Heart Circ Physiol. 1987;252:H721–732. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol Heart Circ Physiol. 2007;293:H2085–2092. doi: 10.1152/ajpheart.00968.2006. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Katusic ZS. Tetrahydrobiopterin and dysfunction of endothelial nitric oxide synthase in coronary arteries. Circulation. 1995;91:139–144. doi: 10.1161/01.cir.91.1.139. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Luscher TF. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res. 1999;43:274–278. doi: 10.1016/s0008-6363(99)00134-0. [DOI] [PubMed] [Google Scholar]
- Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, Channon KM, Alp NJ. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: Insights from cells with TET-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- Cseko C, Bagi Z, Koller A. Biphasic effect of hydrogen peroxide on skeletal muscle arteriolar tone via activation of endothelial and smooth muscle signaling pathways. J Appl Physiol. 2004;97:1130–1137. doi: 10.1152/japplphysiol.00106.2004. [DOI] [PubMed] [Google Scholar]
- Darley-Usmar V, Wiseman H, Halliwell B. Nitric oxide and oxygen radicals: a question of balance. FEBS Lett. 1995;369:131–135. doi: 10.1016/0014-5793(95)00764-z. [DOI] [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- Davis MJ. Determination of volumetric flow in capillary tubes using an optical Doppler velocimeter. Microvasc Res. 1987;34:223–230. doi: 10.1016/0026-2862(87)90055-0. [DOI] [PubMed] [Google Scholar]
- Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol. 2008;586:1161–1168. doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Demaree SR, Lawler JM, Linehan J, Delp MD. Ageing alters aortic antioxidant enzyme activities in Fischer-344 rats. Acta Physiol Scand. 1999;166:203–208. doi: 10.1046/j.1365-201x.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Emsley AM, Jeremy JY, Gomes GN, Angelini GD, Plane F. Investigation of the inhibitory effects of homocysteine and copper on nitric oxide-mediated relaxation of rat isolated aorta. Br J Pharmacol. 1999;126:1034–1040. doi: 10.1038/sj.bjp.0702374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol. 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- Graham DA, Rush JW. Exercise training improves aortic endothelium-dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. J Appl Physiol. 2004;96:2088–2096. doi: 10.1152/japplphysiol.01252.2003. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989;7:645–651. doi: 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- Harrison DG. Endothelial function and oxidant stress. Clin Cardiol. 1997;20(Suppl):II-11–17. [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Koller A, Bagi Z. Nitric oxide and H2O2 contribute to reactive dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol. 2004;287:H2461–2467. doi: 10.1152/ajpheart.00295.2004. [DOI] [PubMed] [Google Scholar]
- Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol. 1990;259:H1063–1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular system. In: Masoro EJ, editor. Handbook of Physiology, section 11, Aging. New York: Oxford University Press; 1995. pp. 413–474. [Google Scholar]
- Maier W, Cosentino F, Lutolf RB, Fleisch M, Seiler C, Hess OM, Meier B, Luscher TF. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol. 2000;35:173–178. doi: 10.1097/00005344-200002000-00001. [DOI] [PubMed] [Google Scholar]
- Marvar PJ, Hammer LW, Boegehold MA. Hydrogen peroxide-dependent arteriolar dilation in contracting muscle of rats fed normal and high salt diets. Microcirculation. 2007;14:779–791. doi: 10.1080/10739680701444057. [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Pharmacol Sci. 2003;92:1–6. doi: 10.1254/jphs.92.1. [DOI] [PubMed] [Google Scholar]
- Meininger CJ, Marinos RS, Hatakeyama K, Martinez-Zaguilan R, Rojas JD, Kelly KA, Wu G. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem J. 2000;349:353–356. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. Regulation of arginine availability and its impact on NO synthesis. Nitric Oxide: Biology and Pathobiology. 2000:187–197. [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Navas FJ, Cordova A. Iron distribution in different tissues in rats following exercise. Biol Trace Elem Res. 2000;73:259–268. doi: 10.1385/BTER:73:3:259. [DOI] [PubMed] [Google Scholar]
- Nitenberg A, Paycha F, Ledoux S, Sachs R, Attali JR, Valensi P. Coronary artery responses to physiological stimuli are improved by deferoxamine but not by L-arginine in non-insulin-dependent diabetic patients with angiographically normal coronary arteries and no other risk factors. Circulation. 1998;97:736–743. doi: 10.1161/01.cir.97.8.736. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Spina RJ, Martin WH, 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med. 2004;37:1064–1072. doi: 10.1016/j.freeradbiomed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol. 2000;279:H2068–2076. doi: 10.1152/ajpheart.2000.279.5.H2068. [DOI] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere P. Citrate synthase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J Clin Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Swei A, Zweifach BW, Schmid-Schonbein GW. In vivo evidence for microvascular oxidative stress in spontaneously hypertensive rats. Hydroethidine microfluorography. Hypertension. 1995;25:1083–1089. doi: 10.1161/01.hyp.25.5.1083. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, Kuo L. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol. 2006;26:2035–2042. doi: 10.1161/01.ATV.0000233334.24805.62. [DOI] [PubMed] [Google Scholar]
- Thomas SR, Chen K, Keaney JF., Jr Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem. 2002;277:6017–6024. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- Tiefenbacher CP. Tetrahydrobiopterin: a critical cofactor for eNOS and a strategy in the treatment of endothelial dysfunction? Am J Physiol Heart Circ Physiol. 2001;280:H2484–2488. doi: 10.1152/ajpheart.2001.280.6.H2484. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei EP, Kontos HA. H2O2 and endothelium-dependent cerebral arteriolar dilation. Implications for the identity of endothelium-derived relaxing factor generated by acetylcholine. Hypertension. 1990;16:162–169. doi: 10.1161/01.hyp.16.2.162. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2002;93:1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- Wu G, Meininger CJ. Impaired arginine metabolism and NO synthesis in coronary endothelial cells of the spontaneously diabetic BB rat. Am J Physiol Heart Circ Physiol. 1995;269:H1312–1318. doi: 10.1152/ajpheart.1995.269.4.H1312. [DOI] [PubMed] [Google Scholar]
- Xu J, Knutson MD, Carter CS, Leeuwenburgh C. Iron accumulation with age, oxidative stress and functional decline. PLoS ONE. 2008;3:e2865. doi: 10.1371/journal.pone.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res. 2007;44:382–390. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]