Abstract
The intracellular signalling kinases Akt/protein kinase B (Akt), protein kinase A (PKA) and adenosine monophosphate-activated protein kinase (AMPK) are phosphorylated in response to increased mechanical force or perfusion rate in cultured endothelial cells or isolated blood vessels. All three kinases phosphorylate endothelial nitric oxide synthase (eNOS) on serine (S) 1177, while Akt and PKA additionally phosphorylate eNOS on S617 and S635 respectively. Although these kinases might contribute to subsequent activation of eNOS during dynamic exercise, the specific mediators of exercise-induced eNOS phosphorylation and activation in vivo are unknown. We determined the impact of 50 min of treadmill running on the phosphorylation of Akt, AMPK, cyclic adenosine monophosphate response element binding protein (CREB – a target of PKA) and eNOS (S 1177, 635 and 617 and threonine (T) 495) in the presence or absence of pharmacological inhibition of PI3 kinase (PI3K) and Akt signalling using wortmannin. Compared to arteries from sedentary mice, eNOS enzyme activity was greater in vessels from treadmill-running animals and was associated with increased phosphorylation of Akt (S473), CREB (S133), AMPK (T172), and eNOS at S1177 and S617 but not at S635 or T495. These data suggest that Akt signalling is a major mediator of eNOS activation. To confirm this, treadmill-running was performed in the presence of vehicle (DMSO) or PI3K inhibition. Compared to results from sedentary mice, vascular Akt phosphorylation and eNOS phosphorylation at S617 during treadmill-running were prevented by wortmannin but not vehicle treatment, whereas exercise-related increases in AMPK and CREB phosphorylation were similar between groups. Arterial eNOS phosphorylation at S1177 increased during exercise after wortmannin treatment relative to values obtained from sedentary animals, but the elevation was blunted by ∼50% compared to results from vehicle-treated mice. These findings indicate that Akt and AMPK contribute importantly to vascular eNOS S1177 phosphorylation during treadmill-running, and that AMPK is sufficient to activate p-eNOS S1177 in the presence of PI3K inhibition.
Frictional forces along the surface of the endothelium exerted by flowing blood are potent stimuli for endothelial nitric oxide (NO) production (Jo et al. 1997). These forces are proportional to the product of blood viscosity and blood velocity at the endothelial wall and collectively have been termed ‘endothelial shear stress’. Endothelial surfaces possess mechanoreceptors that detect shear stress and data concerning the pathways responsible for phosphorylation and activation of endothelial NO synthase (eNOS) are emerging (Davies, 1995; Boo & Jo, 2003; Sessa, 2004) (see Fig. 1). Studies using cultured endothelial cells and isolated blood vessels indicate that mechanical forces and increased perfusion rate, respectively, phosphorylate Akt/protein kinase B (Akt) (Dimmeler et al. 1999; Fulton et al. 1999), protein kinase A (PKA) (Boo et al. 2002, 2003; Shaul, 2002; Boo & Jo, 2003; Fleming & Busse, 2003; Sessa, 2004; Boo, 2006) and/or adenosine monophosphate-activated protein kinase (AMPK) (Fleming et al. 2005; Zhang et al. 2006) leading to eNOS phosphorylation at serine (S) 1177 (Akt, PKA and AMPK), S635 (PKA) and S617 (Akt). Furthermore, data from cultured aortic endothelial cells and human umbilical vein endothelial cells (HUVECs) indicate that while phosphorylated extracellular signal-regulated kinases (ERK) 1 and 2 (ERK 1/2) increase within approximately 10 min of exposure to various profiles of fluid flow/shear stress to phosphorylate eNOS at threonine (T) 495, values return to baseline within 30–60 min (Jo et al. 1997; Bao et al. 2001; Chien, 2007).
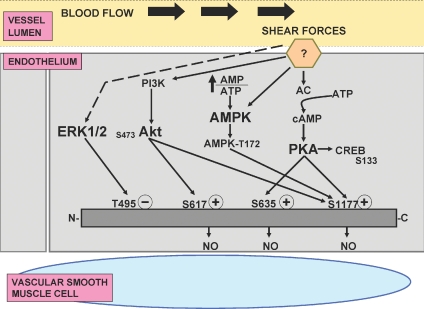
Figure 1.
Proposed regulation of endothelial nitric oxide synthase (eNOS) phosphorylation by Akt, AMPK, PKA and ERK 1/2 after stimulation of unidentified shear force receptor(s) by exercise-induced increases in blood flow Abbreviations: (?), unidentified shear force receptor; PI3K, phosphatidyl-inositol-3-kinase; Akt, Akt/protein kinase B; AC, adenylate cyclase; cAMP, cyclic adenosine monophosphate; ATP, adenosine triphosphate; PKA, protein kinase A; AMP, adenosine monophosphate; AMPK, adenosine monophosphate activated protein kinase; CREB, cyclic AMP response element binding protein; ERK, extracellular signal regulated kinases 1 and 2; NO, nitric oxide; (+), positive regulatory phosphorylation sites, e.g. serine (S) 617, S635, S1177; (–), negative regulatory phosphorylation site, e.g. threonine (T) 495. Continuous lines/arrows indicate increased activation; dotted line/arrow indicates activation that is dependent upon duration of stimulation. See text for full explanation.
To our knowledge it is unknown if the changes in Akt, PKA, AMPK, and/or ERK 1/2 that occur in response to increases in shear stress in cultured endothelial cells and isolated vessels also occur in arteries from animals exposed to a physiological stressor such as dynamic exercise, which produces hyperaemia in metabolically active tissues (Maxwell et al. 1998; Symons et al. 1999, 2003; Musch et al. 2001). Based on results from cultured cell systems and isolated perfused vessels, we hypothesized that 50 min of treadmill-running would evoke sufficient hyperaemia to stimulate phosphorylation of arterial Akt, AMPK and cyclic AMP response element binding protein (CREB; a downstream target of PKA) (Boo et al. 2002; Min et al. 2004; Boo, 2006). Because ERK 1/2 phosphorylation is a transient response to increased shear stress in vitro (Chien, 2007), we also hypothesized that arterial p-ERK 1/2 would not be altered in vessels exposed to 50 min of treadmill-running. Consistent with our hypothesis, p-eNOS S1177, p-Akt, p-CREB, and p-AMPK, but not p-ERK 1/2, were elevated in arteries from animals that completed 50 min of treadmill-running vs. sedentary controls. To elucidate which signalling kinase was primarily responsible for increasing p-eNOS S1177, we examined p-eNOS sites phosphorylated by Akt, AMPK and PKA (i.e. S1177) together with targets specific for Akt (S617) and PKA (S635). A robust increase in p-eNOS S617 but not p-eNOS S635 was observed in arteries from treadmill-running vs. sedentary mice, suggesting that eNOS might not be a substrate of PKA in vascular tissue during dynamic exercise. To determine if Akt phosphorylation is required for increases in p-eNOS S1177 that we observed, mice completed treadmill-running in the absence or presence of phosphoinositol 3-kinase (PI3K) inhibition using wortmannin. While wortmannin prevented increases in vascular p-Akt from occurring in treadmill-running mice, p-CREB and p-AMPK were not affected. As anticipated, vascular p-eNOS S617 did not increase during treadmill-running in the presence of PI3K inhibition, and although p-eNOS S1177 was increased, it was blunted by ∼50% relative to untreated animals. Collectively, these data indicate that Akt and AMPK contribute importantly to vascular p-eNOS S1177 during treadmill-running, and that AMPK activation is sufficient to phosphorylate eNOS on S1177 in the presence of PI3K inhibition.
Methods
Animals and housing
All protocols were approved by the Animal Use and Care Committee at the University of Utah. The University of Utah fully complies with the Association for Assessment and Accreditation of Laboratory Animal Care International. All experiments comply with policies and regulations as stated by Drummond (2009). Male C57BL/6J mice (10–15 weeks old, n= 104, Jackson Laboratories, Bar Harbor, ME, USA) were housed under controlled temperature (23°C) and light conditions (12: 12 h light–dark cycle) and were provided food and water ad libitum.
Treadmill-running
Mice were familiarized with 10 min of treadmill-running at 10–15 m·min−1 and 5% grade for two consecutive days. Following a 4 h fast on Day 3, mice completed an acute exercise protocol (Ex, n= 18) or remained in cages located in the same room as the treadmill (sedentary, SED; n= 18). Ex consisted of running at a 5% grade for each of 5 min at 10 m·min−1, 10 min at 15 m·min−1, 10 min at 18 m·min−1, and 25 min at 20 m·min−1. The total duration of treadmill-running (i.e. 50 min) was based on evidence that a time-dependent effect of shear stress-mediated Akt and eNOS phosphorylation exists in cultured endothelial cells (Boo et al. 2001). In this regard, the maximal effect of shear was produced between 30 and 60 min. The intensity of exercise was based on reports that 20 m·min−1 at 5% grade: (1) requires ∼80% of maximal oxygen consumption in mice (Billat et al. 2005; Schefer & Talan, 1996); and (2) produces significant increases in hindlimb blood flow (Maxwell et al. 1998). Furthermore, in preliminary experiments we observed that p-eNOS to total eNOS increased by > 2.5-fold in aortae from mice that completed 50 min of treadmill-running at 18–22 m·min−1 and 5% grade (n= 2) vs. those that did not exercise (n= 2; data not shown).
Tissue collection and sample preparation
Immediately upon completion of each respective protocol, mice were anaesthetized deeply with 3–5% isoflurane, the chest was opened, and the heart was excised. Next, the entire aorta and both iliac and femoral arteries were dissected free while immersed in ice-cold dissection buffer containing Tris-HCl (50 mm), EDTA (0.10 mm), EGTA (0.10 mm), β-mercaptoethanol (0.1%), phenylmethylsulfonyl fluoride (100 mm), leupeptin (1 mm), pepstatin A (1 mm), and calyculin (1 mm). Iliac and femoral arteries were combined from each mouse into one sample to increase the protein yield and will be referred to as iliac/femoral arteries. Vascular tissue then was transferred immediately to tubes containing 200 μl (aorta) or 150 μl (iliac/femoral arteries) ice-cold homogenization buffer (0.05 m Hepes, 0.01 m sodium pyrophosphate, 0.01 m sodium fluoride, 0.002 m EDTA, 0.002 m sodium orthovanodate, 1% Triton X-100, 10% glycerol). Sigma protease inhibitor cocktail (1: 200; Sigma, St Louis, MO, USA) was added to the homogenization buffer prior to use. Tissue then was sonicated on ice and the resulting homogenate was centrifuged for 15 min at 38 000 g at 4°C. Aliquots of supernatant then were divided and stored at −80°C for later immunoblotting and protein analyses.
Immunoblotting
Protein concentrations were determined using a Thermo-max Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) using bovine serum albumin as a standard. Equal amounts of protein were suspended in a loading buffer (60 mm Tris-HCl, 25% glycerol, 2% SDS, 14.4 mm 2-mercaptoethanol and 0.1% bromophenol blue, or 2% SDS, 6.0 m urea, 62.5 mm Tris-HCl, 160 mm DTT and 0.001% bromophenol blue), incubated for 5 min at 95–100°C, and then resolved in SDS-PAGE. Resolved proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore Immobilon-P Transfer membrane; Millipore Corp., Billerica, MA, USA) at 4°C. Following transfer, the membranes were blocked with 5% non-fat dry milk in phosphate-buffered saline with 1% Tween-20 (PBST) for 30 min at room temperature. Blocked membranes then were probed with the appropriate primary antibody for phosphorylated proteins overnight at 4°C. Next, membranes were washed with PBST (4× 10 min), and incubated with the appropriate secondary antibody conjugated to horseradish peroxidase-conjugated (anti-rabbit IgG (GE Healthcare, Piscataway, NJ, USA); anti-mouse IgG (GE Healthcare Inc, Lake Placid, NY, USA)) for 1 h at room temperature. Antibody binding was visualized using the ECL Plus chemiluminescence kit (GE Healthcare) or the Pierce detection kit (West Femto; Pierce/Thermo Fisher Scientific, Rockford, IL, USA). Membranes were stripped with ReBlotPlus mild solution (1×; Chemicon International, Temecula, CA, USA) and reprobed with the appropriate primary antibody to quantify total protein content. Dilution conditions were: 1: 500, 2% milk/PBST, 8% gel (p-eNOS S1177; Cell Signaling Technology Inc., Danvers, MA, USA); 1: 2500, 2% BSA/TBST, 8% gel (p-eNOS S617; Upstate); 1: 1000, 1% BSA/TBST, 8% gel (p-eNOS T495; Cell Signaling); 1: 1000, 1% BSA/TBST, 8% gel (p-eNOS S635; Cell Signaling); 1: 500, 2% milk/PBST, 8% gel (total eNOS; BD Biosciences, San Jose, CA, USA); 1: 1,000, 5% milk/PBST, 8 or 10% gel (p-Akt S473, Cell Signaling); 1: 1,000, 5% milk/PBST, 8 or 10% gel (total Akt; Cell Signaling); 1: 1,000, 5% milk/PBST, 10% gel (p-AMPKα Thr 172; Cell Signaling); 1: 1,000, 5% milk/PBST, 10% gel (total AMPKα; Cell Signaling); 1: 500, 2% milk/PBST, 10% gel (p-CREB S133; Cell Signaling); 1: 500, 2% milk/PBST, 10% gel (total CREB S133; Cell Signaling); 1:1000, 5% milk/PBST, 10% gel (p-ERK 1/2; Cell Signaling); 1: 1000, 5% milk/PBST, 10% gel (total ERK 1/2, Santa Cruz Biotechnology, Santa Cruz, CA, USA) (Symons et al. 2009).
eNOS enzyme activity
Additional mice that were housed and familiarized as described were used to confirm that our treadmill-running protocol increases vascular eNOS enzyme activity in mice (Fukai et al. 2000). The conversion of [3H]arginine to [3H]citrulline (Bredt & Snyder, 1990) in Sed (n= 4) and Ex (n= 4) was determined in aorta, iliac and femoral arteries combined by following procedures provided by the manufacturer (Calbiochem, San Diego, CA, USA). Results are expressed as eNOS enzyme activity per microgram protein.
Treadmill-running + PI3K inhibition
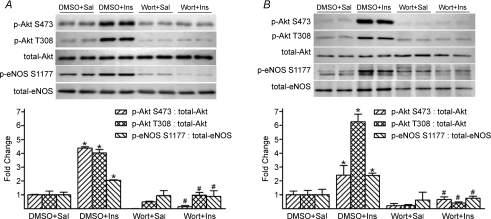
To verify the onset and duration of vascular PI3K inhibition, 1.4 mg wortmannin per kg body wt was dissolved in 0.7% DMSO and administered i.p. (total volume = 10 μl (g body wt)−1) to 24 mice (Kang et al. 2004). Next, 3.8 mU insulin (g body wt)−1 (n= 4) or vehicle (saline, n= 4) was injected into the caudal vein of conscious mice after 10 min, 60 min or 240 min. Five minutes later, animals were anaesthetized, and the entire aorta, iliac, and femoral arteries were excised and prepared for immunoblotting analyses of p-Akt S473, p-Akt T308 and p-eNOS S1177. Identical procedures were performed in 24 additional mice 10 min, 60 min, or 240 min after i.p. administration of the vehicle for wortmannin, i.e. 0.7% DMSO. Results indicated that insulin-stimulated p-Akt S473, p-Akt T308, and p-eNOS S1177 were abolished after 10 and 60 min in arteries from wortmannin vs. DMSO-treated mice, but were partial after 240 min. Based on these results, the 50 min treadmill-running protocol commenced 10 min after i.p. administration of DMSO (n= 4) or wortmannin (n= 4).
Statistical analyses
Intensity (area times density) of the immunoreactive bands was measured using the Scion Image 1.62c program (Frederick, MD, USA). For each protein, activation was quantified by comparing the ratio of phosphorylated (p) protein intensity divided by total protein intensity between Sed and Ex mice. To determine the fold increase in phosphorylation in Ex vs. Sed animals, the following approach was used, as exemplified by Akt. The densitometric ratios for phosphorylated-Akt (p-Akt) to total Akt (Akt) were determined for each Sed animal, and group mean averages were calculated and normalized to 1 by dividing each ratio by the group mean value. Next, the ratio of p-Akt to total Akt was determined for each Ex animal and divided by the group mean p-Akt to Akt ratio in Sed mice, and expressed as fold-change relative to Sed. Mean immunoblotting data and eNOS activity results were compared between Sed and Ex using Student's t-test for unpaired data (SPSS, version 12.0, Chicago, IL, USA, 2003). Statistical significance was accepted when P < 0.05. All values are presented as the mean ±s.e.m.
Results
The group mean treadmill time was 45.8 ± 1.7 min because three Ex mice had difficulty completing the entire 50 min protocol. Given that shear stress-mediated Akt and eNOS phosphorylation are maximal between 30 and 60 min in cultured endothelial cells (Boo et al. 2001), and that results from these three mice were similar to those from animals that completed the entire protocol, all mice were included in the data set.
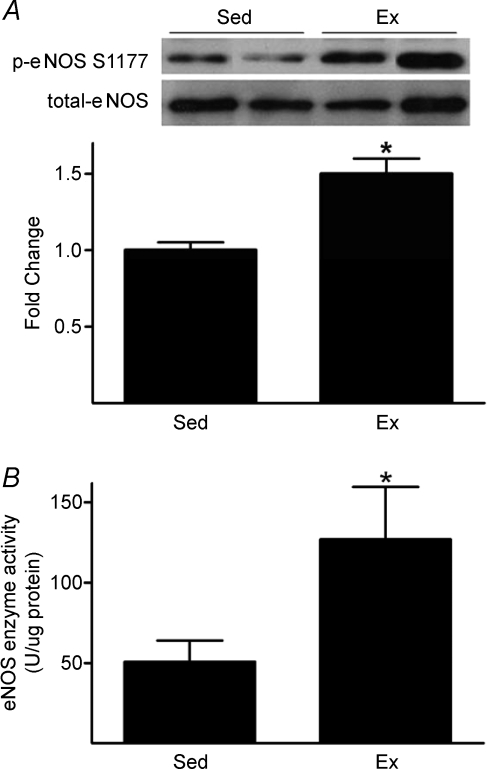
p-eNOS S1177 to total eNOS increased 1.5-fold in iliac/femoral arteries from Ex compared to Sed mice, while no differences existed between groups concerning total eNOS (Fig. 2A). eNOS enzyme activity per μg protein increased more than 2.2-fold in aorta/iliac/femoral artery homogenates from Ex vs. Sed mice (Fig. 2B).
Figure 2.
Treadmill-running increases arterial p-eNOS S1177 (A) and eNOS enzyme activity (B) A, phosphorylated (p)-eNOS S1177 to total eNOS protein content is greater (*P < 0.05) in vessels from mice that completed ∼50 min of treadmill-running (Ex) vs. those exposed to treadmill noise/vibration for 50 min (Sed). Representative immunoblots from 2 mice per group (top) and mean data ± standard error of the mean from 18 femoral/iliac arteries per group (bottom) are shown. Values from Ex mice are expressed as fold change differences from Sed. B, eNOS enzyme activity is greater (*P < 0.05) in aorta/iliac/femoral artery homogenates from Ex vs. Sed mice (n= 4 mice per group).
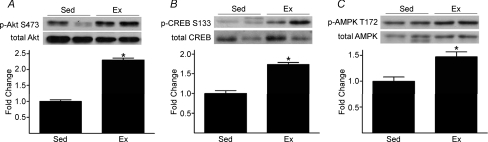
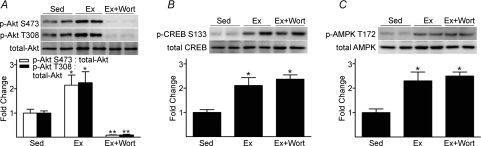
p-Akt S473 to total Akt increased 2.3-fold, p-CREB S133 to total CREB increased 1.7-fold, and p-AMPK T172 to total AMPK increased 1.5-fold, in iliac/femoral arteries from Ex vs. Sed mice (Fig. 3A, B and C). Arterial p-ERK 1/2 to total ERK (arbitrary units) was not different between Ex (0.48 ± 0.13) and Sed (0.76 ± 0.18) mice. This pattern of results was generally similar for aorta (data not shown).
Figure 3.
Treadmill-running increases arterial p-Akt S473 (A), p-CREB S133 (B), and p-AMPK T172 (C) to total protein (P < 0.05) in vessels from Ex vs. Sed mice See Fig. 2 legend for details.
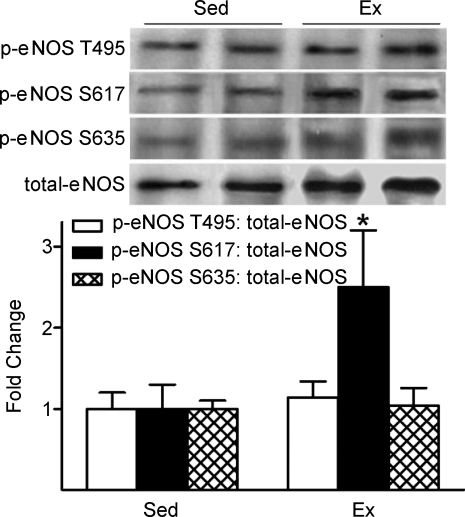
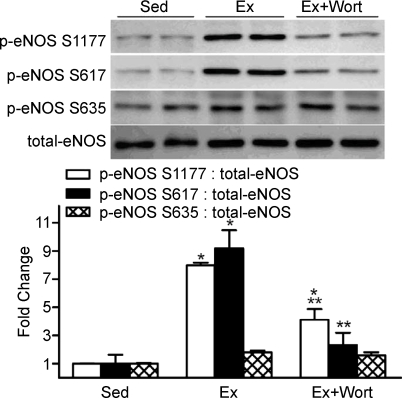
Additional eNOS phosphorylation sites that are specifically activated by ERK, PKA and Akt were also evaluated. While p-eNOS S617 was elevated 2.5-fold in vessels from Ex vs. Sed mice, p-eNOS S635 and p-eNOS T495 were similar between groups (Fig. 4). These data suggest that in our experimental setting, eNOS might be preferentially phosphorylated by Akt but not by PKA or ERK-mediated signalling.
Figure 4.
The influence of treadmill-running on arterial p-eNOS at T495, S617, and S635 p-eNOS S617 increased in vessels from Ex vs. Sed mice, but p-eNOS T495 and p-eNOS S635 were similar between groups. *P < 0.05 vs. Sed.
To more thoroughly evaluate the contribution from Akt to p-eNOS S1177 during dynamic exercise, mice performed treadmill-running in the absence and presence of PI3K inhibition. We initially had to demonstrate that inhibition of this kinase existed throughout the entire exercise protocol. Compared to saline (vehicle) stimulation, insulin-evoked robust increases in p-Akt S473, p-Akt T308 and p-eNOS S1177 10, 60 and 240 min after i.p. DMSO, the vehicle for wortmannin (Fig. 5A and B). While insulin-stimulated p-Akt and p-eNOS were abolished 10 and 60 min after i.p. wortmannin, complete blockade was not evident after 240 min (data not shown). Therefore, the 50 min treadmill-running protocol was initiated 10 min after i.p. administration of DMSO (Ex) or wortmannin (Ex+wort). Increases in p-Akt in Ex vs. Sed mice were predictably absent in Ex+wort animals, but PKA and AMPK signalling were intact in both groups (Fig. 6A, B, and C, respectively). Further, while p-eNOS S635 and p-eNOS S617 did not increase in Ex+wort vs. Sed mice, elevated p-eNOS S1177 persisted (P < 0.05) but was blunted by ∼50% relative to DMSO treated (Ex) animals (Fig. 7).
Figure 5.
Insulin-stimulated arterial p-Akt and p-eNOS is abolished 10-min (A) and 60-min (B) after wortmannin administration A, 10 min after intraperitoneal (i.p.) administration of vehicle (0.7% DMSO) or wortmannin (wort), saline (Sal, n= 4) or 3.8 mU (g body weight)−1 insulin (Ins, n= 4) was injected into the caudal vein of conscious mice. Mice were anaesthetized within 5 min and iliac/femoral arteries were excised. Ins-stimulated increases in p-Akt S473, p-Akt T308 and p-eNOS S1177 in iliac/femoral homogenates were abolished in the presence of wort. *P < 0.05 vs. DMSO+Sal; #P < 0.05 vs. DMSO+Ins. B, 60 min after i.p. administration of DMSO or wort, Ins or Sal (n= 4 per group) was injected into the caudal vein. Results were similar to those described for panel A. These data indicate vascular PI3K is inhibited 10 and 60 min after i.p. administration of wort.
Figure 6.
Treadmill-running evoked increases in arterial p-Akt are abolished by wortmannin, while p-CREB S133 (i.e., PKA) and p-AMPK remain intact Ten minutes after i.p. administration of DMSO or Wort, mice completed 50 min of treadmill-running. Results are expressed as fold differences from Sed; n= 4 mice per group. A, Ex-induced increases in p-Akt S473 and p-Akt T308 relative to total protein content were abolished in the presence of Wort. Ex-induced increases in p-CREB S133 (B) and p-AMPK T172 (C) were similar regardless of treatment with Wort. *P < 0.05 vs. Sed; **P < 0.05 vs. Ex.
Figure 7.
The influence of wortmannin on p-eNOS S1177, S617, and S635 in arteries from treadmill-running and sedentary mice In the same animals described in Fig. 6, Ex-induced increases in p-eNOS S1177 relative to total protein content were blunted, but not abolished, in the presence of Wort. Ex-induced increases in p-eNOS S617 were abolished in the presence of wort. p-eNOS S635 was not elevated during Ex regardless of treatment. *P < 0.05 vs. Sed; **P < 0.05 vs. Ex.
Discussion
The contribution from NO to functional hyperaemia during exercise has been well documented in humans (Joyner & Dietz, 1997) and rodents (Symons et al. 1999). However, the signalling events in the vasculature that mediate NO release are incompletely understood. We sought to determine whether vascular signalling kinases and eNOS S1177 are phosphorylated in response to dynamic exercise. We observed that ∼50 min of treadmill-running increased p-eNOS S1177 and eNOS enzyme activity in aortae and iliac/femoral arteries of mice compared to results obtained from sedentary controls. In the same samples p-Akt, p-AMPK and p-CREB, but not p-ERK 1/2, were increased. Because Akt, AMPK and PKA are upstream activators of eNOS S1177, whereas ERK is thought to be inhibitory via phosphorylation of eNOS T495, these signalling kinases might link flow-induced shear stress to eNOS phosphorylation/activation and thereby contribute to vascular relaxation and functional hyperaemia in metabolically active tissues.
The rationale for conducting this study was two-fold. First, p-eNOS is increased in cultured endothelial cells in response to fluid shear stress via mechanisms dependent on (Dimmeler et al. 1999; Fulton et al. 1999; Boo et al. 2001; Go et al. 2001) or independent of (Boo et al. 2001, 2002; Boo & Jo, 2003; Boo et al. 2003; Fleming et al. 2005) intact Akt signalling. As such, while it is clear that shear stress stimulates eNOS phosphorylation in endothelial cells, the upstream signalling kinases responsible for these events are less certain. Second, to our knowledge, signal transduction mechanisms have not been quantified in blood vessels in response to an acute bout of dynamic exercise, e.g. treadmill-running. Therefore, it is unknown whether results obtained from cultured cells exposed acutely to shear stress can be extrapolated to endothelial cells lining a blood vessel that is subjected to an increase in blood flow in vivo.
Vascular Akt, AMPK and PKA
We hypothesized that signalling via Akt, AMPK and PKA would increase in arteries from mice that completed 50 min of treadmill-running vs. animals that were cage-confined but exposed to the same environmental stimuli. Akt has diverse cellular functions and its many roles are emerging (O’Neil & Abel, 2005). In the context of our experiment, previous data suggest that Akt is an important link between shear stress and p-eNOS S1177 in endothelial cells and perfused arteries (Dimmeler et al. 1999; Fulton et al. 1999; Butt et al. 2000; Go et al. 2001; Boo et al. 2001, 2002). Other studies using endothelial cells, isolated vessels, and/or HUVECs show that shear stress also increases p-eNOS S1177 in an Akt independent manner, i.e. perhaps via AMPK and/or PKA (Boo et al. 2001, 2002, 2003; Boo & Jo, 2003; Fleming et al. 2005; Boo, 2006). AMPK activation couples cellular metabolism with cellular demand (Hardie, 2004a,b;) and is activated by at least two stimuli associated with dynamic exercise, i.e. increased endothelial cell shear stress (Fleming et al. 2005; Zhang et al. 2006) and an elevated AMP to ATP ratio (Chen et al. 1999; Covan et al. 2003; Morrow et al. 2003; Hardie, 2004a,b;). Elevated p-AMPK might be relevant physiologically because data from human and bovine aortic endothelial cells indicate this signalling kinase is an important upstream regulator of p-eNOS S1177 (Morrow et al. 2003; Wu et al. 2007). PKA is a second messenger that controls gene transcription (Kim et al. 2006) and increases p-eNOS S1177 in cultured endothelial cells exposed to 30–60 min of shear stress (Boo, 2006). Collectively, these studies led to our hypotheses that an acute bout of dynamic exercise, i.e. treadmill-running, will increase arterial phosphorylation of Akt, AMPK and PKA in vivo.
Vascular ERK 1/2
In contrast to Akt, AMPK and PKA, we hypothesized that vascular ERK 1/2 would not be different between groups after 50 min of treadmill-running. The rationale for this was based upon reports that shear-induced p-ERK is transient in nature. Thus, while short term (i.e. 5–10 min) upregulation of ERK1/2 occurs in endothelial cells in response to elevated shear stress, values return to baseline after ∼30 min exposure to this stimulus (Jo et al. 1997; Bao et al. 2001; Chien, 2007). One potential consequence of this biphasic response to sustained shear is suppression of monocyte attraction into the vessel wall, which might be atheroprotective (Chien, 2007). Furthermore, ERK 1/2 has been reported to signal downstream to phosphorylate eNOS T495, a site responsible for decreasing eNOS activity (Chen et al. 1999; Bernier et al. 2000; Boo et al. 2001, 2002; Fleming et al. 2001; Rask-Madsen & King, 2005). Because we harvested blood vessels after 50 min, our studies are consistent with the transient nature of ERK1/2 phosphorylation that has been observed in vitro.
eNOS phosphorylation sites
Given the redundancy that is inherent in physiological systems, and the critical importance of p-eNOS S1177 to NO production, it is not surprising that Akt, PKA and AMPK all phosphorylate this site on the eNOS enzyme (Boo & Jo, 2003; Sessa, 2004). After showing these three signalling kinases were increased in vessels from Ex vs. Sed mice, we sought to determine which upstream kinases increased p-eNOS S1177 in vivo. To do so, we examined additional phosphorylation sites on the eNOS enzyme. Although the functional relevance of each known site is controversial and is incompletely defined, it generally is thought that p-S635, p-S617 and p-S1177 increase eNOS activity whereas p-T495 decreases eNOS activity (Sessa, 2004). In vitro evidence currently indicates that Akt phosphorylates S617 and S1177, PKA signals activate S635 and S1177, and AMPK phosphorylates S1177 (Sessa, 2004). Our observation that p-eNOS S617 increased > 2-fold in vessels from Ex vs. Sed mice, whereas p-eNOS S635 was not changed suggests that eNOS might not be targeted by PKA in vivo and that Akt signalling may quantitatively phosphorylate eNOS on S1177 to a greater extent than PKA. It is important to note that the role of PKA-mediated signalling to eNOS might depend on the experimental setting. For example, when endothelial cells were transfected with dominant negative Akt constructs, shear-dependent phosphorylation of eNOS S1177 was PKA dependent (Go et al. 2001), which suggests that PKA signalling could substitute when Akt signalling is defective.
To further explore the role of Akt-mediated signalling to p-eNOS S1177 in the vasculature of treadmill-running mice, animals completed treadmill-running in the absence or presence of PI3K inhibition. In the absence of wortmannin, we recapitulated our previous results that activation of Akt, PKA, AMPK, p-eNOS S1177 and p-eNOS S617, but not p-eNOS S635, occurs in blood vessels from treadmill-running vs. sedentary mice. In the presence of wortmannin, vascular Akt phosphorylation and p-eNOS at S617 did not increase significantly whereas PKA and AMPK phosphorylation remained. Interestingly, while phosphorylation of eNOS S1177 persisted in vessels from treadmill-running mice + wortmannin treatment, the increase was blunted by ∼50%vs. vehicle-treated mice. We interpret these findings to indicate that Akt-mediated signalling partially contributes to vascular p-eNOS S1177 during dynamic exercise, but that AMPK-mediated signalling might be sufficient to activate p-eNOS S1177 in the vasculature of treadmill-running mice.
Experimental considerations
Several issues should be addressed when integrating our findings into the current understanding of eNOS phosphorylation in vascular tissues. First, while we assessed Akt, AMPK, PKA and ERK 1/2, other upstream signalling kinases exist that are capable of modulating eNOS (e.g. PKG, CaMKII, PKC), but their role in the context of fluid shear stress/dynamic exercise has been less extensively studied and so is less well understood (Boo & Jo, 2003). Second, PKA activity was not measured directly; however, the use of p-CREB S133 to estimate PKA activity has been verified in cultured endothelial cells using activators and inhibitors of PKA (Boo et al. 2002). Finally, we assessed Akt, AMPK, PKA, ERK and eNOS in arterial homogenates and recognize that this approach does not allow us to determine whether these signalling events were restricted to endothelium or were occurring in vascular smooth muscle cells as well. In response to insulin, however, we have shown that p-eNOS is activated primarily in the endothelium whereas p-Akt is increased in the vascular smooth muscle compartment as well (Symons et al. 2009).
Summary and perspectives
Exercise training is a non-pharmacological intervention that can improve vascular function of healthy and diseased vessels (Niebauer et al. 1997; Kojda & Harrison, 1999; Laughlin, 2004). Strong evidence that eNOS mRNA and protein expression are increased in endothelial cells exposed to shear stress (Woodman et al. 1999), isolated arterioles subjected to elevated intraluminal flow (Woodman et al. 1997), and aortae and left ventricular tissue from trained vs. untrained mice (Fukai et al. 2000; Kojda et al. 2001; Davis et al. 2002; Lauer et al. 2005) implicates shear forces as important contributors to these vascular adaptations. Our results indicate that at least three signalling kinases, i.e. Akt, PKA, and AMPK, might link the sensation of shear by endothelial mechanoreceptors to acute eNOS phosphorylation at S1177. Because another downstream target of Akt (i.e. p-eNOS S617) but not PKA (p-eNOS S635) was elevated in vessels from Ex vs. Sed mice, we believe Akt is relatively more important than PKA in the context of our experimental setting, i.e. intact blood vessels exposed to increased blood flow. Further, because eNOS S1177 activation persisted in the presence of PI3K inhibition, Akt-mediated signalling might be dispensable for vascular p-eNOS S1177 during dynamic physical activity such as treadmill-running. In this regard, our data suggest that AMPK-mediated signalling might be sufficient to activate p-eNOS S1177 in the vasculature of treadmill-running mice.
It is likely that repeated activation of any or all of the signalling kinases via exercise training contributes, at least in part, to increased arterial eNOS expression that leads to improved vascular function (Bowles et al. 2000; Laughlin, 2004). On the other hand, defects in vascular kinase signalling to eNOS that precipitate decreases in NO bioavailability might be corrected/restored by exercise training. In this regard, evidence exists that insulin-mediated signal transduction to eNOS via Akt is impaired in blood vessels from rats with the metabolic syndrome (Potenza et al. 2005), and p-AMPK and p-eNOS are lower in aortae from obese vs. lean mice (Wu et al. 2007; Symons et al. 2009). In both of these pathophysiological circumstances, acute exercise-induced signalling to eNOS via Akt, AMPK and PKA (as shown in the present study) could bypass specific signalling defects to phosphorylate eNOS and increase NO bioavailability. Further, repeated activation of these signalling kinases via exercise training potentially could increase arterial eNOS expression to an extent that improves vascular function on a more chronic basis. This vascular adaptation has potential benefits including (1) improved exercise capacity via greater vasodilatation of arteries perfusing metabolically active muscle, and (2) reduced susceptibility to vascular disease via decreased platelet aggregation and improved antioxidant, antiproliferative and antiapoptotic properties (Niebauer et al. 1997; Kojda & Harrison, 1999; Kojda & Hambrecht, 2005).
Acknowledgments
This work was funded, in part, by University of Utah College of Health Seed Funds, the University of Utah Research Foundation, and American Heart Association, Western States Affiliate Grant-In-Aid 06-55222Y to J.D.S. E.D.A. is an Established Investigator of the American Heart Association. Funds for student support were provided, in part, by the American Heart Association, Western States Affiliate Summer Student Research Program; American Physiological Society Undergraduate Research Program; University of Utah Undergraduate Research Opportunities Program; and NIH HL091493-01 to J.D.S. Drs Bai Luo and Richard M. McAllister are thanked for their assistance with the vascular NOS assay.
Glossary
Abbreviations
- Akt
protein kinase B
- AMPK
adenosine monophosphate-activated protein kinase
- CREB
cyclic AMP response element binding protein
- ERK
extracellular signal-regulated kinase
- eNOS
endothelial NO synthase
- HUVECs
human umbilical vein endothelial cells
- NO
nitric oxide
- p
phosphorylate
- PKA
protein kinase A
- PI3K
phosphoinositol 3-kinase
- S
serine
- T
threonine
Author contributions
All authors contributed to: conception and design of the experiments; analysis and interpretation of the data; drafting and revising the manuscript; and/or approving the final version of the manuscript to be published. All experiments were completed at the University of Utah.
References
- Bao X, Lu C, Frangos JA. Mechanism of temporal gradients in shear-induced ERK1/2 activation and proliferation in endothelial cells. Am J Physiol Heart Circ Physiol. 2001;281:H22–H29. doi: 10.1152/ajpheart.2001.281.1.H22. [DOI] [PubMed] [Google Scholar]
- Bernier SG, Haldar S, Michel T. Bradykinin-regulated interactions of the mitogen-activated protein kinase pathway with the endothelial nitric-oxide synthase. J Biol Chem. 2000;275:30707–30715. doi: 10.1074/jbc.M005116200. [DOI] [PubMed] [Google Scholar]
- Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol. 2005;98:1258–1263. doi: 10.1152/japplphysiol.00991.2004. [DOI] [PubMed] [Google Scholar]
- Boo YC. Shear stress stimulates phosphorylation of protein kinase A substrate proteins including endothelial nitric oxide synthase in endothelial cells. Exp Mol Med. 2006;38:63–71. doi: 10.1038/emm.2006.8. [DOI] [PubMed] [Google Scholar]
- Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser635 by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol. 2002;283:H1819–H1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol. 2003;285:C499–C508. doi: 10.1152/ajpcell.00122.2003. [DOI] [PubMed] [Google Scholar]
- Boo YC, Sorescu G, Bauer PM, Fulton D, Kemp BE, Harrison DG, Sessa WC, Jo H. Endothelial NO synthase phosphorylated at Ser635 produces NO without requiring intracellular calcium increase. Free Radic Biol Med. 2003;35:729–741. doi: 10.1016/s0891-5849(03)00397-6. [DOI] [PubMed] [Google Scholar]
- Boo YC, Sorescu G, Boyd N, Shiojimas I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms. J Biol Chem. 2001;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- Bowles DK, Woodman CR, Laughlin MH. Coronary smooth muscle and endothelial adaptations to exercise training. Exerc Sport Sci Rev. 2000;28:57–62. [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt E, Bernhardt M, Smolenski A, Kotsonis P, Frolich LG, Sickmann A, Meyer HE, Lohmann SM, Schmidt HHHW. Endothelial nitric oxide synthase (type III) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J Biol Chem. 2000;275:5179–5187. doi: 10.1074/jbc.275.7.5179. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- Covan DL, Hu X, Dong L, Begerson R, Shulman G, Hardie DG, Young LD. Physiological role of AMP-activated protein kinase in the heart: graded activation during exercise. Am J Physiol Endocrinol Metab. 2003;285:E629–E636. doi: 10.1152/ajpendo.00171.2003. [DOI] [PubMed] [Google Scholar]
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–551. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Cai H, McCann L, Fukai T, Harrison DG. Role of c-Src in regulation of endothelial nitric oxide synthase expression during exercise training. Am J Physiol Heart Circ Physiol. 2002;284:H1449–H1453. doi: 10.1152/ajpheart.00918.2002. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:e68–e75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Boo YC, Park H, Maland MC, Patel R, Pritchard KA, Fujio Y, Walsh K, Darley-Usmar V, Jo H. Protein kinase B/Akt activates c-Jun NH2-terminal kinase by increasing NO production in response to shear stress. J Appl Physiol. 2001;91:1574–1581. doi: 10.1152/jappl.2001.91.4.1574. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: the guardian of cardiac energy status. J Clin Invest. 2004a;114:465–468. doi: 10.1172/JCI22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. The AMP-activated protein kinase pathway – new players upstream and downstream. J Cell Sci. 2004b;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. Differential effect of shear stress on extracellular signal regulated kinase and N-terminal jun kinase in endothelial cells. J Biol Chem. 1997;272:1395–1401. doi: 10.1074/jbc.272.2.1395. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol. 1997;83:1785–1796. doi: 10.1152/jappl.1997.83.6.1785. [DOI] [PubMed] [Google Scholar]
- Kang WS, Tamarkin FJ, Wheeler MA, Weiss RM. Rapid up-regulation of endothelial nitric-oxide synthase in a mouse model of Escherichia coli lipopolysaccharide-induced bladder inflammation. J Pharmacol Exp Ther. 2004;310:452–458. doi: 10.1124/jpet.104.066506. [DOI] [PubMed] [Google Scholar]
- Kim D, Vigil D, Anand G, Taylor SS. Structure and dynamics of PKA signalling proteins. Eur J Cell Biol. 2006;85:651–654. doi: 10.1016/j.ejcb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Kojda G, Cheng YC, Burchfield J, Harrison DG. Dysfunctional regulation of endothelial nitric oxide synthase (eNOS) expression in response to exercise in mice lacking one eNOS gene. Circulation. 2001;103:2839–2844. doi: 10.1161/01.cir.103.23.2839. [DOI] [PubMed] [Google Scholar]
- Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res. 2005;67:187–197. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Kojda G, Harrison DG. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:662–671. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Lauer N, Suvorava T, Ruther U, Jacob R, Meyer W, Harrison DG, Kojda G. Critical involvement of hydrogen peroxide in exercise-induced up-regulation of endothelial NO synthase. Cardiovasc Res. 2005;65:254–262. doi: 10.1016/j.cardiores.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Physical activity in prevention and treatment of coronary disease: the battle line is in exercise vascular cell biology. Med Sci Sports Exerc. 2004;36:352–362. doi: 10.1249/01.mss.0000117114.02875.5c. [DOI] [PubMed] [Google Scholar]
- Maxwell AJ, Schauble E, Bernstein D, Cooke JP. Limb blood flow during exercise is dependent on nitric oxide. Circulation. 1998;98:369–374. doi: 10.1161/01.cir.98.4.369. [DOI] [PubMed] [Google Scholar]
- Min KJ, Yang MS, Jou I, Joe EH. Protein kinase A mediates microglial activation induced by plasminogen and gangliosides. Exp Mol Med. 2004;36:461–467. doi: 10.1038/emm.2004.58. [DOI] [PubMed] [Google Scholar]
- Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- Musch T, McAllister R, Symons JD, Stebbins C, Hirai T, Hageman S, Poole D. Effects of nitric oxide synthase inhibition on vascular conductance during high speed treadmill running in rats. Exp Physiol. 2001;86:749–757. doi: 10.1111/j.1469-445x.2001.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Niebauer J, Hambrecht R, Velich T, Hauer K, Marburger C, Kalberer B, Weiss C, von Hodenberg E, Schlierf G, Schuler G, Zimmermann R, Kubler W. Attenuated progression of coronary artery disease after 6 years of multifactorial risk intervention: role of physical exercise. Circulation. 1997;96:2534–2541. doi: 10.1161/01.cir.96.8.2534. [DOI] [PubMed] [Google Scholar]
- O’Neil BT, Abel ED. Akt1 in the cardiovascular system: friend or foe? J Clin Invest. 2005;115:2059–2064. doi: 10.1172/JCI25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1. Am J Physiol Heart Circ Physiol. 2005;289:H813–H822. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:487–496. doi: 10.1161/01.ATV.0000155325.41507.e0. [DOI] [PubMed] [Google Scholar]
- Schefer V, Talan MI. Oxygen consumption in adult and aged C57BL/6J mice during acute treadmill exercise of different intensity. Exp Gerontol. 1996;31:387–392. doi: 10.1016/0531-5565(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Sessa WC. NOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- Shaul PW. Regulation of endothelial nitric oxide. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- Symons JD, Hayashi Y, Ensunsa JL. Improved coronary vascular function evoked by high-intensity treadmill training is maintained in arteries exposed to ischemia and reperfusion. J Appl Physiol. 2003;95:1638–1647. doi: 10.1152/japplphysiol.01168.2002. [DOI] [PubMed] [Google Scholar]
- Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zang Q-J, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signalling to eNOS in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons JD, Stebbins CL, Musch TI. Interactions between angiotensin II and nitric oxide during exercise in normal and heart failure rats. J Appl Physiol. 1999;87:574–581. doi: 10.1152/jappl.1999.87.2.574. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Muller JM, Laughlin MH, Price EM. Induction of nitric oxide synthase mRNA in coronary resistance arteries isolated from exercise-trained pigs. Am J Physiol Heart Circ Physiol. 1997;273:H2575–H2579. doi: 10.1152/ajpheart.1997.273.6.H2575. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Muller JM, Rush JWE, Laughlin MH, Price EM. Flow regulation of ecNOS and CuZn SOD mRNA expression in porcine coronary arterioles. Am J Physiol Heart Circ Physiol. 1999;276:H1058–H1063. doi: 10.1152/ajpheart.1999.276.3.H1058. [DOI] [PubMed] [Google Scholar]
- Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase PP2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T, Jr, Shyy JY. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]