Abstract
Fractal frequency scaling of heart period variability is used as a concise index of overall cardiac control. However, no prior study has assessed within-individual reproducibility of fractal indices of heart period, or reported how the estimated indices respond to autonomic blockade. Therefore, we examined fractal properties of the heart period from ten young, healthy individuals during three separate experimental sessions under control (saline) conditions and twice under combined autonomic blockade (atenolol and atropine sulfate) conditions. Under each condition, R-R intervals were recorded with the subject in the supine and the 40 deg upright tilt positions during 20 min of controlled breathing in each position. We calculated the fractal scaling exponent using detrended fluctuation analysis and estimated confidence intervals of the scaling exponents for each R-R interval time series within each individual. In the control condition, upright tilt significantly increased the scaling exponents (from 0.73 ± 0.11 (±s.d., session 1), 0.72 ± 0.10 (session 2) and 0.75 ± 0.13 (session 3) to 0.82 ± 0.12, 0.82 ± 0.11 and 0.84 ± 0.10; Student's paired t-test, t= 2.79, P= 0.02; t= 2.80, P= 0.02; and t= 2.07, P= 0.07). However, neither the absolute scaling exponents nor their change in response to upright tilt were reproducible (Lin's concordance coefficient less than 0.9, P > 0.1 for all comparisons). Following autonomic blockade, the scaling exponents were significantly increased (supine: 1.08 ± 0.13 and 1.08 ± 0.14; tilt: 1.07 ± 0.21 and 1.08 ± 0.14) for both experimental sessions (two-way repeated-measures ANOVA; F17,1= 40.89, P < 0.001 and F17,1= 42.72, P < 0.001) regardless of position. However, within individuals, the scaling exponents failed to distinguish between control and blockade for half of the subjects in at least one experimental session. Thus, fractal scaling exponents are not reproducible within individuals and do not reliably reflect the autonomic mechanisms responsible for heart period variability. In fact, data from combined blockade suggest that physiological effects of autonomic outflow may mask intrinsic fractal behaviour of the sinoatrial node.
Autonomic mediation of the heart rate is important for cardiovascular regulation, and therefore has major implications for cardiovascular pathologies (Tuck, 1986; Anderson et al. 1989; Kubo et al. 2005; Piepoli & Capucci, 2007). However, the difficulty of obtaining direct measures to quantify autonomic outflow in humans has led to the use of various indirect measures based on heart period variability (Porta et al. 2001; Malliani & Montano, 2002). These indirect estimates are predicated on the general assumption that fluctuations in heart period occurring at different frequencies reflect fluctuations of different autonomic neural outflows (Task Force, 1996). However, in addition to autonomic effects, variability in heart period can also be modulated by other mechanisms, such as intrinsic variability in sinoatrial pacemaker cells and ventricular myocytes (Ponard et al. 2007) or fluctuations in circulating neurohormonal factors (Christophe et al. 1984; Galetta et al. 2008). These may operate at time scales that time- and frequency-domain based approaches cannot capture adequately. As a result, scale-independent measures have been proposed as unique identifiers of the ‘balance’ in the interplay of the numerous mechanisms that modulate variability in heart period.
The use of scale-independent measures is based on the fact that an important hallmark of certain complex, highly non-linear systems is the lack of a characteristic time scale of operation. Instead, the output of healthy systems can demonstrate a type of complex variability associated with distinct classes of non-linear interactions that have long-range correlations. Therefore, quantification of long-range correlations in heart period fluctuations (the ‘output’) may provide valuable insights into the overall, integrated control of the heart. Indeed, more than 25 years ago, Kobayashi & Musha (1982) observed long term fluctuations in heart period that had a power spectral density inversely proportional to frequency (i.e. ∼1/f). Subsequently, it was proposed that, in contrast to traditional time- and frequency-domain analyses, non-linear analyses based on this ‘fractal frequency scaling’ can describe the integrated control of heart period variability independent of the time scale studied (Goldberger, 1991; Yamamoto & Hughson, 1991, 1994; Yamamoto et al. 1995b; Lahiri et al. 2008). Since then, a wealth of research has been conducted using the ‘fractal’ properties of heart period variability to probe cardiac control (Yamamoto et al. 1991; Butler et al. 1994; Yamamoto & Hughson, 1994; Tulppo et al. 2001; Lucy et al. 2003; Merati et al. 2006; Aoyagi et al. 2007). Furthermore, it has been suggested that the absence of a characteristic time scale for operation facilitates the functional adaptive capacity of the cardiovascular system by helping to prevent ‘excessive mode locking’ (Peng et al. 1993). Thus, it has been reasoned, a breakdown in the fractal frequency scaling of heart period variability is diagnostic of disturbances in the overall control of the heart (Lombardi, 2000; Lombardi et al. 2000).
Although fractal measures have prognostic significance in cardiac patients (Huikuri et al. 2000; Stein et al. 2008), they should also be shown to be reproducible across measurement periods within individuals, and consistent and accurate in reflecting disturbances in cardiac control. Surprisingly, no prior study has assessed the within-individual reproducibility of fractal indices of heart period in humans or reported how the estimated fractal indices respond to combined sympathetic and parasympathetic blockade – a profound cardiac autonomic disturbance. Therefore, the present study examined (i) whether fractal properties of the heart period measured over 20 min are reproducible within individuals across different experimental session, and (ii) whether the physiological effects of combined sympathetic and parasympathetic blockade are reliably reflected by fractal indices of heart period.
Methods
Subject characteristics and data collection
Subject characteristics, measurements and study protocol were reported in detail by Taylor et al. (1998). This protocol was approved by the human research committees of the Hunter Holmes McGuire Department of Veterans Affairs Medical Center and the Medical College of Virginia at Virginia Commonwealth University, and conformed with the Declaration of Helsinki. All volunteers gave their written informed consent to participate. Briefly, data were collected from ten healthy subjects (7 men and 3 women, 23–28 years of age) during three separate sessions. Volunteers were non-smokers without histories of cardiovascular or other major diseases, and were not taking any cardioactive medications. Prior to the studies, they were acclimated to laboratory settings, and practiced in controlled breathing. They refrained from alcohol or caffeine intake and strenuous physical activity for 12 h preceding the study sessions. Data were acquired during control conditions (saline administration) for all three sessions, and during combined intravenous autonomic blockade (cardioselective β-adrenergic blockade with 0.2 mg kg−1 atenolol and muscarinic cholinergic blockade with 0.04 mg kg−1 atropine sulfate) for two of these sessions. Drugs were administered in random order.
In each session and for both positions and both conditions, time series of R-R intervals were obtained via ECG lead II with the subject in the supine and passive 40 degrees upright tilt positions during 20 min (in each position) of frequency (0.25 Hz) and tidal volume-controlled breathing. The electrocardiogram was recorded continuously on FM tape for subsequent analog-to-digital conversion, and digitized at a rate of 500 Hz with commercial hardware and software (CODAS, Dataq Instruments, Akron, OH, USA), consistent with the guidelines of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996). Thus, data used for the current study comprised ten R-R interval time series for each subject, representing two conditions (control and combined autonomic blockade) with subjects in two positions (supine and upright tilt) obtained three times for control and twice for combined blockade in separate sessions. All studies were performed in the morning, 2 h postprandial, and separated by 1 week. Session order and position order after drug administration were randomized, and subjects were not told which drugs they would be given. The carefully controlled nature of the data collection, as well as the data length and acquisition (see Appendix) make these time series uniquely suitable for the current study.
Calculation of the fractal scaling exponents and confidence intervals
For each subject, the fractal scaling component was estimated separately for each R-R interval time series via detrended fluctuation analysis (DFA). DFA is widely used in analysis of heart rate variability and has been shown to identify correctly long-range correlations in time series (Peng et al. 1995). We also used approximate entropy (Pincus, 1991; Ho et al. 1997) and sample entropy (Richman & Moorman, 2000; Aboy et al. 2007) to quantify the complexity of heart rate variability; however, results from these indices were qualitatively similar to DFA results. Therefore, we only report the results from DFA. DFA was initially proposed by Peng et al. (1993), and described in detail by Little et al. (2006). A brief description is provided in the Appendix.
Characterizing a component of a complex system with an index of fractal behaviour is predicated on the implicit reductionist assumption that the behaviour and dynamics observed represent behaviour of the overall system (Goldberger, 1991). Though this provides a simple context for interpreting these indices, this assumption does not obviate the statistical error in the estimated fractal index. Prior studies have ignored the magnitude of error in fractal estimates for R-R interval time series; however, we chose to account for this because it markedly impacts the ability to interpret any differences in the index across sessions and conditions. Therefore, we estimated confidence intervals for the scaling exponents for each R-R interval time series for each individual. We describe the derivation of the confidence intervals in the Appendix.
Adequacy and consistency of the data
Although it is feasible to derive a fractal scaling exponent for any time series, there are two major prerequisites for obtaining meaningful estimates. First, the implicit assumption of fractal analyses is that the data actually conform to a standard fractal time series model (Davies & Harte, 1987). In fact, it has been argued that ‘completely meaningless fractal estimates’ can result if this step of signal classification is not taken prior to fractal analysis (Eke et al. 2002). Though power-law (1/f) scaling of the logarithm of time scales and the logarithm of corresponding fluctuations is generally assumed to imply ‘fractality’, this assumption is not always valid when the sampling rate and interval are finite (Avnir et al. 1998). Therefore, we used a conservative test to assess whether R-R interval time series conform to the standard fractal model. The classical model of a fractal time series is based on the dichotomous ‘fractional Brownian motion’ (fBm) and ‘fractal Gaussian noise’ (fGn) process (Mandelbrot & Van Ness, 1968), and this dichotomy has been exploited to develop different analytical tools to derive fractal scaling exponents based on the signal class (Eke et al. 2000, 2002).
However, fBm and fGn processes are two sides of the same coin: the fBm signal is non-stationary with stationary successive increments, which yield fGn signal, and the cumulative sum of an fGn signal is an fBm process (Davies & Harte, 1987; Eke et al. 2002). Therefore, if a time series conforms to either of the signal classes, then either the time series itself (for fGn process) or its successive differences (for fBm process) should be stationary. (Although this alone is not a sufficient condition, it is a necessary condition for the standard fractal model.) To test this, we applied the Priestly–Subba Rao test of stationarity (Priestley & Subba Rao, 1969) to each of the R-R interval time series and their successive differences both in time and frequency domains. The test is described in detail by Priestley & Subba Rao (1969), and the default implementation details employed in the current study are described by Constantine & Percival (2007). We were surprised to find that only 11% of the R-R interval time series (or their successive differences) conformed to the standard model (that is, the time series was neither an fBm nor fGn process, P < 0.05). Therefore, a non-parametric Wilcoxon rank-sum test was used to test possible effects of position (supine or tilt) or experiment day on the outcome of the Priestley–Subba Rao test.
The second assumption of fractal analyses is that the length of the data be adequate to allow reliable calculation of a single fractal scaling exponent. Teich et al. (2001) and Delignieres et al. (2006) suggested that 10–20 min is adequate in most cases to obtain a fractal exponent. Our data length satisfies the second prerequisite. Indeed, the analysis provided in the Appendix shows that increasing our data length to ∼1.5 h would result in a negligible improvement in the accuracy of estimated scaling exponent.
Statistics
To validate a proposed measure, one must first establish its reproducibility from session to session. Therefore, scaling exponents estimated from data acquired under the control supine and upright tilt conditions were compared between the first, second and third experimental sessions using Student's t-test for paired data. In addition, the changes in scaling exponents when subjects moved from the supine to upright tilt positions were calculated and compared with the paired t-test. To assess agreement between estimates on separate sessions, Lin's concordance coefficient (Lin, 1989, 1992) was used to test the null hypothesis (H0) that the estimated scaling exponents are not in agreement (ρc < 0.9). Lin's concordance coefficient is based on Pearson's correlation coefficient (a measure of variation), but includes a bias correction term that takes systematic deviations into account. Furthermore, it is a stronger statistical test compared with least-squares linear regression, since it takes both the intercept and the slope into account simultaneously.
As mentioned, data used for the current study comprised R-R interval time series collected under conditions that broadly represent strikingly different cardiac autonomic states: intact vagal and sympathetic effects that vary with subject position and no autonomic effect regardless of position. To assess whether scaling exponents reliably reflect autonomic control of R-R intervals, we tested the difference in scaling exponents via two-way repeated measures analysis of variance using position (supine vs. tilt), and condition (control vs. combined blockade) as factors. However, to be a reliable diagnostic marker of autonomic control, scaling exponents should discriminate between different autonomic states, not only at the group level, but also at the individual level. Therefore, we also tested whether the distribution of scaling exponents estimated for each individual during different conditions could reliably be distinguished. To that end, we used the distribution of scaling exponents for each time series (calculated as described in the Appendix for construction of confidence intervals) to test the null hypothesis that different fractal exponents calculated during different autonomic states are statistically distinguishable. (That is, the difference between two exponents is greater than zero (one-tailed) at a significance level P= 0.05.) This threshold for statistical significance is based on the conventional use of the same level in standard parametric statistical tests (e.g. one-way ANOVA). In fact, as reported below, in cases where the fractal scaling exponents were not statistically distinguishable, the probability was usually much larger than the adopted criterion of P > 0.05.
Results
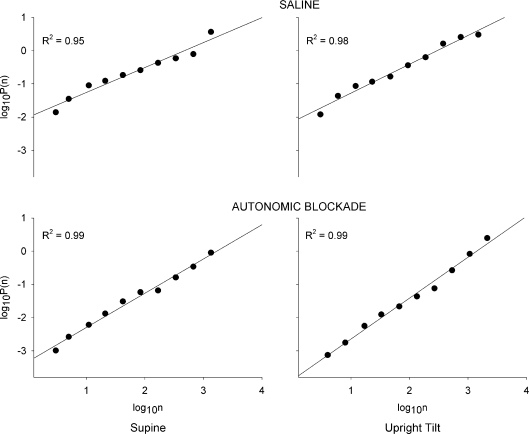
Representative R-R interval time series are shown in Fig. 1. A strong linear relation between the logarithm of the time scales (log10n) and the logarithm of fluctuations (log10P(n)) that extended across more than one time scale was observed for all R-R interval time series (mean coefficient of determination (R2) 0.97 ± 0.02. Representative DFA curves are shown in Fig. 2). However, as mentioned above, 89% of the time series did not conform to the standard fractal model. Furthermore, there was no effect of position or experiment day on this outcome (Wilcoxon's rank-sum test, P > 0.05). However, there was a modest effect of combined blockade. Of the R-R interval time series that did conform to the standard model and therefore met the criteria to be a fractal time series, the majority were observed during combined autonomic blockade (Wilcoxon's rank-sum test, V= 31.5, P= 0.04). In fact, ∼80% of the time series (9 out of 11) that were fractal derived from the combined autonomic blockade state. The lack of conformity could not be completely attributed to the frequency component induced by controlled breathing because even after this component was removed (see online Supplemental Material), 68% of the R-R interval time series still did not conform to the standard model.
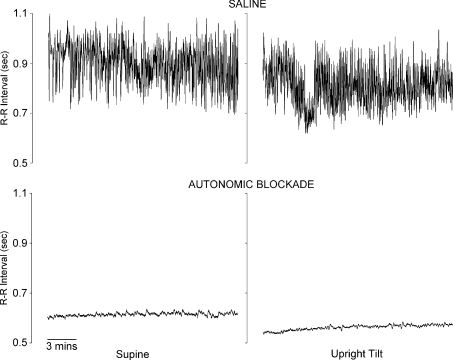
Figure 1.
Example R-R interval time series from one subject in the supine position (left panels) and during upright tilt (right panels), at baseline (saline; upper panels) and after combined vagal and sympathetic blockade (lower panels)
Figure 2. Representative DFA curves from the same subject shown in Fig. 1 at supine position (left panels) and during upright tilt (right panels), at baseline (saline; upper panels) and after combined vagal and sympathetic blockade (lower panels).
Lines in each panel show the linear regression line.
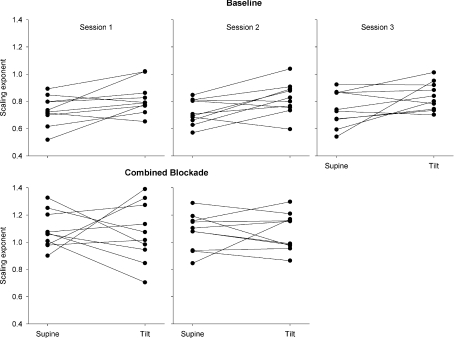
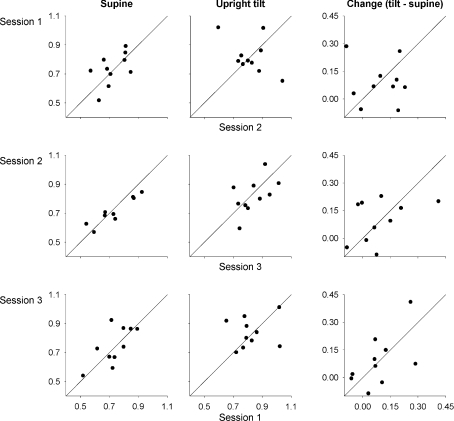
In the supine control condition, the fractal scaling exponent averaged 0.73 ± 0.11, 0.72 ± 0.10 and 0.75 ± 0.13 (mean ±s.d.) for three experimental sessions. Upright tilt increased the fractal scaling exponents to 0.82 ± 0.12, 0.82 ± 0.11 and 0.84 ± 0.10, and at the group level, the scaling exponents were significantly different from supine for the first two sessions (paired t-test, session 1: t= 2.45, P= 0.04, session 2: t= 2.70, P= 0.03) while the difference was not significant for the third session (t= 2.04, P= 0.07). However, the scaling exponents during both supine and upright tilt positions in the control condition were not reproducible across sessions (Table 1 and Figs 3 and 4). Furthermore, though the average increase in the fractal scaling exponent was similar for all three sessions (0.09 ± 0.11, 0.10 ± 0.12, and 0.09 ± 0.14), the changes were even less reproducible than the absolute exponents (Table 1 and Fig. 4). Thus, from day to day, the estimated fractal behaviour in the R-R interval time series was not consistent, and did not demonstrate reproducible responses to modest changes in autonomic state.
Table 1.
Comparison between fractal scaling exponents estimated for different experimental sessions
| Supine |
Upright Tilt |
Change |
|||||
|---|---|---|---|---|---|---|---|
| Session 2 | Session 3 | Session 2 | Session 3 | Session 2 | Session 3 | ||
| Session 1 | b1 | 0.45 | 0.80 | −0.50 | 0.12 | −0.10 | 0.72 |
| b0 | 0.39 | 0.16 | 1.23 | 0.74 | 0.11 | 0.03 | |
| ρc (z) | 0.53 (0.59) | 0.66 (0.80) | −0.49 (−0.54) | 0.14 (0.14) | −0.10 (0.10) | 0.57 (0.64) | |
| p | 0.79 | 0.64 | >0.99 | 0.99 | >0.99 | 0.78 | |
| Session 2 | b1 | — | 1.32 | — | 0.46 | — | 0.51 |
| b0 | — | −0.21 | — | 0.46 | — | 0.05 | |
| ρc (z) | — | 0.87 (1.31) | — | 0.52 (0.58) | — | 0.41 (0.43) | |
| p | — | 0.14 | — | 0.87 | — | 0.91 | |
Last column (Change) represents the comparison of the change in scaling exponents in response to upright tilt. b1 and b0: slope and intercept of the regression line; ρc: Lin's concordance coefficient; z: z-statistic for concordance coefficient; p: probability of scaling exponents in two sessions being discordant (ρc < 0.9).
Figure 3.
Change in scaling exponents from supine to upright tilt position in three sessions during saline administration (upper panels) and in two sessions after combined vagal and sympathetic blockade (lower panels)
Figure 4. Reproducibility of scaling exponents.
First column: in the supine position during saline administration; second column: in the upright tilt position, during saline administration; third column: change in fractal scaling exponents response to upright tilt (tilt – supine), during saline administration. Diagonal lines show x=y (i.e. slope = 1, intersect = 0).
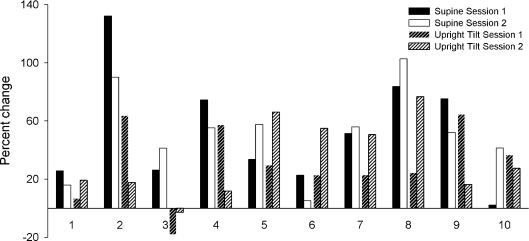
Following combined autonomic blockade, scaling exponents were increased to 1.08 ± 0.13 and 1.08 ± 0.14 in the supine position, and to 1.07 ± 0.21 and 1.08 ± 0.14 in the tilt position. At the group level, combined blockade had a significant effect on the scaling exponent for both experimental sessions (F17,1= 40.89, P < 0.001 and F17,1= 42.72, P < 0.001) that did not depend on position (supine vs. tilt). However, despite this average effect, change in the scaling exponents (expressed as percentage change from control (saline) condition) was highly variable across individuals, positions and experimental sessions (Fig. 5). Furthermore, at the individual level, there remained a considerable overlap with scaling exponents during control conditions. During the first experimental session, fractal scaling exponents from control and combined autonomic blockade conditions were not statistically distinguishable in two subjects in the supine position (subject no. 1: α= 0.83 ± 0.02 (mean ±95%c.i.) during control and α= 0.96 ± 0.13 after combined blockade, P= 0.98; and subject no. 10: α= 0.88 ± 0.01 and α= 0.89 ± 0.01, P= 0.89). This was also true for one subject during the second experimental session (subject no. 6; 0.78 ± 0.03 during control and 0.83 ± 0.04 after combined blockade, P= 0.73). Additionally during the second session, the fractal scaling exponents during control and combined blockade were not statistically distinguishable in two subjects in the upright tilt position (subject no. 3: 0.88 ± 0.01 and 0.85 ± 0.01; P= 0.52; and subject no. 4: 0.87 ± 0.01 and 0.97 ± 0.10; P= 0.88). The subjects with indistinguishable scaling exponents were different in each case; overall, fractal scaling exponents failed to distinguish between saline control and double blockade for half of the subjects for at least one comparison (i.e. supine or tilt, during session 1 or 2).
Figure 5.
Percentage change in individual scaling exponents in response to combined sympathetic and vagal blockade in the supine position and during upright tilt on experimental sessions 1 and 2
Discussion
Prior studies have proposed that the fractal properties of R-R interval time series reflect overall control of the heart, and therefore might be useful as a clinical diagnostic tool to probe the health of the system (Havlin et al. 1999; Goldberger et al. 2002; Naschitz et al. 2002; Signorini, 2004; Krstacic et al. 2007). However, in our study, the scaling exponents calculated for each individual varied substantially across different experimental sessions. Under baseline (saline control) conditions, scaling exponents were not concordant across three experimental sessions, regardless of the subject position. Furthermore, the changes in the fractal scaling exponents in response to upright tilt were not reproducible across different experimental sessions. Day-to-day consistency is crucial for a reliable diagnostic measure. However, the reproducibility of fractal exponents has not been studied previously. This may be due, in part, to the common practice of averaging estimated fractal indices of R-R interval time series across several individuals. Our averages would suggest day-to-day consistency across subjects, but examination of individual values shows that a very high scaling exponent measured on one day can be markedly lower on another day.
Changes in the fractal scaling exponent have been thought to indicate alterations in integrated cardiac control. However, this usage is based on group-level considerations. Indeed, our data confirm that the fractal scaling exponents reflect the physiological effects of complete sympathetic and vagal blockade, but only at the group level; the exponents calculated after autonomic blockade were not statistically distinguishable from those calculated at baseline for half of the individuals half of the time. Thus, for one-quarter of comparisons, fractal scaling exponents failed to reflect a profound change in autonomic state. It is possible that fractal exponents are surrogates for individual physiological differences; however, understanding this would require detailed information on a large number of study subjects, and an analysis to disentangle various relations between individual subject characteristics and fractal indices of heart rate variability. Nonetheless, fractal scaling exponents of heart period do not consistently reflect overall autonomic control of the heart in healthy subjects, and do not reliably indicate the physiological state of the heart, and so should not be considered as reliable diagnostic measures of autonomic state at the individual level.
This may not be surprising if one explores prior reports of fractal indices and cardiac autonomic state. For example, one study (Perkiomaki et al. 2002) suggested that fractal indices do not correlate well with any other index of autonomic control (highest R2 less than 0.50), and that changes in fractal scaling and changes in heart rate after complete cholinergic blockade do not correlate with each other. Furthermore, Yamamoto & Hughson (1994) reported that scaling behaviour following β-adrenergic blockade does not change, Tulppo et al. (2001) suggested that the scaling exponent following noradrenaline infusion decreases, Yamamoto et al. (1995a) and Perkiomaki et al. (2002) found that the scaling exponent following vagal blockade increases, and Mourot et al. (2007) demonstrated that the fractal scaling exponent is unrelated to an increase or decrease in muscle sympathetic nerve activity during autonomic manoeuvres. Thus, the literature does not support a simple relation between fractal scaling and cardiac autonomic effect. Indeed, prior reports and our own results reinforce the conclusion that, although the underlying physiology of the cardiovascular system may be complex, scaling exponents fail to capture significant aspects of this physiology. As a matter of fact, the fractal scaling exponent calculated using DFA is mathematically related to traditional spectral indices of heart period variability (Francis et al. 2002; Willson et al. 2002), which can be unreliable (Sandercock et al. 2005). Francis et al. (2002) observed that the frequency-domain equivalent of DFA is a weighed ratio of spectral powers. This is important to note because even a small change in the numerator or denominator can result in a substantial change in an observed ratio; thus, small day-to-day changes in spectral powers may lead to large day-to-day variability in fractal scaling exponents. As a result, the lack of a relation between fractal scaling exponents and other indices of cardiac autonomic control is not unexpected.
The above considerations contradict the use of fractal indices to probe the ‘health’ of cardiac control. The use of fractal scaling exponents as a diagnostic measure is predicated on the assumption that there is a fractal scaling exponent associated with a ‘healthy’ system, and that this exponent deviates from this value with dysfunction (Task Force, 1996; Goldberger et al. 2002; Ribeiro et al. 2002; Signorini, 2004; Beckers et al. 2006a,b; Merati et al. 2006; Vigo et al. 2007). It is surprising that there is no consensus in the literature on what a ‘healthy’ fractal scaling exponent should be. Mourot et al. (2007) reported a scaling exponent of 0.71 ± 0.13 for healthy subjects during supine rest whereas Struzik et al. (2004) reported a value of 1.18 for healthy subjects using first-order DFA. Fractal scaling exponents have been reported as 0.5 for individuals with heart failure and as 1.0 for healthy individuals (Goldberger et al. 2002). In our study, the fractal scaling exponents calculated for healthy young individuals under baseline conditions showed a great variability, ranging from ∼0.44 to ∼0.91. This is similar to results published by Heffernan et al. (2008). In their study, even though all subjects were ‘apparently healthy without any history of chronic disease’, scaling coefficients at baseline ranged from ∼0.52 to ∼1.36. These values are similar to those reported for older individuals and individuals with congestive heart failure (Goldberger et al. 2002). As a matter of fact, in our study, fractal scaling exponents calculated after complete blockade of autonomic outflow were closer to the presumed ‘healthy’ scaling exponent of 1.0 than those calculated at baseline.
Thus, our results do not support a relation between fractal indices of heart rate variability and the ‘health’ of cardiac autonomic control. This conclusion is in contrast to the finding that as a predictor of mortality following myocardial infarction in a large number of subjects (n= 446), short-term fractal scaling exponent provides 7–12% more predictive power than other traditional measures of heart rate variability (Huikuri et al. 2000). However, in contrast to young healthy individuals, abnormal breathing patterns (e.g. obstructive sleep apnoea; Schafer et al. 1999) or differences in physical activity (physical activity is a major contributor to the ultra low frequency components of heart rate variability; Serrador et al. 1999) in myocardial infarction patients may be reflected in fractal scaling components estimated from 24 h recordings. Moreover, long time intervals used for these analyses may preclude physiological interpretation of the fractal scaling exponents (Pilgram & Kaplan, 1999).
The implicit assumption in application of fractal indices to heart period variability is that the time series conform to the standard statistical model of fractal behaviour (Mandelbrot & Van Ness, 1968). Time series that are consistent with the classical fractional Brownian/Gaussian motion model of fractal, or self-similar scaling can be adequately characterized by the standard deviation and the scaling exponent of the series (Eke et al. 2000). DFA has been suggested as a relatively robust method to quantify the fractal properties of a time series when it cannot be determined whether the time series conforms to the standard model (Eke et al. 2002). However, if a time series is not consistent with this fractal model, its statistical and temporal properties cannot be completely elucidated by a fractal scaling exponent. Using only a single, conservative test, we found a small minority of our data sets might conform to this classical fractal model, despite the strong linear relation between the logarithm of time scales and the logarithm of magnitude of corresponding fluctuations. This result reaffirms the notion that linear power-law scaling does not necessarily imply that the time series is fractal (Avnir et al. 1998).
It could be reasoned that flaws in these data did not allow accurate fractal estimates. Data length is a key issue in fractal estimates; however, our analysis in the Appendix shows that the lengths of our time series are sufficient to allow stable estimates. Along these same lines, it has been suggested that using two or more scaling exponents, a multifractal approach (with longer time series) may better characterize R-R interval time series than a monofractal approach (Ivanov et al. 1999; Iyengar et al. 1996; Meyer & Stiedl, 2003). However, previous work suggests that short-range, long-range and monofractal scaling exponents are not statistically different from each other in young healthy individuals (Iyengar et al. 1996). Indeed, in our data set, the DFA curves in all R-R interval time series were highly linear, suggesting that a multifractal approach would not change our results. Sampling frequency of R-R interval is another crucial element for a reliable estimation of fractal scaling exponents, especially after combined autonomic blockade when noise could be greater than the R-R interval variability itself. In addition, controlled breathing contains a periodic component of 4 s (4–10 beats, depending on the condition) that could affect estimated fractal exponents. However, further analysis (in online Supplemental Material) showed that neither of these factors had a significant effect on our results.
Interestingly, fractal behaviour in the heart period became most apparent following combined autonomic blockade. Roughly 25% of the R-R interval time series observed during combined autonomic blockade were fractal, whereas only 3% of those observed in the control condition had fractal characteristics. This observation may be related to the work of Ponard et al. (2007) who showed fractal behaviour in isolated ventricular myocytes. They inferred that similar intrinsic fractal variability exists in sinoatrial node pacemaker activity and may derive from stochastic fluctuations in transmembrane currents and stochastic gating of intracellular calcium release (Ponard et al. 2007). If our results from combined blockade reflect this same physiology, it would suggest that cardiac autonomic control, rather than contributing to fractal behaviour in heart period, masks intrinsic fractal behaviour that may derive from cardiac pacemaker cell activity. In addition, there may be mechanisms other than autonomic outflow that modify the fractal behaviour of intrinsic pacemaker activity; there are many neuroendocrine effectors that modulate cardiac chronotropy (Christophe et al. 1984; Polikar et al. 1993; Jedrusiak et al. 1995; Galetta et al. 2008) which might also further mask intrinsic fractal patterns. This might be the reason that not all time series from combined autonomic blockade were fractal.
In summary, our results show that a majority of R-R interval time series do not conform to the assumed standard monofractal model of fractional time series and are not purely fractal processes. As a consequence, calculated fractal scaling exponents are not reproducible within individuals, and do not reliably reflect the autonomic state of the heart. As a matter of fact, cardiac autonomic control may mask fractal behaviour that is intrinsic to the physiological function of sinoatrial node pacemaker activity. Thus, our results caution against the use of fractal indices of heart period variability as either physiological measurements or diagnostic tools.
Acknowledgments
This research was supported by the National Institutes of Health, National Institute on Aging grant AG014376 and National Heart Lung and Blood Institute grant HL081693.
Appendix
Calculation of fractal scaling exponents and confidence intervals
To estimate fractal scaling exponent via detrended fluctuation analysis (DFA), first the accumulation of departures in the time series, x(t), from the mean, 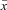 , was calculated by integrating the departure for whole time series from the average value:
, was calculated by integrating the departure for whole time series from the average value:
 |
The integrated series was then divided into non-overlapping segments, and each segment was locally detrended by subtracting the values obtained by linear least-squares regression. The size of each segment (that is, each time scale used in the study) was the same for each time series, and ranged from 4 to approximately 128–256 (∼10% of the length of the series) beats. The amount of fluctuation, P, in each integrated and detrended segment was then calculated by:
 |
The fractal scaling exponent, α, is estimated as the slope of a double logarithmic plot of P as a function of n, i.e. 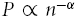 .
.
To estimate the confidence intervals of the calculated fractal scaling exponent for each time series, we followed an empirical Monte Carlo approach to take advantage of the standard model for fractal behaviour. Thus, for the 11% of R-R interval time series that conformed with the standard model, we used an artificial fractional Gaussian noise time series for Monte Carlo simulations. For each of these time series, the scaling exponents were calculated and then 2500 artificial time series were generated with the same scaling exponent (surrogate series) based on the algorithm proposed by Davies and Harte (1987) and described in detail by Delginieres et al. (2006). The scaling exponent for each of the 2500 surrogate time series was then re-calculated. This procedure resulted in a distribution of scaling exponents for each original time series. Each distribution was normal (Kolmogorov–Smirnov test, P > 0.05 in all cases) and so 95% confidence intervals were estimated as 2.5th and 97.5th quantiles of each distribution. For the remaining 89% of R-R interval series that did not conform with the standard model of fractal time series, we estimated confidence intervals via a non-parametric approach. We fitted a long autoregressive model (model order = 50) to each R-R interval time series to obtain a ‘skeleton’ of the series and residuals that are approximately independent and identically distributed. Then, the residuals were shuffled, added to the skeleton, and the fractal exponent for the resulting time series was re-estimated. The second step was repeated 2500 times, and the confidence intervals for each scaling exponent was calculated as described above for the first method.
Adequacy of data length
Adequacy of data length can be verified by determining the change in variability of the scaling exponents in repeated trials with varying data lengths. In practice, however, measurements of variable durations under identical conditions and experimental settings are rarely available. Thus, artificial data sets with the same characteristics as actual data sets can be used to assess changes in the estimated scaling exponents. We generated two separate ensembles of artificial series: one with the same standard deviation and fractal scaling exponent as the shortest R-R interval series at baseline (baseline-surrogates), and one with the same standard deviation and scaling exponent as the shortest R-R interval series during combined autonomic blockade (blockade-surrogates). Each ensemble consisted of 1000 randomly generated fGn/fBm series (as described above for calculation of confidence intervals) and each ensemble was generated several times with time series’ length varying from N= 180 beats (∼3 min) to 4 times the length (with 100 beat increments) of the actual R-R interval time series. We then estimated the fractal scaling exponent for each artificial series. This resulted in 1000 fractal scaling exponents for each N, for baseline- and blockade-surrogates, and we assessed the standard deviation of estimated fractal scaling exponents within each ensemble.
Increasing the length of artificial R-R interval series set to ∼90 min reduced the standard deviation of the estimated exponent only from 0.046 to 0.034 at baseline, and from 0.011 to 0.008 during complete autonomic blockade (see Fig. S1 in the Supplemental Material). This reduction in the standard deviation is substantially smaller than interindividual variation in estimated scaling exponents. Therefore, observed lack of reproducibility and reliability of fractal scaling exponents in our data sets cannot be attributed to the R-R interval time series length.
Author contributions
J.A.T. and D.L.E. designed the study and collected the data. C.O.T. analysed, and C.O.T., M.A.C. and J.A.T. interpreted the results. C.O.T. drafted the manuscript; M.A.C., J.A.T. and D.L.E. revised it critically. All authors have seen and approved the final version of the manuscript to be published. All experiments were done at the Medical College of Virginia at Virginia Commonwealth University.
Supplemental material
References
- Aboy M, Cuesta-Frau D, Austin D, Mico-Tormos P. Characterization of sample entropy in the context of biomedical signal analysis. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:5943–5946. doi: 10.1109/IEMBS.2007.4353701. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- Aoyagi N, Struzik ZR, Kiyono K, Yamamoto Y. Autonomic imbalance induced breakdown of long-range dependence in healthy heart rate. Methods Inf Med. 2007;46:174–178. [PubMed] [Google Scholar]
- Avnir D, Biham O, Lidar D, Malcai O. Is the geometry of nature fractal. Science. 1998;279:39–40. [Google Scholar]
- Beckers F, Verheyden B, Aubert AE. Aging and nonlinear heart rate control in a healthy population. Am J Physiol Heart Circ Physiol. 2006a;290:H2560–H2570. doi: 10.1152/ajpheart.00903.2005. [DOI] [PubMed] [Google Scholar]
- Beckers F, Verheyden B, Couckuyt K, Aubert AE. Fractal dimension in health and heart failure. Biomed Tech (Berl) 2006b;51:194–197. doi: 10.1515/BMT.2006.035. [DOI] [PubMed] [Google Scholar]
- Butler GC, Yamamoto Y, Hughson RL. Heart rate variability to monitor autonomic nervous system activity during orthostatic stress. J Clin Pharmacol. 1994;34:558–562. doi: 10.1002/j.1552-4604.1994.tb02007.x. [DOI] [PubMed] [Google Scholar]
- Christophe J, Waelbroeck M, Chatelain P, Robberecht P. Heart receptors for VIP, PHI and secretin are able to activate adenylate cyclase and to mediate inotropic and chronotropic effects. Species variations and physiopathology. Peptides. 1984;5:341–353. doi: 10.1016/0196-9781(84)90232-8. [DOI] [PubMed] [Google Scholar]
- Constantine W, Percival D. Fractal: Insightful Fractal Time Series Modeling and Analysis. R package version 1.0-2.
- Davies RB, Harte DS. Tests for Hurst effect. Biometrika. 1987;74:95–101. [Google Scholar]
- Delignieres D, Ramdani S, Lemoine L, Torre K, Fortes M, Ninot G. Fractal analyses for ‘short’ time series: A re-assessment of classical methods. J Math Psychol. 2006;50:525–544. [Google Scholar]
- Eke A, Herman P, Bassingthwaighte JB, Raymond GM, Percival DB, Cannon M, Balla I, Ikrenyi C. Physiological time series: distinguishing fractal noises from motions. Pflugers Arch. 2000;439:403–415. doi: 10.1007/s004249900135. [DOI] [PubMed] [Google Scholar]
- Eke A, Herman P, Kocsis L, Kozak LR. Fractal characterization of complexity in temporal physiological signals. Physiol Meas. 2002;23:R1–38. doi: 10.1088/0967-3334/23/1/201. [DOI] [PubMed] [Google Scholar]
- Francis DP, Willson K, Georgiadou P, Wensel R, Davies LC, Coats A, Piepoli M. Physiological basis of fractal complexity properties of heart rate variability in man. J Physiol. 2002;542:619–629. doi: 10.1113/jphysiol.2001.013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetta F, Franzoni F, Fallahi P, Tocchini L, Braccini L, Santoro G, Antonelli A. Changes in heart rate variability and QT dispersion in patients with overt hypothyroidism. Eur J Endocrinol. 2008;158:85–90. doi: 10.1530/EJE-07-0357. [DOI] [PubMed] [Google Scholar]
- Goldberger AL. Is the normal heartbeat chaotic or homeostatic? News Physiol Sci. 1991;6:87–91. doi: 10.1152/physiologyonline.1991.6.2.87. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Hausdorff JM, Ivanov PC, Peng CK, Stanley HE. Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci U S A. 2002;99(Suppl 1):2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlin S, Amaral LA, Ashkenazy Y, Goldberger AL, Ivanov PC, Peng CK, Stanley HE. Application of statistical physics to heartbeat diagnosis. Physica A. 1999;274:99–110. doi: 10.1016/s0378-4371(99)00333-7. [DOI] [PubMed] [Google Scholar]
- Heffernan KS, Sosnoff JJ, Fahs CA, Shinsako KK, Jae SY, Fernhall B. Fractal scaling properties of heart rate dynamics following resistance exercise training. J Appl Physiol. 2008;105:109–113. doi: 10.1152/japplphysiol.00150.2008. [DOI] [PubMed] [Google Scholar]
- Ho KK, Moody GB, Peng CK, Mietus JE, Larson MG, Levy D, Goldberger AL. Predicting survival in heart failure case and control subjects by use of fully automated methods for deriving nonlinear and conventional indices of heart rate dynamics. Circulation. 1997;96:842–848. doi: 10.1161/01.cir.96.3.842. [DOI] [PubMed] [Google Scholar]
- Huikuri HV, Makikallio TH, Peng CK, Goldberger AL, Hintze U, Moller M. Fractal correlation properties of R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation. 2000;101:47–53. doi: 10.1161/01.cir.101.1.47. [DOI] [PubMed] [Google Scholar]
- Ivanov PC, Amaral LA, Goldberger AL, Havlin S, Rosenblum MG, Struzik ZR, Stanley HE. Multifractality in human heartbeat dynamics. Nature. 1999;399:461–465. doi: 10.1038/20924. [DOI] [PubMed] [Google Scholar]
- Iyengar N, Peng CK, Morin R, Goldberger AL, Lipsitz LA. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1078–R1084. doi: 10.1152/ajpregu.1996.271.4.R1078. [DOI] [PubMed] [Google Scholar]
- Jedrusiak J, Brus R, Kostrzewa RM, Slowinski Z. Dopaminergic neuronal systems modulate the central cardiovascular effects of TRH in rats. Pol J Pharmacol. 1995;47:43–52. [PubMed] [Google Scholar]
- Kobayashi M, Musha T. 1/f fluctuation of heartbeat period. IEEE Trans Biomed Eng. 1982;29:456–457. doi: 10.1109/TBME.1982.324972. [DOI] [PubMed] [Google Scholar]
- Krstacic G, Krstacic A, Smalcelj A, Milicic D, Jembrek-Gostovic M. The ‘Chaos Theory’ and nonlinear dynamics in heart rate variability analysis: does it work in short-time series in patients with coronary heart disease? Ann Noninvasive Electrocardiol. 2007;12:130–136. doi: 10.1111/j.1542-474X.2007.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Parker JD, Azevedo ER, Atchison DJ, Newton GE, Picton P, Floras JS. Vagal heart rate responses to chronic beta-blockade in human heart failure relate to cardiac norepinephrine spillover. Eur J Heart Fail. 2005;7:878–881. doi: 10.1016/j.ejheart.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51:1725–1733. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Lin LIK. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- Lin LIK. Assay validation using the concordance correlation coefficient. Biometrics. 1992;48:599–604. [Google Scholar]
- Little M, McSharry P, Moroz I, Roberts S. Nonlinear, biophysically-informed speech pathology detection. 2006;2:1080–1083. Proc IEEE Int Conf Acoust Speech Signal Process ICASSP. [Google Scholar]
- Lombardi F. Chaos theory, heart rate variability, and arrhythmic mortality. Circulation. 2000;101:8–10. doi: 10.1161/01.cir.101.1.8. [DOI] [PubMed] [Google Scholar]
- Lombardi F, Porta A, Marzegalli M, Favale S, Santini M, Vincenti A, De Rosa A. Heart rate variability patterns before ventricular tachycardia onset in patients with an implantable cardioverter defibrillator. Participating Investigators of ICD-HRV Italian Study Group. Am J Cardiol. 2000;86:959–963. doi: 10.1016/s0002-9149(00)01130-9. [DOI] [PubMed] [Google Scholar]
- Lucy SD, Kowalchuk JM, Hughson RL, Paterson DH, Cunningham DA. Blunted cardiac autonomic responsiveness to hypoxemic stress in healthy older adults. Can J Appl Physiol. 2003;28:518–535. doi: 10.1139/h03-040. [DOI] [PubMed] [Google Scholar]
- Malliani A, Montano N. Heart rate variability as a clinical tool. Ital Heart J. 2002;3:439–445. [PubMed] [Google Scholar]
- Mandelbrot BB, Van Ness JW. Fractional brownian motions, fractional noises and applications. SIAM Rev. 1968;10:422–437. [Google Scholar]
- Merati G, Di Rienzo M, Parati G, Veicsteinas A, Castiglioni P. Assessment of the autonomic control of heart rate variability in healthy and spinal-cord injured subjects: contribution of different complexity-based estimators. IEEE Trans Biomed Eng. 2006;53:43–52. doi: 10.1109/TBME.2005.859786. [DOI] [PubMed] [Google Scholar]
- Meyer M, Stiedl O. Self-affine fractal variability of human heartbeat interval dynamics in health and disease. Eur J Appl Physiol. 2003;90:305–316. doi: 10.1007/s00421-003-0915-2. [DOI] [PubMed] [Google Scholar]
- Mourot L, Bouhaddi M, Gandelin E, Cappelle S, Nguyen NU, Wolf JP, Rouillon JD, Hughson R, Regnard J. Conditions of autonomic reciprocal interplay versus autonomic co-activation: effects on non-linear heart rate dynamics. Auton Neurosci. 2007;137:27–36. doi: 10.1016/j.autneu.2007.06.284. [DOI] [PubMed] [Google Scholar]
- Naschitz JE, Sabo E, Naschitz S, Rosner I, Rozenbaum M, Priselac RM, Gaitini L, Zukerman E, Yeshurun D. Fractal analysis and recurrence quantification analysis of heart rate and pulse transit time for diagnosing chronic fatigue syndrome. Clin Auton Res. 2002;12:264–272. doi: 10.1007/s10286-002-0044-8. [DOI] [PubMed] [Google Scholar]
- Peng CK, Mietus J, Haussdorf JM, Havlin S, Stanley HE, Goldberger AL. Long-range anticorrelations and non-Gaussian behaviour of the heartbeat. Phys Rev Lett. 1993;70:1343–1349. doi: 10.1103/PhysRevLett.70.1343. [DOI] [PubMed] [Google Scholar]
- Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- Perkiomaki JS, Zareba W, Badilini F, Moss AJ. Influence of atropine on fractal and complexity measures of heart rate variability. Ann Noninvasive Electrocardiol. 2002;7:326–331. doi: 10.1111/j.1542-474X.2002.tb00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepoli MF, Capucci A. Autonomic nervous system in the genesis of arrhythmias in chronic heart failure: implication for risk stratification. Minerva Cardioangiol. 2007;55:325–333. [PubMed] [Google Scholar]
- Pilgram B, Kaplan DT. Nonstationarity and 1/f noise characteristics in heart rate. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1–R9. doi: 10.1152/ajpregu.1999.276.1.R1. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation. 1993;87:1435–1441. doi: 10.1161/01.cir.87.5.1435. [DOI] [PubMed] [Google Scholar]
- Ponard JG, Kondratyev AA, Kucera JP. Mechanisms of intrinsic beating variability in cardiac cell cultures and model pacemaker networks. Biophys J. 2007;92:3734–3752. doi: 10.1529/biophysj.106.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta A, Guzzetti S, Montano N, Furlan R, Pagani M, Malliani A, Cerutti S. Entropy, entropy rate, and pattern classification as tools to typify complexity in short heart period variability series. IEEE Trans Biomed Eng. 2001;48:1282–1291. doi: 10.1109/10.959324. [DOI] [PubMed] [Google Scholar]
- Priestley MB, Subba Rao T. A test for stationarity of time series. J Royal Stat Soc B. 1969;31:140–149. [Google Scholar]
- Ribeiro AL, Lombardi F, Sousa MR, Lins Barros MV, Porta A, Costa VB, V, Gomes ME, Santana MF, Otavio Costa Rocha M. Power-law behaviour of heart rate variability in Chagas’ disease. Am J Cardiol. 2002;89:414–418. doi: 10.1016/s0002-9149(01)02263-9. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Sandercock GR, Bromley PD, Brodie DA. The reliability of short-term measurements of heart rate variability. Int J Cardiol. 2005;103:238–247. doi: 10.1016/j.ijcard.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology. 1999;92:79–84. doi: 10.1159/000006952. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Finlayson HC, Hughson RL. Physical activity is a major contributor to the ultra low frequency components of heart rate variability. Heart. 1999;82:e9. doi: 10.1136/hrt.82.6.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorini MG. Nonlinear analysis of heart rate variability signal: physiological knowledge and diagnostic indications. Conf Proc IEEE Eng Med Biol Soc. 2004;7:5407–5410. doi: 10.1109/IEMBS.2004.1404511. [DOI] [PubMed] [Google Scholar]
- Stein PK, Le Q, Domitrovich PP. Development of more erratic heart rate patterns is associated with mortality post-myocardial infarction. J Electrocardiol. 2008;41:110–115. doi: 10.1016/j.jelectrocard.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struzik ZR, Hayano J, Sakata S, Kwak S, Yamamoto Y. 1/f scaling in heart rate requires antagonistic autonomic control. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:050901. doi: 10.1103/PhysRevE.70.050901. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Teich MC, Lowen SB, Jost BM, Vibe-Rheymer K. Heart rate variability: Measures and models. In: Akay M, editor. Nonlinear Biomedical Signal Processing, vol. II, Dynamic Analysis and Modeling. New York: IEEE Press; 2001. pp. 159–213. [Google Scholar]
- Tuck ML. The sympathetic nervous system in essential hypertension. Am Heart J. 1986;112:877–886. doi: 10.1016/0002-8703(86)90497-7. [DOI] [PubMed] [Google Scholar]
- Tulppo MP, Makikallio TH, Seppanen T, Shoemaker K, Tutungi E, Hughson RL, Huikuri HV. Effects of pharmacological adrenergic and vagal modulation on fractal heart rate dynamics. Clin Physiol. 2001;21:515–523. doi: 10.1046/j.1365-2281.2001.00344.x. [DOI] [PubMed] [Google Scholar]
- Vigo DE, Castro MN, Dorpinghaus A, Weidema H, Cardinali DP, Siri LN, Rovira B, Fahrer RD, Nogues M, Leiguarda RC, Guinjoan SM. Nonlinear analysis of heart rate variability in patients with eating disorders. World J Biol Psychiatry. 2007:1–7. doi: 10.1080/15622970701261604. [DOI] [PubMed] [Google Scholar]
- Willson K, Francis DP, Wensel R, Coats AJ, Parker KH. Relationship between detrended fluctuation analysis and spectral analysis of heart-rate variability. Physiol Meas. 2002;23:385–401. doi: 10.1088/0967-3334/23/2/314. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Fortrat JO, Hughson RL. On the fractal nature of heart rate variability in humans: effects of respiratory sinus arrhythmia. Am J Physiol Heart Circ Physiol. 1995a;269:H480–H486. doi: 10.1152/ajpheart.1995.269.2.H480. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hughson RL. Coarse-graining spectral analysis: new method for studying heart rate variability. J Appl Physiol. 1991;71:1143–1150. doi: 10.1152/jappl.1991.71.3.1143. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hughson RL. On the fractal nature of heart rate variability in humans: effects of data length and β-adrenergic blockade. Am J Physiol Regul Integr Comp Physiol. 1994;266:R40–R49. doi: 10.1152/ajpregu.1994.266.1.R40. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hughson RL, Peterson JC. Autonomic control of heart rate during exercise studied by heart rate variability spectral analysis. J Appl Physiol. 1991;71:1136–1142. doi: 10.1152/jappl.1991.71.3.1136. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nakamura Y, Sato H, Yamamoto M, Kato K, Hughson RL. On the fractal nature of heart rate variability in humans: effects of vagal blockade. Am J Physiol Regul Integr Comp Physiol. 1995b;269:R830–R837. doi: 10.1152/ajpregu.1995.269.4.R830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.