Abstract
The renal kallikrein–kinin system is involved in the control of the intrarenal circulation and arterial pressure but bradykinin (Bk) effects on perfusion of individual kidney zones have not been examined in detail. Effects of Bk infused into renal artery, renal cortex or medulla on perfusion of whole kidney (RBF, renal artery probe) and of the cortex, outer- and inner medulla (CBF, OMBF, IMBF: laser-Doppler fluxes), were examined in anaesthetized rats. Renal artery infusion of Bk, 0.3–0.6 mg kg−1 h−1, induced no sustained increase in RBF or CBF. OMBF and IMBF increased initially 6 or 16%, respectively; only the IMBF increase (+10%) was sustained. Pre-treatment with l-NAME, 2.4 mg kg−1i.v., prevented the sustained but not initial transient elevation of medullary perfusion. Intracortical Bk infusion, 0.75–1.5 mg kg−1 h−1, did not alter RBF or CBF but caused a sustained 33% increase in IMBF. Intramedullary Bk, 0.3 mg kg−1 h−1, did not alter RBF or CBF but caused sustained increases in OMBF (+10%) and IMBF (+23%). These responses were not altered by pre-treatment with 1-aminobenzotriazole, 10 mg kg−1i.v., a cytochrome P-450 (CYP450) inhibitor, but were prevented or significantly attenuated by l-NAME or intramedullary clotrimazole, 3.9 mg kg−1 h−1, an inhibitor of CYP450 epoxygenase and of calcium-dependent K+ channels (KCa). Thus, cortical vasodilatation induced by Bk is only transient so that the agent is unlikely to control perfusion of the cortex. Bk selectively increases perfusion of the medulla, especially of its inner layer, via activation of the NO system and of KCa channels.
Bradykinin (Bk), a major and functionally most important member of the kinin family, has two sources: it is both a paracrine agent generated by the tissue kallikrein–kinin system and a product of plasma kallikrein. Under most conditions it acts by stimulation of widely distributed constitutive B2 receptors, which leads to activation of mediating systems, mostly of the NO cascade and the still elusive endothelium-derived hyperpolarizing factor (EDHF) (Clements, 2000; Imig, 2005; Moreau et al. 2005; Fleming & Busse, 2006). In the kidney, Bk modifies tubular transport and causes intrarenal vasodilatation (Vio et al. 1992; Navar et al. 1996; Imig, 2005). The latter action appears to be functionally important for modulation by Bk of autoregulation of renal blood flow and is postulated to be involved in the control of arterial pressure (Katori & Majima, 1996; Navar et al. 1996; Nafz et al. 1998; Tornel et al. 2000).
Considering the probable role of the medullary circulation in the long-term control of arterial pressure (Mattson, 2003; Bergström & Evans, 2004; Cowley, 2008), in the past two decades the main interest in renal vascular effects of Bk was focused on its influence on perfusion of the medulla. While the vasodilator influence of Bk in this kidney zone seems clearly established, its possible contribution to the control of perfusion of the cortex or of the whole kidney remains uncertain. The relevant data, especially those from pharmacological studies using bolus injections or very short infusions (Braun et al. 1997; Dabisch et al. 2004) provide no information regarding the stability or transiency of the response. Remarkably, quite different responses to bolus injections as opposed to infusions of Bk were reported (Hoagland et al. 1999a).
In order to re-evaluate the intrarenal vascular action of Bk in different zones of the kidney, in the present study we compared the effects obtained with infusions of the agent into the renal artery and direct infusions into the renal cortex or renal medulla. Simultaneous measurements were conducted of the perfusion of the renal cortex as well as, separately, of the outer and inner medulla. Since quite often the responses to Bk tended to wane, we always continued the infusions until renal perfusion parameters stabilized at a new level. This helped distinguish between the transient and sustained phases of the response.
The long-established knowledge of the role of NO as a mediator of Bk-induced vasodilatation has been more recently supplemented by the sound evidence that non-NO mediation, by multiple factors described as EDHF, is also involved and can be of comparable importance (Roman, 2002; Imig, 2005; Moreau et al. 2005). In the present study, we re-examined the mediatory role of NO and EDHF, in order to characterize the contribution of either factor to the transient and sustained phases of post-Bk vasodilatation. In studies involving inhibition of NO synthesis, precautions were taken to prevent the associated depression of baseline renal haemodynamics. In EDHF studies we focused on its final common pathway of action i.e. the status of calcium-dependent K+ channels and, in addition, examined the contribution of vasoactive products of CYP450-dependent enzymes.
Methods
The experimental procedures were approved by the extramural IV Local Ethical Committee (Warsaw); the standards and regulations used are equivalent to those in the UK (Drummond, 2009). Experiments were designed to examine effects of infusion of bradykinin (Bk, Sigma Chemical Co., St Louis, MO, USA) into the left renal artery or renal medullary or cortical interstitium on ipsilateral total and regional perfusion of the kidney. Effects of bradykinin were also examined in rats pre-treated with agents that were likely to modify the response.
Male Wistar rats weighing 280–330 g, maintained on dry pellet diet and given free access to water, were anaesthetised with intraperitoneal thiopentobarbital (Thiopental, Biochemie GmbH, Vienna, Austria) (100 mg (kg body weight)−1). The experimental techniques, procedures and measurements, including that of the renal artery blood flow (RBF) and laser-Doppler fluxes in the superficial renal cortex (CBF) and in the outer and inner medulla (OMBF and IMBF), were described in detail previously (Bądzyńska et al. 2003; Bądzyńska & Sadowski, 2008). The laser-Doppler flux is a product of the number of blood cells moving and of their mean velocity; for brevity, and in agreement with the accepted usage, the abbreviations used (CBF, OMBF and IMBF) denote fluxes and not flows. The CBF probe (0.5 mm in diameter) was placed on the kidney surface, and OMBF and IMBF probes (0.4 mm in diameter) were placed at depths of 3 and 5 mm from the surface, respectively. In some series, CBF measurements had to be discarded because of problems with probe calibration; in these cases, RBF was taken as a measure of whole cortical perfusion. Earlier studies have shown effectiveness of vasodilator agents infused into the inner medulla of the rat kidney (Lu et al. 1992), in contrast to doubtful value of the procedure in the rabbit (Kalyan et al. 2002). The cannulas for renal artery, intracortical and intramedullary infusions were all 32G.
Experimental infusion protocols
Renal artery infusions of bradykinin (Bk)
(1) In untreated rats (n = 19). Bk dissolved in isotonic saline was infused at a rate of 0.3–0.6 mg kg−1 h−1 until renal perfusion parameters stabilized at a new level; this occurred usually within 20–30 min of the infusion. After termination of the infusion, 20–30 min recovery observations were made. The Bk dose used had no significant effect on mean arterial pressure (MAP). In preliminary experiments, a 5-fold greater Bk dose (1.5 mg kg−1 h−1) was also tested: it still did not affect MAP but the renal haemodynamic responses to Bk were less pronounced than with the smaller, ultimately accepted dose.
(2) In rats pre-treated with intravenous l-NAME (n = 6). Before Bk administration, the rats received l-NAME (Nω-nitro-l-arginine methyl ester, Sigma, Switzerland), 2.4 mg kg−1 infused i.v. during 10 min.
(3) In rats pre-treated with intravenous l-AME and receiving SNP (n= 11). To attenuate the decrease in baseline renal perfusion which follows l-NAME administration, sodium nitroprusside (sodium nitroferricyanide(III), SNP, Sigma-Aldrich) was co-infused with Bk into the renal artery, at about 0.6 mg kg−1 h−1. This manoeuvre was successfully used to nullify such disturbing effects of l-NAME in studies of the responses to Bk in the rat hindlimb vascular bed (Dabisch et al. 2004).
Renal interstitial infusions of Bk
(1) In untreated rats (n = 15)
Bk was infused into the renal cortical tissue (n= 8) via two cannulas placed in the renal cortex, at a total rate of 0.75–1.5 mg kg−1 h−1 (2 ml h−1). The stabilization of perfusion parameters occurred after 30–50 min.
Bk was infused into the medulla (n= 15) at 0.3 mg kg−1 h−1 (1 ml h−1) using a single cannula with the tip placed at or just beneath the outer–inner medullary border. In a parallel series, an inhibitor of bradykinin B2 receptors, HOE 140 (icatibant acetate, Sigma), was infused into the medulla at 0.12 mg kg−1 h−1.
(2) In rats treated with intravenous or intramedullary l-NAME, ABT or clotrimazole. In separate groups, intramedullary infusions of Bk as described above were conducted in rats pre-treated with intravenous l-NAME (2.4 mg kg−1; n= 11), rats receiving l-NAME infusion into the medulla, simultaneously with Bk, at a rate of 0.12 mg kg−1 h−1 (n= 12), and rats pre-treated with intravenous 1-aminobenzatriole (ABT, Fluka, Buchs, The Netherlands) (10 mg kg−1 infused during 10 min; n= 6). In another group (n= 13), we examined the responses to Bk of rats receiving clotrimazole (Sigma-Aldrich). To increase solubility, the substance was transformed into clotrimazole hydrochloride, and 100 μl of 40% 2-hydroxypropyl-β-cyclodextrin (Sigma) was added per 6 ml of saline solvent. Clotrimazole. HCl was infused into the medulla at 3.9 mg kg−1 h−1 (1.5 ml h−1) for about 30 min, which per se did not affect renal perfusion parameters. Subsequently, Bk infusion was started at the usual rate (1 ml h−1) while clotrimazole infusion rate and its solvent infusion rate was reduced to one-third of the initial value, to keep the total volume of the infusion constant at 1.5 ml min−1. During the post-Bk recovery period, the initial clotrimazole infusion rate was restored.
After termination of experiments, the rats were killed with a lethal intravenous dose of sodium thiopenthal. The position of the intrarenal probes and of the infusion needles was checked at the kidney's cross-section.
Statistics
The differences between means were evaluated by repeated measures analysis of variance and by Student's paired t test. For inter-group comparison, the classical one-way ANOVA was used, followed by modified Student's t statistics; Bonferroni's correction was used in case of multiple comparisons. The standard error of the mean (s.e.m.) was used as a measure of data dispersion, and P < 0.05 was accepted as the significance level.
Results
Renal artery infusions of bradykinin
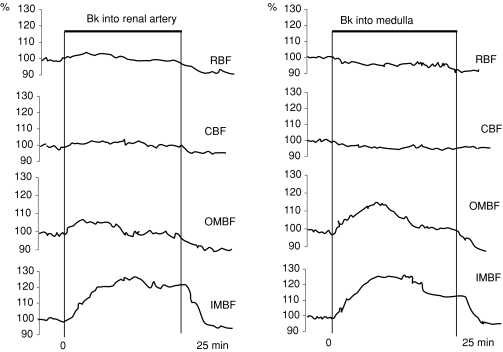
(1) In untreated rats. Bk infusions did not alter MAP. An example of renal haemodynamic responses to Bk is given in the left panel of Fig. 1. It is seen that the responses of RBF and OMBF showed two phases: an initial increase followed by a decrease to baseline; for IMBF the second phase was slightly below the initial phase level. The results are summarized in Fig. 2 (top panel) which shows the averaged values, separately for the initial 10–12 min and the final level. There was an initial 6% increase in RBF, followed by a decrease below baseline whereas CBF did not change at all. OMBF and IMBF increased initially 6% and 16%, respectively. This was followed by a decrease to baseline in the former and by a decrease to a level 10% above baseline in the latter, which was still significantly different from control. In summary, effects of Bk on cortical perfusion (RBF, CBF) and on OMBF, if any, were modest and transient. A sustained increase in perfusion was seen for IMBF only.
Figure 1. Two sample experiments showing intrarenal perfusion responses to bradykinin.
Bradykinin was infused into the renal artery (0.6 mg kg−1 h−1) or into the renal medullary tissue (0.3 mg kg−1 min−1). RBF – total renal blood flow measured by renal artery (Transonic) probe; CBF, OMBF, IMBF – laser-Doppler fluxes measured on kidney surface, and in the outer and inner medulla, respectively.
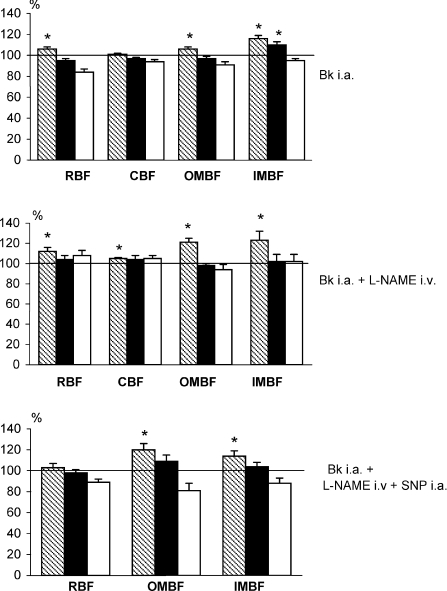
Figure 2. Intrarenal perfusion responses to renal artery infusion of bradykinin.
Bradykinin was infused into the left renal artery (0.3 mg kg−1 min−1) of rats that were either untreated (top, n= 19), pre-treated with l-NAME (middle, n= 6), or with l-NAME combined with renal arterial infusion of sodium nitropruside (SNP, bottom, n= 10–12). Means (%) ±s.e.m. The striped, black and open columns refer to the initial phase of Bk infusion, the final level established during the infusion, and the recovery level after Bk has been withdrawn, respectively. l-NAME was given i.v. as a bolus of 0.6 mg kg−1, followed by infusion at 2.4 mg kg−1 h−1. SNP was given at 0.6 mg kg−1 h−1 into the renal artery. In the l-NAME + SNP group, CBF measurements had to be discarded because of technical problems. Denotations as in Fig. 1. *Significantly higher than pre-bradykinin baseline as tested by paired Student's t test.
(2) In rats pre-treated with intravenous l-NAME. Under l-NAME treatment the initial post-bradykinin increases in RBF, OMBF and IMBF, as seen without pre-treatment, appeared enhanced (Fig. 2, middle panel). However, in the second phase all the perfusion parameters decreased to baseline despite continued infusion. Thus, there was no sustained increase in perfusion of the inner medulla. These results should be interpreted with caution because intravenous l-NAME substantially decreased baseline perfusion parameters and increased arterial blood pressure. Therefore another group of l-NAME-treated rats received, in addition, a renal artery infusion of sodium nitroprusside (SNP), which largely improved baseline perfusion and also reduced the increase in blood pressure (Table 1).
Table 1.
Basal values of MAP and renal perfusion parameters (measured before infusion of bradykinin, pooled data from various groups)
| No treatment (31–39) | l-NAME i.v. (14–19) | l-NAME i.m. (12) | l-NAME i.v.+ SNP i.a. (10) | |
|---|---|---|---|---|
| MAP (mmHg) | 114 ± 2 | 123 ± 2 | 123 ± 3 | 117 ± 2 |
| RBF (ml min−1) | 8.4 ± 0.4 | 4.1 ± 0.2 | 8.1 ± 0.5 | 7.6 ± 0.3 |
| CBF (PU) | 566 ± 15 | 399 ± 13 | – | – |
| OMBF (PU) | 174 ± 7 | 90 ± 3 | 170 ± 18 | 140 ± 7 |
| IMBF (PU) | 141 ± 5 | 105 ± 6 | 132 ± 12 | 120 ± 7 |
(3) In rats receiving intravenous l-NAME and SNP. In this group there was no initial post-bradykinin increase in RBF (Fig. 2, bottom panel). This suggests that the 50% reduction of the baseline value after l-NAME given alone (Table 1) was a necessary condition for this increase. On the other hand, the increases in OMBF and IMBF were significant but limited to the initial phase, similar to those seen with the treatment with l-NAME alone (Fig. 2, middle panel). Overall, l-NAME given with or without SNP did not interfere with the initial transient post-bradykinin increase in medullary perfusion (OMBF and IMBF) but prevented the sustaineds increase.
Since we thought that intravascular route of administration may not be appropriate for Bk, a paracrine agent synthetised largely in the tissue, experiments were performed using infusions into the renal cortex or medulla.
Renal interstitial infusions of bradykinin
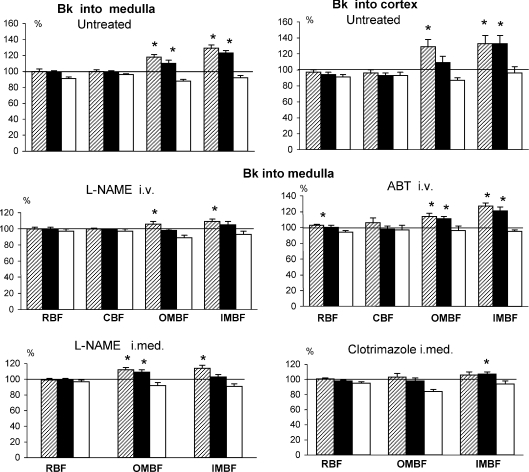
(1) In untreated rats. Cortical interstitial infusions of Bk did not alter RBF or CBF. By contrast, it caused an increase in perfusion of the medulla, which was substantial (+24%) but transient for OMBF, and even more substantial (+28%) but sustained for IMBF (Fig. 3, right upper panel).
Figure 3. Intrarenal perfusion responses to bradykinin infused into the renal medullary or cortical interstitium.
Bradykinin (Bk) was infused into the renal cortex, 0.75–1.50 mg kg−1 h−1 (right hand upper panel) or into the medulla, 0.3–0.6 mg kg−1 h−1 (all the other panels). Means (%) ±s.e.m. The striped, black and open columns refer to the initial phase of Bk infusion, the final level established during the infusion, and the recovery level after Bk has been withdrawn, respectively. The rats were either untreated (n= 15 for intramedullary and n= 8 for intracortical Bk) or pre-treated with l-NAME (2.4 mg kg−1 h−1i.v., n= 11, or 0.12 mg kg−1 h−1 into renal medulla, n= 12), ABT (10 mg kg−1i.v., n= 12) or clotrimazole (3.6 mg kg−1 h−1 into renal medulla, n= 13). In two groups, CBF measurements had to be discarded because of technical problems. *Significantly different from pre-bradykinin baseline as tested by paired Student's t test.
A sample response to intramedullary Bk is shown in the right hand panel of Fig. 1. Compared to effects of renal artery infusions, the initial increase in IMBF was followed by a more substantial decrease in the second phase. Mean responses to Bk for all groups are given in Fig. 3. In untreated rats, intramedullary Bk did not alter RBF or CBF but there was a significant increase in medullary perfusion, both OMBF and IMBF. Intramedullary infusion of bradykinin B2 receptor inhibitor (HOE140) induced significant but minor (less than 1%) decreases in OMBF and IMBF without changing RBF (data not shown). In summary, renal interstitial infusions of Bk did not increase the whole kidney or cortical blood flow, in contrast to a modest transient increase observed with renal artery infusion (Fig. 2). In the medulla, the increases were sustained not only for IMBF but also for OMBF, even though both parameters were lower in the second than in the first phase of Bk infusion.
(2) In rats treated with intravenous or intramedullary l-NAME, intravenous ABT or intramedullary clotrimazole. The treatment with intravenous l-NAME tended to attenuate the initial transient increases in OMBF and IMBF and virtually abolished the sustained (second phase) increase in IMBF. However, with intravenous l-NAME the baseline perfusion of the kidney was greatly reduced (Table 1), which might per se influence the response to Bk. Intramedullary infusion of l-NAME was associated with only minor, if any, reduction of baseline renal perfusion (Table 1). The response to Bk was similar to that seen with intravenous l-NAME, with one exception: the elevation of OMBF remained significant in the second phase of Bk infusion. Taken together, l-NAME treatment abolished or attenuated the Bk-dependent sustained increases in OMBF and IMBF; however, a significant transient elevation of the perfusion was maintained.
In rats treated with intravenous ABT, the Bk-induced increases in renal medullary perfusion were quite comparable with those observed in untreated rats. The increase in RBF was significant but minor (+3%).
Intramedullary clotrimazole greatly attenuated or abolished the Bk-induced increase in medullary perfusion; however, a modest 6% elevation of IMBF (compared to +23% in untreated rats) persisted and was significant in the second phase of infusion (P= 0.043).
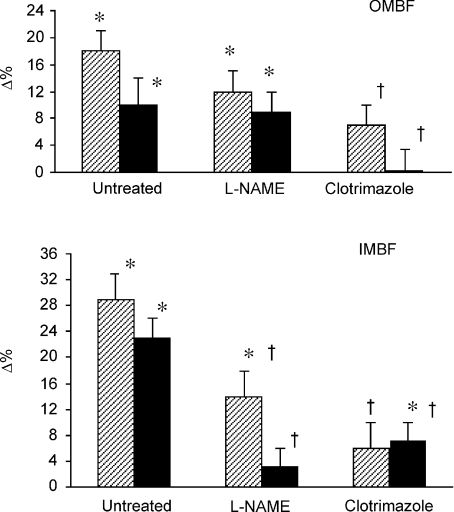
Overall, l-NAME and especially clotrimazole treatment greatly attenuated or abolished the response of medullary perfusion to Bk. The ultimate statistical evaluation of inter-group differences was performed on three groups of rats: untreated, treated with intramedullary l-NAME and treated with clotrimazole. The ABT group was excluded because this treatment was clearly without effect. The group of rats treated with intravenous l-NAME was excluded because this treatment induced profound changes in baseline perfusion, which per se may have been a source of independent inter-group variability. The classical one-way ANOVA was used for evaluation of both absolute and per cent post-bradykinin changes, separately for OMBF and IMBF and separately for the initial and second-phase (sustained) responses. ANOVA showed that the treatments (l-NAME, clotrimazole) significantly altered the responses to Bk, with one exception. The OMBF response to Bk in the second phase of Bk infusion was significantly altered by the treatments when absolute values were considered (F= 3.33, P= 0.047) but not for the data expressed as a percentage of control values (F= 1.99, P < 0.15). Figure 4 compares the Bk-induced increases (Δ%) in OMBF and IMBF of three groups of rats: untreated, treated with intramedullary l-NAME and those treated with intramedullary clotrimazole. Also shown are the results of Student's t test for independent variables, used for two inter-group comparisons (untreated vsl-NAME and untreated vs clotrimazole, the required significance level after Bonferroni's correction: P < 0.025). It is seen that intramedullary l-NAME did not significantly affect the response of OMBF but did reduce the response of IMBF. On the other hand, clotrimazole almost abolished the responses to Bk of both OMBF and IMBF.
Figure 4. A summary of the effects of intramedullary bradykinin on outer- and inner medullary perfusion.
Means (Δ%) ±s.e.m. for rats that were either untreated or treated with intramedullary l-NAME or intramedullary clotrimazole (doses and n values as described for Fig. 3). The striped and black columns refer to the initial phase of Bk infusion and the final level established during the infusion, respectively. *Significantly different from pre-bradykinin baseline as tested by paired t test. †Significantly different from the corresponding change in untreated rats, as tested by unpaired Student's t test.
Discussion
In order to characterise more clearly the influence of Bk on renal regional perfusion, we compared effects seen with different routes of administration: into the renal artery, and into the renal cortical or renal medullary interstitium. With all these routes, systemic vasodilatation and the effect on arterial blood pressure are nullified or minimized. Renal artery infusions result in uniform distribution of the agent throughout the kidney. However, intravascular delivery may be optimal in the case of circulating vasoactive substances, possibly also of Bk generated by plasma kallikrein, whereas paracrine agents synthesised in the tissue reach the vascular smooth muscle from the side of the interstitium. Thus, infusions directly into the tissue (interstitium) may be thought to better mimick Bk diffusion from the major sites of synthesis, e.g. in the cells of the renal connecting or distal tubule (Vio et al. 1992; Clements, 2000). An appropriate alternative to renal artery infusions is administration of agents into the renal cortical interstitium. We showed recently that PGE2 infused into the renal cortex was distributed and acted throughout the kidney, in the same way as seen with renal artery infusions (Bądzyńska & Sadowski, 2008).
Does Bk participate in the control of cortical or whole kidney perfusion?
Due to the increasing interest in the potential contribution of kinins to the control of arterial pressure (Katori & Majima, 1996; Navar et al. 1996; Nafz et al. 1998; Tornel et al. 2000) and considering the postulated role of the renal medulla in this control (Mattson, 2003; Bergström & Evans, 2004; Cowley, 2008), during the past two decades attention was focused on Bk effects on perfusion of the renal medulla (Navar et al. 1996; Clements, 2000; Tornel et al. 2000). However, there is ample evidence indicating that kinins may affect vessel tone throughout the kidney.
Numerous reports from whole kidney studies indicated that exogenous Bk increased total renal blood flow (Webster & Gilmore, 1964; Flamenbaum et al. 1979; Yun et al. 1982; Granger & Hall, 1985; Matsumura et al. 1999; Patel & Smith, 2000) and similar results were reported from experiments with the isolated perfused rat kidney (Gardes et al. 1990; Fulton et al. 1992; Bagate et al. 1997; Pompermayer et al. 2002; Wang et al. 2003). Vasodilator effects of Bk were also demonstrated in isolated preparations of vessels (especially of the glomerular arterioles) that were as a rule harvested from the renal cortex and not from its juxtamedullary layer (Edwards, 1985; Yu et al. 1998; Ren et al. 2002; Wang et al. 2005). The conclusions derived from studies of whole kidney perfusion or studies of isolated outer- or midcortical vessels were usually extrapolated to the vessels determining perfusion of the medulla. The studies showing specifically juxtamedullary arteriolar vasodilatation (Imig et al. 2001) or dilatation of isolated outer medullary descending vasa recta (OMDVR) (Pallone et al. 1998) were relatively few in number.
The vast functional evidence on the action of kinins on perfusion of the whole kidney agrees well with the multiple data indicating the presence of bradykinin B2 receptors throughout the cortex (Vio et al. 1992); taken together these data indicate that the renal cortical vasculature has the potential to respond to Bk. On the other hand, it can be doubted whether Bk, an agent synthesised mostly in the connecting tubule and collecting duct cells, can access the vasculature in the outer or middle cortex. It could also be speculated that the cortical vasculature is affected by Bk generated by plasma kallikrein. Given the uncertainty regarding the genuine role of Bk in the control of intrarenal regional circulation, we thought that examination of the effects obtained with the delivery of the agent to different tissue compartments and to different regions of the kidney might help clarify some fundamental questions.
We found that increases in whole kidney or cortical perfusion induced by Bk were modest and subsided despite continued infusion. Notably, the increases occurred exclusively with renal artery infusion of Bk while intracortical (or intramedullary) infusion was without effect. This finding agrees with the observation that the isolated glomerular afferent arterioles dilated in response to Bk applied from the luminal but not from the perivascular side (Yu et al. 1998). The transiency of Bk-induced vasodilatation is probably not uncommon but was rarely reported (Wang et al. 2003); many experimental protocols, as those using bolus injections, precluded its detection. On the other hand, we saw that renal artery infusion of Bk induced a distinct sustained increase in inner medullary perfusion.
The failure of Bk infused into the renal artery or into the interstitium of the cortex to induce a sustained increase in cortical perfusion suggests that the endogenous kinin, even if it accessed cortical microvessels, be it from the luminal or interstitial side, would not affect local circulation in a sustained manner. An elevation of plasma Bk which follows renal artery infusion might mimic increased release of the agent by plasma kallikrein, e.g. in inflammatory conditions; however, since the vasodilatation was minor and only transient, such an effect is also unlikely to be of functional significance. It could be suspected that at concentrations higher than those induced in this study, Bk could still lead to sustained cortical vasodilatation. However, we saw that increasing the dose 5-fold tended to reduce rather than increase vasodilator responses to renal artery infusions of Bk (data not shown). Others have reported that after increasing Bk dose, renal vasoconstriction instead of vasodilatation was seen (Yu et al. 1998; Hoagland et al. 1999b).
The background of the waning of the vasodilator response to Bk (especially in the cortex), as seen after renal artery infusions, is unclear. When activated by an agonist, rapid desensitization of bradykinin B2 receptor has been described (Moreau et al. 2005). Alternatively, poor response of paracrine systems known to mediate the vasodilatation, such as NO or EDHF, should be considered. The rate of synthesis and the amount of bioavailable NO, a major mediator of Bk action, is much smaller in the cortex than in the medulla and its rapid exhaustion can be imagined and could account for the disappearance of Bk-induced vasodilatation. Our results of renal artery Bk infusions under NO blockade do not support this interpretation. Unlike NO, epoxyeicosatrienoic acids (EETs) are abundant in the rat cortex (Roman, 2002; Moreau et al. 2005) and have established vasodilator potency. In the rabbit cortex these epoxygenase products effectively oppose vasoconstrictor influence of vasopressin V1 receptor stimulation (Rajapakse et al. 2004). Thus, paucity of EETs is an unlikely mechanism of the waning of the response. In a recent study, we found a transient cortical vasodilatation in response to renal artery infusion of prostaglandin E2, followed by sustained decrease in cortical perfusion (Bądzyńska & Sadowski, 2008). Apparently, transient cortical vascular responses to vasodilators are not uncommon, hence the rationale of using prolonged infusions rather than bolus injections. In general, smaller responses to vasodilator agents in the cortical compared to the medullary circulation are not surprising; possible reasons for this phenomenon have been discussed in detail (Evans et al. 2004).
A decrease in cortical or whole kidney perfusion after blockade of B2 receptors would provide an indication of the agent's tonic vasodilator influence in the cortex. However, systemic administration of a kinin antagonist decreased renal papillary blood flow without affecting outer cortical flow or glomerular filtration rate (Roman et al. 1988). Decreases in the total or cortical blood flow after inhibition of B2 receptors were seen only when basal activity of the tissue kinin–kallikrein system had been increased, e.g. by sodium restriction or pre-treatment with inhibitors of kininase II (i.e angiotensin converting enzyme) (Navar et al. 1996; Siragy et al. 1997). It will be noticed that after such inhibition an increase in tissue Bk was only modestly greater in the medulla than in the cortex (Matsuda et al. 1999). Thus, it is likely that Bk participates in the control of the renal cortical circulation under such unusual conditions.
Bk effects on medullary perfusion. Mediatory role of NO, CYP450 and KCa channels
Increases in medullary perfusion in response to Bk were demonstrated with all the three administration routes. They were always sustained in the case of IMBF whereas OMBF remained elevated with intramedullary but not with renal artery or intracortical infusion. In agreement with our observations, intramedullary Bk was reported to increase perfusion of the exposed renal papilla in Munich–Wistar rats (Mattson & Cowley, 1993; Tornel et al. 2000).
It is not clear why Bk had a greater or/and more persistent effect on perfusion of the inner compared to outer medulla, as observed with all the three infusion routes. Given the uncertainty regarding the relative importance of juxtamedullary glomerular arterioles and OMDVR as determinants of perfusion of either sub-zone (Evans et al. 2004), and in the absence of the knowledge of the post-infusion distribution and concentration of Bk in the kidney, speculations based on perfusion measurements alone are unwarranted.
l-NAME pre-treatment did not abolish the initial phase of the post-Bk increase in medullary perfusion, which suggests that this transient and perhaps non-specific response was not or was only in part mediated by NO. On the other hand, the sustained phase of the increase was almost always abolished by l-NAME. There was only one exception: in rats receiving l-NAME infusion into the medulla (but not those given l-NAME i.v.), the OMBF elevation persisted throughout Bk infusion (Figs 3 and 4). Possibly, with infusion into the outer–inner medullary border area, l-NAME did not access the juxtamedullary glomerular arterioles and these vessels were able to release NO in response to Bk. On the whole, our results show that Bk induces a stable increase in the perfusion of the inner medulla and that this action is largely mediated by NO. This suggests that NO mediates the prolonged tonic influence of the kinin while it does not participate in the initial phase of vasodilatation.
There is vast evidence that Bk action is mediated not only by NO but also by EDHF; the latter term is used to describe multiple factors, such as vasodilator EETs, the products of CYP450-dependent epoxygenase (Fulton et al. 1992; Imig et al. 2001; Ren et al. 2002; Imig, 2005; Fleming & Busse, 2006). EETs induce vasodilatation via hyperpolarization and relaxation of vascular smooth muscle cells secondary to activation of Ca2+- dependent K+ channels (KCa) (Fleming & Busse, 2006). To explore the role of EETs in the Bk-induced vasodilatation we re-examined effects of Bk after treatment with ABT, a CYP450 inhibitor that was shown to inhibit both 20-hydroxyeicosatetraenoic acid (20-HETE) and EET synthesis in normal rats (Maier et al. 2000). We chose to first apply non-specific inhibition of CYP450 rather than selective inhibition of EETs synthesis because many of the established inhibitors of epoxygenase (especially antimycotic imidazole derivatives) are in fact direct inhibitors of KCa channels. We found that ABT treatment did not alter the vasodilatation induced by Bk. The dosage used was previously found to significantly decrease RBF in Wistar rats maintained both on standard and on a high-sodium diet (E Kompanowska-Jezierska & M Kuczeriszka unpublished results from our laboratory), suggesting inhibition of vasodilator EETs. However, considering that acute ABT administration may not have sufficiently depleted tissue stores of CYP450-dependent epoxygenase and without direct demonstration of the disappearance of EETs from the tissue, the data are not entirely conclusive.
In a separate experimental series, we found that clotrimazole, an inhibitor of CYP450 epoxygenase but also a potent direct inhibitor of KCa channels (Wu et al. 1999), almost totally abolished (OMBF) or greatly reduced (IMBF) the medullary vascular response to Bk, both its initial and sustained phase (Figs 3 and 4). This indicates that Bk induces vasodilatation by smooth muscle relaxation secondary to activation of KCa channels; to what extent this is mediated by EETs remains uncertain (see above). Imig and co-workers (2001) reported that dilatation of the afferent arteriole of the isolated perfused juxtamedullary nephron induced by Bk was largely inhibited both by miconazole, another imidazole derivative that probably has direct action on KCa channel activity, and also by MS-PPOH, a structurally dissimilar selective epoxygenase inhibitor. Overall, our data indicate that in the kidney of anaesthetized rats the NO system and an increase in the activity of KCa channels contribute to Bk-induced vasorelaxation. There is no doubt, however, that in different animal species, vascular beds and experimental settings, Bk may exert its vasorelaxant action by a variety of complex mechanisms and along multiple biochemical pathways (Wang et al. 2003).
In summary, exogenous bradykinin applied by intravascular route or to the interstitium of the cortex or medulla failed to increase cortical perfusion in a sustained manner; this suggests that the agent has little, if any, role in the physiological control of whole cortex or whole kidney perfusion. By contrast, Bk invariably and substantially increased perfusion of the medulla, especially of its inner subzone. This effect was mediated by the NO system and by activation of KCa channels.
Acknowledgments
We are greatly indebted to Professor Andrzej Lipkowski, Department of Neuropeptides, M. Mossakowski Medical Research Centre, PAN, Warsaw, for his advice regarding the ways to increase the solubility of clotrimazole, to enable its infusions into the renal medulla.
Glossary
Abbreviations
- ABT
1-aminobenzatriole
- Bk
bradykinin
- B2
bradykinin type 2 receptor
- CBF
cortical perfusion (laser-Doppler flux)
- CYP450
cytochrome P-450
- EDHF
endothelium-derived hyperpolarizing factor
- EETs
epoxyeicosatrienoic acids
- 20-HETE
20-hydroxyeicosatetraenoic acid
- HOE 140
icatibant acetate – selective bradykinin B2 receptor antagonist
- IMBF
inner medullary perfusion (laser-Doppler flux)
- KCa
calcium-dependent K+ channel
- MAP
mean arterial pressure
- MS-PPOH
methylsulfonyl-propargyloxyphenylhexanamide – an inhibitor of CYP450 epoxygenases
- NO
nitric oxide
- OMBF
outer medullary perfusion (laser-Doppler flux)
- OMDVR
outer medullary descending vasa recta
- RBF
renal blood flow
- SNP
sodium nitroferricyanide(III)
Author contributions
Both authors contributed comparably to all aspects of the experimental work, evaluation of the results and manuscript preparation.
References
- Bądzyńska B, Grzelec-Mojzesowicz M, Sadowski J. Prostaglandins but not nitric oxide protect renal medullary perfusion in anaesthetised rats receiving angiotensin II. J Physiol. 2003;548:875–880. doi: 10.1113/jphysiol.2002.038075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bądzyńska B, Sadowski J. Opposed effects of PGE2 on perfusion of rat renal cortex and medulla: interactions with the renin–angiotensin system. Exp Physiol. 2008;93:1292–1302. doi: 10.1113/expphysiol.2008.043604. [DOI] [PubMed] [Google Scholar]
- Bagate K, Develioglu L, Michel B, Grima M, Imbs JL, Barthelmebs M. Renal vascular responses of bradykinin in the isolated rat kidney. Arch Mal Coeur Vaiss. 1997;90:1131–1134. [PubMed] [Google Scholar]
- Bergström G, Evans RG. Mechanisms underlying the antihypertensive functions of the renal medulla. Acta Physiol Scand. 2004;181:475–486. doi: 10.1111/j.1365-201X.2004.01321.x. [DOI] [PubMed] [Google Scholar]
- Braun C, Ade M, Unger T, Van Der Woude FJ, Rohmeiss P. Effects of bradykinin and icatibant on renal hemodynamics in conscious spontaneously hypertensive and normotensive rats. J Cardiovasc Pharmacol. 1997;30:446–454. doi: 10.1097/00005344-199710000-00007. [DOI] [PubMed] [Google Scholar]
- Clements JA. Tissue kallikrein-kinin system. In: Fray JCS, Goodman HM, editors. Handbook of Physiology, section 7, chap. 9, The Endocrine System, vol. III, Endocrine Regulation of Water and Electrolyte Balance. N. York, Oxford: Oxford University Press; 2000. pp. 333–376. [Google Scholar]
- Cowley AW. Renal medullary oxidative stress, pressure- natriuresis, and hypertension. Hypertension. 2008;52:777–786. doi: 10.1161/HYPERTENSIONAHA.107.092858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabisch PA, Kerut EK, Liles JT, Wien G, Smith M, Patterson M, Mccoul ED, Sears BW, Saenz R, Kadowitz PJ. Responses to bradykinin are mediated by NO-independent mechanisms in the rat hindlimb vascular bed. Pharmacological Res. 2004;50:575–584. doi: 10.1016/j.phrs.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RM. Response of isolated renal arterioles to acetylcholine, dopamine, and bradykinin. Am J Physiol. 1985;248:F183–F189. doi: 10.1152/ajprenal.1985.248.2.F183. [DOI] [PubMed] [Google Scholar]
- Evans RG, Eppel GA, Anderson WP, Denton KM. Mechanisms underlying the differential control of blood flow in the renal medulla and cortex. J Hypertens. 2004;22:1439–1451. doi: 10.1097/01.hjh.0000133744.85490.9d. [DOI] [PubMed] [Google Scholar]
- Flamenbaum W, Gagnon J, Ramwell P. Bradykinin-induced renal haemodynamic alterations: Renin and prostaglandin relationships. Am J Physiol Renal Physiol. 1979;237:F423–F440. doi: 10.1152/ajprenal.1979.237.6.F433. [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension. 2006;47:629–633. doi: 10.1161/01.HYP.0000208597.87957.89. [DOI] [PubMed] [Google Scholar]
- Fulton D, McGiff JC, Quilley J. Contribution of NO and cytochrome P450 to the vasodilator effect of bradykinin in the rat kidney. Br J Pharmacol. 1992;107:722–725. doi: 10.1111/j.1476-5381.1992.tb14513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardes J, Baussant T, Cirvol P, Menard J, Alhenc-Gelas F. Effect of bradykinin and kininogens in isolated rat kidney vasoconstricted by angiotensin II. Am J Physiol Renal Physiol. 1990;258:F1273–F1281. doi: 10.1152/ajprenal.1990.258.5.F1273. [DOI] [PubMed] [Google Scholar]
- Granger JP, Hall JE. Acute and chronic actions of bradykinin on renal function and arterial pressure. Am J Physiol Renal Physiol. 1985;248:F87–F92. doi: 10.1152/ajprenal.1985.248.1.F87. [DOI] [PubMed] [Google Scholar]
- Hoagland KM, Maddox DA, Martin DS. Intrarenal infusion of bradykinin elicits a pressor response in conscious rats via a B2-receptor mechanism. Can J Physiol Pharmacol. 1999a;77:563–570. [PubMed] [Google Scholar]
- Hoagland KM, Maddox DA, Martin DS. Bradykinin B2-receptors mediate the pressor and hemodynamic effects of intravenous bradykinin in conscious rats. J Auton Nerv Syst. 1999b;75:7–15. doi: 10.1016/s0165-1838(98)00166-0. [DOI] [PubMed] [Google Scholar]
- Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289:F496–F503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J Vas Res. 2001;38:247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- Kalyan A, Eppel GA, Anderson WP, Oliver JJ, Evans RG. Renal medullary interstitial infusion is a flawed technique for examining vasodilator mechanisms in anesthetized rabbits. J Pharmacol Toxicol Methods. 2002;47:153–159. doi: 10.1016/S1056-8719(02)00230-7. [DOI] [PubMed] [Google Scholar]
- Katori M, Majima M. Pivotal role of renal kallikrein-kinin system in the development of hypertension and approaches to new drugs based on this relationship. Jpn J Pharmacol. 1996;70:95–128. doi: 10.1254/jjp.70.95. [DOI] [PubMed] [Google Scholar]
- Lu S, Roman RJ, Mattson DL, Cowley AW. Renal medullary interstitial infusion of diltiazem alters sodium and water excretion in rats. Am J Physiol Regul Integr Comp Physiol. 1992;263:R1064–R1070. doi: 10.1152/ajpregu.1992.263.5.R1064. [DOI] [PubMed] [Google Scholar]
- Maier KG, Henderson L, Narayanan J, Alonso-Galicia M, Roman RJ. Fluorescent HPLC assay for 20-HETE and other P-450 metabolites of arachidonic acid. Am J Physiol Heart Circ Physiol. 2000;279:H863–H871. doi: 10.1152/ajpheart.2000.279.2.H863. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Hayashi K, Arakawa K, Naitoh M, Kubota E, Honda M, Matsumoto A, Suzuki H, Yamamoto T, Kajiya F, Saruta T. Zonal heterogeneity in action of angiotensin-converting enzyme inhibitor on renal microcirculation: role of intrarenal bradykinin. J Am Soc Nephrol. 1999;10:2272–2282. doi: 10.1681/ASN.V10112272. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Tadano K, Yamasaki T. Renal haemodynamic and excretory responses to bradykinin in anaesthetized dogs. Clin Exp Pharmacol Physiol. 1999;26:645–650. doi: 10.1046/j.1440-1681.1999.03098.x. [DOI] [PubMed] [Google Scholar]
- Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Renal Physiol. 2003;284:R13–R27. doi: 10.1152/ajpregu.00321.2002. [DOI] [PubMed] [Google Scholar]
- Mattson DL, Cowley AW., Jr Kinin action on renal papillary blood flow and sodium excretion. Hypertension. 1993;21:961–965. doi: 10.1161/01.hyp.21.6.961. [DOI] [PubMed] [Google Scholar]
- Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38. doi: 10.1254/jphs.srj05001x. [DOI] [PubMed] [Google Scholar]
- Nafz B, Berger K, Rösler C, Persson PB. Kinins modulate the sodium-dependent autoregulation of medullary blood flow. Cardiovasc Res. 1998;40:573–579. doi: 10.1016/s0008-6363(98)00194-1. [DOI] [PubMed] [Google Scholar]
- Navar LG, Inscho EW, Majid DSA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Silldorff EP, Cheung JY. Response of isolated rat descending vasa recta to bradykinin. Am J Physiol Heart Circ Physiol. 1998;43:H752–H759. doi: 10.1152/ajpheart.1998.274.3.H752. [DOI] [PubMed] [Google Scholar]
- Patel A, Smith FG. Renal haemodynamic effects of B2 receptor agonist bradykinin and B2 receptor antagonist HOE140 in conscious lambs. Exp Physiol. 2000;85:811–817. [PubMed] [Google Scholar]
- Pompermayer K, Assreuy J, Vieira MA. Involvement of nitric oxide and potassium channels in the bradykinin-induced vasodilatation in the rat kidney perfused ex situ. Regul Pept. 2002;105:155–162. doi: 10.1016/s0167-0115(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Rajapakse NW, Roman RJ, Falck JR, Oliver JJ, Evans RG. Modulation of V1-receptor mediated renal vasoconstriction by epoxyeicosatrienoic acids. Am J Physiol Regul Integr Comp Physiol. 2004;287:R181–R187. doi: 10.1152/ajpregu.00555.2002. [DOI] [PubMed] [Google Scholar]
- Ren Y, Garvin J, Carretero OA. Mechanism involved in bradykinin-induced efferent arteriole dilation. Kidney Int. 2002;62:544–549. doi: 10.1046/j.1523-1755.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Roman RJ, Kaldunski ML, Scicli AG, Carretero OA. Influence of kinins and angiotensin II on the regulation of papillary blood flow. Am J Physiol Renal Physiol. 1988;255:F690–F698. doi: 10.1152/ajprenal.1988.255.4.F690. [DOI] [PubMed] [Google Scholar]
- Siragy HM, Jaffa AA, Margolius HS. Bradykinin B2 receptor modulates renal prostaglandin E2 and nitric oxide. Hypertension. 1997;29:757–762. doi: 10.1161/01.hyp.29.3.757. [DOI] [PubMed] [Google Scholar]
- Tornel J, Madrid MI, Garcia-Salom M, Wirth KJ, Fenoy FJ. Role of kinins in the control of renal papillary blood flow, pressure natriuresis, and arterial pressure. Circ Res. 2000;86:589–595. doi: 10.1161/01.res.86.5.589. [DOI] [PubMed] [Google Scholar]
- Vio CP, Loyola S, Velarde V. Localization of components of the kallikrein-kinin system in the kidney: relation to renal function. Hypertension. 1992;19:II10–II16. doi: 10.1161/01.hyp.19.2_suppl.ii10. [DOI] [PubMed] [Google Scholar]
- Wang H, Garvin JL, Falck JR, Ren Y, Sankey SS, Carretero OA. Glomerular cytochrome P-450 and cyclooxygenase metabolites regulate efferent arteriole resistance. Hypertension. 2005;46:1175–1179. doi: 10.1161/01.HYP.0000187531.93389.63. [DOI] [PubMed] [Google Scholar]
- Wang X, Trottier G, Loutzenhiser R. Determinants of renal afferent arteriolar actions of bradykinin: evidence that multiple pathways mediate responses attributed to EDHF. Am J Physiol Renal Physiol. 2003;285:F540–F549. doi: 10.1152/ajprenal.00127.2003. [DOI] [PubMed] [Google Scholar]
- Webster ME, Gilmore JP. Influence of kallidin-L0 on renal function. Am J Physiol. 1964;206:714–718. doi: 10.1152/ajplegacy.1964.206.4.714. [DOI] [PubMed] [Google Scholar]
- Wu SN, Li HF, Jan CR, Shen AY. Inhibition of Ca2+-activated K+ current by clotrimazole in rat anterior pituitary GH3 cells. Neuropharmacology. 1999;38:979–989. doi: 10.1016/s0028-3908(99)00027-1. [DOI] [PubMed] [Google Scholar]
- Yu H, Carretero OA, Juncos LA, Garvin L. Biphasic effect of bradykinin on rabbit afferent arterioles. Hypertension. 1998;32:287–292. doi: 10.1161/01.hyp.32.2.287. [DOI] [PubMed] [Google Scholar]
- Yun JCH, Gill JR, Jr, Bartter FC, Kelly GD, Keiser HR. Effect of bradykinin on renal function in dogs treated with indomethacin or propranolol. Renal Physiol. 1982;5:31–43. doi: 10.1159/000172837. [DOI] [PubMed] [Google Scholar]