Abstract
Parietal cell (PC) proton secretion via H+/K+-ATPase requires apical K+ recycling. A variety of K+ channels and transporters are expressed in the PC and the molecular nature of the apical K+ recycling channel is under debate. This study was undertaken to delineate the exact function of KCNQ1 channels in gastric acid secretion. Acid secretory rates and electrophysiological parameters were determined in gastric mucosae of 7- to 8-day-old KCNQ1+/+, +/– and −/− mice. Parietal cell ultrastructure, abundance and gene expression levels were quantified. Glandular structure and PC abundance, and housekeeping gene expression did not differ between the KCNQ1−/− and +/+ mucosae. Microvillar secretory membranes were intact, but basal acid secretion was absent and forskolin-stimulated acid output reduced by ∼90% in KCNQ1−/− gastric mucosa. Application of a high K+ concentration to the luminal membrane restored normal acid secretory rates in the KCNQ1−/− mucosa. The study demonstrates that the KCNQ1 channel provides K+ to the extracellular K+ binding site of the H+/K+-ATPase during acid secretion, and no other gastric K+ channel can substitute for this function.
The coordinated function of the H+/K+-ATPase, as well as K+ and Cl− channels in the apical membrane of the gastric parietal cell, results in acid secretion (Forte et al. 1973, 1990; Sachs et al. 1976; Cuppoletti & Sachs, 1984; Hirst & Forte, 1985; Heitzmann & Warth 2007). Gastric proton secretion by H+/K+-ATPase is coupled to K+ uptake and therefore requires sufficient concentrations of K+ in the lumen of the secretory canaliculi (Aihara et al. 2003; Waldegger, 2003; Forte, 2004). On the other hand, basolateral K+ channels are also essential for acid secretion through maintaining the driving force for electrogenic apical Cl− exit (McLennan et al. 1980).
The K+ channels identified in gastric parietal cells and possibly involved in supporting acid secretion are KCNQ1 (Grahammer et al. 2001; Waldegger, 2003; Heitzmann et al. 2004), the inward rectifiers Kir4.1 (KCNJ10) (Fujita et al. 2002), Kir5.1 (only rat) and Kir2.1 (KCNJ2) (only rabbit) (Malinowska et al. 2004) and ROMK (Kir1.1, KCNJ1) (Aihara et al. 2003). Recent evidence suggests an apical localization of KCC4 on the parietal cell, which could also serve to extrude both K+ and Cl− (Fujii et al. 2009).
KCNQ1 and its subunit KCNE2 are highly expressed in parietal cells (Lambrecht et al. 2005), and both proteins are believed to assemble to form a functional K+ channel (Dedek & Waldegger, 2001; Grahammer et al. 2001; Heitzmann et al. 2004). KCNQ1 channel blockers have been found to inhibit acid secretion to various degrees in a variety of model systems (Grahammer et al. 2001; Heitzmann et al. 2004; Lambrecht et al. 2005), and both KCNQ1- and KCNE2-deficient mice exhibited severe gastric gland hyperplasia, abnormal parietal cell morphology, hypergastrinaemia and loss of acid secretion, providing indirect evidence for an important role of KCNQ1 channels in acid secretion (Lee et al. 2000; Vallon et al. 2005; Roepke et al. 2006). We have recently studied the subcellular localization and trafficking of KCNQ1 channels in rat and rabbit parietal cells (Kaufhold et al. 2008). Interestingly, KCNQ1 was largely located on non-H+/K+-ATPase-containing vesicles, and the majority remained cytoplasmic during stimulation of acid secretion. This latter observation is not unique for agonist-regulated apical K+ channels. However, Kir4.1 channels were much more strongly colocalized with the H+/K+-ATPase during the complete acid secretory cycle. This raised the question whether KCNQ1 channels were one of several, or the only or major K+ recycling channel in the luminal membrane of the parietal cell. This question may be crutial for future acid inhibitory drug development.
The lack of acid secretion in the severely hypertrophied and structurally altered KCNQ1 or KCNE2 knockout mice does not rule out a role of other K+ pathways in apical K+ recycling, because the stomachs of these animals have lost their ability to secrete acid. The severe morphological changes in the gastric mucosa of adult KCNQ1-deficient mice, therefore, make them unsuitable for testing the concept by luminal restoration of K+. We therefore investigated the acid secretory capacity of gastric mucosae in very young KCNQ1−/− mice before they develop severe changes in gastric morphology. The results were striking: KCNQ1 ablation completely prevented basal acid secretion, and even strong stimulation by forskolin elicited almost no secretory response. However, in contrast to the situation in the grossly hypertrophied stomach of adult mice, high luminal K+ could restore normal acid secretory rates in KCNQ1-deficient gastric mucosa of young mice. This proves that despite the many other K+ transporters expressed in parietal cells, the parietal cell KCNQ1 K+ channel is of utmost importance to provide K+ to the extracellular K+ binding site of the H+/K+-ATPase in the initiation of acid secretion, and can be poorly substituted by other K+ transporters. This was found true both in the spontaneously secreting ‘resting state’ mucosa, and after treatment with acid secretagogues.
Methods
Animals
The KCNQ1-deficient mouse strain was originally generated by the group of Karl Pfeifer in the laboratory of Mammalian Genes and Development, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MA, and raised on the original background in the animal facility of Hannover Medical School. All studies were approved by the Committee on Investigations Involving Animals, Hannover Medical School, Germany. Experiments were performed on KCNQ1 (+/+, +/– and −/− littermates), on a C57BL/6-Balb7C background. All mice were 7–8 days of age. The genotypes of the mice were verified by PCR after the experiments.
Measurement of acid secretory rates in isolated gastric mucosa
The experiments were performed as described previously (Kaufhold et al. 2008), with modifications indicated below. After killing the animals by cervical dislocation, the stomach was cleaned and microdissected in ice-cold Ringer solution (145.5 mm NaCl, 4 mm KCl, 1.2 mm CaCl2, 0.05 mm indomethacin) and mounted between two lucite half-chambers (with an exposed surface area of 0.283 cm2) of a water-jacketed Ussing system equipped with a gas-lift system. The serosal solution contained (in mm): 108 NaCl, 22 NaHCO3, 3 KCl, 1.3 MgSO4, 2 CaCl2, 2.25 KH2PO4, 8.9 glucose, 10 sodium pyruvate, with 3 × 10−5m indomethacin and 10−6m TTX to minimize variation due to intrinsic prostanoid and neural tone, respectively, and was gassed with 95% O2–5% CO2, pH 7.4. The mucosal solution contained 154 mm NaCl, was gassed with 100% O2 and kept at constant pH 7.4 with 2 mm NaOH by a pH-stat titration system (Radiometer, Copenhagen, Denmark). The volume of the titrant infused per unit time was used to quantify H+ secretion. These measurements were recorded at 5 min intervals. The rate of luminal H+ secretion (JH+) is expressed as μmol cm−2 h−1. Transepithelial short-circuit current (Isc, reported as μeq cm−2 h−1) and potential difference (PD, expressed in mV) were measured via an automatic voltage clamp (Voltage-Current Clamp, EVC-4000; World Precision Instruments, Berlin, Germany). Transepithelial resistance (Rt, reported as Ω cm2) was calculated according to values of Isc and PD. Basal parameters were measured for a period of 30 min if the stomach showed spontaneous acid secretion, then stimulant (forskolin, 10−5m) was added to the serosal side of the tissue. Changes in gastric acid secretion, Isc, R and PD were determined during the 60 min after the addition of stimulants.
Only stomachs that secreted acid after forskolin stimulation and displayed normal electrophysiological parameters and no signs of leakage (rapid increase of luminal pH, indicative of HCO3− leak from the serosal perfusate) were included in calculations of the time course and magnitude of stimulation of acid secretion by forskolin. In a subset of experiments, the luminal perfusate (154 mm NaCl) was replaced by 154 mm KCl after spontaneous acid secretion had been recorded for a period of 25 min, and subsequent acid secretory rates in the absence of forskolin for 25 min and presence of forskolin for 40 min were determined.
Light and transmission electron microscopy
The stomachs of KCNQ1+/+ and KCNQ1−/− mice were removed and immediately immersed in a fixative solution containing either 2% glutaraldehyde and 2% formaldehyde (freshly prepared from paraformaldehyde) buffered in 0.1 m sodium cacodylate–HCl buffer pH 7.3 or 4% formaldehyde (freshly prepared from paraformaldehyde) in the same buffer. The stomachs were opened along the limiting ridge separating the keratinized forestomach from the glandular stomach. To ascertain the presence of comparable segments in all specimens, small fragments (1–2 mm) from the gastric fundus were prepared all from the same distance from this limiting ridge. The fragments were transferred into fresh fixative solution and stored for at least 4 h at 4°C. For light and transmission electron microscopy, specimens fixed in the presence of glutaraldehyde were washed in 0.1 m sodium cacodylate–HCl pH 7.3, postfixed for 90 min at room temperature (RT) in 2% OsO4 buffered in 0.1 m sodium cacodylate–HCl pH 7.3, subsequently dehydrated in increasing concentrations of ethanol and finally embedded in epoxy resin (Serva, Heidelberg, Germany). Thick plastic sections (1 μm) were prepared with a Reichert Ultracut E ultramicrotome (Leica, Wetzlar, Germany), stained with alkalinized toluidine blue and observed with a light microscope Leitz Orthoplan (Leica) equipped with a CCD camera (Olympus DP50). Thin sections were collected on Formvar-coated copper slot grids, stained with uranyl acetate and lead citrate, and examined using a Zeiss EM 10 CR transmission electron microscope at an acceleration voltage of 80 kV.
Immunofluorescence
Formaldehyde-fixed specimens were dehydrated and embedded as above but without postfixation. Semithin sections (1 μm) of these samples were collected on glass slides pretreated with 3-(triethoxysilyl) propylamide (silane; Merck, Darmstadt, Germany). The sections were etched for 20 min in sodium ethoxide diluted to 50% with absolute ethanol. After washing in PBS, non-specific protein binding was blocked with the application of 5% bovine serum albumin (BSA) diluted in PBS for 30 min at RT. Incubation with a mouse monoclonal anti-H+/K+-ATPase antibody (HK 12.18) diluted 1: 2500 in PBS with the addition of 1% BSA (PBS–BSA) was performed overnight at 4°C in a humid chamber.
After thorough rinsing in PBS the sections were incubated for 60 min at RT with goat anti-mouse IgG conjugated with Alexa 488 (Molecular Probes, Eugene, OR, USA). Laser scanning confocal microscopy was performed using a Zeiss Axiovert 200 M microscope (Zeiss, Oberkochen, Germany) attached to an LSM 510 Meta detector controlled by LSM 5 Image version 3.2 software (Zeiss).
Morphometric analysis
Parietal cells were counted on toluidine blue-stained semithin sections of stomach specimens (three mice per group) using a Leitz Orthoplan light microscope with a 40× objective lens. Only glands that were sectioned perpendicular to their length axis were evaluated.
Quantitative real-time PCR
RNA isolation
RNA from stomachs of 7- to 8-day-old KCNQ1−/− mice and their wild-type (WT) littermates (n= 4) was isolated with a NucleoSpin RNAII total RNA isolation kit (Macherey & Nagel). Reverse transcription was performed with SuperScript III RNaseH− reverse transcriptase (Invitrogen).
Quantitative RT-PCR
PCR primers were designed using the computer program ‘Primer Express’ (Applied Biosystems). Primer sequences were: H+/K+- ATPase forward: 5′-TCTTTCCATCTGTGTCCCTGG-3′, H+/K+-ATPase reverse: 5′-AAGCCGGCAAATGACTGAAT-3′, PCR-product length 150 bp, (H+/K+- ATPase-exchanging, α polypeptide, NM_01873.2). KIR5.1 forward: 5′-CTTGCAGTTTGAAGGAAGCGT-3; KIR5.1 reverse: 5′-GGTGTCAGAATTGCAGGATCC-3′, PCR product length 130 bp (potassium inwardly rectifying channel, subfamily J, member 17, KCNQJ 16, NM_010604.3). AE2 forward: 5′-AACCCCTTGGAGAAAAGACC-3′; AE2 reverse: 5′-CCCATGGCTACAATGAGCAAC-3′, PCR product length 185 bp, (solute carrier family 4, member 2, Slc4a2, NM_009207.2).
Real-time PCR reactions were carried out using Mesa Green qPCRMaster-Mix (Eurogentec) in the Applied Biosystems 7300 Realtime PCR System. PCR extension was performed at 60°C with 40 repeats. Data were analysed using Sequence Detection Software 1.2.3 (Applied Biosystems) and exported to Microsoft Excel. Relative quantification was carried out using ß-actin and ribosomal protein as reference genes.
Statistics
All results are expressed as means ±s.e.m. ΔJH+ refers to stimulated peak responses minus basal levels. Basal values for JH+, Isc and Rt refer to an average taken over a 30 min baseline period. Data were analysed by Student's t tests or by one-way analysis of variance (ANOVA) with the Student–Newman–Keul post hoc test. A P value of less than 0.05 was considered statistically significant.
Results
Body weights of KCNQ1−/− mice and their littermates
Genotype frequencies of KCNQ1 pups from heterozygous matings exhibited a normal Mendelian ratio of 1: 2: 1 with no alteration in the percentage of male or female null mutant mice. At 7–8 days, KCNQ1 null mutants exhibited growth development similar to their wild-type and heterozygous littermates, with no significant difference in body weights between KCNQ1−/− mice and KCNQ1+/+ mice (Table 1).
Table 1.
Body weights of 7-day-old KCNQ1+/+, +/− and −/− pups
| Genotype | Weight (g) |
|---|---|
| KCNQ1+++ (n= 11) | 4.28 ± 0.31 |
| KCNQ1+/– (n= 13) | 5.28 ± 0.66 |
| KCNQ1−/− (n= 11) | 3.85 ± 0.24 |
There was no significant difference in body weights of KCNQ1+/+, +/− and −/− mice.
Effect of forskolin on gastric acid secretion in KCNQ1 knockout mice
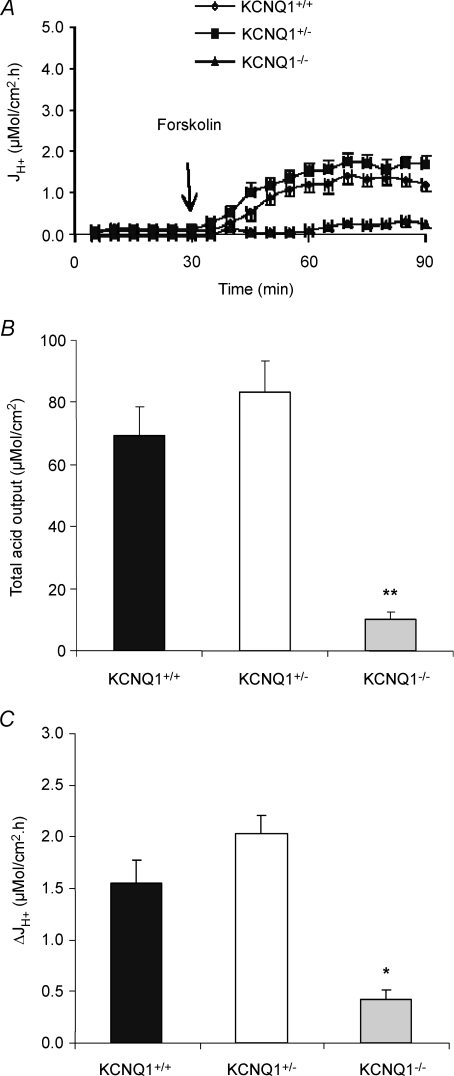
The isolated gastric mucosae from 7- to 8-day-old KCNQ1−/− mice had no spontaneous gastric acid secretion, whereas most KCNQ1+/+ and KCNQ1+/– gastric mucosae secreted acid spontaneously (Fig. 1A). There was a significant reduction in forskolin-induced acid secretion in KCNQ1 knockout mice compared to wild-type and heterozygous littermates (Fig. 1A–C), with a significant delay in the onset of acid secretion. The total acid output 1 h after the addition of forskolin was reduced by ∼90% (Fig. 1A and B), and maximal forskolin-stimulated acid secretory rate obtained at any time point was >80% reduced compared to WT mice (Fig. 1A and C). The results suggest that the loss of KCNQ1 channel interferes very strongly with gastric acid secretion.
Figure 1. Effects of forskolin (10−5m) on gastric acid secretion of gastric mucosa in KCNQ1+/+ (n= 11), +/– (n= 14) and −/− (n= 7) mice.
A, acid secretory rate measured at successive 5 min intervals in response to forskolin added to the serosal bath at 30 min. B, total acid output in 90 min calculated from the area under the curve, and expressed as the mean ±s.e.m. C, the data shown represent the net maximal increases in acid secretory rate observed after the addition of forskolin. In KCNQ1−/− mice, forskolin-stimulated acid secretion was significantly reduced when compared with KCNQ1+/+. **P < 0.001.
Effect of forskolin on PD, Isc and resistance of gastric mucosa in KCNQ1 knockout mice
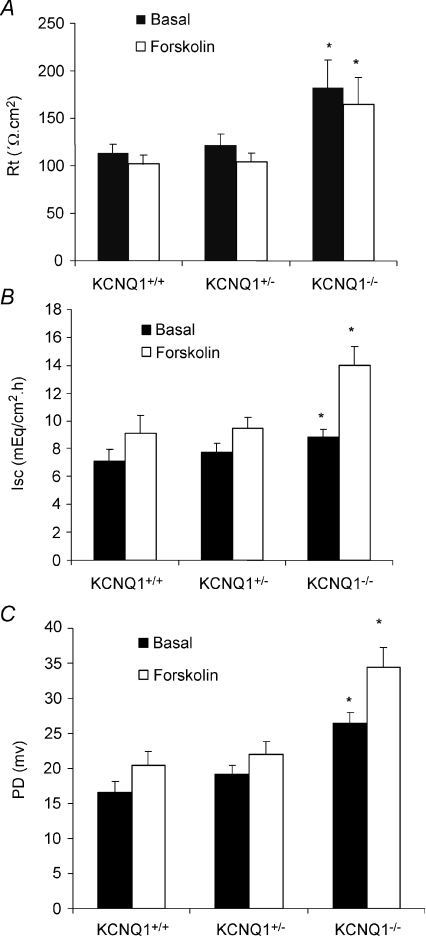
The resting state gastric mucosal transepithelial resistance (Rt) in KCNQ1 knockout mice was significantly increased (Fig. 2A) compared with their littermates, presumably because of a less conductive apical membrane of a gastric epithelial cell type even in the resting state. The addition of forskolin decreased the mucosal resistance by 10–20% in all three groups, but Rt still remained significantly higher in the stimulated KCNQ1−/− mucosae. Basal Isc in KCNQ1−/− gastric mucosa was slightly but not significantly increased compared with KCNQ1+/+ and KCNQ1+/– mucosa (Fig. 2B). In addition, a significant increase in Isc (Fig. 2B), as well as potential difference (Fig. 2C), was seen upon forskolin stimulation, indicative of a stimulation of electrogenic Cl− secretion, and this increase was significantly larger in KCNQ1−/− mucosae. These results suggest that: (1) KCNQ1 channel activity is present also during basal acid secretion, and (2) the electrogenic part of agonist-stimulated anion secretion appears to be increased in KCNQ1-deficient gastric mucosa.
Figure 2. Effects of forskolin (10−5m) on resistance (A), Isc (B) and PD (C) of gastric mucosa in KCNQ1+/+ (n= 11), +/– (n= 14) and −/− (n= 7) mice.
The data shown represent basal value and maximal (B and C) or minimal (A) value after the addition of forskolin. Basal value and maximal response to forskolin in PD and resistance were increased significantly in KCNQ1−/− mice. *P < 0.05. A significant increase in Isc upon forskolin stimulation in KCNQ1−/− mice was noted compared with KCNQ1+/+.
High luminal K+ concentrations restitutes normal acid secretory rates in KCNQ1−/− mucosae
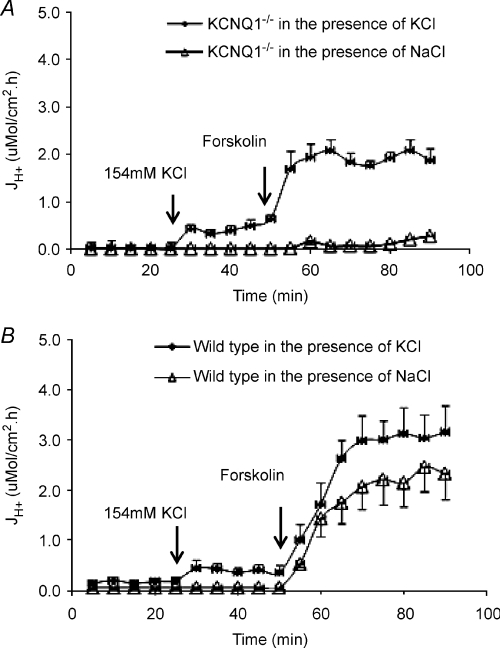
KCNQ1-deficient parietal cells displayed reasonably normal microvillar canalicular membranes when examined by electron microscopy (see below for detailed results). We therefore tested the hypothesis that the primary function of the parietal cell KCNQ1 channels was to supply K+ to the extracellular K+ binding site of the H+/K+-ATPase by replacing isotonic NaCl with KCl in the luminal solution, and recording acid secretory rates. Indeed, the replacement of isotonic NaCl with KCl initiated spontaneous acid secretion and fully restored forskolin-stimulated acid secretory rates to the levels observed in the WT mice (Fig. 3A). In WT gastric mucosa, replacement of isotonic NaCl with isotonic KCl in the luminal solution had only a small effect on spontaneous secretory rates, and increased forskolin-stimulated rates by ∼30% (Fig. 3B). These experiments demonstrate that extracellular K+, applied to the external binding site of the H+/K+-ATPase, fully compensates for the lost function of KCNQ1, both for basal as well as stimulated acid secretion. This virtually rules out any major impact of alternative models of apical K+ exit during basal or stimulated acid secretion, at least in mice of this age.
Figure 3. Acid secretory rate at basal state and in response to forskolin was examined in the presence of perfusate NaCl or KCl in KCNQ1−/− (A) and wild-type (B) mice.
The baseline of acid secretion was increased after 154 mm NaCl perfusate was replaced with 154 mm KCl in KCNQ1−/− (n= 5) and +/+ (n= 7) mice, then the response for forskolin was further increased when compared with the control KCNQ1−/− (n= 5) and +/+ (n= 7) mucosae, which were in the presence of NaCl only as perfusate.
Histological, immunohistochemical and electron microscopical evaluation of KCNQ1-null gastric mucosa
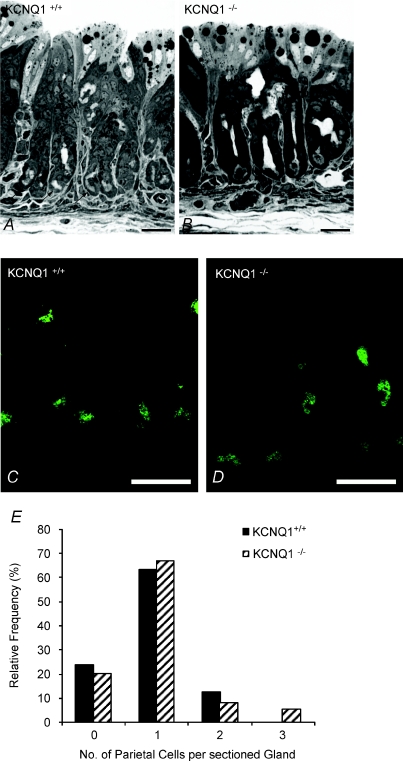
In weanling wild-type animals, the oxyntic mucosal epithelium was composed of numerous mucous neck cells, surface cells, chief cells, endocrine cells and distinctive parietal cells (PCs), which were found along the gland including the gland base (Fig. 4A). Evaluation of parietal cell numbers lining the gastric glands did not reveal significant differences between the wild-type and KCNQ1 knockout mice (Fig. 4B).
Figure 4. Light (A and B) and fluorescence (C and D) microscopy of semi-thin sections of stomach mucosa from KCNQ1 WT and knockout mice.
A and B, in both WT (A) and KCNQ1−/− (B)mucosa, droplets of varying size can be observed in the cells directly lining the lumen (milk). Arrows indicate some of the parietal cells, which, in the young mice, were also found at the base of the glands. C and D, confocal images of semi-thin sections of gastric mucosa from wild-type (C) and knockout animals (D) labelled with a mouse monoclonal against H+/K+-ATPase. Parietal cells are labelled, with no significant difference in the fluorescent intensity between the +/+ and −/− genotypes. The staining is distributed along their apical plasma membrane. Other components of the mucosa remain unlabelled. Bars, 25 μm. E, relative frequency distribution of the number of parietal cells in sections of gastric glands from KCNQ1+/+ and −/− mice.
Laser scanning confocal microscopy of gastric gland sections labelled with an antibody against gastric H+/K+-ATPase revealed that the expression and localization of this enzyme was similar to that of WT littermates. In suitable section planes, H+/K+-ATPase was routinely observed bordering the apical plasma membrane (Fig. 4C).
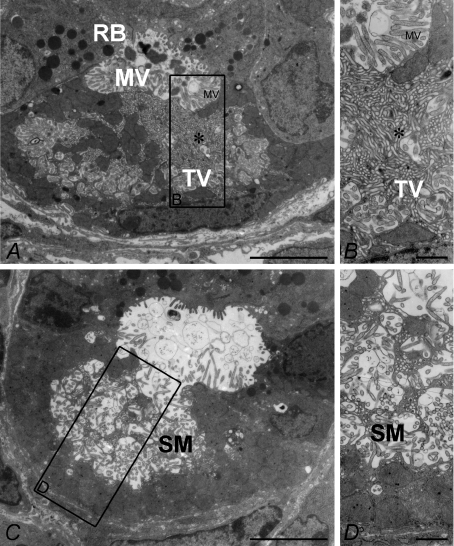
Parietal cell morphology was further studied by electron microscopy (Fig. 5). The morphological features of WT parietal cells showed intracellular canaliculi lined with numerous microvilli. Close to the canaliculi, membranes of the tubulovesicular (TV) system were visible in the resting state mucosa (Fig. 5A and B). Occasional spontaneously secreting PCs were also identified (Fig. 5C and D) in the WT gastric mucosa.
Figure 5. Transmission electron microscopy of stomach mucosa from wild-type oxyntic cells in the resting (A and B) and secreting (C and D) states.
In the resting state, numerous tubulovesicular structures (TV) are seen in the cytoplasm near the resting state mircovilli (MV)-lined apical membrane (enlarged in B). C and D, in the secreting parietal cell, most of these have fused with the apical membrane, and the secretory canaliculi (SM, secretory membrane) are expanded. Bars: A and C, 5 μm; B and D, 0.5 μm.
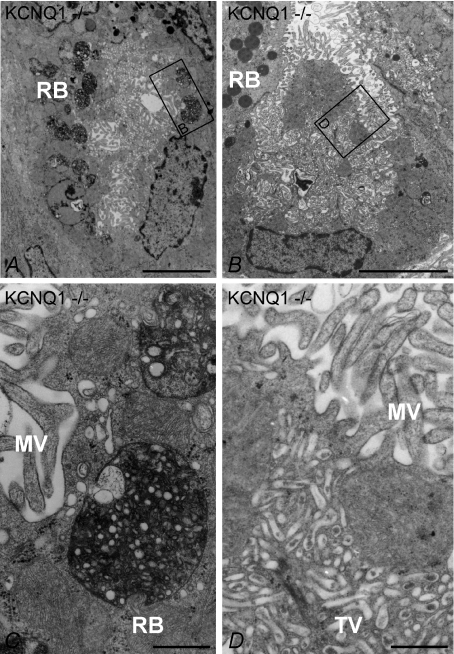
KCNQ1−/− mice showed changes in parietal cell morphology (Fig. 6A–D). The tubulovesicular system was well-developed, although the canaliculi were narrowed and appeared to be obstructed by their microvillus lining (Fig. 6A, B and D). These observations are consistent with the morphology of resting or unstimulated parietal cells in fasted animals described in the past (Ito & Schofield, 1974). In addition, parietal cells of KCNQ1 −/− mice showed significantly more large round bodies (Fig. 6A and C), which could also be found in WT PCs, but less often. They contained membranous and/or vesicular structures surrounded by unstructured electron-dense material and looked like the ‘phagosomes’ or ‘autophagosomes’ observed in rabbit parietal cells after omeprazole treatment (Karam & Forte, 1994). In summary, KCNQ1-deficient parietal cells show degenerative changes as early as 7 days after birth, but at this time, microvilli-lined canalicular structures and tubulovesicles are still present.
Figure 6. Low power (A and B) and high power (C and D) transmission electron microscopy of resting state oxyntic mucosa from KCNQ1−/− mice.
A, parietal cell with microvilli (MV) and a well developed tubulovesicular system (TV). Numerous roundish vesicular structures (RB, roundish bodies) containing material of heterogenous electron density are visible. B, another KCNQ1−/− PC with a vast tubulovesicular system resembling the resting state in wild-type mice and resting state apical membranes (compare with Fig. 5A and B). The framed areas in A and B are reproduced at higher magnification in C and D, respectively. In C the roundish vesicular structures are depicted. They contain amorphous material of varying electron density and/or vesicular structures surrounded by an electron-dense material and resemble auto-phagosomes (Karam & Forte, 1994). D shows the apical part of the parietal cell in (B) at higher magnification. The base of the microvilli (upper part of the image) as well as regions of the well developed tubulovesicular (lower half of the image) system are visible. Bars: A and B, 5 μm; C and D, 0.5 μm.
H+/K+-ATPase, AE2 and Kir 5.1 mRNA expression in KCNQ+/+ and −/− gastric mucosae
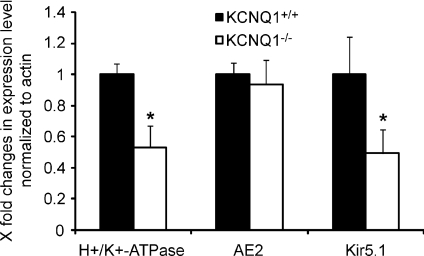
Although no conspicuous changes in H+/K+-ATPase staining and parietal cell number were observed in the WT and KCNQ1-deficient mucosae, we measured the mRNA expression of the H+/K+-ATPase α-unit and the K+ channel Kir5.1, as a marker for tubulovesicular and apical membranes, and for AE2, which is strongly expressed in the parietal cell basolateral membrane (Rossmann et al. 2001) (Fig. 7A–C). The expression levels for H+/K+-ATPase and Kir5.1 were down-regulated to ∼50%, whereas that for AE2 was unchanged in KCNQ1-deficient gastric mucosa compared to wild-type littermates. The exact significance of these differential changes in the expression pattern of apical but not basolateral parietal cell ion transporters is unknown, but probably indicates disturbances of the tubulovesicular and apical compartment. This probably explains why the acid secretory rate was slightly lower in KCNQ1−/− than +/+ mucosae in the presence of high luminal K+.
Figure 7. mRNA expression of H+/K+-ATPase α-unit, the anion exchanger AE2 and the K+ channel Kir5.1.
While H+/K+-ATPase and Kir5.1 localize to the tubolovesicular/apical membrane domain, the anion exchanger AE2 localizes to the basolateral membrane. Since AE2 is very highly expressed in parietal cells compared to other gastric cell types (Hirst & Forte, 1985; Rossmann et al. 2001; Lambrecht et al. 2005), it is a good marker for parietal cell transcriptional activity. There was no difference in AE2 mRNA expression levels between KCNQ1−/− and WT mucosa, confirming the similar parietal cell numbers found in microscopy, but there was a significant decrease in the mRNA expression for the two tubulovesicular/apical genes, suggesting some disturbance of the turnover of apical membrane transporters. n= 4–6. *P < 0.05.
Acid secretion cannot be rescued in adult KCNQ1−/− mice
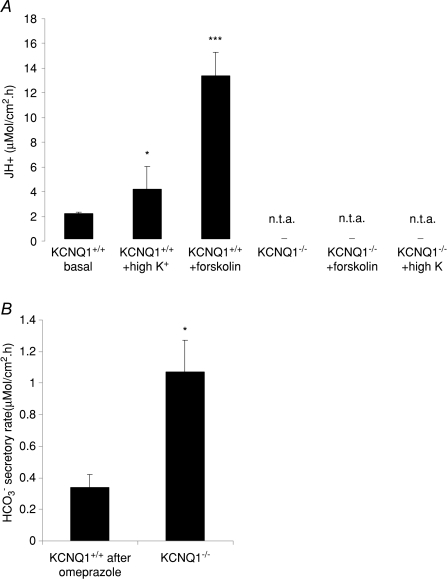
The gastric mucosae of adult KCNQ1−/− mice spontaneously secreted HCO3− rather than acid, and forskolin or high K+ failed to elicit an acid-secretory response (Fig. 8). These data represent, for the first time, a direct assessment of the acid secretory capacity of the adult KCNQ1−/− gastric mucosa, and are consistent with diffuse, but not membrane-associated, expression of H+/K+ ATPase in these stomachs (Lee et al. 2000).
Figure 8. Loss of acid secretion (A) and increase in HCO3− secretion (B) in the gastric mucosa of adult mice (3–6 months).
A, high K+ concentrations increase the basal acid secretory rate in gastric mucosa of adult WT mice but, in contrast to the situation in very young mice, cannot rescue acid secretion in adult KCNQ1−/− mice (n= 11, 5 and 5 for the first three bars and 4, 4 and 3 for the second three bars, n.t.a. indicates no titratable acid). B, in contrast, the hypertrophied gastric mucosa of adult KCNQ1−/− mice secretes significantly more HCO3− than the WT mucosae (after inhibition of acid secretion by 10−5m omeprazole). n= 5. *P < 0.05, ***P < 0.0001.
Discussion
This study investigates the functional role of KCNQ1 K+ channels in the gastric acid secretory process, by utilizing isolated gastric mucosa of very young KCNQ1-defieint and WT mice, before the onset of gastric mucosal hypertrophy and altered glandular ultrastructure. In KCNQ1-deficient mucosa, basal acid secretion was abolished and forskolin-induced acid secretory rates reduced by >90%. Replacement of luminal NaCl with KCl restored normal secretory rates in the KCNQ1-deficient mucosae, supporting the hypothesis that the primary physiological function of the gastric KCNQ1 K+ channel is the supply of K+ to the extracellular K+ binding site of the H+/K+-ATPase during stimulation of acid secretion. Evidently, no other K+ transporter can adequately substitute for this function.
An importance of KCNQ1 for gastric acid secretion has been anticipated several years ago by the observation that adult KCNQ1-deleted animals displayed gross gastric mucosal hypertrophy, elongated glands, degeneration of parietal cells morphology and loss of acid secretion, pointing to a pivotal role of KCNQ1 channels in parietal cell acid secretion (Lee et al. 2000; Vallon et al. 2005). However, the trafficking pattern of these channels remained obscure, with no obvious apical translocation during stimulation (Heitzmann et al. 2004). In addition, other K+ channels (Fujita et al. 2002; Aihara et al. 2003; Malinowska et al. 2004) or transporters (Fujii et al. 2009) with a parietal cell apical location were found, with some functional evidence suggesting a potential involvement in apical K+ recycling during acid secretion. Recent investigations have shown that although the majority of KCNQ1 channels remain cytoplasmic after stimulation of acid secretion, a fraction clearly translocates into the apical membrane (Kaufhold et al. 2008). The present study shows that KCNQ1 deletion causes severe impairment of acid secretion at an early age. The substitution of K+ to the luminal membrane was able to restore virtually normal acid secretory rates, demonstrating that the primary physiological function of the gastric KCNQ1 channel is the supply of K+ to the extracellular K+ binding site of the H+/K+-ATPase during stimulation of acid secretion, with poor substitution by other K+ channels or transporters. This functional rescue of acid secretion by luminal K+ was only possible in the stomachs of very young mice, which still present a near-normal ultrastructure of the microvilli-lined parietal cell canalicular membranes. In adult mice, no acid secretion, and no such functional recovery by luminal K+ was seen, consistent with the observation of diffuse, rather than membrane-associated H+/K+-ATPase expression in the parietal cells of adult KCNQ1-deficient stomach, and severe secondary changes with a complete loss of the normal gastric mucosal morphology (Lee et al. 2000). Consistent with the massive hypertrophy of the glandular stomach with a vast increase in the surface cell area (Lee et al. 2000), we found that the KCNQ1-deficient stomachs secreted significantly more HCO3− than WT stomach after H+/K+-ATPase inhibition.
Interestingly, forskolin-stimulated Isc, as well as PD, was also higher in KCNQ1-deficient than in WT mucosa. This agonist-stimulated increase in PD is dependent on transmucosal Cl− movement (McDaniel et al. 2005). This ‘non-acidic’ component of Cl− secretion has been observed as a transmucosal movement of Cl− in excess of H+ or a short-circuit current (Isc) (Forte & Machen, 1975; Berglindh, 1977; Sachs et al. 1978; Machen & McLennan, 1980; McLennan et al. 1980; Coskun et al. 2002; McDaniel et al. 2005). This increase of Isc response in KCNQ1−/− stomachs reflects an independency of electrogenic Cl− from acid secretion. The transport pathways for non-acidic anion secretion are unknown, but evidence suggests that they are located predominantly in the base of the gastric glands. Basolateral NKCC1 is strongly expressed in parietal cells in the base of the glands, and it has been shown that the genetic ablation of NKCC1 results in a significant decrease of K+, Na+, Cl− and fluid secretion, as well as Isc response to forskolin, while leaving H+ secretion normal (McDaniel et al. 2005).
Compared with KCNQ1 WT and heterozygous mice, a significant increase in gastric mucosal resistance was observed in KCNQ1 knockout mice in the absence of stimuli of acid secretion. This suggests that KCNQ1 channel activity contributes to the gastric epithelial cell membrane potential under basal conditions (as does the complete lack of acid secretion in the KCNQ1-deficient mucosa under basal conditions). Interestingly, after the addition of forskolin, transition to the acid-secreting state was accompanied by a measurable decrease in mucosal resistance, despite only minimal stimulation of acid secretion. The current study therefore suggests that the stimulation of an electrogenic pathway for Cl− secretion, as demonstrated by the increase in Isc, may also cause the drop in transmucosal resistance.
Furthermore, KCNQ1 deletion is accompanied by morphological changes in the parietal cell. These changes consist of hyperproduction of membranous material, which is found below the secretory membrane, and is also found in intracellular bodies. However, compared to the massive ultrastructural changes reported in the adult KCNQ1-deficient stomach, which we could confirm, the changes in the young KCNQ1-deficient parietal cells were mild, with no increase in mucosal thickness and no change in parietal cell number. In addition, normal-appearing canalicular membranes were present, making it feasible, and indeed successful, to directly test and prove the hypothesis that KCNQ1 channels are the primary K+ supply pathway to the H+/K+-ATPase.
The mRNA expression levels for H+/K+-ATPase and another apical K+ channel were decreased in KCNQ1-deficient gastric mucosa, despite preserved parietal cell number, H+/K+-ATPase staining intensity as determined by immunofluorescence, and mRNA expression of the basolateral AE2. This may reflect the futile increase in apical membrane turnover and may indicate early parietal cell damage.
One important new aspect in parietal cell physiology was the recent demonstration of KCC4 K+–2Cl− cotransporters in the parietal cell apical membrane in the resting state (Fujii et al. 2009). The authors speculated that KCC4, located in the resting state apical membrane, provides both K+ for K+ recycling via the H+/K+-ATPase, as well as secretory Cl− during unstimulated (‘resting state’) acid secretion. Upon stimulation, KCNQ1 and other K+ channels, as well as other Cl− channels, translocate into the apical membrane. Several lines of evidence argue against this hypothesis. Firstly, KCNQ1-deficient mucosa had no basal acid secretion. Secondly, resting state KCNQ1-deficient mucosa had a significantly higher electrical resistance than WT mucosa, suggesting that some KCNQ1 channels are in the apical membrane under resting state conditions. Nevertheless, the potential contribution of apical KCC4 to gastric acid secretion is an interesting concept (possibly as a Cl− provision model) and needs verification in the KCC4 knockout mouse model.
In summary, this study demonstrates that KCNQ1 channels are the major apical K+ channels for the provision of K+ to the luminal membrane of the secretory canaliculus in the ‘resting state’ as well as during stimulated acid secretion, and that no other of the parietal cell K+ channels can adequately substitute for this important physiological function. Further studies related to the molecular mechanisms of apical trafficking and activation patterns will increase our understanding on the complex molecular physiology of the parietal cell acid secretory process.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft Sachbeihilfe SE460/9-5 and 9-6 and by the Lower Saxony Ministry of Science and Education. We thank Professor Karl Pfeifer, NIH, Bethesda, for providing the KCNQ1 heterozygote breeding pair, Ulrike Dringenberg, Janina Bonhagen and Brigitte Rausch for their help with breeding and genotyping of mice, and help with the Ussing-chamber experiments.
Author contributions
For this project, P.S, S.G., B.R., A.S. and U.S. designed, performed and interpreted experiments and analysed data, Z.F. and A.K. performed experiments, and P.S., S.G. and U.S. wrote the manuscript.
Author's present address
P. Song: Key Lab of Combined Multi-Organ Transplantation, Ministry of Public Health, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, P.R. China.
References
- Aihara T, Nakamura E, Amagase K, Tomita K, Fujishita T, Furutani K, Okabe S. Pharmacological control of gastric acid secretion for the treatment of acid-related peptic disease: past, present, and future. Pharmacol Ther. 2003;98:109–127. doi: 10.1016/s0163-7258(03)00015-9. [DOI] [PubMed] [Google Scholar]
- Berglindh T. Absolute dependence on chloride for acid secretion in isolated gastric glands. Gastroenterology. 1977;73:874–880. [PubMed] [Google Scholar]
- Coskun T, Baumgartner HK, Chu S, Montrose MH. Coordinated regulation of gastric chloride secretion with both acid and alkali secretion. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1147–G1155. doi: 10.1152/ajpgi.00184.2002. [DOI] [PubMed] [Google Scholar]
- Cuppoletti J, Sachs G. Regulation of gastric acid secretion via modulation of a chloride conductance. J Biol Chem. 1984;259:14952–14959. [PubMed] [Google Scholar]
- Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Arch. 2001;442:896–902. doi: 10.1007/s004240100609. [DOI] [PubMed] [Google Scholar]
- Forte JG. K+ channels in the secretory membrane of the parietal cell. Focus on ‘Gastric parietal cell secretory membrane contains PKA- and acid-activated Kir2.1 K+ channels’. Am J Physiol Cell Physiol. 2004;286:C478–C479. doi: 10.1152/ajpcell.00531.2003. [DOI] [PubMed] [Google Scholar]
- Forte JG, Forte TM, Heinz E. Isolation of plasma membranes from Ehrlich ascites tumor cells. Influence of amino acids on (Na++K+)-ATPase and K+-stimulated phosphatase. Biochim Biophys Acta. 1973;298:827–841. doi: 10.1016/0005-2736(73)90387-8. [DOI] [PubMed] [Google Scholar]
- Forte JG, Hanzel DK, Okamoto C, Chow D, Urushidani T. Membrane and protein recycling associated with gastric HCl secretion. J Intern Med Suppl. 1990;732:17–26. doi: 10.1111/j.1365-2796.1990.tb01467.x. [DOI] [PubMed] [Google Scholar]
- Forte JG, Machen TE. Transport and electrical phenomena in resting and secreting piglet gastric mucosa. J Physiol. 1975;244:33–51. doi: 10.1113/jphysiol.1975.sp010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Takahashi Y, Ikari A, Morii M, Tabuchi Y, Tsukada K, Takeguchi N, Sakai H. Functional association between K+-Cl– cotransporter-4 and H+,K+-ATPase in the apical canalicular membrane of gastric parietal cells. J Biol Chem. 2009;284:619–629. doi: 10.1074/jbc.M806562200. [DOI] [PubMed] [Google Scholar]
- Fujita A, Horio Y, Higashi K, Mouri T, Hata F, Takeguchi N, Kurachi Y. Specific localization of an inwardly rectifying K+ channel, Kir4.1, at the apical membrane of rat gastric parietal cells; its possible involvement in K+ recycling for the H+-K+-pump. J Physiol. 2002;540:85–92. doi: 10.1113/jphysiol.2001.013439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahammer F, Herling AW, Lang HJ, Schmitt-Graff A, Wittekindt OH, Nitschke R, Bleich M, Barhanin J, Warth R. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology. 2001;120:1363–1371. doi: 10.1053/gast.2001.24053. [DOI] [PubMed] [Google Scholar]
- Heitzmann D, Grahammer F, von Hahn T, Schmitt-Graff A, Romeo E, Nitschke R, Gerlach U, Lang HJ, Verrey F, Barhanin J, Warth R. Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells. J Physiol. 2004;561:547–557. doi: 10.1113/jphysiol.2004.075168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzmann D, Warth R. No potassium, no acid: K+ channels and gastric acid secretion. Physiology (Bethesda) 2007;22:335–341. doi: 10.1152/physiol.00016.2007. [DOI] [PubMed] [Google Scholar]
- Hirst BH, Forte JG. Redistribution and characterization of (H++ K+)-ATPase membranes from resting and stimulated gastric parietal cells. Biochem J. 1985;231:641–649. doi: 10.1042/bj2310641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Schofield GC. Studies on the depletion and accumulation of microvilli and changes in the tubulovesicular compartment of mouse parietal cells in relation to gastric acid secretion. J Cell Biol. 1974;63:364–382. doi: 10.1083/jcb.63.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam SM, Forte JG. Inhibiting gastric H+/K+ATPase activity by omeprazole promotes degeneration and production of parietal cells. Am J Physiol Gastrointest Liver Physiol. 1994;266:G745–G758. doi: 10.1152/ajpgi.1994.266.4.G745. [DOI] [PubMed] [Google Scholar]
- Kaufhold MA, Krabbenhoft A, Song P, Engelhardt R, Riederer B, Fahrmann M, Klocker N, Beil W, Manns M, Hagen SJ, Seidler U. Localization, trafficking, and significance for acid secretion of parietal cell Kir4.1 and KCNQ1 K+ channels. Gastroenterology. 2008;134:1058–1069. doi: 10.1053/j.gastro.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Lambrecht NW, Yakubov I, Scott D, Sachs G. Identification of the K efflux channel coupled to the gastric H-K-ATPase during acid secretion. Physiol Genomics. 2005;21:81–91. doi: 10.1152/physiolgenomics.00212.2004. [DOI] [PubMed] [Google Scholar]
- Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, et al. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest. 2000;106:1447–1455. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel N, Pace AJ, Spiegel S, Engelhardt R, Koller BH, Seidler U, Lytle C. Role of Na-K-2Cl cotransporter-1 in gastric secretion of nonacidic fluid and pepsinogen. Am J Physiol Gastrointest Liver Physiol. 2005;289:G550–G560. doi: 10.1152/ajpgi.00095.2005. [DOI] [PubMed] [Google Scholar]
- Machen TE, McLennan WL. Na+-dependent H+ and Cl– transport in in vitro frog gastric mucosa. Am J Physiol Gastrointest Liver Physiol. 1980;238:G403–G413. doi: 10.1152/ajpgi.1980.238.5.G403. [DOI] [PubMed] [Google Scholar]
- McLennan WL, Machen TE, Zeuthen T. Ba2+ inhibition of electrogenic Cl– secretion in vitro frog and piglet gastric mucosa. Am J Physiol Gastrointest Liver Physiol. 1980;239:G151–G160. doi: 10.1152/ajpgi.1980.239.3.G151. [DOI] [PubMed] [Google Scholar]
- Malinowska DH, Sherry AM, Tewari KP, Cuppoletti J. Gastric parietal cell secretory membrane contains PKA- and acid-activated Kir2.1 K+ channels. Am J Physiol Cell Physiol. 2004;286:C495–C506. doi: 10.1152/ajpcell.00386.2003. [DOI] [PubMed] [Google Scholar]
- Roepke TK, Anantharam A, Kirchhoff P, Busque SM, Young JB, Geibel JP, Lerner DJ, Abbott GW. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem. 2006;281:23740–23747. doi: 10.1074/jbc.M604155200. [DOI] [PubMed] [Google Scholar]
- Rossmann H, Bachmann O, Wang Z, Shull GE, Obermaier B, Stuart-Tilley A, Alper SL, Seidler U. Differential expression and regulation of AE2 anion exchanger subtypes in rabbit parietal and mucous cells. J Physiol. 2001;534:837–848. doi: 10.1111/j.1469-7793.2001.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs G, Chang HH, Rabon E, Schackman R, Lewin M, Saccomani G. A nonelectrogenic H+ pump in plasma membranes of hog stomach. J Biol Chem. 1976;251:7690–7698. [PubMed] [Google Scholar]
- Sachs G, Spenney JG, Lewin M. H+ transport: regulation and mechanism in gastric mucosa and membrane vesicles. Physiol Rev. 1978;58:106–173. doi: 10.1152/physrev.1978.58.1.106. [DOI] [PubMed] [Google Scholar]
- Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, Rexhepaj R, Gerlach U, Rong Q, Pfeifer K, Lang F. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci U S A. 2005;102:17864–17869. doi: 10.1073/pnas.0505860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldegger S. Heartburn: cardiac potassium channels involved in parietal cell acid secretion. Pflugers Arch. 2003;446:143–147. doi: 10.1007/s00424-003-1048-5. [DOI] [PubMed] [Google Scholar]